Abstract
Background:
The aim of this study is to compare the antenatal care, body weight, and weight gain in pregnancy between the adolescent and adult pregnancies and, thus, examine the impact of adolescence on the studied parameters.
Methods:
This prospective study includes 300 pregnant women who were the patients of University Clinical Center Tuzla, Clinic for Gynecology and Obstetrics from January 2011 to December 2014. The women were divided into two groups: an experimental group consisted of 150 adolescent pregnant women aged 13–19 years and a control group consisted of 150 adult pregnant women aged 20–35 years. The following parameters were analyzed: age of pregnant women, number of antenatal controls in pregnancy, prepregnancy body weight, weight gain in pregnancy, parity, and obstetric history data.
Results:
A significantly higher number of adolescent pregnant women belongs to a subgroup from one to two examinations during pregnancy (P < 0.000013) and to subgroups from three to five examinations (P < 0.000001). A significantly smaller number of adolescent pregnant women performed their first antenatal control in the first 2 lunar months (P < 0.01). A subgroup with optimal body weight (from 51 to 69 kg) are the most prevalent among adolescent pregnant women (P < 0.000001). A significantly larger number of adolescent pregnant women had an optimal weight gain of 7.8 to 12.99 kg (P < 0.001).
Conclusions:
The adolescent pregnant women have suboptimal antenatal care, which could lead to adverse maternal and birth outcomes, but have optimal body weight and weight gain during pregnancy.
Keywords: Pregnancy in adolescence, prenatal care, weight gain
Introduction
Antenatal care is a program of health care measures taken during pregnancy and represents the complete coverage of all pregnant women by a sufficient number of checkups, with appropriate supervision in pregnancy. The main aim of these health care measures is the birth of a living and healthy baby, the reduction in the risk of stillbirths and pregnancy complications while maintaining the mother's health.[1] The optimal number of examinations in a normal pregnancy should be between eight and ten, but the number of visits to a gynecologist may be higher, which depends on risks and course of pregnancy.[1] WHO reported that eight or more contacts for antenatal care can reduce perinatal deaths by up to 8 per 1000 births when compared to four visits.[1] The main reasons for not undergoing and avoiding checkups in adolescent pregnancy are emotional crises, lack of self-esteem, neglect of the future newborn, worry for the future and depression, lower socioeconomic status, lower education level, husband with lower education level, life in rural areas, and not being exposed to mass media.[2] Because of poor antenatal care, they tend to deliver lower birth weight infants, premature infants, preterm and premature preterm rupture of membranes, infant mortality, and morbidity rates are also higher in compared to adult women.[3,4,5,6]
The proper intrauterine growth of a fetus depends on the mother's body weight before pregnancy, the weight gain in pregnancy, and the rate at which this weight gain occurs during pregnancy. Counseling about healthy eating and keeping physically active during pregnancy is recommended for pregnant women to stay healthy and to prevent excessive weight gain during pregnancy.[1] Weight gain has a certain regularity; in the first 6 months, a pregnant woman should gain one kilogram per month, and the remaining 3 months by two kilograms per month. Weight gain less than 7.80 kg is pathological, as is weight gain greater than 13 kg. The optimal weight value of pregnant women belongs to the subgroup 51 to 69 kg.[7] In the research we conducted, we used standard anthropometric values of our authors, adjusted for the age of adolescent pregnant women.[8]
Despite the vulnerability of young girls during pregnancy, the literature on antenatal care utilization and weight gain among adolescents is scarce[2] and, therefore, our study will contribute to improving knowledge about this group of patients.
The aim of this study is to compare the antenatal care, body weight, and weight gain in pregnancy between the adolescent and adult pregnancies and, thus, examine the impact of adolescence on the studied parameters.
Methods
This prospective study included 300 pregnant women (13 to 35 years of age) who were the patients of University Clinical Center Tuzla, Clinic for Gynecology and Obstetrics from January 2011 to December 2014.
