Abstract
Foodomics, emergent field of metabolomics, has been applied to study food system processes, and it may be useful to understand sensorial food properties, among others, through foods metabolites profiling. Thus, as beer volatile components represent the major contributors for beer overall and peculiar aroma properties, this work intends to perform an in-depth profiling of lager beer volatile metabolites and to generate new data that may contribute for molecules’ identification, by using multidimensional gas chromatography. A set of lager beers were used as case-study, and 329 volatile metabolites were determined, distributed over 8 chemical families: acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. From these, 96 compounds are reported for the first time in the lager beer volatile composition. Around half of them were common to all beers under study. Clustering analysis allowed a beer typing according to production system: macro- and microbrewer beers. Monoterpenic and sesquiterpenic compounds were the chemical families that showed wide range of chemical structures, which may contribute for the samples’ peculiar aroma characteristics. In summary, as far as we know, this study presents the most in-depth lager beer volatile composition, which may be further used in several approaches, namely, in beer quality control, monitoring brewing steps, raw materials composition, among others.
Keywords: lager beer, volatile metabolites, foodomics, beer typing, HS-SPME, GC×GC-ToFMS
1. Introduction
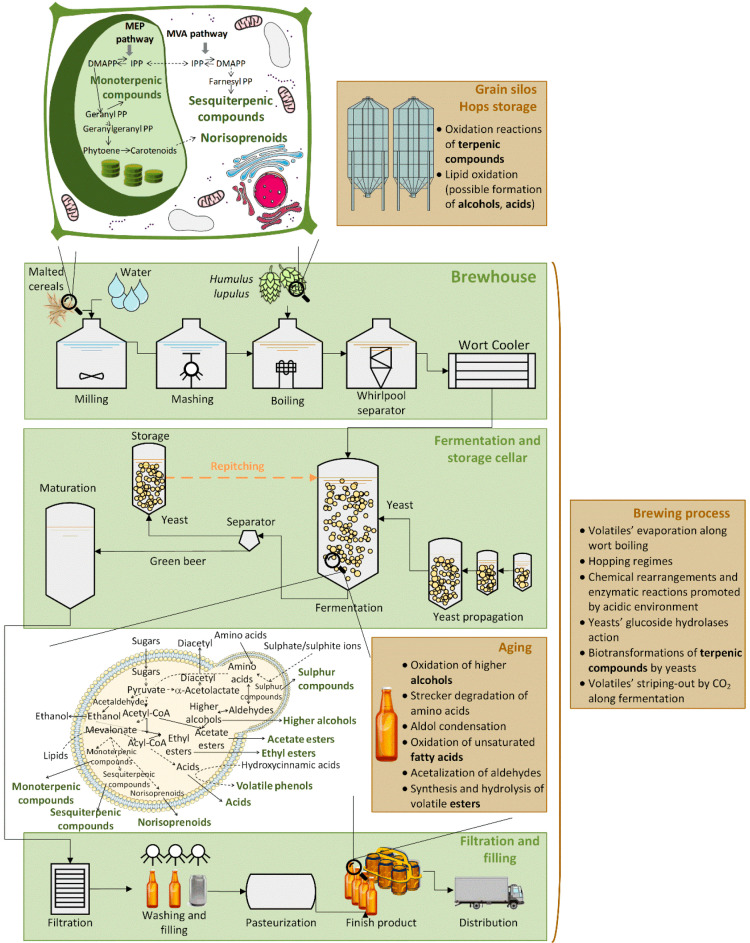
Beer represents a broadly popular and widespread alcoholic beverage, and according to World Health Organization (WHO) statistics, it is the most consumed beverage type per capita in Europe (40.0%) and WHO Region of the Americas (53.8%) [1]. Hundreds of different beer brands are available in the market, being lager beers the main type to be produced and consumed worldwide [2], and consequently the most studied. Beer attractiveness arises from its pleasant organoleptic and nutritional attributes regarding a moderate consumption. Indeed, taste and flavor are the main factors which contribute for beer quality, thus conditioning the consumers’ acceptance. Overall and peculiar aroma properties of beer are dependent on its volatile components, whose origin and/or change can be attributed to several brewing steps, from raw materials until consumption. One particular source of these volatile components is mainly related with raw materials (cereals and hops) [3] and yeasts metabolism [4,5] (Figure 1). Plants secondary metabolites, such as terpenic compounds and norisoprenoids are the main chemical families from the raw materials that may be present in the beer metabolome. Moreover, the main yeast metabolites arise from diverse chemical families, such as acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols [4,5]. Furthermore, chemical changes, which may occur in raw materials’ storage [6,7] and throughout the brewing process, as well as the phenomena of beer aging [8] may promote changes in the beer’s volatile composition.
Figure 1.
Schematic representation proposed to explain sources of the target analytes under study (acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols), taking into account that they can be formed from raw materials and/or produced and biotransformed along brewing, also the main associated metabolic pathways were highlighted (magnifying glass) [3,4,5,6,7,8,9,10]. MEP pathway: methylerythritol 4-phosphate pathway; MVA pathway: mevalonate pathway.
Foodomics is an emergent field of metabolomics, which displays the profile of food metabolites and may assess food quality, safety, authenticity, or traceability. Food metabolome represents the gathering of small molecules (molecular weight lower than 2 kDa) called metabolites, that are present in foods and mostly derive from animals, plants, and microorganism’s metabolism. Moreover, food metabolites may be changed along food process, storage, microorganisms, or chemical contaminations. Each food has its own characteristics according to the presence and abundance of certain metabolites (which may be or not specific of one more origins) or even depending on the metabolites’ combination [11,12]. Thus, metabolomics might be helpful to comprehend the relation of the composition with the sensory and nutritional quality of foods. Metabolomics have been used to differentiate beers according to their type [13,14], cereals type [15,16], hops [17], or yeasts footprinting [18,19,20,21]. Moreover, beer volatile metabolites were tracked along the brewing process [22,23], different storage conditions [24,25], or dealcoholization process [26].
Foods volatile fraction gives functional information on sample-related variables (e.g., raw materials, processing or transformation technologies, storage conditions, etc.), and gas-phase extraction are good approaches to obtain an accurate chemical characterization. In this context, green solid-phase microextraction (SPME) combined with comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-ToFMS) showed a huge potential to study the complex beer volatile composition [27]. In fact, the orthogonal analytes separation of GC×GC produces structured chromatograms, in which chemically-related analytes are distributed in a 2D “chemical map”, showing the diversity of the chemical structures of volatile and semi-volatile molecules [27]. This methodology was also applied to study the lager beer terpenic profiles, in which was achieved a beer terpen-typing according to producer system (macro- and microbrewers) [28]. Nevertheless, to go further to in-depth disclose beer aroma, several challenges have to be overcome in order to have high-quality and robust data, namely, the complexity and diversity of beer products, the chemical diversity of volatile and semi-volatile components, wide concentration dynamic range, difficulties of molecules identification, particularly due to the lack of standards, presence of unknow molecules, scarce databases from ToFMS, among others.
Considering the current state-of-the-art, the full potential of GC×GC for study the beer volatile composition is still far from being exploited. In fact, a detailed study of the lager beers’ chemical profiling can be obtained through GC×GC, studying an enlarged number of chemical families, for instance with aroma relevance. In this sense, this work intends to perform an in-depth profiling of lager beer volatile metabolites, using multidimensional gas chromatography. The acquisition of high-quality and robust GC×GC data will also allow to generate new chemical data to contribute to molecules’ identification, endeavouring to overcome the challenges presented previously. This goal was pursued with a set of 8 selected chemical families, namely, acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. Lager beers (from different countries, batches from the same brewery, breweries, aging times) were used as case-study.
2. Materials and Methods
The sampling, reporting of chemical analysis, and data pre-treatment, processing, and interpretation were performed according to the Metabolomics Standards Initiative (MSI) [29].
2.1. Materials and Reagents
Sodium hydroxide was supplied by Panreac (Barcelona, Spain). The retention index probe (an n-alkanes series of C8 to C20 straight-chain alkanes, in n-hexane) was purchased from Fluka (Buchs, Switzerland). The solid phase microextraction (SPME) holder for manual sampling and the fiber coating used were acquired from Supelco (Aldrich, Bellefonte, PA, USA), which included a 1 cm StableFlex™ fused silica fiber that was coated with partially cross-linked 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB). According to the producer’s recommendations, the SPME fiber was initially conditioned at 250 °C for 30 min in the GC injector and daily for 10 min at 250 °C.
2.2. Samples
A total of 18 lager (Pilsner type) beers were analyzed in this study (Table 1):
A total of 15 beers from 9 macrobreweries (P1–P9)—macro-bbs, which were available on the Portuguese market, from different countries (Portugal, France, Spain, and Germany) and batches, with alcohol contents from 4.2–5.2%, with shelf-life from 1–10 months;
A total of 3 beers from 3 microbreweries (P10–P12)—micro-bbs, which were available on the Portuguese market, produced in Portugal, with alcohol contents from 5.0–5.5%, with shelf-life from 4–10 months.
Table 1.
List of lager beers analyzed in this study and respective available characteristics.
| Sample | Category of Beer | Composition | Alcohol Content (%) | Shelf-Life at Analysis (Months) |
Country of Production | |||
|---|---|---|---|---|---|---|---|---|
| Malt | Unmalted Cereals | Hops | Others | |||||
| P1-1 | Macrobrewer | Barley | Corn and barley | Extract | Glucose syrup | 5.2 | 3 | Portugal |
| P1-2 | Macrobrewer | Barley | Corn and barley | Extract | Glucose syrup | 5.2 | 5 | Portugal |
| P1-3 | Macrobrewer | Barley | Corn and barley | Extract | Glucose syrup | 5.2 | 2 | Portugal |
| P2-1 | Macrobrewer | Barley | Maize or rice and barley | Extract | – | 5.0 | 10 | Portugal |
| P2-2 | Macrobrewer | Barley | Maize or rice and barley | Extract | – | 5.0 | 8 | Portugal |
| P3-1 | Macrobrewer | Barley | – | Extract | – | 5.0 | 8 | France |
| P3-2 | Macrobrewer | Barley | – | Extract | – | 5.0 | 5 | France |
| P4-1 | Macrobrewer | Barley | Maize and barley | Extract | E150c and E450 | 4.8 | 7 | Portugal |
| P5-1 | Macrobrewer | Barley | Corn and barley | Extract | – | 5.1 | 3 | Portugal |
| P6-1 | Macrobrewer | Barley | Corn and barley | Extract | E150c and E405 | 5.0 | 1 | Portugal |
| P7-1 | Macrobrewer | Barley | Maize or rice and barley | Extract | – | 4.2 | 9 | Portugal |
| P8-1 | Macrobrewer | Barley | Corn and barley | Extract | E150c and E405 | 5.0 | 3 | Spain |
| P8-2 | Macrobrewer | Barley | Corn and barley | Extract | E150c and E405 | 5.0 | 3 | Spain |
| P9-1 | Macrobrewer | Barley | – | Extract | – | 5.0 | 6 | Germany |
| P9-2 | Macrobrewer | Barley | – | Extract | – | 5.0 | 5 | Germany |
| P10-1 | Microbrewer (fresh) | Barley | – | Pellet | – | 5.0 | 6 | Portugal |
| P11-1 | Microbrewer (fresh) | Barley | – | Pellet | – | 5.5 | 4 | Portugal |
| P12-1 | Microbrewer (pasteurized) | Barley | – | Pellet | – | 5.1 | 10 | Portugal |
Note: E150c—Ammonia caramel; E405—Propylene glycol alginate; E450—di-phosphates.
