Abstract
A task force of the International Parkinson and Movement Disorder Society (MDS) recently published a tremor classification scheme that is based on the nosologic principle of two primary axes for classifying an illness: clinical manifestations (Axis 1) and etiology (Axis 2). An Axis 1 clinical syndrome is a recurring group of clinical symptoms, signs (physical findings), and possibly laboratory results that suggests the presence of at least one underlying Axis 2 etiology. Syndromes must be defined and used consistently to be of value in finding specific etiologies and effective treatments. The MDS task force concluded that essential tremor is a common neurological syndrome that has never been defined consistently by clinicians and researchers. The MDS task force defined essential tremor as a syndrome of bilateral upper limb action tremor of at least 3 years duration, with or without tremor in other locations (e.g., head, voice, or lower limbs), in the absence of other neurological signs (e.g., dystonia, parkinsonism, myoclonus, ataxia, peripheral neuropathy, and cognitive impairment). Deviations from this definition should not be labeled as essential tremor. Patients with additional questionably-abnormal signs or with signs of uncertain relevance to tremor are classified as essential tremor plus. The MDS classification scheme encourages a thorough unbiased phenotyping of patients with tremor, with no assumptions of etiology, pathology, pathophysiology, or relationship to other neurological disorders. The etiologies, pathology, and clinical course of essential tremor are too heterogeneous for this syndrome to be viewed as a disease or a family of diseases.
Keywords: essential tremor, classification, diagnostic axes, tremor, syndrome
Introduction
An international task force on tremor was convened by the International Parkinson and Movement Disorder Society (MDS) in 2011 to review the 1998 MDS consensus statement on tremor, which was devoted to the classification of pathologic tremors (1). The Task Force found that the 1998 consensus did not use a consistent approach to tremor classification. Tremor classifications were variably based on presumed anatomical origin (e.g., cerebellar tremor), presumed etiology (e.g., neuropathic tremor), and clinical phenomenology (e.g., primary writing tremor, isolated voice tremor). The Task Force was concerned that essential tremor (ET) was often viewed as a specific disease, rather than a clinical syndrome, and that ET was not defined and diagnosed consistently in the clinic or in research. A revised classification scheme (2) emerged from a comprehensive review of the literature and 5 years of intense discussion that included four 1-h meetings, a 2-day conference, several teleconferences, and numerous e-mail exchanges and document drafts. The revised classification scheme is based on the nosologic principle of two primary axes for classifying an illness: clinical manifestations (Axis 1) and etiology (Axis 2) (3). The clinical manifestations in Axis 1 include symptoms, signs, and laboratory results that characterize the tremor disorder.
Essential Tremor Is a Syndrome
A syndrome is a recurring group of Axis 1 clinical symptoms, signs (physical findings), and possibly laboratory results that suggests the presence of at least one underlying etiology (4). The Task Force acknowledged the existence of many useful Axis 1 tremor syndromes and broadly defined two groups of tremor syndromes: those in which tremor is the only abnormal sign (isolated tremor syndromes) and those in which tremor occurs in combination with one or more additional signs such as dystonia or ataxia (combined tremor syndromes). ET was originally viewed as “a tremor diathesis that was often familial and occurred in isolation of other neurologic signs” (5). The Task Force concluded that this view of ET is still valid and formally defined ET as an isolated tremor syndrome of bilateral upper limb action tremor of at least 3 years duration, with or without tremor in other locations (e.g., head, voice, or lower limbs). This definition of ET differs from the widely-used TRIG criteria (Tremor Investigation Group) only in the required 3-year history of tremor, instead of 5 years (1). The MDS definition of ET characterizes the vast majority of people with ET, most of whom have not seen a physician for their tremor (6, 7). These people have a long-standing, relatively-mild ET syndrome (8, 9) with strong heritability (10).
