Abstract
In the last few decades, heparan sulfate (HS) proteoglycans (HSPGs) have been an intriguing subject of study for their complex structural characteristics, their finely regulated biosynthetic machinery, and the wide range of functions they perform in living organisms from development to adulthood. From these studies, key roles of HSPGs in tumor initiation and progression have emerged, so that they are currently being explored as potential biomarkers and therapeutic targets for cancers. The multifaceted nature of HSPG structure/activity translates in their capacity to act either as inhibitors or promoters of tumor growth and invasion depending on the tumor type. Deregulation of HSPGs resulting in malignancy may be due to either their abnormal expression levels or changes in their structure and functions as a result of the altered activity of their biosynthetic or remodeling enzymes. Indeed, in the tumor microenvironment, HSPGs undergo structural alterations, through the shedding of proteoglycan ectodomain from the cell surface or the fragmentation and/or desulfation of HS chains, affecting HSPG function with significant impact on the molecular interactions between cancer cells and their microenvironment, and tumor cell behavior. Here, we overview the structural and functional features of HSPGs and their signaling in the tumor environment which contributes to tumorigenesis and cancer progression.
Keywords: tumor microenvironment, extracellular matrix, heparan sulfate proteoglycans, remodeling, signaling
1. Introduction
The tumor microenvironment consists of a heterogeneous population of cells such as proliferating tumor cells and infiltrating inflammatory cells, the tumor stroma, blood vessels, secreted factors, and extracellular matrix (ECM) components, all together contributing to cancer development and progression. Complex and dynamic interactions between tumor cells and their microenvironment, involving cell-cell and cell-ECM contacts and the activity of soluble factors that enable these contacts, are essential to promote tumor growth, invasion, and metastasis [1,2,3]. Hence, due to the compelling role of tumor microenvironment in carcinogenesis, therapeutic strategies targeting tumor microenvironment components that interfere with the complex crosstalk between tumor cells, host cells, and their surrounding ECM are being explored [4,5,6].
The ECM constituents form a highly dynamic network that plays both structural and functional roles of key importance for development and tissue homeostasis. The composition of ECM may differ among tissues and continuously undergo remodeling both in physiological and pathological conditions [7,8,9]. The main ECM components include fibrillar proteins such as collagen, elastin, fibronectin, and laminins, glycosaminoglycans (GAGs), proteoglycans (PGs), and other glycoproteins. The interaction between ECM components and cell surface receptors and/or matrix effectors activates signal transduction cascades underlying cell differentiation, proliferation, survival, adhesion, migration, and other biological processes relevant to cancer biology [8].
Among ECM components, heparan sulfate (HS) proteoglycans (HSPGs) emerged as critical determinants in ECM assembly and functions both in health and disease [10,11]. The ubiquitously expressed HSPGs comprise diverse families of HS chains bearing protein cores that include syndecans, glypicans, perlecan, agrin, and collagen type XVIII. While perlecan, agrin, and collagen type XVIII are directly secreted in the ECM once synthesized, the transmembrane syndecans and glycosylphosphatidylinositol-anchored (GPI)-anchored glypicans are cell surface-bound HSGPs, but they can be cleaved by proteinases or heparanases, and their truncated forms can be distributed in the ECM. The sulfated moieties enable HSPGs to interact with other ECM components and a variety of ligands such as morphogens, growth factors, enzymes, cytokines, chemokines, etc. [12,13,14,15]. However, not only the sulfated pattern of HS chains dictates the binding specificity of HSPGs, but their protein core can also bind ligands, and the ECM secreted HSPG types contain functionally independent ligand-binding domains [11,12,13,16]. The HSPG binding ability is essential for regulating the distribution, availability, and signaling activity of the ligands.
The main activity attributed to HSPGs is to serve as co-receptors for morphogens/growth factors, thus enhancing signaling activation of their respective receptor, however, HSPGs can act as receptors themselves and can transactivate receptors in adjacent cells [10,11,12,13,15,17]. In addition, HSPGs are involved in endocytosis and vesicular trafficking [18]. By acting as intermediaries between ECM and intracellular signaling pathways, HSPGs regulate a variety of physiological and pathological processes including tissue development, morphogenesis, cell proliferation, apoptosis, adhesion, motility, wound healing, inflammation, and tumorigenesis [10,11,17,19,20,21,22,23,24,25,26,27,28].
Altered expression and structural variability of HSPGs have been associated with an extensive remodeling of tumor microenvironment where HSPGs not only contribute to the formation of a structural framework for tumor growth but are also involved in the regulation of cell-matrix and cell-cell interactions, and cell signaling [29,30,31,32,33,34,35]. They are able to modulate cancer cell phenotype, the development of drug resistance, and tumor stroma angiogenesis [36,37,38,39,40,41]. Differential expression and structure/activity modifications of HSPGs have been found in several cancers and may correlate with either inhibitory or tumor-promoting activity. This review focuses on the structural and functional alterations of HSPGs in tumor microenvironment that have a significant impact on tumor growth and progression. We also discuss the advancements in the development of cancer therapies targeting HSPGs.
2. Structural Features, Biosynthesis, and Enzymatic Modification of HSPGs Regulating Cancer Promotion and Progression
The HSPGs are glycosylated proteins characterized by a core protein carrying covalently attached HS chains (Table 1). Thirteen genes encode HSPG core proteins. They include the genes encoding for cell surface-tethered 4 syndecan (SDC1-4) and 6 glypican (GPC1-6) isoforms, and 3 encoding for the basement membrane- and -ECM localized perlecan, agrin, and collagen type VIII [11]. Syndecan isoforms are transmembrane glycoproteins with the extracellular domain harboring HS chains and chondroitin sulfate chains, and highly conserved transmembrane and cytoplasmic domains which mediate multimerization and interactions with intracellular proteins, respectively. Glypicans are proteins anchored to the cell membrane by GPI, and with HS chains attached near the juxtamembrane region. Perlecan, agrin, and collagen type XVIII are localized in the ECM, including the basement membrane zone [11,16,42].
Table 1.
Heparan sulfate proteoglycan (HSPG) nomenclature, human genes, schematic structure, cellular localization.
| HSPG | Encoding Gene | Schematic Structure | Cellular Localization |
|---|---|---|---|
| Syndecan-1 | SDC1 |
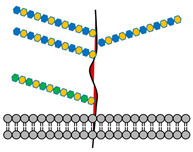
|
Cell surface |
| Syndecan-2 | SDC2 | ||
| Syndecan-3 | SDC3 | ||
| Syndecan-4 | SDC4 | ||
| Glypican-1 | GPC1 |
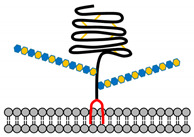
|
|
| Glypican-2 | GPC2 | ||
| Glypican-3 | GPC3 | ||
| Glypican-4 | GPC4 | ||
| Glypican-5 | GPC5 | ||
| Glypican-6 | GPC6 | ||
| Perlecan | PRCAN |
|
ECM, Basement membrane |
| Agrin | AGRN |
|
|
| Collagen type VIII | COL8A1 |
|
In HSPGs, the HS chains are constituted by a long unbranched backbone of disaccharide units of D-glucosamine and uronic acid (D-glucuronic and L-uronic acids) carrying negatively charged carboxylated or N- and O-sulfated groups generated through tightly regulated post-translational reactions in the Golgi apparatus upon the arrival of the core protein from the endoplasmic reticulum [17]. The HSPG biosynthetic process starts with the attachment of a specific serine residue of the core protein to a tetrasaccharide linker (glucuronic acid-galactose-galactose-xylose) bearing HS chains; this reaction is catalyzed by xylosyltransferase (XTLY). Exostosin (EXT) enzymes catalyze the elongation of HS chains through the alternate addition of glucuronic acid and N-acetylglucosamine. Then, the HS backbone undergoes modifications involving N-deacetylation and N-sulfation of glucosamine, C-5 epimerization of glucuronic acid to iduronic acid, 2-O-sulfation and 3-O-sulfation of uronic acid and glucosamine, respectively, and 6-O-sulfation of N-acetylated or N-sulfated glucosamine residues. Additional modifications occur at the cell surface or ECM through the action of 6-O-endo-sulfatases and/or the endoglycosidase heparanase. The controlled biosynthesis and post-synthetic modifications of HS chains provide an enormous potential of heterogeneity and structural variability of HS chains which accounts for a wide variety of HSPG interactions with regulatory proteins and, in turn, for their biological activities [12,13,14,15,43]. Several studies have demonstrated that there are cell and tissue-specific changes in the HS chain synthetic pathway in cancer cells and tissues in vitro and in vivo, thus suggesting a close involvement of HS chain biosynthetic machinery in carcinogenesis [30,44,45]. These changes may concern either the expression and/or activity of HS synthetic and modifying enzymes, or changes in the HSPGs protein core.
The genetic loss of NDST4, a member of the N-deacetylase/N-sulfotransferase (NDST) family, correlates with an advanced pathological stage and poor survival in colorectal carcinomas [46]. Interestingly, depending on the metastatic nature of the tumor and its localization, differential expression in the transcription of genes involved in the epimerization and sulfation of uronic acid, and glucosamine sulfation were detected in left- and right-sided colorectal cancers [31]. Defective HS-3-O-sulfation due to methylation-associated repression of HS glucosamine 3-O-sulfotransferase gene (3-OST) results in being associated with chondrosarcoma progression [47], whereas hypermethylation of the 3-OST gene is associated with poor survival in non-small cell lung cancer [48]. In addition, HS-2-O-sulfotransferase (2-OST) results in being essential for the proliferation and invasion of prostate cancer cells [49]. Overexpression of HS glucosamine 6-O-sulfotransferase-2 (6-OST) has been reported in colorectal cancer and gastric cancer, while it results in being downregulated in glioma [50,51,52].
Mutations in EXT1 or EXT2, members of the EXT family of glycosyltransferases are responsible for hereditary multiple osteochondromas that may degenerate into chondro- or osteo-sarcomas [53]. Furthermore, mutations in EXT2 have been detected in breast tumor patients, and thyroid cancer [54,55,56]. Epigenetic inactivation of EXT1 by promoter hyper-methylation preventing HS chain synthesis is observed in leukemia and non-melanoma skin cancer [57,58]. An antiproliferative effect of D-glucuronyl C5-epimerase (GLCE) has been ascertained in breast and small lung cancer cells [59,60,61], whereas increased GLCE expression has been associated with advanced stages of prostate tumors [62,63]. Although many other examples of the dysregulation of HS biosynthetic and post-synthetic modifying enzymes in carcinogenesis have been reported (Table 2), the complex changes of their expression in different cancers remains still to be explored.
Table 2.
HS biosynthetic and modifying enzymes involved in cancer development and progression.
| Enzyme | Gene | Type(s) of Cancer | Reference(s) |
|---|---|---|---|
| Xylosyltransferase1/2 (XYLT1/2) |
XYLT1-2 | Breast cancer/bone metastasis Salivary gland tumors |
[64] [65] |
| β-1,4-Galactosyltransferase (b4Gal-T1-7) |
B4GALT1-7 | Breast cancer Colon cancer Liver cancer Leukemia Lung cancer Neuroblastoma Renal carcinoma |
[66] [67] [68] [69] [70] [71] [72] |
| β-1,3-Glucuronyltransferase3 (GlcAT-I) |
B3GAT3 | Liver cancer | [73] |
| Exostosin like glycosyltransferase (EXTL1-3) |
EXTL1-3 | Breast cancer Hepatocarcinoma |
[55] [74] |
| Exostosin1/2 (EXT1/2) |
EXT1-2 | Breast cancer Chondrosarcoma Osteochondroma Hepatocarcinoma Glioma Leukemia Thyroid tumor |
[54,55] [75,76] [53,75,76] [77] [52] [57,58] [56] |
| N-deacetylase/N-sulfotransferase (1-4) (NDST1-4) |
NDST1-4 | Colorectal cancer Melanoma |
[31,46] [78] |
| Glucuronyl C5-epimerase (GLCE) |
GLCE | Breast cancer Lung cancer Prostate cancer |
[59,60] [61] [62,63] |
| Hexuronyl 2-O-sulfotransferase (2-OST) |
HS2ST | Breast cancer Multiple myeloma Prostate cancer |
[79] [30] [49] |
| Glucosaminyl 6-O-sulfotransferase (6-OST) |
HS6ST | Colorectal cancer Gastric cancer Glioma Ovarian cancer Pancreatic cancer |
[50] [51] [52] [80,81] [82] |
| Glucosaminyl 3-O-sulfotransferase (3-OST) |
HS3ST | Breast cancer Chondrosarcoma Colorectal cancer Leukemia Lung cancer Pancreatic cancer |
[83] [47,83] [84] [85] [48] [86] |
| Endo-6-O-sulfatase1/2 (SULF1/2) |
SULF1-2 | Breast cancer Cervical cancer Liver tumors Ovarian cancer Other cancers |
[87] [88] [89] [87] [90,91] |
| Heparanase (HPSE1/2) |
HPSE1-2 | Bladder cancer Brain tumors Breast cancer Gastric cancer Head and neck cancers Hepatocarcinoma Mesothelioma Myeloma Ovarian cancer Pancreatic cancer Sarcoma |
[92] [93] [94,95] [96] [97] [98] [99] [100,101] [102] [103] [104] |
In addition to the differential expression and/or activity of the enzymes involved in the biosynthesis or post-synthetic modification of HS chains, HSPG core proteins may also affect cancer development and progression, either by preventing or promoting these processes [10,11,36,39,40]. The alterations in the expression levels of HSPGs depend on their location and may represent a hallmark of the metastatic or non-metastatic nature of the tumor. For example, while SDC1 results in being overexpressed in left-sided colorectal tumors independently from the presence of metastasis, it results in being upregulated only in metastatic right-sided colorectal cancers [31,105]. However, a significant reduction of cell surface tethered SDC1 and an increase of shed SDC1 in the ECM has been observed as a function of tumor progression and aggressiveness, suggesting the involvement of post-transcriptional mechanisms in SDC1 expression in this type of tumor. Differential regulation of SDC1 expression as well as of the other SDC isoforms, GPCs, and the other HSPGs has been found in several tumors (Table 3) [105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153].
Table 3.