2.3. Beer Volatile Metabolites’ Determination by HS-SPME/GC×GC-ToFMS
HS-SPME and GC×GC-ToFMS experimental parameters and decarbonation procedure were used as reported in a previous study [27] that was developed to characterize beer volatile composition. Summarily, 10 mL of beer were degassed overnight at 4 °C (static procedure) and then were placed into a 20 mL glass vial. Then, vial was capped and placed in a thermostated bath adjusted to 40.0 ± 0.1 °C, along with NaCl (2 g) and stirring bar (2 cm × 0.5 cm). The PDMS/DVB SPME fiber was inserted in the vial headspace for 30 min.
After the adsorption and absorption of beer volatile metabolites, the SPME fiber was manually introduced (30 s) into the LECO Pegasus 4D (LECO, St. Joseph, MI, USA) GC×GC-ToFMS injection port at 250 °C. This system consists of an Agilent GC 7890A GC (Agilent Technologies, Inc. Wilmington, DE, USA), with a dual stage jet cryogenic modulator (licensed from Zoex), a secondary oven, and a mass spectrometer with ToF analyzer. Equity-5 column (30 m × 0.32 mm I.D., 0.25 μm film thickness, Supelco, Inc., Bellefonte, PA, USA) was used as 1D column, and a DB-FFAP (0.79 m × 0.25 mm I.D., 0.25 μm film thickness, J&W Scientific Inc., Folsom, CA, USA) was used as a 2D column. The evaluation of the separation general quality and manual identification of peaks was performed through contour plots. For identification purposes, the mass spectrum and retention times (1D and 2D) of each component were compared to standards; the mass spectrum with those reported in mass spectral libraries, namely, in-house library of standards and two commercial databases (Wiley 275 and US National Institute of Science and Technology (NIST) V. 2.0–Mainlib and Replib). The identification was complemented by the experimentally determined linear retention index (RI) values through the use of van den Dool and Kratz equation [30]. RI determination was performed with the use of a C8-C20 n-alkanes series, whose values were compared with those reported in the bibliography for chromatographic columns similar to the above stated 1D column (Supplementary Table S1). The relative content of each volatile component in beer was estimated through the DTIC (Deconvoluted Total Ion Current) GC×GC area data that was expressed as arbitrary units (a. u.).
2.4. Statistical Analysis
Full data matrix consisted of 54 observations (18 beer samples, each one by three independent replicates) and 329 variables (peak areas of volatile metabolites). The list of all these volatile metabolites can be assessed in Supplementary Table S1, which besides the identification and chromatographic information also includes the GC chromatographic peak areas of the three independent replicates of each beer sample. Cytoscape v3.5.1 49 (The Cytoscape Consortium, San Diego, CA, USA) was used to build the systematization of 329 volatile metabolites detected in lager beer in study (Section 3.2), using the median of the GC peak area in all lager beer under study. The hierarchical cluster analysis (HCA) and heatmap was carried out using MetaboAnalyst 3.0 (web software, The Metabolomics Innovation Centre (TMIC), Edmonton, AB, Canada) [31]; data were autoscaled and normalized by the maximum, and the squared Euclidean distances and the Ward’s minimum variance as the clustering algorithm were used. Box plots were performed using the GraphPad Prism version 8 for Windows (trial version GraphPad Software, San Diego, CA, USA), and t-test was used to observe the significant statistical differences.
3. Results and Discussion
3.1. Chromatogram Contour Plot Analysis
In order to characterize the volatile metabolites present in beer, eight chemical families were selected according to the available literature [3,4,5] (Figure 1): acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. From these chemical families, monoterpenic and sesquiterpenic compounds were previously analyzed in our laboratory [28]. This previous targeted metabolomics approach [28] was focused on the in-depth coverage of beer terpenic compounds, in which a beer terpen-typing was achieved due to samples’ category clustering. Nevertheless, as this current study intends to characterize all the volatile metabolites present in lager beer, these chemical families (monoterpenic and sesquiterpenic compounds) could not be ignored, and therefore, they were also included in this study, being key elements of the beer volatile composition.
This targeted metabolomics approach, achieved by the high throughput and sensitive methodology based on HS-SPME/GC×GC-ToFMS, allowed the detection of 329 volatile metabolites from the 8 selected chemical families previously enumerated. As monoterpenic and sesquiterpenic compounds were previously reported [28], the remaining detected volatile metabolites were identified, being possible the putative identification of 181 volatile metabolites, from which 96 are reported for the first time in the lager beer volatile composition, which corresponds to an increase of ca. 53% of new chemical information. The putative metabolites’ identification was accomplished through a set of parameters that included the standards’ co-injection (when available), mass spectra comparison (home-made and commercial databases), calculation of retention index (RIcalc) and their comparison with retention index from literature (RIlit) for columns of 5% phenylpolysilphenylene-siloxane (or equivalent), and analysis of the metabolites retention times according to the structured chromatogram principle (i.e., similar chemical structures are displayed in the same 2D chromatographic space, being a unique and distinctive criteria for metabolites identification by GC×GC-ToFMS) (Supplementary Table S1).
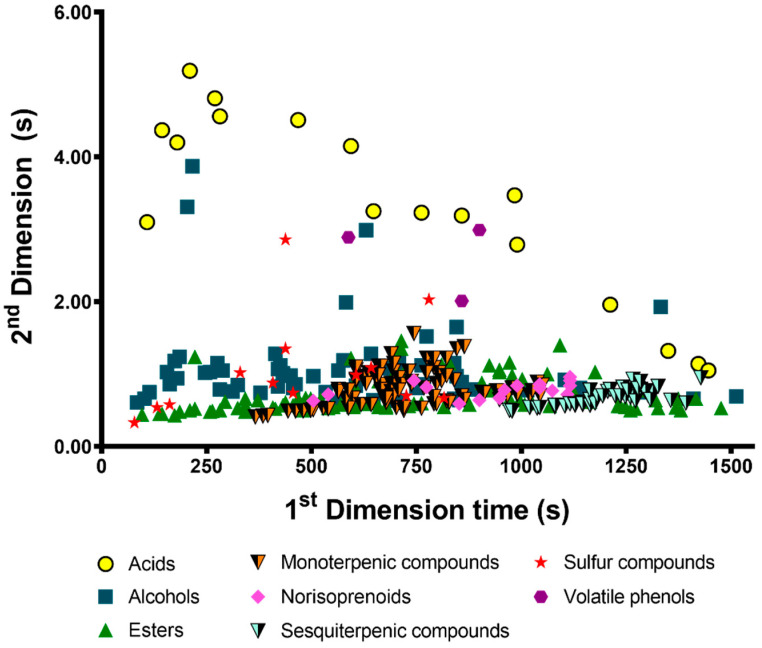
The distribution of the retention time’ coordinates of the 329 volatile metabolites is illustrated in the peak apex representation, which can be observed in Figure 2. This figure shows a practical example of the structured chromatogram, in which each metabolite is displayed in the chromatographic space according its physicochemical properties. Indeed, the orthogonal separation achieved by non-polar/polar (NP/P) set of columns that were employed, allows the volatile metabolites’ separation through their volatility (1D) and polarity (2D). For instance, esters tend to be the least polar compounds, presenting the global lower retention time for the second dimension (2tR); while acids, which present higher polarity, registered higher 2tR value (Figure 2 and Supplementary Table S1).
Figure 2.
GC×GC peak apex plot with retention time coordinates of the 329 detected analytes in lager beer.
The analytical variability of HS-SPME/GC×GC-ToFMS analysis was evaluated based on the data reproducibility, expressed through relative standard deviation (% RSD) of each analyte. DTIC GC×GC area was used to estimate its relative content. The % RSD of the detected volatile metabolites was examined, being achieved a median value of 20.9% for all metabolites, whereas the % RSD varied between 0.1% and 157%, minimum and maximum (Supplementary Table S1), respectively, considering each metabolite by itself. Furthermore, 52.4% of the peaks had % RSD lower than 20% (recommended limit for analytical variability of target bioanalysis by FDA [32]), and % RSD higher than 50% was only verified for 8.7% of the peaks, which indicated a good reproducibility.