The Value of Clinical Syndromes
Syndromes are useful only to the extent that they facilitate the discovery of useful treatments and specific etiologies, and by this standard, the syndrome of ET has been disappointing. The Task Force debated extensively whether ET should be defined more broadly or more narrowly, but ultimately, no conclusion was possible because the syndrome of ET has never been defined and used consistently (11). ET has been used loosely to include tremor syndromes ranging from enhanced physiologic tremor to action tremor in patients with neurological diseases such as Parkinson disease (12). Louis (13) has referred to ET as a “family of diseases” with an “evolving definition” (13) and “premotor stage” (14). The validity of these concepts is questionable. The pathologic and etiologic heterogeneities of ET are so great that the concept of “family” has no validity. An “evolving definition” of ET is precisely what the MDS Task Force wanted to avoid. The Task Force encouraged the definition of additional tremor syndromes within Axis 1 if these syndromes are believed to be useful in defining cohorts of patients that lead to the identification of specific Axis 2 etiologies. However, a clinical syndrome must be defined and used consistently to be of value in the discovery of useful treatments and specific etiologies. Inconsistent “evolving” definitions of ET make published studies difficult or impossible to reconcile. Misdiagnosis is understandably common (15–17), even among movement disorder specialists (18).
ET is defined as an isolated tremor syndrome in which tremor is the only permissible neurologic sign. A major problem has been that specialists differ in their thresholds for identifying dystonia, Parkinsonism, ataxia, and other neurological signs. Mild neurological abnormalities are commonly missed or dismissed in the evaluation of patients with possible ET. Questionable signs of dystonia such as a mild head tilt, spooning posture of the extended hands (19), and index finger extension (20) occur too commonly in normal people to be used confidently in clinical diagnosis. Jerkiness and asymmetry are features of dystonic tremor (21), but these characteristics have never been operationally defined. Impaired tandem gait in ET patients is often interpreted as a cerebellar sign, but this common test has never been properly validated, making interpretation difficult, particularly in the elderly (22). The Task Force concluded that questionably abnormal clinical manifestations should be consistently documented and that ET plus should be the classification of patients who fulfill the criteria for ET but have one or more of these “soft” signs of uncertain significance (2). The classification ET plus encourages clinicians to document all deviations from the ET syndrome that are of questionable significance (e.g., spooning of the hands, unsteady tandem gait) or questionable relevance to tremor (e.g., mild cognitive impairment).
Retrospective reviews of outpatient clinical cohorts have shown that 40% or more of patients previously diagnosed as ET are reclassified as ET plus or a combined tremor syndrome when the new MDS classification scheme is applied (23–25). For example, 20 of the last 34 patients undergoing DBS surgery for ET at our center were reclassified as ET plus due to the following Axis 1 features: rest tremor or questionable rest tremor (n = 9), questionable dystonic posturing (n = 14), jerky tremor (n = 7), asymmetry in upper limb tremor exceeding 1 point on the Essential Tremor Rating Assessment Scale (n = 8) (26), rapid progression (n = 6), strained voice (n = 3), and impaired tandem gait (n = 7). These changes in diagnosis cannot be attributed to a drastic change in the definition of ET because the new definition of ET differs from the old TRIG definition only in the required duration of tremor (3 vs. 5 years) and differs from the old MDS consensus criteria only in the exclusion of isolated head tremor and the required 3-year history of tremor. Instead, the changes in diagnosis are primarily due to the new classification ET plus, which places great emphasis on documenting additional signs of uncertain abnormality and relevance to tremor. Previously, these additional signs were frequently overlooked, not documented, or wrapped into the diagnosis ET.
There is already evidence that the deeper phenotyping inherent in ET plus is worthwhile. Merchant et al. (27) found that patients with signs of ataxia were more likely to develop rapid tolerance to thalamic deep brain stimulation, and Picillo et al. (28) found that patients with ET plus were more likely to develop dystonia from thalamic neurosurgery. Geneticists are also beginning to embrace this approach to tremor classification (29).
