Abstract
Background:
Impaired fasting plasma glucose (IFG) as well as diabetes mellitus (DM) may influence the presence of another metabolic syndrome (MetS) components resulting in the different risk of cardiovascular (CV) morbidity and mortality. This study aimed to determine the impact of IFG as well as DM on the 10-year CV risk using Thai CV risk score and primary prevention in complying with CV risk score in these patients.
Methods:
This cross-sectional study was conducted at the internal medicine clinic, Pathum Thani Hospital, Thailand. The study was approved by the hospital ethics committee and written informed consent was obtained from all patients. Patients having MetS according to the criteria of the International Diabetes Federation were enrolled while those with a history of CVD were excluded. The 10-year CV risk was assessed using the Thai CV risk score.
Results:
The total of 112 patients were enrolled in the study. They were in old age and female sex was a significantly higher proportion (61.70% vs 35.50%, P = 0.013). Of these, 72.32% had IFG or DM. Proportions of patients with moderate and high CV risk score were significantly greater in IFG/DM group and only 34.48% and 79.31% of patients with moderate or high CV risk score received aspirin and statin. IFG or DM significantly elevated CV risk score (OR = 6.66, 95% CI = 2.29, 19.58).
Conclusions:
IFG/DM significantly elevated CV risk score in these patients with the strongest impact. The assessment of CV risk is highly recommended for primary prevention and long-term CVD benefit.
Keywords: Cardiovascular diseases, diabetes mellitus, metabolic syndrome, risk assessment
Introduction
The metabolic syndrome (MetS) is the multiplex risk factor of cardiovascular disease (CVD). It was estimated that 25% of the adult population worldwide have MetS similar to 24% of the Thai population.[1,2] These patients carried three times higher risk of CVD compared to those without MetS.[1] Impaired fasting plasma glucose (IFG) was found in 7.1% of patients with MetS and kept increasing with age up to 77.2% in the aging population.[3,4,5] IFG is the risk factor for diabetes mellitus (DM) and CVD and increased risk of cardiovascular mortality as well as all-cause mortality.[6]
Genetic factors were postulated as the common risk factors of CVD and DM. Impaired intracellular transport of cholesterol in type 2 DM was caused by Apo B gene mutations, while the atherosclerotic plaque was associated with high-mobility group A (HMGA1), a group of chromosomal proteins. Moreover, there was a correlation between DNA methylation and obesity as well as CV risk. There were many gene expressions associated with vascular damages. Reactive oxygen species induced epigenetic modifications are associated with endothelial dysfunction and vascular damages. Abnormal microRNA (miRNA) expression also led to endothelial dysfunction, loss of vascular smooth muscle cell contractility and proliferative; and migratory properties, accelerating the onset of CVD. In addition, abnormal long noncoding RNA (lncRNAs) expression led to microvascular and macrovascular damages, promoting endothelial dysfunction. Furthermore, the downregulation of miRNA increased platelet reactivity and aggregation while the upregulation of SREBP-1 and FAAS genes by miRNA caused abnormal cholesterol homeostasis, increased fatty acid, and triglyceride synthesis. Reduction of miR-26a increased fatty acid synthesis and obesity-related metabolic complications. IFG and type 2 DM were found to be significantly associated with single nucleotide polymorphisms (SNPs). In the IFG and type 2 DM patients, HbA1c, homeostatic model assessment for insulin resistance (HOMA-IR), 8-epi-prostaglandin F2α (8-epi-PGF2α) and plasma malondialdehyde (MDA) showed significant positive correlations with weighted genetic risk score (wGRS).[7]
The study in the Korean population showed that the wGRS constructed from SNP has been associated with the fasting plasma glucose (FPG) levels, HbA1c levels and type 2 DM. Furthermore, it was strongly and significantly associated with the concentrations of urinary 8-epi-PGF2α and MDA, the lipid peroxidation biomarker and oxidative stress.[8]
Several estimators for the 10-year cardiovascular (CV) risk were constructed especially, in European countries. However, it was overestimated in the Thai population due to different risk profiles among ethnics. Consequently, the Thai CV risk score was developed. Therefore, this study aimed to determine the impact of IFG as well as DM on the 10-year CV risk using Thai CV risk score and primary prevention in complying with CV risk score in these patients.
