Abstract
Background:
Previous studies show that aberrant synthesis of Hyaluronan accelerates tumor growth, angiogenesis, and metastasis. The fibroblasts are probably responsible for most of the hyaluronic acid (HA) accumulation in tumor microenvironment after radiotherapy. Our goal is to investigate and compare radiation and lactate effects on HA levels in supernatant and exosome isolated from supernatant of primary mouse fibroblast cell culture.
Methods:
Fibroblast cells were prepared from skin of C57BL6 mouse. These cells were divided into three groups (no treatment, cells treated with 10 mM ammonium lactate, and irradiated cells). Then supernatant was harvested from FBS-free culture media after 48 h. Exosomes were purified by differential centrifugation (300 × g for 10 min, 2000 × g for 30 min, 16500 g for 30 min) and were pelleted by ultracentrifugation (150,000 × g for 180 min). Size of exosomes was determined using a Zetasizer. HA concentration measured using a HA ELISA Kit. Data were analyzed using one-way ANOVA.
Results:
There was a significant increase in HA-coated exosomes isolated from supernatants of irradiated cells compared to untreated cell and cells treated with 10 mM ammonium lactate (P < 0.001). As well, there was a significant increase in the HA concentration in the supernatants of cells treated with 10 mM ammonium lactate relative to untreated cells and irradiated cells (P < 0.05).
Conclusions:
It seems that routine radiation therapy leads to massive shedding of HA-coated exosomes by normal fibroblast cells and thus exosomes-HA may contribute to tumor promotion and induce of the premetastatic niche.
Keywords: Exosomes, hyaluronic acid, radiation
Introduction
The tumor initiation, growth, invasion, and metastasis are an outcome of a complex process between the host and cancer cells. In addition to immune cells and cells of the vasculature, fibroblast cells have a significant role in different steps in cancer development. The recent studies show that fibroblasts receive and provide pro-tumoral paracrine signaling. Therefore, these cells are involved in tumor initiation, early progression, and various steps of the metastatic process.[1,2,3]
Hyaluronan (or hyaluronic acid, HA) as a component of the extracellular matrix is an un-sulfated anionic linear glycosaminoglycan polymer. It is composed of repeating glucuronic acid and N-acetyl-glucosamine disaccharide units.[4] Multiple physiological roles have been suggested for HA, including embryogenesis, tissue remodeling, and wound healing.[5] As well, HA is involved in tumorigenesis promotion and progression. Hence, this polysaccharide allows cancer cell proliferation, malignant transformation, migration, angiogenesis, invasion, and metastasis.[6,7,8,9] High expression of HA is a predictive marker of tumors malignancy. The stromal fibroblasts are the main producers of HA at tumor microenvironment.[10]
The cell-derived microvesicles are phospholipid bilayer-enclosed vesicles that are secreted into biological fluids by different cell types.[11,12] Exosomes, nanovesicles of 30–100 nm in diameter, are released by exocytosis of multivesicular bodies by direct budding of the plasma membrane. EVs participate in intercellular communication by carrying different membrane and cytosolic molecules, such as proteins, lipids and genetic material (RNA, DNA, miRNA).[13,14] The elevated EVs counts are associated with metastasis, thrombosis and angiogenesis. The exosomes implicate as serious messengers in tumor progression and metastasis.[15] Exosomes have recently served as novel delivery vehicles for targeted transferring various molecules to tumor cells. Therefore, identification of cancer markers, for example CD44 ligand, HA, can be used for targeted therapeutic strategies.[16,17]
Some studies report that cancer cells produce lactate by anaerobic metabolism and the lactate induces HA expression by tumor stroma.[16,18,19] On the other word, most common cancer types can be treated by radiation therapy to control cancer cell growth. However, ionizing radiation influences both normal and cancerous cells.[17,20] Although it seems that radiation delay tumor progression, its effects on tumor stromal cells are not clear yet. In current study, we evaluated and compared radiation and lactate effects on HA levels as a pro-cancerous factor in culture supernatant of primary mouse fibroblast cells and exosome isolated from culture supernatant of primary mouse fibroblast cells.
