Abstract
(E)-β-caryophyllene (BCP) is a natural sesquiterpene hydrocarbon present in hundreds of plant species. BCP possesses several important pharmacological activities, ranging from pain treatment to neurological and metabolic disorders. These are mainly due to its ability to interact with the cannabinoid receptor 2 (CB2) and the complete lack of interaction with the brain CB1. A systematic analysis of plant species with essential oils containing a BCP percentage > 10% provided almost 300 entries with species belonging to 51 families. The essential oils were found to be extracted from 13 plant parts and samples originated from 56 countries worldwide. Statistical analyses included the evaluation of variability in BCP% and yield% as well as the statistical linkage between families, plant parts and countries of origin by cluster analysis. Identified species were also grouped according to their presence in the Belfrit list. The survey evidences the importance of essential oil yield evaluation in support of the chemical analysis. The results provide a comprehensive picture of the species with the highest BCP and yield percentages.
Keywords: plant species, essential oil, yield, percentages of (E)-β-caryophyllene, Belfrit list, plant part, geographical origin
1. Introduction
The endogenous cannabinoid system (ECS) plays an important role in the immune response to an infection. At present, two cannabinoid (CB) receptors are described: cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2), both G-protein coupled receptors [1]. The CB2 receptor represents the peripheral CB, due to its expression on circulating immune cells. However, studies have also found CB2 expression in the brain, such as cerebellum and microglial cells [2]. The CB2 receptor is involved in the attenuation of inflammatory immune responses. CB2 receptor pathway activation entails the suppression of cytokine release from immune cells and thereby dampening of the inflammatory response (immunosuppression) [3].
(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene hydrocarbon which is present in the essential oil of several plant species [4]. The Research Institute for Fragrance Materials (RIFM) evaluated BCP safety and the molecule has been approved by the Food and Drug Administration and by the European Food Safety Authority as a flavoring agent, which can be used in cosmetic and food additives [5]. Reports on oral sub-chronic toxicity support the safety of BCP for its proposed use also in medical food products [5]. BCP has been reported to be active against several disorders, with particular reference to cancer, chronic pain and inflammation [2]. Non-clinical BCP toxicity and an absence of adverse effects have been described [6]. Moreover, BCP can act as a selective agonist of CB2 [1], it activates peroxisome proliferator-activated receptor-α (PPAR α) [7] and has been recently involved in the prevention of lipid accumulation and in the improvement of glucose uptake [8]. Therefore, BCP is a plant-derived bioactive molecule able to improve health and prevent lifestyle diseases. Moreover, the specificity of BCP for the CB2 receptor, mainly expressed in peripheral tissues, and its inability to bind CB1, which is predominantly expressed at the level of the central nervous system, implies that its action is devoid of the known psychoactive effects associated with the activation of CB1 [1,2,9,10]. In this context, BCP is an interesting alternative to the use of Cannabis.
Owing to the growing importance of BCP, it was interesting to evaluate the occurrence of this important endocannabinoid in plant species used for the extraction of essential oils. Therefore, the aim of this work was to look for plant natural sources of BCP in order to provide the pharmaceutical, nutraceutical and aroma industries a summary of plant species, parts used for extraction and geographical origin of plants producing BCP. Moreover, additional information was provided with regards to the content and yield of BCP as well as the occurrence of selected species in the Belfrit list [11], which includes botanicals allowed in food supplements and ensures compliance of botanicals in terms of quality and safety.
2. Results and Discussion
The database search (performed in July 2020) for the term caryophyllene provided 5867 entries. The search was then refined by selecting all papers with a chemical composition description. This selection provided 2604 entries, which were individually analyzed in order to select papers providing information on BCP percentage > 10%. Papers were then analyzed and the species binomial name, the plant family, the country of origin of samples and the plant part extracted were reported along with the BCP percentage and yield percentage. The total number of selected species was 295 (Table 1). Table 1 also lists the presence of the species in the Belfrit list [11].
Table 1.
Occurrence of (E)-β-caryophyllene (BCP) in different plant species. n.a., data not available, the essential oil (E.O.) yield is expressed as volume/weight percentage.
| Family | Genus | Species and Auth | Geogr. Origin of Sample | Belfrit List | Part Used | E.O. Yield% | BCP% | Code | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Anacardiaceae | Rhus | coriaria L. | Iran | YES | fruits | 0.55 | 34.3 | 249 | [12] |
| Anacardiaceae | Spondias | pinnata (Linn. F.) Kurz | Egypt | NO | leaves | 2.00 | 49.9 | 268 | [13] |
| Annonaceae | Annona | muricata L. | Bénin | YES | leaves | 0.10 | 13.6 | 30 | [14] |
| Annonaceae | Annona | densicoma Mart. | Brazil | NO | leaves | 0.10 | 14.4 | 31 | [15] |
| Annonaceae | Annona | senegalensis Pers. | Burkina Faso | NO | leaves | 0.73 | 19.1 | 32 | [16] |
| Annonaceae | Annona | squamosa L. | India | YES | leaves | 0.12 | 22.9 | 33 | [17] |
| Annonaceae | Artabotrys | hexapetalus (L. f.) Bhandare | Vietnam | NO | flowers | 0.94 | 11.4 | 38 | [18] |
| Annonaceae | Cananga | odorata (Lam.) Hook.f. and Thomson | Australia | YES | leaves | 0.30 | 52.0 | 62 | [19] |
| Annonaceae | Cleistopholis | glauca Pierre ex Engler and Diels | Ivory Coast | NO | leaves | 0.19 | 26.2 | 81 | [20] |
| Annonaceae | Fissistigma | rubiginosum Merr. | Vietnam | NO | leaves | 0.30 | 28.1 | 125 | [21] |
| Annonaceae | Goniothalamus | multiovulatus Ast | Vietnam | NO | stems | 0.21 | 35.7 | 135 | [22] |
| Annonaceae | Melodorum | sp. (Dunal) Hook.f. and Thomson | Australia | NO | leaf | 0.15 | 26.7 | 182 | [23] |
| Annonaceae | Miliusa | horsfieldii (Bennett) Baillon ex Pierre | Australia | NO | leaves | 0.1 | 20.2 | 188 | [24] |
| Annonaceae | Mitrephora | zippeliana Miq. | Australia | NO | leaves | 0.30 | 18.1 | 189 | [19] |
| Annonaceae | Polyalthia | oliveri Engl. | Ivory Coast | NO | leaves | 0.13 | 31.4 | 237 | [25] |
| Annonaceae | Pseuduvaria | hylandii Jessup | Australia | NO | leaves | 0.50 | 24.1 | 242 | [26] |
| Annonaceae | Uvariodendron | calophyllum R. E. Fries | Cameroon | NO | stem barks | 0.52 | 32.5 | 284 | [27] |
| Apiaceae | Berula | erecta (Hudson) Coville subsp. erecta | Serbia | NO | aerial parts | 0.01 | 14.9 | 52 | [28] |
| Apiaceae | Bilacunaria | anatolica A. Duran | Turkey | NO | aerial parts | 0.14 | 10.3 | 54 | [29] |
| Apiaceae | Centella | asiatica L. | South Africa | YES | aerial parts | 0.06 | 19.1 | 75 | [30] |
| Apiaceae | Conium | maculatum L. | Iran | NO | aerial parts | 0.20 | 15.3 | 85 | [31] |
| Apiaceae | Dorema | aucheri Boiss. | Iran | NO | leaves | 0.40 | 35.7 | 108 | [32] |
| Apiaceae | Eryngium | vesiculosum Labill. | Australia | NO | aerial parts | n.a. | 20.3 | 116 | [33] |
| Apiaceae | Ferula | glauca L. | Iran | NO | leaves | 0.07 | 24.9 | 123 | [34] |
| Apiaceae | Grammosciadium | pterocarpum Boiss. | Turkey | NO | aerial parts | n.a. | 15.3 | 136 | [35] |
| Apiaceae | Hippomarathrum | microcarpum (M. Bieb.) B. Fedtsch | Iran | NO | aerial parts | 0.85 | 15.75 | 145 | [36] |
| Apiaceae | Hippomarathrum | boissieri Reuter et Hausskn | Turkey | NO | aerial parts | 0.40 | 25.6 | 146 | [37] |
| Apiaceae | Laser | trilobum (L.) Borkh. | Iran | NO | aerial parts | 1.80 | 22.4 | 165 | [38] |
| Apiaceae | Oenanthe | divaricata (R. Br.) Mabb. | Spain | NO | aerial parts | 0.20 | 15.3 | 206 | [39] |
| Apiaceae | Ostericum | viridiflorum (Turcz.) Kitagawa | China | NO | aerial parts | 0.03 | 24.3 | 210 | [40] |
| Apiaceae | Pimpinella | kotschyana Boiss. | Iran | NO | seeds | 5.16 | 49.9 | 224 | [41] |
| Apiaceae | Prangos | uloptera DC. | Iran | NO | aerial parts | 0.70 | 18.2 | 240 | [42] |
| Apiaceae | Zosima | absinthifolia Link | Iran | NO | aerial parts | 0.20 | 22.2 | 295 | [43] |
| Apocynaceae | Allamanda | cathartica L. | Brazil | NO | flowers | n.a. | 15.7 | 21 | [44] |
| Apocynaceae | Aspidosperma | cylindrocarpon Muell. Arg. | Brazil | NO | leaves | 0.03 | 14.3 | 45 | [45] |
| Apocynaceae | Tabernaemontana | catharinensis A. DC. | Brazil | NO | leaves | 0.30 | 56.9 | 272 | [46] |
| Araliaceae | Schefflera | stellata (Gaertn.) Harms | India | NO | leaves | 0.10 | 19.2 | 260 | [47] |
| Aristolochiaceae | Aristolochia | elegans Mast. | Argentina | NO | leaves | n.a. | 27.8 | 36 | [48] |
| Aristolochiaceae | Aristolochia | fordiana Hemsl | China | NO | aerial parts | 0.19 | 11.1 | 37 | [49] |
| Asteraceae | Achillea | asplenifolia Vent. | Serbia | NO | aerial parts | 0.10 | 17.6 | 4 | [50] |
| Asteraceae | Achyrocline | alata (D.C.) | Brazil | NO | leaf and flowers | 4.00 | 16.0 | 5 | [51] |
| Asteraceae | Acroptilon | repens (L.) | Iran | NO | aerial parts | 0.11 | 10.0 | 6 | [52] |
| Asteraceae | Ageratum | fastigiatum (Gardn.) R. M. King et H. Rob | Brazil | NO | branches | 0.20 | 34.9 | 13 | [53] |
| Asteraceae | Ageratum | conyzoides L. | Portugal | NO | flowers | 0.17 | 24.6 | 14 | [54] |
| Asteraceae | Anthemis | altissima L. | Iran | NO | flowers | 0.03 | 25.3 | 34 | [55] |
| Asteraceae | Artemisia | verlotiorum Lamotte | France | YES | aerial parts | 0.20 | 12.7 | 39 | [56] |
| Asteraceae | Artemisia | parviflora Roxb | India | NO | aerial parts | 0.20 | 15.3 | 40 | [57] |
| Asteraceae | Artemisia | roxburghiana Besser var. purpurascens (Jacq.) Hook | India | NO | aerial parts | 0.85 | 18.4 | 41 | [58] |
| Asteraceae | Artemisia | capillaris Thunb | South Korea | YES | aerial parts | n.a. | 11.1 | 42 | [59] |
| Asteraceae | Artemisia. | stricta Edgew. f. stricta Pamp | India | NO | aerial parts | 0.46 | 13.4 | 43 | [60] |
| Asteraceae | Artemisia. | lavandulaefolia DC | South Korea | NO | aerial parts | n.a. | 16.1 | 44 | [61] |
| Asteraceae | Aspilia | africana (Pers.) C. D. Adams | Nigeria | NO | leaves | 0.02 | 10.8 | 46 | [62] |
| Asteraceae | Baccharis. | articulata (Lam.) Pers | Argentina | NO | aerial parts | n.a. | 16.8 | 48 | [63] |
| Asteraceae | Bidens | pilosa L. | Cameroon | NO | leaves | n.a. | 27.1 | 53 | [64] |
| Asteraceae | Centaurea | zlatiborensis Zlatkovic, Novakovic and Janackovic | Serbia | NO | flowers | n.a. | 28.3 | 73 | [65] |
| Asteraceae | Centaurea | appendicigera C. Koch | Turkey | NO | aerial parts | 0.18 | 17.5 | 74 | [66] |
| Asteraceae | Centratherum | punctatum Cass | Nigeria | NO | leaves | n.a. | 16.6 | 76 | [67] |
| Asteraceae | Chromolaena | odorata L. | Togo | NO | aerial parts | 0.50 | 25.2 | 78 | [68] |
| Asteraceae | Conyza | bonariensis (L.) Cronquist | Brazil | NO | aerial parts | 0.20 | 14.4 | 87 | [69] |
| Asteraceae | Cyanthillium | cinereum (L.) H. Rob | Ivory Coast | NO | roots | n.a. | 17.0 | 100 | [70] |
| Asteraceae | Dendranthema | indicum (L.) Des Moul. | China | NO | aerial parts | 0.08 | 13.8 | 106 | [71] |
| Asteraceae | Emilia | sonchifolia (L.) DC. | India | NO | aerial parts | n.a. | 22.7 | 110 | [72] |
| Asteraceae | Epaltes | alata Steetz | Niger | NO | leaves | 0.30 | 24.0 | 111 | [73] |
| Asteraceae | Eremanthus | erythropappus (DC.) MacLeish | Brazil | NO | leaves | 0.12 | 29.3 | 113 | [74] |
| Asteraceae | Erigeron | ramosus (Walt.) B.S.P. | Korea | NO | flowers | 0.40 | 24.0 | 114 | [75] |
| Asteraceae | Eriocephalus | luederitzianus O.Hoffm. | South Africa | NO | aerial parts | 0.10 | 13.3 | 115 | [76] |
| Asteraceae | Eupatorium | triplinerve Vahl | India | NO | leaves | 0.40 | 14.7 | 120 | [77] |
| Asteraceae | Flourensia | campestris | Argentina | NO | aerial parts | 0.02 | 15.3 | 127 | [78] |
| Asteraceae | Helichrysum | indutum Humbert | Madagascar | NO | aerial parts | 0.19 | 33.1 | 141 | [79] |
| Asteraceae | Helichrysum | kraussii Sch. Bip. | South Africa | NO | aerial parts | n.a. | 30.7 | 142 | [80] |
| Asteraceae | Helichrysum | melaleucum Rchb. ex Holl. | Spain | NO | aerial parts | 0.10 | 35.4 | 143 | [39] |
| Asteraceae | Koanophyllon | villosum (Sw.) King et Robins | Cuba | NO | aerial parts | 0.45 | 17.0 | 160 | [81] |
| Asteraceae | Laggera | oloptera (DC.) C. D. Adams | Cameroon | NO | leaves | 0.05 | 20.4 | 161 | [82] |
| Asteraceae | Microglossa | pyrrhapappa var. pyrrhopappa (A. Rich) Agnew | Kenya | NO | leaves | 0.40 | 20.3 | 185 | [83] |
| Asteraceae | Mikania | cordata (Burm.f.) B.L. Robinson var. cordata | Ivory Coast | NO | leaves | 0.63 | 11.8 | 187 | [84] |
| Asteraceae | Oyedaea | verbesinoides DC. | Venezuela | NO | leaves | 0.05 | 27.1 | 211 | [85] |
| Asteraceae | Perymenium | grande Hemsl. var. nelsonii (Robins. and Greenm.) Fay | Costa Rica | NO | leaves | 0.30 | 30.5 | 217 | [86] |
| Asteraceae | Petasites | japonicus (Siebold and Zucc.) Maxim. | Japan | NO | leaves | 0.02 | 21.9 | 218 | [87] |
| Asteraceae | Pluchea | carolinensis (Jacq.) Sweet | Martinique | NO | leaves | 0.11 | 21.1 | 236 | [88] |
| Asteraceae | Porophyllum | obscurum (Spreng.) D.C. | Argentina | NO | leaves | 0.30 | 14.1 | 238 | [89] |
| Asteraceae | Solidago | decurrens Lour | China | NO | leaves | 0.37 | 15.4 | 266 | [90] |
| Asteraceae | Tagetes | patula L. | Austria | NO | flowers | 0.15 | 53.5 | 273 | [91] |
| Asteraceae | Tagetes | erecta L. | Iran | YES | flowers | 0.35 | 35.2 | 274 | [92] |
| Asteraceae | Tanacetum | punctatum (Desr.) Grierson | Iran | NO | aerial parts | 0.1 | 21.1 | 275 | [93] |
| Asteraceae | Tarchonanthus | trilobus var. galpinii (Hutch. and E.Phillips) Paiva | South Africa | NO | leaves | 0.14 | 30.4 | 276 | [94] |
| Asteraceae | Vernonia | chalybaea Mart. | Brazil | NO | aerial parts | 0.10 | 39.1 | 287 | [95] |
| Asteraceae | Vernonia | scorpioides (Lam.) Pers. | Brazil | NO | aerial parts | 0.10 | 30.6 | 288 | [96] |
| Asteraceae | Xanthium | strumarium L. | Pakistan | NO | leaves | n.a. | 17.5 | 291 | [97] |
| Asteraceae, | Leptocarpha | rivularis DC. | Chile | NO | aerial parts | 0.15 | 21.1 | 168 | [98] |
| Atherospermataceae | Daphnandra | repandula (F.Muell.) F.Muell. | Australia | NO | aerial parts | 0.20 | 12.2 | 105 | [99] |
| Boraginaceae | Cordia | leucocephala Moric | Brazil | NO | leaves | 0.04 | 39.0 | 91 | [100] |
| Boraginaceae | Cordia | multispicata Cham. | Brazil | NO | leaves | 0.25 | 56.6 | 92 | [101] |
| Burseraceae | Bursera | aromatica (Proctor) | Jamaica | NO | leaves | 0.03 | 21.7 | 59 | [102] |
| Burseraceae | Bursera | microphylla A. Gray | USA | NO | oleo-gum-resin | 2.10 | 72.9 | 60 | [103] |
| Burseraceae | Canarium | parvum Leen. | Vietnam | NO | leaves | 0.20 | 18.7 | 63 | [104] |
| Burseraceae | Dacryodes | edulis (G. Don) H. J. Lam | Nigeria | NO | leaves | 0.08 | 26.0 | 103 | [105] |
| Burseraceae | Protium | heptaphyllum (Aubl.) March. | Brazil | YES | leaves | 0.30 | 18.6 | 241 | [106] |
| Cannabaceae | Cannabis | sativa L. ssp. spontanea | Austria | YES | aerial parts | n.a. | 16.2 | 64 | [107] |
| Cannabaceae | Cannabis | sativa L. | Italy | YES | flowers | 0.10 | 23.8 | 65 | [108] |
| Cannabaceae | Humulus | lupulus L. | USA | YES | aerial parts | n.a. | 22.0 | 148 | [109] |
| Caryophyllaceae | Dianthus | caryophyllus L. | Iran | YES | aerial parts | n.a. | 34.8 | 107 | [110] |
| Cephalotaxaceae | Cephalotaxus | harringtonia K.Koch subsp. harringtonia | India | NO | twigs | 0.01 | 21.1 | 77 | [111] |
| Clusiaceae | Clusia | nemorosa G. Mey | Brazil | NO | fruits | 0.30 | 48.6 | 83 | [112] |
| Clusiaceae | Garcinia | atroviridis Griff. ex T. Anders. | Malaysia | NO | fruits | n.a. | 23.8 | 128 | [113] |
| Clusiaceae | Kielmeyera | rugosa Choisy | Brazil | NO | fruits | n.a. | 16.4 | 158 | [114] |
| Clusiaceae | Pentadesma | butyracea Sabine | Benin | NO | barks | 0.08 | 74.0 | 214 | [115] |
| Clusiaceae | Psorospermum | corymbiferum Hochr | Nigeria | NO | leaves | 0.02 | 46.8 | 245 | [116] |
| Convolvulaceae | Convolvulus | persicus L. | Iran | NO | aerial parts | 0.04 | 47.0 | 86 | [117] |
| Cupressaceae | Cedrus | atlantica G. Manetti | Algeria | NO | twigs | 0.02 | 11.4 | 72 | [118] |
| Cupressaceae | Juniperus | macrocarpa Sibth. and Sm. (Jom) | Turkey | NO | fruits | n.a. | 29.6 | 156 | [119] |
| Cupressaceae | Thuja | orientalis L. | Egypt | NO | aerial parts | 2.60 | 24.0 | 281 | [120] |
| Cyperaceae | Cyperus | glomeratus L. | Serbia | NO | rhizomes and roots | 0.06 | 12.6 | 102 | [121] |
| Ehretiaceae | Varronia | curassavica Jacq. | Brazil | NO | leaves | 0.6 | 41.2 | 285 | [122] |
| Ehretiaceae | Varronia | schomburgkii (DC.) Borhidi | French Guiana | NO | aerial parts | 0.06 | 47.0 | 286 | [123] |
| Euphorbiaceae | Acalypha | fruticosa Forssk | India | NO | leaves | 1.40 | 42.0 | 2 | [124] |
| Euphorbiaceae | Alchornea | tiliifolia (Benth.) Muell. | Vietnam | NO | aerial parts | n.a. | 10.7 | 20 | [125] |
| Euphorbiaceae | Croton | rhamnifolioides Pax and Hoffm | Brazil | NO | leaf | 0.21 | 33.3 | 94 | [126] |
| Euphorbiaceae | Croton | glandulosus L. | Brazil | NO | aerial parts | 0.12 | 53.2 | 95 | [127] |
| Euphorbiaceae | Croton | pulegiodorus Baill. | Brazil | NO | aerial parts | 5.00 | 20.9 | 96 | [128] |
| Euphorbiaceae | Phyllanthus | muellerianus (O. Kuntze) Exell | Nigeria | NO | leaves | 0.12 | 41.9 | 223 | [129] |
| Fabaceae | Bauhinia | rufa Steud. | Brazil | NO | leaves | 0.01 | 15.8 | 50 | [130] |
| Fabaceae | Bowdichia | virgilioides Kunt | Brazil | YES | seeds | 2.20 | 44.1 | 57 | [131] |
| Fabaceae | Caesalpinia | decapetala (Roth) Alston | Japan | NO | aerial parts | 0.07 | 17.2 | 61 | [132] |
| Fabaceae | Copaifera | langsdorffii Desf. | Brazil | YES | oleoresins | 28.00 | 72.0 | 88 | [133] |