The Limitations of Clinical Syndromes
The classifications ET and ET plus are purely clinical, and it is recognized that experts will disagree on the Axis 1 classification of patients, particularly those patients who are older and have greater tremor severity (18, 30). The presence of one questionably-abnormal sign, such as three missteps in a 10-step tandem walk, may not be deemed sufficient to exclude an older person from a therapeutic trial of ET but will likely reach the threshold for ET plus in a 20-year old with no other medical problems. A patient that is completely unable to tandem walk and is also unsteady when walking should be classified as having a combined tremor-ataxia syndrome, not ET or ET plus. Similarly, spooning hand posturing alone could be a normal variant, but spooning in combination with jerky asymmetric upper limb tremor [≥1 point on the Essential Tremor Rating Assessment Scale (26)] may be regarded as too suggestive of dystonic tremor to be classified as ET or ET plus. True rest tremor occurs in <15% of clinic patients (31) and in <5% of people in the general population who otherwise fulfill criteria for ET (32). Therefore, the MDS Task Force concluded that patients meeting the criteria for ET except for the presence of rest tremor should be classified as ET plus. These uncertainties illustrate that many aspects of the neurological exam are still in need of validation and standardization. Clinical constructs such as jerkiness, unsteadiness, and asymmetry need to be operationally defined and quantified, possibly with the aid of quantitative motion analysis and clinical electrophysiology (21).
One criticism of the new MDS classification scheme is that ET and ET plus are diagnostic placeholders, not final diagnoses or specific diseases (33). However, this is true of all medical conditions that are defined solely in terms of clinical manifestations (Axis 1) and not etiology (Axis 2). Clinical syndromes (e.g., acquired immunodeficiency syndrome, AIDS) are useful only to the extent that they facilitate the discovery of specific etiologies (human immunodeficiency virus, HIV) and effective treatments (antiretroviral drugs). A disease is not discovered until the underlying etiology is identified. Furthermore, a patient's syndrome or condition may change as the disease progresses. Thus, ET and ET plus may evolve into a more complex (combined) tremor syndrome before an Axis 2 etiology is discovered. Such patients are then classified with their Axis 2 etiology and current Axis 1 tremor syndrome and are said to have antecedent ET or ET plus (Figure 1).
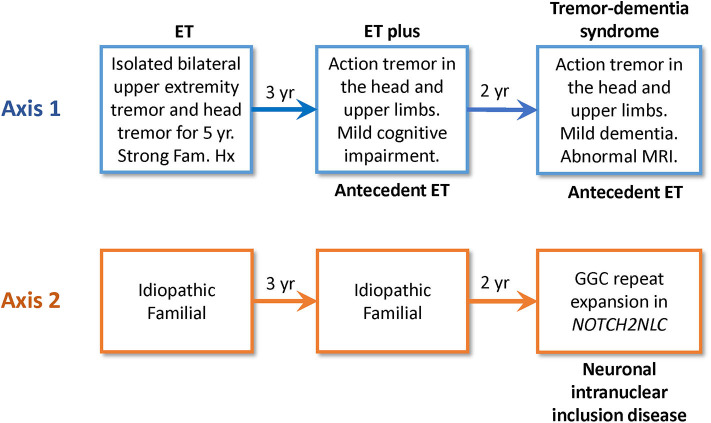
Figure 1.
This flow diagram illustrates how Axis 1 and 2 classifications may change over time. This clinical scenario is based on the work of Chen et al. (40). A 54-year-old Chinese man presented with a 5-year history of tremor in the head and upper limbs. His family history was consistent with autosomal dominant inheritance. His initial Axis 1 classification was ET, and his Axis 2 classification was idiopathic familial. Over time, his Axis 1 classification changed from ET to ET plus mild cognitive impairment, and his Axis 1 classification ultimately changed to a combined tremor-dementia syndrome with antecedent ET. His MRI brain revealed diffusion-weighted abnormality in the subcortical U-fibers of both frontal lobes, and genetic testing revealed a GGC repeat expansion in NOTCH2NLC. Thus, his Axis 2 diagnosis was ultimately neuronal intranuclear inclusion disease that presented initially as ET.
ET can be a stable syndrome throughout a person's life, given the presence of this syndrome in many patients with a decades-long history of tremor. The stipulated 3-year history of tremor is an attempt, admittedly arbitrary, to increase the likelihood of a stable clinical syndrome. It is widely acknowledged that longitudinal studies are needed to determine the degree to which the ET syndrome is stable (34) and to determine the significance of a stable ET syndrome in terms of underlying etiology and pathophysiology.