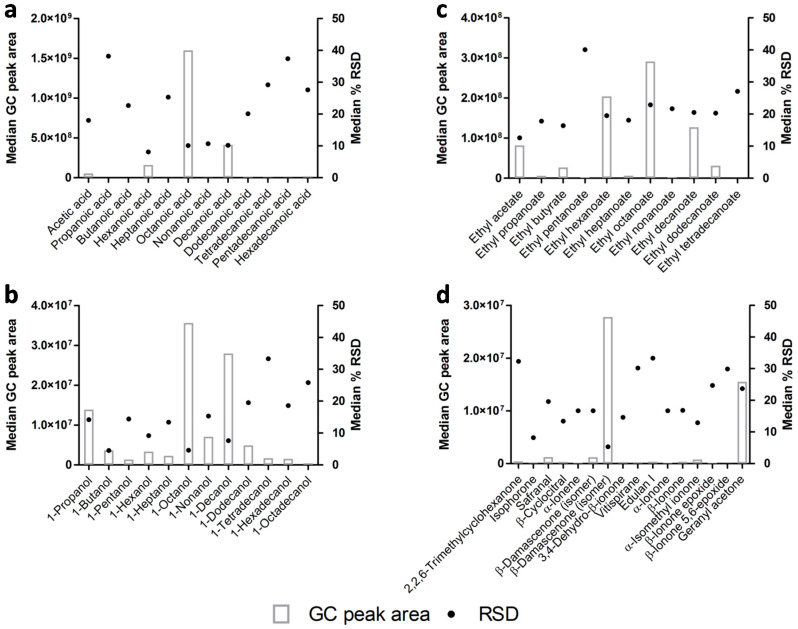
Homologous groups from the same chemical family (that are structurally related) were selected to evaluate the data reproducibility related with the volatile metabolites’ molecular weight and also with respective GC peak areas. Acids, 1-alcohols, ethyl esters, and norisoprenoids were selected as examples (Figure 3). Figure 3 shows the % RSD (grey square) and respective GC peak area (black circle) of the previously mentioned volatile metabolites. Volatile metabolites were displayed in x-axis according to molecular weight (from lower to higher), only norisoprenoids presented volatile metabolites with equal molecular weights. In the case of acids (Figure 3a) and 1-alcohols (Figure 3b), there was observed higher RSD values for the volatile metabolites with higher molecular weight, possibly due to the increase of their hydrophobicity and more affinity with the matrix, which may perturb their extraction. For the esters, no relationship was observed between the variability of the data and the chemical characteristics of the molecules, namely the molecular weight (Figure 3c). Nonetheless, it is important to mention that it was possible to observe a relation between the % RSD and the detected GC peak areas, wherein lower RSD is observed for volatile metabolites with higher GC peak area (Figure 3), and several examples can be observed in Figure 3, for instance 10% for octanoic acid (Figure 3a), 5% for 1-octanol (Figure 3b), 8% for 1-decanol (Figure 3b), and 5% for β-damascenone (Figure 3d).
Figure 3.
Analytical variability of HS-SPME/GC×GC-ToFMS analysis of beer volatile metabolites, considering the GC peak area (grey rectangle) and RSD (black circle) for selected homologous groups, namely (a) acids, (b) 1-alcohols, (c) ethyl esters, and (d) norisoprenoids. Volatile metabolites were displayed by increasing order of molecular weight on x-axis, except norisoprenoids that contain volatile metabolites with equal molecular weights.
3.2. Profiling the Lager Beer Volatile Metabolites
The quality and trait of fermented foods, such as beer, can be monitored by metabolomics, e.g., evaluation of the changes of the metabolic profiles during fermentation and prediction of fermented foods quality, among others [33]. The comprehensive characterization of the beer volatile metabolites, a metabolite profiling strategy, was selected taking into account two potential sources of volatile metabolites in beer: raw materials (cereals and hops) and yeasts (Figure 1). In fact, monoterpenic and sesquiterpenic compounds may arise from raw materials, once they are reported to be synthetized through the mevalonate (MVA) and methylerythritol 4-phosphate (MEP, only expressed in plants metabolism) metabolic pathways [9]. Cereal and hops may also contribute to the presence of norisoprenoids in beer volatile composition, through the carotenoids’ degradation in plants [10]. Yeasts metabolism is the main responsible for the unique aroma profiles of beer, through the production of a wide range of volatile metabolites, particularly the target chemical families that were selected for this study: acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, and sulfur compounds. Indeed, they may arise from metabolism of carbohydrates and amino acids [5,10,34,35,36,37]; from biosynthesis of monoterpenic compounds, sesquiterpenic compounds, and fatty acids [38,39,40,41]; and also from de novo synthesis of sulfur compounds [34].
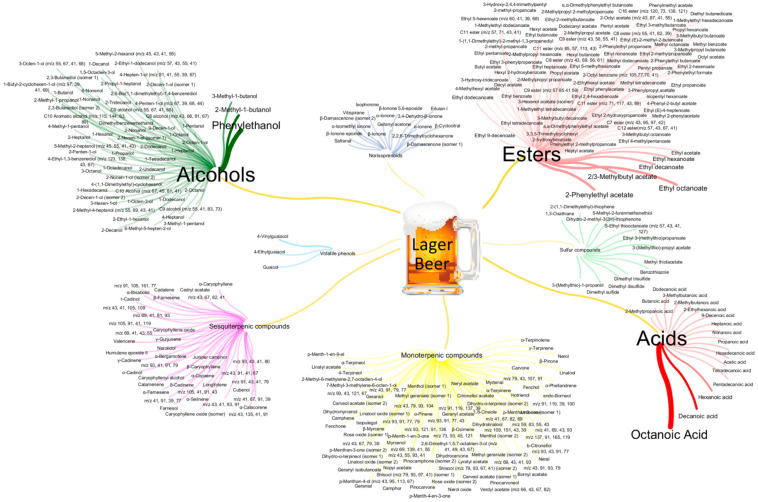
The 329 detected volatile metabolites were systematized in Figure 4, in which the detected volatile metabolites (target nodes) were displayed and organized according to the respective chemical families (nodes).
Figure 4.
Systematization of 329 volatile metabolites detected in lager beer in study, distributed over 8 chemical families. Nodes correspond to chemical families and the target nodes are the detected metabolites. Edge thickness is linked to the amount (median GC peak area) of each metabolite, as well as the size of the nodes’ name.
The chemical families with higher number of detected metabolites present in Figure 4 were esters and monoterpenic compounds (ca. 90 metabolites each), followed by alcohols (60), sesquiterpenic compounds (42), acids (17), norisoprenoids (16), sulfur compounds (13), and volatile phenols (3). Moreover, the amount of each metabolite (median GC chromatographic peak area in all lager beer under study) is reflected in the edge’ thickness, and also in the size of the volatile metabolites’ name (Figure 4), i.e., the higher GC chromatographic peak area is proportional to the highest volatile metabolites’ names and edge thickness (e.g., octanoic acid and phenylethanol were the volatile metabolites with the highest GC chromatographic peak area, considering all the lager beers under study).
The variety and diversity of the detected chemical structures from the 8 targeted families, even within each chemical family and also the wide range of detected amount (variation of GC chromatographic peak areas from 104 to 109) was only possible due to the methodology used. In fact, the orthogonal mechanism and ToF analyzer of GC×GC-ToFMS increases the chromatographic and spectral resolution and also the sensitivity, allowing the simultaneous analysis of major and trace analytes of beer within a single analysis.
From the detected volatile metabolites, it was possible to observe that 168 were present in all lager beers under study being called as common volatile metabolites. They were listed in Table 2 and represent ca. 51% of the total detected metabolites. An in-depth analysis of these 168 common metabolites allowed to construct Table 3, in which it is possible to compare the number and percentage of the common volatile metabolites within each chemical family. Table 3 shows that the number of common volatile metabolites varied between 22% and 100% from the total number of detected volatile metabolites depending on the chemical family. In fact, the chemical families can be ordered according to the increase of the percentage of common volatile metabolites: monoterpenic compounds < sesquiterpenic compounds < norisoprenoids < acids < alcohols < esters < sulfur compounds < volatile phenols. Therefore, the majority of monoterpenic and sesquiterpenic compounds were not present in all lager beers (only 22.0% and 33.3% of the total detected volatile metabolites, respectively), and they may contribute for the highest diversity and peculiar characteristics of the volatile composition of the lager beers under study. While volatile phenols and sulfur compounds were almost present in all lager beers (100% and 76.9% of the total detected volatile metabolites, respectively).
Table 2.
List of the common volatile metabolites detected in all lager beers under study, using HS-SPME/GC×GC-ToFMS, including relevant chromatographic data used to assess compounds identification and those compounds that were previously reported on lager beer. More details, including chromatographic data, are available in Table S1. Bold names represent the major detected volatile metabolites.