The women were divided into two groups: the first group consisted of 150 adolescent pregnant women aged 13–19 years (experimental group) and the second group consisted of 150 adult pregnant women aged 20–35 years (control group). Inclusion criteria for the study group were as follows: age under 19 at the time of conception, singleton pregnancy, being healthy before pregnancy, delivering at University Clinical Center Tuzla, Clinic for Gynecology and Obstetrics. Exclusion criteria were: age over 19 at the time of conception, multiple pregnancies, women with pathological conditions before pregnancies (genital and extragenital diseases), women who during the follow-ups showed maternal age over 35 years of age, pregnancies less than 24 weeks of gestation, pregnancies with fetal abnormalities, and delivering at other healthcare institutions.
Our study received an ethical approval from the Research Ethical Committee of the University Clinical Center and Faculty of Medicine, University of Tuzla, Bosnia and Herzegovina.
The data from the course of pregnancy was collected on the basis of available medical documentation (pregnancy booklet, mother's disease history, and partograms). The following parameters were analyzed: age of pregnant women, number of antenatal controls in pregnancy, body weight on the day of delivery, and weight gain in pregnancy. The body weight of pregnant women was measured immediately upon arrival at the doctor's office with the mechanical weight, where the weight was rounded to the nearest 10 g. Pregnant women were divided into five subgroups according to the number of antenatal controls (zero, one-two, three-five, six-eight, > eight controls), into three subgroups according to body weight (<50, 51–69, 70–125 kg), and into four subgroups according to weight gain in pregnancy (<7.80, 7.80–12.99, 13.00–17.79, >17.80 kg).
Statistical analysis
The t-test (statistical comparison test), the χ2 test (frequency comparison test), and the z-test (proportional test) were used. The data is expressed using percentages. Statistically significant difference was set to less than 5%.
Results
This prospective study included 300 pregnant women (13 to 35 years of age). Women divided into two groups: the first group consisted of 150 adolescent pregnant women aged 13–19 years (experimental group) and the second group consisted of 150 adult pregnant women aged 20–35 years (control group).
We analyzed the number of antenatal controls during pregnancy in both groups of pregnant women and presented the results in Figure 1. There is a statistically significant difference in these distributions for all subgroups of antenatal control examinations (χ2 = 582.24, P < 0.000001). Using the z-test, we found a statistically significant difference in proportions for each subgroup individually.
Figure 1.
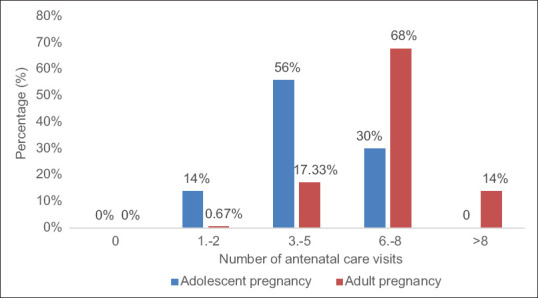
Percentage distribution of antenatal controls of examined pregnant women
A significantly higher number of adolescent pregnant women belongs to the subgroup from one to two examinations during pregnancy (z = 4.43; P < 0.000013) and to the subgroups from three to five examinations (z = 6.949; P < 0.000001). The pregnant women from the control group had a significantly higher number of optimal examinations (z = −4.752; P < 0.000003) compared to adolescent pregnant women.
A significantly smaller number of adolescent pregnant women performed their first antenatal control in the first 2 lunar months compared to pregnant women in the control group (z = −2.573; P < 0.01). In the group of adolescent pregnant women, there are more pregnant women with the first examination in the fourth lunar month (z = 3.535; P < 0.0004) and the sixth lunar month (z = 1.984; P < 0.048), which is statistically significant.
The distribution of body weight of pregnant women was shown in Table 1. There is a statistically significant difference in these distributions for all subgroups of body weight (χ2 = 189.88; P < 0.000001). Using a z-test, we found a statistically significant difference in proportions for each subgroup individually.
Table 1.
Body weight of pregnant women
| Body weight (kg) | Adolescent pregnancy n (%) | Adult pregnancy n (%) | P |
|---|---|---|---|
| <=50 | 9 (6) | 1 (0.67) | P<0.01 |
| 51-69 | 88 (58.67) | 33 (22) | P<0.000001 |
| 70-125 | 53 (35.33) | 116 (77.33) | P<0.000001 |
| Total | 150 (100) | 150 (100) |
With the Z-test, we established statistical significance for each subgroup individually. In the group of adolescent pregnant women, there were significantly more pregnant women weighing less than 50 kg (z = 2.573; P < 0.01). Pregnant women in the 51 to 69 kg subgroup are the most prevalent among adolescent pregnant women, which represents the subgroup with optimal body weight (z = 6.472; P < 0.000001). Pregnant women in the control group (z = 7.333; P < 0.000001) were statistically significantly more present in the 70 to 125 kg subgroup.