Methods
Cell preparation and culture
Fresh animal skin specimens were obtained from C57BL6 mouse aged from 6 to 8 weeks. Mouse were killed and placed on 70% alcohol for 15 min. The specimens were washed with sterile phosphate-buffered saline (PBS), and the subcutaneous tissues were carefully removed. Then the skins were cut into small pieces (1–2 mm3). The skin specimens were digested with 0.1% Dispase (Sigma, United States) at 4°C overnight, the epidermal layers were removed and the remaining dermal parts were further digested with 0.1% collagenase I (Sigma, United States) at 37°C for another 4 h. The digested tissues were centrifuged and suspended in high-glucose DMEM (Bio Idea, Tehran, Iran). Tissue fragments were cultured in DMEM supplemented with 10% FBS (Bio Idea, Tehran, Iran), 300 μg/m1 L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Bio Idea, Tehran, Iran) for about 7 days until Fibroblasts grew out of the tissue. Then, cells were removed and cultured in T25 flask at 37°C in a humidified atmosphere with5% CO2. When cultured cells reached more than 90% of confluence, the culture medium were removed and replaced with FBS free medium. These cells were divided into three groups: group 1 (control, no treatment), group 2 (cells treated with 10 mM ammonium lactate for 48 h) and group 3 (irradiated cells). The cell culture supernatant was collected from each group after 48 h.
Irradiation procedure
Irradiation was done using a 6 MV linear accelerator Siemens by a clinically calibrated irradiation field of 20 × 20 cm. The irradiation dose was 6 Gy. For plating after irradiation, cell culture flasks were filled with FBS free medium and irradiated horizontally in an upright position. For plating before irradiation, the plates were placed horizontally.[21] After radiation, cell culture supernatant was harvested after 48 h.
Exosome purification
The supernatant was cleared of cells, debris and non-exosome vesicles by some sequential centrifugations (300 × g for 10 min, 2000 × g for 30 min, 16500 × g for 30 min). Exosomes were pelleted by ultracentrifugation (Beckman Coulter, Ti90 rotor) at 150,000 × g for 180 min. The exosome pellet was washed once in a large volume of PBS by ultracentrifugation at 150,000 × g for 180 min and resuspended in 200 μl PBS.
Size determination
Exosomes were diluted 1:50 in PBS and then poured into the 4-sided clear cuvette to determine size of exosomes using a Zetasizer (Malvern Zen 3600 Instruments, UK, Figure 1).
Figure 1.
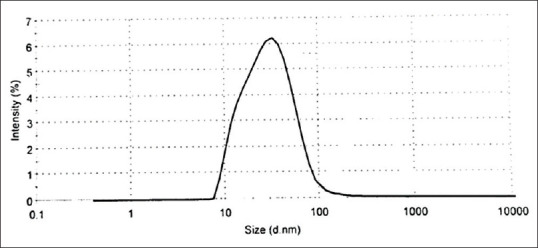
The size of particles in the pellets was determined using a Zetasizer. The z-averageparticlesize was 37.62 nm in diameter
Measurement of HA by ELISA
The HA concentration in supernatant and exosomes isolated from supernatant of each group was measured using a HA ELISA Kit (R&D Systems, USA). The ELISA was performed according to the manufacturer's instructions.
Statistics
Experimental results were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using one-way ANOVA (P < 0.05) to determine statistically significant differences among groups. IBM SPSS Statistics version 21 was used for data analysis.
Results
Confirmation of plasma-derived exosomes
The exosomes isolated from cell culture supernatant, as illustrated in Figure 1, were evaluated by Zetasizer. The Zetasizer demonstrated the z-average of peak size of exosomes was 37.62 nm in diameter.
The effects of radiation and ammonium lactate on HA concentration
HA levels in the cell culture supernatants and exosomes isolated from cell culture supernatants were determined by ELISA in each group. HA levels were evaluated after 48 h in the FBS-free media. There was a significant difference in HA concentration in cell culture supernatants and exosomes isolated from cell culture supernatants (P = 0.048 and P = 0.0001, one-way ANOVA). The significant increase was observed in HA-coated exosomes isolated from supernatants of irradiated cells compared to untreated cell and cells treated with 10 mM ammonium lactate (P = 0.0001, LSD Post-Hoc). As well, there was a significant increase in the HA concentration in the supernatants of cells treated with 10 mM ammonium lactate compared to untreated cells and irradiated cells (P = 0.026 and P = 0.037, LSD Post-Hoc).
Furthermore, the HA-coated exosomes isolated from supernatants was less than the cell culture supernatants in untreated cell and cells treated with 10 mM ammonium lactate (P = 0.0001 and P = 0.007). However, the HA-coated exosomes isolated from supernatants was more than the cell culture supernatants in the irradiated cell (P = 0.015). Data are shown in Figure 2.
