Abstract
In this study, orange seed proteins were hydrolyzed by Alcalase enzyme at different enzyme concentrations 1–3% (v/w) and hydrolysis times (2–5 h), to obtain bioactive peptides showing antioxidant, Angiotensin-converting enzyme (ACE) -inhibitory, and hypoglycemic activities. The highest biological activities (p < 0.05) were achieved by using a hydrolysis time of 5 h and an enzyme concentration of 2%. Orange seed protein hydrolysate (OSPH) was prepared under these conditions, and peptides were isolated and purified by using size-exclusion chromatography and high-performance liquid chromatography, respectively. The fractions that showed the highest biological activities were analyzed by mass spectrometry in tandem, and a total of 63 peptide sequences were found. Moreover, the effect of simulated gastrointestinal digestion on the bioactivity of the fractions was studied, and the novel peptide sequences generated were also identified. Overall, despite there being some differences in the profile of peptide sequences obtained, the main results showed non-significant differences in the analyzed bioactivities after simulated gastrointestinal digestion.
Keywords: orange seed, bioactive peptides, antioxidant, ACE-inhibitory, antidiabetic activity, gastrointestinal digestion
1. Introduction
Throughout the years, studies have proved that health and nutrition are extremely interrelated. Not only is food the supplier of the necessary nutrients for the correct functionality of the metabolism, but certain compounds such as hydrolyzed proteins and peptides can also stimulate and modulate specific and desirable physiological reactions in the body [1]. Bioactive peptides sequence present in the parent’s proteins needs to be further hydrolyzed to have a function; in fact, processes such as proteolytic microorganisms fermentation, plant or microorganism-extracted enzymes proteolysis, and gastrointestinal digestion can release them and activate their function [2,3]. As the use of microbial proteases is simple, non-expensive, and completely safe, it is the preferred methodology for hydrolysis processes compared with the other commercial proteases from plant and animal origin [4]. The resulting hydrolyzed proteins and peptides have been described to exert an important biological role, including antioxidant [5], antimicrobial [6], anticancer [5], antidiabetic [7], and antihypertensive activities [8]. They also influence the cardiovascular, immune, nervous, and gastrointestinal systems [8,9].
Generally, the hydrolysis of protein reduces its molecular weight, increases the ionic groups’ number, and enhances the access to hydrophilic regions [10]. To show their activities, peptides must reach their target sites mainly through the bloodstream [11]. Based on the results of experiments on the effect of gastrointestinal digestion conditions in vitro condition on the antioxidant and antimicrobial activity of hydrolyzed flaxseed proteins, it was found that gastrointestinal digestion had very little effect on the antimicrobial activity of peptides; however, this effect has been greater on antioxidant activity [12]. In a study conducted by Orsini et al. (2011), Amaranth hydrolysates were digested under simulated gastrointestinal digestion. After evaluating the antioxidant activity, they saw that the digested samples showed a good percentage of ABTS radical inhibition activity and inhibition of reactive oxygen species [13]. The results of the assay showed that simulated gastrointestinal digestion increased the Angiotensin-converting enzyme (ACE) -inhibitory activity of bean and lentil hydrolysates [14].
Hydrolyzed proteins prepared from less economically valued sources, especially food industry by-products, can be considered as a rich reservoir of bioactive peptides to be applied as nutritional supplements or functional enhancers for the production of functional foods. In this sense, Siavaraze Citrus sinensis of citrus species and Rutaceae family are commonly used in the juice production industry. Iran produces around 2,700,000 tons of citrus fruits and ranks seventh in the fruit production. Citrus species are used fresh or processed to juice; consequently, large amounts of seeds are discharged at processing plants. The wastes of the juice industry, including peels, seeds, and pulps, represent about 50% of the raw processed fruit. This potentially valuable resource, otherwise processed, can aggravate disposal problems [15]. Orange seed flour, having been defatted, is considered a by-product containing about 17.9–26.5% of protein that is possible to be implemented as a valuable and economical source for proteins extraction and hydrolysis [16]. The objective of the current study was to investigate the optimum circumstances for enzyme-driven hydrolysis of orange seed protein concentrate, using Alcalase enzyme for the production of hydrolyzed proteins showing the highest antioxidant, antihypertensive, and hypoglycemic activities. Having been separated by size-exclusion chromatography (SEC) and HPLC-RP, Alcalase hydrolysate was then investigated, and those fractions showing the highest bioactivity were analyzed, using mass spectrometry in tandem (MS/MS), in order to identify the active peptides.