In our study, we compared the weight gain of adolescent pregnant women in relation to the control group of pregnant women and presented the results in Table 2.
Table 2.
Weight gain in pregnancy
| Weght gain in pregnancy (kg) | Adolescent pregnancy n (%) | Adult pregnancy n (%) | P |
|---|---|---|---|
| <7.80 | 6 (4) | 3 (2) | P<0.001 |
| 7.80-12.99 | 95 (63.33) | 68 (45.33) | P<0.001 |
| 13.00-17.79 | 48 (32) | 47 (31.33) | NS |
| >17.80 | 1 (0.67) | 32 (21.34) | P<0.000001 |
| Total | 150 (100) | 150 (100) |
NS* nonsignificant
The frequency comparison test showed a statistically significant difference in the distribution of body weight gain in adolescent pregnant women in relation to the control group (χ2 = 43.773; P < 0.000001).
The proportionality test indicated statistical significance for each individual weight gain subgroup. A significant number of pregnant women in the control group had a weight gain greater than 17.8 kg (z = −5.72; P < 0.000001), and a significantly larger number of adolescent pregnant women had an optimal weight gain of 7.8 to 12.99 kg (z = 3.129; P < 0.001).
Discussion
Numerous authors report data on inadequate antenatal care of adolescent pregnant women, related to the subsequent arrival at the first check-up or the suboptimal number of total examinations in pregnancy, leading to adverse pregnancy outcomes.[9,10,11,12] The data from developed countries indicates that properly administered antenatal care, with timely selection and monitoring of pregnancy with risk factors, leads to a healthy pregnancy and a childbirth in adolescent pregnancy.[13,14]
According to our results, suboptimal antenatal care was observed in the group of adolescent pregnant women, since 60% of adolescent pregnant women received up to five examinations during pregnancy and none had more than eight examinations. Globally, only 64% of women receive antenatal care four or more times through their pregnancy in the WHO report.[1] In high-income countries, all women have at least four antenatal care visits. In 2015, only 40% of all pregnant adolescents in low-income countries had recommended antenatal care visits, which correlates with our results.[15] Mersal et al. reported that more than 70% of participants in their study had made only 1 visit to the antenatal care clinic, which is lower than in our study.[3]
A significantly lower number of adolescent pregnant women undergo the first examination in the first 2 lunar months, and in the same group a significantly higher number of pregnant women with the first examination in the fourth and sixth lunar months, which correlates with global world data.[16] WHO recommends pregnant women to have their first contact in the first 12 weeks gestation, with subsequent contacts taking place at 20, 26, 30, 34, 36, 38, and 40 weeks gestation.[1] The highest rates of late or no prenatal care were reported in adolescent pregnancy. In 2017, 27% of births to females under age 15, and 11% of births ages 15 to 19 are in category late or no prenatal care. This proportion drops with increasing age, reaching a low of 5% for women in their thirties and 6% among women in their forties.[16]
Govender et al. reported that 54% pregnant adolescents undergo their first antenatal care visit above 20 weeks, 43% attend only between zero and three visits, but 2% never attend antenatal care visit prior to birth.[17] In the study of Medhi et al., >80% adolescent mothers received quality antenatal care, which is higher than in our study.[18] In the study of Tilghman and Lovette, 69% pregnant adolescents aged 15–19 years old received care beginning at the first trimester of pregnancy, and 64% received early and adequate prenatal care, which is higher than in our study.[19] Significantly higher numbers of adolescent pregnant women belong to subgroup from one to two examinations and subgroup from three to five examinations, while pregnant women from the control group have significantly greater numbers of optimal examinations, indicating poor antenatal protection for adolescent pregnant women, which correlates with the study of Govender et al.[17]
Adolescents could have similar pregnancy outcome to that of adult women with good psychosocial support, appropriate and adequate antenatal care. Some studies could not find any adverse pregnancy outcome among adolescent mothers with provision of high-quality antenatal care.[3,18]
In research by Leppalahti et al.[11] and Bildircin et al.,[20] they report normal body weight, optimal weight gain in adolescent pregnancy, as well as adverse outcome accompanied by a significantly higher incidence of anemia, preterm and premature preterm rupture of membranes, preeclampsia, and low birth weight.[5,21]
Our study showed that majority of adolescent pregnancies belonged to the class with optimal body weight (P < 0.000001), while the obese class was dominated by pregnant women from the control group (P < 0.000001). The results of the analysis of body weight gain in pregnancy showed that a significantly higher number of adolescent pregnant women had the optimal weight gain in pregnancy (P < 0.001), and a significant number of pregnant women in the control group had a weight gain greater than 17.80 kg. The results represent a pathological phenomenon (P < 0.000001), which is in agreement with the studies of the above authors.