| Fabaceae | Copaifera | multijuga Hayne | Brazil | NO | oleoresins | n.a. | 57.5 | 89 | [134] |
| Fabaceae | Copaifera | reticulata Ducke | Brazil | NO | oleoresins | n.a. | 68.0 | 90 | [135] |
| Fabaceae | Dalea | carthagenensis L. | Colombia | NO | leaves | 0.15 | 20.7 | 104 | [136] |
| Fabaceae | Eperua | duckeana Cowan | Brazil | NO | leaves | n.a. | 31.8 | 112 | [137] |
| Fabaceae | Glycyrrhiza | triphylla Fisch. and C.A.Mey | Iran | NO | aerial parts | 0.50 | 25.4 | 134 | [138] |
| Fabaceae | Psoralea | bituminosa L | Italy | NO | leaves | 0.10 | 23.2 | 244 | [139] |
| Fabaceae | Rynchosia | minima DC. | Kenya | NO | aerial parts | 0.10 | 30.4 | 252 | [140] |
| Flacourtiaceae | Casearia | decandra Jacq. | Brazil | NO | leaves | 0.20 | 13.0 | 67 | [141] |
| Flacourtiaceae | Casearia | sylvestris Swart. | Brazil | NO | leaves | 0.60 | 27.5 | 68 | [142] |
| Geraniaceae | Geranium | wallichianum D. Don ex Sweet | India | NO | aerial parts | n.a. | 15.9 | 130 | [143] |
| Gramineae | Elyonurns | muticus (Sprengel) O.Kuntze | Brazil | NO | leaves | 0.45 | 17.9 | 109 | [144] |
| Gramineae | Melinis | minutiflora P. Beauv | Kenya | NO | aerial parts | 0.01 | 24.2 | 180 | [145] |
| Hernandiaceae | Hernandia | nymphaeifolia (C.Presl) Kubitzki | Australia | NO | leaves | 0.01 | 43.8 | 144 | [146] |
| Hypericaceae | Hypericum | brasiliense Choisy | Brazil | NO | aerial parts | 0.10 | 29.5 | 150 | [147] |
| Hypericaceae | Hypericum | perforatum L. | Iran | YES | aerial parts | n.a. | 25.05 | 151 | [148] |
| Hypericaceae | Vismia | baccifera subsp. dealbata (Kunth) Ewan | Venezuela | NO | leaves | 0.07 | 45.7 | 289 | [149] |
| Juglandaceae | Juglans | regia L. | India | YES | leaves | 0.02 | 15.5 | 155 | [150] |
| Lamiaceae | Aegiphila | lhotzkiana Cham. | Brazil | NO | leaves | 0.02 | 27.5 | 9 | [151] |
| Lamiaceae | Ajuga | parviflora Benth. | India | NO | aerial parts | n.a. | 22.4 | 18 | [152] |
| Lamiaceae | Ajuga | comata Stapf. | Iran | NO | aerial parts | n.a. | 30.9 | 19 | [153] |
| Lamiaceae | Ballota | nigra L. | Algeria | YES | aerial parts | n.a. | 24.6 | 49 | [154] |
| Lamiaceae | Clerodendrum | polycephalum Baker | Nigeria | NO | leaves | 0.16 | 28.9 | 82 | [155] |
| Lamiaceae | Colquhounia | coccinea Wall. | India | NO | flower | 0.20 | 53.2 | 84 | [156] |
| Lamiaceae | Cunila | incana Benth. | Brazil | NO | aerial parts | 0.72 | 11.3 | 98 | [157] |
| Lamiaceae | Cyclotrichium. | strussii Bornm | Iran | NO | aerial parts | 0.37 | 16.9 | 101 | [158] |
| Lamiaceae | Glechoma | hederacea L. | Lithuania | NO | aerial parts | 0.05 | 14.2 | 131 | [159] |
| Lamiaceae | Glechon | marifolia Benth. | Brazil | NO | leaves | 1.40 | 32.2 | 132 | [160] |
| Lamiaceae | Hoslundia | opposita Vahl. | Ivory Coast | NO | leaves | 0.04 | 24.8 | 147 | [161] |
| Lamiaceae | Hymenocrater | calycinus (Boiss.) Benth. | Iran | NO | aerial parts | 0.20 | 32.8 | 149 | [162] |
| Lamiaceae | Hyptidendron | canum (Pohl ex Benth.) Harley | Brazil | NO | leaves | 0.82 | 41.6 | 152 | [163] |
| Lamiaceae | Hyptis | mutabilis (Rich.) Briq. | Argentina | NO | aerial parts | n.a. | 59.4 | 153 | [164] |
| Lamiaceae | Hyptis | suaveolens (L.) Poit. | Bénin | YES | fruits | 0.10 | 43.7 | 154 | [165] |
| Lamiaceae | Lallenmantia | iberica (M. Bieb.) Fisch and CA Mey | Turkey | NO | aerial parts | n.a. | 18.3 | 162 | [166] |
| Lamiaceae | Leonotis | ocymifolia (Burm.f.) M.Iwarsson | South Africa | NO | leaves | 0.06 | 30.8 | 166 | [167] |
| Lamiaceae | Leonurus | sibiricus L. | Argentina | NO | aerial parts | n.a. | 35.2 | 167 | [164] |
| Lamiaceae | Leucas | aspera (Willd.) Link | India | NO | aerial parts | 0.30 | 34.2 | 169 | [168] |
| Lamiaceae | Leucas | indica (L.) R.Br | India | NO | aerial parts | n.a. | 51.1 | 170 | [169] |
| Lamiaceae | Marrubium | bourgaei subsp. caricum P.H.Davis | Tunisia | NO | aerial parts | 0.07 | 23.2 | 175 | [170] |
| Lamiaceae | Marsypianthes | chamnedrys (Vahl) Kuntze | Brazil | NO | aerial parts | n.a. | 15.1 | 176 | [171] |
| Lamiaceae | Melissa | romana Miller | Italy | NO | aerial parts | 0.30 | 15.8 | 181 | [172] |
| Lamiaceae | Mentha | longifolia (L.) Hudson | Iran | NO | aerial parts | 0.41 | 23.2 | 183 | [173] |
| Lamiaceae | Micromeria | myrtifolia Boiss. and Hohen. | Turkey | NO | aerial parts | 0.20 | 40.8 | 186 | [174] |
| Lamiaceae | Mosla | soochowensis Matsuda | China | NO | aerial parts | 0.05 | 12.8 | 191 | [175] |
| Lamiaceae | Nepeta | fissa C.A. Mey | Iran | NO | aerial parts | 0.25 | 33.1 | 200 | [176] |
| Lamiaceae | Nepeta | curviflora Boiss. | Lebanon | NO | aerial parts | 0.30 | 50.2 | 201 | [177] |
| Lamiaceae | Ocimum | tenuiflorum L. | India | YES | aerial parts | 0.33 | 30.0 | 203 | [178] |
| Lamiaceae | Origanum | majorana L. | Algeria | YES | aerial parts | 1.20 | 26.0 | 207 | [179] |
| Lamiaceae | Orthodon | dianfhera Maxim. | Vietnam | NO | aerial parts | 0.20 | 52.9 | 208 | [180] |
| Lamiaceae | Orthosiphon | pallidus Royle, ex Benth | India | NO | aerial parts | n.a. | 17.4 | 209 | [181] |
| Lamiaceae | Perilla | frutescens var. japonica (Hassk.) H.Hara | China | YES | leaves | 0.11 | 37.2 | 215 | [182] |
| Lamiaceae | Phlomis | crinita Cav. ssp. mauritanica Munby | Tunisia | NO | aerial parts | 0.10 | 40.8 | 220 | [183] |
| Lamiaceae | Phlomis | rigida Labill. | Turkey | NO | aerial parts | 0.05 | 38.7 | 221 | [184] |
| Lamiaceae | Platostoma | menthoides (L.) A. J. Paton | Sri Lanka | NO | aerial parts | 0.50 | 37.0 | 233 | [185] |
| Lamiaceae | Plectranthus | rugosus Wall. | India | NO | leaves | n.a. | 38.4 | 234 | [186] |
| Lamiaceae | Pycnostachys | eminii Gürke | Ethiopia | NO | leaves | 0.13 | 21.6 | 246 | [187] |
| Lamiaceae | Rosmarinus | officinalis L | Lebanon | YES | aerial parts | 0.09 | 12.9 | 251 | [188] |
| Lamiaceae | Salvia | palaefolia Kunth | Colombia | NO | aerial parts | 0.06 | 32.2 | 253 | [189] |
| Lamiaceae | Salvia | bracteata Banks and Soland | Iran | NO | aerial parts | 0.28 | 41.4 | 254 | [190] |
| Lamiaceae | Salvia | hydrangea DC. ex Benth. | Iran | NO | aerial parts | 0.20 | 33.4 | 255 | [191] |
| Lamiaceae | Salvia | nemorosa L. | Iran | NO | aerial parts | 0.12 | 41.6 | 256 | [192] |
| Lamiaceae | Salvia | virgata Jacq. | Iran | NO | aerial parts | 0.48 | 46.6 | 257 | [193] |
| Lamiaceae | Salvia | canariensis L. | Spain | NO | aerial parts | 4.00 | 30.2 | 258 | [194] |
| Lamiaceae | Salvia | montbretii Benth. | Turkey | NO | aerial parts | 0.10 | 32.8 | 259 | [195] |
| Lamiaceae | Scutellaria | havanensis Jacq. | Cuba | NO | leaves | 0.18 | 75.6 | 261 | [196] |
| Lamiaceae | Scutellaria | brevibracteata Stapf. subsp. pannosula | Turkey | NO | aerial parts | n.a. | 36.4 | 262 | [197] |
| Lamiaceae | Sideritis | clandestina subsp. peloponnesiaca (Boiss. and Heldr.) Baden | Greece | NO | aerial parts | 1.00 | 16.4 | 263 | [198] |
| Lamiaceae | Sideritis | phlomoides Boiss. and Bal. | Turkey | NO | aerial parts | 0.20 | 30.7 | 264 | [199] |
| Lamiaceae | Stachys | viticina Boiss. | Turkey | NO | aerial parts | 0.20 | 62.3 | 269 | [200] |
| Lamiaceae | Teucrium | arduini L. | Croatia | NO | aerial parts | 0.35 | 35.4 | 277 | [201] |
| Lamiaceae | Teucrium | flavum L. | Iran | NO | leaves | 0.20 | 30.7 | 278 | [202] |
| Lamiaceae | Teucrium | siculum (Raf.) Guss. | Italy | NO | aerial parts | 0.10 | 30.9 | 279 | [203] |
| Lamiaceae | Teucrium | turredanum Losa and Rivas-Goday | Spain | NO | aerial parts | 0.60 | 32.0 | 280 | [204] |
| Lamiaceae | Viticipremna | queenslandica Munir | Australia | NO | leaves | n.a. | 33.6 | 290 | [205] |
| Lamiaceae | Ziziphora | taurica M.Bieb. subsp. taurica | Turkey | NO | aerial parts | 0.80 | 24.8 | 294 | [206] |
| Lauraceae | Aiouea | costaricensis (Mez) Kosterm. | Costa Rica | NO | leaf | 0.10 | 12.0 | 17 | [207] |
| Lauraceae | Alseodaphne | peduncularis Meisn | Malaysia | NO | leaves | n.a. | 24.0 | 27 | [208] |
| Lauraceae | Aniba | riparia (Nees) Mez | Brazil | NO | leaves | 0.30 | 16.9 | 29 | [209] |
| Lauraceae | Beilschmiedia | penangiana Gamble | Malaysia | NO | aerial parts | 0.10 | 12.6 | 51 | [210] |
| Lauraceae | Cassytha | pubescens R.Br. | Australia | NO | aerial parts | 0.10 | 30.9 | 69 | [211] |
| Lauraceae | Cinnamomum | tamala (Ham) Nees and Eberm. | Pakistan | NO | leaves | 0.03 | 25.3 | 79 | [212] |
| Lauraceae | Litsea | helferi Hook.f. | Vietnam | NO | leaves | 0.30 | 14.2 | 172 | [213] |
| Lauraceae | Nectandra | lanceolata Ness | Brazil | NO | leaves | 0.20 | 32.5 | 198 | [214] |
| Lauraceae | Neolitsea | foliosa (Nees) Gamble var. caesia (Meisner) Gamble | India | NO | leaves | 0.10 | 35.3 | 199 | [215] |
| Lauraceae | Ocotea | duckei Vattimo-Gil | Brazil | NO | leaves | 0.70 | 60.5 | 204 | [216] |
| Lauraceae | Ocotea | splendens (Meisn.) Baill | Brazil | NO | leaves | 0.35 | 51.0 | 205 | [217] |
| Lauraceae | Persea | americana Mill. | Nigeria | YES | leaves | 0.20 | 43.9 | 216 | [218] |
| Lauraceae | Phoebe | porphyria (Griseb.) Mez. | Argentina | NO | aerial parts | 0.15 | 19.3 | 222 | [219] |
| Magnoliaceae | Magnolia | obovata Thunb. | Japan | NO | leaves | 0.05 | 23.7 | 173 | [220] |
| Malvaceae | Pachira | glabra Pasq. | Nigeria | NO | leaves | 0.71 | 14.5 | 212 | [221] |
| Malvaceae | Triumfetta | rhomboidea Jacq. | Burkina-Faso | NO | aerial parts | 0.02 | 24.2 | 282 | [222] |
| Meliaceae | Aglaia | odorata Lour. | Thailand | NO | stem | 0.07 | 10.2 | 15 | [223] |
| Meliaceae | Aphanamixis | polystachya (Wall.) R.Parker | Bangladesh | NO | wood | n.a. | 19.4 | 35 | [224] |
| Meliaceae | Cedrela | fissilis Vellozo | Brazil | NO | leaves | 0.06 | 26.3 | 70 | [225] |
| Meliaceae | Guarea | macrophylla Vahl. ssp. tuberculata Vellozo | Brazil | NO | leaves | 0.15 | 10.0 | 137 | [226] |
| Moraceae | Ficus | benjamina L. | Nigeria | NO | leaves | n.a. | 17.0 | 124 | [227] |
| Myricaceae | Morella | pensylvanica (Mirbel) Kartesz | Canada | NO | aerial parts | 0.15 | 14.5 | 190 | [228] |
| Myristicaceae | Gymnacranthera | canarica (King) Warb. | India | NO | leaves | 0.01 | 23.4 | 138 | [229] |
| Myristicaceae | Knema | kunstleri Warb. | Malaysia | NO | aerial parts | 0.12 | 23.2 | 159 | [230] |
| Myristicaceae | Myristica | malabarica Lam. | India | NO | leaves | 0.05 | 27.3 | 197 | [229] |
| Myrtaceae | Blepharocalyx | salicifolius O.Berg | Brazil | NO | leaves | 0.90 | 22.9 | 55 | [231] |
| Myrtaceae | Eucalyptus | leptophleba F. Muell. | Australia | NO | leaves | 0.01 | 11.4 | 118 | [232] |
| Myrtaceae | Eugenia | stipitata McVaugh ssp. sororia | Portugal | NO | leaves | 0.35 | 22.7 | 119 | [233] |
| Myrtaceae | Feijoa | sellowiana Berg. | France | NO | fruits | 0.10 | 12.0 | 121 | [234] |
| Myrtaceae | Marlierea | silvatica Kiaersk | Brazil | NO | leaves | 0.30 | 25.4 | 174 | [235] |
| Myrtaceae | Melaleuca | sphaerodendra var. microphylla (Virot) Craven and J.W. Dawson | New Caledonia | NO | leaves | 0.10 | 28.8 | 178 | [236] |
| Myrtaceae | Myrcia | cuprea (O. Berg) Kiaersk. | Brazil | NO | aerial parts | 0.10 | 39.1 | 194 | [237] |
| Myrtaceae | Myrcianthes | pseudo-mato (Legr.) Mc. Vaugh | Argentina | NO | leaves | 0.30 | 18.9 | 195 | [238] |
| Myrtaceae | Myrciaria | tenella (DC.) Berg | Brazil | NO | leaves | 0.40 | 25.1 | 196 | [239] |
| Myrtaceae | Ochrosperma | lineare (C.T. White) Trudgen | Australia | NO | aerial parts | 0.30 | 11.6 | 202 | [240] |
| Myrtaceae | Plinia | edulis (Vell.) Sobral | Brazil | NO | leaves | 0.10 | 21.2 | 235 | [241] |
| Myrtaceae | Psidium | striatulum DC. | Brazil | NO | leaves | 0.10 | 28.6 | 243 | [242] |
| Myrtaceae | Syzygium | aromaticum L. | Morocco | YES | buds | 8.58 | 27.5 | 270 | [243] |
| Myrtaceae | Syzygium | grande (Wight) Walp. | Vietnam | NO | stem | 0.12 | 29.3 | 271 | [244] |
| Myrtaceae | Uromyrtus | australis A. J. Scott | Australia | NO | leaves | 0.12 | 20.7 | 283 | [245] |
| Papilionaceae | Meristotropis | xanthioides Vassilez | Iran | NO | aerial parts | 3.20 | 11.8 | 184 | [246] |
| Phyllanthaceae | Actephila | excelsa (Dazl.) Muell. | Vietnam | NO | leaves | 0.15 | 11.2 | 7 | [247] |
| Pinaceae | Abies | nephrolepis (Khingan fir) | South Korea | NO | needles | 0.40 | 10.8 | 1 | [248] |
| Pinaceae | Pinus | pinaster Aiton | Morocco | YES | needles | 0.38 | 22.2 | 225 | [249] |
| Pinaceae | Pinus | armandii Franch. | Scotland | NO | needles | n.a. | 36.3 | 226 | [250] |
| Pinaceae | Pinus | bungeana Zucc. | South Korea | NO | needles | 0.31 | 27.2 | 227 | [251] |
| Pinaceae | Pinus | halepensis Mill. | Turkey | NO | needles | n.a. | 25.9 | 228 | [252] |
| Piperaceae | Piper | tuberculatum var. tuberculatum (Micq.) CDC | Brazil | NO | leaves | n.a. | 26.3 | 229 | [253] |
| Piperaceae | Piper | guineense Schumach. and Thonn. | Cameroon | NO | seeds | 1.1 | 57.6 | 230 | [254] |
| Piperaceae | Piper | nigrum L. | India | YES | seeds | n.a. | 45.3 | 231 | [255] |
| Piperaceae | Piper | maingayi Hk. F. | Malaysia | NO | seeds | 0.21 | 39.6 | 232 | [256] |
| Piperaceae | Pothomorphe | peltata (L.) Miq. | Brazil | NO | leaves | 0.20 | 68.0 | 239 | [257] |
| Plantaginaceae | Adenosma | indianum (Lour.) Merr. | China | NO | aerial parts | 0.29 | 10.32 | 8 | [258] |
| Podocarpaceae | Afrocarpus | mannii (Hook.f.) C.N.Page | S. Tomé e Principe | NO | leaves | 0.15 | 13.1 | 12 | [259] |
| Ptaeroxylaceae | Cedrelopsis | grevei H. Baillon | Madagascar | NO | barks | n.a. | 10.6 | 71 | [260] |
| Rosaceae | Agrimonia | eupatoria L. | Iran | YES | flowers | 1.20 | 42.8 | 16 | [261] |
| Rosaceae | Rosa | canina L. | Tunisia | YES | flowers | 1.40 | 32.0 | 250 | [262] |
| Rubiaceae | Cruciata | laevipes Opiz | Italy | YES | aerial parts | 0.70 | 19.0 | 97 | [263] |
| Rubiaceae | Geophila | repens (L.) I.M. Johnst | China | NO | aerial parts | 0.07 | 23.3 | 129 | [264] |
| Rutaceae | Aegle | marmelos (L.) Corr. | Nepal | YES | leaves | 0.29 | 29.6 | 10 | [265] |
| Rutaceae | Amyris | elimifera L. | Cuba | NO | leaves | 0.60 | 37.8 | 28 | [266] |
| Rutaceae | Atalantia | buxifolia (Poir.) Oliv. | China | NO | leaves | 0.36 | 25.8 | 47 | [267] |
| Rutaceae | Boenninghausenia | albiflora Reichb. | India | NO | flowers | 0.20 | 13.1 | 56 | [268] |
| Rutaceae | Citrus | garrawayi F.M.Bailey | Australia | NO | leaves | 0.20 | 17.6 | 80 | [269] |
| Rutaceae | Feroniella | lucida (Scheff.) Swing | Thailand | NO | leaves | 0.12 | 26.6 | 122 | [270] |
| Rutaceae | Flindersia | pimenteliana F.Muell. | Australia | NO | leaves | 0.03 | 16.9 | 126 | [271] |
| Rutaceae | Haplophyllum | villosum (M. B.) G. Don | Iran | NO | aerial parts | 0.22 | 13.1 | 139 | [272] |
| Rutaceae | Medicosma | obovata T.G. Hartley | Australia | NO | aerial parts | 0.40 | 17.2 | 177 | [273] |
| Rutaceae | Melicope | peninsularis T.G. Hartley | Australia | NO | leaves | 0.10 | 49.0 | 179 | [274] |
| Rutaceae | Murraya | paniculata L. | Brazil | NO | leaves | 0.03 | 57.6 | 192 | [275] |
| Rutaceae | Murraya | koenigii (L.) Spreng | India | YES | leaves | 0.1 | 45.9 | 193 | [276] |
| Rutaceae | Pamburus | missionis (Wight) Swingle | India | NO | leaves | 0.05 | 25.4 | 213 | [277] |
| Rutaceae | Spiranthera | odoratissima A. St. Hil. | Brazil | NO | leaves | n.a. | 23.8 | 267 | [278] |
| Rutaceae | Zanthoxylum | veneficum F.M.Bailey | Australia | NO | leaves | 0.10 | 36.3 | 292 | [279] |
| Sapindaceae | Acer | truncatum Bunge | China | NO | leaves | n.a. | 12.9 | 3 | [280] |
| Schisandraceae | Kadsura | coccinea (Lem.) A.C. Smith | China | NO | roots | 0.20 | 24.9 | 157 | [281] |
| Scrophulariaceae | Buddleia | asiatica Lour. | India | NO | leaves | 0.30 | 15.8 | 58 | [282] |
| Scrophulariaceae | Capraria | biflora L. | Brazil | NO | leaves | 0.09 | 29.6 | 66 | [283] |
| Solanaceae | Solanum | stipulaceum Roem and Schult | Brazil | NO | flowers | 0.08 | 25.8 | 265 | [284] |
| Verbenaceae | Aloysia | virgata Juss. | Cuba | NO | aerial parts | n.a. | 15.4 | 22 | [285] |
| Verbenaceae | Lantana | montevidensis Briq | Brazil | NO | leaves | 0.13 | 31.5 | 163 | [286] |
| Verbenaceae | Lantana | camara L. | Madagascar | NO | aerial parts | 0.08 | 43.61 | 164 | [287] |
| Verbenaceae | Lippia | myriocephala Schltdl. et Cham. | Costa Rica | NO | leaves | 0.08 | 16.1 | 171 | [288] |
| Verbenaceae | Petitia | domingensis Jacq. | Cuba | NO | flowers | n.a. | 35.7 | 219 | [289] |
| Zingiberaceae | Aframomum | corrorima (Braun) P.C.M. Jansen | Ethiopia | NO | leaves | 0.50 | 60.7 | 11 | [290] |
| Zingiberaceae | Alpinia | purpurata (Viell.) | Fiji | NO | flowers | 0.05 | 24.2 | 23 | [291] |
| Zingiberaceae | Alpinia | conchigera Griff. | Malaysia | NO | rhizomes | 0.14 | 10.0 | 24 | [292] |
| Zingiberaceae | Alpinia | mutica Roxb. | Vietnam | NO | fruit | 0.17 | 22.6 | 25 | [293] |
| Zingiberaceae | Alpinia | pinnanensis T. L. Wu and Senjen | Vietnam | NO | fruit | 0.23 | 11.4 | 26 | [294] |
| Zingiberaceae | Costus | afer Ker–Grawl | Nigeria | NO | leaves | n.a. | 12.3 | 93 | [295] |
| Zingiberaceae | Curcuma | longa L. | India | YES | rhizomes | 2.20 | 9.8 | 99 | [296] |
| Zingiberaceae | Etlingera | elatior (Jack) R. M. Smith | Malaysia | NO | leaves | 0.70 | 10.7 | 117 | [297] |
| Zingiberaceae | Globba | schomburgkii Hook. f. | India | NO | aerial parts | 0.01 | 31.7 | 133 | [298] |
| Zingiberaceae | Hedychium | coronarium Koen. | Brazil | YES | leaves | 0.68 | 43.0 | 140 | [299] |
| Zingiberaceae | Renealmia | breviscapa Poepp. and Endl. | Brazil | NO | rhizomes | 0.01 | 62.3 | 247 | [300] |
| Zingiberaceae | Renealmia | alpinia (Rottb.) Maas | Brazil | NO | leaves | 0.50 | 22.9 | 248 | [301] |
| Zingiberaceae | Zingiber | nimmonii Dalzell | India | NO | rhizomes | 0.04 | 42.2 | 293 | [302] |
In general, the 295 species belonged to 51 families and were reported from 56 countries worldwide. The essential oil containing BCP was extracted from 13 different plant parts. Out of 295 species, 34 were found to be listed in the Belfrit list, whereas for 51 species no data were available on the yield percentage. In many cases, the researchers used a small amount of plant parts (ranging from a few g to 200–300 g) from which it was impossible to evaluate the oil yield. However, in the majority of the other cases the yield was provided and hence reported (Table 1).