Differential expression of individual HSPGs in cancer.
| HSPG | Changes in Expression Levels | Type(s) of Cancer | Reference(s) |
|---|---|---|---|
| SDC1 | Increased | Bladder cancer, breast cancer, colorectal cancer, multiple myeloma, ovarian cancer, pancreatic ductal adenocarcinoma, squamous cell carcinoma | [29,31,35,105,108,109] |
| Reduced | Cancer stem cell, colorectal cancer, endometrial cancer, hepatocellular carcinoma, mesothelioma, non-small-cell lung cancer, prostate cancer, sarcoma | [35,108,110,111] | |
| SDC2 | Increased | Bladder cancer, breast cancer, colorectal cancer, glioma, lung cancer, melanoma, prostate cancer | [112,113] |
| Reduced | Osteosarcoma | [114] | |
| SDC3 | Increased | Bladder cancer, ovarian cancer, renal cell carcinoma | [115,116,117] |
| Reduced | Neuroblastoma | [35] | |
| SDC4 | Increased | Ovarian cancer, papillary thyroid carcinoma | [115,118] |
| Reduced | Neuroblastoma | [35] | |
| GPC1 | Increased | Breast cancer, esophageal squamous cell carcinoma, glioma, pancreatic cancer | [119,120,121,122,123] |
| Reduced | Colorectal cancer, neuroblastoma | [35,105] | |
| GPC2 | Increased | Neuroblastoma, medulloblastoma, retinoblastoma | [124,125] |
| GPC3 | Increased | Liver cancer, lung squamous cell carcinoma, neuroblastoma, ovarian cancer, testicular germ cell tumor, thyroid cancer, yolk sac tumor | [125,126,127,128,129] |
| Reduced | Breast cancer, colorectal cancer, mesothelioma, non-small-cell lung cancer, neuroblastoma, renal cell carcinoma | [35,105,125,130] | |
| GPC4 | Increased | Colorectal cancer, pancreatic cancer | [31,131] |
| Reduced | Breast cancer, ovarian carcinoma | [125,132,133] | |
| GPC5 | Increased | Rhabdomyosarcoma | [35,134,135] |
| Reduced | Breast cancer, glioma, hepatocellular carcinoma, lung cancer, pancreatic cancer, prostate cancer | [136,137,138] | |
| GPC6 | Increased | Gastric cancer, melanoma | [139,140] |
| Reduced | Colorectal cancer, ovarian cancer, retinoblastoma | [105,141,142] | |
| Perlecan | Increased | Hepatocellular carcinoma, melanoma, pancreatic cancer, prostate cancer | [35,38,143,144,145,146] |
| Reduced | Breast cancer, colorectal cancer, lung cancer, ovarian cancer, fibrosarcoma | [35,38,105,143,144,147] | |
| Agrin | Increased | Cholangiocarcinoma, glioma, hepatocellular carcinoma, lung cancer, oral squamous cell carcinoma, rectal cancer | [38,148,149,150,151,152] |
| Collagen type VIII | Increased | Breast cancer, lung cancer, melanoma, ovary, pancreatic cancer, prostate cancer | [35,38,153] |
| Reduced | Colorectal cancer | [105] |
High levels of SDC1 have been detected in squamous cell carcinomas such as those from cervix uteri and esophagus, in invasive urothelial cancer, and lung cancer [108]. Overexpression of SDC1 correlates with tumor aggressiveness and poor survival in triple-negative breast carcinoma [109]. Both SDC1 and SDC4 are overexpressed in papillary thyroid carcinoma [118]. Conversely, reduced expression of SDC1 has been found in mesothelioma, non-small-cell lung cancer, prostate cancer, and sarcoma [35,108,110,111]. SDC2 expression is upregulated in breast, colon, and pancreatic cancers, and melanomas, whereas high levels of SDC2 in neuroendocrine tumors correlate with a better survival of patients [112,113]. On the contrary, a tumor-suppressor function for SDC2 correlated to apoptosis dysregulation in osteosarcoma has been suggested [114]. Elevated expression levels of SDC3 have been reported in bladder and ovarian cancer, and renal cell carcinoma [115,116,117], whereas low levels of SDC3, SDC4, GPC1, and GPC3 are expressed in neuroblastoma [35].
Overexpression of GPC1 is a hallmark of breast cancer, esophageal squamous cell carcinoma, and gliomas [119,120,121]. The upregulation of GPC1 and GPC4 is found in pancreatic cancer [122,123]. High expression of GPC2 has been detected in neuroblastoma and other pediatric cancers such as medulloblastoma and retinoblastoma [124,125]. While CPG3 results in being overexpressed in liver cancer, lung squamous cell carcinoma, neuroblastoma, ovarian cancer, testicular germ cell tumor, thyroid cancer, yolk sac tumor and other cancers, reduced levels of GPC3 have been found in breast cancer, colorectal cancer, mesothelioma, non-small-cell lung cancer, neuroblastoma, and renal cell carcinoma [35,105,125,126,127,128,129,130]. Overexpression of GPC4 mRNA has been detected in metastatic colorectal cancer, where GPC1, GPC3 and GPC6, perlecan, and collagen type VIII result in being downregulated [31,35,105]. While GPC5 expression is downregulated in breast cancer, glioma, hepatocellular carcinoma, lung cancer, pancreatic cancer, prostate cancer, it results in being upregulated in rhabdomyosarcoma [35,134,135,136,137,138]. Overexpression of GPC6 is associated with gastric adenocarcinoma and metastatic progression of cutaneous melanoma [140]. Increased expression levels of perlecan have been found in hepatocellular carcinoma, melanoma, pancreatic and prostate cancer, whereas the upregulation of the expression of agrin has been demonstrated in oral squamous cell carcinoma, hepatocellular carcinoma, cholangiocarcinoma, lung carcinoma, oral squamous cell carcinoma, and rectal cancer [35,38,144,145,146,148,149,150,151,152]. Reduced levels of perlecan correlate with the progression of breast cancer, colorectal cancer, lung cancer, ovarian cancer, and fibrosarcoma [35,38,105,143,144,147]. Finally, type VIII collagen results in being elevated in melanoma, lung, breast, ovary, prostate, and pancreatic cancers [35,38,153].
Noticeably, in some cases, the HS chain and the protein core of an HSPG may have a distinct impact on the same tumor. For example, in Lewis lung carcinoma, clones with a low metastatic potential contain high levels of SDC2, whereas, in highly metastatic clones, SDC2 overexpression reduces the invasive potential of cells due to the binding of HS chains to the fibronectin [112]. The expression patterns of HSPGs in tumor cells and microenvironment in some cases correlate with those of ligands that require HSPGs to elicit their cellular responses. [33,34,35,36,38,39,40,41,106,107]. The aberrant expression of specific HSPGs in the various types of cancers significantly affects HSPG-ligand binding and subsequent signaling, thus determining the malignancy of the tumor phenotype. Therefore, HSPGs can serve as cancer-type-specific biomarkers, prognostic factors, and therapeutic targets.
It has been well established that cell surface and ECM secreted HSPGs may undergo a cleavage process known as “shedding” which regulates the amount of HSPGs tethered to the cell surface and that present in the pericellular microenvironment [10,11,12,13,14]. The enzymes involved in the HSPG shedding depend on the type of HSPG and include the endoglycosidase heparanase and endosulfatases that modify the structure of HS chains; matrix metalloproteinases (MMPs) and ADAMs, composed of a disintegrin and MMP proteases, for SDCs shedding; the extracellular lipase Notum that cleaves the GPI anchor of GPCs; and other proteases that cleave the core proteins of ECM secreted HSPGs [33,34,37,39,42,154,155]. The cleaved HSPG products released in the tumor microenvironment may have a significant impact on cancer cell behavior [91]. The proteolysis of the SDC juxtamembrane region releases their whole ectodomains in the ECM [29]. Soluble SDC1 promotes the growth of myeloma tumors in vivo, while shed SDC2 enhances colon, lung, and breast cancer progression [11,91,100,101,156,157]. SDC-1 shedding is associated with increased mitogenic activity and invasive potential of pancreatic cancer cells, whereas shedding of SDC4 in human endothelial cells promotes wound healing, angiogenesis, and inflammation [156,157]. Furthermore, SDC1 shedding has been shown to trigger a switch from a proliferative to an invasive phenotype of breast cancer cells [158]. The cleavage of GPC1 by ADAM17 plays a role in the adhesion, proliferation and migration of oral squamous cell carcinoma cells [159]. At the basement membrane of the cells, perlecan can undergo shedding through heparanase, MMPs, and other proteases [145]. The C-terminal fragment of perlecan, known as endorepellin, resulting from the proteolytic cleavage of perlecan, may undergo further proteolysis that leads to the release of the C-terminal endorepellin fragment LG3 whose levels are reduced in breast cancer [160]. LG3 and other endorepellin fragments have been found in the secretome of colon and pancreatic cancers [161,162]. On the other hand, the proteases cathepsin L and elastase cleave the N-terminal hinge domain of collagen type VIII, releasing the 22-kDa fragment endostatin which is known to inhibit the progression of several types of malignant tumors, including melanomas, fibrosarcomas, and hemangioendothelioma [163,164]. Both MMPs and the serine protease cleave the HSPG agrin giving rise to 100-, 90-, and 22-kDa fragments which are involved in cancer growth [38].
The above reported are only few examples of the broad impact of HSPG structural features in cancer development and progression. Interestingly, the complexity of structural properties of HSPGs translates in a variety of biological activities that may either positively or negatively regulate tumor initiation and progression.
3. Functional Properties of HSPGs in Tumor Microenvironment
The sulfated HS side chains bearing multiple negative charges, but also protein cores, allow HSPGs to bind and interact with a broad variety of signaling effectors in the tumor microenvironment [165]. These HSPG-ligand interactions serve multiple functions including the modulation of ligand distribution and function, the restriction of ligand range of action on target cells, the prevention of ligand degradation, the generation of morphogen gradients, the proper presentation of growth factors to their cognate receptors, the transactivation of receptors in adjacent cells, the promotion of endocytosis and vesicular trafficking, etc. [7,8,10,11,12,13,14,15,17,18]. In addition to a well-established role in development [20,23,26,104,165,166,167], HSPG-ligand interactions play major roles in tumor stroma and tumor microenvironment by regulating cellular proliferation, differentiation, adhesion, migration, apoptosis, angiogenesis, inflammation, invasion, and metastasis [3,22,24,25,28,33,34,35,36,37,38,39,40,107,143,165,168] (Figure 1).
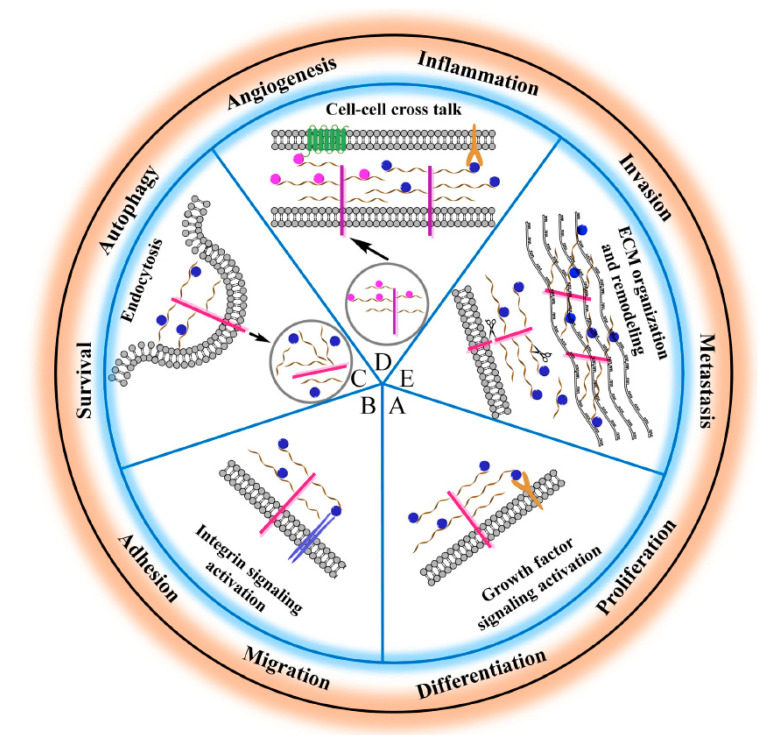
Figure 1.
Schematic representation of the main HSPG functions relevant to cancer cell biology. (A,D) HSPGs serve as a signaling co-receptor for growth factor activity, allowing a proper presentation of them to their cognate receptors, on the same or adjacent cells. In panel D, transcellular transport of a ligand (i.e., chemokine) bound to HS chains and its presentation at the cell surface is also shown. (B,D) HGPGs bind integrins modulating their downstream signaling that regulates cytoskeleton organization as well as cell adhesion, spreading and sensing mechanical stress. (C) HSPGs act as endocytic receptors and undergo constitutive as well as ligand-induced endocytosis: exosomes, cell-penetrating peptides, polycation–nucleic acid complexes, lipoproteins, growth factors, and morphogens enter cells through this mechanism. Internalized cargo can be sorted for lysosomal degradation, escape into the cytosol, or recycle back to the plasma membrane. (E) HSPGs are critical determinants of extracellular matrix (ECM) assembly and remodeling. If the HSPGs perlecan, agrin, and collagen type XVIII are directly secreted in the ECM, cell surface-tethered HSPGs (syndecans and glypicans) undergo proteolytic cleavage of their ectodomains or to cleavage of HS chains by heparanases and their truncated forms can be distributed in the ECM. Here, HSPGs act as a reservoir of growth factors and supply them to target cells when needed. Otherwise, they may act as a barrier for growth factors, by preventing their passive diffusion over longer distances, instead of confining them to the vicinity of producing cells. Overall, HSPGs control fundamental cellular processes (i.e., cell adhesion, migration, etc.) whose dysregulation underlies tumor development and progression.
3.1. HSPG-Regulated Mechanisms in Cell-Matrix and Cell-Cell Interactions
One of the most studied molecular mechanisms of ligand-receptor complex formation and signaling activation mediated by HSPGs is related to the action of fibroblast growth factor (FGF) family members and their tyrosine kinase receptors (FGFR) [10,11,12,13,14,15,169]. The HS chain of HSPGs binds the FGF ligand and receptor forming a ternary complex that promotes FGFR dimerization, and in turn activates signaling. Depending on the tumor type, HSPG-regulated FGF binding and receptor dimerization triggers the activation of four main signaling pathways, including mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and protein kinase C (PKC) pathways [15,35,125,170]. However, other HSPG-mediated FGF/FGFR downstream signaling, such as Jun N-terminal kinase (JNK), ribosomal protein S6 kinase 2 (RSK2), and Rho GTPase pathways, have been described to play a role in some cancers [35,125,171,172,173].
Commonly, the MAPK/ERK signaling cascade activated by FGFs is implicated in cell growth and differentiation, the PI3K/AKT signaling cascade in cell survival and cell fate determination, and PKC in cell polarity [174]. For example, these pathways are involved in SDC1 activation of FGF2-FGFR1 complex formation and downstream signaling leading to malignant transformation in lymphomas, breast, and prostate cancer [16,106,107,175,176]. However, in breast cancer, while membrane-bound SDC1 promotes cell proliferation and inhibits invasion through FGF2 mediated MAPK signaling, soluble SDC1 deriving from proteolytic cleavage of membrane-bound SDC1 may trigger a switch from a proliferative to an invasive phenotype through Rho GTPase pathways [159]. The shedding of SDC1 serves an important role in the regulation of FGF2 signaling activation of the PI3K/Akt pathway that promotes epithelial-mesenchymal transition, invasion, and metastasis of pancreatic cancer cells [177]. In gliomas, GPC1 contributes to enhance mitogenic signaling via forming a ternary complex with FGF2 and the FGFR and activating both MAPK/ERK and PI3K/AKT pathways [178,179]. In rhabdomyosarcomas, GPC5 enhances FGF2 signaling that leads to mesodermal cell proliferation without inducing myogenic differentiation [134]. Furthermore, GPC5 regulates lung cancer development through a complex pathway network, including FGF-mediated activation of MAPK, PI3K, and STAT pathways [180]. The HS chains of perlecan are known to bind FGF2 promoting receptor activation, and mitogenic and pro-angiogenic signaling in different tumors, whereas the protein core of perlecan is implicated in FGF7 binding and activation of its receptor and downstream MAPK signaling leading to human colon carcinoma cell growth [38,146,181].