2. Materials and Methods
2.1. Materials
Seeds meal of orange fruit (Siavaraze Citrus sinensis) with 3.6 ± 0.32% protein, 54.2 ± 12% lipid, 10.13 ± 0.65% moisture, and 2.5 ± 0.23 ash was purchased from Kosar Cultivation and Technology factory, Gorgan, Iran. Alcalase enzyme (protease from Bacillus lichenformis, 2.4 U/g) and all other chemicals were obtained from Merck and were of analytical grade.
2.2. Productions of Orange Seed Proteins Concentrate
Orange seed proteins concentrate was produced based on the method which was described by Horax et al. [17]. Distilled water was added to the orange seed flour, with the ratio of 10:1, at room temperature; they were then mixed, and pH was adjusted to 10, using 1 mol/L NaOH. The sample was subsequently stirred for 1 h, at ambient temperature, and centrifuged at 12,000 rpm, for 15 min (Avanti J-26S XP, Beckman, Brea, CA, USA). Then the pH of supernatant was adjusted to 3 by 1 mol/L HCl, and it was kept at room temperature for 30 min. The suspension was then centrifuged at 12,000 rpm, at the same temperature for 15 min. The produced pellets were washed with 20 mL distilled water and then freeze-dried (SCANVAC, Labogene ApS, Alleryd, Denmark).
2.3. Optimization of Enzymatic Hydrolysis
The enzymatic hydrolysis was conducted on protein solution with concentration of 0.05% w/v, using Alcalase enzyme at different concentrations, namely 1, 1.5, and 3% (enzyme to substrate ratio); a temperature of 55 °C; and a hydrolysis time of 2, 3.5, and 5 h, in a shaker incubator (Radleys, Essex, UK). The samples included the following: Sample 1, 0.01 E/S-2 h; Sample 2, 0.02 E/S-2 h; Sample 3, 0.03 E/S-2 h; Sample 4, 0.01 E/S-3.5 h; Sample 5, 0.02 E/S-3.5 h; Sample 6, 0.03 E/S-3.5 h; Sample 7, 0.01 E/S-5 h; Sample 8, 0.02 E/S-5 h; and Sample 9, 0.03 E/S-5 h. After deactivating the enzyme at 85 °C for 15 min, we centrifuged the sample (Suprema 25, TOMY, Tokyo, Japan) at 4 °C, for 20 min, at 12,000 rpm. The supernatant was separated and later lyophilized to be stored at −20 °C for further analysis. The measurement of the antioxidant DPPH scavenging activity and ferric-reducing power activities; the ACE-inhibitory activity; and the hypoglycemic α-amylase- and α-glucosidase-inhibitory activities were carried out and recorded for each treatment. Those conditions showing the highest bioactivity were selected and used for the production of orange seed protein hydrolysate [18].
