Abstract
Adiponectin, an adipokine secreted by adipocytes, is a well-known homeostatic factor for regulating glucose levels, lipid metabolism, and insulin sensitivity through its anti-inflammatory, anti-fibrotic, and antioxidant effects. All these metabolic processes are mediated via two adiponectin receptors, AdipoR1 and AdipoR2. In addition, adiponectin is one of the hormones with the highest plasma concentrations. Weight loss or caloric restriction leads to increasing adiponectin levels, and this increase is associated with increased insulin sensitivity. Therefore, the adiponectin pathway can play a crucial role in the development of drugs to treat type 2 diabetes mellitus and other obesity-related diseases affected by insulin resistance like cancers or cardiovascular diseases. Adiponectin appears to increase insulin sensitivity by improving glucose and lipid metabolisms. The objective of this review is to analyze current knowledge concerning adiponectin and, in particular, its role in physiology and pathophysiology.
Keywords: Adipokines, adiponectin, AdipoR, obesity, type 2 diabetes mellitus
Introduction
Adiponectin was characterized, in 1995, as a protein abundantly secreted by 3T3-L1 adipocytes and present at high plasma concentrations in mice. Adiponectin is also referred to as ACRP30, AdipoQ, apM1, or GBP28. Four different teams working differently discovered that it is produced by the white adipose tissue almost at the same time.[1,2,3,4] Of all this nomenclature, the name adiponectin (ApN) is the most widely accepted. Numerous studies have made it possible to establish the determining role of ApN in energy homeostasis, the metabolisms of lipids and carbohydrates, in particular in muscle and liver, as well as its anti-inflammatory and anti-atherogenic properties.[5] ApN has also been detected in skeletal muscle,[5] cardiomyocytes,[6] osteoblasts,[7] lymphocytes,[8] adrenal gland,[9] placenta,[10] testis,[11] ovary,[12] pituitary gland,[13] and liver tissue.[14] The objective of this review is to analyze current knowledge concerning ApN and, in particular, its role in physiology and pathophysiology.
Adiponectin Structure and Biology
Structure of adiponectin
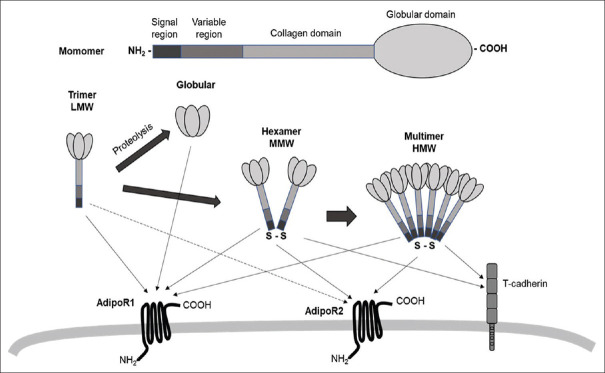
ApN is a specific protein in the adipose tissue of 247 amino acids with a molecular weight of 30 kDa in mice and 244 amino acids with a molecular weight of 28 kDa in humans.[1,4] The protein structure of ApN includes four parts: a signal region at the NH2-terminus, a variable region that is species specific, a collagenous domain and a globular domain at the COOH-terminus.[15]
Circulating ApN oligomers are present in plasma in three multimeric forms [Figure 1]. The assembly is carried out beforehand in the endoplasmic reticulum by post-translational modifications, such as hydroxylations and glycosylations of the ApN monomer. The globular domain allows the formation of low molecular weight trimers by hydrophobic bond, and the interactions at the level of the collagen domain by disulfide bonds allow the formation of medium molecular weight hexamers (association of two trimers), and high molecular weight multimers (4 to 6 trimers). ApN is also present in plasma in its globular form alone, resulting from proteolysis, but in very small quantities.[16] The different forms of ApN have different biological properties and probably different tissue targets.
Figure 1.