The essential oil yield of 243 species ranged from 0.001 to 8.58%, whereas the BCP percentage of all selected species ranged from 9.8 (the threshold minimum level for species selection) to 75.6% (Table 2), providing an average percentage of 0.42% for yield and 27.4% for BCP. As shown in Table 2, variability was higher for yield percentages than for BCP percentage. The reason for the yield and BCP variability depends on several factors, including plant part, the quantity of plant material distilled and, most of all, the genetic variability and phenotypic plasticity of plants [303,304,305,306].
Table 2.
General statistics on BCP and yield percentages of plant species listed in Table 1.
| Specification | Essential Oil Yield | Percentage of BCP |
|---|---|---|
| Number of cases | 243 | 295 |
| Range | ||
| Minimum | 0.00 | 9.8 |
| Maximum | 8.58 | 75.6 |
| Mean | 0.42 | 27.4 |
| S.E.M. | 0.06 | 0.8 |
| S.D. | 0.87 | 13.6 |
| C.V. % | 2.09 | 0.5 |
S.E.M., standard error of the mean; S.D., standard deviation; C.V., coefficient of variation.
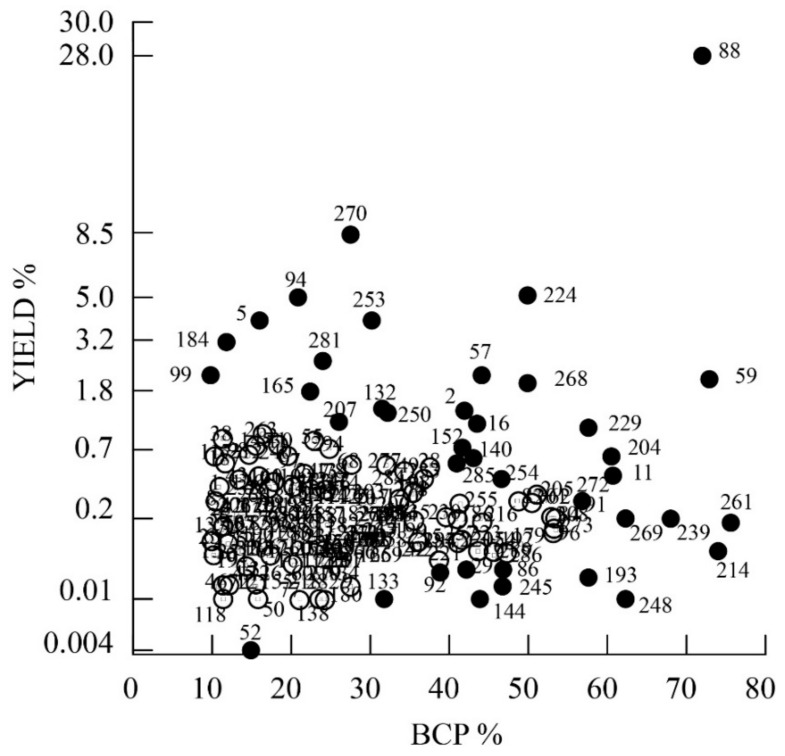
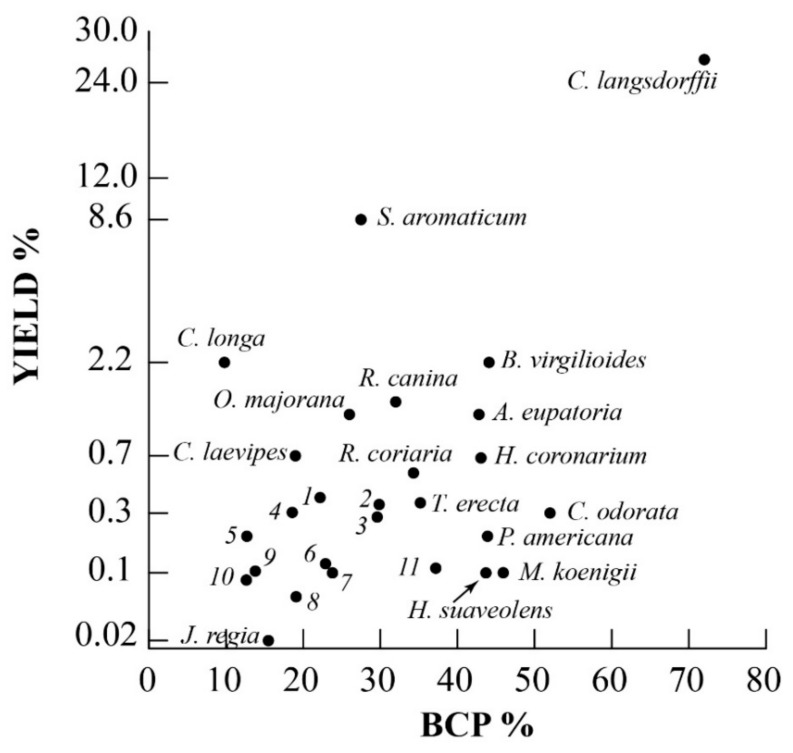
In order to look for plant species with the highest BCP and yield percentages, a scatter plot was obtained, as depicted in Figure 1. The highest yield and BCP percentages were found for Copaifera langsdorffii. High BCP percentages but with decreasing yields were found for Bursera microphylla, Scutellaria havanensis and Pentadesma butyracea. Copaifera species, popularly known as copaiba oil, are widely used in Brazilian popular medicine and the genus is known for its high essential oil yield and BCP content [135,307,308]. The genus Bursera belongs to the plant family Burseraceae and contains several aromatic spices producing oleo-gum resins, such as the traditional incenses, frankincense and myrrh [309]. Pentadesma butyracea (Clusiaceae) is a dense forest species which is found in the center and north of Benin forests whose bark, rough and deeply cracked, exudes a thick resinous juice, of reddish yellow color [115]. The Scutellaria genus (Lamiaceae) consists of plants which are widely distributed throughout the world; S. butyracea is an endemic plant native from Havana and is ethnomedically used for several purposes because of its BCP content [196].
Figure 1.
Scatter plot of BCP percentage vs. yield percentage. The yield axis is represented as a power of 0.3 scale in order to evidence species with yields ranging from 0.004 to 3%. Numbers correspond to plant species listed in Table 1. Filled circles outline the species outside the central group of all other species (hollow circles).
High yields with lower BCP percentages were found for Acalypha fruticosa, Achyrocline alata, Agrimonia eupatoria, Bowdichia virgilioides, Bursera microphylla, Croton pulegiodorus, Curcuma longa, Glechon marifolia, Laser trilobum, Meristotropis xanthioides, Origanum majorana, Pimpinella kotschyana, Piper guineense, Rosa canina, Salvia canariensis, Spondias pinnata, Syzygium aromaticum and Thuja orientalis. All other species had a yield ranging from 0.004 to 1% and a BCP content ranging from 9.8 to 55 % (Figure 1).
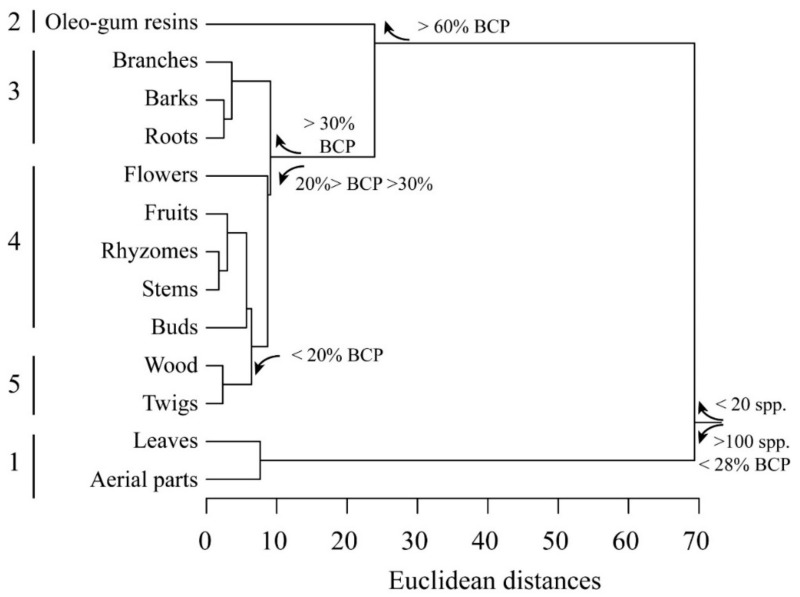
The plant part that contained the highest content of BCP was then analyzed. In order to evidence the statistical linkage between the plant parts, a cluster analysis was calculated by considering as category the plant part and as variables the number of species, the BCP% and the yield% reported in Table 1 (Figure 2). Euclidean distances were calculated by using the average linkage method. Five clusters were evidenced: the first cluster was made by plant parts reported in more than 100 species and was dominated by leaves and aerial parts, which contained in general a BCP percentage lower than 28%. The other four clusters were made by plant parts reported in less than 16 species. These four clusters were further subdivided according to their BCP content (Figure 2). As expected, the highest BCP percentage was found in oleo-gum resins (cluster 2), followed by roots, barks and branches (cluster 3). Flowers and buds (cluster 4) showed a high yield, whereas twigs and woods (cluster 5) had both low yields and BCP percentages (Figure 2).
Figure 2.
Cluster analysis of BCP and yield percentages according to the plant part used for extraction. Euclidean distances are calculated with average linkage method. Five clusters are evident (see text for explanation).
Table 3 summarizes the statistical analysis of BCP and yield percentages reported from different plant parts.
Table 3.
Average percentages of BCP and yields from plant parts as reported in plant species listed in Table 1. (±S.E.M.); n.c., not computable; E.O., essential oil.
| Plant Part | Number of Species | BCP % | E.O. Yield % |
|---|---|---|---|
| Aerial Parts | 115 | 25.19 (±1.10) | 0.42 (±4.85) |
| Barks | 3 | 39.03 (±18.59) | 0.30 (±0.22) |
| Branches | 1 | 34.90 (±n.c.) | 0.20 (± n.c.) |
| Buds | 1 | 27.50 (± n.c.) | 8.58 (± n.c.) |
| Flowers | 16 | 29.29 (±3.11) | 0.41 (±0.13) |
| Fruits | 9 | 26.93 (±4.43) | 0.24 (±0.07) |
| Leaves | 128 | 27.58 (±1.15) | 0.30 (±0.04) |
| Oleo-gum resin | 4 | 66.13 (±4.54) | 15.50 (±8.30) |
| Rhyzomes | 5 | 27.38 (±10.65) | 0.49 (±0.43) |
| Roots | 7 | 39.77 (±5.37) | 1.77 (±0.92) |
| Stems | 3 | 25.07 (±7.66) | 0.13 (±0.04) |
| Twigs | 2 | 16.25 (±4.85) | 0.02 (±0.01) |
| Wood | 1 | 19.40 (± n.c.) | 0.42 (±n.c.) |
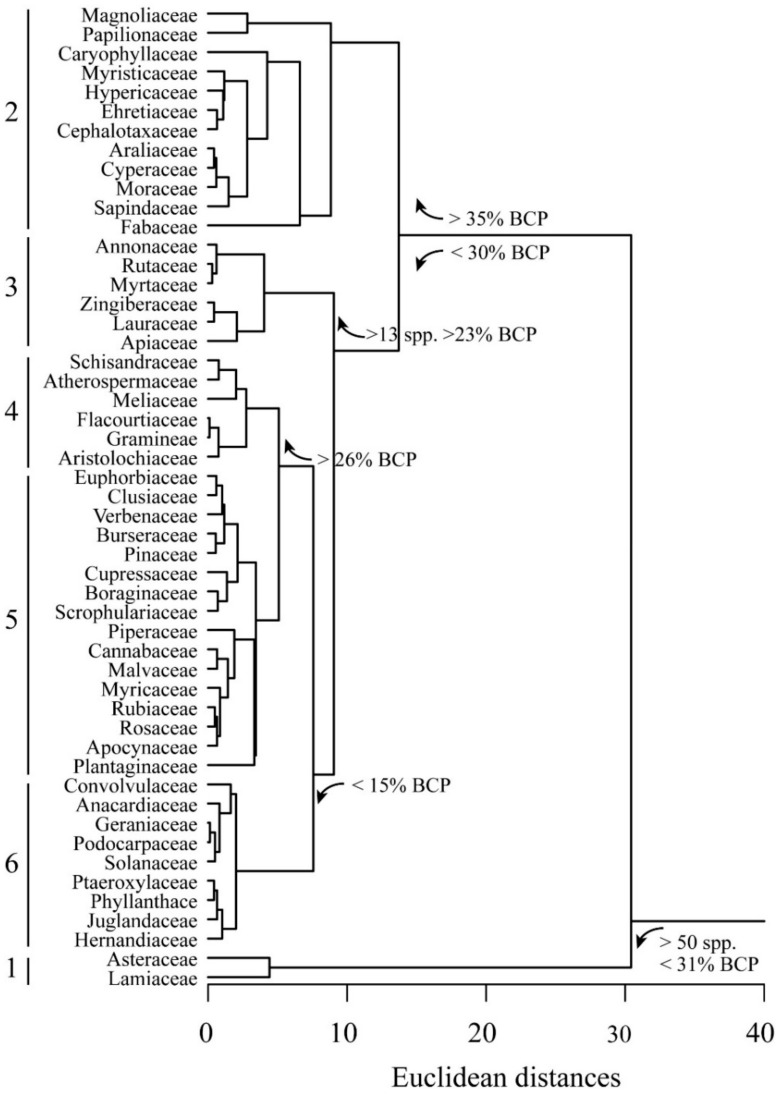
The next analysis was at the familial level. A cluster analysis was calculated with average linkage method by using data of Table 1 by considering as a category the plant families and the species number, yield% and BCP% as variables. The results of the cluster analysis show the presence of 6 clusters (Figure 3). The first cluster is made by the Asteraceae and the Lamiaceae which consist of a number of species > 50 and a BCP% < 31. The second cluster gathers all families whose species have a BCP% > 35%; in this cluster, the Magnoliaceae and the Papilionaceae are separated in a subcluster because of their high BCP% and low yield%, whereas the Fabaceae (which include the above mentioned C. langsdorffii) are separated in a subcluster because of their high yield %. The third cluster is made by families with a number of species > 13 and a BCP% > 23%; here, the Lauraceae, the Apiaceae and the Zingiberaceae are separated in a subcluster because of their higher BCP%. The genus Ocotea is one of the largest of the Lauraceae family, with approximately 350 species distributed throughout tropical and subtropical America. O. splendens, as many other Ocotea species [212] is characterized by a high percentage of BCP [217]. In the Apiaceae family, the species P. kotschyana spreads widely through Anatoly, Iran (northwest, west and center) and north of Iraq and contains BCP in all plant parts [41]. The family Zingiberaceae is well known for producing essential oils that are used to prevent and control several diseases; the species R. breviscapa was found to possess a high percentage of BCP [300]. The fourth cluster is made by families with a BCP% > 26 and a subcluster separates the Atherospermaceae, the Flacourtiaceae and the Meliaceae because of their BCP%. The fifth cluster is made by families with a BCP% < 25 and the Plantaginaceae are separated in a subcluster because of their relatively higher yield%. Finally, the sixth cluster is made by plant families with a low BCP percentage and a subcluster separates the Hernandiaceae, the Juglandaceae, the Phyllanthace and the Ptaeroxylaceae because of their BCP content lower than 11%.
Figure 3.
Cluster analysis of BCP and yield percentages according to the plant families. Euclidean distances are calculated with average linkage method. Six clusters are evident (see text for explanation).
Table 4 describes the statistical data related to plant families.
Table 4.
Average percentages of BCP and yields from plant families belonging to the plant species reported in Table 1. (±S.E.M.); n.c., not computable; n.a., not available; E.O., essential oil.