2.4. Determination of Hydrolysis Degree
The hydrolysis degree was calculated according to the method adopted by Kaewka et al. (2009) [19]. A total of 10 mL protein hydrolysate and 10 mL 10% trichloroacetic acid (TCA) were mixed and centrifuged at 4 °C, for 10 min, at 10,000 rpm. Nitrogen content in supernatant and total nitrogen were measured by Kjeldahl method, and the degree of hydrolysis was calculated by using the following formula:
| Hydrolysis degree (%) = (Total N in supernatant/Total N in whole sample) × 100. | (1) |
2.5. Assay of DPPH Radical Scavenging Activity Measurement
In total, 100 μL of protein hydrolysate was blended with 500 μL of ethanol (96%) and 125 μL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (0.02% in ethanol, (v/v)); samples were then incubated for 60 min, at ambient temperature and in a dark place. Absorbance of samples was observed at 517 nm [20]. Distilled water and butylated hydroxytoluene (1 μg/μL) acted as negative and positive controls, respectively. DPPH radical scavenging activity was calculated through the following equation:
| Antioxidant activity (%) = (Abs of negative control − Abs of sample)/(Abs of negative control) × 100. | (2) |
2.6. Assay of Ferric-Reducing Antioxidant Power Measurement
A total of 250 μL of protein hydrolysate was mixed with the same volume of 200 mM sodium phosphate buffer, at a pH 6.6, and the same volume of 10 mg/mL of potassium ferricyanide. Next, the mixture was incubated during 20 min, at 50 °C, in the dark. Then, 250 μL of 100 mg/mL of trichloroacetic acid was added to the solution. After centrifuge at 1650 rpm for 10 min, we removed 500 μL of supernatant and added 500 μL of bi-distilled water and 100 μL of ferric chloride (1 mg/mL). Then, the sample was incubated for 10 min, at room temperature, and the absorbance was measured at 700 nm. Bi-distilled water and butylated hydroxytoluene (1 μg/μL) were used as negative and positive controls, respectively. There is a positive correlation between sample absorbance and ferric-reducing power [21].
2.7. Assay of the ACE-Inhibitory Activity Measurement
The assay was developed according to Sentandreu and Toldrá (2006) with some modifications. Briefly, 50 μL of protein hydrolysate was blended with 50 μL of 150 mM Tris-base buffer (pH 8.3), which contained 3 mU/mL of ACE, and then 200 μL of 150 mM Tris-base buffer (pH 8.3), which contained 1.125 M of NaCl, and then 10 mM of Abz-Gly-Phe(NO2)-Pro was added. Finally, the final product was incubated at 37 °C, for 60 min. The action of releasing o-aminobenzoylglycine as a result of ACE generated a fluorescence that was read at 355 and 405 nm as excitation and emission wavelengths, respectively. The fluorescence generated in the reaction was measured each 15 min along the incubation time. ACE-inhibitory activity was calculated as follows:
| ACE-inhibitory activity (%) = ((Abs Sample T60 − Abs Sample T0)/(Abs Control T60 − Abs Control T0)) × 100. | (3) |
Based on the results in pretests, the dilution of 1:100 (sample: distilled water) used for this assay [22].
2.8. Assay of α-Amylase-Inhibitory Activity
The α-amylase-inhibitory activity was assessed by using a commercial kit (SPINREACT, ref Amylase-LQ, Girona, Spain). The formation rate of 2-chloro-4-nitrophenol, which was observed photometrically, is positively correlated with the catalytic concentration of α-amylase present in the sample. Then 80 μL of protein hydrolysate solution was added to 40 μL of α-amylase enzyme (from porcine pancreas, A3176, 1 μU), and they were mixed accordingly. Then the reaction mixture was incubated at 37 °C, for 10 min. After that, 160 μL of the 2-chloro-4-nitrophenyl-α-D-maltotrioside (CNPG3) containing ((MES pH = 6, 100 mM), (CNPG3, 2.25 mM), (sodium clorhidre, 350 mM), (calcium acetate, 6 mM), (potassium thiocyanate, 900 mM), (sodium azide 0.95 gr/L)) was added, and then the reaction mixture was stored at 37 °C, for 5 min. Finally, the optical density was recorded at 405 nm. Phosphate buffer 20 mM (pH 6.8) and Acarbose (2 mg/mL phosphate buffer) were considered as blank and positive controls, respectively (SPINREACT, Girona, Spain). The α-amylase-inhibitory activity was determined as follows:
