Abstract
Breast milk from a single mother was collected during a 28-week lactation period. Bacterial diversity was studied by amplicon sequencing analysis of the V3-V4 variable region of the 16S rRNA gene. Firmicutes and Proteobacteria were the main phyla detected in the milk samples, followed by Actinobacteria and Bacteroidetes. The proportion of Firmicutes to Proteobacteria changed considerably depending on the sampling week. A total of 411 genera or higher taxons were detected in the set of samples. Genus Streptococcus was detected during the 28-week sampling period, at relative abundances between 2.0% and 68.8%, and it was the most abundant group in 14 of the samples. Carnobacterium and Lactobacillus had low relative abundances. At the genus level, bacterial diversity changed considerably at certain weeks within the studied period. The weeks or periods with lowest relative abundance of Streptococcus had more diverse bacterial compositions including genera belonging to Proteobacteria that were poorly represented in the rest of the samples.
Keywords: breast milk, biodiversity, lactic acid bacteria, late lactation, metagenomics
1. Introduction
Human milk is considered to be an important source of bacteria for the newborn. Many of these bacteria may be human commensals or have potential probiotic effects [1]. Lactic acid bacteria, such as Lactobacillus fermentum, L. gasseri, L. rhamnosus, isolated from human breast milk, can be regarded as potential probiotic bacteria [2,3,4]. Previous studies have suggested that commensal coagulase-negative staphylococci and viridans streptococci found in breast milk can reduce the acquisition of undesired pathogens by infants exposed to hospital environments [5]. In addition, some of the bacterial strains found in human milk may have a large potential to improve the mother’s health [6]. Furthermore, bacteria ingested during breastfeeding contribute to the development of the infant gut microbiome [7]. The benefits of breastfeeding also extend to a reduction of respiratory and gastrointestinal tract infections and to a correct education of the immune system, with a concomitant reduction of the risks to develop several diseases such as obesity, diabetes, or inflammatory bowel diseases [7,8,9].
In addition to classical studies based on isolation and identification of bacteria from human milk [5], culture-independent studies have provided a large amount of information on the human milk microbiota. The culture-independent approaches based on amplification and sequencing of variable regions within the 16S rDNA gene, allow the detection in a single step of both aerobic and anaerobic bacteria as well as bacteria that, in spite of being in a low proportion in the population, may play significant roles. Recent review papers have summarized the major findings of previous studies on the microbiota from human milk samples based on culture-independent approaches [10,11]. Many of the studies have focused on the influence of different factors such as the mother’s diet and health status, maternal age, child delivery method, probiotic use, HIV infection, administration of antibiotics or collection/feeding method, and involve samples from several subjects. Such studies led to the proposal of a core microbiota for the human milk or at least a list of the bacterial genera most frequently found [11]. Nevertheless, the influence of the lactation stage has been studied to a much less extent. Cabrera-Rubio et al. [12] analyzed the milk samples from 18 women at three sampling points, e.g., within 2 days after mothers gave birth in the maternity hospital (colostrum) and at 1 and 6 months after delivery at home. The main results obtained after 16S rRNA gene amplification and pyrosequencing indicated that the human milk microbiome changes over lactation: Weissella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus were predominant in colostrum samples, whereas in 1- and 6-month milk samples, the typical inhabitants of the oral cavity (e.g., Veillonella, Leptotrichia, and Prevotella) increased significantly. Khodayar-Pardo et al. [13] applied quantitative polymerase chain reaction (PCR) to study the microbial composition of milk samples collected from 322 mothers within the first month of exclusive breastfeeding and reported that total bacteria, Bifidobacterium and Enterococcus spp. counts, increased throughout the lactation period. Most of these studies, however, do not report individual variations in the microbiota during the lactation period. Nevertheless, it is suspected that different changes may occur at the individual level due to different factors. The aim of the present study was to investigate the microbiota during the mid-to-late lactation period in breast milk from a single mother and to analyze possible changes in bacterial diversity during the period.
2. Materials and Methods
2.1. Sample Collection
Written informed consent was obtained, in accordance with the Declaration of Helsinki. Samples were taken from a single 38-year-old Latin American female donor, suffering from asthma and overweight, during a lactating period between weeks 21 and 48, inclusive, after cesarean delivery. Samples were taken three times in the day before baby lactation. The hands of the volunteer were cleaned with soap and covered with sterile gloves. Then, the nipples and surrounding areola were cleaned with cotton soaked with 70% ethanol. The first drops of milk were discarded, and then 5–7 mL of milk was extracted from each breast manually using a sterile manual pump (Philips Avent SCF330/20; Philips Ibérica, Madrid, Spain). The milk was transferred to sterile falcon test tubes, stored at 4 °C, and transported on ice to the laboratory within the next 12 h, where it was stored at −20 °C until analysis.