| 1tR (s) a | 2tR (s) a | Compound | CAS Number | MSI Level b | RI Calc.c |
RI Lit d |
Previously Reported on Lager Beer |
|---|---|---|---|---|---|---|---|
| Monoterpenic compounds | |||||||
| 606 | 0.91 | Linalool | 78-70-6 | 1 | 1101 | 1101 | [28,42,43,44,45] |
| 624 | 0.97 | Fenchol | 1632-73-1 | 2 | 1113 | 1118 | [28,45,46] |
| 636 | 1.02 | Myrcenol | 543-39-5 | 2 | 1122 | 1103 | [28,47] |
| 696 | 1.15 | Borneol | 507-70-0 | 1 | 1163 | 1166 | [28,46] |
| 708 | 0.95 | Menthol | 1490-04-6 | 1 | 1172 | 1174 | [28] |
| 714 | 0.86 | (−)-Terpinen-4-ol | 20126-76-5 | 1 | 1176 | 1180 | [28,45,48] |
| 732 | 1.04 | α-Terpineol | 98-55-5 | 1 | 1192 | 1195 | [28,42] |
| 774 | 1.22 | 7-Methyl-3-methylene-6-octen-1-ol | 13066-51-8 | 2 | 1219 | 1222 | [28] |
| 792 | 1.09 | (+)-β-Citronellol | 106-22-9 | 1 | 1233 | 1234 | [28,42,43,45,47] |
| 792 | 1.22 | Nerol | 106-25-2 | 1 | 1233 | 1237 | [28] |
| 864 | 0.65 | Bornyl acetate | 76-49-3 | 2 | 1287 | 1288 | [28] |
| 912 | 0.74 | Methyl geraniate (isomer) | 2349-14-6 | 1 | 1323 | 1324 | [28,47] |
| 948 | 0.65 | Citronellol acetate | 150-84-5 | 2 | 1353 | 1354 | [28,47] |
| 966 | 0.70 | Neryl acetate | 141-12-8 | 2 | 1364 | 1362 | [28,42] |
| 444 | 0.49 | β-Myrcene | 123-35-3 | 1 | 988 | 988 | [28,42,43,45,47] |
| 582 | 0.67 | Fenchone | 7787-20-4 | 2 | 1084 | 1093 | [28] |
| 666 | 0.79 | (1R)-(+)-Camphor | 464-49-3 | 1 | 1142 | 1147 | √ [28] |
| 810 | 0.91 | p-Menth-4-en-3-one | 5113-66-6 | 2 | 1246 | 1251 | √ [28] |
| 618 | 0.58 | Rose oxide (isomer) | 16409-43-1 | 1 | 1109 | 1114 | √ [28,46] |
| 720 | 0.54 | m/z 91, 119, 39, 100 | – | 3 | 1180 | – | √ [28] |
| Sesquiterpenic compounds | |||||||
| 1206 | 0.76 | Nerolidol | 7212-44-4 | 1 | 1571 | 1573 | [28,43,47,49] |
| 1212 | 0.79 | Caryophyllenyl alcohol | – | 2 | 1577 | 1569 | [28,43,45] |
| 1242 | 0.82 | m/z 43, 67, 82, 41 | – | 3 | 1610 | – | [28] |
| 1260 | 0.68 | Cubenol | 21284-22-0 | 2 | 1634 | 1643 | [28] |
| 1272 | 0.75 | τ-Cadinol | 5937-11-1 | 2 | 1651 | 1651 | [28,42] |
| 1080 | 0.57 | α-Caryophyllene (α-Humulene) | 6753-98-6 | 1 | 1456 | 1456 | [28,42,45] |
| 1104 | 0.56 | γ-Gurjunene | 22567-17-5 | 2 | 1476 | 1472 | [28] |
| 1164 | 0.57 | δ-Cadinene | 483-76-1 | 2 | 1530 | 1524 | [28,42,45] |
| 1164 | 0.65 | Calamenene | 72937-55-4 | 2 | 1530 | 1528 | [28,42] |
| 1266 | 0.93 | m/z 41, 67, 91, 39 | – | 3 | 1643 | – | [28] |
| 1164 | 0.78 | m/z 91, 43, 41, 79 | – | 3 | 1530 | – | [28] |
| 1236 | 0.70 | m/z 93, 43, 41, 80 | – | 3 | 1601 | – | [28] |
| 1308 | 0.65 | m/z 43, 135, 41, 91 | – | 3 | 1701 | – | [28] |
| 1392 | 0.60 | m/z 69, 41, 43, 55 | – | 3 | 1851 | – | [28] |
| Norisoprenoids | |||||||
| 504 | 0.63 | 2,2,6-Trimethylcyclo-hexanone | 2408-37-9 | 2 | 1034 | 1051 | – |
| 744 | 0.91 | Safranal | 116-26-7 | 2 | 1197 | 1201 | – |
| 774 | 0.82 | β-Cyclocitral | 432-25-7 | 2 | 1219 | 1225 | – |
| 852 | 0.59 | Vitispirane | 65416-59-3 | 2 | 1278 | 1289 | – |
| 900 | 0.64 | Edulan I | 41678-29-9 | 2 | 1314 | 1314 | – |
| 960 | 0.78 | β-Damascenone (isomer) | 23726-93-4 | 2 | 1360 | 1383 | [43,45,46,47,49,50,51] |
| 990 | 0.84 | β-Damascenone (isomer) | 23726-93-4 | 2 | 1383 | 1383 | [43,45,46,47,49,50,51] |
| 1074 | 0.77 | Geranyl acetone | 689-67-8 | 1 | 1451 | 1455 | [47] |
| 1110 | 0.72 | α-Isomethyl ionone | 127-51-5 | 2 | 1481 | 1487 | – |
| Esters | |||||||
| Aliphatics | |||||||
| 96 | 0.44 | Ethyl acetate | 141-78-6 | 2 | 611 | 611 | [23,42,46,47,50,52,53] |
| 138 | 0.45 | Ethyl propanoate | 105-37-3 | 1 | 685 | 696 | [50] |
| 174 | 0.43 | Ethyl 2-methylpropanoate | 97-62-1 | 2 | 748 | 751 | [51] |
| 186 | 0.48 | 2-Methylpropyl acetate | 110-19-0 | 2 | 769 | 769 | [42,43,45,47,50,52,53] |
| 210 | 0.50 | Ethyl butanoate | 105-54-4 | 1 | 806 | 806 | [23,42,43,45,46,47,49,50,51,52,53] |
| 222 | 0.52 | Butyl acetate | 123-86-4 | 2 | 816 | 819 | [53] |
| 222 | 1.24 | Ethyl 2-hydroxypropanoate | 97-64-3 | 2 | 817 | 819 | [48] |
| 258 | 0.48 | Ethyl 2-methylbutanoate | 7452-79-1 | 1 | 848 | 851 | [52,53] |
| 264 | 0.49 | Ethyl 3-methylbutanoate | 108-64-5 | 2 | 853 | 857 | [43,49] |
| 294 | 0.62 | 2/3-Methylbutyl acetate | 123-92-2 | 2 | 879 | 877 | [23,42,43,45,46,47,49,50,52,53] |
| 324 | 0.53 | Ethyl pentanoate | 539-82-2 | 2 | 905 | 906 | [47,49,51] |
| 342 | 0.57 | Pentyl acetate | 628-63-7 | 2 | 917 | 916 | [54] |
| 342 | 0.66 | C7 ester (m/z 43, 95, 97, 42) | - | 3 | 917 | - | – |
| 372 | 0.64 | Ethyl (E)-2-methyl-2-butenoate | 5837-78-5 | 2 | 938 | 943 | – |
| 408 | 0.54 | Ethyl 4-methylpentanoate | 25415-67-2 | 2 | 963 | 967 | [51] |
| 414 | 0.52 | Pentyl propanate | 624-54-4 | 2 | 967 | 969 | [54] |
| 432 | 0.57 | C8 ester (m/z 43, 69, 56, 61) | - | 3 | 980 | - | – |
| 456 | 0.57 | Ethyl hexanoate | 123-66-0 | 1 | 996 | 996 | [23,42,43,45,46,47,48,49,50,51,52,53] |
| 480 | 0.59 | Hexyl acetate | 142-92-7 | 1 | 1013 | 1006 | [23,43,45,46,47,48,49,50,53] |
| 522 | 0.65 | Ethyl 2-hexenoate | 1552-67-6 | 2 | 1042 | 1045 | – |
| 552 | 0.55 | Ethyl 5-methylhexanoate | 10236-10-9 | 2 | 1063 | 1072 | [45,47] |
| 570 | 0.58 | C9 ester (m/z 43, 56, 55, 41) | - | 3 | 1075 | - | – |
| 600 | 0.55 | Propyl hexanoate | 626-77-7 | 2 | 1096 | 1101 | – |
| 600 | 0.57 | Ethyl heptanoate | 106-30-9 | 1 | 1096 | 1095 | [23,43,45,48,50] |
| 624 | 0.59 | Heptyl acetate | 112-06-1 | 2 | 1113 | 1113 | [23,43,45,46,47,48,50] |
| 642 | 0.60 | Methyl octanoate | 111-11-5 | 2 | 1125 | 1130 | – |
| 660 | 0.55 | 2-Octyl acetate (m/z 43, 87, 41, 55) | 2051-50-5 | 3 | 1138 | - | – |
| 660 | 0.90 | C9 ester (m/z 57 85 41 59) | - | 3 | 1138 | - | – |
| 678 | 0.54 | 2-Methylpropyl hexanoate | 105-79-3 | 2 | 1150 | 1154 | – |
| 678 | 0.56 | 2-Ethylhexyl acetate | 103-09-3 | 2 | 1150 | 1159 | – |
| 744 | 0.59 | Ethyl octanoate | 106-32-1 | 1 | 1196 | 1196 | [23,43,45,46,47,49,50,51,52,53,54] |
| 762 | 0.60 | Octyl acetate | 112-14-1 | 2 | 1210 | 1211 | [23,43,45,47,49] |
| 876 | 0.58 | Ethyl nonanoate | 123-29-5 | 1 | 1296 | 1295 | [43,45,47] |
| 924 | 1.12 | C11 ester (m/z 71, 117, 43, 89) | - | 3 | 1333 | - | – |
| 948 | 1.03 | C11 ester (m/z 57, 71, 43, 41) | - | 3 | 1351 | - | – |
| 972 | 0.66 | C12 ester (m/z 57, 43, 67, 41) | - | 3 | 1369 | - | – |
| 978 | 0.93 | 3-Hydroxy-2,4,4-trimethylpentyl 2-methyl-propanoate | 74367-34-3 | 2 | 1373 | 1364 | [43] |
| 996 | 0.64 | Ethyl 9-decenoate | 67233-91-4 | 2 | 1387 | 1388 | [43,45,47,50] |
| 1008 | 0.58 | Ethyl decanoate | 110-38-3 | 1 | 1396 | 1396 | [23,42,43,45,46,48,49,50,52,53,54] |
| 1068 | 0.56 | 3-Methylbutyl octanoate | 2035-99-6 | 2 | 1446 | 1447 | [43] |
| 1164 | 0.62 | Methyl dodecanoate | 111-82-0 | 2 | 1530 | 1526 | – |
| 1176 | 1.03 | 3-Hydroxytridecanoate | 107141-15-1 | 2 | 1542 | 1539 | – |
| 1230 | 0.56 | Ethyl dodecanoate | 106-33-2 | 2 | 1595 | 1594 | [42,43,46,49,50,53] |
| 1236 | 0.56 | 1-(1,1-Dimethylethyl)-2-methyl-1,3-propanediyl 2-methyl-propanoate | 74381-40-1 | 2 | 1601 | 1607 | – |
| 1260 | 0.50 | 1-Methylethyl dodecanoate | 10233-13-3 | 2 | 1634 | 1632 | – |
| 1296 | 0.78 | Hexyl 2-hydroxybenzoate | 6259-76-3 | 2 | 1684 | 1678 | – |
| 1326 | 0.53 | Methyl tetradecanoate | 124-10-7 | 2 | 1731 | 1726 | – |
| 1368 | 0.54 | Ethyl tetradecanoate | 124-06-1 | 2 | 1801 | 1801 | [43] |
| 1374 | 0.66 | C16 ester (m/z 120, 73, 138, 121) | - | 3 | 1814 | - | – |
| 1380 | 0.50 | 1-Methylethyl tetradecanoate | 110-27-0 | 2 | 1826 | 1834 | – |
| Aromatics | |||||||