Samano et al. reported that the proportion of overweight and obese pregnant adolescents was 11.5% and 10.1%, and frequency for underweight was 5%, which is similar to our data.[22]
In the study of Cift et al., they reported that adolescent pregnant women gained less weight during pregnancy, on average 7.3 kg, compared to adult pregnancies, which correlates with our results.[23] According to Samano et al., about 42% and 23% of pregnant adolescents had low and high gestational weight gain, in contrast to our results that the majority pregnant adolescent had optimal weight gain.[22] Prepregnancy body mass index (BMI) is an important determinant of many outcomes of pregnancy, so the new guidelines for weight gain during pregnancy continue to be presented according to prepregnancy BMI category. High or low weight gain during pregnancy was associated with an increased risk of low birth weight and preterm delivery.[22]
Some of the limitations of our study include relatively small sample size in examined groups, limited access to socioeconomic status data of patients, and inclusion of single-center. However, this is the first prospective study in Bosnia and Herzegovina relating to antenatal care and weight gain in adolescent pregnancy.
All the obtained results indicated the optimum physical condition of adolescent pregnant women but also the significant obesity in the control group of pregnant women with a tendency toward the possible occurrence of metabolic, diabetes, and hypertension disorders.
Conclusions
Adolescent pregnant women have suboptimal antenatal care, which could lead to adverse maternal and birth outcomes. Adolescent mothers have optimal body weight and optimal weight gain during pregnancy. Because of that, it is needed to put an emphasis on improving adolescents' education and removing financial barriers to their access to maternal health services.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organisation. New guidelines on antenatal care for a positive pregnancy expirience. 2018. [Last accessed on 2019 Sep 20]. Available from: https://wwwwhoint/reproductivehealth/news/antenatal-care/en/
- 2.Banke-Thomas OE, Banke-Thomas AO, Ameh CA. Factors influencing utilisation of maternal health services by adolescent mothers in Low-and middle-income countries: A systematic review. BMC Pregnancy Childbirth. 2017;17:65. doi: 10.1186/s12884-017-1246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mersal FA, Esmat OM, Khalil GM. Effect of prenatal counselling on compliance and outcomes of teenage pregnancy. East Mediterr Health J. 2013;19:10–7. [PubMed] [Google Scholar]
- 4.Cerovac A, Grgic G, Ljuca Dž. Mode of delivery in preterm births-bosnian and herzegovinian experience. Mater Sociomed. 2018;30:290–3. doi: 10.5455/msm.2018.30.290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marković S, Bogdanović G, Cerovac A. Premature and preterm premature rupture of membranes in adolescent compared to adult pregnancy. Med Glas (Zenica) 2020;17:18–22. doi: 10.17392/1052-20. [DOI] [PubMed] [Google Scholar]
- 6.Nevačinović E, Cerovac A, Bogdanović G, Grgić G. Perinatal characteristics and prevalence of low birth weight infants in the Federation of Bosnia and Herzegovina: Prospective multicentric study. Med Glas (Zenica) 2019;16:92–7. doi: 10.17392/987-19. [DOI] [PubMed] [Google Scholar]
- 7.Kurjak A, Matijević R. Fetalni rast. In: Kurjak A, editor. Ginekologija i Perinatologija 2nd ed Zagreb. Znanstvena Biblioteka: 2003. pp. 66–106. [Google Scholar]