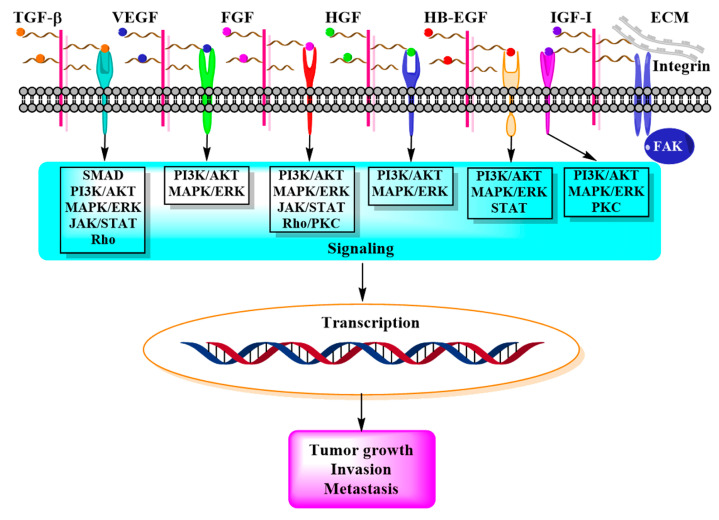
In addition to FGF, HSPGs bind several other growth factors such as hepatocyte growth factor (HGF), epidermal growth factor (EGF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), transforming growth factor (TGF) beta, vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 receptor (IGF1R), and modulate their signaling in a context-dependent fashion [13,15] (Figure 2).
Figure 2.
Schematic representation of the interaction between HSPGs, growth factors, and receptors, and main downstream signaling pathways that lead to tumor development and progression.
The HSPG-mediated signaling activation of HGF released in the tumor microenvironment and of its receptor c-MET promotes ECM remodeling, inflammation, migration, angiogenesis, and invasion [182,183,184]. For example, in myeloma, shed SDC1 promotes HGF paracrine signaling that involves MAPK and PI3K cascade activation resulting in enhanced cell proliferation and survival [176,185,186]. In pancreatic cancer, HSPG-mediated activation of HGF/c-MET signaling induces proliferation and migration of tumor cells through the activation of ERK1/2 but not the AKT pathway [187]. Dysregulation of HSPG-regulated HGF/c-MET signaling in tumor microenvironment plays a key role in hepatocarcinoma [188]. Strong evidence demonstrates a role for loss of HB-EGF in the tumor microenvironment in neuroblastoma pathogenesis [189]. Indeed, HSPG-mediated binding of soluble HB-EGF with EGF receptor activates ERK1/2 and STAT3 signaling pathways, resulting in neuroblast differentiation and decreased proliferation [189]. Both SDC4 and GPC1 play a role in the EGF receptor signaling activation involving PI3K/AKT, MAPK/ERK, and JAK/STAT pathways that affect the proliferative, invasive, and migratory abilities of colon cancer cells [190]. Furthermore, SDC1 affects AKT and STAT3 signaling pathways activated by the EGF receptor in breast cancer stem cells from triple-negative breast cancer [191]. On the other hand, the HS chains of shed SCD1 bind HB-EGF, and thereby activate MAPK/ERK downstream signaling in colorectal cancer [177].
The shedding of HS chains from SDC1 in hepatocarcinoma cells facilitates lymphatic endothelial cell proliferation through VEGF-C induced ERK signaling pathway [98]. In myeloma, SDC1-mediated activation of the VEGF receptor on adjacent endothelial cells promotes AKT and ERK signaling and stimulates tumor angiogenesis [192]. Similar VEGF activation by SDC1 occurs in melanoma and ovarian carcinoma [193]. In pathologic lymphangiogenesis, association between SDC4, VEGF-C, and VEGF receptor-3 triggers activation of ERK and AKT pathways leading to mitogenic and survival responses [194]. The binding of shed perlecan to VEGF promotes activation of VEGF2 receptor signaling thus sustaining cell survival via the AKT pathway and tumor angiogenesis in hepatoblastoma [195].
In pancreatic cancer cells, GPC1 interaction with TGF-β1 promotes SMAD pathway activation resulting in cell growth inhibition [196,197]. However, TGF-β signaling may play a dual role in both pro-tumorigenic and tumor-suppressive of pancreatic cancer, depending on tumor stage and microenvironment [198]. Indeed, besides SMAD activation, TGF-β signaling can also be transduced through the non-canonical pathways that include PI3K/AKT, JNK, MAPK, and Rho GTPase pathways [199]. In glioblastoma, the stem-like population glioma-initiating cells rely on TGF-β for self-renewal, through activation of the JAK-STAT pathway [199]. In hepatocellular carcinoma, GPC3 regulates TGF-β2 signaling that involves both SMAD and MAPK/ERK pathways [200]. In fibrosarcoma, SDC2 mediates TGFβ2 transcriptional regulation via Smad signaling that affects cell adhesion [112,201]. In the same type of cancer, SDC2 also mediates IGF-I-induced activation of the ERK pathway facilitating cell migration [202]. A significant role of SDC4 on IGF-I receptor activation, together with the involvement of integrins and estrogen receptors, leading to MAPK, PI3K/AKT, and/or PKC signaling pathways, in the breast cancer cell aggressiveness has been established [203]. Furthermore, HSPG-mediated association of IGF-I with β1 integrin modulates adhesion and migration of human multiples of myeloma cells via phosphorylation of FAK and paxillin, and activation of ERK and PI3K/AKT signaling [204].
In addition to acting as co-receptors for growth factors, HSGPs provide a unique functional activity to the processes of cell-matrix and cell-cell adhesion relevant to cancer initiation and progression [40]. Indeed, HSPGs are able to bind matrix proteins such as fibronectin, laminin, thrombospondin, and collagens, and to modulate integrin activation either by direct binding or exposing the binding sites of matrix proteins for integrin engagement, thus affecting focal adhesion assembly/disassembly and intracellular signaling that regulates cell adhesion, spreading, and sensing mechanical stress [7,8,10,11,12,13,165,205,206,207]. The ectodomain and HS chains of SDC1, through αvβ3 integrin, induce ECM fiber alignment that promotes the directional migration and invasion of breast carcinoma cells [208]. A ternary complex formed by SDC1 ectodomain, IGF1 receptor, and αvβ3 integrin transduces angiogenic signals [209]. The interaction of the extracellular domain of SDC1 with αvβ3 and αvβ5 integrins regulates angiogenesis and tumorigenesis in human mammary carcinoma cells, and myeloma [192,210]. On the other hand, the interaction of the SDC1 cytoplasmic domain with the laminin receptor α6β4 integrin regulates ErbB2 tyrosine kinase activation leading to human squamous carcinoma cell spreading [211]. The protein core of SDC1 supports α2β1 integrin-mediated cell adhesion to collagen, thus negatively regulating carcinoma cell migration and invasion [111,212].
In addition to SDC1, also SDC2 acts as a co-receptor of α2β1 integrin, thus playing an important role in regulating actin cytoskeleton organization and focal adhesion kinase signaling [16,213]. Such cooperation between SDC2 and α2β1 integrin represents a possible mechanism underlying the tumorigenic activity of colon cancer cells [214]. This property correlates with the induction of differentiation toward a migratory mesenchymal phenotype of colorectal cancer-derived HT-29 M6 epithelial cells [214]. In malignant breast cancer cells, SDC2 interaction with β1 integrin promotes the invasive capacity of the cells by regulating the Rho GTPase activity [215]. SDC2 also cooperates with α5β1 integrin for regulation of actin-cytoskeletal organization in cell adhesion to fibronectin in Lewis lung carcinoma-derived metastatic cells, thus affecting their invasive capacity [216]. The integrin-dependent focal adhesion kinase (FAK) regulates SDC2 induced tumorigenic activity of HT1080 fibrosarcoma and melanoma cells [217,218]. Furthermore, SDC2 enhances FAK phosphorylation and the downstream extracellular signal-regulated kinase (ERK) activity in colon cancer cells [219]. The involvement of SDC4 interaction with β1 integrin in the development and metastasis of renal carcinomas has been demonstrated [186]. While SDC4 interaction with α6β4 integrin mediates mammary carcinoma cell migration [175], downregulation of SDC4 by FGF2-dependent dephosphorylation of FAK promotes the migration of melanoma cells [220,221]. Activation of FAK by SDC4 in epithelial tumor cells resulting in the transmission of mechano-transduction signals is important for cell spreading, actin cytoskeleton assembly, and cell contractility [222]. A ternary complex formed by SDC4, α5β1 integrin, and endothelial surface glycoprotein Thy-1 supporting cell-cell adhesion modulates mechano-signaling in melanoma cells [223]. Finally, it has been shown that α-dystroglycan and β1 integrin act as receptors for perlecan in oral precancerous lesions prior to the invasion, and the perlecan-induced signals to these receptors trigger cell differentiation and proliferation of oral carcinoma cells [224]. On the other hand, endorepellin, the C-terminal domain of perlecan, by simultaneously engaging α2β1 integrin and VEGF receptor 2 inhibits tumor angiogenesis [225]. The basal lamina and ECM localized HSPG agrin interact with αvβ1 integrin activating mechanotransduction signaling which promotes human liver cancer [149].
3.2. HSPG-Regulated Mechanisms in Tumor Microenvironment Remodeling
Multiple evidence demonstrates that HSPGs require proteolytic enzymes for ECM remodeling and for modulating cell signaling in tumor microenvironment. Such an interplay between proteolytic enzymes and HSPGs greatly contributes to the cancer pathogenesis [8,33,37,42,226]. In particular, the metalloproteinases MMPs, ADAMSs, ADAMS with thrombospondin motifs (ADAMTSs), and cathepsins are among the proteinases that cooperate with HSPGs in all the stages of cancer development and progression, although in a cell- and tissue-specific manner. In addition to the role of metalloproteinases in shedding which releases the ectodomain of cell surface-tethered HSPGs into the extracellular milieu with the already described impact on tumor cells, HSPGs contain docking sites for these proteases which allow the formation of complexes and their allosteric activation. Indeed, SDC2 acts as a docking receptor for pro-MMP-7 in colon cancer cells, promoting pro-MMP-7 processing into the active MMP-7, and subsequent cleavage of MMP-7 substrate E-cadherin, which, in turn, results in enhanced cell migration [219,227]. Similarly, GPCs associate with secreted MMP-9 to mediate motility of colon adenocarcinoma cells [228]. The binding of SDC4 to ADAMTSs promotes their activation, and subsequent tumorigenic signaling [229]. Furthermore, HS chains of HSPGs can simultaneously interact with an active MMP and a substrate, forming a trimeric complex [230]. For example, the binding of SDC1 to ADAMTS-4 and MMP-17 triggers the activation of ADAMTS-4 [231]. HSPGs also interact with the cathepsin family of proteases that play key roles in several human diseases, including inflammation and cancer [232,233,234,235,236,237]. In tumor microenvironment, the interaction between HS side chains of HSPGs and secreted cathepsins regulates the stability and activity of these proteases, by protecting them from alkaline pH-induced de-activation, facilitating their autocatalytic activation, and promoting conformational changes in their structure that enhance their affinity for substrates [234,236,237]. The HSPGs perlecan and collagen XVIII serve as substrates for specific cathepsins resulting in the generation of endorepellin and endostatin, respectively, whose activity in tumor microenvironment remodeling and cancer progression has been well established [163,164].
Finally, in tumor microenvironment, HSPGs are involved in compartment exchanges between cells through extracellular vesicles (EVs), thus regulating communication between malignant and stromal cells in tumor development [168]. It has been proposed that EV-associated HSPGs may function as a dynamic reservoir of signaling molecules with potential implications in the exchange of ligands between EVs and tumor target cells [238]. The release of EV within the tumor microenvironment represents a mechanism by which cell-to-cell transfer of bioactive molecules occurs with a broad impact on tumor growth, angiogenesis, and invasion [239].
In conclusion, HSPGs may regulate tumor microenvironment and cancer cell behavior through either binding growth factors or their interaction with other effectors, resulting in different types of downstream intracellular signaling that contribute to tumor promotion and progression.
4. Heparan Sulfate Proteoglycans as Therapeutic Targets for Cancer
Since already few years, HSPGs have been explored as potential targets for the treatment of cancers. However, due to the polyhedric nature of these molecules in terms of both structure and functions, different strategies have been developed to target HSPGs for cancer therapy. Specific domains of proteoglycan core and/or HS chains as well as HSPG synthetizing and remodeling enzymes represent potential therapeutic targets [205]. Among the explored approaches, there is the use of high-affinity antibodies recognizing functional epitopes of HSPGs, HS mimetic compounds, cationic proteins which interact with the highly anionic sulfate and carboxylate moieties of HS chains, natural and synthetic peptides, small organic molecules that may affect either HSPG-protein interactions and subsequent signaling or the HSPG biosynthetic machinery [4,5,6,29,32,37,155,156,165,239,240,241,242,243]. Some examples of HSPG targeting-based therapeutics for cancer treatment are reported in Table 4.
Table 4.
Selected examples of HSPG targeting-based therapeutics for cancers.
| Type of Drug | Target | Type(s) of Cancer | Reference(s) |
|---|---|---|---|
| Anti-GPC1 monoclonal antibody | Glypican-1 | Esophageal squamous cell carcinoma | [244] |
| Monoclonal antibody HS20 | Glypican-3 HS chain |
Hepatocellular carcinoma | [245,246] |
| Human single-domain antibody specific for GPC2 | Glypican-2 | Neuroblastoma | [247] |
| Human recombinant antibody OC-46F2 | Syndecan-1 ectodomain | Melanoma Ovarian carcinoma |
[193] [248] |
| Antibody-pyrrolobenzodiazepine conjugate | Glypican-2 | Neuroblastoma | [249] |
| Antibody-auristatin F conjugate | Glypican-1 | Uterine cervical squamous cell carcinoma | [250] |
| HS mimetics G2.2 | HSPG induced MAPK activation | Colon cancer stem cells | [251,252] |
| HS mimetics OTR4120 and OTR4131 | HSPGs-mediated RANTES signaling | Hepatocellular carcinoma | [253] |
| Peptidic HS mimetics Synstatin |
Syndecan-1/integrin/IGF1 complex formation | Mammary tumors Hepatocellular carcinoma |
[210,226] [254] |
| Xylosides | HSPG biosynthesis | Glioma Lung cancer |
[165,255,256] [257] |
| HS mimetics RK-682 | Heparanase | Bladder cancer | [92,258,259] |
| HS mimetics PG545 (Pixatimod) | Heparanase | Mesothelioma Lymphoma Breast cancer |
[260] [261] [262] |
| HS mimetics SST0001 (Roneparstat) | Heparanase | Sarcoma Myeloma |
[263,264] [101] |
| HS mimetics M402 (Necuparanib) | Heparanase | Pancreatic cancer | [251,263,265] |
| HS mimetics PI-88 (Mupafostat) | Heparanase and Endoglucosamine 6-sulfatase | Hepatocellular carcinoma | [251,263] |
| Monoclonal antibodies 9E8 and H1023 | Heparanase | Lymphoma Myeloma |
[266] [266] |
| Triazolo-thiadiazoles | Heparanase | Hepatocellular carcinoma Lung cancer |
[267] [267] |
| Phenyl sulfonyl compound OKN-007 | Sulfatase 2 | Hepatocellular carcinoma Glioblastoma |
[268] [269] |
| Proteasome inhibitor (Bortezomib) | Sulfatase 2 | Breast cancer | [270] |
Several antibodies targeting distinct HSPG domains have been developed to date. An anti-GPC1 monoclonal antibody has shown potent antitumor activity in esophageal squamous cell carcinoma [244], whereas a human monoclonal antibody against GPC3, HS20, destroying Wnt3a and GPC3 interaction and subsequent signaling, exhibits elevated antitumor activity in liver cancer [245,246]. Two forms of antibody therapeutics targeting GPC2 have been successfully developed for neuroblastoma treatment [247]. The human antibody OC-46F2, specific for the ectodomain domain of SDC1, has proved to inhibit tumor growth in experimental human models of melanoma and ovarian carcinoma by blocking angiogenesis [193,248]. In some cases, antibody-drug-conjugates (ADC) consisting of a highly cytotoxic small-molecule covalently linked to a monoclonal antibody that recognizes a cell surface antigen have been developed. Indeed, a GPC2-targeted ADC obtained by conjugating a GPC2 directed antibody with pyrrolobenzodiazepine dimers resulted in being effective in neuroblastoma [249]. Furthermore, an ADC composed of an anti-GPC1 antibody conjugated with auristatin F, an anti-tubulin compound that inhibits cell division, has shown to be effective in uterine cervical squamous cell carcinoma [250].