Adiponectin structure and receptors.Monomeric ApN consists of a globular domain, a collagenous domain, a species-specific domain, and a signal peptide. Oligomerization facilitates the formation of the trimers (LMW), hexamers (MMW), and multimer (HMW). S-S: disulfide bond.ApN interacts with ApN receptors: AdipoR1, AdipoR2, and T-Cadherin. The dotted line between AdipoR2 and globular ApN reflects that AdipoR2 is a relatively low-affinity receptor for globular ApN
Synthesis of adiponectin
Unlike other adipokines, ApN has an inverse relationship with obesity.[17] Several studies have shown that weight loss, including a reduction in body fat, is accompanied by an increase in circulating ApN levels.[18] The increase in circulating ApN induced by weight loss is not homogeneous but is in favor of the HMW form and to the detriment of the LMW and MMW forms.[19] This is important, considering that the high molecular weight form is currently considered to be the active form of ApN, or at least more active than the LMW and MMW forms.[20,21] During puberty, a notable decrease in the circulating ApN concentration is observed in males.[22] In addition, circulating ApN levels also vary by ethnicity[23] and are positively associated with age.[24] Arai et al. (2006) showed that centenarians had higher circulating ApN levels than younger people with the same BMI.[25] The body fat of the subjects was not measured in this study, however. The circadian variation profile of ApN shows that concentrations fluctuate by about 20% over 24 hours, with a slight decrease in rates overnight until a minimum in the early morning.[26] This daily variation seems to be greater in women than in men and would not differ between thin and obese subjects.[27]
Structure of adiponectin receptors
To exert its biological effects, ApN must bind to its specific receptors. The AdipoR1 and AdipoR2 receptors were first identified by Yamauchi et al. (2003),[28] and T. Cadherin [Figure 1], a member of the cadherin family, has also been identified by Hugh et al. (2004) as a receptor of hexamers and HMW adiponectin oligomers.[29]
The AdipoR1 and AdipoR2 receptor genes are located on chromosomes 1, locus 1p36.13-q41, and 12, locus 12p13.31, respectively. AdipoR1 and AdipoR2 are members of the progestin and AdipoQ receptor superfamily, which possesses seven transmembrane domains. They are topologically integral membrane proteins with intracellular N-terminus and extracellular C-terminus, which is the reverse topology of all other G-protein coupled receptors.[28]
The AdipoR1 receptor has a higher affinity for the globular form, while the AdipoR2 receptor preferentially binds to the high molecular weight form [Figure 1]. It was initially shown that the AdipoR1 receptor was mainly expressed in skeletal muscles and AdipoR2 in the liver.[28] Subsequently, the expression of these receptors has been identified in other tissues, such as the myocardium, macrophages, brain tissue, endothelial cells, lymphocytes, and adipose tissue,[30] or in pancreatic β cells where the level of expression of AdipoR2 is even equivalent to that of its expression in the liver, and the level of expression of AdipoR1 higher than that of its muscle expression.[31] Studies in mice have confirmed that these two receptors are the main ApN receptors in vivo and that they mediate the effects of ApN.[32] Thus, KO mice for these two receptors develop glucose intolerance and hyperinsulinemia, showing their major involvement in carbohydrate homeostasis and insulin sensitivity. These effects appear to be receptor-specific, with in particular the involvement of AdipoR1 in the activation of AMPK while AdipoR2 is involved in the activation of PPARα.[30] More generally, a study to invalidate these receptors has shown that the involvements of the AdipoR1 and AdipoR2 receptors in metabolism are very different.[33] In particular, they showed that AdipoR1 KO mice increase their adipose mass and insulin sensitivity, and decrease their glucose tolerance and energy expenditure. Conversely, AdipoR2 KO mice show better sensitivity to insulin and glucose, maintain normal body weight even under a fatty diet, spend more energy and improve their dyslipidemia.