Etiologies of the Essential Tremor Syndrome
ET has an additive heritability of at least 75%, so environmental factors probably play a small and still undefined role (35). Large families with apparent Mendelian dominant inheritance are common, but after more than 25 years of extensive searching, only four genes with rare causative mutations have been discovered: fused in sarcoma gene (FUS) (36), GGC repeat expansion in the Notch 2 N-terminal like C gene (NOTCH2NLC) (37), HtrA Serine Peptidase 2 gene (HTRA2) (38), and teneurin transmembrane protein 4 gene (TENM4) (39). There is little doubt that others will be discovered. However, these rare causative mutations are not found in most ET patients. Moreover, studies of families with these mutations illustrate the important fact that ET is frequently not a stable phenotype. ET can be the initial phenotype of neuronal intranuclear inclusion disease (GGC repeat expansion in the NOTCH2NLC gene) (40) but may evolve into a more complex syndrome with dementia, parkinsonism, ataxia, convulsions, neuropathy, or autonomic dysfunction (41) (Figure 1). ET may exist for years before a patient with the HTRA2 p.G399S allele develops Parkinsonism (38). ET is also an early but temporary phenotype of hereditary dystonia (e.g., ANO3) (42), hereditary ataxia (e.g., SCA12) (43), and PARK-parkin disease (44). Progression of these diseases ultimately produces complex combined tremor syndromes. In summary, ET is a syndrome or phenotype with many genetic etiologies. Monogenic inheritance appears to be rare, and polygenic or epigenetic inheritance may be a factor, even in families with rare causative gene mutations (39). The genetic heterogeneity of ET seems inconsistent with the notion that ET is “a family of diseases.”
Purkinje cell pathology is found in some but not all ET patients (45–47). However, comparable Purkinje cell loss is also found in diseases that do not cause tremor, such as Huntington disease (48) and Alzheimer disease (49). It is unclear whether distinctive cerebellar pathology is associated with ET (50), and it is also unclear whether the reported Purkinje cell pathology is tremorogenic. The notion that ET is a “Purkinjopathy” belies the etiologic, pathologic, and pathophysiologic complexity of ET (51). Purkinje cell pathology is no justification for regarding ET as “a family of diseases.”
Pathophysiology of Essential Tremor Syndrome
ET is produced by abnormal oscillation and neuronal entrainment in the cerebellothalamocortical pathway. However, this is true for all forms of pathologic tremor (52). The cerebellum and thalamocortical loop have direct or indirect connections with virtually all motor pathways of the nervous system. Therefore, the source of oscillation in a patient with ET need not be the cerebellum or the thalamocortical loop, and the source may vary among etiologies of ET. Cerebellar Purkinje cells and neurons in the thalamocortical loop have intrinsic membrane properties that are conducive to oscillation (53, 54), and the cerebellum and thalamocortical loop have network properties that could amplify oscillation and promote neuronal entrainment of oscillation originating nearly anywhere in the nervous system (54–56). Oscillation in the cerebellothalamocortical pathway will produce tremor if there is sufficient neuronal entrainment. It is likely that virtually all etiologies of ET produce oscillation in the cerebellothalamocortical pathway. Therefore, the etiologic heterogeneity of ET and syndromic classification of ET should not deter us from conducting therapeutic trials that target the mechanisms of oscillation in the cerebellothalamocortical pathway.
Subtypes of the Essential Tremor Syndrome
It is possible that the current definition of ET is too broad to identify etiologies and effective treatments. Researchers and clinicians are free to define subtypes of ET, such as late-onset ET (e.g., onset after age 65), familial (e.g., one or more first-degree relatives with ET), sporadic, and tremor predominantly (not exclusively) in the head or voice. However, data from one subtype may not be applicable to all patients with ET. The reasons are obvious. Elderly patients with late-onset action tremor are far more likely to have undiagnosed subclinical neurological comorbidities than young healthy adults (57), and they are more likely to have comorbid systemic illnesses that cause enhanced physiologic tremor, which is easily mistaken for mild ET (6). Familial and sporadic cases are likely to differ in their likelihood of harboring risk genes. Patients with predominant head or voice tremor may be more likely to have a form of dystonia.
It is also possible that the current definition of ET is too narrow to identify etiologies and effective treatments. The MDS classification scheme permits the definition of additional Axis 1 tremor syndromes in which the criteria for ET are met except for the existence of one or more additional Axis 1 features (e.g., gait ataxia). To avoid confusion, these combined tremor syndromes should not be referred to as subtypes or variants of ET.