Both saccharidic and non-saccharidic HS mimetics have shown to affect tumor cells and components of tumor microenvironment through different mechanisms, including the inhibition of cell surface-tethered HSPG signaling and HSPG-mediated cell adhesion, spreading, and angiogenesis [165,251]. Small HS mimetics molecules result in being effective in various types of cancers either administered alone or in combination regimens and are characterized by good safety and tolerability profiles [242]. A sulfated non-saccharide mimetics of heparin hexasaccharide, G2.2, inhibits colon cancer stem cells [252]. The HS mimetics OTR4120 and OTR4131 exhibit anti-tumoral effects in human hepatocellular carcinoma by interfering with HSPGs-mediated RANTES signaling [253]. Synstatin, a short peptide mimicking the SDC1 ectodomain responsible for αvβ3 or αvβ5 integrin/IGF1 complex formation and receptor activation, has been proved to be effective in mammary tumors and hepatocellular carcinoma [210,226,254]. Another approach in cancer therapy uses HS mimetics in conjunction with inhibitors of the exosites of proteases (i.e., cathepsins), thus interfering with HS/proteinase binding and proteinase catalytic activities [254].
In addition, targeting HSPG biosynthetic and post-translational modifying enzymes such as endosulfatases and heparanase represents an effective therapeutic intervention for cancer treatment [266,267,268,269,270]. An approach is represented by the manipulation of HSPG synthesis using xylosides that, competing with core proteins for HS binding, promote the secretion of xyloside-primed HS chains and core proteins with reduced, or completely lacking, HS chains [165]. The reduced glycosylation of cell surface proteoglycans affects HSPG-dependent growth factor and chemokine signaling, thus inhibiting angiogenesis, tumor growth, and invasion. Treatment with xylosides also attenuates EV-mediated intercellular transfer of signaling molecules regulated by HSPGs, resulting in a reduction of cancer cell migration and invasion [238,239]. On the other hand, different modalities for targeting EV-mediated intercellular communications have been proved to represent a useful strategy to prevent tumor progression and metastasis [271]. In addition, HS mimetics as well as antibodies, and other modulators have been developed to target heparanase and sulfatases involved in the regulation of HSPGs in tumor microenvironment [92,101,165,251,260,261,262,263,264,265,266,267,268,269,270]. Indeed, the HS mimetics PI-88, PG545, and M402 have been shown to exert anti-angiogenic and antimetastatic effects by inhibiting heparanase in several types of cancers [89,224,225,226,227,228,229,230]. Furthermore, heparanase neutralizing monoclonal antibodies attenuate myeloma and lymphoma tumor growth and dissemination [155,251,261,262,265,266]. Recently, a novel class of triazole-thiadiazole small molecules with heparanase inhibitory activity has shown the ability to reduce the metastatic potential of hepatocellular carcinoma [267]. In addition to heparanase, sulfatases that remove the O-sulfate group from HS chains have been explored as targets for cancer therapy [91]. The human sulfatase 2 (SULF2) inhibitor 2,4-disulfophenyl-N-tert-butylnitrone (OKN-007) exhibits antitumoral activity in hepatocellular carcinoma and glioblastoma by affecting TGFbeta1/SMAD signaling, and cell proliferation and angiogenesis, respectively [268,269]. On the other hand, proteasomal inhibitors such as MG132, Lactacystin, and Bortezomib treatment abolish SULF2 expression in multiple breast cancer cell lines [270]. Inhibition of human sulfatase 1 (SULF1) inhibits the malignant phenotype of gallbladder carcinoma cells by hindering the cell response to growth factors [272]. Thus, the modulation of tumor microenvironment by affecting the structure and/or activity of HSPGs represents an effective therapeutic strategy for preventing tumor growth and progression.
5. Concluding Remarks
A huge amount of data demonstrates that HSPGs are key players in tumor growth, invasion, and metastasis, due to their capability to influence tumor microenvironment and, in turn, tumor cell fate. Indeed, these multifunctional molecules by interacting with matrix effectors, cell surface receptors, and enzymes are involved in the complex network of cell-cell and cell-matrix interactions that dictate tumor cell behavior. The extensive remodeling of tumor microenvironment during cancer development and progression is associated with changes in the expression levels of HSPGs as well as in structural and functional alterations of HSPGs that affect cancer cell phenotype. Advances in understanding the molecular mechanisms underlying HSPG structural and functional variability in malignancy has provided promising HSPG-based therapeutic approaches for cancer treatment. HSPG targeting-based tumor treatment may involve the use of: (i) antibodies targeting selected HSPG epitopes or synthetic molecules that interfere with the functional binding of HSPGs with ligands such as growth factors or integrins and other receptors, thus affecting the downstream signaling and the related cellular processes such as adhesion, proliferation, migration, and invasion; (ii) small molecules that interfere with EV-mediated intercellular transfer of signaling molecules regulated by HSPGs; (iii) specific inhibitors or proteinase inhibitors that prevent HSPG shedding; (iv) drugs that regulate the expression levels of HSPGs in tumor microenvironment. However, as the knowledge on the multifaceted roles of HSPGs in tumor microenvironment progresses, innovative HSPG structure/function targeting strategies are explored to fight cancer.
Acknowledgments
We apologize to all the authors whose work could not be cited due to space limitations.
Abbreviations
| ADAM | a disintegrin and MMP protease |
| ADAMTS | ADAMS with thrombospondin motifs |
| ADC | antibody-drug-conjugates |
| AKT | protein kinase B |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| ERK | extracellular signal-regulated kinase |
| EXT | Exostosin |
| EXTL | N-acetylglucosaminyltransferase |
| EV | extracellular vesicle |
| FAK | focal adhesion kinase |
| FGF | fibroblast growth factor |
| FGFR | fibroblast growth factor receptor |
| GAG | glycosaminoglycan |
| GalT | galactosyltransferase |
| GlcAT | glucuronyltransferase |
| GLCE | D-glucuronyl C5-epimerase |
| GPC | glypican |
| GPI | glycosylphosphatidylinositol |
| HB-EGF | heparin-binding epidermal growth factor-like |
| HGF | hepatocyte growth factor |
| HPSE | heparanase |
| HS | heparan sulfate |
| HSPG | heparan sulfate proteoglycan |
| HS3ST2 | heparan sulfate glucosamine 3-O-sulfotransferase-2 |
| HS6ST2 | heparan sulfate glucosamine 6-O-sulfotransferase-2 |
| IGF1 | insulin-like growth factor-1 |
| JAK | Janus kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| NDST | N-deacetylase/N-sulfotransferase |
| OST | heparan sulfate-O-sulfotransferase |
| PI3K | phosphatidylinositol 3-kinase |
| PKC | protein kinase C |
| PDGF | platelet-derived growth factor |
| PG | proteoglycans |
| SDC | syndecan |
| STAT | signal transducer and activator of transcription protein |
| SULF | endo-6-O-sulfatase |
| TGF | transforming growth factor |
| XYLT | xylosyltransferase |
| VEGF | vascular endothelial growth factor |
Author Contributions
Conceptualization, V.D.P. and L.M.P.; writing—original draft preparation, V.D.P. and L.M.P.; writing—review and editing, V.D.P. and L.M.P.; supervision, L.M.P.; funding acquisition, L.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the POR Campania FESR 2014–2020 “SATIN” grant from Regione Campania, Italy.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ungefroren H., Sebens S., Seidl D., Lehnert H., Hass R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011;9:18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J. Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 3.Walker C., Mojares E., Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer L., Reinhardt D.P. Special issue: Extracellular matrix: Therapeutic tools and targets in cancer treatment. Adv. Drug Deliv. Rev. 2016;97:1–3. doi: 10.1016/j.addr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Roma-Rodrigues C., Mendes R., Baptista P.V., Fernandes A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baghban R., Roshangar L., Jahanban-Esfahlan R., Seidi K., Ebrahimi-Kalan A., Jaymand M., Kolahian S., Javaheri T., Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020;18:59. doi: 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Manou D., Caon I., Bouris P., Triantaphyllidou I.E., Giaroni C., Passi A., Karamanos N.K., Vigetti D., Theocharis A.D. The complex interplay between extracellular matrix and cells in tissues. Methods Mol. Biol. 2019;1952:1–20. doi: 10.1007/978-1-4939-9133-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Theocharis A.D., Manou D., Karamanos N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019;286:2830–2869. doi: 10.1111/febs.14818. [DOI] [PubMed] [Google Scholar]
- 10.Sarrazin S., Lamanna W.C., Esko J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011;3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iozzo R.V., Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirn-Safran C., Farach-Carson M.C., Carson D.D. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell Mol. Life Sci. 2009;66:3421–3434. doi: 10.1007/s00018-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billings P.C., Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: Mechanisms and mysteries. Connect. Tissue Res. 2015;56:272–280. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neill T., Schaefer L., Iozzo R.V. Decoding the matrix: Instructive roles of proteoglycan receptors. Biochemistry. 2015;54:4583–4598. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie M., Li J.P. Heparan sulfate proteoglycan—A common receptor for diverse cytokines. Cell Signal. 2019;54:115–121. doi: 10.1016/j.cellsig.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Afratis N.A., Nikitovic D., Multhaupt H.A., Theocharis A.D., Couchman J.R., Karamanos N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017;284:27–41. doi: 10.1111/febs.13940. [DOI] [PubMed] [Google Scholar]
- 17.Li J.P., Kusche-Gullberg M. Heparan sulfate: Biosynthesis, structure, and function. Int. Rev. Cell Mol. Biol. 2016;325:215–273. doi: 10.1016/bs.ircmb.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Christianson H.C., Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Brooks R., Williamson R., Bass M. Syndecan-4 independently regulates multiple small GTPases to promote fibroblast migration during wound healing. Small GTPases. 2012;3:73–79. doi: 10.4161/sgtp.19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu C., Griffiths L.R., Haupt L.M. Exploiting heparan sulfate proteoglycans in human neurogenesis-controlling lineage specification and fate. Front. Integr. Neurosci. 2017;11:28. doi: 10.3389/fnint.2017.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agere S.A., Kim E.Y., Akhtar N., Ahmed S. Syndecans in chronic inflammatory and autoimmune diseases: Pathological insights and therapeutic opportunities. J. Cell Physiol. 2018;233:6346–6358. doi: 10.1002/jcp.26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Callaghan P., Zhang X., Li J.P. Heparan sulfate proteoglycans as relays of neuroinflammation. J. Histochem. Cytochem. 2018;66:305–319. doi: 10.1369/0022155417742147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz N.B., Domowicz M.S. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018;592:3791–3805. doi: 10.1002/1873-3468.13026. [DOI] [PubMed] [Google Scholar]
- 24.Marchand M., Monnot C., Muller L., Germain S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2019;89:147–156. doi: 10.1016/j.semcdb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Collins L.E., Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019;105:81–92. doi: 10.1002/JLB.3RU0618-246R. [DOI] [PubMed] [Google Scholar]
- 26.De Pasquale V., Pavone L.M. Heparan sulfate proteoglycans: The sweet side of development turns sour in mucopolysaccharidoses. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:165539. doi: 10.1016/j.bbadis.2019.165539. [DOI] [PubMed] [Google Scholar]
- 27.Gopal S. Syndecans in inflammation at a glance. Front. Immunol. 2020;11:227. doi: 10.3389/fimmu.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H., Wu Q., Sun A., Liu X., Fan Y., Deng X. Cancer cell glycocalyx and its significance in cancer progression. Int. J. Mol. Sci. 2018;19:2484. doi: 10.3390/ijms19092484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbouri D., Afratis N., Gialeli C., Vynios D.H., Theocharis A.D., Karamanos N.K. Syndecans as modulators and potential pharmacological targets in cancer progression. Front. Oncol. 2014;4:4. doi: 10.3389/fonc.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hull E.E., Montgomery M.R., Leyva K.J. Epigenetic regulation of the biosynthesis & enzymatic modification of heparan sulfate proteoglycans: Implications for tumorigenesis and cancer biomarkers. Int. J. Mol. Sci. 2017;18:E1361. doi: 10.3390/ijms18071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crespo A., García-Suárez O., Fernández-Vega I., Solis-Hernandez M.P., García B., Castañón S., Quirós L.M. Heparan sulfate proteoglycans undergo differential expression alterations in left sided colorectal cancer, depending on their metastatic character. BMC Cancer. 2018;18:687. doi: 10.1186/s12885-018-4597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka Y., Tateishi R., Koike K. Proteoglycans are attractive biomarkers and therapeutic targets in hepatocellular carcinoma. Int. J. Mol. Sci. 2018;19:3070. doi: 10.3390/ijms19103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theocharis A.D., Karamanos N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019;75–76:220–259. doi: 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Bartolini B., Caravà E., Caon I., Parnigoni A., Moretto P., Passi A., Vigetti D., Viola M., Karousou E. Heparan sulfate in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1245:147–161. doi: 10.1007/978-3-030-40146-7_7. [DOI] [PubMed] [Google Scholar]