T-cadherin is a glycoprotein involved in cell adhesion and a potential receptor for ApN. It is colocalized at the cellular level with ApN and is expressed in tissues like aorta, heart, or skeletal muscle, but almost none T-cadherin can be found in liver.[34] Only the high molecular weight complexes of ApN are capable of binding T-cadherin, which implies that post-translational modifications of ApN are essential for its binding. It is therefore conceivable that T-cadherin, which is an extracellular protein without an intracellular domain, can act as an ApN binding protein rather than as a co-receptor.[29] It has been shown by Fukuda et al. (2017) that T-cadherin has a role in adiponectin binding, especially the 130-kDa prodomain-bearing where T-cadherin is preferentially localized on the cell surface and bound more adiponectin than its 100-kDa form.[35] exosomes that contain adiponectin and T-cadherin are released in response to adiponectin in cells expressing T-cadherin.[36]
Regulation of adiponectin receptor expression
Various factors regulate the expression of ApN receptors, which are thus expressed differently depending on the tissue. In the adipose tissue of transgenic mice for the ApN gene, with a targeted overexpression of ApN in adipose tissue, the level of expression of the AdipoR2 receptor is increased, but not that of AdipoR1.[37] Furthermore, in adipose tissue, the expression levels of ApN receptors are associated with the level of tissue expression of ApN.[38] The expression levels of AdipoR1 and AdipoR2 are inversely correlated with body fat and obesity.[39,40] Unlike the expression of ApN itself, there is no sexual dimorphism in the expression of ApN receptors in adipose tissue.[40] McAinch et al. (2006)showed that ApN up-regulates the AdipoR1 receptor in differentiated primary skeletal muscle cells from normal-weighted subjects, but not those from diabetic, obese, or having lost weight.[41] The level of expression of the AdipoR2 receptor was not changed in this study. AdipoR1 and AdipoR2 receptors expression levels are also associated with age and aerobic capacity in skeletal muscle.[42] Unlike adipose tissue, the expression of ApN receptors in muscle depends on gender, with men expressing more ApN receptors,[42] this dimorphism thus appears to be tissue specific. Interestingly, circulating ApN levels are lower in men than women, which can be explained by the level of receptor expression in muscle. Finally, a study has shown that the expression of the three receptors is highly correlated, and positively associated with the expression of PPARδ in human myocytes.[43]
Adiponectin: Role in Physiology and Pathophysiology
Carbohydrate metabolism and insulin sensitization
Numerous studies have shown the existence of an inverse relationship between circulating ApN concentrations and insulin resistance in several pathologies with high cardiovascular risk such as obesity, metabolic syndrome, and T2DM.[44,45] The question is to know the meaning of this interaction. Mice disabled for the ApN gene develop hepatic, but not global resistance to insulin, with a 65% increase in hepatic glucose production. On a diet rich in saturated fatty acids, they also develop carbohydrate intolerance, which can be corrected by acute administration of recombinant ApN, without modification of muscle glucose uptake. Following this administration, the hepatic expression of the enzymes of gluconeogenesis, phosphoenol-carboxykinase and glucose-6-phosphatase is increased, with no change in insulinemia, which is a sign of insulin sensitization.[46] In addition, the notion of an insulin-sensitizing activity of ApN is reinforced by the observation in individuals with extreme insulin resistance of a high ApN concentration.[47]
In mice, injection of recombinant ApN induces an increase in circulating insulin levels.[48] Indeed, it has been demonstrated that the treatment of pancreatic β cells with ApN induces an increase in insulin exocytosis, accompanied by an increase in expression of Pdx-1 and MafA genes, co-activators of transcription of the insulin gene.[49] ApN promotes the consumption of glucose by stimulating the membrane translocation of GLUT4 in muscle cells and adipocytes following the phosphorylation of AMPK.[50,51] This phenomenon is associated with the activation of the Rab5 protein by the APPL1 protein. Indeed, Rab5 is a GTPase involved in the biogenesis of endosomes, whose role seems crucial during the translocation of GLUT4 from endosomes to the plasma membrane.[52] ApN also inhibits the formation of glucose and glycogen. In liver cells, glycogenolysis and gluconeogenesis are slowed down by ApN, following the decrease in the expression of two key enzymes in these pathways, glucose-6-phosphatase and PEPCK.[53] In muscle cells, glycogen production is also reduced by ApN, following activation of AMPK.[51] ApN induces a drop in blood sugar by its hypoglycemic action, it helps protect the body against the onset of T2DM.[51]
Lipid metabolism
Mice homozygously disabled for the ApN gene exhibit hepatic steatosis in the long term, but not in the short term. Conversely, mice transfected with ApN gene have a reduced hepatic triglyceride content and, fed a diet rich in fat, limit the hepatic accumulation of triglycerides and lipid derivatives. Conversely, ApN promotes the differentiation of adipocytes, their sensitivity to insulin, and their accumulation of triglycerides.[54] It therefore appears that ApN diverts fatty acids from ectopic (non-subcutaneous) lipid deposits towards deposits of young subcutaneous adipocytes, which generate less insulin resistance.[55] Having found no mention in studies of the in vitro effects of ApN on lipoproteins, it is difficult for us to distinguish whether dyslipidemia linked to hypoadiponectinemia results from a direct hepatic effect of the hormone or from an indirect effect of insulin resistance.