| 594 | 1.22 | Methyl benzoate | 93-58-3 | 2 | 1093 | 1096 | – |
| 702 | 1.07 | Ethyl benzoate | 93-89-0 | 2 | 1167 | 1172 | [43,49] |
| 714 | 1.35 | Methyl 2-phenylacetate | 101-41-7 | 2 | 1176 | 1179 | – |
| 714 | 1.46 | 2-Phenylethyl formate | 104-62-1 | 2 | 1176 | 1174 | – |
| 810 | 1.13 | Ethyl phenylacetate | 101-97-3 | 1 | 1246 | 1248 | [43,45,47] |
| 828 | 1.15 | 2-Phenylethyl acetate | 103-45-7 | 2 | 1260 | 1260 | [23,42,43,45,46,47,48,49,50,51,52,53] |
| 906 | 0.88 | α,α-Dimethylphenylethyl acetate | 151-05-3 | 2 | 1319 | 1320 | – |
| 948 | 1.06 | 2-Phenylethyl propanoate | 122-70-3 | 2 | 1351 | 1357 | – |
| 972 | 1.16 | 3-Phenylpropyl acetate | 122-72-5 | 2 | 1369 | 1388 | – |
| 1002 | 0.93 | Phenylethyl 2-methylpropanoate | 103-48-0 | 2 | 1392 | 1395 | [46,49] |
| 1062 | 1.00 | 2-Phenylethyl butanoate | 103-52-6 | 2 | 1441 | 1441 | [45,46,47] |
| 1092 | 1.40 | Ethyl 3-phenylpropenoate | 103-36-6 | 2 | 1466 | 1480 | [43,50] |
| 1320 | 0.64 | 2-Octyl benzoate (m/z 105,77,70, 41) | 6938-51-8 | 3 | 1721 | - | – |
| 1416 | 0.66 | 3,3,5-Trimethylcyclohexyl 2-hydroxybenzoate | 118-56-9 | 2 | 1901 | 1904 | – |
| Acids | |||||||
| 108 | 3.10 | Acetic acid | 64-19-7 | 2 | 637 | 631 | [23,43,45,47] |
| 270 | 4.81 | 3-Methylbutanoic acid | 503-74-2 | 2 | 862 | 861 | [43,48] |
| 468 | 4.51 | Hexanoic acid | 142-62-1 | 1 | 1007 | 997 | [23,43,45,46,47,48,50] |
| 762 | 3.23 | Octanoic Acid | 124-07-2 | 2 | 1212 | 1210 | [23,43,45,46,47,48,49,50,53] |
| 858 | 3.19 | Nonanoic acid | 112-05-0 | 2 | 1284 | 1288 | [43,47,53] |
| 990 | 2.79 | Decanoic acid | 334-48-5 | 2 | 1384 | 1387 | [43,45,46,47,53] |
| 1212 | 1.96 | Dodecanoic acid | 143-07-7 | 2 | 1578 | 1609 | [43,53] |
| 1350 | 1.32 | Tetradecanoic acid | 544-63-8 | 2 | 1772 | 1788 | – |
| 1422 | 1.14 | Pentadecanoic acid | 1002-84-2 | 1 | 1915 | 1878 | – |
| 1446 | 1.05 | Hexadecanoic acid | 57-10-3 | 1 | 1965 | 1964 | – |
| Alcohols | |||||||
| Aliphatics | |||||||
| 84 | 0.61 | 1-Propanol | 71-23-8 | 2 | 591 | 589 | [23,42] |
| 102 | 0.66 | 2-Methyl-1-propanol | 78-83-1 | 2 | 622 | 613 | [23,42,43,45,46,47,50,51,52,53] |
| 114 | 0.75 | 1-Butanol | 71-36-3 | 2 | 643 | 639 | – |
| 156 | 1.03 | 3-Methyl-1-butanol | 123-51-3 | 1 | 718 | 718 | [23,42,43,45,46,47,48,49,50,51,52,53] |
| 162 | 0.86 | 2-Methyl-1-butanol | 137-32-6 | 2 | 728 | 728 | [42,50,52] |
| 174 | 1.18 | 4-Penten-1-ol (m/z 67, 39, 68, 44) | 821-09-0 | 3 | 749 | - | – |
| 204 | 3.31 | 2,3-Butanediol (isomer) | 513-85-9 | 2 | 803 | 796 | [45] |
| 216 | 3.87 | 2,3-Butanediol (isomer) | 513-85-9 | 2 | 814 | 796 | [45] |
| 246 | 1.02 | 4-Methyl-1-pentanol | 626-89-1 | 2 | 838 | 843 | – |
| 258 | 1.03 | 3-Methyl-1-pentanol | 589-35-5 | 2 | 848 | 851 | – |
| 288 | 1.05 | 1-Hexanol | 111-27-3 | 1 | 875 | 880 | [23,43,45,46,47,48,49,50] |
| 324 | 0.85 | 2-Heptanol | 543-49-7 | 2 | 905 | 907 | – |
| 420 | 1.07 | 1-Heptanol | 111-70-6 | 2 | 975 | 970 | [43,45,46,47,48,50] |
| 432 | 1.01 | 1-Octen-3-ol | 3391-86-4 | 1 | 980 | 982 | [45,52] |
| 450 | 0.98 | 6-Methyl-5-hepten-2-ol | 4630-06-2 | 2 | 996 | 996 | – |
| 456 | 0.81 | 3-Octanol | 589-98-0 | 1 | 996 | 1001 | – |
| 462 | 0.86 | 2-Octanol | 123-96-6 | 2 | 1001 | 1004 | [46] |
| 504 | 0.97 | 2-Ethyl-1-hexanol | 104-76-7 | 2 | 1030 | 1034 | [43,45,48] |
| 558 | 0.63 | 2-Octen-1-ol | 22104-78-5 | 2 | 1067 | 1074 | – |
| 564 | 1.05 | 1-Octanol | 111-87-5 | 1 | 1072 | 1074 | [23,43,45,46,47,48,49,50,53] |
| 606 | 0.84 | 2-Nonanol | 628-99-9 | 2 | 1101 | 1107 | [43,45] |
| 684 | 1.08 | C9 alcohol (m/z 55, 67, 41, 68) | - | 3 | 1159 | - | – |
| 702 | 0.63 | 2-Nonen-1-ol (isomer) | 22104-79-6 | 2 | 1167 | 1151 | – |
| 708 | 1.00 | 1-Nonanol | 143-08-8 | 2 | 1172 | 1174 | [43] |
| 714 | 0.64 | 2-Nonen-1-ol (isomer) | 22104-79-6 | 2 | 1175 | 1179 | – |
| 840 | 1.15 | 9-Decen-1-ol | 13019-22-2 | 2 | 1269 | 1272 | [43] |
| 846 | 0.97 | 1-Decanol | 112-30-1 | 1 | 1274 | 1265 | [43,45,46,47,49,50] |
| 888 | 0.79 | 2-Undecanol | 1653-30-1 | 1 | 1305 | 1309 | [43,45] |
| 1014 | 0.78 | 2-Dodecanol | 10203-28-8 | 1 | 1401 | 1413 | – |
| 1104 | 0.92 | 1-Dodecanol | 112-53-8 | 1 | 1476 | 1480 | [43,47,48] |
| 1410 | 0.66 | 1-Hexadecanol | 36653-82-4 | 2 | 1889 | 1884 | – |
| Aromatics | |||||||
| 582 | 1.99 | Dimethylbenzenemethanol | 617-94-7 | 2 | 1085 | 1086 | – |
| 630 | 2.99 | Phenylethanol | 60-12-8 | 1 | 1119 | 1122 | [23,42,43,45,46,47,48,50,51,52,53] |
| 642 | 1.28 | 4-Ethyl-1,3-benzenediol (m/z 123, 138, 43, 67) | 2896-60-8 | 3 | 1126 | - | – |
| 774 | 1.52 | C10 Aromatic alcohol (m/z 115, 144, 63, 89) | - | 3 | 1219 | - | – |
| 1332 | 1.93 | 2,6-Bis(1,1-dimethylethyl)-1,4-benzenediol | 1020-31-1 | 2 | 1743 | 1683 | – |
| Cyclics | |||||||
| 846 | 1.65 | 1-Butyl-2-cyclohexen-1-ol (m/z 97, 39, 41, 69) | 88116-46-5 | 3 | 1274 | - | – |
| Sulfur compounds | |||||||
| 78 | 0.33 | Dimethyl sulfide | 75-18-3 | 2 | 580 | 526 | [52] |
| 162 | 0.58 | Dimethyl disulfide | 624-92-0 | 2 | 727 | 731 | [52] |
| 330 | 1.02 | 1,3-Oxathiane | 646-12-8 | 2 | 909 | 913 | – |
| 408 | 0.88 | Dimethyl trisulfide | 3658-80-8 | 2 | 963 | 969 | [52] |
| 438 | 2.86 | 3-(Methylthio)-1-propanol | 505-10-2 | 2 | 985 | 989 | [43,46,50,51,52] |
| 456 | 0.74 | 5-Methyl-2-furanmethanethiol | 59303-05-8 | 2 | 996 | 995 | – |
| 606 | 0.99 | Ethyl 3-(methylthio)propanoate | 13327-56-5 | 2 | 1101 | 1098 | – |
| 642 | 1.09 | 3-(Methylthio)-propyl acetate | 16630-55-0 | 2 | 1126 | 1125 | – |
| 780 | 2.03 | Benzothiazole | 95-16-9 | 2 | 1224 | 1230 | – |
| 816 | 0.67 | 2-(1,1-Dimethylethyl)-thiophene | 1689-78-7 | 2 | 1251 | 1251 | – |
| Volatile phenols | |||||||
| 588 | 2.89 | Guaicol | 90-05-1 | 1 | 1090 | 1095 | [49] |
| 858 | 2.01 | 4-Ethylguaiacol | 2785-89-9 | 2 | 1283 | 1290 | [50] |
| 900 | 2.99 | 4-Vinylguaiacol | 7786-61-0 | 2 | 1316 | 1333 | [43,46,47,50,51] |
a Retention times for first (1tR) and second (2tR) dimensions in seconds. b Level of metabolite identification according to Sumner et al. [29]: (1) Identified compounds; (2) Putatively annotated compounds; (3) Putatively characterized compound classes; (4) Unknown compounds. c RI: Retention Index obtained through the modulated chromatogram. d RI, Retention Index reported in the literature for Equity-5 GC column or equivalents.
Table 3.
Number and percentage of the common analytes within each chemical family, as well as the relative chromatographic area of the common and non-common analytes, considering the macro- and microbrewer beers.
| Chemical Family | Number of Common Analytes | Number of Common Analytes within Each Chemical Family (%) | Chromatographic Area of the Common Analytes | Chromatographic Area of the Non-Common Analytes |
|---|---|---|---|---|
| Acids | 10 | 58.8 | >Microbrewer ** | >Microbrewer * |
| Alcohols | 37 | 61.7 | Equal | >Microbrewer *** |
| Esters | 64 | 73.4 | >Microbrewer *** | >Microbrewer *** |
| Monoterpenic compounds | 20 | 22.0 | >Microbrewer **** | >Microbrewer **** |
| Norisoprenoids | 9 | 56.3 | Equal | >Microbrewer *** |
| Sesquiterpenic compounds | 14 | 33.3 | >Microbrewer **** | >Microbrewer **** |
| Sulfur compounds | 10 | 76.9 | >Macrobrewer *** | >Macrobrewer *** |
| Volatile phenols | 3 | 100 | Equal | – |
| TOTAL | 167 | 50.8 | >Microbrewer **** | >Microbrewer **** |
Significant statistical differences are observed for p < 0.05 (*), p < 0.01 (**), p = 0.001 (***), p < 0.0001 (****), using t-test in GraphPad prism.