| Family | Number of Species | BCP% | E.O. Yield% |
|---|---|---|---|
| Anacardiaceae | 2 | 13.25 (±2.65) | n.a. |
| Annonaceae | 15 | 22.17 (±1.26) | 0.20 (±0.05) |
| Apiaceae | 16 | 30.96 (±4.15) | 0.37 (±0.14) |
| Apocynaceae | 3 | 17.63 (±3.05) | 0.26 (±0.10) |
| Araliaceae | 1 | 39.00 (n.c.) | 0.04 (n.c.) |
| Aristolochiaceae | 2 | 26.65 (±3.75) | 0.21 (±0.13) |
| Asteraceae | 50 | 27.94 (±1.92) | 0.47 (±0.14) |
| Atherospermaceae | 1 | 32.20 (n.c.) | 0.06 (n.c.) |
| Boraginaceae | 2 | 22.95 (±10.15) | 0.15 (±0.10) |
| Burseraceae | 5 | 24.20 (±4.83) | 0.14 (±0.02) |
| Cannabaceae | 3 | 20.24 (±5.14) | 0.27 (±0.14) |
| Caryophyllaceae | 1 | 46.60 (n.c.) | 0.48 (n.c.) |
| Cephalotaxaceae | 1 | 41.60 (n.c.) | 0.82 (n.c.) |
| Clusiaceae | 5 | 25.85 (±6.84) | 0.29 (±0.19) |
| Convolvulaceae | 1 | 15.10 (n.c.) | n.a. |
| Cupressaceae | 3 | 23.83 (±9.60) | 1.59 (±0.84) |
| Cyperaceae | 1 | 38.40 (n.c.) | n.a. |
| Ehretiaceae | 2 | 41.95 (±15.65) | 1.10 (n.c.) |
| Euphorbiaceae | 6 | 25.60 (±15.42) | 0.42 (±0.46) |
| Fabaceae | 11 | 36.92 (±6.15) | 3.89 (±3.45) |
| Flacourtiaceae | 2 | 27.75 (±3.15) | n.a. |
| Geraniaceae | 1 | 13.10 (n.c.) | 0.22 (n.c.) |
| Gramineae | 2 | 27.90 (±13.50) | 0.19 (±0.09) |
| Hernandiaceae | 1 | 9.80 (n.c.) | 2.20 (n.c.) |
| Hypericaceae | 3 | 41.10 (±15.86) | 0.13 (±0.05) |
| Juglandaceae | 1 | 10.00 (n.c.) | 0.15 (n.c.) |
| Lamiaceae | 57 | 31.03 (±2.03) | 0.41 (±0.17) |
| Lauraceae | 13 | 29.33 (±3.14) | 0.38 (±0.18) |
| Magnoliaceae | 1 | 56.90 (n.c.) | 0.30 (n.c.) |
| Malvaceae | 2 | 19.70 (±5.20) | 0.11 (±0.04) |
| Meliaceae | 4 | 30.55 (±9.27) | 0.14 (±0.03) |
| Moraceae | 1 | 37.80 (n.c.) | 0.60 (n.c.) |
| Myricaceae | 1 | 18.10 (n.c.) | 0.30 (n.c.) |
| Myristicaceae | 3 | 42.93 (±10.61) | 1.35 (±0.85) |
| Myrtaceae | 15 | 23.49 (±2.17) | 0.27 (±0.08) |
| Papilionaceae | 1 | 52.00 (n.c.) | 0.30 (n.c.) |
| Phyllanthace | 1 | 10.70 (n.c.) | n.a. |
| Pinaceae | 5 | 23.22 (±5.33) | 0.20 (±0.06) |
| Piperaceae | 5 | 19.70 (±2.26) | 0.23 (±0.07) |
| Plantaginaceae | 1 | 20.90 (n.c.) | 5.00 (n.c.) |
| Podocarpaceae | 1 | 12.90 (n.c.) | n.a. |
| Ptaeroxylaceae | 1 | 11.30 (n.c.) | 0.72 (n.c.) |
| Rosaceae | 2 | 18.00 (±6.60) | 0.10 (±0.08) |
| Rubiaceae | 2 | 17.15 (±0.25) | 0.03 (n.c.) |
| Rutaceae | 15 | 22.97 (±2.69) | 0.27 (±0.06) |
| Sapindaceae | 1 | 36.30 (n.c.) | n.a. |
| Schisandraceae | 1 | 32.00 (n.c.) | 1.40 (n.c.) |
| Scrophulariaceae | 2 | 21.75 (±0.65) | 0.10 (n.c.) |
| Solanaceae | 1 | 12.20 (n.c.) | 0.20 (n.c.) |
| Verbenaceae | 5 | 24.70 (±6.58) | 1.59 (±1.20) |
| Zingiberaceae | 13 | 28.61 (±4.25) | 0.22 (±0.06) |
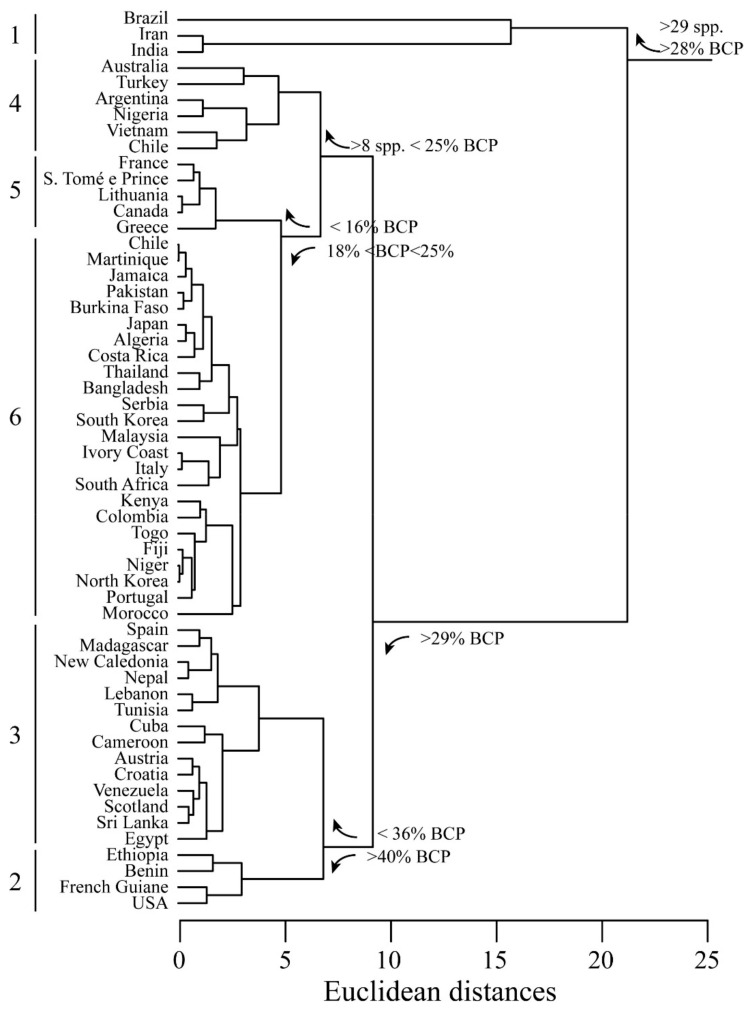
The next analysis aimed to evidence the geographical areas from which the plant species listed in Table 1 were collected. A cluster analysis was calculated with average linkage method, considering the country of origin as a category of their species number, yield% and BCP% as variables. The results of the cluster analysis show the presence of 6 clusters (Figure 4). The first cluster gathers countries with the highest number of species and a BCP percentage higher than 28%; here, a subcluster separates Brazil from India and Iran because of the higher number of species, in agreement with the literature data [310]. The second and third clusters identify countries where BCP has the highest percentages, whereas the fourth cluster gathers countries with a number of species higher than 8. The fifth cluster is made by countries where the BCP content is the lowest, whereas the sixth cluster is made by two subclusters with BCP percentages ranging from 18 to 25%. One of these subclusters is made by countries (Colombia, Fiji, Kenya, Morocco, Niger, North Korea, Portugal and Togo) where the species had a BCP percentage higher than 24% (Figure 4).
Figure 4.
Cluster analysis of BCP and yield percentages according to the country of origin of extracts. Euclidean distances are calculated with average linkage method. Six clusters are evident (see text for explanation).
Table 5 summarizes the statistics related to countries of origin.
Table 5.
Average percentages of BCP and yields from countries from which plant species reported in Table 1 were sampled. (±S.E.M.); n.c., not computable; n.a., not available; E.O., essential oil.
| Country | Number of Species | BCP% | E.O. Yield% |
|---|---|---|---|
| Algeria | 3 | 20.67 (±4.65) | 0.61 (±0.59) |
| Argentina | 8 | 25.85 (±5.41) | 0.19 (±0.07) |
| Australia | 18 | 25.70 (±2.98) | 0.18 (±0.04) |
| Austria | 2 | 34.85 (±18.65) | 0.15 (n.c.) |
| Bangladesh | 1 | 19.40 (n.c.) | n.a. |
| Benin | 3 | 43.77 (±17.44) | 0.09 (±0.01) |
| Brazil | 56 | 33.01 (±2.20) | 1.08 (±0.59) |
| Burkina Faso | 2 | 21.65 (±2.55) | 0.38 (±0.36) |
| Cameroon | 4 | 34.40 (±8.12) | 0.56 (±0.30) |
| Canada | 1 | 14.50 (n.c.) | 0.15 (n.c.) |
| Chile | 1 | 21.10 (n.c.) | 0.15 (n.c.) |
| China | 11 | 19.26 (±2.54) | 0.18 (±0.04) |
| Colombia | 2 | 26.45 (±5.75) | 0.11 (±0.05) |
| Costa Rica | 3 | 19.53 (±5.61) | 0.16 (±0.07) |
| Croatia | 1 | 35.40 (n.c.) | 0.35 (n.c.) |
| Cuba | 5 | 36.30 (±10.85) | 0.41 (±0.12) |
| Egypt | 2 | 36.95 (±12.95) | 2.30 (±0.30) |
| Ethiopia | 2 | 41.15 (±19.55) | 0.32 (±0.19) |
| Fiji | 1 | 24.20 (n.c.) | 0.05 (n.c.) |
| France | 2 | 12.35 (±0.35) | 0.15 (±0.05) |
| French Guian | 1 | 47.00 (n.c.) | 0.06 (n.c.) |
| Greece | 1 | 16.40 (n.c.) | 1.00 (n.c.) |
| India | 29 | 27.00 (±2.32) | 0.34 (±0.11) |
| Iran | 30 | 28.69 (±2.02) | 0.67 (±0.22) |
| Italy | 5 | 22.54 (±2.55) | 0.26 (±0.12) |
| Ivory Coast | 5 | 22.24 (±3.48) | 0.25 (±0.13) |
| Jamaica | 1 | 21.70 (n.c.) | 0.03 (n.c.) |
| Japan | 3 | 20.93 (±1.94) | 0.05 (±0.02) |
| Kenya | 3 | 24.97 (±2.94) | 0.17 (±0.12) |
| Lebanon | 2 | 31.55 (±18.65) | 0.20 (±0.11) |
| Lithuania | 1 | 14.20 (n.c.) | 0.05 (n.c.) |
| Madagascar | 3 | 29.10 (±9.74) | 0.14 (±0.06) |
| Malaysia | 7 | 20.56 (±3.98) | 0.25 (±0.11) |
| Martinique | 1 | 21.10 (n.c.) | 0.11 (n.c.) |
| Morocco | 2 | 24.85 (±2.65) | 4.48 (±4.10) |
| Nepal | 1 | 29.60 (n.c.) | 0.29 (n.c.) |
| New Caledonia | 1 | 28.80 (n.c.) | 0.10 (n.c.) |
| Niger | 1 | 24.00 (n.c.) | 0.30 (n.c.) |
| Nigeria | 10 | 25.87 (±4.39) | 0.19 (±0.09) |
| North Korea | 1 | 24.00 (n.c.) | 0.40 (n.c.) |
| Pakistan | 2 | 21.40 (±3.90) | 0.03 (n.c.) |
| Portugal | 2 | 23.65 (±0.95) | 0.26 (±0.09) |
| S. Tomé e Prince | 1 | 13.10 (n.c.) | 0.15 (n.c.) |
| Scotland | 1 | 36.30 (n.c.) | n.a. |
| Serbia | 4 | 18.35 (±3.47) | 0.05 (±0.03) |
| South Africa | 5 | 24.86 (±3.65) | 0.09 (±0.02) |
| South Korea | 4 | 16.30 (±3.83) | 0.36 (±0.05) |
| Spain | 4 | 28.23 (±4.44) | 1.23 (±0.93) |
| Sri Lanka | 1 | 37.00 (n.c.) | 0.50 (n.c.) |
| Thailand | 2 | 18.40 (±8.20) | 0.10 (±0.03) |
| Togo | 1 | 25.20 (n.c.) | 0.50 (n.c.) |
| Tunisia | 3 | 32.00 (±5.08) | 0.52 (±0.44) |
| Turkey | 14 | 29.21 (±3.51) | 0.25 (±0.08) |
| USA | 2 | 47.45 (±25.45) | 2.10 (n.c.) |
| Venezuela | 2 | 36.40 (±9.30) | 0.06 (±0.01) |
| Vietnam | 11 | 22.38 (±4.01) | 0.28 (±0.08) |
In order to separate which species containing BCP were also represent in the Belfrit list, a scatter plot was obtained by selecting BCP% and yield% as variables (Figure 5). C. langdorffii, S. aromaticum, C. longa and B. virgilioides were characterized by a yield ranging from 2 to 28%, with varying percentages of BCP; on the other hand, high percentages of BCP but lower yields% were found for A. eupatoria, H. coronarium, C. odorata, P. americana and M. keonigi. All other species showed both lower yields and BCP percentage.
Figure 5.
Scatter plot of BCP% and yield% of plant species present in the Belfrit list. The yield axis is scaled as a power of 0.2 in order to evidence species with yields ranging from 0.02 to 8.6%. 1, Pinus pinaster Aiton; 2, Ocimum tenuiflorum L.; 3, Aegle marmelos (L.) Corr.; 4, Protium heptaphyllum (Aubl.) March.; 5, Artemisia verlotiorum Lam rinus officinalis L.; 6, Annona squamosa L.; 7, Cannabis sativa L.; 8, Centella asiatica L.; 9, Annona muricata L.; 10, Rosmarinus officinalis L; 11, Perilla frutescens var. japonica (Hassk.) H. Hara.
3. Materials and Methods
3.1. Systematic Analysis of BCP-Containing Plant Species
After a preliminary search by using different databases, the work was performed by using Clarivate Analytics Web of Science as a database (http://apps.webofknowledge.com). The basic search criterion was on the general search for the molecule (caryophyllene), then the exclusion criteria were the presence of BCP and a percentage of BCP in the reported results higher than 10%. Papers reporting the occurrence of BCP where then downloaded and saved as a pdf for further reading and collection of information.
3.2. Statistical Analysis
The binomial name of the species (including the author), the family of belonging, the plant part used, the country of origin of the sample, the yield and the BCP percentages were inserted in a database by using Systat® 10 software (Systat Software Inc., San Jose, California, U.S.A.). Data were organized in columns and used for further processing. Average values along with ranges, standard deviation (S.D.), standard error of the mean (S.E.M.) and coefficient of variation (C.V.) were calculated by considering as grouping categories either the species, families, country of origin or plant part used. As a classification statistical method, a cluster analysis was calculated by considering for each category the total number of species, the BCP percentage and the yield percentage by using Systat® 10 software. Euclidean distances were calculated with the average linkage method. Data were plotted as either scatter plots of yield percentage vs. BCP percentage or dendrograms showing the different clusters according to the calculated distance.
4. Conclusions
The attractiveness of BCP, a natural sesquiterpene present in the essential oil of different plant species, arises from its pharmacological feature as a CB2 receptor agonist. This characteristic, along with the lack of interaction with the CB1, makes BCP an interesting plant endocannabinoid with the advantage of lacking any psychotropic effect, as is typical of some Cannabis extracts [8,311,312].
This systematic analysis of published literature on plant species containing BCP in their essential oils identified the species with the highest yield and BCP content and allowed to select which species are also present in the Belfrit list (i.e., potentially attractive for pharmaceutical and nutraceutical industries).
This survey also evidenced the common practice of many authors to ignore the importance of providing the yield of the distilled essential oil, which represent a basic starting point for all industrial applications of the plant species under study. This problem was often correlated with the low amount of plant material distilled. Although interesting from a chemical-analytical point of view, the sole chemical analysis of the essential oil is not useful if performed on a single plant or a few plants, because it does not provide any information on the population genetic variability, being mainly affected by phenotypic plasticity, which is responsible for individual variations inside a population [305].
This work identified some top species like C. langsdforffii, C. odorata, H. lupulus, P. nigrum and S. aromaticum, which provide a high percentage of BCP along with interesting yields. These species, upon a skillful molecular fractionation to remove undesired/toxic monoterpenes, may provide high percentages of BCP that can be used for the preparation of new drugs or dietary supplements aimed to improve health, prevent lifestyle diseases and act as a valid support for chronical diseases such as pain, metabolic and neurological disorders.
Abbreviations
| BCP | (E)-β-caryophyllene |
Funding
This research was funded by the University of Turin, local research grant number to M.E.M.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Gertsch J., Leonti M., Raduner S., Racz I., Chen J.-Z., Xie X.-Q., Altmann K.-H., Karsak M., Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francomano F., Caruso A., Barbarossa A., Fazio A., La Torre C., Ceramella J., Mallamaci R., Saturnino C., Iacopetta D., Sinicropi M.S. Beta-caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019;9:9–19. doi: 10.3390/app9245420. [DOI] [Google Scholar]
- 3.Meza A., Lehmann C. Betacaryophyllene–A phytocannabinoid as potential therapeutic modality for human sepsis? Med. Hypotheses. 2018;110:68–70. doi: 10.1016/j.mehy.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Sharma C., Al Kaabi J.M., Nurulain S.M., Goyal S.N., Kamal M.A., Ojha S. Polypharmacological properties and therapeutic potential of beta-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016;22:3237–3264. doi: 10.2174/1381612822666160311115226. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt D., Levy R., Carroll B. Toxicological evaluation of -caryophyllene oil: Subchronic toxicity in rats. Int. J. Toxicol. 2016;35:558–567. doi: 10.1177/1091581816655303. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira G.L.D., Machado K.C., Machado K.C., da Silva A., Feitosa C.M., Almeida F.R.D. Non-clinical toxicity of beta-caryophyllene, a dietary cannabinoid: Absence of adverse effects in female swiss mice. Regul. Toxicol. Pharmacol. 2018;92:338–346. doi: 10.1016/j.yrtph.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Wu C., Jia Y., Lee J.H., Jun H.J., Lee H.S., Hwang K.Y., Lee S.J. Trans-caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-alpha. Bioorg. Med. Chem. Lett. 2014;24:3168–3174. doi: 10.1016/j.bmcl.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 8.Geddo F., Scandiffio R., Antoniotti S., Cottone E., Querio G., Maffei M.E., Bovolin P., Gallo M.P. Pipenig (r)-fl, a fluid extract of black pepper (Piper nigrum L.) with a high standardized content of trans-beta-caryophyllene, reduces lipid accumulation in 3t3-l1 preadipocytes and improves glucose uptake in c2c12 myotubes. Nutrients. 2019;11:2788. doi: 10.3390/nu11112788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhopeshwarkar A., Mackie K. Cb2 cannabinoid receptors as a therapeutic target-what does the future hold? Mol. Pharmacol. 2014;86:430–437. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuyama S., Mizoguchi H., Kuwahata H., Komatsu T., Nagaoka K., Nakamura H., Bagetta G., Sakurada T., Sakurada S. Involvement of peripheral cannabinoid and opioid receptors in -caryophyllene-induced antinociception. Eur. J. Pain. 2013;17:664–675. doi: 10.1002/j.1532-2149.2012.00242.x. [DOI] [PubMed] [Google Scholar]
- 11.Commission E., Decree Regulating the Use of Vegetable Substances and Preparations in Food Supplements, Replacing the Decree of the Minister for Health of 9 July 2012 Communication from the Commission—TRIS/(2017) 01619 2017. Notification Number: 2017/276/I. [(accessed on 6 September 2020)]; Available online: https://ec.europa.eu/growth/tools-databases/tris/en/search/?trisaction=search.detail&year=2017&num=276.