| α-amylase-inhibitory activity (%) = ((Abs control − Abs sample)/Abs control) × 100. | (4) |
2.9. Assay of α-Glucosidase-Inhibitory Activity
The α-glucosidase-inhibition activity was measured using a kinetic method with a commercial kit (Sigma-Aldrich, α-glucosidase Activity Assay Kit, St. Louis, MO, USA). In this assay, α-glucosidase activity was recorded through a reaction in which α-glucosidase hydrolysis of ρ-nitrophenyl-α-D-glucopyranoside results in the formation of a colorimetric substance, which subsequently can be read at 405 nm. One unit of α-glucosidase was explained to be the amount of enzyme that facilitates the hydrolysis of 1 μmol of substrate per minute at pH = 7. For the analysis, 20 μL of protein hydrolysate solution was mixed with 50 μL of α-glucosidase enzyme (from Saccharomyces cerevisiae, 1 mg/mL). The reaction medium was incubated at 37 °C, for 5 min, and then 200 μL of the Master Reaction Mix containing 200 μL of assay buffer (pH 7) and 8 μL of α-NPG substrate were added to the medium and incubated at 37 °C, for 60 min. Acarbose (2 mg/mL) was used as positive control. The α-glucosidase-inhibitory activity was obtained from the following equation:
| α-glucosidase-inhibitory activity (%) = ((Abs control − Abs sample)/Abs control) × 100. | (5) |
2.10. SEC Separation of Hydrolyzed Protein
To estimate the distribution of the peptide components in the orange seed proteins hydrolysate, a SEC separation was used based on Lassoued et al. (2015) and Jemil et al. (2016) [23,24]. For this purpose, 5 g of protein hydrolysate was stirred with 35 mL of HCl (0.01 mol/L), using a magnetic stirrer. Then three volumes of ethanol was added and mixed. The solution was then stored at 4 °C, overnight, for deproteinization. The final mixture was centrifuged at 12,000 rpm, for 20 min, at 4 °C. The ethanol was removed from the sample, using a rotary evaporator. Ultimately, the deproteinized solutions were lyophilized (SCANVAC, Labogene ApS, Alleryd, Denmark). After that, 1 g of the deproteinized powder was added to 10 mL of 0.01 N HCl solution and then filtered through 0.45 μm. Then 5 mL of the filtered solution was loaded on a Sephadex G25 column (Amersham Biosciences, Uppsala, Sweden). The separation process of peptide components was performed by providing a flow rate of 15 mL/h of filtered and degassed 0.01 N HCl, and peptide fractions were automatically accumulated in 5 mL volumes. The absorbance intensity was recorded at 254 and 280 nm, using an Ultraviolet–Visible spectrophotometer (Agilent Cary 60, Agilent Technologies, Palo Alto, CA, USA). Finally, antioxidant activity, ACE-inhibitory activity, and α-amylase- and α-glucosidase-inhibitory activities were performed separately for all fractions. Peptide fractions that showed the highest activity were pooled, lyophilized, and kept at −20 °C, to be used in further purifications.
2.11. Isolation of Most Active Fractions Using RP-HPLC
The peptide components separated by SEC showing the highest antioxidant activity, α-amylase, α-glucosidase, and ACE-inhibitory activity, were excessively purified, using reverse-phase HPLC. The pooled peptide fractions were mixed and filtered through a 0.45 μm filter, and 30 μL was analyzed in an HPLC system (Agilent 1100, Agilent Technologies, Palo Alto, CA, USA), which was equipped with a Symmetry C18 column (4.6 × 250 mm, 5 μm) (Waters Co., Milford, MA, USA). Two solvents, namely solvent A with TFA in bi-distilled water (0.1%, v/v) and solvent B with TFA (0.085%, v/v) in acetonitrile (ACN:bi-distilled water, (60:40, v/v)), were used as mobile phases. Both mobile phases A and B were purified with a 0.45 μm filter and degassed prior to application. Peptides were first eluted with 100% solvent A for 2 min, followed by a linear gradient from 0 to 50% of solvent B, during 50 min, at a flow rate of 1 mL/min. The obtained fractions (1 mL) were measured at 214 nm, lyophilized, and assayed for their bioactivity [23,25].