2.2. DNA Extraction
Thawed milk samples from the same day were mixed thoroughly and centrifuged at 16,000× g for 7 min in a refrigerated centrifuge 5424 R (Eppendorf, Corp., Hamburg, Germany). After removal of the supernatants, total DNA from the remaining pellets was extracted with a QIAamp Stool DNA Fast Mini Kit (Qiagen, Madrid, Spain) following the manufacturer instructions. The quality and quantity of the extracted DNA was determined by QuantiFluor® ONE dsDNA system (Promega, Madison, WI, USA). The DNA was stored at −20 °C until analysis.
2.3. DNA Sequencing and Analysis
The 16S rDNA V3-V4 regions were amplified following the Illumina Metagenomics Sequencing Library Preparation protocol (Illumina, Inc., San Diego, CA, USA). Illumina adapter overhang nucleotide sequences were added to the gene-specific sequences. The following 16S rDNA gene amplicon PCR primer sequences were used: forward primer: 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG; reverse primer: 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC [14]. Microbial genomic DNA (5 ng/μL in 10 mM Tris pH 8.5) was used to initiate the protocol. After 16S rDNA gene amplification, the multiplexing step was performed using Nextera XT Index Kit (Illumina). Then, 1 μL of the PCR product was run on a Bioanalyzer DNA 1000 chip to verify the size (expected size ~550 bp). After size verification, the libraries were sequenced using a 2 × 300 pb paired-end run on a MiSeq Sequencer according to the manufacturer’s instructions (Illumina). Quality assessment was performed by the use of prinseq-lite program [15]. The sequence data were analyzed using qiime2 pipeline [16]. Denoising, paired-ends joining, and chimera depletion were performed starting from paired ends data using DADA2 pipeline [17]. Taxonomic affiliations were assigned using the Naive Bayesian classifier integrated in quiime2 plugins and the SILVA_release_132 database. Statistical analysis was carried out with SPSS software version 24 (IBM Corp., Foster City, CA, USA).
3. Results
3.1. Characteristics of Sequence Reads
The numbers of reads assigned to operational taxonomic units (OTUs) and the alpha diversity indicators are shown in Table 1. The number of assigned reads ranged from 140 to 1,375,641. A few samples yielded very low numbers of reads (e.g., samples corresponding to weeks 11, 15, 19, and 26 of the sampling period) and yielded a very low number of observations (between 3 and 9). In addition, samples 11 and 26 showed an abnormal bacterial composition. Therefore, these samples were excluded from the analysis.