Methods
Study design and study participants
This cross-sectional study was conducted at the internal medicine clinic at the outpatient department, Pathum Thani Hospital, Thailand. The study was approved by the hospital ethics committee (0032.203.3/17159). Study information was provided by verbal and participant information sheets before obtaining written informed consent. None of the participants' names, initials, or hospital numbers were mentioned elsewhere except in the logbook.
We enrolled patients having the MetS according to the criteria of the International Diabetes Federation (IDF) 2006.[1] Patients previously diagnosed with CVD were excluded.
The primary objective of the study was to determine the impact of IFG as well as DM on the 10-year CV risk. According to the previous study, impaired fasting plasma glucose (IFG) was found in 7.1% of patients with MetS.[3] Therefore the prevalence of 7% is estimated to obtain from this study, with a 95% confidence interval (CI) and the precision to be within 5% of the true value with expected 10% loss to follow-up.
The sample size calculation is stated as below:
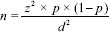
whereas:
n = sample size
z = 1.96 (α = 0.05)
p = the prevalence of IFG
d = error allowance
A required sample size of at least 110 MetS patients was enrolled in our study.
Study Procedures
A simple random sampling method was used in the present study. The patients who met the eligible criteria were randomly selected. A face-to-face interview was conducted to obtain sociodemographic characteristics. Clinical characteristics were obtained from the medical records and laboratory data were obtained from laboratory reports within 3 months preceding the interview. The 10-year CV risk was assessed using Thai CV risk score, the online calculator; available from https://med.mahidol.ac.th/cardio_vascular_risk/thai_cv_risk_score/tcvrs_en.html. The model variables consist of age, sex, total cholesterol, DM, BP, and smoking. Waist circumstance was considered if total cholesterol was not available. The risk categories used to describe the probability of CVD within the next 10 years were as follow: <10%: low; 10– <20%: moderate; 20– <30%: high risk; 30– <40%: very high risk and >40%: extremely high risk.
Statistical analysis
Data analysis was carried out using a statistical software package SPSS 15.0 (SPSS, Chicago, IL). Categorical variables were summarized as frequencies and percentages and then analyzed using the Chi-square test or Fisher's exact test. Continuous variables were summarized as mean and 95% CI or median and interquartile range (IQR) values and compared using t-test or the Mann Whitney U-test where appropriate. All variables with a statistically significant relationship with IFG/DM were included in the logistic regression model and the variables with statistical significance indicated “the association” to the 10-year CV risk score. All tests for significance were two-sided and P < 0.05 was considered the statistical significance.
Results
Table 1 describes the sociodemographic characteristics of patients. A total of 112 patients were enrolled in the study. Patients with IFG/DM were slightly older than those without IFG/DM (60.51 vs 58.58 years old, P = 0.589) and female patients were predominant and significantly higher in proportion in IFG/DM group (50 (61.70%) vs 11 (35.50%), P = 0.013). Proportion of patients with unemployment (50 (61.70%) vs 10 (32.30%), P = 0.005) and having low income were significantly higher in IFG/DM group (52 (64.20%) vs 11 (35.50%), P = 0.006).
Table 1.