- 12.Zhaleh M., Sohrabi N., Zangeneh M.M., Zangeneh A., Moradi R., Zhaleh H. Chemical composition and antibacterial effects of essential oil of Rhus Coriaria fruits in the west of Iran (Kermanshah) J. Essent. Oil Bear. Plants. 2018;21:493–501. doi: 10.1080/0972060X.2018.1462739. [DOI] [Google Scholar]
- 13.Sameh S., Al-Sayed E., Labib R.M., Singab A.N.B. Comparative metabolic profiling of essential oils from Spondias Pinnata (linn. F.) kurz and characterization of their antibacterial activities. Ind. Crop Prod. 2019;137:468–474. doi: 10.1016/j.indcrop.2019.05.060. [DOI] [Google Scholar]
- 14.Kossouoh C., Moudachirou M., Adjakidje V., Chalchat J.C., Figueredo G. Essential oil chemical composition of Annona Muricata L. Leaves from Benin. J. Essent. Oil Res. 2007;19:307–309. doi: 10.1080/10412905.2007.9699288. [DOI] [Google Scholar]
- 15.Andrade E.H.A., Oliveira J., Zoghbi M.D.B. Volatiles of Anaxagorea Dolichocarpa Spreng. & sandw. and Annona Densicoma mart. growing wild in the state of para, Brazil. Flavour Frag. J. 2007;22:158–160. [Google Scholar]
- 16.Nebie R.H.C., Yameogo R.T., Belanger A., Sib F.S. Chemical composition of leaf essential oil of Annona Senegalensis pers. from Burkina Faso. J. Essent. Oil Res. 2005;17:331–332. doi: 10.1080/10412905.2005.9698922. [DOI] [Google Scholar]
- 17.Garg S.N., Gupta D. Composition of the leaf oil of Annona squamosa L. from the north Indian plains. J. Essent. Oil Res. 2005;17:257–258. doi: 10.1080/10412905.2005.9698894. [DOI] [Google Scholar]
- 18.Phan G.M., Phan S.T., Konig W.A. Chemical composition of the flower essential oil of Artabotrys Hexapetalus (L. F.) bhandare of Vietnam. J. Essent. Oil Res. 2007;19:523–524. doi: 10.1080/10412905.2007.9699321. [DOI] [Google Scholar]
- 19.Brophy J., Goldsack R., Forster P. Essential oils from the leaves of some queensland Annonaceae. J. Essent. Oil Res. 2004;16:95–100. doi: 10.1080/10412905.2004.9698660. [DOI] [Google Scholar]
- 20.Ouattara Z.A., Boti J.B., Ahibo C.A., Tomi F., Casanova J., Bighelli A. Chemical composition of the leaf oil of Cleistopholis Glauca Pierre ex engler & diels from Cote d’Ivoire. J. Essent. Oil Res. 2012;24:471–474. [Google Scholar]
- 21.Hoferl M., Dai D.N., Thang T.D., Jirovetz L., Schmidt E. Leaf essential oils of six vietnamese species of Fissistigma (Annonaceae) Nat. Prod. Commun. 2013;8:663–665. doi: 10.1177/1934578X1300800529. [DOI] [Google Scholar]
- 22.Thang T.D., Dai D.N., Ogunwande I.A. Identification of the volatile compounds in the leaf and stem bark of three Goniothalamus species from Vietnam. J. Essent. Oil Bear. Plants. 2016;19:743–749. doi: 10.1080/0972060X.2014.958563. [DOI] [Google Scholar]
- 23.Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the queensland species of Melodorum (Annonaceae) J. Essent. Oil Res. 2004;16:483–486. doi: 10.1080/10412905.2004.9698777. [DOI] [Google Scholar]
- 24.Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the australian species of Miliusa (Annonaceae) J. Essent. Oil Res. 2004;16:253–255. doi: 10.1080/10412905.2004.9698714. [DOI] [Google Scholar]
- 25.Ouattara Z.A., Boti J.B., Ahibo C.A., Bekro Y.A., Casanova J., Tomi F., Bighelli A. Composition and chemical variability of Ivoirian Polyalthia Oliveri leaf oil. Chem. Biodivers. 2016;13:293–298. doi: 10.1002/cbdv.201500066. [DOI] [PubMed] [Google Scholar]
- 26.Brophy J.J., Goldsack R.J., Hook J.M., Fookes C.J.R., Forster P.I. The leaf essential oils of the australian species of Pseuduvaria (Annonaceae) J. Essent. Oil Res. 2004;16:362–366. doi: 10.1080/10412905.2004.9698743. [DOI] [Google Scholar]
- 27.Boyorn F.F., Zollo P.H.A., Agnaniet H., Menut C., Bessiere J.M. Aromatic plants of tropical Central Africa. Xl. Essential oils from Uvariodendron Calophylium re fries growing in cameroon. J. Essent. Oil Res. 2005;17:128–129. [Google Scholar]
- 28.Lazarevic J., Radulovic N., Palic R., Zlatkovic B. Chemical analysis of volatile constituents of Berula Erecta (Hudson) Coville Subsp Erecta (Apiaceae) from Serbia. J. Essent. Oil Res. 2010;22:153–156. doi: 10.1080/10412905.2010.9700290. [DOI] [Google Scholar]
- 29.Kurkcuoglu M. Essential oil composition from fruits and aerial parts of Bilacunaria anatolica a. Duran (Apiaceae) endemic in Turkey. J. Essent. Oil Bear. Plants. 2016;19:379–383. doi: 10.1080/0972060X.2015.1029991. [DOI] [Google Scholar]
- 30.Oyedeji O.A., Afolayan A.J. Chemical composition and antibacterial activity of the essential oil of Centella Asiatica growing in South Africa. Pharm. Biol. 2005;43:249–252. doi: 10.1080/13880200590928843. [DOI] [Google Scholar]
- 31.Masoudi S., Esmaeili A., Khalilzadeh M.A., Rustaiyan A., Moazami N., Akhgar M.R., Varavipoor M. Volatile constituents of Dorema Aucheri boiss., Seseli Libanotis (L.) w. D. Koch var. Armeniacum Bordz. and conium Maculatum L. Three umbelliferae herbs growing wild in Iran. Flavour Frag. J. 2006;21:801–804. doi: 10.1002/ffj.1722. [DOI] [Google Scholar]
- 32.Akbarian A., Rahimmalek M., Sabzalian M.R. Variation in essential oil yield and composition of Dorema Aucheri Boiss., an endemic medicinal plant collected from wild populations in natural habitats. Chem. Biodivers. 2016;13:1756–1766. doi: 10.1002/cbdv.201600160. [DOI] [PubMed] [Google Scholar]
- 33.Pala-Paul J., Brophy J.J., Goldsack R.J., Copeland L.M., Perez-Alonso M.J., Velasco-Negueruela A. Essential oil composition of the seasonal Heterophyllous leaves of Eryngium Vesiculosum from Australia. Aust. J. Bot. 2003;51:497–501. doi: 10.1071/BT03030. [DOI] [Google Scholar]
- 34.Sahebkar A., Iranshahi M. Volatile constituents of the genus ferula (Apiaceae): A review. J. Essent. Oil Bear. Plants. 2011;14:504–531. doi: 10.1080/0972060X.2011.10643969. [DOI] [Google Scholar]
- 35.Kucukboyaci N., Demirci B., Adiguzel N., Bani B., Baser K.H.C. Volatile compounds from the aerial part and fruits of Grammosciadium Pterocarpum boiss. growing in Turkey. J. Essent. Oil Res. 2015;27:177–181. doi: 10.1080/10412905.2015.1014117. [DOI] [Google Scholar]
- 36.Khalilzadeh M.A., Tajbakhsh M., Gholami F.A., Hosseinzadeh M., Dastoorani P., Norouzi M., Dabiri H.A. Composition of the essential oils of Hippomarathrum Microcarpum (m. Bieb.) b. Fedtsch. And Physospermum Cornubiense (L.) dc. from Iran. J. Essent. Oil Res. 2007;19:567–568. doi: 10.1080/10412905.2007.9699333. [DOI] [Google Scholar]
- 37.Baser K.H.C., Ozek T., Aytac Z. Essential oil of Hippomarathrum Boissieri Reuter et Hausskn. J. Essent. Oil Res. 2000;12:231–232. doi: 10.1080/10412905.2000.9699505. [DOI] [Google Scholar]
- 38.Masoudi S., Ameri N., Rustaiyan A., Moradalizadeh M., Azar P.A. Volatile constituents of three umbelliferae herbs: Azilia Eryngioedes (pau) hedge et lamond, Laser Trilobum (L.) borkh. and Falcaria Falcarioides (bornm. Et wolff) growing wild in Iran. J. Essent. Oil Res. 2005;17:98–100. doi: 10.1080/10412905.2005.9698843. [DOI] [Google Scholar]
- 39.Pino J.A., Fernandes P., Marbot R., Rosado A., Fontinha S.S. Leaf oils of Helichrysum Melaleucum Rchb. Ex Holl, Oenanthe Divaricata (r. Br.) mabb. and Persea Indica (L.) spreng. from Madeira. J. Essent. Oil Res. 2004;16:487–489. doi: 10.1080/10412905.2004.9698778. [DOI] [Google Scholar]
- 40.Zhang H.M., Guo S.S., Fan B., Du S.S., Wang Y.Y., Deng Z.W. Evaluation of efficacy of the essential oil from Ostericum Viridiflorum (turcz.) kitagawa in control of stored product insects. Environ. Sci. Pollut. Res. 2019;26:1406–1413. doi: 10.1007/s11356-018-3728-x. [DOI] [PubMed] [Google Scholar]
- 41.Askari F., Teimouri M., Sefidkon F. Chemical composition and antimicrobial activity of Pimpinella Kotschyana boiss. Oil in Iran. J. Essent. Oil Bear. Plants. 2011;14:124–130. doi: 10.1080/0972060X.2011.10643911. [DOI] [Google Scholar]
- 42.Mazloomifar H., Bigdeli M., Saber-Tehrani M., Rustaiyan A., Masoudi S., Ameri N. Essential oil of Prangos Uloptera dc. From Iran. J. Essent. Oil Res. 2004;16:415–416. doi: 10.1080/10412905.2004.9698758. [DOI] [Google Scholar]
- 43.Shafaghat A. Comparison of chemical composition of essential oil and n-hexane extracts of Zosimia absinthifolia (vent.) link. J. Essent. Oil Bear. Plants. 2011;14:490–493. doi: 10.1080/0972060X.2011.10643606. [DOI] [Google Scholar]
- 44.Maia J.G.S., Zoghbi M.B., Andrade E.H.A., Carreira L.M.M. Volatiles from flowers of Thevetia Peruviana (pers.) k. Schum. and Allamanda cathartics linn. (Apocynaceae) J. Essent. Oil Res. 2000;12:322–324. doi: 10.1080/10412905.2000.9699526. [DOI] [Google Scholar]
- 45.Cornelio M.L., Lago J.H.G., Moreno P.R.H. Volatile oil composition of Aspidosperma Cylindrocarpon muelL. Arg. Leaves. J. Essent. Oil Res. 2005;17:310–311. doi: 10.1080/10412905.2005.9698913. [DOI] [Google Scholar]
- 46.Boligon A.A., Schwanz T.G., Piana M., Bandeira R.V., Frohlich J.K., de Brum T.F., Zadra M., Athayde M.L. Chemical composition and antioxidant activity of the essential oil of Tabernaemontana Catharinensis a. Dc. Leaves. Nat. Prod. Res. 2013;27:68–71. doi: 10.1080/14786419.2011.653971. [DOI] [PubMed] [Google Scholar]
- 47.Sabulal B., George V., Pradeep N.S., Dan M. Volatile oils from the root, stem and leaves of Schefflera Stellata (gaertn.) harms (araliaceae): Chemical characterization and antimicrobial activity. J. Essent. Oil Res. 2008;20:79–82. doi: 10.1080/10412905.2008.9699428. [DOI] [Google Scholar]
- 48.Vila R., Mundina M., Muschietti L., Priestap H.A., Bandoni A.L., Adzet T., Canigueral S. Volatile constituents of leaves, roots and stems from aristolochia elegans. Phytochemistry. 1997;46:1127–1129. doi: 10.1016/S0031-9422(97)00400-7. [DOI] [Google Scholar]
- 49.Su X.D., Gao Y., Xiang Y.X., Lai P.X., Xing X. Chemical composition and biological activities of the essential oil from Aristolochia Fordiana hemsl. Rec. Nat. Prod. 2019;13:346–354. [Google Scholar]
- 50.Simic N., Palic R., Vajs V., Milosavljevic S., Djokovic D. Composition and antibacterial activity of Achillea Asplenifolia essential oil. J. Essent. Oil Res. 2002;14:76–78. doi: 10.1080/10412905.2002.9699770. [DOI] [Google Scholar]
- 51.Rodrigues R.A.F., Queiroga C.L., Rodrigues M.V.N., Foglio M.A., Sartoratto A., Montanari I. Study of the variation of the composition of the essential oil of leaves and flowers of Achyrocline Alata (dc) along a period of the day. J. Essent. Oil Res. 2002;14:280–281. doi: 10.1080/10412905.2002.9699854. [DOI] [Google Scholar]
- 52.Norouzi-Arasi H., Yavari I., Chalabian F., Kiarostami V., Ghaffarzadeh F., Nasirian A. Chemical constituents and antimicrobial activities of the essential oil of Acroptilon Repens (L.) dc. Flavour Frag. J. 2006;21:247–249. doi: 10.1002/ffj.1568. [DOI] [Google Scholar]
- 53.Del-Vechio-Vieira G., Sousa O.V., Yamamoto C.H., Kaplan M.A.C. Chemical composition and antimicrobial activity of the essential oils of Ageratum Fastigiatum (asteraceae) Rec. Nat. Prod. 2009;3:52–57. [Google Scholar]
- 54.Martins A.P., Salgueiro U.R., Goncalves M.J., Vila R., Canigueral S., Tomi F., Casanova J. Essential oil composition and antimicrobial activity of ageratum conyzoides from s. Tome and principe. J. Essent. Oil Res. 2005;17:239–242. doi: 10.1080/10412905.2005.9698888. [DOI] [Google Scholar]
- 55.Javidnia K., Miri R., Kamalinejad M., Sarkarzadeh H., Jamalian A. Chemical composition of the essential oils of anthemis altissima L. Grown in Iran. Flavour Frag. J. 2004;19:213–216. doi: 10.1002/ffj.1277. [DOI] [Google Scholar]
- 56.Juteau F., Masotti V., Viano J., Bessiere J.M. Chemical variation in the oil of Artemisia Verlotiorum lamotte of french origin harvested at a vegetative stage and during flowering. J. Essent. Oil Res. 2005;17:254–256. doi: 10.1080/10412905.2005.9698893. [DOI] [Google Scholar]
- 57.Rana V.S., Juyal A.P., Blazquez M.A., Bodakhe S.H. Essential oil composition of Artemisia Parviflora aerial parts. Flavour Frag. J. 2003;18:342–344. doi: 10.1002/ffj.1239. [DOI] [Google Scholar]
- 58.Haider F., Kumar N., Banerjee S., Naqvi A.A., Bagchi G.D. Effect of altitude on the essential oil constituents of Artemisia Roxburghiana besser var. Purpurascens (jacq.) hook. J. Essent. Oil Res. 2009;21:303–304. doi: 10.1080/10412905.2009.9700177. [DOI] [Google Scholar]
- 59.Cha J.D., Jeong M.R., Jeong S.I., Moon S.E., Kim J.Y., Kil B.S., Song Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia Scoparia and a-capillaris. Planta Med. 2005;71:186–190. doi: 10.1055/s-2005-837790. [DOI] [PubMed] [Google Scholar]
- 60.Manika N., Chanotiya C.S., Darokar M., Singh S., Das Bagchi G. Compositional characters and antimicrobial potential of Artemisia Stricta edgew. F. Stricta pamp. Essential oil. Rec. Nat. Prod. 2016;10:40–46. [Google Scholar]
- 61.Cha J.D., Jeong M.R., Choi H.J., Jeong S., Moon S.E., Yun S., Kim Y.H., Kil B.S., Song Y.H. Chemical composition and antimicrobial activity of the essential oil of Artemisia Lavandulaefolia. Planta Med. 2005;71:575–577. doi: 10.1055/s-2005-864164. [DOI] [PubMed] [Google Scholar]
- 62.Gbolade A.A., Dzamic A., Marin P.D., Ristic M. Essential oil constituents of Aspilia Africana (pers.) c. D. Adams leaf from Nigeria. J. Essent. Oil Res. 2009;21:348–350. doi: 10.1080/10412905.2009.9700188. [DOI] [Google Scholar]
- 63.Zunino M.P., Newton M.N., Maestri D.M., Zygadlo J.A. Essential oils of three baccharis species. Planta Med. 1998;64:86–87. doi: 10.1055/s-2006-957378. [DOI] [PubMed] [Google Scholar]
- 64.Zollo P.H.A., Kuiate J.R., Menut C., Lamaty G., Bessiere J.M., Chalchat J.C., Garry R.P. Aromatic plants of tropical central Africa. Part xx. The occurrence of 1-phenylhepta-1,3,5-triyne in the essential oil of bidens pilosa L. From Cameroon. Flavour Frag. J. 1995;10:97–100. doi: 10.1002/ffj.2730100208. [DOI] [Google Scholar]
- 65.Novakovic J., Rajcevic N., Garcia-Jacas N., Susanna A., Marin P.D., Janackovic P. Capitula essential oil composition of seven centaurea species (sect. Acrocentron, asteraceae)—Taxonomic implication and ecological significance. Biochem. Syst. Ecol. 2019;83:83–90. doi: 10.1016/j.bse.2019.01.010. [DOI] [Google Scholar]
- 66.Yayli N., Yasar A., Albay C., Asamaz Y., Coskuncelebi K., Karaoglu S. Chemical composition and antimicrobial activity of essential oils from Centaurea Appendicigera and Centaurea Helenioides. Pharm. Biol. 2009;47:7–12. doi: 10.1080/13880200802397970. [DOI] [Google Scholar]
- 67.Ogunwande I.A., Olawore N.O., Usman L. Composition of the leaf oil of Centratherum Punctatum cass. Growing in Nigeria. J. Essent. Oil Res. 2005;17:496–498. doi: 10.1080/10412905.2005.9698976. [DOI] [Google Scholar]
- 68.Koba K., Nenonene A.Y., Catherine G., Raynaud C., Chaumont J.P., Sanda K., Laurence N. Chemical composition and cytotoxic activity of essential oil of Chromolaena Odorata L. Growing in Togo. J. Essent. Oil Bear. Plants. 2011;14:423–429. doi: 10.1080/0972060X.2011.10643597. [DOI] [Google Scholar]
- 69.Maia J.G.S., da Silva M.H.L., Zoghbi M.D.B., Andrade E.H.A. Composition of the essential oils of Conyza Bonariensis (L.) cronquist. J. Essent. Oil Res. 2002;14:325–326. doi: 10.1080/10412905.2002.9699871. [DOI] [Google Scholar]
- 70.Boue G.B., Boti J.B., Tonzibo Z.F., Paoli M., Bighelli A. New trans-beta-bergamotene derivatives in the root and the flower essential oils of Cyanthillium Cinereum (L.) h. Rob. From cote d’Ivoire. Nat. Prod. Res. 2019;33:2795–2800. doi: 10.1080/14786419.2018.1502766. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W.J., You C.X., Yang K., Wang Y., Su Y., Geng Z.F., Du S.S., Wang C.F., Deng Z.W., Wang Y.Y. Bioactivity and chemical constituents of the essential oil from Dendranthema Indicum (L.) des moul. Against two stored insects. J. Oleo Sci. 2015;64:553–560. doi: 10.5650/jos.ess14231. [DOI] [PubMed] [Google Scholar]
- 72.Joshi R.K. Volatile constituents of Emilia Sonchifolia from India. Nat. Prod. Commun. 2018;13:1355–1356. doi: 10.1177/1934578X1801301030. [DOI] [Google Scholar]
- 73.Idrissa M., Djibo A.K., Khalid I., Marie B.J. The essential oil of Epaltes Alata (compositae) Flavour Frag. J. 2005;20:203–204. doi: 10.1002/ffj.1395. [DOI] [Google Scholar]
- 74.Pinto A.P.R., Seibert J.B., dos Santos O.D.H., Vieira S.A., do Nascimento A.M. Chemical constituents and allelopathic activity of the essential oil from leaves of Eremanthus Erythropappus. Aust. J. Bot. 2018;66:601–608. doi: 10.1071/BT18138. [DOI] [Google Scholar]
- 75.Rahman A., Hossain M.A., Kang S.C. Control of phytopathogenic fungi by the essential oil and methanolic extracts of Erigeron Ramosus (walt.) bsp. Eur. J. Plant Pathol. 2010;128:211–219. doi: 10.1007/s10658-010-9645-6. [DOI] [Google Scholar]
- 76.Viljoen A.M., Njenga E.W., van Vuuren S.F., Bicchi C., Rubiolo P., Sgorbini B. Essential oil composition and in vitro biological activities of seven namibian species of eriocephalus L. (asteraceae) J. Essent. Oil Res. 2006;18:124–128. doi: 10.1080/10412905.2006.12067133. [DOI] [Google Scholar]
- 77.Gupta D., Charles R., Garg S.N. Chemical composition of the essential oil from the leaves of Eupatorium Triplinerve vahl. J. Essent. Oil Res. 2004;16:473–475. doi: 10.1080/10412905.2004.9698774. [DOI] [Google Scholar]
- 78.Silva M.P., Piazza L.A., Lopez D., Rivilli M.J.L., Turco M.D., Cantero J.J., Tourn M.G., Scopel A.L. Phytotoxic activity in Flourensia Campestris and isolation of (-)-hamanasic acid a as its active principle compound. Phytochemistry. 2012;77:140–148. doi: 10.1016/j.phytochem.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 79.Rabehaja D.J.R., Bezert G., Rakotonandrasana S.R., Ramanoelina P.A.R., Andrianjara C., Bighelli A., Tomi F., Paoli M. Chemical composition of aerial parts essential oils from six endemic Malagasy Helichrysum species. Plants. 2020;9:14. doi: 10.3390/plants9020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bougatsos C., Meyer J.J.M., Magiatis P., Vagias C., Chinou I.B. Composition and antimicrobial activity of the essential oils of Helichrysum Kraussii sch. Bip. And h-rugulosum less. From South Africa. Flavour Frag. J. 2003;18:48–51. doi: 10.1002/ffj.1152. [DOI] [Google Scholar]
- 81.Pino J.A., Marbot R., Payo A., Chao D., Herrera P., Marti M.P. Leaf oil of Koanophyllon Villosum (sw.) king et robins. J. Essent. Oil Res. 2005;17:427–428. doi: 10.1080/10412905.2005.9698951. [DOI] [Google Scholar]
- 82.Kuiate J.R., Bessiere J.M., Zollo P.H.A. Composition of the essential oils from three laggera spp. From Cameroon. Flavour Frag. J. 2002;17:105–108. doi: 10.1002/ffj.1057. [DOI] [Google Scholar]
- 83.Mwangi J.W., Thoithi G.N., Juliani H.R., Zygadlo J.A. Composition of the essential oil of Microglossa Pyrrhopappa (a. Rich) agnew var. Pyrrhopappa from Kenya. J. Essent. Oil Res. 2001;13:229–230. doi: 10.1080/10412905.2001.9699677. [DOI] [Google Scholar]
- 84.Pelissier Y., Marion C., Kone D., Brunel J.F., Fofana H., Bessiere J.M. Volatile constituents of the leaves of Mikania Cordata (burm.F.) b.L. Robinson var. Cordata (asteraceae) J. Essent. Oil Res. 2001;13:31–32. doi: 10.1080/10412905.2001.9699596. [DOI] [Google Scholar]
- 85.Villarreal S., Solorzano M., Velasco J., Diaz T., Rojas L.B., Usubillaga A., Ramirez-Gonzalez I. Composition and in vitro antibacterial activity of essential oil of Oyedaea Verbesinoides dc from Venezuela. J. Essent. Oil Bear. Plants. 2008;11:643–648. doi: 10.1080/0972060X.2008.10643681. [DOI] [Google Scholar]
- 86.Ciccio J.F., Chaverri C. Chemical composition of the leaf and branch oils of Perymenium Grande hemsl. Var. Nelsonii (robins. & greenm.) fay (asteraceae-heliantheae) from Costa Rica. Rec. Nat. Prod. 2012;6:371–375. [Google Scholar]