Table 1.
N° of reads and alpha diversity indexes at genus level of breast milk samples at different weeks (W) of the sampling period.
| Sample | N° Reads | N° Observations | Chao1 | Shannon-Weaver | Simpson |
|---|---|---|---|---|---|
| W1 | 111,968.00 | 107.00 | 107.00 | 2.90 | 0.89 |
| W2 | 64,302.00 | 91.00 | 91.0 | 3.32 | 0.94 |
| W3 | 106,190.00 | 95.00 | 95.00 | 1.60 | 0.52 |
| W4 | 125,198.00 | 165.00 | 165.00 | 3.37 | 0.93 |
| W5 | 145,841.00 | 115.00 | 115.00 | 1.87 | 0.70 |
| W6 | 132,594.00 | 71.00 | 71.00 | 2.08 | 0.83 |
| W7 | 145,004.00 | 163.00 | 163.00 | 2.89 | 0.84 |
| W8 | 122,052.00 | 65.00 | 65.00 | 1.24 | 0.47 |
| W9 | 151,193.00 | 110.00 | 110.00 | 2.94 | 0.89 |
| W10 | 86,107.00 | 140.00 | 140.00 | 3.49 | 0.94 |
| W11 | 3245.00 | 9.00 | 9.00 | 1.34 | 0.66 |
| W12 | 55,527.00 | 65.00 | 65.00 | 2.02 | 0.78 |
| W13 | 138,427.00 | 118.00 | 118.00 | 2.27 | 0.81 |
| W14 | 141,003.00 | 93.00 | 93.00 | 1.86 | 0.65 |
| W15 | 143.00 | 3.00 | 3.00 | 0.32 | 0.14 |
| W16 | 102,600.00 | 102.00 | 102.00 | 2.45 | 0.80 |
| W17 | 98,764.00 | 81.00 | 81.00 | 2.00 | 0.68 |
| W18 | 107,346.00 | 1370.00 | 130.00 | 3.31 | 0.92 |
| W19 | 140.00 | 3.00 | 3.00 | 0.62 | 0.37 |
| W20 | 68,575.00 | 90.00 | 90.00 | 2.69 | 0.85 |
| W21 | 1,375,641.00 | 119.00 | 119.00 | 1.92 | 0.68 |
| W22 | 89,705.00 | 92.00 | 92.00 | 1.65 | 0.58 |
| W23 | 116,236.00 | 104.00 | 104.00 | 1.82 | 0.62 |
| W24 | 100,113.00 | 102.00 | 102.00 | 2.34 | 0.76 |
| W25 | 97,925.00 | 109.00 | 109.00 | 2.52 | 0.86 |
| W26 | 61,413.00 | 6.00 | 6.00 | 0.01 | 0.00 |
| W27 | 105,221.00 | 119.00 | 119.00 | 2.72 | 0.87 |
| W28 | 36,336.00 | 111.00 | 111.00 | 2.77 | 0.81 |
3.2. Bacterial Diversity in Breast Milk Samples
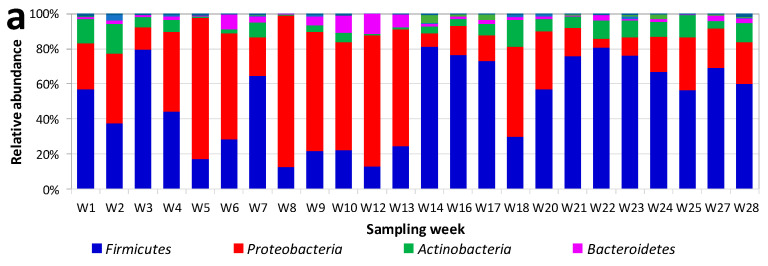
Firmicutes and Proteobacteria were the main phyla detected in the milk samples (Figure 1a). The proportion of Firmicutes to Proteobacteria changed considerably depending on the sampling week. Firmicutes were most abundant in samples from weeks 1, 3, 7, 14–17, 20–25, and 27–28 of the sampling period. However, Proteobacteria were predominant in the rest of the samples. Actinobacteria were the third most important group in most of the samples, followed by Bacteroidetes. Bacteroidetes had higher relative abundances than Actinobacteria in samples corresponding to weeks 6 and 10–13. The above-mentioned phyla represented between 98.0% and 99.8% of the OTUs.
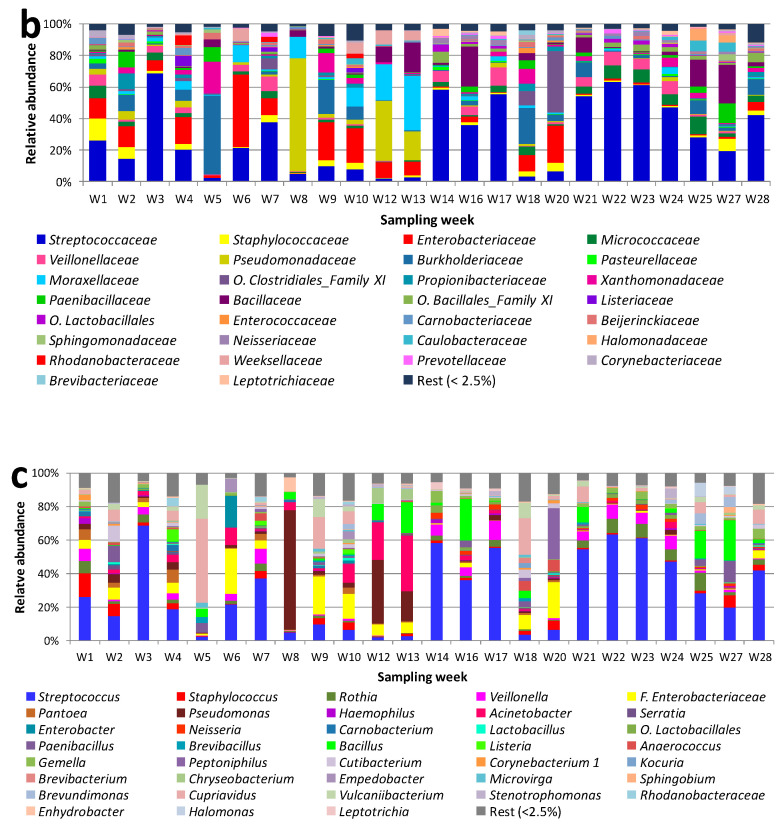
Figure 1.