ApN increases the transport of fatty acids into muscle cells, stimulating the expression of fatty acid translocase. It also promotes the catabolism of fatty acids by inducing the activity and expression of many enzymes involved in the β-oxidation process.[50] In particular, via AMPK, ApN inactivates ACC by phosphorylation. This enzyme catalyzes the production of malonylcoA, an inhibitor of CPT-1. This protein transports fatty acids to the mitochondria.[56] By lifting the inhibition of CPT-1, ApN promotes mitochondrial transport of fatty acids where they are degraded by the enzymes of β-oxidation. ApN also regulates the transcription of many genes involved in lipid catabolism, such as ACO, FABP3, and CPT-1 by inducing the expression of the transcription factor PPARα.[57]
ApN also promotes the accumulation of triglycerides in adipocytes.[54] In the liver, on the other hand, it reduces the transport of fatty acids and the accumulation of triglycerides.[58] ApN inhibits the expression of around thirty hepatic genes encoding proteins involved in the transport of fatty acids and lipogenesis.[59] ApN therefore controls lipid metabolism by promoting the transport of fatty acids and β-oxidation in muscle cells, by inhibiting hepatic lipogenesis and by stimulating the storage function of adipose tissue. It therefore induces a decrease in circulating lipid levels, exerting a lipid-lowering role in the body.
Cardiovascular effects
In the cardiovascular system, the effects of ApN are not limited to carbohydrate metabolism alone. A role is indeed recognized for it in cardiovascular pathology in particular. Atherosclerosis is the result of a complex process in which the adhesion of circulating monocytes to endothelial cells, their differentiation into macrophagic cells, the accumulation of cholesterol within them, and their transformation into foam cells are key steps. Various receptors such as receptors of the R scavenger family and numerous adhesion molecules including VCAM-1 or ICAM-1 play important roles in it. ApN could suppress the expression of the scavenger type A receptor at the macrophagic level and, in this way, inhibit the lipid accumulation and the transformation into foamy cells of circulating monocytes.[60] Likewise, it would antagonize the action of TNFα. The latter, via the NF-κB pathway, stimulates the transcription of endothelial adhesion molecules and represents a factor in the pro-inflammatory reaction.[61] We reported above that plasma ApN levels were significantly lowered in obese patients, who are also known to be at high cardiovascular risk. Measuring the plasma ApN level could therefore constitute an index to be taken into account in the assessment of coronary risk. In addition, there is a sexual dimorphism: the women have higher ApN levels than men and menopause does not affect their levels.[62,63,64] Oophorectomy does not change plasma ApN levels and estrogen replacement has no effect on them either. In contrast, castrated male mice expressed significantly decreased ApN levels, while, in cell cultures, adding testosterone reduced both circulating ApN and ApN secretion.[63,65] This treatment also induces insulin resistance. In contrast, oophorectomy does not cause changes in ApN levels in female mice. Therefore, ApN may be involved in increasing the cardiovascular risk in men.
In addition, there is a close link between insulin resistance and atherosclerosis. ApN levels are further reduced in patients with T2DM complicated by atherosclerosis. Recently, a modulating effect on vascular remodeling has also been suggested by the suppressive activity of ApN on proliferation and migration of human aortic smooth muscle cells. A mouse model deficient in ApN showed a neointimal formation, in response to an external vascular lesion, twice as large than that observed in normal mice.[66] This type of mouse exhibited moderate insulin resistance accompanied by glucose intolerance. Furthermore, the treatment of ApN-deficient mice with an ApN-producing adenovirus attenuated neointimal proliferation. ApN therefore seems to be a cytokine with an “anti-insulin resistance” and “anti-atherosclerosis” effect.