- 35.Knelson E.H., Nee J.C., Blobe G.C. Heparan sulfate signaling in cancer. Trends Biochem. Sci. 2014;39:277–288. doi: 10.1016/j.tibs.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackhall F.H., Merry C.L., Davies E.J., Jayson G.C. Heparan sulfate proteoglycans and cancer. Br. J. Cancer. 2001;85:1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanderson R.D., Yang Y., Kelly T., MacLeod V., Dai Y., Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: Growth regulation and the prospect of new cancer therapies. J. Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 38.Iozzo R.V., Zoeller J.J., Nyström A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol. Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagarajan A., Malvi P., Wajapeyee N. Heparan sulfate and heparan sulfate proteoglycans in cancer initiation and progression. Front. Endocrinol. 2018;9:483. doi: 10.3389/fendo.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur S.P., Cummings B.S. Role of glypicans in regulation of the tumor microenvironment and cancer progression. Biochem. Pharmacol. 2019;168:108–118. doi: 10.1016/j.bcp.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75–76:1–11. doi: 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park P.W. Isolation and functional analysis of syndecans. Methods Cell Biol. 2018;143:317–333. doi: 10.1016/bs.mcb.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Wang F., Sheng J. “Coding” and “Decoding”: Hypothesis for the regulatory mechanism involved in heparan sulfate biosynthesis. Carbohydr. Res. 2016;428:1–7. doi: 10.1016/j.carres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Suhovskih A.V., Domanitskaya N.V., Tsidulko A.Y., Prudnikova T.Y., Kashuba V.I., Grigorieva E.V. Tissue-specificity of heparan sulfate biosynthetic machinery in cancer. Cell Adh. Migr. 2015;9:452–459. doi: 10.1080/19336918.2015.1049801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzeng S.T., Tsai M.H., Chen C.L., Lee J.X., Jao T.M., Yu S.L., Yen S.J., Yang Y.C. NDST4 is a novel candidate tumor suppressor gene at chromosome 4q26 and its genetic loss predicts adverse prognosis in colorectal cancer. PLoS ONE. 2013;8:e67040. doi: 10.1371/journal.pone.0067040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bui C., Ouzzine M., Talhaoui I., Sharp S., Prydz K., Coughtrie M.W., Fournel-Gigleux S. Epigenetics: Methylation-associated repression of heparan sulfate 3-O-sulfotransferase gene expression contributes to the invasive phenotype of H-EMC-SS chondrosarcoma cells. FASEB J. 2010;24:436–450. doi: 10.1096/fj.09-136291. [DOI] [PubMed] [Google Scholar]
- 48.Hwang J.A., Kim Y., Hong S.H., Lee J., Cho Y.G., Han J.Y., Kim Y.H., Han J., Shim Y.M., Lee Y.S., et al. Epigenetic inactivation of heparan sulfate (glucosamine) 3-O-sulfotransferase 2 in lung cancer and its role in tumorigenesis. PLoS ONE. 2013;8:e79634. doi: 10.1371/journal.pone.0079634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson B.W., Datta S. Role of heparan sulfate 2-O-sulfotransferase in prostate cancer cell proliferation, invasion, and growth factor signaling. Prostate. Cancer. 2011;2011:893208. doi: 10.1155/2011/893208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatabe S., Kimura H., Arao T., Kato H., Hayashi H., Nagai T., Matsumoto K., DE Velasco M., Fujita Y., Yamanouchi G., et al. Overexpression of heparan sulfate 6-O-sulfotransferase-2 in colorectal cancer. Mol. Clin. Oncol. 2013;1:845–850. doi: 10.3892/mco.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin Y., He J., Du J., Zhang R.X., Yao H.B., Shao Q.S. Overexpression of HS6ST2 is associated with poor prognosis in patients with gastric cancer. Oncol. Lett. 2017;14:6191–6197. doi: 10.3892/ol.2017.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ushakov V.S., Tsidulko A.Y., de La Bourdonnaye G., Kazanskaya G.M., Volkov A.M., Kiselev R.S., Kobozev V.V., Kostromskaya D.V., Gaytan A.S., Krivoshapkin A.L., et al. Heparan sulfate biosynthetic system is inhibited in human glioma due to EXT1/2 and HS6ST1/2 down-regulation. Int. J. Mol. Sci. 2017;18:2301. doi: 10.3390/ijms18112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erickson L.A., Inwards C.Y. Multiple hereditary osteochondromas. Mayo Clin. Proc. 2019;94:1388–1389. doi: 10.1016/j.mayocp.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Yoneda A., Lendorf M.E., Couchman J.R., Multhaupt H.A. Breast and ovarian cancers: A survey and possible roles for the cell surface heparan sulfate proteoglycans. J. Histochem. Cytochem. 2012;60:9–21. doi: 10.1369/0022155411428469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sembajwe L.F., Katta K., Grønning M., Kusche-Gullberg M. The exostosin family of glycosyltransferases: mRNA expression profiles and heparan sulphate structure in human breast carcinoma cell lines. Biosci. Rep. 2018;38:BSR20180770. doi: 10.1042/BSR20180770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitt S.C., Hernandez R.A., Nehs M.A., Gawande A.A., Moore F.D., Jr., Ruan D.T., Cho N.L. Identification of novel oncogenic mutations in thyroid cancer. J. Am. Coll. Surg. 2016;222:1036–1043.e2. doi: 10.1016/j.jamcollsurg.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 57.Ropero S., Setien F., Espada J., Fraga M.F., Herranz M., Asp J., Benassi M.S., Franchi A., Patiño A., Ward L.S., et al. Epigenetic loss of the familial tumor-suppressor gene exostosin-1 (EXT1) disrupts heparan sulfate synthesis in cancer cells. Hum. Mol. Genet. 2004;13:2753–2765. doi: 10.1093/hmg/ddh298. [DOI] [PubMed] [Google Scholar]
- 58.Liu N.W., Huang X., Liu S., Lu Y. EXT1, Regulated by MiR-665, promotes cell apoptosis via ERK1/2 signaling pathway in acute lymphoblastic leukemia. Med. Sci. Monit. 2019;25:6491–6503. doi: 10.12659/MSM.918295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prudnikova T.Y., Mostovich L.A., Domanitskaya N.V., Pavlova T.V., Kashuba V.I., Zabarovsky E.R., Grigorieva E.V. Antiproliferative effect of D-glucuronyl C5-epimerase in human breast cancer cells. Cancer Cell Int. 2010;10:27. doi: 10.1186/1475-2867-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belyavskaya V.A., Prudnikova T.Y., Domanitskaya N.V., Litviakov N.V., Maksimov V.N., Cherdyntseva N.V., Grigorieva E.V. GLCE rs3865014 (Val597Ile) polymorphism is associated with breast cancer susceptibility and triple-negative breast cancer in Siberian population. Gene. 2017;628:224–229. doi: 10.1016/j.gene.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 61.Grigorieva E.V., Prudnikova T.Y., Domanitskaya N.V., Mostovich L.A., Pavlova T.V., Kashuba V.I., Zabarovsky E.R. D-glucuronyl C5-epimerase suppresses small-cell lung cancer cell proliferation in vitro and tumour growth in vivo. Br. J. Cancer. 2011;105:74–82. doi: 10.1038/bjc.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg E.E., Prudnikova T.Y., Zabarovsky E.R., Kashuba V.I., Grigorieva E.V. D-glucuronyl C5-epimerase cell type specifically affects angiogenesis pathway in different prostate cancer cells. Tumour Biol. 2014;35:3237–3245. doi: 10.1007/s13277-013-1423-6. [DOI] [PubMed] [Google Scholar]
- 63.Prudnikova T.Y., Soulitzis N., Kutsenko O.S., Mostovich L.A., Haraldson K., Ernberg I., Kashuba V.I., Spandidos D.A., Zabarovsky E.R., Grigorieva E.V. Heterogeneity of d-glucuronyl C5-epimerase expression and epigenetic regulation in prostate cancer. Cancer Med. 2013;2:654–661. doi: 10.1002/cam4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potapenko I.O., Haakensen V.D., Lüders T., Helland A., Bukholm I., Sørlie T., Kristensen V.N., Lingjaerde O.C., Børresen-Dale A.L. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol. Oncol. 2010;4:98–118. doi: 10.1016/j.molonc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., Zhang Y.N. Roles of proteoglycans in the tumourigenesis and development of adenoid cystic carcinoma and pleomorphic adenoma of the salivary gland: A systematic review. Chin. J. Dent. Res. 2020;23:11–25. doi: 10.3290/j.cjdr.a44332. [DOI] [PubMed] [Google Scholar]
- 66.Tang W., Li M., Qi X., Li J. β1,4-galactosyltransferase V modulates breast cancer stem cells through Wnt/β-catenin signaling pathway. Cancer Res. Treat. 2020 doi: 10.4143/crt.2020.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poeta M.L., Massi E., Parrella P., Pellegrini P., De Robertis M., Copetti M., Rabitti C., Perrone G., Muda A.O., Molinari F., et al. Aberrant promoter methylation of beta-1,4 galactosyltransferase 1 as potential cancer-specific biomarker of colorectal tumors. Genes Chromosomes Cancer. 2012;51:1133–1143. doi: 10.1002/gcc.21998. [DOI] [PubMed] [Google Scholar]
- 68.Lee A., Chick J.M., Kolarich D., Haynes P.A., Robertson G.R., Tsoli M., Jankova L., Clarke S.J., Packer N.H., Baker M.S. Liver membrane proteome glycosylation changes in mice bearing an extra-hepatic tumor. Mol. Cell Proteom. 2011;10:M900538-MCP200. doi: 10.1074/mcp.M900538-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H., Ma H., Wei W., Ji D., Song X., Sun J., Zhang J., Jia L. B4GALT family mediates the multidrug resistance of human leukemia cells by regulating the hedgehog pathway and the expression of p-glycoprotein and multidrug resistance-associated protein 1. Cell Death Dis. 2013;4:e654. doi: 10.1038/cddis.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X., Jiang J., Shen H., Wang H., Zong H., Li Z., Yang Y., Niu Z., Liu W., Chen X., et al. Elevated beta-1,4-galactosyltransferase I in highly metastatic human lung cancer cells. Identification of E1AF as important transcription activator. J. Biol. Chem. 2005;280:12503–12516. doi: 10.1074/jbc.M413631200. [DOI] [PubMed] [Google Scholar]
- 71.Chang H.H., Chen C.H., Chou C.H., Liao Y.F., Huang M.J., Chen Y.H., Wang W.J., Huang J., Hung J.S., Ho W.L., et al. β-1,4-Galactosyltransferase III enhances invasive phenotypes via β1-integrin and predicts poor prognosis in neuroblastoma. Clin. Cancer Res. 2013;19:1705–1716. doi: 10.1158/1078-0432.CCR-12-2367. [DOI] [PubMed] [Google Scholar]
- 72.Xie H., Zhu Y., An H., Wang H., Zhu Y., Fu H., Wang Z., Fu Q., Xu J., Ye D. Increased B4GALT1 expression associates with adverse outcome in patients with non-metastatic clear cell renal cell carcinoma. Oncotarget. 2016;7:32723–32730. doi: 10.18632/oncotarget.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y.L., Ding C., Sun L. High expression B3GAT3 is related with poor prognosis of liver cancer. Open Med. 2019;14:251–258. doi: 10.1515/med-2019-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nadanaka S., Hashiguchi T., Kitagawa H. Aberrant glycosaminoglycan biosynthesis by tumor suppressor EXTL2 deficiency promotes liver inflammation and tumorigenesis through Toll-like 4 receptor signaling. FASEB J. 2020;34:8385–8401. doi: 10.1096/fj.201902076R. [DOI] [PubMed] [Google Scholar]
- 75.Busse-Wicher M., Wicher K.B., Kusche-Gullberg M. The exostosin family: Proteins with many functions. Matrix Biol. 2014;35:25–33. doi: 10.1016/j.matbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Veraldi N., Parra A., Urso E., Cosentino C., Locatelli M., Corsini S., Pedrini E., Naggi A., Bisio A., Sangiorgi L. Structural features of heparan sulfate from multiple osteochondromas and chondrosarcomas. Molecules. 2018;23:3277. doi: 10.3390/molecules23123277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong S., Wu Y., Yu S., Yang Y., Lu L., Fan S. Increased EXT1 gene copy number correlates with increased mRNA level predicts short disease-free survival in hepatocellular carcinoma without vascular invasion. Medicine. 2018;97:e12625. doi: 10.1097/MD.0000000000012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baljinnyam E., Umemura M., De Lorenzo M.S., Iwatsubo M., Chen S., Goydos J.S., Iwatsubo K. Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment. Cell Melanoma Res. 2011;24:680–687. doi: 10.1111/j.1755-148X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 79.Vijaya Kumar A., Brézillon S., Untereiner V., Sockalingum G.D., Kumar Katakam S., Mohamed H.T., Kemper B., Greve B., Mohr B., Ibrahim S.A., et al. HS2ST1-dependent signaling pathways determine breast cancer cell viability, matrix interactions, and invasive behavior. Cancer Sci. 2020;111:2907–2922. doi: 10.1111/cas.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole C.L., Rushton G., Jayson G.C., Avizienyte E. Ovarian cancer cell heparan sulfate 6-O-sulfotransferases regulate an angiogenic program induced by heparin-binding epidermal growth factor (EGF)-like growth factor/EGF receptor signaling. J. Biol. Chem. 2014;289:10488–10501. doi: 10.1074/jbc.M113.534263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen Y., Ruan L., Lian C., Li R., Tu Z., Liu H. Discovery of HB-EGF binding peptides and their functional characterization in ovarian cancer cell lines. Cell Death Discov. 2019;5:82. doi: 10.1038/s41420-019-0163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song K., Li Q., Peng Y.B., Li J., Ding K., Chen L.J., Shao C.H., Zhang L.J., Li P. Silencing of hHS6ST2 inhibits progression of pancreatic cancer through inhibition of Notch signalling. Biochem. J. 2011;436:271–282. doi: 10.1042/BJ20110297. [DOI] [PubMed] [Google Scholar]
- 83.Denys A., Allain F. The emerging roles of heparan sulfate 3-O-sulfotransferases in cancer. Front. Oncol. 2019;9:507. doi: 10.3389/fonc.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tokuyama Y., Takahashi T., Okumura N., Nonaka K., Kawaguchi Y., Yamaguchi K., Osada S., Gazdar A., Yoshida K. Aberrant methylation of heparan sulfate glucosamine 3-O-sulfotransferase 2 genes as a biomarker in colorectal cancer. Anticancer Res. 2010;30:4811–4818. [PubMed] [Google Scholar]
- 85.Zhang L., Song K., Zhou L., Xie Z., Zhou P., Zhao Y., Han Y., Xu X., Li P. Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and proliferation by induction of VEGF in acute myeloid leukemia cells. J. Cell Biochem. 2015;116:1101–1112. doi: 10.1002/jcb.25066. [DOI] [PubMed] [Google Scholar]
- 86.Song K., Li Q., Jiang Z.Z., Guo C.W., Li P. Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3B1, a novel epithelial-mesenchymal transition inducer in pancreatic cancer. Cancer Biol. Ther. 2011;12:388–398. doi: 10.4161/cbt.12.5.15957. [DOI] [PubMed] [Google Scholar]
- 87.Khurana A., Beleford D., He X., Chien J., Shridhar V. Role of heparan sulfatases in ovarian and breast cancer. Am. J. Cancer Res. 2013;3:34–45. [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang T., Chen Z.H., Chen Z., Tan D. SULF2 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells through the ERK/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020;53:e8901. doi: 10.1590/1414-431x20198901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graham K., Murphy J.I., Dhoot G.K. SULF1/SULF2 reactivation during liver damage and tumour growth. Histochem. Cell Biol. 2016;146:85–97. doi: 10.1007/s00418-016-1425-8. [DOI] [PubMed] [Google Scholar]
- 90.Bret C., Moreaux J., Schved J.F., Hose D., Klein B. SULFs in human neoplasia: Implication as progression and prognosis factors. J. Transl. Med. 2011;9:72. doi: 10.1186/1479-5876-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammond E., Khurana A., Shridhar V., Dredge K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front. Oncol. 2014;4:195. doi: 10.3389/fonc.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tatsumi Y., Miyake M., Shimada K., Fujii T., Hori S., Morizawa Y., Nakai Y., Anai S., Tanaka N., Konishi N., et al. Inhibition of heparanase expression results in suppression of invasion, migration and adhesion abilities of bladder cancer cells. Int. J. Mol. Sci. 2020;21:3789. doi: 10.3390/ijms21113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong A., Spyrou A., Forsberg-Nilsson K. Involvement of heparan sulfate and heparanase in neural development and pathogenesis of brain tumors. Adv. Exp. Med. Biol. 2020;1221:365–403. doi: 10.1007/978-3-030-34521-1_14. [DOI] [PubMed] [Google Scholar]