- 87.Miyazawa M., Teranishi A., Ishikawa Y. Components of the essential oil from Petasites Japonicus. Flavour Frag. J. 2003;18:231–233. doi: 10.1002/ffj.1203. [DOI] [Google Scholar]
- 88.Kerdudo A., Gonnot V., Ellong E.N., Boyer L., Chandre F., Adenet S., Rochefort K., Michel T., Fernandez X. Composition and bioactivity of Pluchea Carolinensis (jack.) g. Essential oil from Martinique. Ind. Crop Prod. 2016;89:295–302. doi: 10.1016/j.indcrop.2016.04.076. [DOI] [Google Scholar]
- 89.Labuckas D.O., Zygadlo J.A., Espinar L.A. Constituents of the volatile oil of Porophyllum Obscurum (spreng.) dc. Flavour Frag. J. 1999;14:107–108. doi: 10.1002/(SICI)1099-1026(199903/04)14:2<107::AID-FFJ799>3.0.CO;2-E. [DOI] [Google Scholar]
- 90.Zhu X.W., Zhang X.H., Chen J.H., Zhu X.W., Tan J.C., Chen H.X., Wan F.H. Chemical composition of leaf essential oil from Solidago Decurrens lour. J. Essent. Oil Res. 2009;21:354–356. doi: 10.1080/10412905.2009.9700190. [DOI] [Google Scholar]
- 91.Szarka S., Hethelyi E., Lemberkovics E., Kuzovkina I.N., Banyai P., Szoke E. Gc and gc-ms studies on the essential oil and thiophenes from Tagetes Patula L. Chromatographia. 2006;63:S67–S73. doi: 10.1365/s10337-006-0729-6. [DOI] [Google Scholar]
- 92.Sefidkon F., Salehyar S., Mirza M., Dabiri M. The essential oil of Tagetes Erecta L. Occurring in Iran. Flavour Frag. J. 2004;19:579–581. doi: 10.1002/ffj.1360. [DOI] [Google Scholar]
- 93.Shafaghat A. Antibacterial activity and sesquiterpenoid contents of the essential oil of Tanacetum Punctatum (desr.) grierson. J. Essent. Oil Bear. Plants. 2012;15:270–275. doi: 10.1080/0972060X.2012.10644046. [DOI] [Google Scholar]
- 94.Nanyonga S.K., Opoku A.R., Lewu F.B., Oyedeji A.O. The chemical composition, larvicidal and antibacterial activities of the essential oil of Tarchonanthus Trilobus var galpinii. J. Essent. Oil Bear. Plants. 2013;16:524–530. doi: 10.1080/0972060X.2013.831572. [DOI] [Google Scholar]
- 95.Sobrinho A.C.N., dos Santos H.S., de Morais S.M., Cavalcante C.S.D., de Souza E.B., de Sousa H.A., Albuquerque M., Fontenelle R.O.D. Antifungal and antioxidant activities of Vernonia Chalybaea mart. Ex dc. Essential oil and their major constituent beta-caryophyllene. Braz. Arch. Biol. Technol. 2020;63:11. [Google Scholar]
- 96.Albuquerque M., Lemos T.L.G., Pessoa O.D.L., Nunes E.P., Nascimento R.F., Silveira E.R. Chemical composition of the essential oil from Vernonia Scorpioides (asteraceae) Flavour Frag. J. 2007;22:249–250. doi: 10.1002/ffj.1782. [DOI] [Google Scholar]
- 97.Parveen Z., Mazhar S., Siddique S., Manzoor A., Ali Z. Chemical composition and antifungal activity of essential oil from Xanthium Strumarium L. Leaves. Indian J. Pharm. Sci. 2017;79:316. doi: 10.4172/pharmaceutical-sciences.1000232. [DOI] [Google Scholar]
- 98.Uquiche E.L., Toro M.T., Quevedo R.A. Supercritical extraction with carbon dioxide and co-solvent from Leptocarpha Rivularis. J. Appl. Res. Med. Aromat. Plants. 2019;14:8. doi: 10.1016/j.jarmap.2019.100210. [DOI] [Google Scholar]
- 99.Brophy J.J., Forster P.I., Goldsack R.J. Characterization of essential oils from the leaves of the genus daphnandra (atherospermataceae) J. Essent. Oil Res. 2016;28:339–347. doi: 10.1080/10412905.2016.1161565. [DOI] [Google Scholar]
- 100.Diniz J.C., Viana F.A., de Oliveira O.F., Silveira E.R., Pessoa O.D.L. Chemical composition of the leaf essential oil of Cordia Leucocephala moric from north-east of Brazil. J. Essent. Oil Res. 2008;20:495–496. doi: 10.1080/10412905.2008.9700068. [DOI] [Google Scholar]
- 101.Das Gracas M., Zoghbi B., Andrade E.H.A., Pereira R.A., Oliveira J. Volatiles of the Cordia Multispicata cham.: A weed medicinal Brazilian plant. J. Essent. Oil Res. 2010;22:543–545. doi: 10.1080/10412905.2010.9700395. [DOI] [Google Scholar]
- 102.Junor G.A.O., Porter R.B.R., Yee T.H., Waugh T. The volatile constituents from the leaves, bark and fruits of Bursera Aromatica (proctor) found in Jamaica. J. Essent. Oil Res. 2010;22:19–22. doi: 10.1080/10412905.2010.9700257. [DOI] [Google Scholar]
- 103.Tucker A.O., Maciarello M.J., Brown R.C., Landrum L.R., Lafferty D. Essential oils from the oleo-gum-resins of elephant tree or torote (Bursera Microphylla a. Gray, burseraceae) from Arizona. J. Essent. Oil Res. 2009;21:57–58. doi: 10.1080/10412905.2009.9700109. [DOI] [Google Scholar]
- 104.Thang T.D., Dai D.N., Luong N.X., Ogunwande I.A. Constituents of essential oils from the leaves, stem barks and resins of Canarium Parvum leen., and Canarium Tramdenanum dai et yakovl. (burseracea) grown in vietnam. Nat. Prod. Res. 2014;28:461–466. doi: 10.1080/14786419.2013.873435. [DOI] [PubMed] [Google Scholar]
- 105.Onocha P.A., Ekundayo O., Oyelola O., Laakso I. Essential oils of Dacryodes Edulis (g.Don) h. J. Lam (african pear) Flavour Frag. J. 1999;14:135–139. doi: 10.1002/(SICI)1099-1026(199903/04)14:2<135::AID-FFJ788>3.0.CO;2-L. [DOI] [Google Scholar]
- 106.Bandeira P.N., Machado M.I.L., Cavalcanti F.S., Lemos T.L.G. Essential oil composition of leaves, fruits and resin of Protium Heptaphyllum (aubl.) march. J. Essent. Oil Res. 2001;13:33–34. doi: 10.1080/10412905.2001.9699597. [DOI] [Google Scholar]
- 107.Novak J., Franz C. Composition of the essential oils and extracts of two populations of Cannabis Sativa L. Ssp spontanea from Austria. J. Essent. Oil Res. 2003;15:158–160. doi: 10.1080/10412905.2003.9712100. [DOI] [Google Scholar]
- 108.Benelli G., Pavela R., Petrelli R., Cappellacci L., Santini G., Fiorini D., Sut S., Dall’Acqua S., Canale A., Maggi F. The essential oil from industrial hemp (Cannabis Sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crop Prod. 2018;122:308–315. doi: 10.1016/j.indcrop.2018.05.032. [DOI] [Google Scholar]
- 109.Goncalves J., Figueira J., Rodrigues F., Camara J.S. Headspace solid-phase microextraction combined with mass spectrometry as a powerful analytical tool for profiling the terpenoid metabolomic pattern of hop-essential oil derived from saaz variety. J. Sep. Sci. 2012;35:2282–2296. doi: 10.1002/jssc.201200244. [DOI] [PubMed] [Google Scholar]
- 110.Poralijan V., Rad A.S. Extraction of eugenol from carnation: A quantitative and qualitative analysis by aqueous and ethanolic solvents. J. Essent. Oil Bear. Plants. 2016;19:1495–1502. doi: 10.1080/0972060X.2016.1211962. [DOI] [Google Scholar]
- 111.Mendiratta A., Dayal R., Bartley J.R. Gc/ms analysis of essential oils of needles and twigs of Cephalotaxus Harringtonia (knight ex forbes) koch var. Harringtonia. J. Essent. Oil Res. 2005;17:308–309. doi: 10.1080/10412905.2005.9698912. [DOI] [Google Scholar]
- 112.De Oliveira J.C.S., Neves I.A., da Camara C.A.G., Schwartz M.O.E. Volatile constituents of the fruits of Clusia Nemorasa G. Mey. From different region of Atlantic coast restingas of pernambuco (northeast of Brazil) J. Essent. Oil Res. 2008;20:219–222. doi: 10.1080/10412905.2008.9699996. [DOI] [Google Scholar]
- 113.Tan W.N., Wong K.C., Khairuddean M., Eldeen I.M., Asmawi M.Z., Sulaiman B. Volatile constituents of the fruit of Garcinia Atroviridis and their antibacterial and anti-inflammatory activities. Flavour Frag. J. 2013;28:2–9. doi: 10.1002/ffj.3118. [DOI] [Google Scholar]
- 114.Andrade M.S., Sampaio T.S., Nogueira P.C.L., Ribeiro A.S., Bittrich V., Amaral M.D.E. Volatile compounds of the leaves, flowers and fruits of Kielmeyera Rugosa choisy (clusiaceae) Flavour Frag. J. 2007;22:49–52. doi: 10.1002/ffj.1751. [DOI] [Google Scholar]
- 115.Alitonou G., Avlessi F., Sohounhloue D.C.K., Bessiere J.M., Menut C. Chemical and biological investigation on volatile constituents of Pentadesma Butyracea sabine (clusiaceae) from Benin. J. Essent. Oil Res. 2010;22:138–140. doi: 10.1080/10412905.2010.9700285. [DOI] [Google Scholar]
- 116.Zubair M.F., Oladosu I.A., Olawore N.O. Chemical composition of the leaf oil of Psorospermum Corymbiferum hochr. Growing in Africa. J. Essent. Oil Res. 2010;22:529–530. doi: 10.1080/10412905.2010.9700390. [DOI] [Google Scholar]
- 117.Dehghan H., Sarrafi Y., Salehi P. Chemical composition of the essential oil of Convolvulus Persicus L. J. Essent. Oil Bear. Plants. 2015;18:592–595. doi: 10.1080/0972060X.2014.986539. [DOI] [Google Scholar]
- 118.Boudarene L., Rahim L., Baaliouamer A., Meklati B.Y. Analysis of algerian essential oils from twigs, needles and wood of Cedrus Atlantica g. Manetti by gc/ms. J. Essent. Oil Res. 2004;16:531–534. doi: 10.1080/10412905.2004.9698790. [DOI] [Google Scholar]
- 119.Tort N.S., Demiray H., Guvensen A., Dereboylu A.E. Chemical composition of essential oils of berries of Juniper Us Macrocarpa sibth. & sm. From Turkey. Bangladesh J. Bot. 2019;48:339–343. [Google Scholar]
- 120.Elsharkawy E.R., Aljohar H., Donia A. Comparative study of antioxidant and anticancer activity of Thuja Orientalis growing in Egypt and Saudi Arabia. Br. J. Pharm. Res. 2017;15:9. doi: 10.9734/BJPR/2017/32387. [DOI] [Google Scholar]
- 121.Lazarevic J., Radulovic N., Palic R., Zlatkovic B. Chemical composition of the essential oil of Cyperus Glomeratus L. (cyperaceae) from Serbia. J. Essent. Oil Res. 2010;22:578–581. doi: 10.1080/10412905.2010.9700404. [DOI] [Google Scholar]
- 122.Queiroz T.B., da Fonseca F.S.A., Mendes A.D.R., Azevedo A.M., Martins E.R. Chemical diversity of accessions of the in vivo germplasm bank of Varronia Curassavica (jacq.) Acta Sci. Agron. 2020;42:11. doi: 10.4025/actasciagron.v42i1.42726. [DOI] [Google Scholar]
- 123.Scotto C.I., Burger P., el Khil M.K., Ginouves M., Prevot G., Blanchet D., Delprete P.G., Fernandez X. Chemical composition and antifungal activity of the essential oil of Varronia Schomburgkii (dc.) borhidi (cordiaceae) from plants cultivated in French Guiana. J. Essent. Oil Res. 2017;29:304–312. doi: 10.1080/10412905.2017.1278729. [DOI] [Google Scholar]
- 124.Deepaa C.V., Chalchat J.C., John J.A. Chemical composition of the essential oil from the leaves of Acalypha Fruticosa. J. Essent. Oil Bear. Plants. 2012;15:609–613. doi: 10.1080/0972060X.2012.10644095. [DOI] [Google Scholar]
- 125.Nguyen A.D., Tran D.T., Hong V., Nguyen X.D. Volatile constituents of the leaf oil of Alchornea Tiliifolia (benth.) muell. (family euphorbiaceae) from Vietnam. J. Essent. Oil Res. 2009;21:1–2. [Google Scholar]
- 126.Da Camara C.A.G., de Moraes M.M., de Melo J.P.R., da Silva M.M.C. Chemical composition and acaricidal activity of essential oils from Croton Rhamnifolioides pax and hoffm. In different regions of a caatinga biome in northeastern Brazil. J. Essent. Oil Bear. Plants. 2017;20:1434–1449. doi: 10.1080/0972060X.2017.1416677. [DOI] [Google Scholar]
- 127.De Oliveira L.F., Damasceno C.S., Campos R., de Souza A.M., Mendes G., Dias J.D.G., Miguel O.G., Miguel M.D. Chemical composition of the volatile oil of Croton Glandulosus linnaeus and its allelopathic activity. Nat. Prod. Res. 2020;4:1–4. doi: 10.1080/14786419.2020.1727468. [DOI] [PubMed] [Google Scholar]
- 128.Doria G.A.A., Silva W.J., Carvalho G.A., Alves P.B., Cavalcanti S.C.H. A study of the larvicidal activity of two croton species from northeastern Brazil against Aedes Aegypti. Pharm. Biol. 2010;48:615–620. doi: 10.3109/13880200903222952. [DOI] [PubMed] [Google Scholar]
- 129.Eresanya O.I., Avoseh O.N., Ogunwande I.A., Lawal O.A., Giwa-Ajeniya A.F. Chemical constituents of essential oil from the leaves of Phyllanthus Muellerianus (o. Kuntze) exell. J. Essent. Oil Bear. Plants. 2019;22:865–870. doi: 10.1080/0972060X.2019.1623721. [DOI] [Google Scholar]
- 130.Da Silva K.L.C., da Silva M.M.C., de Moraes M.M., da Camara C.A.G., Santos M.L., Fagg C.W. Chemical composition and acaricidal activity of essential oils from two species of the genus bauhinia that occur in the cerrado biome in Brazil. J. Essent. Oil Res. 2020;32:93–101. doi: 10.1080/10412905.2019.1662338. [DOI] [Google Scholar]
- 131.Rodrigues M.O., Alves P.B., Nogueira P.C.L., Machado S.M.F., Moraes V.R.S., Ribeiro A.D., Silva E.S., Feitosa J.G.R. Volatile constituents and antibacterial activity from seeds of Bowdichia Virgilioides kunt. J. Essent. Oil Res. 2009;21:286–288. doi: 10.1080/10412905.2009.9700172. [DOI] [Google Scholar]
- 132.Miyazawa M., Nagata T., Nakahashi H., Takahashi T. Characteristic odor components of essential oil from Caesalpinia Decapetala. J. Essent. Oil Res. 2012;24:441–446. doi: 10.1080/10412905.2012.703475. [DOI] [Google Scholar]
- 133.De Oliveira L.G.S., Ribeiro D.A., Saraiva M.E., de Macedo D.G., Macedo J.G.F., Pinheiro P.G., da Costa J.G.M., Souza M.M.D., de Menezes I.R.A. Chemical variability of essential oils of Copaifera Langsdorffii desf. In different phenological phases on a savannah in the northeast, Ceara, Brazil. Ind. Crop Prod. 2017;97:455–464. doi: 10.1016/j.indcrop.2016.12.031. [DOI] [Google Scholar]
- 134.Veiga V.F., Rosas E.C., Carvalho M.V., Henriques M., Pinto A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera Cearensis Huber Ex Ducke, Copaifera Reticulata ducke and Copaifera Multijuga hayne—A comparative study. J. Ethnopharmacol. 2007;112:248–254. doi: 10.1016/j.jep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Zoghbi M.D.B., Andrade E.H.A., Martins-da-Silva R.C.V., Trigo J.R. Chemical variation in the volatiles of Copaifera Reticulata ducke (leguminosae) growing wild in the states of para and amapa, Brazil. J. Essent. Oil Res. 2009;21:501–503. doi: 10.1080/10412905.2009.9700228. [DOI] [Google Scholar]
- 136.Munoz-Acevedo A., Gonzalez M.D., Stashenko E.E. Volatile fractions and essential oils of the leaves and branches of Dalea Carthagenensis (jacq.) jf macbr. From northern region of Colombia. J. Essent. Oil Bear. Plants. 2019;22:774–788. doi: 10.1080/0972060X.2019.1623720. [DOI] [Google Scholar]
- 137.Leandro L.M., Da Veiga V.F., Sales A.P.B., Pessoa C.D. Chemical composition and cytotoxic activity of essential oils from the leaves and stems of Eperua Duckeana cowan. Boletin Latinoamericano Y Del Caribe de Plantas. 2015;14:42–47. [Google Scholar]
- 138.Shakeri A., Akhtari J., Soheili V., Taghizadeh S.F., Sahebkar A., Shaddel R., Asili J. Identification and biological activity of the volatile compounds of Glycyrrhiza Triphylla fisch & camey. Microb. Pathog. 2017;109:39–44. doi: 10.1016/j.micpath.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 139.Bertoli A., Menichini F., Noccioli C., Morelli L., Pistelli L. Volatile constituents of different organs of Psoralea bituminosa L. Flavour Frag. J. 2004;19:166–171. doi: 10.1002/ffj.1315. [DOI] [Google Scholar]
- 140.Mwangi J.W., Thoithi G.N., Kibwage I.O., Demo M.S., Oliva M.M., Zunino M.R., Zygadlo J.A. Essential oil of Rynchosia Minima dc. From kenya: Composition and antibacterial properties. J. Essent. Oil Res. 2005;17:230–231. doi: 10.1080/10412905.2005.9698885. [DOI] [Google Scholar]
- 141.Stefanello M.E.A., Wisniewski A., Simionatto E.L., Cervi A.C. Essential oil composition of Casearia Decandra jacq. J. Essent. Oil Res. 2010;22:157. doi: 10.1080/10412905.2010.9700291. [DOI] [Google Scholar]
- 142.Sousa F.G., Schneider N.F.Z., Mendes C.E., de Moura N.F., Denardin R.B.N., Matuo R., Mantovani M.S. Clastogenic and anticlastogenic effect of the essential oil from Casearia Sylvestris swart. J. Essent. Oil Res. 2007;19:376–378. doi: 10.1080/10412905.2007.9699309. [DOI] [Google Scholar]
- 143.Tewari K., Pande C., Tewari G., Kharkwal G.C., Punetha D. Volatile constituents of Geranium Wallichianum d. Don ex sweet. From north-western Himalayan region. J. Indian Chem. Soc. 2015;92:123–125. [Google Scholar]
- 144.Scramin S., Saito M.L., Pott A., Marques M.O.M. Essential oil of Elyonurus Muticus (sprengel) o.Kuntze (gramineae) J. Essent. Oil Res. 2000;12:298–300. doi: 10.1080/10412905.2000.9699520. [DOI] [Google Scholar]
- 145.Kimani S.M., Chhabra S.C., Lwande W., Khan Z.R., Hassanali A., Pickett J.A. Airborne volatiles from Melinis Minutiflora p. Beauv., a non-host plant of the spotted stem borer. J. Essent. Oil Res. 2000;12:221–224. doi: 10.1080/10412905.2000.9699502. [DOI] [Google Scholar]
- 146.Brophy J.J., Goldsack R.J., Forster P.I. Leaf essential oils of the australian species of gyrocarpus and hernandia (hernandiaceae) J. Essent. Oil Res. 2000;12:717–722. doi: 10.1080/10412905.2000.9712199. [DOI] [Google Scholar]
- 147.Abreu L.N., Reis M.G., Marsaioli A.J., Mazzafera P. Essential oil composition of Hypericum Brasiliense choise. Flavour Frag. J. 2004;19:80–82. doi: 10.1002/ffj.1319. [DOI] [Google Scholar]
- 148.Ghiasvand A., Shadabi S., Hajipour S., Nasirian A., Borzouei M., Hassani-Moghadam E., Hashemi P. Comparison of ultrasound-assisted headspace solid-phase microextraction and hydrodistillation for the identification of major constituents in two species of hypericum. J. Chromatogr. Sci. 2016;54:264–270. doi: 10.1093/chromsci/bmv136. [DOI] [PubMed] [Google Scholar]
- 149.Buitrago A., Rojas L.B., Rojas J., Buitrago D., Usubillaga A., Morales A. Comparative study of the chemical composition of the essential oil of Vismia Baccifera var. Dealbata (guttiferae) collected in two different locations in Merida-Venezuala. J. Essent. Oil Bear. Plants. 2009;12:651–655. doi: 10.1080/0972060X.2009.10643769. [DOI] [Google Scholar]
- 150.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. And its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 151.Luciano J.H.S., Barros M.C.P., Lima M.A.S., do Nascimento R.F., Silveira E.R. Volatile composition of leaves from Aegiphila Lhotzkiana cham. Flavour Frag. J. 2005;20:537–538. doi: 10.1002/ffj.1465. [DOI] [Google Scholar]
- 152.Singh P., Prakash O., Pant A.K. Essential oil composition of Ajuga parviflora benth. Growing in western Himalayan region of Uttarakhand (India) J. Essent. Oil Bear. Plants. 2015;18:697–701. doi: 10.1080/0972060X.2014.890074. [DOI] [Google Scholar]
- 153.Karami A. Essential oil composition of Ajuga comata stapf. From southern Zagros, Iran. Nat. Prod. Res. 2017;31:359–361. doi: 10.1080/14786419.2016.1236094. [DOI] [PubMed] [Google Scholar]
- 154.Sebaa N.A., Zatla A.T., Dib M.E.A., Tabti B., Costa J., Muselli A. Antifungal activity of essential oil and hydrosol extract of Ballota nigra L. and their protective effects against the black rot of tomatoes. Curr. Nutr. Food Sci. 2019;15:662–671. doi: 10.2174/1573401314666180515114935. [DOI] [Google Scholar]
- 155.Ogundajo A.L., Owoyele O.A., Ogunwande I.A., Owolabi M.S. Chemical composition of essential oil from the leaves of Clerodendrum polycephalum baker growing in Nigeria. J. Essent. Oil Bear. Plants. 2016;19:119–124. doi: 10.1080/0972060X.2014.905759. [DOI] [Google Scholar]
- 156.Bhaft R., Padalia R.C., Pande C. Chemical composition of the essential oil of Colquhounia coccinea Wall. J. Essent. Oil Res. 2009;21:74–75. [Google Scholar]
- 157.Agostini G., Souza-Chies T.T., Agostini F., Atti-Serafini L., Echeverrigaray S. Essential oil composition of Cunila incana benth. (lamiaceae) J. Essent. Oil Res. 2010;22:432–434. doi: 10.1080/10412905.2010.9700365. [DOI] [Google Scholar]
- 158.Nori-Shargh D., Baharvand B. Volatile constituents analysis of Cyclotrichium strussii bornm. From Iran. J. Essent. Oil Res. 2006;18:261–262. doi: 10.1080/10412905.2006.9699081. [DOI] [Google Scholar]
- 159.Judzentiene A., Stoncius A., Budiene J. Chemical composition of the essential oils from Glechoma hederacea plants grown under controlled environmental conditions in Lithuania. J. Essent. Oil Res. 2015;27:454–458. doi: 10.1080/10412905.2015.1039663. [DOI] [Google Scholar]