Sociodemographic characteristics of patients with MetS (n=112)
| Characteristics | Impaired fasting glucose/diabetes mellitus | ||
|---|---|---|---|
| Yes (%); n=81 | No (%); n=31 | P | |
| Age (mean; 95% CI) | 60.51; 58.19, 62.82 | 58.58; 55.16, 62.00 | 0.589 |
| Female | 50 (61.70) | 11 (35.50) | 0.013 |
| BMI (median; IQR) | 29.78; 26.64-34.68 | 27.11; 24.98-31.22 | 0.123 |
| Occupation: housewife/retired | 50 (61.70) | 10 (32.30) | 0.005 |
| Low income* | 52 (64.20) | 11 (35.50) | 0.006 |
| Low education† | 78 (89.70) | 21 (84.00) | 0.482 |
| Married | 60 (69.00) | 19 (76.00) | 0.497 |
| Smoker | 4 (4.90) | 10 (32.30) | 0.511 |
| Alcohol | 26 (32.10) | 15 (48.80) | 0.109 |
| Family history of CVD | 6 (7.40) | 2 (6.50) | 1.000 |
*Less than national income per capita, †Less than basic education, CI=Confident interval, IQR=Inter quartile range
Table 2 described the 10-year CV risk score of patients with metabolic syndrome. Proportions of patients with moderate and high CV risk scores were significantly greater in IFG/DM group. Figure 1 illustrates primary prevention by aspirin or statins stratified by the 10-year CV risk score. Of 58 patients with a moderate or high CV risk score, 20 patients (34.48%) and 46 patients (79.31%) received ASA and statin, respectively. Table 3 described multiple logistic regression analysis of the variables associated with the 10-year CV risk score. IFG/DM showed the strongest association with CV risk in patients with MetS (OR = 6.69, 95% CI = 2.28, 19.58) followed by raised BP or HPT component (OR = 4.42, 95% CI = 1.48, 13.18).
Table 2.
The 10-year CV risk score of patients with MetS
| The 10-year CV risk score | IFG/DM | P | |
|---|---|---|---|
| Yes (%); n=81 | No (%); n=31 | ||
| CV risk score | 0.001 | ||
| <10% | 31 (38.30) | 23 (74.20) | <0.001* |
| 10-<20% | 39 (48.10) | 8 (25.80) | 0.040* |
| 20-<30% | 11 (13.60) | 0 | 0.030* |
| Male | 31 (60.78) | 20 (39.22) | 0.062 |
| <10% | 11 (35.48) | 12 (60.00) | 0.090† |
| 10- <20% | 14 (45.16) | 8 (40.00) | 0.720† |
| 20- <30% | 6 (19.35) | 0 | 0.040† |
| Female | 50 (81.97) | 11 (18.03) | 0.002 |
| <10% | 20 (40.00) | 11 (100.00) | <0.001† |
| 10-<20% | 25 (50.00) | 0 | <0.001† |
| 20-<30% | 5 (10.00) | 0 | 0.280† |
*Adjusted P; Bonferroni corrected P<0.008 indicated significance. †Adjusted P; Bonferroni corrected P<0.004 indicated significance
Figure 1.
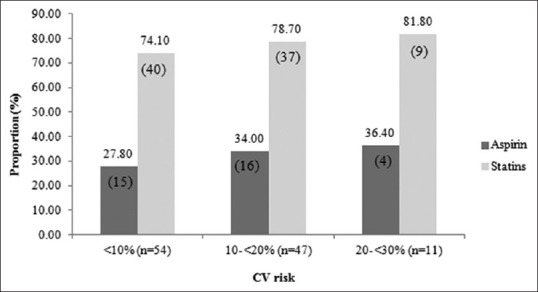
Primary prevention by aspirin or statins stratified by the 10-year CV risk score
Table 3.
Multiple logistic regression analysis of the variables associated with the 10-year CV risk score in patients with MetS
| Variables | Odd ratio (95% CI) | P |
|---|---|---|
| Raised FPG/DM | 6.69 (2.28, 19.58) | 0.001 |
| Raised BP/treatment of HPT | 4.42 (1.48, 13.18) | 0.006 |
| Male | 4.35 (14.29, 1.35) | 0.005 |
| Low income | 0.85 (0.11, 6.90) | 0.090 |
CI=Confident interval
Discussion
In patients with MetS, IFG or DM was associated with a moderate and high CV risk score. It was reported that the measurement of 10-year CV risk has been shown to decrease CV morbidity and mortality in US adults with diabetes.[9]
Though the assessment of CV risk is encouraged for all patients carrying risk factors, it is rarely performed in routine practice due to excessive workload. To overcome this situation, patients at highest risk should be identified. Since MetS components are the multiplex risk factors of CVD, the impact of each MetS component on CV risk should be identified to develop the screening criteria for CV risk assessment.