Discussion
Syndromes must be defined and used consistently to be of value in clinical care and research. The ET syndrome has never been defined and used consistently. This has made the sizeable literature on ET difficult to interpret because readers must carefully examine each paper for differences in definition that can affect outcome.
The new ET and ET plus classifications do not invalidate earlier studies that carefully defined the axis 1 clinical characteristics of their patient populations, but the results of older studies may need some reinterpretation in the context of the new MDS classification scheme. The main problem with many older studies is that clinicians and researchers commonly used ad hoc definitions of ET, and neurological signs of uncertain significance (abnormality) and uncertain relevance to tremor (e.g., mild cognitive impairment in an elderly patient with ET) were often not documented or simply wrapped in a diagnosis of ET. Even patently abnormal signs other than tremor were deemed as permissible within some definitions of ET (58, 59). Furthermore, some studies included isolated head tremor, isolated voice tremor and tremor of <1 year duration (60).
ET plus is a new classification, not a specific syndrome. Clinicians are encouraged to carefully document the additional Axis 1 manifestations beyond tremor when using the classification ET plus. ET plus may include a variety of neurologic signs that are questionably abnormal or questionably relevant to the patient's tremor disorder. Specific ET plus syndromes are permissible within the new classification scheme, as long as the syndromes are defined and used consistently.
A syndrome should not be expanded or changed unless there is good reason to believe that the newly defined syndrome will be a better tool for the discovery of underlying etiology or effective treatment. Changing the definition of a syndrome like ET creates confusion in the comparison of new and old clinical studies. The new MDS definition of the ET syndrome does not differ significantly from the old and widely-used TRIG criteria (1) and is completely compatible with the original concept of ET (5). The new classification ET plus provides us with a tremor classification in which new syndromes can be defined, without disturbing the definition of ET. Subtypes of ET are permissible with the caveat that data from this subtype may not apply to the broad ET patient population.
People with ET and ET plus may be included in the same study cohort if this is believed to facilitate the study objectives. The new MDS definitions of ET and ET plus make no assumptions about underlying etiology or response to treatment. Patients with ET and ET plus may or may not have the same underlying etiology. Furthermore, it is clear that cerebellothalamocortical oscillation is a cornerstone of all forms of tremor, so the notion of ET being a syndrome should not deter one from pursuing new treatments. Careful phenotyping and classification under the new classification scheme will permit post hoc exploratory data analyses, and the results can be confirmed or refuted in subsequent studies.
In conclusion, the MDS classification scheme provides much-needed rigor to the classification of ET and puts ET in the proper perspective of being a clinical syndrome, not a specific disease. The classification ET plus facilitates a deeper phenotyping of patients without assumptions of etiology or causality. This should facilitate gene discovery, given the likely polygenic inheritance of ET in most patients. These views do not belittle ET, rather they properly acknowledge the importance of thorough Axis 1 phenotyping, unencumbered by any assumptions of etiology, pathology, or pathophysiology.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author is solely responsible for the writing and content of this manuscript.
Conflict of Interest
RE was chair of the Tremor Task Force of the International Parkinson and Movement Disorder Society. He has served as a consultant for Cadent, Cavion, Cydan, Five Microns, Jazz, Merz, Osmotica, Praxis Precision Medicines, and Sage in the design and execution of clinical trials for essential tremor. He serves on advisory boards for the International Essential Tremor Foundation and the Neuroscience Research Foundation of Kiwanis International, Illinois-Eastern Iowa District, and he receives research grant support from this foundation. This article is related to a companion perspective: Do We Belittle Essential Tremor by Calling it a Syndrome Rather than a Disease? Yes. Both Articles were prepared independent of each other.