Bacterial diversity of breast milk samples at Phylum (a), Family (b) and Genus (c) levels. The different sampling weeks (W) are represented.
The 30 families that had relative abundances ≥ 2.5% are shown in Figure 1b. These covered between 88.1 and 98.2 of OTUs. Firmicutes were represented mainly by Fam. Streptococcaceae. This group was found at relative abundances in a range from 2.0% to 68.8%. The following families were represented in many of the samples: Staphylococcaceae, Bacillaceae, Paenibacillaceae, and Veillonellaceae. Members of O. Clostridiales Family XI were detected only in a few of the samples. Unidentified members of O. Lactobacillales and Carnobacteriaceae were also relevant in some samples, with relative abundances ranging from ca. 1.5% to ca. 4.5%. Lactobacillaceae only were represented in two samples (W2, W10), with relative abundances between 1.0% and 1.2%. Enterobacteriaceae were highly represented in most of the samples, ranking sometimes in first position in relative abundance. Members of Pseudomonadaceae, Moraxellaceae, and Xanthomonadaceae were also relevant groups among the Proteobacteria. Microccaceae and Weeksellaceae were the main representatives among Actinobacteria and Bacteroidetes, respectively.
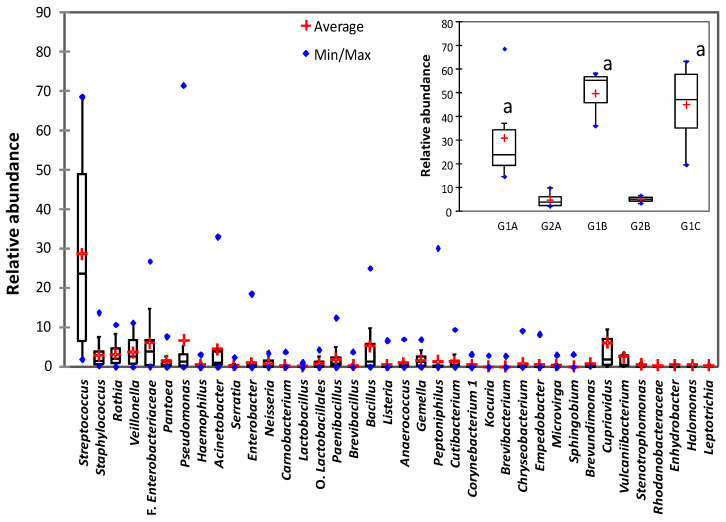
The 38 genera with relative abundances ≥ 2.5% (representing between 81.7 and 97.5 of OTUs) are shown in Figure 1c. Genus Streptococcus was detected in all the samples (being the most abundant OTU in 14 samples), although there were large differences in its relative abundances between samples. For example, genus Streptococcus had relative abundances that were above 50% in samples from weeks 3, 14, 17, and 21–23. By contrast, the lowest relative abundances for this genus were detected in samples from weeks 5, 8–10, 12–13, 18, and 20. Box-plot representation of the relative abundances of the main genera across the samples (Figure 2) indicated that Streptococcus was the main genus in the milk (Figure 2). Data on the relative abundance of Streptococcus were clustered in three groups of high relative abundances with two intercalated periods of low relative abundances (Figure 2, insert) and then analyzed by univariate statistical analysis (ANOVA, Tukey’s test, Kruskal–Wallis, Dunn’s post hoc). The results revealed that the three main groups of samples (G1A, G1B, G1C) had significantly higher relative abundances (p < 0.05) than the low-abundance samples. Apparently, groups G1B and G1C had higher relative abundances than group G1A (which would suggest an increase in the relative abundance of Streptococcus by the end of the sampling period). However, the differences between the three groups were not statistically significant (p > 0.05) for any of the univariate analyses carried out.
Figure 2.
Box-plot representation of the relative abundances of the 38 main bacterial genera detected in the milk samples. Insert: Box-plot representation of the relative abundances of gen. Streptococcus during the sampling period. Data were grouped by weeks according to relative abundance. Groups with high relative abundance: G1A (weeks 1–4, 6–7), G1B (weeks 14, 16–17), G1C (weeks 21–25, 27–28). Groups with low relative abundance: G2A (weeks 5, 8–10, 12–13), G2B (weeks 18, 20). a statistically significant differences (p < 0.05) with G2A.