Therefore, manipulations of the ApN gene in mice have clarified its involvement in vascular and carbohydrate homeostasis. In the absence of ApN, the response to an external arterial injury is exacerbated, with thickening of the intima and excessive proliferation of smooth muscle cells.[67] Conversely, administration of ApN reduces the extent of atherosclerotic lesions that appear spontaneously in apolipoprotein E-deficient mice.[68] More recent studies suggest that ApN may also act as an antithrombotic factor and protect against ischemic damage to the myocardium.[69,70] In humans, epidemiological studies clearly point to low circulating levels of ApN as a risk factor for cardiovascular disease, while high levels are associated with a reduced risk of myocardial infarction.[67]
Adiponectin, obesity, insulin resistance, and T2DM
Conditions associating insulin resistance, T2DM and obesity, as in the case of lipodystrophy, present collapsed serum ApN levels.[58] Studies carried out in knockout mice for the ApN gene (mice genetically deprived of the expression of this protein) provide different elements as to the role played by this adipocytokine. In KO mice, we can measure lower plasma acid clearance and levels of FATP-1, as well as increased TNFα levels in adipose tissue and in plasma. When these mice are put on a diet rich in glucose and lipids, they acquire insulin resistance, probably linked to a decrease in the substrate of the intracellular insulin type 1 receptor, thus causing reduction of intracellular uptake of glucose by insulin-sensitive tissues (mainly skeletal muscle).[58] The restitution of ApN expression in this KO mouse corrects these various metabolic abnormalities. ApN therefore appears to be a hormone sensitizing the action of insulin and a decrease in its production could be linked to the pathophysiology of insulin resistance. In human, many clinical studies indicate that increased ApN is a negative predictor of the development of insulin resistance and T2DM in BMI-adjusted subjects populations.[71,72,73] A lowered plasma ApN level is a risk factor for progression of T2DM. The mechanisms by which ApN improves insulin sensitivity are unknown. Studies in lipoatrophic mice and in animal obesity models suggest that ApN improves tissue sensitivity to insulin by lowering the plasma concentration of free fatty acids. The action of ApN could also involve stimulating the activity of AMPK.[50] Insulin resistance causes stress in the endoplasmic reticulum, where the adiponectin multimer is formed, which in turn activates the unfolded protein response, and then suppresses adiponectin synthesis.[74] In addition, obesity-induced inflammation and oxidative stress inhibit adiponectin maturation and secretion.[75]
In addition, the insulin-sensitizing effects of new anti-diabetic molecules such as the PPARγ nuclear receptor agonists (thiazolinediones) are accompanied by an increase in adiponectinemia. Carriers of the Pro12Ala variant of the PPARγ gene, which we know is protective against T2DM, have also high adiponectinemia. Conversely, diabetic, insulin-resistant, and hypertensive patients with mutants with a negative dominant effect of PPARγ have collapsed adiponectinemia. Thus, in humans, hypoadiponectinemia is a risk factor for T2DM.[76] These results are confirmed by studies in animal models showing unambiguously that insulin resistance and a high susceptibility to atherosclerosis accompany ApN deficiency. Conversely, the administration of ApN in lipodystrophic or obese mice corrects their insulin resistance.[58] Recent genome studies have highlighted the ApN gene on the long arm of chromosome 3 (3q27). At this locus, a susceptibility to diabetes and dysmetabolic syndrome has been localized. Indeed, polymorphisms of this gene are associated with insulin resistance as well as a predisposition to T2DM, most certainly via an alteration in his expression which results in lowered ApN plasma levels.[77] Therefore, the ApN gene has been proposed as a gene for susceptibility to T2DM. Studies in Pima Indians and in the general population also indicate that subjects with high concentrations of ApN are less at risk of developing T2DM than those with low concentrations.[78] Genetic analyzes at the ApN locus on chromosome 3q27 also support the implication of low ApN levels in increasing the risk of T2DM.[46] Thus, the decrease in adiponectinemia is now identified as a contributing factor in the pathogenesis of insulin resistance, T2DM, and cardiovascular pathologies.