- 8.Berberović LJ, Hadžiselimović R, Dizdarević M. Antropometrijske mjere. In: Dizdarević S, editor. Medicinska Antropologija Sarajevo. Svjetlost: 2009. pp. 67–84. [Google Scholar]
- 9.Malabarey OT, Balayla J, Klam SL. Pregnancies in young adolescent mothers: A population-based study on 37 million births. J Pediatr Adolesc Gynecol. 2012;25:98–102. doi: 10.1016/j.jpag.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Feldman J. Best practice for adolescent prenatal care: Application of an attachment theory perspective to enhance prenatal care and diminish birth risks. Child Adol Soc Work J. 2012;29:151–66. [Google Scholar]
- 11.Leppalahti S, Gissler M, Mentula M, Heikinheimo O. Is teenage pregnancy an obstetric risk in a welfare society.A population–based study in Finland? BMJ. 2013;3:322–35. doi: 10.1136/bmjopen-2013-003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsikouras P, Dafopoulos A, Trypsianis G, Vrachnis N, Bouchlariotou S. Pregnancies and their obstetric outcome in two selected age groups of teenage women in Greece. J Matern Fetal Neonatal Med. 2012;25:1606–11. doi: 10.3109/14767058.2011.648242. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age – A cohort study. BJOG. 2014;121:261–8. doi: 10.1111/1471-0528.12311. [DOI] [PubMed] [Google Scholar]
- 14.Wallace ME, Harville EW. Predictors of healthy birth outcome in adolescents: A positive deviance approach. J Pediatr Adolesc Gynecol. 2012;25:314–21. doi: 10.1016/j.jpag.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation. Maternal mortality. 2018. [Last accessed on 2019 Sep 19]. Available from: https://wwwwhoint/news-room/fact-sheets/detail/maternal-mortality .
- 16.Child Trends. Late or no prenatal care. 2019. [Last accessed on 2019 May 05]. Retrieved from: https://wwwchildtrendsorg/indicators/late-or-no-prenatal-care .
- 17.Govender T, Reddy P, Ghuman S. Obstetric outcomes and antenatal access among adolescent pregnancies in KwaZulu-Natal, South Africa. S Afr Fam Pract. 2018;60:1–7. [Google Scholar]
- 18.Medhi R, Das B, Das A, Ahmed M, Bawri S, Rai S. Adverse obstetrical and perinatal outcome in adolescent mothers associated with first birth: A hospital-based case control study in a tertiary care hospital in North-East India. Adolesc Health Med Ther. 2016;7:37–42. doi: 10.2147/AHMT.S91853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilghman J, Lovette A. Prenatal care: The adolescent's perspective. J Perinat Educ. 2008;17:50–3. doi: 10.1624/105812408X298390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bildircin FD, Kurtoglu E, Kokcu A, Isik Y, Ozkarci M. Comparision of perinatal outcome between adolescent and adult pregnancies. J Matern Fetal Neonatal Med. 2014;27:121–6. doi: 10.3109/14767058.2013.829816. [DOI] [PubMed] [Google Scholar]
- 21.Marković S, Cerovac A, Kunosić S, Ramić S, Bećirović E. Stereological analysis of terminal villi, intervillous space and fibrinoid of adolescent placentas and birth weight of newborns. Med Glas (Zenica) 2020;17:60–5. doi: 10.17392/1055-20. [DOI] [PubMed] [Google Scholar]
- 22.Sámano R, Chico-Barba G, Martínez-Rojano H, Godínez E, Rodríguez-Ventura AL, Ávila-Koury G, et al. Pre-pregnancy body mass index classification and gestational weight gain on neonatal outcomes in adolescent mothers: A follow-up study. PLoS One. 2018;13:e0200361. doi: 10.1371/journal.pone.0200361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Çift T, Korkmazer E, Temur M, Bulut B, Korkmaz B, Ozdenoǧlu O, et al. Adolescent pregnancies: Complications, birth outcomes and the possible solutions. Ginekol Pol. 2017;88:393–7. doi: 10.5603/GP.a2017.0073. [DOI] [PubMed] [Google Scholar]