The prevalence of the IFG/DM component was higher in our study compared to the previous study according to IDF criteria (72.32% vs ~50%) because BMI cutoff for the Thai population was lower.[10,11] IFG/DM was the greatest CV risk consistent with earlier studies.[12,13] Hyperglycemia can cause cardiovascular complications.14 Therefore, well-controlled FPG should be encouraged for long-term cardiovascular benefit especially, in DM patients.[15] IFG was significantly associated with total CHD events with a stronger association in females compared to males contrasting to our study due to different definitions of MetS.[15] However, other previous study showed that IFG increased the risk of IHD in males but not in females which supported our findings.[16] Similarly, Thai males possessed greater risk factors of CVD than females such as smoking, drinking, and physical inactivity.[17] However, the results did show that MetS and DM, the strong risk factors of CVD, highlighted the need for CV risk assessment, regardless of other risk factors.[18]
In the previous study, the level of education showed the inverse relationship with Framingham's risk score while obesity significant increased Framingham's risk score different from this study.[19] Different diagnostic criteria for DM and MetS may be responsible for this discrepancy. Income level has been consistently associated with elevated CVD risk in European and Asian countries.[20] Financial barriers to obtaining healthcare critically influenced especially, in the socioeconomically disadvantaged group.[20] However, the universal health coverage provided to Thai people with low socioeconomic status can decrease the inequalities in obtaining healthcare services.
The 10-year CV risk assessment is strongly recommended in CVD naïve patients with MetS especially, in those having IFG/DM. Low-dose aspirin was recommended in those with a moderate or high CV risk score.[21] In addition, CVD naïve patients with the following criteria; 40–75 years of age, ≥1 CVD risk factors and 10-year CVD risk ≥10%; were recommended low-to-moderate dose of statin.[22] Interestingly, more than 60% and 20% of our patients with moderate or high CV risk score did not receive aspirin and statin, respectively. It is urgent to encourage healthcare professionals for routine CV risk assessment. Training patients for self-assessment could reduce healthcare professionals' workload and enhance primary prevention for CVD. In addition, a significant association between unemployed elderly patients and IFG/DM was found due to insulin resistance from less physical activity.[23]
It is challenging to establish the assessment of the 10-year CV risk as to the routine practice and the guidance for primary prevention. In addition, lifestyle therapy such as diet and physical activity should be implemented since they simultaneously reduce all components of MetS.[24] A systematic review of implementing these practices should be conducted. Furthermore, studies in different levels of the healthcare setting and the attitude of healthcare professionals toward primary prevention of CVD are fruitful.
It is important to be aware of some limitations. Recall bias was most likely since some information was obtained through interviews. Regarding gender differences, the impact of IFG/DM on CV risk score should be considered separately. Due to the selection bias, the impact of some variables on CV risk score may be misestimated.
Conclusions
In conclusion, IFG/DM was the strongest predictor of CV risk in CVD naïve patients with MetS. Thus, it can be the appropriate screening criteria for CV risk assessment in routine practice especially, in an ambulatory setting with excessive workload.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to express a special thanks to Dr. Arthit Ourairat, President of Rangsit University. We also thank Mrs. Suwipa Lueangaram for her support in this study.
References
- 1.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. c2006. [Last cited on 2018 Aug 5]. Available form: http://wwwidf org/webdata/docs/IDF_Meta_def_finalpdf Published .
- 2.Aekplakorn W, Chongsuvivatwong V, Tatsanavivat P, Suriyawongpaisal P. Prevalence of metabolic syndrome defined by the International diabetes federation and National cholesterol education program criteria among Thai adults. Asia Pac J Public Health. 2011;23:792–800. doi: 10.1177/1010539511424482. [DOI] [PubMed] [Google Scholar]
- 3.Morales DD, Punzalan FE, Paz-Pacheco E, Sy RG, Duante CA. Metabolic syndrome in the Philippine general population: Prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Dis Res. 2008;5:36–43. doi: 10.3132/dvdr.2008.007. [DOI] [PubMed] [Google Scholar]
- 4.Yen YF, Hu HY, Lin IF, Lai YJ, Su VY, Pan SW, et al. Associations of metabolic syndrome and its components with mortality in the elderly: A cohort study of 73,547 Taiwanese adults. Medicine. 2015;94:1–9. doi: 10.1097/MD.0000000000000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidaka T, Hayakawa T, Kakamu T, Kumagai T, Hiruta Y, Hata J, et al. Prevalence of metabolic syndrome and its components among Japanese workers by clustered business category. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0153368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation [Internet] c2006. [Last cited on 2018 Jan 31]. Available form: http://wwwwhoint/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_newpdf .