Another genus represented in all breast milk samples was Staphylococcus (Figure 1c), with relative abundances from 0.3% to 14%. The lactic acid bacteria Carnobacterium and Lactobacillus had very low relative abundances. Aerobic endospore formers (Paenibacillus, Brevibacillus, and Bacillus) were detected in many of the samples, in some cases with high relative abundances. Gemella was also detected in many samples, and Listeria was detected in three samples (reaching 6.8% in one sample).
Most of the Enterobacteriales belonged to Fam. Enterobacteriaceae (Others). Members of the genera Pantoea, Serratia, and Enterobacter were also relevant in some samples (Figure 1c). Members of the genera Acinetobacter, Haemophilus, and Neisseria were detected in some samples. Pseudomonas had low relative abundances, except for three samples (weeks 8, 12, and 13). Several samples showed high relative abundances of members of family Burkholderiaceae (Cupriavidus), with remarkably high values at week 5. All samples with a high relative abundance of Cupriavidus also had higher relative abundances of Vulcaniibacterium (Xanthomonadaceae). Among Actinobacteria, the main representative was Rothia (with relative abundances between 3% and 10% in many samples).
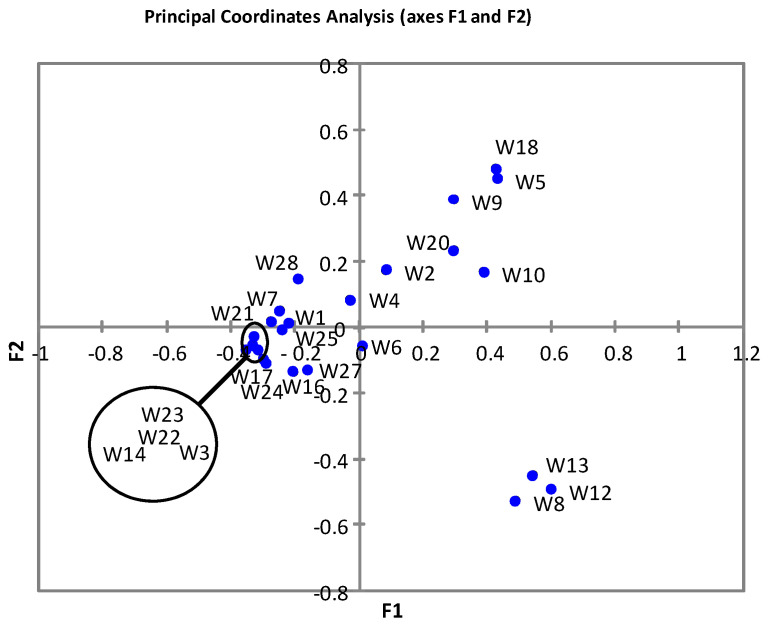
PCoA (Figure 3) revealed a main cluster of samples (all of them having a mid-to high relative abundance of OTUs belonging to genus Staphylococcus), with at least two minor clusters (samples W8, W12, W13) characterized by a very low relative abundance of Streptococcus and high relative abundances of Pseudomonas/Acinetobacter/Bacillus, and samples W5, W9, W10, W18 (also having very low relative abundances of Streptococcus and higher relative abundances of Cupriavidus/Vulcaniibacterium together with other bacterial groups).
Figure 3.
Principal coordinates analysis of breast milk samples taken at different weeks (W) during the sampling period.
4. Discussion
Results from the present study provided information on the changes in bacterial diversity in human milk from a single individual during the late lactating period. By focusing on a single donor, the present study avoided the confounding effect of data from different subjects and allowed to follow this mother continuously. Although the data may be more difficult to analyze, the study revealed a sample variability that otherwise may be unnoticed in studies involving a compendium of samples. Many previous studies have addressed the microbial composition of human milk, but usually from a compendium of samples taken from different individuals and taken at early to mid lactating period. Zimmermann and Curtis [11] identified 44 studies investigating 3105 breast milk samples from 2655 women, and reported that the most frequently found genera were Staphylococcus, Streptococcus, Lactobacillus, Pseudomonas, Bifidobacterium, Corynebacterium, Enterococcus, Acinetobacter, Rothia, Cutibacterium, Veillonella, and Bacteroides. Another review paper reported that Streptococcus and Staphylococcus appear to be widely predominant in human milk without regard to differences in geographic location or analytic methods [10].