- 160.Venturi C.R., Danielli L.J., Klein F., Apel M.A., Montanha J.A., Bordignon S.A.L., Roehe P.M., Fuentefria A.M., Henriques A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015;53:682–688. doi: 10.3109/13880209.2014.936944. [DOI] [PubMed] [Google Scholar]
- 161.Tonzibo Z.F., Coffy A.A., Chalachat J.C., N’Guessan Y.T. Chemical composition of essential oils of hoslundia opposita vahl. From Ivory Coast. Flavour Frag. J. 2006;21:789–791. doi: 10.1002/ffj.1715. [DOI] [Google Scholar]
- 162.Firouznia A., Rustaiyan A., Nadimi M., Masoudi S., Bigdeli M. Composition of the essential oil of Hymenocrater calycinus (boiss.) benth. From Iran. J. Essent. Oil Res. 2005;17:527–529. doi: 10.1080/10412905.2005.9698984. [DOI] [Google Scholar]
- 163.Fiuza T.S., Saboia-Morais S.M.T., Paula J.R., Bara M.T.F., Tresvenzol L.M.F., Ferreira H.D., Ferri P.H. Composition and chemical variability in the essential oils of Hyptidendron canum (pohl ex benth.) harley. J. Essent. Oil Res. 2010;22:159–163. doi: 10.1080/10412905.2010.9700292. [DOI] [Google Scholar]
- 164.Dambolena J.S., Zunino M.P., Lucini E.I., Zygadlo J.A., Rotman A., Ahumada O., Biurrun F. Essential oils of plants used in home medicine in north of Argentina. J. Essent. Oil Res. 2009;21:405–409. doi: 10.1080/10412905.2009.9700204. [DOI] [Google Scholar]
- 165.Kossouoh C., Moudachirou M., Adjakidje V., Chalchat J.C., Figueredo G. A comparative study of the chemical composition of the leaves and fruits deriving the essential oil of Hyptis suaveolens (L.) poit. From Benin. J. Essent. Oil Res. 2010;22:507–509. doi: 10.1080/10412905.2010.9700384. [DOI] [Google Scholar]
- 166.Yuce E., Bagci E. Study of the essential oil composition of Lallenmantia iberica (m. Bieb.) fisch and ca mey. (lamiaceae) from Turkey. Asian J. Chem. 2012;24:4817–4818. [Google Scholar]
- 167.Oyedeji O.A., Afolayan A. Comparative study of the essential oil composition and antimicrobial activity of leonotis leonurus and l-ocymifolia in the eastern Cape, South Africa. S. Afr. J. Bot. 2005;71:114–116. doi: 10.1016/S0254-6299(15)30160-5. [DOI] [Google Scholar]
- 168.Joshi R.K. Leucas aspera (willd.) link essential oil from India: Beta-caryophyllene and 1-octen-3-ol chemotypes. J. Chromatogr. Sci. 2016;54:295–298. doi: 10.1093/chromsci/bmv173. [DOI] [PubMed] [Google Scholar]
- 169.Joshi R.K. Gc/ms analysis of the essential oil of leucas indica from India. Nat. Prod. Commun. 2014;9:1607–1608. doi: 10.1177/1934578X1400901119. [DOI] [PubMed] [Google Scholar]
- 170.Demirci B., Baser K.H.C., Kirimer N. Composition of the essential oil of Marrubium bourgaei ssp caricum p.H. Davis. J. Essent. Oil Res. 2004;16:133–134. doi: 10.1080/10412905.2004.9698674. [DOI] [Google Scholar]
- 171.Matos F.J.D., Machado M.I.L., Craveiro A.A., Alencar J.W., Meneses F.D. Essential oil composition of Marsypianthes chamaedrys (vahl) kuntze grown in northeast Brazil. J. Essent. Oil Res. 2001;13:45–46. doi: 10.1080/10412905.2001.9699601. [DOI] [Google Scholar]
- 172.Miceli A., Negro C., Tommasi L. Essential oil of Melissa romana (miller) grown in southern Apulia (Italy) J. Essent. Oil Res. 2006;18:473–475. doi: 10.1080/10412905.2006.9699144. [DOI] [Google Scholar]
- 173.Nori-Shargh D., Norouzi-Arasi H., Mohammadi S., Mirza M., Jaimand K. Volatile component of Mentha longifolia (L.) huds. From Iran. J. Essent. Oil Res. 2000;12:111–112. doi: 10.1080/10412905.2000.9712056. [DOI] [Google Scholar]
- 174.Sarikurkcu C., Ceylan O., Zeljkovic S.C. Micromeria myrtifolia: Essential oil composition and biological activity. Nat. Prod. Commun. 2019;14:3. doi: 10.1177/1934578X19851687. [DOI] [Google Scholar]
- 175.Chen X.B., Chen R., Luo Z.R. Chemical composition and insecticidal properties of essential oil from aerial parts of mosla soochowensis against two grain storage insects. Trop. J. Pharm. Res. 2017;16:905–910. doi: 10.4314/tjpr.v16i4.23. [DOI] [Google Scholar]
- 176.Talebi S.M., Nohooji M.G., Yarmohammadi M., Khani M., Matsyura A. Effect of altitude on essential oil composition and on glandular trichome density in three nepeta species (N. Sessilifolia, N. Heliotropifolia and N. Fissa) Mediterr. Bot. 2019;40:81–93. doi: 10.5209/MBOT.59730. [DOI] [Google Scholar]
- 177.Senatore F., Arnold N.A., Piozzi F. Composition of the essential oil of nepeta curviflora boiss. (lamiaceae) from Lebanon. J. Essent. Oil Res. 2005;17:268–270. doi: 10.1080/10412905.2005.9698898. [DOI] [Google Scholar]
- 178.Raina A.P., Kumar A., Dutta M. Chemical characterization of aroma compounds in essential oil isolated from “holy basil” (Ocimum tenuiflorum L.) grown in India. Genet. Resour. Crop Evol. 2013;60:1727–1735. doi: 10.1007/s10722-013-9981-4. [DOI] [Google Scholar]
- 179.Brada M., Saadi A., Wathelet J.P., Lognay G. The essential oils of Origanum majorana L. and Origanum floribundum munby in Algeria. J. Essent. Oil Bear. Plants. 2012;15:497–502. doi: 10.1080/0972060X.2012.10644078. [DOI] [Google Scholar]
- 180.Van Hac L., Luong N.X., Dung N.X., Klinkby N., Leclercq P.A. Volatile constituents of the essential oil of orthodon dianthera maxim. (syn. Mosla dianthera maxim.) from Vietnam. J. Essent. Oil Res. 2001;13:18–20. doi: 10.1080/10412905.2001.9699591. [DOI] [Google Scholar]
- 181.Joshi R.K. Gc-ms analysis of the volatile constituents of orthosiphon pallidus royle, ex benth. Nat. Prod. Res. 2020;34:441–444. doi: 10.1080/14786419.2018.1534849. [DOI] [PubMed] [Google Scholar]
- 182.Ghimire B.K., Yoo J.H., Yu C.Y., Kim S.H., Chung I.M. Profiling volatile and phenolic compound composition and characterization of the morphological and biological activities of perilla frutescence britton var. Japonica accessions. Acta Physiol. Plant. 2019;41:16. doi: 10.1007/s11738-019-2890-1. [DOI] [Google Scholar]
- 183.Amor I.L.B., Neffati A., Ben Sgaier M., Bhouri W., Boubaker J., Skandrani I., Bouhlel I., Kilani S., Ben Ammar R., Chraief I., et al. Antimicrobial activity of essential oils isolated from phlomis crinita cav. Ssp mauritanica munby. J. Am. Oil Chem. Soc. 2008;85:845–849. doi: 10.1007/s11746-008-1272-4. [DOI] [Google Scholar]
- 184.Demirci B., Baser K.H.C., Dadandi M.Y. Composition of the essential oils of phlomis rigida labill. and Phlomis samia L. J. Essent. Oil Res. 2006;18:328–331. doi: 10.1080/10412905.2006.9699103. [DOI] [Google Scholar]
- 185.Tennakoon T., Abeysekera A.M., de Silva K.T.D., Padumadasa C., Wijesundara D.S.A. Essential oil composition of Platostoma menthoides (L.) a. J. Paton whole plant. J. Essent. Oil Bear. Plants. 2016;19:1516–1520. doi: 10.1080/0972060X.2016.1224688. [DOI] [Google Scholar]
- 186.Tiwari A., Padalia R.C., Mathela C.S. Sesquiterpene rich essential oil from Plectranthus Rugosus wall. J. Essent. Oil Bear. Plants. 2008;11:58–61. doi: 10.1080/0972060X.2008.10643598. [DOI] [Google Scholar]
- 187.Hussien J., Hymete A., Rohloff J. Volatile constituents and biological activities of Pycnostachys Abyssinica and Pycnostachys Eminii extracts. Pharm. Biol. 2010;48:1384–1391. doi: 10.3109/13880209.2010.486406. [DOI] [PubMed] [Google Scholar]
- 188.Apostolides N.A., El Beyrouthy M., Dhifi W., Najm S., Cazier F., Najem W., Labaki M., AbouKais A. Chemical composition of aerial parts of Rosmarinus Officinalis L. Essential oil growing wild in Lebanon. J. Essent. Oil Bear. Plants. 2013;16:274–282. doi: 10.1080/0972060X.2013.764189. [DOI] [Google Scholar]
- 189.Garcia-Rojas A., Fontecha-Garcia J., Peralta-Bohorquez A.F., Quijano-Celis C.E., Morales G., Pino J.A. Composition of the essential oil from leaves and fruits of Salvia Palaefolia kunth grown in Colombia. J. Essent. Oil Res. 2010;22:369–370. doi: 10.1080/10412905.2010.9700348. [DOI] [Google Scholar]
- 190.Sefidkon F., Hooshidary R., Jamzad Z. Chemical variation in the essential oil of Salvia Bracteata banks & soland from Iran. J. Essent. Oil Bear. Plants. 2007;10:265–272. [Google Scholar]
- 191.Barazandeh M.M. Volatile constituents of the oil of Salvia Hydrangea dc. Ex benth. From Iran. J. Essent. Oil Res. 2004;16:20–21. doi: 10.1080/10412905.2004.9698639. [DOI] [Google Scholar]
- 192.Mirza M., Sefidkon F. Essential oil composition of two salvia species from Iran, Salvia Nemorosa L. and Salvia Reuterana boiss. Flavour Frag. J. 1999;14:230–232. doi: 10.1002/(SICI)1099-1026(199907/08)14:4<230::AID-FFJ816>3.0.CO;2-L. [DOI] [Google Scholar]
- 193.Sefidkon F., Mirza M. Chemical composition of the essential oils of two salvia species from Iran, Salvia Virgata jacq. and Salvia Syriaca L. Flavour Frag. J. 1999;14:45–46. doi: 10.1002/(SICI)1099-1026(199901/02)14:1<45::AID-FFJ774>3.0.CO;2-S. [DOI] [Google Scholar]
- 194.Vallejo M.C.G., Moujir L., Burillo J., Guerra L.L., Gonzalez M., Penate R.D., San Andres L., Luis J.G., Blanco F.L., de Galarreta C.M.R. Chemical composition and biological activities of the essential oils of Salvia Canariensis. Flavour Frag. J. 2006;21:277–281. doi: 10.1002/ffj.1583. [DOI] [Google Scholar]
- 195.Abak F., Yildiz G., Atamov V., Kurkcuoglu M. Composition of the essential oil of Salvia Montbretii benth. From Turkey. Rec. Nat. Prod. 2018;12:426–431. doi: 10.25135/rnp.51.17.12.080. [DOI] [Google Scholar]
- 196.Delange D.M., Rico C.L.M., Canavaciolo V.G., Leyes E.A.R., Perez R.S. Volatile constituents from leaves of endemic Scutellaria Havanensis jacq. In Cuba. J. Essent. Oil Bear. Plants. 2013;16:368–371. doi: 10.1080/0972060X.2013.794045. [DOI] [Google Scholar]
- 197.Yilmaz G., Iek M., Demirci B., Baser K.H.C. Essential oil compositions of subspecies of Scutellaria Brevibracteata stapf from Turkey. J. Essent. Oil Res. 2019;31:255–262. doi: 10.1080/10412905.2019.1579762. [DOI] [Google Scholar]
- 198.Dimaki V.D., Iatrou G., Lamari F.N. Effect of acidic and enzymatic pretreatment on the analysis of mountain tea (sideritis spp.) volatiles via distillation and ultrasound-assisted extraction. J. Chromatogr. A. 2017;1524:290–297. doi: 10.1016/j.chroma.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 199.Kirimer N., Tabanca N., Tumen G., Duman H., Baser K.H.C. Composition of the essential oils of four endemic sideritis species from Turkey. Flavour Frag. J. 1999;14:421–425. doi: 10.1002/(SICI)1099-1026(199911/12)14:6<421::AID-FFJ852>3.0.CO;2-Z. [DOI] [Google Scholar]
- 200.Goren A.C., Piozzi F., Akcicek E., Kilic T., Carikci S., Mozioglu E., Setzer W.N. Essential oil composition of twenty-two stachys species (mountain tea) and their biological activities. Phytochem. Lett. 2011;4:448–453. doi: 10.1016/j.phytol.2011.04.013. [DOI] [Google Scholar]
- 201.Kremer D., Bolaric S., Ballian D., Bogunic F., Stesevic D., Karlovic K., Kosalec I., Vokurka A., Rodriguez J.V., Randic M., et al. Morphological, genetic and phytochemical variation of the endemic Teucrium arduini L. (lamiaceae) Phytochemistry. 2015;116:111–119. doi: 10.1016/j.phytochem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 202.Baher Z.F., Mirza M. Volatile constituents of Teucrium Flavum L. From Iran. J. Essent. Oil Res. 2003;15:106–107. doi: 10.1080/10412905.2003.9712082. [DOI] [Google Scholar]
- 203.Candela R.G., Ilardi V., Badalamenti N., Bruno M., Rosselli S., Maggi F. Essential oil compositions of Teucrium Fruticans, t. Scordium subsp. Scordioides and t. Siculum growing in Sicily and Malta. Nat. Prod. Res. 2020;10:1–10. doi: 10.1080/14786419.2019.1709193. [DOI] [PubMed] [Google Scholar]
- 204.Blazquez M.A., Perez I., Boira H. Essential oil analysis of Teucrium Libanitis and t-turredanum by gc and gc-ms. Flavour Frag. J. 2003;18:497–501. doi: 10.1002/ffj.1256. [DOI] [Google Scholar]
- 205.Brophy J.J., Goldsack R.J., Forster P.I. The leaf essential oils of Viticipremna Queenslandica (lamiaceae) J. Essent. Oil Res. 2008;20:403–404. doi: 10.1080/10412905.2008.9700040. [DOI] [Google Scholar]
- 206.Konyalioglu S., Ozturk B., Meral G.E. Comparison of chemical compositions and antioxidant activities of the essential oils of two Ziziphora Taxa from Anatolia. Pharm. Biol. 2006;44:121–126. doi: 10.1080/13880200600592244. [DOI] [Google Scholar]
- 207.Chaverri C., Ciccio J.F., Diaz C. Chemical composition of Aiouea Costaricensis (lauraceae) essential oils from Costa Rica and their cytotoxic activity on cell lines. J. Essent. Oil Res. 2010;22:524–529. doi: 10.1080/10412905.2010.9700389. [DOI] [Google Scholar]
- 208.Salleh W., Ahmad F. Antioxidant and anticholinesterase activities of essential oil of Alseodaphne Peduncularis meisn. Turk. J. Pharm. Sci. 2016;13:347–350. doi: 10.4274/tjps.2016.11. [DOI] [Google Scholar]
- 209.Luz A.I.R., da Silva J.D., Zoghbi M.D.B., Andrade E.H.A., Maia J.G.S. Essential oil from Aniba Riparia (nees) mez. J. Essent. Oil Res. 2002;14:218–219. doi: 10.1080/10412905.2002.9699827. [DOI] [Google Scholar]
- 210.Salleh W., Ahmad F., Khong H.Y., Zulkifli R.M. Comparative study of the essential oils of three beilschmiedia species and their biological activities. Int. J. Food Sci. Technol. 2016;51:240–249. doi: 10.1111/ijfs.12962. [DOI] [Google Scholar]
- 211.Brophy J.J., Goldsack R.J., Forster P.I. The essential oils of some australian Cassytha Species (lauraceae) J. Essent. Oil Res. 2009;21:543–546. doi: 10.1080/10412905.2009.9700239. [DOI] [Google Scholar]
- 212.Ahmed A., Choudhary M.I., Farooq A., Demirci B., Demirci F., Baser K.H.C. Essential oil constituents of the spice Cinnamomum Tamala (ham.) nees & eberm. Flavour Frag. J. 2000;15:388–390. [Google Scholar]
- 213.Son L.C., Dai D.N., Thang T.D., Huyen D.D., Ogunwande I.A. Analysis of the essential oils from five vietnamese litsea species (lauraceae) J. Essent. Oil Bear. Plants. 2014;17:960–971. doi: 10.1080/0972060X.2014.935068. [DOI] [Google Scholar]
- 214.Danielli L.J., Pippi B., Soares K.D., Duarte J.A., Maciel A.J., Machado M.M., Oliveira L.F.S., Bordignon S.A.L., Fuentefria A.M., Apel M.A. Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex rottb. species essential oils. Ind. Crop Prod. 2017;102:7–15. doi: 10.1016/j.indcrop.2017.03.013. [DOI] [Google Scholar]
- 215.John A.J., Karunakaran V.P., George V. Chemical composition and antibacterial activity of leaf oil of Neolitsea foliosa (nees) gamble var. caesia (meisner) gamble. J. Essent. Oil Res. 2007;19:498–500. doi: 10.1080/10412905.2007.9699962. [DOI] [Google Scholar]
- 216.Barbosa J.M., Cunha R.M., Dias C.S., Athayde P.F., Silva M.S., Da-Cunha E.V.L., Machado M.I.L., Craveiro A.A., Medeiros I.A. Gc-ms analysis and cardiovascualr activity of the essential oil of Ocotea Duckei. J. Pharmacogn. 2008;18:37–41. [Google Scholar]
- 217.Yamaguchi K.K.D., Alcantara J.M., Lima E.S., da Veiga V.F. Chemical composition and platelet aggregation activity of essential oils of two species of the Genus Ocotea (lauraceae) J. Essent. Oil Bear. Plants. 2013;16:518–523. doi: 10.1080/0972060X.2013.855364. [DOI] [Google Scholar]
- 218.Ogunbinu A.O., Ogunwande I.A., Flamini G., Cioni P.L. Volatile compounds of Persea Americana mill from Nigeria. J. Essent. Oil Bear. Plants. 2007;10:133–138. doi: 10.1080/0972060X.2007.10643531. [DOI] [Google Scholar]
- 219.Lopez M.L., Zunino M.P., Zygadlo J.A., Lopez A.G., Lucini E.I., Facillaci S.M. Aromatic plants of yungas. Part ii. Chemical composition of the essential oil of Phoebe Porphyria (griseb.) mez. (lauraceae) J. Essent. Oil Res. 2004;16:129–130. doi: 10.1080/10412905.2004.9698672. [DOI] [Google Scholar]
- 220.Miyazawa M., Nakashima Y., Nakahashi H., Hara N., Nakagawa H., Usami A., Chavasiri W. Volatile compounds with characteristic odor of essential oil from Magnolia Obovata leaves by hydrodistillation and solvent-assisted flavor evaporation. J. Oleo Sci. 2015;64:999–1007. doi: 10.5650/jos.ess15114. [DOI] [PubMed] [Google Scholar]
- 221.Lawal O.A., Ogunwande I.A., Salvador A.F., Sanni A.A., Opoku A.R. Pachira Glabra Pasq. essential oil: Chemical constituents, antimicrobial and insecticidal activities. J. Oleo Sci. 2014;63:629–635. doi: 10.5650/jos.ess13179. [DOI] [PubMed] [Google Scholar]
- 222.Mevy J.P., Bessiere J.M., Rabier J., Dherbomez M., Ruzzier M., Millogo J., Viano J. Composition and antimicrobial activities of the essential oil of Triumfetta Rhomboidea jacq. Flavour Frag. J. 2006;21:80–83. doi: 10.1002/ffj.1511. [DOI] [Google Scholar]
- 223.Joycharat N., Thammavong S., Voravuthikunchai S.P., Plodpai P., Mitsuwan W., Limsuwan S., Subhadhirasakul S. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata lour. Nat. Prod. Res. 2014;28:2169–2172. doi: 10.1080/14786419.2014.924934. [DOI] [PubMed] [Google Scholar]
- 224.Rahman M.S., Ahad A., Saha S.K., Hong J., Kim K.H. Antibacterial and phytochemical properties of Aphanamixis polystachya essential oil. Anal. Sci. Technol. 2017;30:113–121. [Google Scholar]
- 225.Lago J.H.G., de Avila P., de Aquino E.M., Moreno P.R.H., Ohara M.T., Limberger R.P., Apel M.A., Henriques A.T. Volatile oils from leaves and stem barks of Cedrela fissilis (meliaceae): Chemical composition and antibacterial activities. Flavour Frag. J. 2004;19:448–451. doi: 10.1002/ffj.1347. [DOI] [Google Scholar]
- 226.Ribeiro W.H.F., Arriaga A.M.C., Andrade-Neto M., Vasconcelos J.N., Santiago G.M.P., Nascimento R.F. Composition of the essential oil of Guarea macrophylla vahl. ssp tuberculata (meliaceae) from northeast of Brazil. J. Essent. Oil Res. 2006;18:95–96. doi: 10.1080/10412905.2006.9699397. [DOI] [Google Scholar]
- 227.Ogunwande I.A., Jimoh R., Ajetunmobi A.A., Avoseh N.O., Flamini G. Essential oil composition of Ficus benjamina (moraceae) and Irvingia barteri (irvingiaceae) Nat. Prod. Commun. 2012;7:1673–1675. doi: 10.1177/1934578X1200701233. [DOI] [PubMed] [Google Scholar]
- 228.St-Gelais A., Roger B., Alsarraf J., Legault J., Masse D., Pichette A. Aromas from Quebec. Vi. Morella pensylvanica from the Magdalen Islands: A (-)-alpha-bisabolol-rich oil featuring a new bisabolane ether. J. Essent. Oil Res. 2018;30:319–329. doi: 10.1080/10412905.2018.1470039. [DOI] [Google Scholar]
- 229.Sabulal B., Kurup R., Sumitha B., George V. Chemical composition of the leaf oils of Myristica malabarica lam. and Gymnacranthera canarica (king) warb. J. Essent. Oil Res. 2007;19:323–325. doi: 10.1080/10412905.2007.9699293. [DOI] [Google Scholar]
- 230.Salleh W., Anuar M.Z.A., Khamis S., Nafiah M.A., Sul’ain M.D. Chemical investigation and biological activities of the essential oil of Knema kunstleri warb. from Malaysia. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1669027. [DOI] [PubMed] [Google Scholar]
- 231.Limberger R.P., Sobral M.E.G., Zuanazzi J.A.S., Moreno P.R.H., Schapoval E.E.S., Henriques A.T. Biological activities and essential oil composition of leaves of Blepharocalyx salicifolius. Pharm. Biol. 2001;39:308–311. doi: 10.1076/phbi.39.4.308.5915. [DOI] [Google Scholar]
- 232.Bignell C.M., Dunlop P.J., Brophy J.J., Jackson J.F. Volatile leaf oils of some queensland and northern australian species of the Genus eucalyptus. (series ii). Part i. Subgenus symphyomyrtus, section adnataria: (a) series oliganthae, (b) series ochrophloiae, (c) series moluccanae, (d) series polyanthemae, (e) series paniculatae, (f) series melliodorae and (g) series porantheroideae. Flavour Frag. J. 1997;12:19–27. [Google Scholar]
- 233.Medeiros J.R., Medeiros N., Medeiros H., Davin L.B., Lewis N.G. Composition of the bioactive essential oils from the leaves of eugenia stipitata mcvaugh ssp sororia from the Azores. J. Essent. Oil Res. 2003;15:293–295. doi: 10.1080/10412905.2003.9712145. [DOI] [Google Scholar]