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. (1998) 13(Suppl 3):2–23. 10.1002/mds.870131303 [DOI] [PubMed] [Google Scholar]
- 2.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMahon B, Pugh TF, Causes entities of disease In: Clark DW, MacMahon B. editors. Preventive Medicine. Boston, MA: Little, Brown; (1967). p. 26–33. [Google Scholar]
- 4.Calvo F, Karras BT, Phillips R, Kimball AM, Wolf F. Diagnoses, syndromes, and diseases: a knowledge representation problem. AMIA Annu Symp Proc. (2003) 2003:802. [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED, Broussolle E, Goetz CG, Krack P, Kaufmann P, Mazzoni P. Historical underpinnings of the term essential tremor in the late 19th century. Neurology. (2008) 71:856–9. 10.1212/01.wnl.0000325564.38165.d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenning GK, Kiechl S, Seppi K, Muller J, Hogl B, Saletu M, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. (2005) 4:815–20. 10.1016/S1474-4422(05)70226-X [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. 10.1002/mds.22838 [DOI] [PubMed] [Google Scholar]
- 8.Lorenz D, Poremba C, Papengut F, Schreiber S, Deuschl G. The psychosocial burden of essential tremor in an outpatient- and a community-based cohort. Eur J Neurol. (2011) 18:972–9. 10.1111/j.1468-1331.2010.03295.x [DOI] [PubMed] [Google Scholar]
- 9.Bain PG, Findley LJ, Thompson PD, Gresty MA, Rothwell JC, Harding AE, et al. A study of hereditary essential tremor. Brain. (1994) 117(Pt 4):805–24. 10.1093/brain/117.4.805 [DOI] [PubMed] [Google Scholar]
- 10.Lorenz D, Frederiksen H, Moises H, Kopper F, Deuschl G, Christensen K. High concordance for essential tremor in monozygotic twins of old age. Neurology. (2004) 62:208–11. 10.1212/01.WNL.0000103236.26934.41 [DOI] [PubMed] [Google Scholar]
- 11.Elble RJ. Essential tremor is a useful concept? No. Mov Disord Clin Pract. (2017) 4:663–5. 10.1002/mdc3.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden CD, Obeso JA, Rothwell JC, Benign essential tremor is not a single entity In: Yahr MD. editor. Current Concepts of Parkinson's Disease Related Disorders. Amsterdam: Excerpta Medica; (1983). p. 31–46. [Google Scholar]
- 13.Louis ED. The evolving definition of essential tremor: what are we dealing with? Parkinsonism Relat Disord. (2018) 46(Suppl 1):S87–S91. 10.1016/j.parkreldis.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenka A, Benito-Leon J, Louis ED. Is there a premotor phase of essential tremor? Tremor Other Hyperkinet Mov. (2017) 7:498 10.5334/tohm.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. (2006) 63:1100–4. 10.1001/archneur.63.8.1100 [DOI] [PubMed] [Google Scholar]
- 16.Schrag A, Munchau A, Bhatia KP, Quinn NP, Marsden CD. Essential tremor: an overdiagnosed condition? J Neurol. (2000) 247:955–9. 10.1007/s004150070053 [DOI] [PubMed] [Google Scholar]
- 17.Amlang CJ, Trujillo Diaz D, Louis ED. Essential tremor as a “waste basket” diagnosis: diagnosing essential tremor remains a challenge. Front Neurol. (2020) 11:172. 10.3389/fneur.2020.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor-some controversial aspects. Mov Disord. (2011) 26:18–23. 10.1002/mds.23289 [DOI] [PubMed] [Google Scholar]
- 19.Kim CY, Louis ED. “Spooning”: a subtle sign of limb dystonia. Tremor Other Hyperkinet Mov. (2018) 8:607. 10.5334/tohm.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vives-Rodriguez A, Louis ED. Index finger pointing (likely a subtle form of hand dystonia): prevalence across movement disorders. Front Neurol. (2018) 9:542. 10.3389/fneur.2018.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govert F, Becktepe J, Balint B, Rocchi L, Brugger F, Garrido A, et al. Temporal discrimination is altered in patients with isolated asymmetric and jerky upper limb tremor. Mov Disord. (2020) 35:306–15. 10.1002/mds.27880 [DOI] [PubMed] [Google Scholar]
- 22.Margolesky J, Singer C. How tandem gait stumbled into the neurological exam: a review. Neurol Sci. (2018) 39:23–9. 10.1007/s10072-017-3108-1 [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Yang X, Zhao Q, Ning P, Shen Q, Wang H, et al. Clinical characteristics of patients with essential tremor or essential tremor plus. Acta Neurol Scand. (2020) 141:335–41. 10.1111/ane.13209 [DOI] [PubMed] [Google Scholar]