Results from the present study also identified main genera reported in previous studies (Streptococcus, Staphylococcus, Pseudomonas, Acinetobacter, Rothia, Cutibacterium, and Veillonella). Furthermore, the microbial composition of several samples from the present study resembled, in a certain way, that of the children salivary microbiome reported by other authors [18] (with the following common genera: Streptococcus, Veillonella, Rothia, Leptotrichia, Haemophilus, and Neisseria). These results would suggest colonization of mammary glands by bacteria from the baby’s mouth. As a matter of fact, human milk is considered to be colonized by bacteria from the mother’s gut and skin or the infant’s mouth [19,20].
Streptococcus was the predominant OTU in 14 of the samples. Remarkably, those samples with lower relative abundances of Streptococcus had different microbial compositions. While the sequence reads obtained in the present study only allowed identification at the genus level, a previous study reported the presence of the following species of Streptococcus in human milk: S. mitis, S. infantis, S. cristatus, S. salivarius, S. mutans, S. sanguinis, S. gordonii, and S. sanguinosus [21]. Streptococci may produce different types of antimicrobial substances including hydrogen peroxide, organic acids, and bacteriocins [22,23]. The obtained results would suggest an ecological role of Streptococcus in the control of microbial populations in breast milk. Staphylococcus was also detected in all breast milk samples, with relative abundances reaching up to 14% in one sample. It has been suggested that commensal coagulase-negative staphylococci and viridans streptococci from breast milk could reduce the acquisition of undesired pathogens by infants, especially when exposed to hospital environments [5,24].
Contrary to other studies, Lactobacillus had low relative abundance in the studied milk samples, and other related genera (Enterococcus, Weissella, Leuconostoc) represented less than 2%. This could be related to the late lactation period. Carnobacterium was represented in two samples (2.1–3.8%). Carnobacterium has seldom been reported in human milk, although this bacterium could be a new, largely unexplored candidate for novel probiotic bacteria from human milk. One study reported that the presence of Carnobacterium in milk was associated with cesarean delivery [12], which is in agreement with the delivery procedure in the present study. It is believed that cesarean delivery influences the milk microbiota because of the differential exposure of the newborn to skin and environmental bacteria instead of vaginal microbiota and because the skin and oral cavity of newborns act as sources of colonization of the bacteria in breast milk [12].
Aerobic endosporeformers of the genera Bacillus and Paenibacillus also had high relative abundances in several milk samples and contributed (together with Streptococcus) to the increase of Firmicutes during late lactation. Remarkably, genus Bacillus reached ca. 25% relative abundance in two samples (weeks 16 and 27). The presence of Bacillus in breast milk samples has also been reported in several previous studies [21,25,26,27,28]. Patel et al. [28] reported both the presence of Bacillus and Paenibacillus in human milk, but Bacillus was associated with subacute or acute mastitis. However, during the sampling period of the present study, the mother did not report any signs of mastitis. Members of genus Bacillus are ubiquitous, sporulating, saprophytic microorganisms that can readily contaminate human milk during its collection or storage [29]. Among them, B. cereus is a matter of concern in stored breast milk (specially in breast milk banks) since it can produce food poisoning toxins and may also cause severe illness in neonates [29,30,31]. On the other hand, selected strains of Bacillus species are commercialized as human probiotics [32] and strains of Bacillus coaulans and Bacillus clausii are being investigated for pediatric and infant formula applications [33,34,35].
Results from the present study indicate large variations in the microbiota of the breast milk from a single woman during the sampling period. Tentatively, some of the changes could be explained by environmental contamination of the milk. The baby was already in the weaning period and crawling. Therefore, transfer of microbiota from the baby to the mother’s breast could account for some of the contaminants observed, including Enterobacteria. Previous work has suggested that the environment, utensils, and water could contribute considerably to the microbiota detected in breast milk [6]. A second way of contamination could be during milk expression by the breast pump. It has been reported that breast pumps may be difficult to decontaminate and increase the bacterial contamination of the milk [36]. And, finally, contamination could also occur during sample processing and analysis operations. Additionally, previous studies reported that contaminant DNA (sample collection and preparation, laboratory environment and reagents, personnel…) and cross contamination (e.g., during sequencing runs) can influence the results of next-generation sequencing approaches [37,38]. However, none of the variations reported in the present study were observed in other studies carried out in the same lab and using the same reagents and therefore it seems unlikely that they may be due to lab contamination. In addition, the negative controls included in the Illumina amplification and sequencing protocol clearly ruled out the possibility of contamination at this step.