A set of 12 volatile metabolites were the major ones in lager beers that corresponded to ca. 81% of the total area of the targeted chemical families, namely, 3 acids—octanoic acid, decanoic acid, and hexanoic acid; 3 alcohols—phenylethanol, 2-methyl-1-butanol, and 3-methyl-1-butanol; and 6 esters—2-phenylethyl acetate, 2/3-methylbutyl acetate, ethyl octanoate, ethyl decanoate, ethyl hexanoate, and ethyl acetate. These 12 volatile metabolites were highlighted in bold in Table 2, which contributed highly to the highest total amount of the acids, alcohols, and esters. Indeed, all of these previously mentioned metabolites are frequently detected in beer volatile composition [46,50], being their main source related with yeast metabolism [55,56]. Moreover, these results are in agreement with those reported by Ruvalcaba et al. [49], which showed a similar set of metabolites that had the highest concentration in beers, namely, 3-methyl-1-butanol, ethyl octanoate, ethyl hexanoate, 3-methylbutyl acetate, 2-phenylethyl acetate, and octanoic acid.
The beer profiling can be helpful for the simultaneous screenings and/or follow-up of different aspects, such as the monitoring of the beer aroma (reported in Beer Flavor Wheel [57]) once these targeted chemical families have a huge impact on beer aroma properties, e.g., alcohol (n-propanol, 2-methyl-1-propanol), sour apple (ethyl hexanoate, ethyl octanoate), rose (phenylethanol, phenylethyl acetate), cheese (butanoic, hexanoic, octanoic acids), or fruity (β-damascenone, ethyl decanoate) aromas, among others. Furthermore, the detected volatile metabolites may be used to monitor the yeast metabolism [4,5,23,35], for instance the yeasts’ aminoacids uptake [35], e.g., uptake of leucine promotes the formation of 3-methyl butanol (one of the major volatile metabolites present in all lager beers under study). It also allows the detection of different off-flavors that may be monitored in the final product or along brewing process, namely, dimethyl sulfide (vegetables aroma, formed from malt during wort boiling) [58]; acids (cheese aromas) and sulfur-containing metabolites (putrid aromas), which can be associated to be produced by bacteria spoilage (e.g., Pediococcus spp., Lactobacilli spp., Megasphaera spp., Pectinatus spp.) [9,59]; 4-vinylguaiacol (clove-like aroma) that can be produced by wild yeasts spoilage (Brettanomyces spp., Candida spp., Cryptococcus spp., Hansenula spp., Pichia spp.) [9,59]; or β-damascenone (apple, peach, fruity aromas) that might be produced by carotenoid degradation or glycosides hydrolysis during aging [59].
3.3. Lager Beer Typing
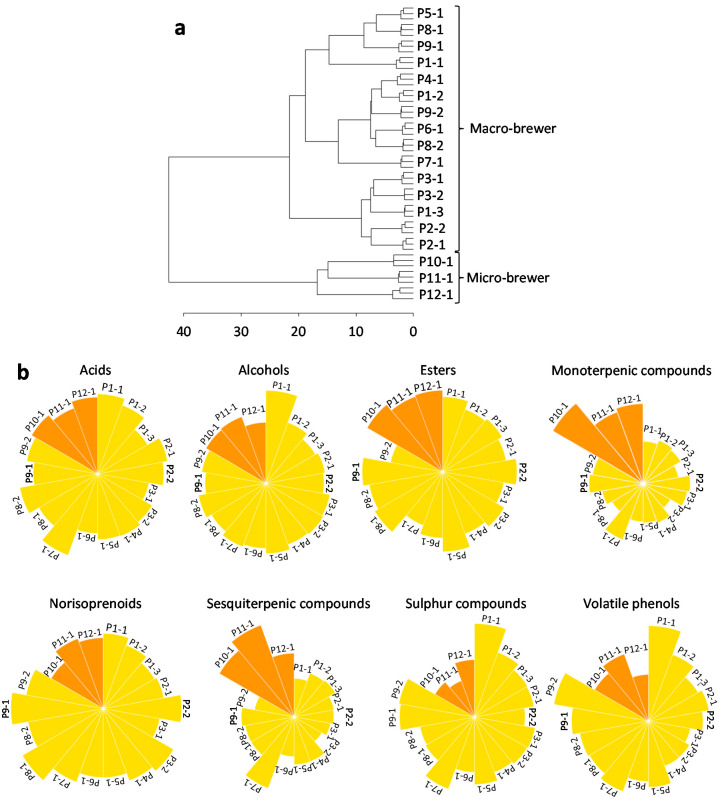
A HCA was applied to the chromatographic data of the 329 detected volatile metabolites (data presented in Supplementary Table S1) from the lager beers under study, in order to characterize this data set by the clusters formation (natural grouping). The obtained dendrogram (Figure 5a) revealed the presence of two main clusters: commercial pasteurized macro-bbs and micro-bbs (including fresh and pasteurized beers). The detailed heatmap can be visualized in Figure S1. The production system was the main differentiating factor among the lager beers under study, and this type of clustering was previously reported only based on the monoterpenic and sesquiterpenic components [28].
Figure 5.
GC chromatographic data of the 329 detected volatile metabolites, from lager beers under study, were submitted to HCA (a), in which lager beers were grouped according to the production system: macrobrewer beers (P1–P9) and microbrewer beers (P10–12). The mean of GC chromatographic areas from each chemical family (b) is displayed for each lager beer (orange—microbrewer beers; yellow—macrobrewer beers).
It is noteworthy to perceive that each lager beer has its own specificities and inherent brewing process, and consequently, lower intra-variability among replicates (good clustering of the 3 replicates in Figure 5a and Figure S1) and higher inter-variability between the 18 lager beers under study from the 12 different producers was observed. Several factors contributed to the lager beers’ clustering, such as different raw materials, brewing (e.g., fermentation, clarification, filling, and pasteurization), and/or also the aging that varied for each lager beer under study. Moreover, as previously stated [28], microbreweries tend to have less brewing steps (e.g., clarification and possible stabilization of beer) and use different raw materials, these factors being possibly the main explanation for the clustering of the samples according to the producer system.
An in-depth analysis of the data was performed to understand the behavior of each chemical family among the lager beers under study. The mean of GC chromatographic areas of each chemical family from each lager beer under study is displayed in Figure 5b. Moreover, the chromatographic area of the common and non-common volatile analytes was also evaluated and compared considering the macro-bbs and micro-bbs, for each chemical family under study (Table 3 and Supplementary Figures S2–S12). Globally, micro-bbs have the highest GC chromatographic areas for most of the detected analytes (common and non-common analytes among all lager beers under study). The exceptions were observed for sulfur compounds that registered the highest GC chromatographic areas for macro-bbs (Figure 5b and Figure S8), and for common alcohols (Figure S3), common norisoprenoids (Figure S6), and common volatile phenols (Figure S8) there were not observed differences statistically significant between macro-bbs and micro-bbs. Giannetti et al. [45] showed different volatile profiles of industrial and craft Pilsner-type beers, particularly terpenic compounds and esters that had highest concentrations in craft beers, when compared with industrial beers, which are in agreement with our results.
Furthermore, as esters are important beer volatiles (have low thresholds and contribute for beers overall aroma [56]), a detailed analysis was performed for different ester classes, namely, short-chain (fruity aromas [16]), long-chain (oily aromas [16]), and acetate esters (fruity aromas [16]). Short-chain and long-chain esters showed highest GC chromatographic areas for micro-bbs (Figure S11), while acetate esters showed no statistical differences between macro-bbs and micro-bbs (Figure S12). Thus, acetate esters are not contributing for the differentiation of the esters profile between macro-bbs and micro-bbs.
Two main clusters according to production system (macro-bbs and micro-bbs) were only verified for alcohols and monoterpenic compounds (statistical analysis was performed to each chemical family, data not shown), being possibly that these two chemical families were the main contributors for the two main clusters that was observed when all the detected metabolites were used (Figure 5a and Figure S1). Furthermore, the global highest content of volatile metabolites was achieved for beer P1-1, particularly due to the higher relative peak area of 2-phenylethanol and 2-phenylethyl acetate (both volatile metabolites that may result from the yeasts’ degradation of phenylalanine [35] and also belong to the set of major volatile metabolites present in the lager beers under study), whereas the highest number of detected volatile metabolites was verified for beer P11-2 (Supplementary Table S1).
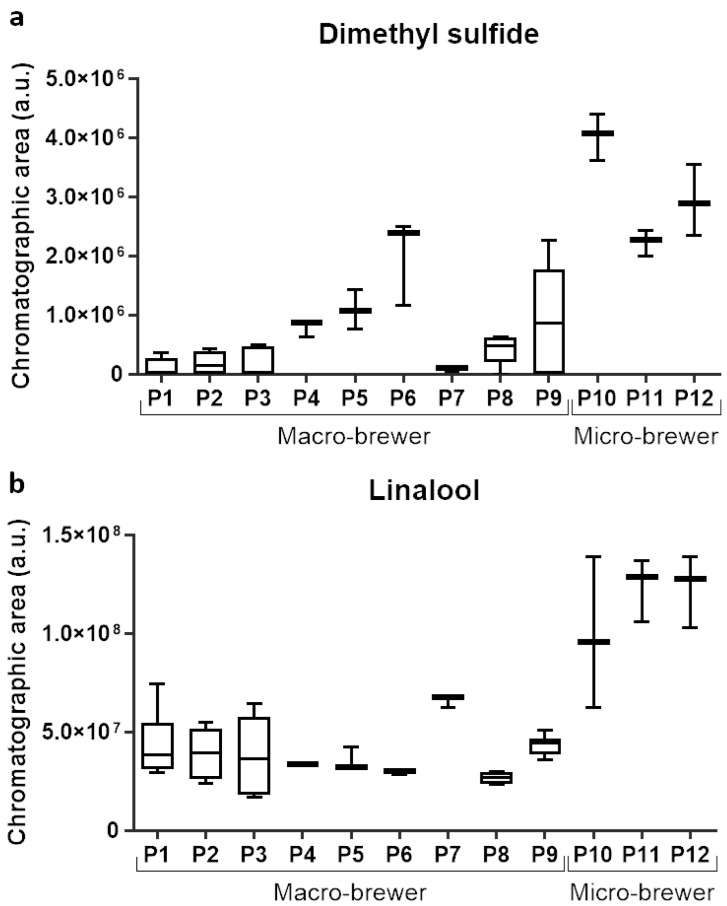
This methodology can be also used to control and monitor the content of particular volatile metabolites, for instance that may be off-flavors or used for quality control. In this case, Figure 6a shows the amount of dimethyl sulfide in all samples under study, in which micro-bbs (P10–P12) had highest median GC chromatographic areas of dimethyl sulfide. This particular volatile metabolite is considered an off-flavor due to its vegetable aroma (if it is present in amount above its sensorial perception limit) and whose origin is reported to be from malt, and this volatile is used for the quality control of the wort boiling step [58]. These results show that it may not have such a good monitoring during wort boiling, which consequently may lead to higher contents of this compound in the lager micro-bbs under study.