- 24.Pandey S, Bhattad S. Questionable dystonia in essential tremor plus: a video-based assessment of 19 patients. Mov Disord Clin Pract. (2019) 6:722–3. 10.1002/mdc3.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajalingam R, Breen DP, Lang AE, Fasano A. Essential tremor plus is more common than essential tremor: insights from the reclassification of a cohort of patients with lower limb tremor. Parkinsonism Relat Disord. (2018) 56:109–10. 10.1016/j.parkreldis.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 26.Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos JL, et al. Reliability of a new scale for essential tremor. Mov Disord. (2012) 27:1567–9. 10.1002/mds.25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchant SH, Kuo SH, Qiping Y, Winfield L, McKhann G, Sheth S, et al. Objective predictors of ‘early tolerance’ to ventral intermediate nucleus of thalamus deep brain stimulation in essential tremor patients. Clin Neurophysiol. (2018) 129:1628–33. 10.1016/j.clinph.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picillo M, Paramanandam V, Morgante F, Algarni M, Olszewska DA, Munhoz RP, et al. Dystonia as complication of thalamic neurosurgery. Parkinsonism Relat Disord. (2019) 66:232–6. 10.1016/j.parkreldis.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Yau WY, O'Connor E, Chen Z, Vandrovcova J, Wood NW, Houlden H. GGC repeat expansion in NOTCH2NLC is rare in European patients with essential tremor. Brain. (2020) 143:e57. 10.1093/brain/awaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajan R, Pandey S, Anandapadmanabhan R, Srivastava AK. Interrater and intrarater agreement on the 2018 consensus statement on classification of tremors. Mov Disord. (2018) 33:1966–7. 10.1002/mds.27513 [DOI] [PubMed] [Google Scholar]
- 31.Whaley NR, Putzke JD, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord. (2007) 13:333–9. 10.1016/j.parkreldis.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Louis ED, Hernandez N, Michalec M. Prevalence and correlates of rest tremor in essential tremor: cross-sectional survey of 831 patients across four distinct cohorts. Eur J Neurol. (2015) 22:927–32. 10.1111/ene.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasano A, Lang AE, Espay AJ. What is “essential” about essential tremor? A diagnostic placeholder. Mov Disord. (2018) 33:58–61. 10.1002/mds.27288 [DOI] [PubMed] [Google Scholar]
- 34.Hopfner F, Haubenberger D, Galpern WR, Gwinn K, Van't Veer A, White S, et al. Knowledge gaps and research recommendations for essential tremor. Parkinsonism Relat Disord. (2016) 33:27–35. 10.1016/j.parkreldis.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diez-Fairen M, Bandres-Ciga S, Houle G, Nalls MA, Girard SL, Dion PA, et al. Genome-wide estimates of heritability and genetic correlations in essential tremor. Parkinsonism Relat Disord. (2019) 64:262–7. 10.1016/j.parkreldis.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merner ND, Girard SL, Catoire H, Bourassa CV, Belzil VV, Riviere JB, et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet. (2012) 91:313–9. 10.1016/j.ajhg.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun QY, Xu Q, Tian Y, Hu ZM, Qin LX, Yang JX, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. (2020) 143:222–33. 10.1093/brain/awz372 [DOI] [PubMed] [Google Scholar]
- 38.Unal Gulsuner H, Gulsuner S, Mercan FN, Onat OE, Walsh T, Shahin H, et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci U S A. (2014) 111:18285–90. 10.1073/pnas.1419581111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hor H, Francescatto L, Bartesaghi L, Ortega-Cubero S, Kousi M, Lorenzo-Betancor O, et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum Mol Genet. (2015) 24:5677–86. 10.1093/hmg/ddv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Lu L, Wang B, Hua X, Wan B, Sun M, et al. Essential tremor as the early symptom of NOTCH2NLC gene-related repeat expansion disorder. Brain. (2020) 143:e56. 10.1093/brain/awaa142 [DOI] [PubMed] [Google Scholar]