ApN and its signal in cancer
Obesity, a disease characterized by significant excess of body fat, is a risk factor for many conditions like T2DM, cardiovascular diseases, as well as malignancies leading to cancers. Obesity decreases ApN while it is now established that ApN helps against cancer by limiting tumor development and the metabolic dysfunctions that they can cause.[79] ApN indeed reduces cancer cells migration and invasion abilities, stops their growth and proliferation, and helps in triggering apoptosis in them.[80] ApN helps fighting endometrial cancer, and, as a corollary, a low ApN level is associated with a faster development of the illness.[81] The analysis of serum of patients with ovarian cancer also show low levels of ApN.[82] AdipoR1 and AdipoR2 are expressed in epithelial ovarian cancer cell, granulosa tumor cell, cancerous epithelial, and ovarian tissues.[83,84,85] A positive AdipoR1 expression in patients with epithelial ovarian cancer is associated with a better life expectancy than a negative AdipoR1 expression.[84] ApN decreased epithelial ovarian cancer cell proliferation independently from apoptosis.[85] Thyroid cancer cells express both AdipoR1 and AdipoR2, while papillary thyroid carcinoma cell lines express a significantly lower number of receptors than normal thyrocytes.[86] In prostate cancers, ApN and its receptors are known to be importantly involved but so far the results are partly contradictory.[87] AdipoR1 and AdipoR2 receptor isoforms expression has thus been measured at a low level in prostate cancerous neoplastic tissue.[88] ApN levels of patients with advanced prostate cancer were measured at a higher level than those at an earlier stage of the illness. Furthermore, AdipoR2 expression has also been reported to be directly associated with prostate cancer progression and metastatization.[89] Recently, scientists have shown that treatment with adiponectin significantly inhibits the proliferation of human pancreatic cancer cells. Suppression of adiponectin receptors abolished the antiproliferative effect of adiponectin and clearly promoted the development of human pancreatic cancer xenografts in nude mice.[90] ApN also inhibits the proliferation of cancer cells in the colon and changes the cell cycle in the G1/S transition phase.[91]
Conclusions
Adiponectin is an adipokine produced and secreted by adipocytes. The biological actions of adiponectin are mediated through interactions with its receptor subtypes, AdipoR1, AdipoR2, and T-cadherin. ApN exerts multiple protective effects on various cell types, such as insulin-sensitizing, anti-inflammation, anti-proliferation, or anti-atherosclerotic actions and suppression of carcinogenesis. ApN is also a relatively abundant serum protein in human. Its levels are decreased in various pathological states including insulin resistance, T2DM, obesity, metabolic syndrome, or cardiovascular diseases. Many studies have shown the protective role of ApN in obesity-associated diseases and cancer. ApN modulates several signaling pathways to exert its physiological and protective functions. Figure 2 shows the involvement of APN in the signaling of its target cells. Focusing on ApN for new therapeutic strategies is full of promise regarding the aforementioned protective action against metabolic diseases.
Figure 2.
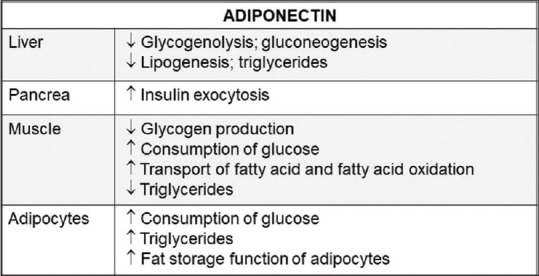
Summary of the involvement of Adiponectin in the signalling of its target cells
Abbreviations
ApN Adiponectin
ACRP30 Adipocyte complement-related protein of 30 kDa
ADIPOQ Adiponectin, C1Q and Collagen Domain Containing
APM1 Adipose most abundant gene transcript 1
GBP28 Gelatin-binding protein 28
LMW Low molecular weight
MMW Medium molecular weight
HMW High molecular weight
AdipoR1 Adiponectin receptor 1
AdipoR2 Adiponectin receptor 2
BMI Body mass index)
KO Knockout
AMPK AMP-activated protein kinase
PPAR Peroxisome proliferator-activated receptor
T2DM Type 2 diabetes mellitus
GLUT4 Glucose transporter type 4
PEPCK Phosphoenolpyruvate carbox kinase
CPT-1 Carnitine palmitoyl transferase-1
ACO Acetyl-CoA oxidase
ACC Acetyl-CoA carboxylase
FABP3 Fatty-acid binding-protein 3
VCAM-1 Vascular cell adhesion molecule-1
ICAM-1 Intercellular adhesion molecule-1
TNFα Tumor necrosis factor alpha
NF-κB Nuclear factor kappa B
FATP-1 Fatty acid transport proteins-1
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 5.Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: In vivo and in vitro studies. Endocrinology. 2004;145:5589–97. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- 6.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 7.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Crawford LJ, Peake R, Price S, Morris TC, Irvine AE. Adiponectin is produced by lymphocytes and is a negative regulator of granulopoiesis. J Leukoc Biol. 2010;88:807–11. doi: 10.1189/jlb.1109723. [DOI] [PubMed] [Google Scholar]