- 94.Vornicova O., Naroditsky I., Boyango I., Shachar S.S., Mashiach T., Ilan N., Vlodavsky I., Bar-Sela G. Prognostic significance of heparanase expression in primary and metastatic breast carcinoma. Oncotarget. 2017;9:6238–6244. doi: 10.18632/oncotarget.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyango I., Barash U., Fux L., Naroditsky I., Ilan N., Vlodavsky I. Targeting heparanase to the mammary epithelium enhances mammary gland development and promotes tumor growth and metastasis. Matrix Biol. 2018;65:91–103. doi: 10.1016/j.matbio.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Tang B., Yang S. Involvement of heparanase in gastric cancer progression and immunotherapy. Adv. Exp. Med. Biol. 2020;1221:351–363. doi: 10.1007/978-3-030-34521-1_13. [DOI] [PubMed] [Google Scholar]
- 97.Doweck I., Feibish N. Opposing effects of heparanase and heparanase-2 in head & neck cancer. Adv. Exp. Med. Biol. 2020;1221:847–856. doi: 10.1007/978-3-030-34521-1_37. [DOI] [PubMed] [Google Scholar]
- 98.Yu S., Lv H., Zhang H., Jiang Y., Hong Y., Xia R., Zhang Q., Ju W., Jiang L., Ou G., et al. Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway. Biochem. Biophys Res. Commun. 2017;485:432–439. doi: 10.1016/j.bbrc.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 99.Barash U., Lapidot M., Zohar Y., Loomis C., Moreira A., Feld S., Goparaju C., Yang H., Hammond E., Zhang G., et al. Involvement of heparanase in the pathogenesis of mesothelioma: Basic aspects and clinical applications. J. Natl. Cancer Inst. 2018;110:1102–1114. doi: 10.1093/jnci/djy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Purushothaman A., Sanderson R.D. Heparanase: A dynamic promoter of myeloma progression. Adv. Exp. Med. Biol. 2020;1221:331–349. doi: 10.1007/978-3-030-34521-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramani V.C., Zhan F., He J., Barbieri P., Noseda A., Tricot G., Sanderson R.D. Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma. Oncotarget. 2016;7:1598–1607. doi: 10.18632/oncotarget.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng H., Ruan J., Zhao P., Chen S., Pan L., Liu J. Heparanase is involved in proliferation and invasion of ovarian cancer cells. Cancer Biomark. 2015;15:525–534. doi: 10.3233/CBM-150459. [DOI] [PubMed] [Google Scholar]
- 103.Wang C., Wei Y., Wang G., Zhou Y., Zhang J., Xu K. Heparanase potentiates the invasion and migration of pancreatic cancer cells via epithelial-to-mesenchymal transition through the Wnt/β-catenin pathway. Oncol. Rep. 2020;44:711–721. doi: 10.3892/or.2020.7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cassinelli G., Zaffaroni N., Lanzi C. The heparanase/heparan sulfate proteoglycan axis: A potential new therapeutic target in sarcomas. Cancer Lett. 2016;382:245–254. doi: 10.1016/j.canlet.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Fernández-Vega I., García-Suárez O., García B., Crespo A., Astudillo A., Quirós L.M. Heparan sulfate proteoglycans undergo differential expression alterations in right sided colorectal cancer, depending on their metastatic character. BMC Cancer. 2015;15:742. doi: 10.1186/s12885-015-1724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raman K., Kuberan B. Chemical tumor biology of heparan sulfate proteoglycans. Curr. Chem. Biol. 2010;4:20–31. doi: 10.2174/187231310790226206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jenkins L.M., Horst B., Lancaster C.L., Mythreye K. Dually modified transmembrane proteoglycans in development and disease. Cytokine Growth Factor Rev. 2018;39:124–136. doi: 10.1016/j.cytogfr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kind S., Merenkow C., Büscheck F., Möller K., Dum D., Chirico V., Luebke A.M., Höflmayer D., Hinsch A., Jacobsen F., et al. Prevalence of syndecan-1 (CD138) expression in different kinds of human tumors and normal tissues. Dis. Markers. 2019;2019:4928315. doi: 10.1155/2019/4928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen T.L., Grizzle W.E., Zhang K., Hameed O., Siegal G.P., Wei S. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am. J. Clin. Pathol. 2013;140:468–474. doi: 10.1309/AJCPZ1D8CALHDXCJ. [DOI] [PubMed] [Google Scholar]
- 110.Poblete C.E., Fulla J., Gallardo M., Muñoz V., Castellón E.A., Gallegos I., Contreras H.R. Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int. J. Oncol. 2014;44:647–654. doi: 10.3892/ijo.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishikawa T., Kramer R.H. SDC1 negatively modulates carcinoma cell motility and invasion. Exp. Cell Res. 2010;316:951–965. doi: 10.1016/j.yexcr.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mytilinaiou M., Nikitovic D., Berdiaki A., Kostouras A., Papoutsidakis A., Tsatsakis A.M., Tzanakakis G.N. Emerging roles of syndecan 2 in epithelial and mesenchymal cancer progression. IUBMB Life. 2017;69:824–833. doi: 10.1002/iub.1678. [DOI] [PubMed] [Google Scholar]
- 113.Hua R., Yu J., Yan X., Ni Q., Zhi X., Li X., Jiang B., Zhu J. Syndecan-2 in colorectal cancer plays oncogenic role via epithelial-mesenchymal transition and MAPK pathway. Biomed. Pharm. 2020;121:109630. doi: 10.1016/j.biopha.2019.109630. [DOI] [PubMed] [Google Scholar]
- 114.Orosco A., Fromigué O., Bazille C., Entz-Werle N., Levillain P., Marie P.J., Modrowski D. Syndecan-2 affects the basal and chemotherapy-induced apoptosis in osteosarcoma. Cancer Res. 2007;67:3708–3715. doi: 10.1158/0008-5472.CAN-06-4164. [DOI] [PubMed] [Google Scholar]
- 115.Marzioni D., Lorenzi T., Mazzucchelli R., Capparuccia L., Morroni M., Fiorini R., Bracalenti C., Catalano A., David G., Castellucci M., et al. Expression of basic fibroblast growth factor, its receptors and syndecans in bladder cancer. Int. J. Immunopathol. Pharmacol. 2009;22:627–638. doi: 10.1177/039463200902200308. [DOI] [PubMed] [Google Scholar]
- 116.Davies E.J., Blackhall F.H., Shanks J.H., David G., McGown A.T., Swindell R., Slade R.J., Martin-Hirsch P., Gallagher J.T., Jayson G.C. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin. Cancer Res. 2004;10:5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 117.Yamada Y., Arai T., Kojima S., Sugawara S., Kato M., Okato A., Yamazaki K., Naya Y., Ichikawa T., Seki N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 2018;109:2919–2936. doi: 10.1111/cas.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reyes I., Reyes N., Suriano R., Iacob C., Suslina N., Policastro A., Moscatello A., Schantz S., Tiwari R.K., Geliebter J. Gene expression profiling identifies potential molecular markers of papillary thyroid carcinoma. Cancer Biomark. 2019;24:71–83. doi: 10.3233/CBM-181758. [DOI] [PubMed] [Google Scholar]
- 119.Matsuda K., Maruyama H., Guo F., Kleeff J., Itakura J., Matsumoto Y., Lander A.D., Korc M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001;61:5562–5569. [PubMed] [Google Scholar]
- 120.Li J., Chen Y., Zhan C., Zhu J., Weng S., Dong L., Liu T., Shen X. Glypican-1 promotes tumorigenesis by regulating the PTEN/Akt/β-catenin signaling pathway in esophageal squamous cell carcinoma. Dig. Dis. Sci. 2019;64:1493–1502. doi: 10.1007/s10620-019-5461-9. [DOI] [PubMed] [Google Scholar]
- 121.Saito T., Sugiyama K., Hama S., Yamasaki F., Takayasu T., Nosaka R., Onishi S., Muragaki Y., Kawamata T., Kurisu K. High expression of glypican-1 predicts dissemination and poor prognosis in glioblastomas. World Neurosurg. 2017;105:282–288. doi: 10.1016/j.wneu.2017.05.165. [DOI] [PubMed] [Google Scholar]
- 122.Kleeff J., Ishiwata T., Kumbasar A., Friess H., Büchler M.W., Lander A.D., Korc M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J. Clin. Investig. 1998;102:1662–1673. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bosse K.R., Raman P., Zhu Z., Lane M., Martinez D., Heitzeneder S., Rathi K.S., Kendsersky N.M., Randall M., Donovan L., et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell. 2017;32:295–309.e12. doi: 10.1016/j.ccell.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li N., Spetz M.R., Ho M. The role of glypicans in cancer progression and therapy. J. Histochem. Cytochem. 2020:22155420933709. doi: 10.1369/0022155420933709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zynger D.L., McCallum J.C., Luan C., Chou P.M., Yang X.J. Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: Immunohistochemical investigation with analysis of histological growth patterns. Histopathology. 2010;56:750–757. doi: 10.1111/j.1365-2559.2010.03553.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhou F., Shang W., Yu X., Tian J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med. Res. Rev. 2018;38:741–767. doi: 10.1002/med.21455. [DOI] [PubMed] [Google Scholar]
- 128.Moek K.L., Fehrmann R.S.N., van der Vegt B., de Vries E.G.E., de Groot D.J.A. Glypican 3 overexpression across a broad spectrum of tumor types discovered with functional genomic mRNA profiling of a large cancer database. Am. J. Pathol. 2018;188:1973–1981. doi: 10.1016/j.ajpath.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 129.Wang D., Gao Y., Zhang Y., Wang L., Chen G. Glypican-3 promotes cell proliferation and tumorigenesis through up-regulation of β-catenin expression in lung squamous cell carcinoma. Biosci. Rep. 2019;39:BSR20181147. doi: 10.1042/BSR20181147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valsechi M.C., Oliveira A.B., Conceicao A.L., Stuqui B., Candido N.M., Provazzi P.J., de Araujo L.F., Silva W.A., de Freitas Calmon M., Rahal P. GPC3 reduces cell proliferation in renal carcinoma cell lines. BMC Cancer. 2014;14:631. doi: 10.1186/1471-2407-14-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao J., Ma J., Sun L., Li J., Qin T., Zhou C., Cheng L., Chen K., Qian W., Duan W., et al. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/β-catenin pathway in pancreatic cancer cells. J. Cell Biochem. 2018;119:9498–9512. doi: 10.1002/jcb.27266. [DOI] [PubMed] [Google Scholar]
- 132.Varma R.R., Hector S.M., Clark K., Greco W.R., Hawthorn L., Pendyala L. Gene expression profiling of a clonal isolate of oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Oncol. Rep. 2005;14:925–932. doi: 10.3892/or.14.4.925. [DOI] [PubMed] [Google Scholar]
- 133.Munir J., Van Ngu T., Na Ayudthaya P.D., Ryu S. Downregulation of glypican-4 facilitates breast cancer progression by inducing cell migration and proliferation. Biochem. Biophys. Res. Commun. 2020;526:91–97. doi: 10.1016/j.bbrc.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 134.Williamson D., Selfe J., Gordon T., Lu Y.J., Pritchard-Jones K., Murai K., Jones P., Workman P., Shipley J. Role for amplification and expression of glypican-5 in rhabdomyosarcoma. Cancer Res. 2007;67:57–65. doi: 10.1158/0008-5472.CAN-06-1650. [DOI] [PubMed] [Google Scholar]
- 135.Li F., Shi W., Capurro M., Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J. Cell Biol. 2011;192:691–704. doi: 10.1083/jcb.201008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y., Xu K., He M., Fan G., Lu H. Overexpression of glypican 5 (GPC5) inhibits prostate cancer cell proliferation and invasion via suppressing Sp1-mediated EMT and activation of Wnt/β-catenin signaling. Oncol. Res. 2018;26:565–572. doi: 10.3727/096504017X15044461944385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan Q., Zhang Y., Li J., Cao G., Yang W. High expression of microRNA-4295 contributes to cell proliferation and invasion of pancreatic ductal adenocarcinoma by the down-regulation of Glypican-5. Biochem. Biophys. Res. Commun. 2018;497:73–79. doi: 10.1016/j.bbrc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 138.Liu T., Zhang X., Sha K., Liu X., Zhang L., Wang B. miR-709 up-regulated in hepatocellular carcinoma, promotes proliferation and invasion by targeting GPC5. Cell Prolif. 2015;48:330–337. doi: 10.1111/cpr.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dinccelik-Aslan M., Gumus-Akay G., Elhan A.H., Unal E., Tukun A. Diagnostic and prognostic significance of glypican 5 and glypican 6 gene expression levels in gastric adenocarcinoma. Mol. Clin. Oncol. 2015;3:584–590. doi: 10.3892/mco.2015.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y., Li M., Shats I., Krahn J.M., Flake G.P., Umbach D.M., Li X., Li L. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS ONE. 2019;14:e0218067. doi: 10.1371/journal.pone.0218067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Januchowski R., Zawierucha P., Rucinski M., Nowicki M., Zabel M. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. Biomed. Res. Int. 2014;2014:365867. doi: 10.1155/2014/365867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lau C.S., Yu C.B., Wong H.K., Fan D.S., Mak H.T., Wong K.W., Lam D.S., Pang C.P., Choy K.W. Allelic imbalance at 13q31 is associated with reduced GPC6 in Chinese with sporadic retinoblastoma. Br. J. Ophthalmol. 2010;94:357–362. doi: 10.1136/bjo.2009.158832. [DOI] [PubMed] [Google Scholar]
- 143.Elgundi Z., Papanicolaou M., Major G., Cox T.R., Melrose J., Whitelock J.M., Farrugia B.L. Cancer metastasis: The role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front. Oncol. 2020;9:1482. doi: 10.3389/fonc.2019.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang X., Couchman J.R. Perlecan and tumor angiogenesis. J. Histochem. Cytochem. 2003;51:1393–1410. doi: 10.1177/002215540305101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cruz L.A., Tellman T.V., Farach-Carson M.C. Flipping the molecular switch: Influence of perlecan and its modifiers in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1245:133–146. doi: 10.1007/978-3-030-40146-7_6. [DOI] [PubMed] [Google Scholar]
- 146.Ghiselli G., Eichstetter I., Iozzo R.V. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem. J. 2001;359:153–163. doi: 10.1042/bj3590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nackaerts K., Verbeken E., Deneffe G., Vanderschueren B., Demedts M., David G. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int. J. Cancer. 1997;74:335–345. doi: 10.1002/(SICI)1097-0215(19970620)74:3<335::AID-IJC18>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 148.Batmunkh E., Tátrai P., Szabó E., Lódi C., Holczbauer A., Páska C., Kupcsulik P., Kiss A., Schaff Z., Kovalszky I. Comparison of the expression of agrin, basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Hum. Pathol. 2007;38:1508–1515. doi: 10.1016/j.humpath.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 149.Chakraborty S., Njah K., Pobbati A.V., Lim Y.B., Raju A., Lakshmanan M., Tergaonkar V., Lim C.T., Hong W. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 150.Rivera C., Zandonadi F.S., Sánchez-Romero C., Soares C.D., Granato D.C., González-Arriagada W.A., Paes Leme A.F. Agrin has a pathological role in the progression of oral cancer. Br. J. Cancer. 2018;118:1628–1638. doi: 10.1038/s41416-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li D., Gu Q., Xie Z., Shen Q., Li H. Clinical significance of nuclear localisation of agrin in lung adenocarcinoma. Pol. J. Pathol. 2019;70:198–204. doi: 10.5114/pjp.2019.90396. [DOI] [PubMed] [Google Scholar]
- 152.Wang Z.Q., Sun X.L., Wang Y.L., Miao Y.L.J. Agrin promotes the proliferation, invasion and migration of rectal cancer cells via the WNT signaling pathway to contribute to rectal cancer progression. J. Recept. Signal Transduct. 2020;30:1–8. doi: 10.1080/10799893.2020.1811325. [DOI] [PubMed] [Google Scholar]