5. Conclusions
In conclusion, results from the present study reveal major changes in the microbiota of breast milk from a single mother during the late breastfeeding period. The main changes include a decrease in the relative abundance of Staphylococcus and an increase of other microbial groups belonging to phylum Proteobacteria. Factors such as environmental contamination and changes in the baby habits (weaning and crawling) could account for the observed differences during the sampling period.
Acknowledgments
Wendy Marin acknowledges the financial support of ISA University and MESCYT for pursuing her Ph.D.
Author Contributions
W.M.-G. Conceptualization, investigation, writing-review & editing. M.J.G. Methodology, supervision, writing-review & editing. R.P.-P. Investigation. R.L. Conceptualization, supervision, writing-review & editing. A.G. Conceptualization, funding acquisition, formal analysis, writing—original draft, review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The University of Jaén (grant Estructura AGR-230) provided financial support for this research. Action co-financed by the European Union through the Operational Program of European Regional Development Fund (ERDF) of Valencia Region (Spain) 2014–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Heikkila M.P., Saris P.E. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003;95:471–478. doi: 10.1046/j.1365-2672.2003.02002.x. [DOI] [PubMed] [Google Scholar]
- 2.Asan-Ozusaglam M., Gunyakti A. Lactobacillus fermentum strains from human breast milk with probiotic properties and cholesterol-lowering effects. Food Sci. Biotechnol. 2018;28:501–509. doi: 10.1007/s10068-018-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Rubio R., Mira-Pascual L., Mira A., Collado M.C. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health. 2016;7:54–60. doi: 10.1017/S2040174415001397. [DOI] [PubMed] [Google Scholar]
- 4.Jost T., Lacroix C., Braegger C., Chassard C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015;73:426–437. doi: 10.1093/nutrit/nuu016. [DOI] [PubMed] [Google Scholar]
- 5.Fernández L., Langa S., Martín V., Maldonado A., Jiménez E., Martín R., Rodríguez J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Fernández L., Rodríguez J.M. Human milk microbiota: Origin and potential uses. Nestle Nutr. Inst. Workshop Ser. 2020;94:75–85. doi: 10.1159/000505031. [DOI] [PubMed] [Google Scholar]
- 7.Pannaraj P.S., Li F., Cerini C., Bender J.M., Yang S., Rollie A., Adisetiyo H., Zabih S., Lincez P.J., Bittinger K., et al. Association between breast milk bacterial communities and establishment and development of the infant gut. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäckhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H., et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Li N., Pang B., Liu G., Zhao X., Xu X., Jiang C., Yang B., Liu Y., Shi J. Lactobacillus rhamnosus from human breast milk shows therapeutic function against foodborne infection by multi-drug resistant Escherichia coli in mice. Food Funct. 2020;11:435–447. doi: 10.1039/C9FO01698H. [DOI] [PubMed] [Google Scholar]
- 10.Fitzstevens J.L., Smith K.C., Hagadorn J.I., Caimano M.J., Matson A.P., Brownell E.A. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 2017;32:354–364. doi: 10.1177/0884533616670150. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann P., Curtis N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020;81:17–47. doi: 10.1016/j.jinf.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera-Rubio R., Collado M.C., Laitinen K., Salminen S., Isolauri E., Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012;96:544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 13.Khodayar-Pardo P., Mira-Pascual L., Collado M.C., Martínez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014;34:599–605. doi: 10.1038/jp.2014.47. [DOI] [PubMed] [Google Scholar]
- 14.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., Fierer N. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashima I., Theodorea C.F., Thaweboon B., Thaweboon S., Scannapieco F.A., Nakazawa F. Exploring the salivary microbiome of children stratified by the oral hygiene index. PLoS ONE. 2017;12:e0185274. doi: 10.1371/journal.pone.0185274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino H., Kushiro A., Ishikawa E., Kubota H., Gawad A., Sakai T., Oishi K., Martin R., Ben-Amor K., Knol J., et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE. 2013;8:e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014;5:779–784. doi: 10.3945/an.114.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boix-Amoros A., Collado M.C., Mira A. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front. Microbiol. 2016;7:492. doi: 10.3389/fmicb.2016.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wescombe P.A., Heng N.C., Burton J.P., Chilcott C.N., Tagg J.R. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 2009;4:819–835. doi: 10.2217/fmb.09.61. [DOI] [PubMed] [Google Scholar]