- 7.Rosa SD, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Daniela P, et al. Type 2 diabetes mellitus and cardiovascular disease: Genetic and epigenetic links. Front Endocrinol (Lausanne) 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M, Kim M, Huang L, Jee SH, Le JH. Genetic risk score of common genetic variants for impaired fasting glucose and newly diagnosed type 2 diabetes influences oxidative stress. Sci Rep. 2018;8:7828. doi: 10.1038/s41598-018-26106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes 2018. Diabetes Care. 2018;41:S3–157. doi: 10.2337/dc18-Sppc01. [DOI] [PubMed] [Google Scholar]
- 10.Pongchaiyakul C, Nguyen TV, Kosulwat V, Rojroongwasinkul N, Charoenkiatkul S, Pongchaiyakul C, et al. Defining obesity by body mass index in the Thai population: An epidemiologic study. Asia Pac J Clin Nutr. 2006;15:293–9. [PubMed] [Google Scholar]
- 11.Sharma SK, Ghimire A, Radhakrishnan J, Thapa L, Shrestha NR, Paudel N, et al. Prevalence of hypertension, obesity, diabetes, and metabolic syndrome in Nepal. Int J Hypertens. 2011;2011:1–9. doi: 10.4061/2011/821971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in beaver dam. Diabetes care. 2002;25:1790–4. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 13.Berezin A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab Syndr. 2016;10:S176–83. doi: 10.1016/j.dsx.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 15.Rachas A, Raffaitin C, Barberger-Gateau P, Helmer C, Ritchie K, Tzourio C, et al. Clinical usefulness of the metabolic syndrome for the risk of coronary heart disease does not exceed the sum of its individual components in older men and women.The Three-City (3C) study. Heart. 2012;98:650–5. doi: 10.1136/heartjnl-2011-301185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HK, Kim CH, Kim EH, Bae SJ, Choe J, Park JY, et al. Impaired fasting glucose and risk of cardiovascular disease in Korean men and women: The Korean heart study. Diabetes Care. 2013;36:328–35. doi: 10.2337/dc12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putadechakum S, Leelahakul V, Rodjinda P, Phanachet P, Roongpisuthipong C. Cardiovascular risk factors and 10-year risk for coronary heart disease in Thai adults. Int J Sci Res. 2014;4:1–7. [Google Scholar]
- 18.Peykari N, Saeedi Moghaddam S, Djalalinia S, Kasaeian A, Sheidaei A, Mansouri A, et al. High fasting plasma glucose mortality effect: A comparative risk assessment in 25-64 years old Iranian population. Int J Prev Med. 2016;7:75. doi: 10.4103/2008-7802.182732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udenze IC, Amadi CE. Cardiovascular disease risk assessment in Nigerian adults with type 2 diabetes and metabolic syndrome using the Framingham's risk score. Int J Non-Commun Dis. 2018;3:15–20. [Google Scholar]
- 20.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes. Circulation. 2018;137:2166–78. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3671–89. doi: 10.1210/jc.2008-0222. [DOI] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K. Statin use for the primary prevention of cardiovascular disease in adults, US Preventive Services Task Force Recommendation statement. JAMA. 2016;316:1997–2007. doi: 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 23.Zhan Y, Yu J, Chen R, Gao J, Ding R, Fu Y, et al. Socioeconomic status and metabolic syndrome in the general population of China: A cross-sectional study. BMC Public Health. 2012;12:921. doi: 10.1186/1471-2458-12-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma P, Srivastava RK, Jain D. Association of lifestyle risk factors with metabolic syndrome components: A cross-sectional study in Eastern India. Int J Prev Med. 2018;9:6. doi: 10.4103/ijpvm.IJPVM_236_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