- 41.Sone J, Mori K, Inagaki T, Katsumata R, Takagi S, Yokoi S, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. (2016) 139:3170–86. 10.1093/brain/aww249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamelou M, Charlesworth G, Cordivari C, Schneider SA, Kagi G, Sheerin UM, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord. (2014) 29:928–34. 10.1002/mds.25802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hearn E, Holmes SE, Calvert PC, Ross CA, Margolis RL. SCA-12: Tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology. (2001) 56:299–303. 10.1212/WNL.56.3.299 [DOI] [PubMed] [Google Scholar]
- 44.Stark RS, Walch J, Kagi G. The phenotypic variation of a Parkin-related Parkinson's disease family and the role of heterozygosity. Mov Disord Clin Pract. (2019) 6:700–3. 10.1002/mdc3.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch Neurol. (2005) 62:1004–7. 10.1001/archneur.62.6.1004 [DOI] [PubMed] [Google Scholar]
- 46.Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. (2014) 29:496–500. 10.1002/mds.25845 [DOI] [PubMed] [Google Scholar]
- 47.Rajput AH, Robinson CA, Rajput A. Purkinje cell loss is neither pathological basis nor characteristic of essential tremor. Parkinsonism Relat Disord. (2013) 19:490–1. 10.1016/j.parkreldis.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 48.Singh-Bains MK, Mehrabi NF, Sehji T, Austria MDR, Tan AYS, Tippett LJ, et al. Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann Neurol. (2019) 85:396–405. 10.1002/ana.25413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavroudis I, Petridis F, Kazis D, Njau SN, Costa V, Baloyannis SJ. Purkinje cells pathology in Alzheimer's disease. Am J Alzheimers Dis Other Demen. (2019) 34:439–49. 10.1177/1533317519859200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louis ED, Kerridge CA, Chatterjee D, Martuscello RT, Diaz DT, Koeppen AH, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol. (2019) 138:859–76. 10.1007/s00401-019-02043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louis ED. Essential tremor: a common disorder of Purkinje neurons? Neuroscientist. (2016) 22:108–18. 10.1177/1073858415590351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elble RJ. Tremor disorders. Curr Opin Neurol. (2013) 26:413–9. 10.1097/WCO.0b013e3283632f46 [DOI] [PubMed] [Google Scholar]
- 53.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. (1988) 242:1654–64. 10.1126/science.3059497 [DOI] [PubMed] [Google Scholar]
- 54.Brown AM, White JJ, van der Heijden ME, Lin T, Sillitoe RV. Cerebellar Purkinje cell microcircuits are essential for tremor. bioRxiv. (2019). 10.1101/770321 [DOI] [Google Scholar]
- 55.Benagiano V, Rizzi A, Lorusso L, Flace P, Saccia M, Cagiano R, et al. The functional anatomy of the cerebrocerebellar circuit: a review and new concepts. J Comp Neurol. (2018) 526:769–89. 10.1002/cne.24361 [DOI] [PubMed] [Google Scholar]
- 56.Pedrosa DJ, Quatuor EL, Reck C, Pauls KA, Huber CA, Visser-Vandewalle V, et al. Thalamomuscular coherence in essential tremor: hen or egg in the emergence of tremor? J Neurosci. (2014) 34:14475–83. 10.1523/JNEUROSCI.0087-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopfner F, Ahlf A, Lorenz D, Klebe S, Zeuner KE, Kuhlenbaumer G, et al. Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov Disord. (2016) 31:1560–6. 10.1002/mds.26708 [DOI] [PubMed] [Google Scholar]
- 58.Chouinard S, Louis ED, Fahn S. Agreement among movement disorder specialists on the clinical diagnosis of essential tremor. Mov Disord. (1997) 12:973–6. 10.1002/mds.870120621 [DOI] [PubMed] [Google Scholar]
- 59.Jankovic J. Essential tremor: a heterogenous disorder. Mov Disord. (2002) 17:638–44. 10.1002/mds.10221 [DOI] [PubMed] [Google Scholar]
- 60.Benito-Leon J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES). J Alzheimers Dis. (2011) 23:727–35. 10.3233/JAD-2011-101572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.