2.12. Simulated in Vitro Gastrointestinal Digestion
The in vitro gastrointestinal digestion assay was done according to Minekus et al. (2014), with some modifications. This method was designed to imitate the physiological conditions of the human digestive system in two phases, namely the gastric phase and intestinal phase. In the gastric phase, 2 mL of 0.01 mol/L HCl was added to 500 mg of RP-HPLC fractions (fractions showing the highest bioactivity values). Porcine pepsin (2000 U/ mL in 0.01 mol/mol HCl) and CaCl2 were added, to have a final concentration of 0.075 mM. Samples were incubated during 3 h, to simulate digestion at 37 °C, while they were constantly stirred, and the enzyme became inactivated by increasing the pH to 7, using 1 mol/mol NaOH. For imitating the intestinal period, different digestive enzymes, including trypsin (from hog pancreas, 100 U/mL), chymotrypsin (from bovine pancreas, 25 U/mL), porcine pancreatic α-amylase (200 U/mL), porcine pancreatic lipase (2000 U/mL), and porcine bile extract (10 mM), were added. A final concentration of 0.3 mM CaCl2 was also used. After 2 h, at 37 °C, the digestion process was over by heating at 95 °C for 2 min. The sample was deproteinized by adding 3 volumes of ethanol and keeping the sample at 4 °C for 20 h. After that, it was centrifuged at 12,000× g, at 4 °C, for 10 min. Eventually, the supernatant was dehydrated in a rotatory evaporator and then lyophilized [26].
2.13. Free Amino Acid Analysis
The amino acid analysis was done according to a method proposed by Flores et al. (1997), with some modifications. Then 1 mL of distilled water was added to 0.1 g of deproteinized protein hydrolysate sample, to dissolve it, and then 100 μL of this solution was blended with 850 μL of distilled water and 50 μL of internal standard (including 10 mM N-Leu). After that, 200 μL of the mixture was evaporated and dried, and then 15 μL of drying material (including methanol:sodium acetate 1 mol/mol: TEM, 2:2:1) was included and dried again after vortexing. Then 15 μL of derivatization material (methanol:distilled water:TEA:PITC, 7:1:1:1) was added and shaken vigorously. It stayed fixed for 20 min and then evaporated and dried again. In the next step, it was dissolved in 1 mL of 5 mM Di-sodium phosphate and centrifuged at 12,000× g, at 4 °C, for 10 min. Finally, the supernatant was separated and kept in a special vial for HPLC analysis. The content of amino acid within the supernatant was clarified by an Agilent 1200 HPLC system (Agilent Technologies, Palo Alto, CA, USA), which was equipped with a photodiode array detector (254 nm). The used column was a Symmetry C18 column (3.9 × 300 mm, 5 μm). In total 1 mM of each measured amino acid was used as standard. The solvent mixture had two eluents, solvent (A), including 70 mM sodium acetate, which was adjusted to pH 6.55 with 10% acetic acid and 2.5% acetonitrile; and solvent (B), including acetonitrile:water:methanol (45:40:15, v/v/v) [27].