- 9.Paschke L, Zemleduch T, Rucinski M, Ziolkowska A, Szyszka M, Malendowicz LK. Adiponectin and adiponectin receptor system in the rat adrenal gland: Ontogenetic and physiologic regulation, and its involvement in regulating adrenocortical growth and steroidogenesis. Peptides. 2010;31:1715–24. doi: 10.1016/j.peptides.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90:4276–86. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- 11.Caminos JE, Nogueiras R, Gaytan F, Pineda R, Gonzalez CR, Barreiro ML, et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149:3390–402. doi: 10.1210/en.2007-1582. [DOI] [PubMed] [Google Scholar]
- 12.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133:719–31. doi: 10.1530/REP-06-0244. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–10. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Gomez V, Lu L, Yang X, Wu X, Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:233–7. doi: 10.1111/j.1440-1746.2008.05548.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–7. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 18.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: Mechanisms and functional implications. Biochem J. 2008;409:623–33. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 22.Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: A cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007;92:1857–62. doi: 10.1210/jc.2006-2310. [DOI] [PubMed] [Google Scholar]
- 23.Sulistyoningrum DC, Gasevic D, Lear SA, Ho J, Mente A, Devlin AM. Total and high molecular weight adiponectin and ethnic-specific differences in adiposity and insulin resistance: A cross-sectional study. Cardiovasc Diabetol. 2013;12:170. doi: 10.1186/1475-2840-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 25.Arai Y, Nakazawa S, Kojima T, Takayama M, Ebihara Y, Shimizu KI, et al. High adiponectin concentration and its role for longevity in female centenarians. Geriatr Gerontol Int. 2006;6:32–9. [Google Scholar]
- 26.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 27.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 29.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–22. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 33.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda K, Fujishima Y, Maeda N, Mori T, Hirata A, Sekimoto R, et al. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology. 2015;156:934–46. doi: 10.1210/en.2014-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda S, Kita S, Obata Y, Fujishima Y, Nagao H, Masuda S, et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J Biol Chem. 2017;292:7840–9. doi: 10.1074/jbc.M117.780734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight. 2018;3:e99680. doi: 10.1172/jci.insight.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauche IB, Ait El Mkadem S, Rezsohazy R, Funahashi T, Maeda N, Miranda LM, et al. Adiponectin downregulates its own production and the expression of its AdipoR2 receptor in transgenic mice. Biochem Biophys Res Commun. 2006;345:1414–24. doi: 10.1016/j.bbrc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Kim MJ, Maachi M, Debard C, Loizon E, Clement K, Bruckert E, et al. Increased adiponectin receptor-1 expression in adipose tissue of impaired glucose-tolerant obese subjects during weight loss. Eur J Endocrinol. 2006;155:161–5. doi: 10.1530/eje.1.02194. [DOI] [PubMed] [Google Scholar]
- 39.Bluher M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin Sci (Lond) 2016;130:1603–14. doi: 10.1042/CS20160005. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: Effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- 41.McAinch AJ, Steinberg GR, Mollica J, O'Brien PE, Dixon JB, Macaulay SL, et al. Differential regulation of adiponectin receptor gene expression by adiponectin and leptin in myotubes derived from obese and diabetic individuals. Obesity (Silver Spring) 2006;14:1898–904. doi: 10.1038/oby.2006.221. [DOI] [PubMed] [Google Scholar]
- 42.Storgaard H, Poulsen P, Ling C, Groop L, Vaag AA. Relationships of plasma adiponectin level and adiponectin receptors 1 and 2 gene expression to insulin sensitivity and glucose and fat metabolism in monozygotic and dizygotic twins. J Clin Endocrinol Metab. 2007;92:2835–9. doi: 10.1210/jc.2006-1812. [DOI] [PubMed] [Google Scholar]
- 43.Ordelheide AM, Heni M, Gommer N, Gasse L, Haas C, Guirguis A, et al. The myocyte expression of adiponectin receptors and PPARδ is highly coordinated and reflects lipid metabolism of the human donors. Exp Diabetes Res. 2011;2011:692536. doi: 10.1155/2011/692536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 45.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–60. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 47.Semple RK, Cochran EK, Soos MA, Burling KA, Savage DB, Gorden P, et al. Plasma adiponectin as a marker of insulin receptor dysfunction: Clinical utility in severe insulin resistance. Diabetes Care. 2008;31:977–9. doi: 10.2337/dc07-2194. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–35. doi: 10.1007/s00125-008-0944-9. [DOI] [PubMed] [Google Scholar]
- 49.Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623–31. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 51.Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132–9. doi: 10.1007/s00125-004-1609-y. [DOI] [PubMed] [Google Scholar]