Figure 2.
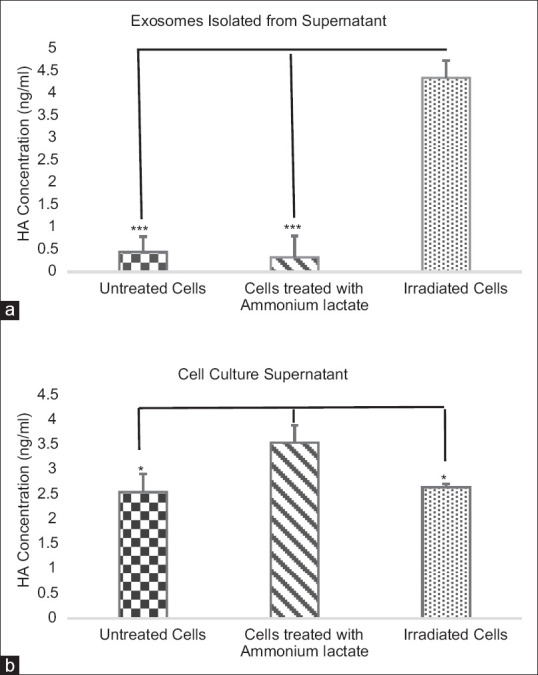
Effect of radiation and ammonium lactate on the HA concentration. After 48 h, the media were harvested and HA concentration was measured by ELISA. Statistical relationships were shown in cells treated with ammonium lactate and irradiated cells compared with untreated cells in exosomes isolated from supernatant (a) and supernatant (b). Results were expressed as the mean ± standard deviation (SD). ***P < 0.001 and *P < 0.05 showed significant differences
Discussion
In current study, we observed an increase in HA-coated exosomes isolated from cell culture supernatant after radiation of mouse fibroblast cell. A review study indicates that radiation-derived exosomes promote tumor, reduce survival, and lead to radio-resistance. Furthermore, radiation can change the contents of exosomes.[22] Therefore, release of HA-coated exosomes from mouse fibroblast cell after radiation may promote tumor and reduce survival.
As it was mentioned many tumors increase HA content and release of EVs. HA–EVs indicate the missing link between HA and cancer. Previous findings demonstrate that HA is present in the stroma and around tumor cells in many epithelial cancer types like breast, ovarian, and prostate cancers.[23,24,25] Some studies show the apoptotic efficiency of chemotherapy drugs is decreased by tumor-associated HA.[26,27] The massive shedding EVs by tumor cells could explain that the induction of the premetastatic niche is depend on the HA accumulation and the subsequent attachment to CD44-positive adjacent stroma, or tissues at more distant sites.[28,29] Therefore, increased HA-coated exosomes by irradiated fibroblast cells might be responsible for HA accumulation and preparation of the premetastatic niche.
Some studies show that cancer cells in malignant tumors like breast cancer depend on high-glucose uptake and the elevated intracellular glucose level lead to the increased production of HA.[18,30] Thus, high blood glucoseand HA accumulation in the tumor–stroma of breast cancer predict progression and metastasis of the tumor.[31,32]
We observed increased concentration of HA in mouse fibroblast cells treated with 10 mM ammonium lactate. Similarly, some studies demonstrate that HA and CD44 expression are regulated by lactate levels in fibroblasts.[16] As well, tumor cells can provide a lactate-rich environment that stimulates HA production by the surrounding fibroblasts. Therefore, lactate as a well-known stimulus of HA expression could promote malignant progression.[16,33]
There were some limitations in the current study. It is necessary to treat C57B16 mouse with ammonium lactate and gamma radiation and then investigate HA-coated exosomes. In addition, a study should be done for better evaluation of effect of HA-coated exosomes secreted from normal C57B16 mouse fibroblasts on tumor growth and metastasis in vitro. As well, we could use HA-coated exosomes as a vehicle for delivery of anti-tumor agent.
Conclusions
Despite critical role of radiation at cancer therapy, routine radiation therapy may contribute to shedding of massive exosomes coated with HA by normal fibroblast cells. These HA-coated exosomes could influence tumor promotion and induce of the premetastatic niche. In addition, lactate could increase HA concentration in tumor microenvironment that it might be led to tumor progression. However, it is necessary to evaluate radiation effect on HA expression in tumor microenvironment in vivo.
Financial support and sponsorship
Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by Research Department of Isfahan University of Medical Sciences (194227).