- 153.Hansen N.U., Willumsen N., Sand J.M., Larsen L., Karsdal M.A., Leeming D.J. Type VIII collagen is elevated in diseases associated with angiogenesis and vascular remodeling. Clin. Biochem. 2016;49:903–908. doi: 10.1016/j.clinbiochem.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 154.Vlodavsky I., Gross-Cohen M., Weissmann M., Ilan N., Sanderson R.D. Opposing functions of heparanase-1 and heparanase-2 in cancer progression. Trends Biochem. Sci. 2018;43:18–31. doi: 10.1016/j.tibs.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindahl U., Li J.P. Heparanase—Discovery and targets. Adv. Exp. Med. Biol. 2020;1221:61–69. doi: 10.1007/978-3-030-34521-1_2. [DOI] [PubMed] [Google Scholar]
- 156.Bertrand J., Bollmann M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019;176:67–81. doi: 10.1111/bph.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi S., Choi Y., Jun E., Kim I.S., Kim S.E., Jung S.A., Oh E.S. Shed syndecan-2 enhances tumorigenic activities of colon cancer cells. Oncotarget. 2015;6:3874–3886. doi: 10.18632/oncotarget.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vuong T.T., Reine T.M., Sudworth A., Jenssen T.G., Kolset S.O. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J. Histochem. Cytochem. 2015;63:280–292. doi: 10.1369/0022155415568995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nikolova V., Koo C.Y., Ibrahim S.A., Wang Z., Spillmann D., Dreier R., Kelsch R., Fischgrabe J., Smollich M., Rossi L.H., et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis. 2009;30:397–407. doi: 10.1093/carcin/bgp001. [DOI] [PubMed] [Google Scholar]
- 160.Chang J.W., Kang U.B., Kim D.H., Yi J.K., Lee J.W., Noh D.Y., Lee C., Yu M.H. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteom. Clin. Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 161.Gonzalez E.M., Reed C.C., Bix G., Fu J., Zhang Y., Gopalakrishnan B., Greenspan D.S., Iozzo R.V. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 162.Gronborg M., Kristiansen T.Z., Iwahori A., Chang R., Reddy R., Sato N., Molina H., Jensen O.N., Hruban R.H., Goggins M.G., et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol. Cell Proteom. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 163.Poluzzi C., Iozzo R.V., Schaefer L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv. Drug Deliv. Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferreras M., Felbor U., Lenhard T., Olsen B.R., Delaissé J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/S0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 165.Karamanos N.K., Piperigkou Z., Theocharis A.D., Watanabe H., Franchi M., Baud S., Brézillon S., Götte M., Passi A., Vigetti D., et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018;118:9152–9232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 166.Matsuo I., Kimura-Yoshida C. Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130545. doi: 10.1098/rstb.2013.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Häcker U., Nybakken K., Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat. Rev. Mol. Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 168.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Venero M., Kramer K.L., Piotrowski T. Heparan sulfate proteoglycans regulate FGF signaling and cell polarity during collective cell migration. Cell. Rep. 2015;10:414–428. doi: 10.1016/j.celrep.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Turner N., Grose R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 171.Kang S., Elf S., Dong S., Hitosugi T., Lythgoe K., Guo A., Ruan H., Lonial S., Khoury H.J., Williams I.R., et al. Fibroblast growth factor receptor 3 associates with and tyrosine phosphorylates p90 RSK2, leading to RSK2 activation that mediateshematopoietic transformation. Mol. Cell Biol. 2009;29:2105–2117. doi: 10.1128/MCB.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seitz T., Freese K., Dietrich P., Thasler W.E., Bosserhoff A., Hellerbrand C. Fibroblast Growth Factor 9 is expressed by activated hepatic stellate cells and promotes progression of hepatocellular carcinoma. Sci. Rep. 2020;10:4546. doi: 10.1038/s41598-020-61510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zacharopoulou N., Tsapara A., Kallergi G., Schmid E., Tsichlis P.N., Kampranis S.C., Stournaras C. The epigenetic factor KDM2B regulates cell adhesion, small rho GTPases, actin cytoskeleton and migration in prostate cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:587–597. doi: 10.1016/j.bbamcr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 174.Katoh M., Nakagama H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014;34:280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 175.Jastrebova N., Vanwildemeersch M., Lindahl U., Spillmann D. Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling. Biol. Chem. 2010;285:26842–26851. doi: 10.1074/jbc.M109.093542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Szatmári T., Ötvös R., Hjerpe A., Dobra K. Syndecan-1 in cancer: Implications for cell signaling, differentiation, and prognostication. Dis. Markers. 2015;2015:796052. doi: 10.1155/2015/796052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang X., Zuo D., Chen Y., Li W., Liu R., He Y., Ren L., Zhou L., Deng T., Wang X., et al. Shed syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colorectal cancer. Br. J. Cancer. 2014;111:1965–1976. doi: 10.1038/bjc.2014.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Su G., Meyer K., Nandini C.D., Qiao D., Salamat S., Friedl A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006;168:2014–2026. doi: 10.2353/ajpath.2006.050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qiao D., Meyer K., Friedl A. Glypican 1 stimulates S phase entry and DNA replication in humanglioma cells and normal astrocytes. Mol. Cell Biol. 2013;33:4408–4421. doi: 10.1128/MCB.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 180.Li Y., Yang P. GPC5 gene and its related pathways in lung cancer. J. Thorac. Oncol. 2011;6:2–5. doi: 10.1097/JTO.0b013e3181fd6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou Z., Wang J., Cao R., Morita H., Soininen R., Chan K.M., Liu B., Cao Y., Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 182.Accornero P., Pavone L.M., Baratta M. The scatter factor signaling pathways as therapeutic associated target in cancer treatment. Curr. Med. Chem. 2010;17:2699–2712. doi: 10.2174/092986710791859261. [DOI] [PubMed] [Google Scholar]
- 183.Spina A., De Pasquale V., Cerulo G., Cocchiaro P., Della Morte R., Avallone L., Pavone L.M. HGF/c-MET Axis in tumor microenvironment and metastasis formation. Biomedicines. 2015;3:71–88. doi: 10.3390/biomedicines3010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pavone L.M., Cattaneo F., Rea S., De Pasquale V., Spina A., Sauchelli E., Mastellone V., Ammendola R. Intracellular signaling cascades triggered by the NK1 fragment of hepatocyte growth factor in human prostate epithelial cell line PNT1A. Cell Signal. 2011;23:1961–1971. doi: 10.1016/j.cellsig.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 185.Derksen P.W., de Gorter D.J., Meijer H.P., Bende R.J., van Dijk M., Lokhorst H.M., Bloem A.C., Spaargaren M., Pals S.T. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003;17:764–774. doi: 10.1038/sj.leu.2402875. [DOI] [PubMed] [Google Scholar]
- 186.Ramani V.C., Yang Y., Ren Y., Nan L., Sanderson R.D. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J. Biol. Chem. 2011;286:6490–6499. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pothula S., Xu Z., Goldstein D., Biankin A.V., Pirola R.C., Wilson J.S., Apte M.V. Hepatocyte growth factor inhibition: A novel therapeutic approach in pancreatic cancer. Br. J. Cancer. 2016;114:269–280. doi: 10.1038/bjc.2015.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.García-Vilas J.A., Medina M.A. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J. Gastroenterol. 2018;24:3695–3708. doi: 10.3748/wjg.v24.i33.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gaviglio A.L., Knelson E.H., Blobe G.C. Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation. FASEB J. 2017;31:1903–1915. doi: 10.1096/fj.201600828R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ellina M.-J., Bouris P., Aletras A.J., Theocharis A.D., Kletsas D., Karamanos N. K. EGFR and HER2 exert distinct roles on colon cancer cell functional properties and expression of matrix macromolecules. Biochim. Biophys. Acta. 2014;1840:2651–2661. doi: 10.1016/j.bbagen.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 191.Ibrahim S.A., Gadalla R., El-Ghonaimy E.A., Samir O., Mohamed H.T., Hassan H., Greve B., El-Shinawi M., Mohamed M.M., Gotte M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer. 2017;16:57. doi: 10.1186/s12943-017-0621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Purushothaman A., Uyama T., Kobayashi F., Yamada S., Sugahara K., Rapraeger A.C., Sanderson R.D. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Orecchia P., Conte R., Balza E., Petretto A., Mauri P., Mingari M.C., Carnemolla B. A novel human anti-syndecan-1 antibody inhibits vascular maturation and tumour growth in melanoma. Eur. J. Cancer. 2013;49:2022–2033. doi: 10.1016/j.ejca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 194.Johns S.C., Yin X., Jeltsch M., Bishop J.R., Schuksz M., El Ghazal R., Wilcox-Adelman S.A., Alitalo K., Fuster M.M. Functional importance of a proteoglycan coreceptor in pathologic lymphangiogenesis. Circ. Res. 2016;119:210–221. doi: 10.1161/CIRCRESAHA.116.308504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kadenhe-Chiweshe A., Papa J., McCrudden K.W., Frischer J., Bae J.-O., Huang J., Fisher J., Lefkowitch J.H., Feirt N., Rudge J., et al. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol. Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- 196.Li J., Kleeff J., Kayed H., Felix K., Penzel R., Büchler M.W., Korc M., Friess H. Glypican-1 antisense transfection modulates TGF-beta-dependent signaling in Colo-357 pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2004;320:1148–1155. doi: 10.1016/j.bbrc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 197.Hezel A.F., Deshpande V., Zimmerman S.M., Contino G., Alagesan B., O’Dell M.R., Rivera L.B., Harper J., Lonning S., Brekken R.A., et al. TGF-β and αvβ6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012;72:4840–4845. doi: 10.1158/0008-5472.CAN-12-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shen W., Tao G., Zhang Y., Cai B., Sun J., Tian Z. TGF-β in pancreatic cancer initiation and progression: Two sides of the same coin. Cell Biosci. 2017;7:39. doi: 10.1186/s13578-017-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lund M.E., Campbell D.H., Walsh B.J. The role of glypican-1 in the tumour microenvironment. Adv. Exp. Med. Biol. 2020;1245:163–176. doi: 10.1007/978-3-030-40146-7_8. [DOI] [PubMed] [Google Scholar]
- 200.Sun C.K., Chua M.-S., He J., So S.K. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011;13:735–747. doi: 10.1593/neo.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mytilinaiou M., Bano A., Nikitovic D., Berdiaki A., Voudouri K., Kalogeraki A., Karamanos N.K., Tzanakakis G.N. Syndecan-2 is a key regulator of transforming growth factor beta 2/Smad2-mediated adhesion in fibrosarcoma cells. IUBMB Life. 2013;65:134–143. doi: 10.1002/iub.1112. [DOI] [PubMed] [Google Scholar]
- 202.Mytilinaiou M., Nikitovic D., Berdiaki A., Papoutsidakis A., Papachristou D.J., Tsatsakis A., Tzanakakis G.N. IGF-I regulates HT1080 fibrosarcoma cell migration through a syndecan-2/Erk/ezrin signaling axis. Exp. Cell Res. 2017;361:9–18. doi: 10.1016/j.yexcr.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 203.Afratis N.A., Bouris P., Skandalis S.S., Multhaupt H.A., Couchman J.R., Theocharis A.D., Karamanos N.K. IGF-IR cooperates with ERα to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ECM molecules. Sci. Rep. 2017;7:40138. doi: 10.1038/srep40138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tai Y.T., Podar K., Catley L., Tseng Y.H., Akiyama M., Shringarpure R., Burger R., Hideshima T., Chauhan D., Mitsiades N., et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3’-kinase/AKT signaling. Cancer Res. 2003;63:5850–5858. [PubMed] [Google Scholar]
- 205.Morgan M.R., Humphries M.J., Bass M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Soares M.A., Teixeira F.C., Fontes M., Arêas A.L., Leal M.G., Pavão M.S., Stelling M.P. Heparan sulfate proteoglycans may promote or inhibit cancer progression by interacting with integrins and affecting cell migration. Biomed. Res. Int. 2015;2015:453801. doi: 10.1155/2015/453801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.De Pasquale V., Pezone A., Sarogni P., Tramontano A., Schiattarella G.G., Avvedimento V.E., Paladino S., Pavone L.M. EGFR activation triggers cellular hypertrophy and lysosomal disease in NAGLU-depleted cardiomyoblasts, mimicking the hallmarks of mucopolysaccharidosis IIIB. Cell Death Dis. 2018;9:40. doi: 10.1038/s41419-017-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang N., Friedl A. Syndecan-1-induced ECM fiber alignment requires integrin αvβ3 and syndecan-1 ectodomain and heparan sulfate chains. PLoS ONE. 2016;11:e0150132. doi: 10.1371/journal.pone.0150132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rapraeger A.C. Synstatin: A selective inhibitor of the syndecan-1-coupled IGF1R-αvβ3 integrin complex in tumorigenesis and angiogenesis. FEBS J. 2013;280:2207–2215. doi: 10.1111/febs.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Beauvais D.L.M., Ell B.J., McWhorter A.R., Rapraeger A.C. Syndecan-1 regulates alphavbeta3 and alphavbeta5 Integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J. Exp. Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang H., Leavitt L.A., Ramaswamy R., Rapraeger A.C. Interaction of syndecan and alpha6beta4 integrin cytoplasmic domains: Regulation of ErbB2-mediated integrin activation. J. Biol. Chem. 2010;285:13569–13579. doi: 10.1074/jbc.M110.102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liebersbach B.F., Sanderson R.D. Expression of syndecan-1 inhibits cell invasion into type I collagen. J. Biol. Chem. 1994;269:20013–20019. [PubMed] [Google Scholar]
- 213.Choi S., Kim Y., Park H., Han I.-O., Chung E., Lee S.-Y., Kim Y.-B., Lee J.W., Oh E.S., Yi J.Y. Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem. Biophys. Res. Commun. 2009;384:231–235. doi: 10.1016/j.bbrc.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 214.Contreras H.R., Fabre M., Granés F., Casaroli-Marano R., Rocamora N., Herreros A.G., Reina M., Vilaró S. Syndecan-2 expression in colorectal cancer-derived HT-29 M6 epithelial cells induces a migratory phenotype. Biochem. Biophys. Res. Commun. 2001;286:742–751. doi: 10.1006/bbrc.2001.5459. [DOI] [PubMed] [Google Scholar]
- 215.Lim H.C., Couchman J.R. Syndecan-2 regulation of morphology in breast carcinoma cells is dependent on RhoGTPases. Biochim. Biophys. Acta. 2014;1840:2482–2490. doi: 10.1016/j.bbagen.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 216.Munesue S., Kusano Y., Oguri K., Itano N., Yoshitomi Y., Nakanishi H., Yamashina I., Okayama M. The role of syndecan-2 in regulation of actin- cytoskeletal organization of Lewis lung carcinoma-derived metastatic clones. Biochem. J. 2002;363:201–209. doi: 10.1042/bj3630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Park H., Han I., Kwon H.J., Oh E.S. Focal adhesion kinase regulates syndecan-2-mediated tumorigenic activity of HT1080 fibrosarcoma cells. Cancer Res. 2005;65:9899–9905. doi: 10.1158/0008-5472.CAN-05-1386. [DOI] [PubMed] [Google Scholar]