- 52.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–23. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 53.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–79. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–76. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 56.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012;109:14568–73. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 61.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 62.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–76. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 63.Gui Y, Silha JV, Murphy LJ. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res. 2004;12:1481–91. doi: 10.1038/oby.2004.185. [DOI] [PubMed] [Google Scholar]
- 64.Yu H, Chhabra KH, Thompson Z, Jones GL, Kiran S, Shangguan G, et al. Hypothalamic POMC deficiency increases circulating adiponectin despite obesity. Mol Metab. 2020;35:100957. doi: 10.1016/j.molmet.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 66.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis.The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 68.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 69.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, et al. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Thromb Vasc Biol. 2006;26:224–30. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 70.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–40. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 72.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T. Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: Two-year follow-up study in Japanese population. J Clin Endocrinol Metab. 2004;89:87–90. doi: 10.1210/jc.2003-031163. [DOI] [PubMed] [Google Scholar]
- 74.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59:2809–16. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soares AF, Guichardant M, Cozzone D, Bernoud-Hubac N, Bouzaidi-Tiali N, Lagarde M, et al. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–9. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 77.Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, et al. Association of adiponectin mutation with type 2 diabetes: A candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–8. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- 78.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 79.Vansaun MN. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926–32. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schott S, Schneeweiss A, Sohn C. Breast cancer and diabetes mellitus. Exp Clin Endocrinol Diabetes. 2010;118:673–7. doi: 10.1055/s-0030-1254116. [DOI] [PubMed] [Google Scholar]
- 81.Rzepka-Gorska I, Bedner R, Cymbaluk-Ploska A, Chudecka-Glaz A. Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women. Eur J Gynaecol Oncol. 2008;29:594–7. [PubMed] [Google Scholar]
- 82.Jin JH, Kim HJ, Kim CY, Kim YH, Ju W, Kim SC. Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet Gynecol Sci. 2016;59:279–85. doi: 10.5468/ogs.2016.59.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tiwari A, Ocon-Grove OM, Hadley JA, Giles JR, Johnson PA, Ramachandran R. Expression of adiponectin and its receptors is altered in epithelial ovarian tumors and ascites-derived ovarian cancer cell lines. Int J Gynecol Cancer. 2015;25:399–406. doi: 10.1097/IGC.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Yu Z, Fang L, Liu F, Jiang K. Expression of adiponectin receptor-1 and prognosis of epithelial ovarian cancer patients. Med Sci Monit. 2017;23:1514–21. doi: 10.12659/MSM.899990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffmann M, Gogola J, Ptak A. Adiponectin reverses the proliferative effects of estradiol and IGF-1 in human epithelial ovarian cancer cells by downregulating the expression of their receptors. Horm Cancer. 2018;9:166–74. doi: 10.1007/s12672-018-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitsiades N, Pazaitou-Panayiotou K, Aronis KN, Moon HS, Chamberland JP, Liu X, et al. Circulating adiponectin is inversely associated with risk of thyroid cancer: In vivo and in vitro studies. J Clin Endocrinol Metab. 2011;96:E2023–8. doi: 10.1210/jc.2010-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu MB, Xu H, Hu JM, Zhu WH, Yang T, Jiang HW, et al. Genetic polymorphisms in leptin, adiponectin and their receptors affect risk and aggressiveness of prostate cancer: Evidence from a meta-analysis and pooled-review. Oncotarget. 2016;7:81049–61. doi: 10.18632/oncotarget.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: A case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–13. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 89.Rider JR, Fiorentino M, Kelly R, Gerke T, Jordahl K, Sinnott JA, et al. Tumor expression of adiponectin receptor 2 and lethal prostate cancer. Carcinogenesis. 2015;36:639–47. doi: 10.1093/carcin/bgv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang J, Fan Y, Zhang W, Shen Y, Liu T, Yao M, et al. Adiponectin suppresses human pancreatic cancer growth through attenuating the beta-catenin signaling pathway. Int J Biol Sci. 2019;15:253–64. doi: 10.7150/ijbs.27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim AY, Lee YS, Kim KH, Lee JH, Lee HK, Jang SH, et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24:1441–52. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]