2.14. Identification of Peptides, Using Mass Spectrometry in Tandem
The nano LC–MS/MS analysis was done with a nano-LC Ultra 1D Plus system (Eksigent of AB Sciex, CA, USA), which was coupled to a quadrupole-time-of-flight (Q ToF) TripleTOF® 5600 that was equipped with a nanoelectrospray ionization source (AB Sciex Instruments, Framingham, MA, USA). The system parameters used were selected according to Mora et al. (2015) [28]. The peptide components of lyophilized orange seed protein hydrolysate obtained by RP-HPLC were dissolved in 20 μL of trifluoroacetic acid (TFA 0.1%) and concentrated and purified with Zip Tip C-18 tips (Millipore, Billerica, MA, USA). Then 5 μL of the eluted sample was injected into the mass spectrometer. Samples were concentrated beforehand on a C18 trap column (3 μ, 350 μm × 0.5 mm) (Eksigent of AB Sciex, Redwood City, CA, USA), using TFA 0.1% v/v as the mobile phase, at a flow rate of 4 μL/min. After 5 min of this concentration step, the trap column was automatically put in line with a nano-HPLC capillary column (3 μm, 75 μm × 12.3 cm, C18) from Nikkyo Technos Co, Ltd. (Tokyo, Japan), to elute the compounds. The mobile phase A was 1% formic acid in water, and mobile phase B was formed by 0.1% formic acid in 100% acetonitrile. The chromatographic conditions consisted of linear gradients from 5 to 35% of solvent B during 90 min, and then from 35 to 65% for 10 min of solvent B at a flow rate of 3 μL per minute and a temperature of 30 °C. The outlet of the capillary column was straightly attached to a nanoelectrospray ion source. The operating conditions of the Ion trapping mass spectrometry were as follows: positive polarity, capillary column with temperature of 200 °C, voltage 4.5 V, and nitrogen was used as a collision gas. The first scan was a mass scan of a mass ratio of 400 to 2500. Automatic spectrometry and generation of peak list were performed by using Mascot Distiller software. Search in databases was carried out by Mascot Daemon software in combination with Mascot interface software 2.2. In terms of searching parameters, Mascot searches were done by selecting none specific enzyme, none modifications, and mass tolerance measurement 100 ppm in the MS state and 0.6 Dalton for MS/MS ions. Identification of the origin of protein for peptides was done by using the UniProt database and NCBInr database. The BIOPEP-UWM database was used to search for similarities between the sequences identified in this study and previously published bioactive peptide sequences [15,29,30]. Only identification results showing a confidence higher of 90% were reported. The BIOPEP-UWM™ database of bioactive peptides has newly become a popular tool in the research on bioactive peptides, especially on these derived from foods and being constituents of diets that inhibit the development of chronic diseases [30].
2.15. Statistical Analysis
Analysis of data collected from experiments on the protein hydrolysate in optimized conditions was carried out through a completely random design by SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Each experiment was repeated in triplicate. The Duncan’s test was also utilized at 5% significance level for comparing the mean values. Excel software (Microsoft Excel Worksheet (.xlsx), Redmond, WA, USA, 2013) was used to draw charts.
3. Results and Discussion
3.1. Antioxidant Activity
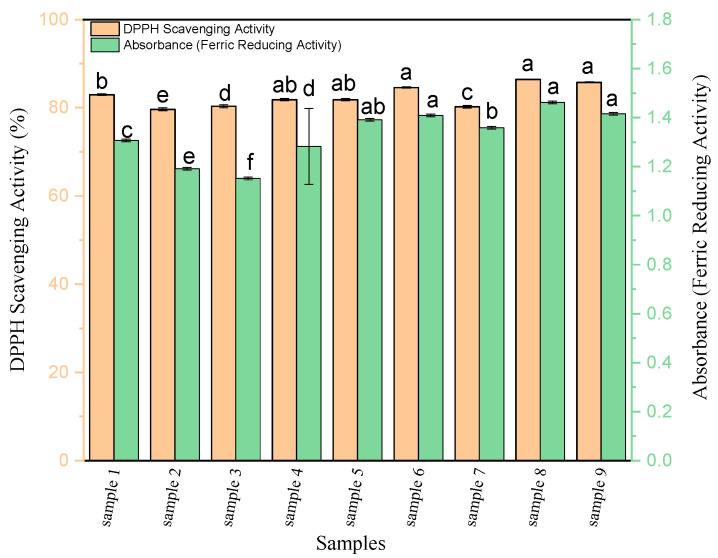
The capacity of orange seed protein hydrolysate (OSPH) as an antioxidant was evaluated based on DPPH radical scavenging activity and ferric-reducing power, and the results are presented in Figure 1: Sample 1, 0.01 E/S-2 h; Sample 2, 0.02 E/S-2 h; Sample 3, 0.03 E/S-2 h; Sample 4, 0.01 E/S-3.5 h; Sample 5, 0.02 E/S-3.5 h; Sample 6, 0.03 E/S-3.5 h; Sample 7, 0.01 E/S-5 h; Sample 8: 0.02 E/S-5 h; and Sample 9, 0.03 E/S-5 h.