Figure 6.
Box plots of the GC chromatographic area of specific metabolites, namely, dimethyl sulphide (a) and linalool (b), monitored in each beer producer under study: P1–P9 are macrobrewer, and P10–P12 are microbrewer.
Linalool, hop aroma indicator in beer [7], can also be monitored. The GC chromatographic area of linalool present in the lager beers under study is represented in Figure 6b, which showed that the median values for micro-bbs (P10–P12) were higher than macro-bbs (P1–P9). Micro-bbs employ different types of raw materials, for instance hop pellets, which when comparing with macro-bbs that use hop extracts, will influence the content of this analyte and may support this variation. A similar result was previously reported when comparing the linalool content between craft and industrial beers, it being higher for the first ones [45].
4. Conclusions
A comprehensive study of lager beer volatile composition was provided by profiling 329 volatile metabolites (wherein 181 were putatively identified, and 96 were reported for the first time), which were distributed over eight chemical families with potential impact on beer aroma, namely, acids (17), alcohols (60), esters (87), monoterpenic compounds (91), norisoprenoids (16), sesquiterpenic compounds (42), sulfur compounds (13), and volatile phenols (3). As far as we know, this study represents the most in-depth and detailed profiling of the lager beer volatile composition, which was only possible due to the combination of the extraction technique (SPME), which allows a direct analysis of beer without the addition of any organic solvent (fulfils the criteria for the green analytical chemistry techniques), with the high sensitivity, chromatographic resolution and high throughput technique, such as the GC×GC-ToFMS. The chromatographic data generated through the modulated chromatogram system can be further used as support of analytes’ identification. This detailed chemical profiling of lager beers allowed the simultaneous determination of a wide range of metabolites, namely, the major components such as acids, alcohols, and esters and the trace ones, like monoterpenic and sesquiterpenic compounds and norisoprenoids and sulfur compounds. Furthermore, data can be explored by univariate analysis to monitor, for instance, the content of target volatile metabolites, e.g., linalool and dimethyl sulfide. In fact, the combination of GC×GC-ToFMS with SPME may represent a useful tool for a streamlined evaluation of beer characteristics by constructing a multiple attribute methodology (MAM) workflow taking advantage of their sensitivity and high throughput attributes. According to the current green analytical chemistry concerns, such type of methodology that provides from a single analysis data from multi-analytes is preferred to methods using one analyte at a time.
Clustering analysis allowed a beer typing according to the beer production system: macro- and microbrewer beers. In fact, around half (ca. 51%) of the detected metabolites were common to all lager beers under study. Except for sulfur compounds, all the chemical families had the highest GC chromatographic areas for micro-bbs. Moreover, no statistical differences were observed for common alcohols, norisoprenoids, or volatile phenols between macro-bbs and micro-bbs. Monoterpenic and sesquiterpenic compounds were not present in all lager beers (only 22.0% and 33.3% of the total detected volatile metabolites, respectively), showing a wide range of chemical structures, which may give the beers unique characteristics. Furthermore, a set of 12 compounds (3 acids, 3 alcohols, and 6 esters) were the major volatile metabolites present in lager beers (ca. 81% of the total area of the targeted chemical families), their origin being associated to yeast fermentation, and they are also the main reported and monitored metabolites in beer volatile composition.
In summary, the generated data contributes to enlarge the knowledge on lager beer volatile metabolites that can be further applied and exploited to obtain relevant information in various contexts, such as the analysis of beer aroma (not only of lager beers but also of other beer styles) and subsequent used for their quality control, beer typing (e.g., understand similarities and differences between different beers, different brewing steps), monitor brewing steps (e.g., fermentation), and detection of off-flavors, among others.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/9/1276/s1. Figure S1: Heatmap and dendrogram representation of 329 volatile components from lager beers under study. Figure S2: Box plots of the chromatographic area of acids for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S3: Box plots of the chromatographic area of alcohols for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S4: Box plots of the chromatographic area of esters for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S5. Box plots of the chromatographic area of monoterpenic compounds for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S6: Box plots of the chromatographic area of norisoprenoids for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S7: Box plots of the chromatographic area of sesquiterpenic compounds for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S8: Box plots of the chromatographic area of sulfur for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S9: Box plots of the chromatographic area of sulfur for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Figure S10: Box plots of the total chromatographic area of all detected analytes for macrobrewer and microbrewer beers. Figure S11: Box plots of the short-chain (left, C6–C12) and long-chain (right, C14 and C16) esters for macrobrewer and microbrewer beers. Figure S12: Box plots of the chromatographic area of acetate esters for macrobrewer and microbrewer beers, considering the common (left) and non-common (right) analytes to all lager beers under study. Table S1: Full data set used for statistics processing including the detected metabolites in lager beers using HS-SPME/GC×GC-ToFMS, with relevant chromatographic data used to assess compounds identification.
Author Contributions
C.M. performed the experimental work, validation, and formal analysis; wrote original version and edited the final version and prepared illustrations. T.B. and A.A. contributed to supervision, data discussion, writing—review and editing. S.M.R. was responsible for conceptualization, writing, review and editing of the final version, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Funding is acknowledged from the FCT/MEC for the financial support to the QOPNA(UID/QUI/00062/2019), LAQV-REQUIMTE (UIDB/50006/2020) and CESAM (UID/AMB/50017/2019), through national funds and where applicable co-financed by the FEDER, within the PT2020 Partnership Agreement. C. Martins thanks the FCT for the PhD grant (SFRH/BD/77988/2011) through the program POPH/FSE.
Conflicts of Interest
The authors declare no conflict of interest. Super Bock Group did not fund this work and had no role in the design of the study; in the collection of samples, analyses, or interpretation of data, or in the decision to publish the results.
References
- 1.World Health Organization . In: Global Status Report on Alcohol and Health 2018. Poznyak V., Rekve D., editors. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 2.Swinnen J.F.M., editor. The Economics of Beer. Oxford University Press; New York, NY, USA: 2011. [Google Scholar]
- 3.Gonçalves J.L., Figueira J.A., Rodrigues F.P., Ornelas L.P., Branco R.N., Silva C.L., Câmara J.S. A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chem. 2014;160:266–280. doi: 10.1016/j.foodchem.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 4.Alves Z., Melo A., Figueiredo A.R., Coimbra M.A., Gomes A.C., Rocha S.M. Exploring the Saccharomyces cerevisiae volatile metabolome: Indigenous versus commercial strains. PLoS ONE. 2015;10:e0143641. doi: 10.1371/journal.pone.0143641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodolo E.J., Kock J.L.F., Axcell B.C., Brooks M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008;8:1018–1036. doi: 10.1111/j.1567-1364.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoff S., Lund M.N., Petersen M.A., Jespersen B.M., Andersen M.L. Quality of pilsner malt and roasted malt during storage. J. Inst. Brew. 2014;120:331–340. doi: 10.1002/jib.144. [DOI] [Google Scholar]
- 7.Almaguer C., Schönberger C., Gastl M., Arendt E.K., Becker T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014;120:289–314. doi: 10.1002/jib.160. [DOI] [Google Scholar]
- 8.Vanderhaegen B., Neven H., Verachtert H., Derdelinckx G. The chemistry of beer aging—A critical review. Food Chem. 2006;95:357–381. doi: 10.1016/j.foodchem.2005.01.006. [DOI] [Google Scholar]
- 9.Boulton C. Encyclopaedia of Brewing. Wiley-Blackwell; West Sussex, UK: 2013. [Google Scholar]
- 10.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuadros-Rodríguez L., Ruiz-Samblás C., Valverde-Som L., Péez-Castaño E., González-Casado A. Chromatographic fingerprinting: An innovative approach for food “identitation” and food authentication—A tutorial. Anal. Chim. Acta. 2016;909:9–23. doi: 10.1016/j.aca.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Putignani L., Dallapiccola B. Foodomics as part of the host-microbiota-exposome interplay. J. Proteom. 2016;147:3–20. doi: 10.1016/j.jprot.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Andrés-Iglesias C., Blanco C.A., Blanco J., Montero O. Mass spectrometry-based metabolomics approach to determine differential metabolites between regular and non-alcohol beers. Food Chem. 2014;157:205–212. doi: 10.1016/j.foodchem.2014.01.123. [DOI] [PubMed] [Google Scholar]
- 14.Stefanuto P.-H., Perrault K.A., Dubois L.M., L’Homme B., Allen C., Loughnane C., Ochiai N., Focant J.-F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A. 2017;1507:45–52. doi: 10.1016/j.chroma.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 15.Lachenmeier D.W., Frank W., Humpfer E., Schäfer H., Keller S., Mörtter M., Spraul M. Quality control of beer using high-resolution nuclear magnetic resonance spectroscopy and multivariate analysis. Eur. Food Res. Technol. 2005;220:215–221. doi: 10.1007/s00217-004-1070-7. [DOI] [Google Scholar]