- 218.Lee J.H., Park H., Chung H., Choi S., Kim Y., Yoo H., Kim T.Y., Hann H.J., Seong I., Kim J., et al. Syndecan-2 regulates the migratory potential of melanoma cells. J. Biol. Chem. 2009;284:27167–27175. doi: 10.1074/jbc.M109.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jang B., Jung H., Choi S., Lee Y.H., Lee S.T., Oh E.S. Syndecan-2 cytoplasmic domain up-regulates matrix metalloproteinase-7 expression via the protein kinase Cγ–mediated FAK/ERK signaling pathway in colon cancer. J. Biol. Chem. 2017;292:16321–16332. doi: 10.1074/jbc.M117.793752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Erdem M., Erdem S., Sanli O., Sak H., Kilicaslan I., Sahin F., Telci D. Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma. Urol. Oncol. 2014;32:e13–e20. doi: 10.1016/j.urolonc.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 221.Chalkiadaki G., Nikitovic D., Berdiaki A., Sifaki M., Krasagakis K., Katonis P., Karamanos N.K., Tzanakakis G.N. Fibroblast growth factor-2 modulates melanoma adhesion and migration through a syndecan-4-dependent mechanism. Int. J. Biochem. Cell Biol. 2009;41:1323–1331. doi: 10.1016/j.biocel.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 222.Huang C.-P., Cheng C.-M., Su H.-L., Lin Y.-W. Syndecan-4 promotes epithelial tumor cells spreading and regulates the turnover of PKCα activity under mechanical stimulation on the elastomeric substrates. Cell Physiol. Biochem. 2015;36:1291–1304. doi: 10.1159/000430297. [DOI] [PubMed] [Google Scholar]
- 223.Fiore V.F., Ju L., Chen Y., Zhu C., Barker T.H. Dynamic catch of a Thy-1-α5β1+syndecan-4 trimolecular complex. Nat. Commun. 2014;5:4886. doi: 10.1038/ncomms5886. [DOI] [PubMed] [Google Scholar]
- 224.Ahsan M.S., Yamazaki M., Maruyama S., Kobayashi T., Ida-Yonemochi H., Hasegawa M., Henry Ademola A., Cheng J., Saku T. Differential expression of perlecan receptors, α-dystroglycan and integrin β1, before and after invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. 2011;40:552–559. doi: 10.1111/j.1600-0714.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 225.Douglass S., Goyal A., Iozzo R.V. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect. Tissue Res. 2015;56:381–391. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Theocharis A.D., Gialeli C., Bouris P., Giannopoulou E., Skandalis S.S., Aletras A.J., Iozzo R.V., Karamanos N.K. Cell-matrix interactions: Focus on proteoglycan-proteinase interplay and pharmacological targeting in cancer. FEBS J. 2014;281:5023–5042. doi: 10.1111/febs.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ryu H.Y., Lee J., Yang S., Park H., Choi S., Jung K.C., Lee S.T., Seong J.K., Han I.O., Oh E.S. Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J. Biol. Chem. 2009;284:35692–35701. doi: 10.1074/jbc.M109.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Koyama Y., Naruo H., Yoshitomi Y., Munesue S., Kiyono S., Kusano Y., Hashimoto K., Yokoi T., Nakanishi H., Shimizu S., et al. Matrix metalloproteinase-9 associated with heparan sulphate chains of GPI-anchored cell surface proteoglycans mediates motility of murine colon adenocarcinoma cells. J. Biochem. 2008;143:581–592. doi: 10.1093/jb/mvn006. [DOI] [PubMed] [Google Scholar]
- 229.Lambert J., Makin K., Akbareian S., Johnson R., Alghamdi A.A.A., Robinson S.D., Edwards D.R. ADAMTS-1 and syndecan-4 intersect in the regulation of cell migration and angiogenesis. J. Cell Sci. 2020;133:jcs235762. doi: 10.1242/jcs.235762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Van Doren S.R., Marcink T.C., Koppisetti R.K., Jurkevich A., Fulcher Y.G. Peripheral membrane associations of matrix metalloproteinases. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1964–1973. doi: 10.1016/j.bbamcr.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gao G., Plaas A., Thompson V.P., Jin S., Zuo F., Sandy J.D. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J. Biol. Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 232.Novinec M., Lenarčič B., Turk B. Cysteine cathepsin activity regulation by glycosaminoglycans. Biomed. Res. Int. 2014;2014:309718. doi: 10.1155/2014/309718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cocchiaro P., De Pasquale V., Della Morte R., Tafuri S., Avallone L., Pizard A., Moles A., Pavone L.M. The multifaceted role of the lysosomal protease cathepsins in kidney disease. Front. Cell Dev. Biol. 2017;5:114. doi: 10.3389/fcell.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vidak E., Javoršek U., Vizovišek M., Turk B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells. 2019;8:E264. doi: 10.3390/cells8030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.De Pasquale V., Moles A., Pavone L.M. Cathepsins in the pathophysiology of mucopolysaccharidoses: New perspectives for therapy. Cells. 2020;9:979. doi: 10.3390/cells9040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vizovišek M., Fonović M., Turk B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix Biol. 2019;75–76:141–159. doi: 10.1016/j.matbio.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 237.Khaket T.P., Kwon T.K., Kang S.C. Cathepsins: Potent regulators in carcinogenesis. Pharmacol. Ther. 2019;198:1–19. doi: 10.1016/j.pharmthera.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 238.Cerezo-Magaña M., Bång-Rudenstam A., Belting M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin. Cancer Biol. 2020;62:99–107. doi: 10.1016/j.semcancer.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 239.Choi D., Lee T.H., Spinelli C., Chennakrishnaiah S., D’Asti E., Rak J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017;67:11–22. doi: 10.1016/j.semcdb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 240.Walimbe T., Panitch A. Proteoglycans in biomedicine: Resurgence of an underexploited class of ECM molecules. Front. Pharmacol. 2020;10:1661. doi: 10.3389/fphar.2019.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.De Pasquale V., Sarogni P., Pistorio V., Cerulo G., Paladino S., Pavone L.M. Targeting heparan sulfate proteoglycans as a novel therapeutic strategy for mucopolysaccharidoses. Mol. Methods Clin. Dev. 2018;10:8–16. doi: 10.1016/j.omtm.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morla S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 2019;20:1963. doi: 10.3390/ijms20081963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Weiss R.J., Esko J.D., Tor Y. Targeting heparin and heparan sulfate protein interactions. Org. Biomol. Chem. 2017;15:5656–5668. doi: 10.1039/C7OB01058C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Harada E., Serada S., Fujimoto M., Takahashi Y., Takahashi T., Hara H., Nakatsuka R., Sugase T., Nishigaki T., Saito Y., et al. Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma. Oncotarget. 2017;8:24741–24752. doi: 10.18632/oncotarget.15799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gao W., Kim H., Feng M., Phung Y., Xavier C.P., Rubin J.S., Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. 2014;60:576–587. doi: 10.1002/hep.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhu A.X., Gold P.J., El-Khoueiry A.B., Abrams T.A., Morikawa H., Ohishi N., Ohtomo T., Philip P.A. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013;19:920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 247.Li N., Gao W., Zhang Y.-F., Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4:741–754. doi: 10.1016/j.trecan.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Orecchia P., Balza E., Pietra G., Conte R., Bizzarri N., Ferrero S., Mingari M.C., Carnemolla B. L19-IL2 immunocytokine in combination with the anti-syndecan-1 46F2SIP antibody format: A new targeted treatment approach in an ovarian carcinoma model. Cancers. 2019;11:1232. doi: 10.3390/cancers11091232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Li N., Fu H., Hewitt S.M., Dimitrov D.S., Ho M. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc. Natl. Acad. Sci. USA. 2017;114:E6623–E6631. doi: 10.1073/pnas.1706055114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Matsuzaki S., Serada S., Hiramatsu K., Nojima S., Matsuzaki S., Ueda Y., Ohkawara T., Mabuchi S., Fujimoto M., Morii E., et al. Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int. J. Cancer. 2018;142:1056–1066. doi: 10.1002/ijc.31124. [DOI] [PubMed] [Google Scholar]
- 251.Lanzi C., Cassinelli G. Heparan sulfate mimetics in cancer therapy: The challenge to define structural determinants and the relevance of targets for optimal activity. Molecules. 2018;23:2915. doi: 10.3390/molecules23112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Boothello R.S., Patel N.J., Sharon C., Abdelfadiel E.I., Morla S., Brophy D.F., Lippman H.R., Desai U.R., Patel B.B. A unique non saccharide mimetic of heparin hexasaccharide inhibits colon cancer stem cells via p38 MAP kinase activation. Mol. Cancer Ther. 2019;18:51–61. doi: 10.1158/1535-7163.MCT-18-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sutton A., Friand V., PapyGarcia D., Dagouassat M., Martin L., Vassy R., Haddad O., Sainte-Catherine O., Kraemer M., Saffar L., et al. Glycosaminoglycans and their synthetic mimetics inhibit RANTES-induced migration and invasion of human hepatoma cells. Mol. Cancer Ther. 2007;6:2948–2958. doi: 10.1158/1535-7163.MCT-07-0114. [DOI] [PubMed] [Google Scholar]
- 254.Metwaly H.A., El-Gayar A.M., El-Shishtawy M.M. Inhibition of the signaling pathway of syndecan-1 by synstatin: A promising anti-integrin inhibitor of angiogenesis and proliferation in HCC in rats. Arch. Biochem. Biophys. 2018;652:50–58. doi: 10.1016/j.abb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 255.Chua J.S., Kuberan B. Synthetic xylosides: Probing the glycosaminoglycan biosynthetic machinery for biomedical applica- tions. Acc. Chem. Res. 2017;50:2693–2705. doi: 10.1021/acs.accounts.7b00289. [DOI] [PubMed] [Google Scholar]
- 256.Raman K., Kuberan B. Click-xylosides mitigate glioma cell invasion in vitro. Mol. BioSyst. 2010;6:1800–1802. doi: 10.1039/c0mb00020e. [DOI] [PubMed] [Google Scholar]
- 257.Tao X., Yin Y., Lian D., Gu H., Chen W., Yang L., Yin G., Liu P., Li L., Wei Y., et al. Puerarin 6″-O-xyloside suppresses growth, self-renewal and invasion of lung cancer stem-like cells derived from A549 cells via regulating Akt/c-Myc signalling. Clin. Exp. Pharmacol. Physiol. 2020;47:1311–1319. doi: 10.1111/1440-1681.13294. [DOI] [PubMed] [Google Scholar]
- 258.Pisano C., Vlodavsky I., Ilan N., Zunino F. The potential of heparanase as a therapeutic target in cancer. Biochem. Pharmacol. 2014;89:12–19. doi: 10.1016/j.bcp.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Veraldi N., Zouggari N., de Agostini A. The challenge of modulating heparan sulfate turnover by multitarget heparin derivatives. Molecules. 2020;25:390. doi: 10.3390/molecules25020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dredge K., Brennan T.V., Hammond E., Lickliter J.D., Lin L., Bampton D., Handley P., Lankesheer F., Morrish G., Yang Y., et al. A phase I study of the novel immunomodulatory agent PG545 (Pixatimod) in subjects with advanced solid tumours. Br. J. Cancer. 2018;118:1035–1041. doi: 10.1038/s41416-018-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Weissmann M., Bhattacharya U., Feld S., Hammond E., Ilan N., Vlodavsky I. The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action. Matrix Biol. 2019;77:58–72. doi: 10.1016/j.matbio.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 262.Hammond E., Brandt R., Dredge K. PG545, a heparan sulfate mimetic, reduces heparanase expression in vivo, blocks spontaneous metastases and enhances overall survival in the 4T1 breast carcinoma model. PLoS ONE. 2012;7:e52175. doi: 10.1371/journal.pone.0052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mohan C.D., Hari S., Preetham H.D., Rangappa S., Barash U., Ilan N., Nayak S.C., Gupta V.K., Vlodavsky I., Rangappa K.S. Targeting heparanase in cancer: Inhibition by synthetic, chemically modified, and natural compounds. Iscience. 2019;15:360–390. doi: 10.1016/j.isci.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cassinelli G., Favini E., Dal Bo L., Tortoreto M., De Maglie M., Dagrada G., Pilotti S., Zunino F., Zaffaroni N., Lanzi C. Antitumor efficacy of the heparan sulfate mimic Roneparstat (SST0001) against sarcoma models involves multi-target inhibition of receptor tyrosine kinases. Oncotarget. 2016;7:47848–47863. doi: 10.18632/oncotarget.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhou H., Roy S., Cochran E., Zouaoui R., Chu C.L., Duffner J., Zhao G., Smith S., Galcheva-Gargova Z., Karlgren J., et al. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS ONE. 2011;6:e21106. doi: 10.1371/journal.pone.0021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Weissmann M., Arvatz G., Horowitz N., Feld S., Naroditsky I., Zhang Y., Ng M., Hammond E., Nevo E., Vlodavsky I., et al. Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc. Natl. Acad. Sci. USA. 2016;113:704–709. doi: 10.1073/pnas.1519453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Baburajeev C.P., Mohan C.D., Rangappa S., Mason D.J., Fuchs J.E., Bender A., Barash U., Vlodavsky I., Rangappa K.S. Identification of novel class of triazolo-thiadiazoles as potent inhibitors of human heparanase and their anticancer activity. BMC Cancer. 2017;17:235. doi: 10.1186/s12885-017-3214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zheng X., Gai X., Han S., Moser C.D., Hu C., Shire A.M., Floyd R.A., Roberts L.R. The human sulfatase 2 inhibitor 2,4-disulfonylphenyl-tert-butylnitrone (OKN-007) has an antitumor effect in hepatocellular carcinoma mediated via suppression of TGFB1/SMAD2 and Hedgehog/GLI1 signaling. Genes Chromosome Cancer. 2013;52:225–236. doi: 10.1002/gcc.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Coutinho de Souza P., Mallory S., Smith N., Saunders D., Li X.-N., McNall-Knapp R.Y., Fung K.M., Towner R.A. Inhibition of pediatric glioblastoma tumor growth by the anti-cancer agent OKN-007 in orthotopic mouse xenografts. PLoS ONE. 2015;10:e0134276. doi: 10.1371/journal.pone.0134276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Khurana A., Jung-Beom D., He X., Kim S.-H., Busby R.C., Lorenzon L., Villa M., Baldi A., Molina J., Goetz M.P., et al. Matrix detachment and proteasomal inhibitors diminish Sulf-2 expression in breast cancer cell lines and mouse xenografts. Clin. Exp. Metastasis. 2013;30:407–415. doi: 10.1007/s10585-012-9546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Clancy J., D’Souza-Schorey C. Extracellular vesicles in cancer: Purpose and promise. Cancer J. 2018;24:65–69. doi: 10.1097/PPO.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yi B., Qiu Y., Ji W., Wei M., Liu C., Peng Z., Zhang Y., Quan Z., Tang Z., Su C. Desulfation of cell surface HSPG is an effective strategy for the treatment of gallbladder carcinoma. Cancer Lett. 2016;381:349–358. doi: 10.1016/j.canlet.2016.08.002. [DOI] [PubMed] [Google Scholar]