Figure 1.
DPPH scavenging activity (%) and ferric-reducing power activity of tomato protein hydrolysate (OSPH) under different conditions. Data are mean ± SD of three replications and the values for each activity with different letter are significantly different (p < 0.05).
The enzyme-to-substrate ratio and hydrolysis time showed significant effects (p < 0.05) on the DPPH scavenging activity and ferric-reducing power of OSPH. As the results show, longer times of hydrolysis (5 h in Samples 7, 8, and 9) result in the best antioxidant activities, whereas no clear trends were observed at increasing E/S ratios. A remarkable increase (p < 0.05) in the antioxidant activity was detected, using an enzyme concentration of 2% and hydrolysis time of 5 h with 86.4 ± 0.07% and 1.467 ± 0.1 in the DPPH radical scavenging activity and ferric-reducing power, respectively. With regard to the antioxidant, results in this study revealed that the production of antioxidant peptides increases by increasing the enzyme concentration, which is in accordance with Guérard et al. (2002) [31]. Jamdar et al. (2010) and Je et al. (2009) reported that increased hydrolysis time and enzyme concentration had an increasing effect on the antioxidant activity in peanut and tuna liver protein hydrolysates, respectively [31,32,33]. Based on amino acids analysis, hydrophobic amino acids (aromatic or branched) in the peptide sequences and the presence of one or more residues of His, Pro, Cys, Tyr, Trp, Phe, or Met amino acids increase antioxidant activity [34]. Trp plays the most important role in DPPH scavenging activity, and this is probably due to its hydrogenating role [35].
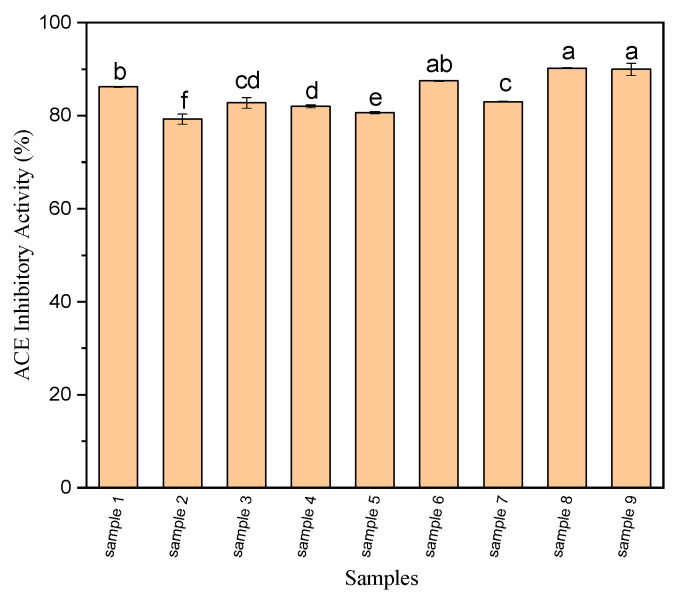
3.2. ACE-Inhibitory Activity
As it is shown in Figure 2, an increase in hydrolysis time and enzyme concentration affected significantly the ACE-inhibitory activity of samples. Similar to antioxidant activity, longer times of hydrolysis (5 h in Samples 7, 8, and 9) results in the best antioxidant activities, whereas no clear trends were observed at increasing enzyme/substrate (E/S) ratios. A significant increase in the ACE-inhibitory activity (p < 0.05) was observed, using an enzyme/substrate (E/S) ratio of 2% and a hydrolysis time of 5 h (90.2 ± 0.1%). In a previous study, an increase in the amount of peptides with molecular weight smaller than 3 kDa was observed with increasing hydrolysis times; consequently, the ACE-inhibitory activity also increased [36]. ACE-inhibitory activity seems to be related to the number of hydrophobic amino acids that are available in the peptide sequences probably because of the fact that the active site of ACE by hydrophobic peptides is more reachable [24,37,38,39]. Regarding the amino acids composition, amino acids His, Pro, Ser, Glu, and Tyr were important amino acids in peptides with ACE-inhibitory activity. Studies have revealed that hydrophobic amino acid residues such as Tyr, Ala, Leu, Val, Phe, or Trp can take action as competitive ACE-inhibitors, as they selectively bind the catalytic sites of ACE [37]. Particularly, it has been discussed that aromatic amino acids presence, such as phenylalanine, at any of the three spots that is closest to the C-terminal is the most appropriate situation [37]. Moreover, peptides that are the most potent antihypertensive ones include positively charged amino acids such as arginine and lysine at their C-terminal spot [39].