- 16.Romero-Medina A., Estarrón-Espinosa M., Verde-Calvo J.R., Lelièvre-Desmas M., Escalona-Buendía H.B. Renewing traditions: A sensory and chemical characterisation of mexican pigmented corn beers. Foods. 2020;9:886. doi: 10.3390/foods9070886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inui T., Tsuchiya F., Ishimaru M., Oka K., Komura H. Different beers with different hops. Relevant compounds for their aroma characteristics. J. Agric. Food Chem. 2013;61:4758–4764. doi: 10.1021/jf3053737. [DOI] [PubMed] [Google Scholar]
- 18.Pope G.A., Mackenzie D.A., Defernez M., Aroso M.A.M.M., Fuller L.J., Mellon F.A., Dunn W.B., Brown M., Goodacre R., Kell D.B., et al. Metabolic footprinting as a tool for discriminating between brewing yeasts. Yeast. 2007;24:667–679. doi: 10.1002/yea.1499. [DOI] [PubMed] [Google Scholar]
- 19.Krogerus K., Magalhães F., Vidgren V., Gibson B. New lager yeast strains generated by interspecific hybridization. J. Ind. Microbiol. Biotechnol. 2015;42:769–778. doi: 10.1007/s10295-015-1597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callejo M.J., García Navas J.J., Alba R., Escott C., Loira I., González M.C., Morata A. Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur. Food Res. Technol. 2019;245:1229–1238. doi: 10.1007/s00217-019-03244-w. [DOI] [Google Scholar]
- 21.Paiva A.C., Oliveira D.S., Hantao L.W. A bottom-up approach for data mining in bioaromatization of beers using flow-modulated comprehensive two-dimensional gas chromatography/mass spectrometry. Separations. 2019;6:46. doi: 10.3390/separations6040046. [DOI] [Google Scholar]
- 22.Spevacek A.R., Benson K.H., Bamforth C.W., Slupsky C.M. Beer metabolomics: Molecular details of the brewing process and the differential effects of late and dry hopping on yeast purine metabolism. J. Inst. Brew. 2016;122:21–28. doi: 10.1002/jib.291. [DOI] [Google Scholar]
- 23.Alves V., Gonçalves J., Figueira J.A., Ornelas L.P., Branco R.N., Câmara J.S., Pereira J.A.M. Beer volatile fingerprinting at different brewing steps. Food Chem. 2020;326:126856. doi: 10.1016/j.foodchem.2020.126856. [DOI] [PubMed] [Google Scholar]
- 24.Heuberger A.L., Broeckling C.D., Lewis M.R., Salazar L., Bouckaert P., Prenni J.E. Metabolomic profiling of beer reveals effect of temperature on non-volatile small molecules during short-term storage. Food Chem. 2012;135:1284–1289. doi: 10.1016/j.foodchem.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 25.Hughey C.A., McMinn C.M., Phung J. Beeromics: From quality control to identification of differentially expressed compounds in beer. Metabolomics. 2016;12:1–13. doi: 10.1007/s11306-015-0885-5. [DOI] [Google Scholar]
- 26.Andrés-Iglesias C., Blanco C.A., García-Serna J., Pando V., Montero O. Volatile compound profiling in commercial lager regular beers and derived alcohol-free beers after dealcoholization by vacuum distillation. Food Anal. Methods. 2016;9:3230–3241. doi: 10.1007/s12161-016-0513-7. [DOI] [Google Scholar]
- 27.Martins C., Brandão T., Almeida A., Rocha S.M. Insights on beer volatile profile: Optimization of solid-phase microextraction procedure taking advantage of the comprehensive two-dimensional gas chromatography structured separation. J. Sep. Sci. 2015;38:2140–2148. doi: 10.1002/jssc.201401388. [DOI] [PubMed] [Google Scholar]
- 28.Martins C., Brandão T., Almeida A., Rocha S.M. Unveiling the lager beer volatile terpenic compounds. Food Res. Int. 2018;114:199–207. doi: 10.1016/j.foodres.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Sumner L., Amberg A., Barret D., Beale M., Beger R., Daykin C., Fan T.W.-M., Fiehn O., Goodacre R., Griffin J.L., et al. Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Den Dool H., Kratz D.J.A. Generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 31.Xia J., Sinelnikov I.V., Han B., Wishart D.S. MetaboAnalyst 3.0- making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Researc. Center for Veterinary Medicin Guidance for Industry: Bioanalytical Method Validation. Draft Guidance. [(accessed on 27 June 2019)]; Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.
- 33.Kim S., Kim J., Yun E.J., Kim K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016;37:16–23. doi: 10.1016/j.copbio.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Cordente A.G., Curtin C.D., Varela C., Pretorius I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012;96:601–618. doi: 10.1007/s00253-012-4370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazelwood L.A., Daran J.M., van Maris A.J.A., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez-Bustamante E., Sánchez S. Microbial production of C13-norisoprenoids and other aroma compounds via carotenoid cleavage. Crit. Rev. Microbiol. 2007;33:211–230. doi: 10.1080/10408410701473306. [DOI] [PubMed] [Google Scholar]
- 37.Chemler J.A., Yan Y., Koffas M.A.G. Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb. Cell Fact. 2006;5:20. doi: 10.1186/1475-2859-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui M.S., Thodey K., Trenchard I., Smolke C.D. Advancing secondary metabolite biosynthesis in yeast with synthetic biology tools. FEMS Yeast Res. 2012;12:144–170. doi: 10.1111/j.1567-1364.2011.00774.x. [DOI] [PubMed] [Google Scholar]
- 39.Takoi K., Koie K., Itoga Y., Katayama Y., Shimase M., Nakayama Y., Watari J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010;58:5050–5058. doi: 10.1021/jf1000524. [DOI] [PubMed] [Google Scholar]
- 40.Lambrechts M.G., Pretorius I.S. Yeast and its importance to wine aroma. S. Afr. J. Enol. Vitic. 2000;21:97–129. doi: 10.21548/21-1-3560. [DOI] [Google Scholar]
- 41.King A.J., Dickinson J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003;3:53–62. doi: 10.1016/S1567-1356(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 42.Dresel M., Praet T., Van Opstaele F., Van Holle A., Naudts D., De Keukeleire D., De Cooman L., Aerts G. Comparison of the analytical profiles of volatiles in single-hopped worts and beers as a function of the hop variety. Brew. Sci. 2015;68:8–28. [Google Scholar]
- 43.Tsuji H., Mizuno A. Volatile compounds and the changes in their concentration levels during storage in beers containing varying malt concentrations. J. Food Sci. 2010;75:C79–C84. doi: 10.1111/j.1750-3841.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 44.Castro L.F., Ross C.F., Vixie K.R. Optimization of a solid phase dynamic extraction (SPDE) method for beer volatile profiling. Food Anal. Methods. 2015;8:2115–2124. doi: 10.1007/s12161-015-0104-z. [DOI] [Google Scholar]
- 45.Giannetti V., Boccacci Mariani M., Torrelli P., Marini F. Flavour component analysis by HS-SPME/GC–MS and chemometric modeling to characterize Pilsner-style Lager craft beers. Microchem. J. 2019;149:103991. doi: 10.1016/j.microc.2019.103991. [DOI] [Google Scholar]
- 46.Riu-Aumatell M., Miró P., Serra-Cayuela A., Buxaderas S., López-Tamames E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014;57:196–202. doi: 10.1016/j.foodres.2014.01.016. [DOI] [Google Scholar]
- 47.Rendall R., Reis M.S., Pereira A.C., Pestana C., Pereira V., Marques J.C. Chemometric analysis of the volatile fraction evolution of Portuguese beer under shelf storage conditions. Chemom. Intell. Lab. Syst. 2015;142:131–142. doi: 10.1016/j.chemolab.2015.01.015. [DOI] [Google Scholar]
- 48.Harayama K., Hayase F., Kato H. New method for analyzing the volatiles in beer. Biosci. Biotechnol. Biochem. J. 1994;58:2246–2247. doi: 10.1271/bbb.58.2246. [DOI] [Google Scholar]
- 49.Ruvalcaba J.E., Durán-Guerrero E., Barroso C.G., Castro R. Development of head space sorptive extraction method for the determination of volatile compounds in beer and comparison with stir bar sorptive extraction. Foods. 2020;9:255. doi: 10.3390/foods9030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Bencomo J.J., Muñoz-González C., Martín-Álvarez P.J., Lázaro E., Mancebo R., Castañé X., Pozo-Bayón M.A. Optimization of a HS-SPME-GC-MS procedure for beer volatile profiling using response surface methodology: Application to follow aroma stability of beers under different storage conditions. Food Anal. Methods. 2012;5:1386–1397. doi: 10.1007/s12161-012-9390-x. [DOI] [Google Scholar]
- 51.Fritsch H.T., Schieberle P. Identification based on quantitative measurements and aroma recombination of the character impact odorants in a Bavarian Pilsner-type beer. J. Agric. Food Chem. 2005;53:7544–7551. doi: 10.1021/jf051167k. [DOI] [PubMed] [Google Scholar]
- 52.Vera L., Aceña L., Guasch J., Boqué R., Mestres M., Busto O. Characterization and classification of the aroma of beer samples by means of an MS e-nose and chemometric tools. Anal. Bioanal. Chem. 2011;399:2073–2081. doi: 10.1007/s00216-010-4343-y. [DOI] [PubMed] [Google Scholar]
- 53.Rossi S., Sileoni V., Perretti G., Marconi O. Characterization of the volatile profiles of beer using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Sci. Food Agric. 2014;94:919–928. doi: 10.1002/jsfa.6336. [DOI] [PubMed] [Google Scholar]
- 54.Castro L.F., Ross C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction. J. Inst. Brew. 2015;121:197–203. doi: 10.1002/jib.219. [DOI] [Google Scholar]
- 55.Brányik T., Vicente A.A., Dostálek P., Teixeira J.A. A review of flavour formation in continuous beer fermentations. J. Inst. Brew. 2008;114:3–13. doi: 10.1002/j.2050-0416.2008.tb00299.x. [DOI] [Google Scholar]
- 56.Pires E.J., Teixeira J.A., Brányik T., Vicente A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014;98:1937–1949. doi: 10.1007/s00253-013-5470-0. [DOI] [PubMed] [Google Scholar]
- 57.Meilgaard M.C., Dalgliesh C.E., Clapperton J.F. Beer flavour terminology. J. Inst. Brew. 1979;85:38–42. doi: 10.1002/j.2050-0416.1979.tb06826.x. [DOI] [Google Scholar]
- 58.Eßlinger H.M., editor. Handbook of Brewing. John Wiley & Sons; Hoboken, NJ, USA: 2009. [Google Scholar]
- 59.Preedy V.R., editor. Beer in Health and Disease Prevention. Elsevier Inc.; Burlington, MA, USA: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.