References
- 1.Östman A, Augsten M. Cancer-associated fibroblasts and tumor growth - bystanders turning into key players. Curr Opin Genet Dev. 2009:1967–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Pietras K, Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 3.Strell C, Rundqvist H, Östman A. Fibroblasts-a key host cell type in tumor initiation, progression, and metastasis. Ups J Med Sci. 2012;117:187–95. doi: 10.3109/03009734.2012.654859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slevin M, Krupinski J, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- 5.Constant S, Huang S, Wisniewski L, Mas C. Advanced human in vitro models for the discovery and development of lung cancer therapies. Drug Discovery and Development-From Molecules to Medicine In Tech. 2015 [Google Scholar]
- 6.Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99:1720–5. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourguignon LYW, Hongbo Z, Shao L, Chen YW. CD44 interaction with Tiam1 promotes Rac1 signaling and hyaluronic acid- mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–38. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- 8.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–63. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 9.Golshani R, Lopez L, Estrella V, Kramer M, Iida N, Lokeshwar VB. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008;68:483–91. doi: 10.1158/0008-5472.CAN-07-2140. [DOI] [PubMed] [Google Scholar]
- 10.Knudson W, Biswas C, Li XQ, Nemec RE, Toole BP. The role and regulation of tumour-associated hyaluronan. Ciba Found Symp. 1989;143:150–9. doi: 10.1002/9780470513774.ch10. [DOI] [PubMed] [Google Scholar]
- 11.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:1–60. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van DerPol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 14.Rilla K, Siiskonen H, Tammi M, Tammi R. Hyaluronan-coated extracellular vesicles- A novel link between hyaluronan and cancer. Adv Cancer Res. 2014;123:121–48. doi: 10.1016/B978-0-12-800092-2.00005-8. [DOI] [PubMed] [Google Scholar]
- 15.Odintsova E, Van Niel G, Conjeaud H, Raposo G, Iwamoto R, Mekada E, et al. Metastasis suppressor tetraspanin CD82/KAI1 regulates ubiquitylation of epidermal growth factor receptor. J Biol Chem. 2013;288:26323–34. doi: 10.1074/jbc.M112.439380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: The Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 17.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci. 2012;9:193–9. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auvinen P, Tammi R, Parkkinen J, Tammi M, Šgren U, Johansson R, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–36. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponting J, Kumar S, Pye D. Co-localisation of hyaluronan and hyaluronectin in normal and neoplastic breast tissues. Int J Oncol. 1993;2:889–93. doi: 10.3892/ijo.2.6.889. [DOI] [PubMed] [Google Scholar]
- 20.Prasanna PGS, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: Where are the Gaps? Transl Cancer Res. 2012;1:35–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Buch K, Peters T, Nawroth T, Sänger M, Schmidberger H, Langguth P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay - A comparative study. Radiat Oncol. 2012;7:1. doi: 10.1186/1748-717X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y. Exosomes in cancer radioresistance. Front Oncol. 2019 doi: 10.3389/fonc.2019.00869. doi: 103389/fonc 201900869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–5. [PubMed] [Google Scholar]
- 24.Josefsson A, Adamo H, Hammarsten P, Granfors T, Stattin P, Egevad L, et al. Prostate cancer increases hyaluronan in surrounding nonmalignant stroma, and this response is associated with tumor growth and an unfavorable outcome. Am J Pathol. 2011;179:1961–8. doi: 10.1016/j.ajpath.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipponen P, Aaltomaa S, Tammi R, Tammi M, Šgren U, Kosma VM. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur J Cancer. 2001;37:849–56. doi: 10.1016/s0959-8049(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardelli C, Ween MP, Lokman NA, Tan IA, Pyragius CE, Oehler MK. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer. 2013;13:476. doi: 10.1186/1471-2407-13-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toole BP, Slomiany MG. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244–50. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(PDF) Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. [PMC free article] [PubMed] [Google Scholar]
- 29.Jung T, Castellana D, Klingbeil P, Hernández IC, Vitacolonna M, Orlicky DJ, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenza GL. Tumor metabolism: Cancer cells give and take lactate. J Clin Investig. 2008;118:3835–7. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auvinen P, Rilla K, Tumelius R, Tammi M, Sironen R, Soini Y, et al. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat. 2014;143:277–86. doi: 10.1007/s10549-013-2804-7. [DOI] [PubMed] [Google Scholar]
- 32.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 33.Miletti-González KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–7. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]