- 23.Herrero E.R., Slomka V., Bernaerts K., Boon N., Hernandez-Sanabria E., Passonial B.B., Quirynen M., Teughels W. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 2016;47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Park B., Iwase T., Liu G.Y. Intranasal application of S. epidermidis prevents colonization by methicillin-resistant Staphylococcus aureus in mice. PLoS ONE. 2011;6:e25880. doi: 10.1371/journal.pone.0025880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding M., Qi C., Yang Z., Jiang S., Bi Y., Lai J., Sun J. Geographical location specific composition of cultured microbiota and Lactobacillus occurrence in human breast milk in China. Food Funct. 2019;10:554–564. doi: 10.1039/C8FO02182A. [DOI] [PubMed] [Google Scholar]
- 26.Dahaban N.M., Romli M.F., Roslan N.R., Kong S.S., Cheah F.C. Bacteria in expressed breastmilk from mothers of premature infants and maternal hygienic status. Breastfeed. Med. 2013;8:422–423. doi: 10.1089/bfm.2012.0109. [DOI] [PubMed] [Google Scholar]
- 27.Cacho N.T., Harrison N.A., Parker L.A., Padgett K.A., Lemas D.J., Marcial G.E., Li N., Carr L.E., Neu J., Lorca G.L. Personalization of the microbiota of donor human milk with mother’s own milk. Front. Microbiol. 2017;8:1470. doi: 10.3389/fmicb.2017.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S.H., Vaidya Y.H., Patel R.J., Pandit R.J., Joshi C.G., Kunjadiya A.P. Culture independent assessment of human milk microbial community in lactational mastitis. Sci. Rep. 2017;7:7804. doi: 10.1038/s41598-017-08451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigourd V., Barnier J.P., Ferroni A., Nicloux M., Hachem T., Magny J.F., Lapillonne A., Frange P., Nassif X., Bille E. Recent actuality about Bacillus cereus and human milk bank: A new sensitive method for microbiological analysis of pasteurized milk. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1297–1303. doi: 10.1007/s10096-018-3249-z. [DOI] [PubMed] [Google Scholar]
- 30.Moro G.E., Billeaud C., Rachel B., Calvo J., Cavallarin L., Christen L., Escuder-Vieco D., Gaya A., Lembo D., Wesolowska A., et al. Processing of donor human milk: Update and recommendations from the European Milk Bank Association (EMBA) Front. Pediatr. 2019;7:49. doi: 10.3389/fped.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewin A., Delage G., Bernier F., Germain M. Banked human milk and quantitative risk assessment of Bacillus cereus infection in premature infants: A simulation study. Can. J. Infect. Dis. Med. Microbiol. 2019:6348281. doi: 10.1155/2019/6348281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee N.K., Kim W.S., Paik H.D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019;28:1297–1305. doi: 10.1007/s10068-019-00691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food Drug Administration GRAS Notice (GRN) No. 660. Notice to US Food and Drug Administration that Bacillus coagulans GBI-30, 6086 is Generally Recognized as Safe for Use in Non-Exempt Term Infant Formula. [(accessed on 16 July 2020)];2016 Available online: https://www.fda.gov/media/100025/download.
- 34.Guo Q., Goldenberg J.Z., Humphrey C., El Dib R., Johnston B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019:CD004827. doi: 10.1002/14651858.CD004827.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Akker C.H.P., van Goudoever J.B., Szajewska H., Embleton N.D., Hojsak I., Reid D., Shamir R. ESPGHAN Working Group for Probiotics, Prebiotics & Committee on Nutrition. Probiotics for preterm infants: A strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018;67:103–122. doi: 10.1097/MPG.0000000000001897. [DOI] [PubMed] [Google Scholar]
- 36.Boo N.Y., Nordiah A.J., Alfizah H., Nor-Rohaini A.H., Lim V.K. Contamination of breast milk obtained by manual expression and breast pumps in mothers of very low birthweight infants. J. Hosp. Infect. 2001;49:274–281. doi: 10.1053/jhin.2001.1117. [DOI] [PubMed] [Google Scholar]
- 37.Eisenhofer R., Minich J.J., Marotz C., Cooper A., Knight R., Weyrich L.A. Contamination in low microbial biomass microbiome studies: Issues and recommendations. Trends Microbiol. 2019;27:105–117. doi: 10.1016/j.tim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Salter S.J., Cox M.J., Turek E.M., Calus S.T., Cookson W.O., Moffatt M.F., Turner P., Parkhill J., Loman N.J., Walker A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]