- 234.Fernandez X., Loiseau A.M., Poulain S., Lizzani-Cuvelier L., Monnier Y. Chemical composition of the essential oil from feijoa (feijoa sellowiana berg.) peel. J. Essent. Oil Res. 2004;16:274–275. doi: 10.1080/10412905.2004.9698719. [DOI] [Google Scholar]
- 235.Limberger R.P., Simoes-Pires C.A., Sobral M., Henriques A.T. Essential oils of marlierea species. J. Essent. Oil Res. 2004;16:479–482. doi: 10.1080/10412905.2004.9698776. [DOI] [Google Scholar]
- 236.Hnawia E., Brophy J.J., Craven L.A., Lebouvier N., Cabalion P., Nour M. An examination of the leaf essential oils of the Endemic melaleuca (myrtaceae) species of New Caledonia. J. Essent. Oil Res. 2012;24:273–278. doi: 10.1080/10412905.2012.676776. [DOI] [Google Scholar]
- 237.Zoghbi M.D., Andrade E.H.A., da Silva M.H.L., Carreira L.M.M., Maia J.G.S. Essential oils from three myrcia species. Flavour Frag. J. 2003;18:421–424. doi: 10.1002/ffj.1242. [DOI] [Google Scholar]
- 238.Demo M.S., Oliva M.M., Zunino M.R., Lopez M.L., Zygadlo J.A. Aromatic plants from Yungas. Part iv: Composition and antimicrobial activity of myrcianthes pseudo-mato essential oil. Pharm. Biol. 2002;40:481–484. doi: 10.1076/phbi.40.7.481.14689. [DOI] [Google Scholar]
- 239.Apel M.A., Lima M.E.L., Sobral M., Young M.C.M., Cordeiro I., Schapoval E.E.S., Henriques A.T., Moreno P.R.H. Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm. Biol. 2010;48:433–438. doi: 10.3109/13880200903164386. [DOI] [PubMed] [Google Scholar]
- 240.Southwell I.A., Russell M.F., Smith R.L., Vinnicombe A. Ochrosperma lineare, a new source of methyl chavicol. J. Essent. Oil Res. 2003;15:329–330. doi: 10.1080/10412905.2003.9698602. [DOI] [Google Scholar]
- 241.Apel M.A., Sobral M., Zuanazzi J.A., Henriques A.T. Essential oil composition of four plinia species (myrtaceae) Flavour Frag. J. 2006;21:565–567. doi: 10.1002/ffj.1638. [DOI] [Google Scholar]
- 242.Da Silva J.D., Luz A.I.R., da Silva M.H.L., Andrade E.H.A., Zoghbi M.D., Maia J.G.S. Essential oils of the leaves and stems of four psidium spp. Flavour Frag. J. 2003;18:240–243. doi: 10.1002/ffj.1219. [DOI] [Google Scholar]
- 243.El Ghallab Y., Al Jahid A., Eddine J.J., Said A.A.H., Zarayby L., Derfoufi S. Syzygium aromaticum l.: Phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Adv. Tradit. Med. 2020;20:153–158. doi: 10.1007/s13596-019-00416-7. [DOI] [Google Scholar]
- 244.Huong L.T., Hung N.V., Chac L.D., Dai D.N., Ogunwande I.A. Essential oils from Syzygium grande (wight) walp. and syzygium sterrophyllum merr. et perry. J. Essent. Oil Bear. Plants. 2017;20:1620–1626. doi: 10.1080/0972060X.2017.1409658. [DOI] [Google Scholar]
- 245.Brophy J.J., Goldsack R.J., Forster P.I. The essential oils of the australian species of uromyrtus (myrtaceae) Flavour Frag. J. 1996;11:133–138. doi: 10.1002/(SICI)1099-1026(199603)11:2<133::AID-FFJ564>3.0.CO;2-2. [DOI] [Google Scholar]
- 246.Rustaiyan A., Khalilzadeh M.A., Eslami B., Masoudi S., Tajbakhsh M. Volatile constituents of Meristotropis xanthioides vassilez. and Lotus michauxianus ser. from Iran. J. Essent. Oil Res. 2006;18:631–632. doi: 10.1080/10412905.2006.9699187. [DOI] [Google Scholar]
- 247.Dai D.N., Thang T.D., Thin D.B., Ogunwande I.A. Chemical composition of the leaf oil of actephila excelsa from Vietnam. Nat. Prod. Commun. 2014;9:1359–1360. doi: 10.1177/1934578X1400900934. [DOI] [PubMed] [Google Scholar]
- 248.Yu E.J., Kim T.H., Kim K.H., Lee H.J. Characterization of aroma-active compounds of Abies nephrolepis (khingan fir) needles using aroma extract dilution analysis. Flavour Frag. J. 2004;19:74–79. doi: 10.1002/ffj.1314. [DOI] [Google Scholar]
- 249.Hmamouchi M., Hamamouchi J., Zouhdi M., Bessiere J.M. Chemical and antimicrobial properties of essential oils of five Moroccan pinaceae. J. Essent. Oil Res. 2001;13:298–302. doi: 10.1080/10412905.2001.9699699. [DOI] [Google Scholar]
- 250.Tsitsimpikou C., Petrakis P.V., Ortiz A., Harvala C., Roussis V. Volatile needle terpenoids of six pinus species. J. Essent. Oil Res. 2001;13:174–178. doi: 10.1080/10412905.2001.9699652. [DOI] [Google Scholar]
- 251.Jeon J.H., Lee H.S. Volatile components of essential oils extracted from pinus species. J. Essent. Oil Bear. Plants. 2012;15:750–754. doi: 10.1080/0972060X.2012.10644115. [DOI] [Google Scholar]
- 252.Yener H.O., Saygideger S.D., Sarikurkcu C., Yumrutas O. Evaluation of antioxidant activities of essential oils and methanol extracts of pinus species. J. Essent. Oil Bear. Plants. 2014;17:295–302. doi: 10.1080/0972060X.2014.895164. [DOI] [Google Scholar]
- 253.Facundo V.A., de Morais S.M. Essential oil of Piper tuberculatum var. Tuberculatum pip (micq.) cdc leaves. J. Essent. Oil Res. 2005;17:304–305. doi: 10.1080/10412905.2005.9698910. [DOI] [Google Scholar]
- 254.Jirovetz L., Buchbauer G., Ngassoum M.B., Geissler M. Aroma compound analysis of Piper nigrum and piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J. Chromatogr. A. 2002;976:265–275. doi: 10.1016/S0021-9673(02)00376-X. [DOI] [PubMed] [Google Scholar]
- 255.Menon A.N., Padmakumari K.P., Jayalekshmy A., Gopalakrishnan M., Narayanan C.S. Essential oil composition of four popular Indian cultivars of black pepper (Piper nigrum L.) J. Essent. Oil Res. 2000;12:431–434. doi: 10.1080/10412905.2000.9699558. [DOI] [Google Scholar]
- 256.Sirat H.M., Thai O.B., Ahmad F. Chemical composition of the essential oil of Piper maingayi hk. F. J. Essent. Oil Res. 2010;22:323–324. doi: 10.1080/10412905.2010.9700336. [DOI] [Google Scholar]
- 257.Moraes M.S., Machado S.R., Marques M.O.M. Essential oil of the Pothomorphe peltata (L.) miq. J. Essent. Oil Res. 2004;16:15–16. doi: 10.1080/10412905.2004.9698637. [DOI] [Google Scholar]
- 258.Zeng Z., Meng C.Y., Ye X.N., Zeng Z. Analysis of volatile components of adenosma Indianum (lour.) merr. by steam distillation and headspace solid-phase microextraction. J. Chem. 2013;2013:1–7. doi: 10.1155/2013/545760. [DOI] [Google Scholar]
- 259.Martins A.P., Salgueiro L.R., Cavaleiro C., da Cunha A.P., Tomi F., Casanova J. Chemical composition of the oil of Afrocarpus mannii, an endemic species from s.tome e principe. J. Essent. Oil Res. 2001;13:431–433. doi: 10.1080/10412905.2001.9699717. [DOI] [Google Scholar]
- 260.Cavalli J.F., Tomi F., Bernardini A.F., Casanova J. Composition and chemical variability of the bark oil of Cedrelopsis grevei h. baillon from Madagascar. Flavour Frag. J. 2003;18:532–538. doi: 10.1002/ffj.1263. [DOI] [Google Scholar]
- 261.Navaei M.N., Mirza M. A comparative study of the essential oils of Agrimonia eupatoria both cultivated and wild growing conditions in Iran. J. Essent. Oil Bear. Plants. 2009;12:369–373. doi: 10.1080/0972060X.2009.10643733. [DOI] [Google Scholar]
- 262.Ghazghazi H., Miguel M.G., Weslati M., Hasnaoui B., Sebei H., Barroso J.G., Pedro L.G., Figueiredo A.C. Chemical variability of the essential oils from Rosa canina L. and Rosa sempervirens L. Flowers collected at Tunisia. J. Essent. Oil Res. 2012;24:475–480. doi: 10.1080/10412905.2012.703509. [DOI] [Google Scholar]
- 263.Tava A., Biazzi E., Ronga D., Avato P. Identification of the volatile components of Galium verum L. and Cruciata leavipes opiz from the western Italian Alps. Molecules. 2020;25:11. doi: 10.3390/molecules25102333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rao H.J.Z., Lai P.X., Gao Y. Chemical composition, antibacterial activity, and synergistic effects with conventional antibiotics and nitric oxide production inhibitory activity of essential oil from Geophila repens (l.) imjohnst. Molecules. 2017;22:13. doi: 10.3390/molecules22091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pant P., Sut S., Castagliuolo I., Gandin V., Maggi F., Gyawali R., Stefano D. Sesquiterpene rich essential oil from nepalese bael tree (Aegle marmelos (l.) correa) as potential antiproliferative agent. Fitoterapia. 2019;138:6. doi: 10.1016/j.fitote.2019.104266. [DOI] [PubMed] [Google Scholar]
- 266.Pino J.A., Rosado A., Bello A., Urquiola A., Garcia S. Essential oil of Amyris elimifera L. from Cuba. J. Essent. Oil Res. 2000;12:39–40. doi: 10.1080/10412905.2000.9712037. [DOI] [Google Scholar]
- 267.Pang X., Almaz B., Qi X.J., Wang Y., Feng Y.X., Geng Z.F., Xi C., Du S.S. Bioactivity of essential oil from Atalantia buxifolia leaves and its major sesquiterpenes against three stored-product insects. J. Essent. Oil Bear. Plants. 2020;23:38–50. doi: 10.1080/0972060X.2020.1729245. [DOI] [Google Scholar]
- 268.Padalia R.C., Verma R.S., Chauhan A. Compositional variations in volatile constituents of Boenninghausenia albiflora reichb. from western Himalaya. Natl. Acad. Sci. Lett. 2013;36:635–640. doi: 10.1007/s40009-013-0183-6. [DOI] [Google Scholar]
- 269.Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the australian species of citrus (rutaceae) J. Essent. Oil Res. 2001;13:264–268. doi: 10.1080/10412905.2001.9699690. [DOI] [Google Scholar]
- 270.Supudompol B. Composition and anti-mycobacterial activity of the essential oil of Feroniella lucida (scheff.) swing. J. Essent. Oil Res. 2009;21:561–562. doi: 10.1080/10412905.2009.9700245. [DOI] [Google Scholar]
- 271.Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the australian species of flindersia (rutaceae) J. Essent. Oil Res. 2005;17:388–395. doi: 10.1080/10412905.2005.9698939. [DOI] [Google Scholar]
- 272.Azadi B., Khaef S., Ziarati P. Chemical composition of Haplophyllum villosum (m. B.) g. don essential oil. J. Essent. Oil Bear. Plants. 2014;17:1161–1164. doi: 10.1080/0972060X.2014.958556. [DOI] [Google Scholar]
- 273.Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the australian species of medicosma (rutaceae) J. Essent. Oil Res. 2004;16:161–166. doi: 10.1080/10412905.2004.9698683. [DOI] [Google Scholar]
- 274.Brophy J.J., Goldsack R.J., Forster P.I. Composition of the leaf oils of the australian species of euodia and melicope (rutaceae) J. Essent. Oil Res. 2004;16:286–293. doi: 10.1080/10412905.2004.9698723. [DOI] [Google Scholar]
- 275.Neta M.C.S., Vittorazzi C., Guimaraes A.C., Martins J.D.L., Fronza M., Endringer D.C., Scherer R. Effects of beta-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm. Biol. 2017;55:190–197. doi: 10.1080/13880209.2016.1254251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Padmakumari K.P. Volatile constituents from the leaves and flowers of Murraya koenigii (linn.) spreng. J. Essent. Oil Bear. Plants. 2009;12:722–727. doi: 10.1080/0972060X.2009.10643781. [DOI] [Google Scholar]
- 277.Pavithra P.S., Mehta A., Verma R.S. Induction of apoptosis by essential oil from p. Missionis in skin epidermoid cancer cells. Phytomedicine. 2018;50:184–195. doi: 10.1016/j.phymed.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 278.Cabral F.D., Alves C.C.F., Cabral R.S.C., Willrich G.B., Crotti A.E.M., MIranda M.L.D. Chemical constituents of essential oils extracted from the leaves and flowers of Spiranthera odoratissima a. st. hil. (rutaceae) Rec. Nat. Prod. 2019;13:172–175. doi: 10.25135/rnp.95.18.07.117. [DOI] [Google Scholar]
- 279.Brophy J.J., Goldsack R.J., Forster P.I., Hutton I. Composition of the leaf oils of the australian and lord howe island species of zanthoxylum (rutaceae) J. Essent. Oil Res. 2000;12:285–291. doi: 10.1080/10412905.2000.9699517. [DOI] [Google Scholar]
- 280.Song X.H., Li H., Li C.R., Xu J.W., Hu D.M. Effects of vocs from leaves of Acer truncatum bunge and Cedrus deodara on human physiology and psychology. Urban For. Urban Green. 2016;19:29–34. doi: 10.1016/j.ufug.2016.06.021. [DOI] [Google Scholar]
- 281.Rehman J.U., Wang M., Yang Y.P., Liu Y.B., Li B., Qin Y., Wang W., Chittiboyina A.G., Khan I.A. Toxicity of Kadsura coccinea (lem. ) a. C. Sm. Essential oil to the bed bug, Cimex lectularius L. (hemiptera: Cimicidae) Insects. 2019;10:11. doi: 10.3390/insects10060162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Garg S.C., Dengre S.L. Composition of the essential oil from the leaves of Buddleia asiatica lour. Flavour Frag. J. 1992;7:125–127. doi: 10.1002/ffj.2730070306. [DOI] [Google Scholar]
- 283.Fonseca A.M., Pessoa O.D.L., Lemos T.L.G., Nascimento R.F. Constituents of the essential oil of capraria biflora from northeast Brazil. J. Essent. Oil Res. 2006;18:158–159. doi: 10.1080/10412905.2006.9699052. [DOI] [Google Scholar]
- 284.Osorio A.M.B., Silva T.M., Duarte L.P., Ferraz V.P., Pereira M.T., Mercadante-Simoes M.O., Evangelista F.C.G., Sabino A.P., Alcantara A.F.C. Essential oil from flowers of solanum stipulaceum: Composition, effects of gamma-radiation, and antileukemic activity. J. Braz. Chem. Soc. 2015;26:2233–2240. [Google Scholar]
- 285.Pino J.A., Marbot R., Fuentes V. Essential oil of Aloysia virgata juss. from Cuba. J. Essent. Oil Res. 2004;16:44–45. doi: 10.1080/10412905.2004.9698649. [DOI] [Google Scholar]
- 286.Sousa E.O., Rodrigues F.F.G., Coutinho H.D.M., Campos A.R., Lima S.G., Costa J.G.M. Chemical composition and aminoglycosides synergistic effect of Lantana montevidensis briq. (verbenaceae) essential oil. Rec. Nat. Prod. 2011;5:60–64. [Google Scholar]
- 287.Randrianalijaona J.A., Ramanoelina P.A.R., Rasoarahona J.R.E., Gaydou E.M. Seasonal and chemotype influences on the chemical composition of Lantana camara L. essential oils from Madagascar. Anal. Chim. Acta. 2005;545:46–52. doi: 10.1016/j.aca.2005.04.028. [DOI] [Google Scholar]
- 288.Vila R., Iglesias J., Canigueral S., Ciccio J.F. Composition of the essential oil from leaves of lippia myriocephala from Costa Rica. J. Essent. Oil Res. 2004;16:177–179. doi: 10.1080/10412905.2004.9698688. [DOI] [Google Scholar]
- 289.Baez D., Pino J.A., Morales D. Floral scent composition in Petitia domingensis jacq. Analyzed by hs-spme. J. Essent. Oil Bear. Plants. 2012;15:782–784. doi: 10.1080/0972060X.2012.10644120. [DOI] [Google Scholar]
- 290.Eyob S., Appelgren M., Rohloff J., Tsegaye A., Messele G. Traditional medicinal uses and essential oil composition of leaves and rhizomes of korarima (Aframomum corrorima (braun) p.C.M. Jansen) from southern Ethiopia. S. Afr. J. Bot. 2008;74:181–185. doi: 10.1016/j.sajb.2007.10.007. [DOI] [Google Scholar]
- 291.Ali S., Sotheeswaran S., Tuiwawa M., Smith R.M. Comparison of the composition of the essential oils of alpinia and hedychium species—Essential oils of fijian plants, part 1. J. Essent. Oil Res. 2002;14:409–411. doi: 10.1080/10412905.2002.9699904. [DOI] [Google Scholar]
- 292.Wong K.C., Lee B.C., Lam N.F., Ibrahim P. Essential oils of the rhizomes of Alpinia conchigera griff. and Alpinia latilabris ridl. Flavour Frag. J. 2005;20:431–433. doi: 10.1002/ffj.1458. [DOI] [Google Scholar]
- 293.Huong L.T., Dai D.N., Thang T.D., Bach T.T., Ogunwande I.A. The essential oils of the leaf, pseudostem root and fruit of Alpinia mutica roxb. J. Essent. Oil Bear. Plants. 2016;19:2049–2055. doi: 10.1080/0972060X.2016.1244018. [DOI] [Google Scholar]
- 294.Huong L.T., Dai D.N., Thang T.D., Bach T.T., Ogunwande I.A. Analysis of the volatile constituents of alpinia pinnanensis. J. Essent. Oil Bear. Plants. 2017;20:264–271. doi: 10.1080/0972060X.2017.1298474. [DOI] [Google Scholar]
- 295.Taiwo A.O., Bolanle A.A. The leaf essential oil of costus afer ker-grawl from Nigeria. Flavour Frag. J. 2003;18:309–311. doi: 10.1002/ffj.1186. [DOI] [Google Scholar]
- 296.Raina V.K., Srivastava S.K., Jain N., Ahmad A., Syamasundar K.V., Aggarwal K.K. Essential oil composition of Curcuma longa L. Cv. Roma from the plains of northern India. Flavour Frag. J. 2002;17:99–102. doi: 10.1002/ffj.1053. [DOI] [Google Scholar]
- 297.Wong K.C., Sivasothy Y., Boey P.L., Osman H., Sulaiman B. Essential oils of Etlingera elatior (jack) r. M. Smith and Etlingera littoralis (koenig) giseke. J. Essent. Oil Res. 2010;22:461–466. doi: 10.1080/10412905.2010.9700372. [DOI] [Google Scholar]
- 298.Raj G., George V., Dan M., Sethuraman M.G. Essential oil composition of Globba schomburgkii hook. F. and Globba ophioglossa wight. J. Essent. Oil Res. 2010;22:220–222. doi: 10.1080/10412905.2010.9700307. [DOI] [Google Scholar]
- 299.Dos Santos B.C.B., Barata L.E.S., Marques F.A., Baroni A.C.M., Karnos B.A.C., de Oliveira P.R., Guerrero P.G. Composition of leaf and rhizome essential oils of Hedychium coronarium koen. from Brazil. J. Essent. Oil Res. 2010;22:305–306. doi: 10.1080/10412905.2010.9700331. [DOI] [Google Scholar]
- 300.Gevu K.V., Limag H.R.P., Neves B.A., Mello E.O., Taveirag G.B., Carvalhoa L.P., Carvalho M.G., Gomes V.M., Melo E.J.T., Da Cunha M. Chemical composition and anti-candida and anti-trypanosoma cruzi activities of essential oils from the rhizomes and leaves of Brazilian species of renealmia l. Fil. Rec. Nat. Prod. 2019;13:268–280. doi: 10.25135/rnp.105.18.08.125. [DOI] [Google Scholar]
- 301.Maia J.G.S., Andrade E.H.A., Carreira L.M.M., da Silva M.H.L. Essential oil composition of Renealmia alpinia (rottb.) maas. J. Essent. Oil Bear. Plants. 2007;10:10–14. doi: 10.1080/0972060X.2007.10643512. [DOI] [Google Scholar]
- 302.Sabulal B., Dan M., Anil J.J., Kurup R., Pradeep N.S., Valsamma R.K., George V. Caryophyllene-rich rhizome oil of zingiber nimmonii from south India: Chemical characterization and antimicrobial activity. Phytochemistry. 2006;67:2469–2473. doi: 10.1016/j.phytochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 303.Barra A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009;4:1147–1154. doi: 10.1177/1934578X0900400827. [DOI] [PubMed] [Google Scholar]
- 304.Gad H.A., El-Ahmady S.H., Abou-Shoer M.I., Al-Azizi M.M. Application of chemometrics in authentication of herbal medicines: A review. Phytochem. Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 305.Maffei M.E. Plant Bioactive Molecules. Cambridge Scholars Publishing; Newcastle upon Tyne, UK: 2018. [Google Scholar]
- 306.Soni U., Brar S., Gauttam V.K. Effect of seasonal variation on secondary metabolites of medicinal Plants. Int. J. Pharmacol. Sci. Res. 2015;6:3654–3662. [Google Scholar]
- 307.Xavier F.H., Maciuk A., Morais A.R.D., Alencar E.D., Garcia V.L., do Egito E.S.T., Vauthier C. Development of a gas chromatography method for the analysis of copaiba oil. J. Chromatogr. Sci. 2017;55:969–978. doi: 10.1093/chromsci/bmx065. [DOI] [PubMed] [Google Scholar]
- 308.Sousa J.P.B., Brancalion A.P.S., Souza A.B., Turatti I.C.C., Ambrosio S.R., Furtado N., Lopes N.P., Bastos J.K. Validation of a gas chromatographic method to quantify sesquiterpenes in copaiba oils. J. Pharm. Biomed. Anal. 2011;54:653–659. doi: 10.1016/j.jpba.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 309.Junor G.A.O., Porter R.B.R., Yee T.H. Chemical composition of essential oils from the aerial parts of jamaican bursera lunanii spreng. J. Essent. Oil Res. 2010;22:602–605. doi: 10.1080/10412905.2010.9700410. [DOI] [Google Scholar]
- 310.Temel M., Tinmaz A.B., Ozturk M., Gunduz O. Production and trade of medicinal and aromatic plants in the world and Turkey. Ksu Tarim Ve Doga Dergisi-Ksu J. Agric. Nat. 2018;21:198–214. [Google Scholar]
- 311.Klauke A.L., Racz I., Pradier B., Markert A., Zimmer A.M., Gertsch J., Zimmer A. The cannabinoid cb(2) receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014;24:608–620. doi: 10.1016/j.euroneuro.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 312.Chicca A., Caprioglio D., Minassi A., Petrucci V., Appendino G., Taglialatela-Scafati O., Gertsch J. Functionalization of beta-caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chem. Biol. 2014;9:1499–1507. doi: 10.1021/cb500177c. [DOI] [PubMed] [Google Scholar]