Figure 2.
Angiotensin-converting enzyme (ACE) inhibitory activity (%) of OSPH produced under different conditions. Data are mean ± SD of three replications and the values with different letter are significantly different (p < 0.05).
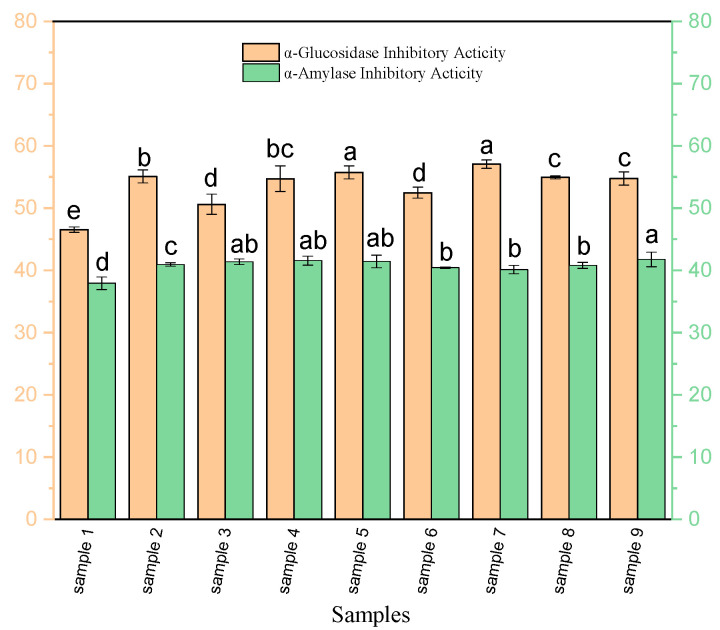
3.3. α-Amylase- and α-Glucosidase-Inhibitory Activity
The measurement of these activities was done for each treatment (Figure 3), and the conditions which showed the greatest inhibitory activity were selected. Best results were observed by using an enzyme concentration of 3% and hydrolysis time of 5 h for the α-amylase-inhibitory activity (41.7 ± 1.1%) and an enzyme concentration of 1% and hydrolysis time of 5h for the α-glucosidase-inhibitory activity (57.0 ± 0.6%). The α-glucosidase-inhibitory activity of OSPH in this study was higher than that of whey protein hydrolysates reported by Lacroix and Li-Chan., (2013) [40]. Regarding the amino acid composition, Ser, Asp, and Glu were important amino acids in peptides with α-amylase and α-glucosidase-inhibitory activities. It has been proved that peptides with aromatic residues (Phe, Trp, and Tyr) are considered as key factors in α-amylase- and α-glucosidase-inhibitory activities [41].
Figure 3.
Overall α-amylase- and α-glucosidase-inhibitory activity (%) of OSPH under different conditions. Data are mean ± SD of three replications and the values for each activity with different letter are significantly different (p < 0.05).
Meinert et al. (2015), Selamassakul et al. (2018), Soleymanzadeh et al. (2019), and Moayedi et al. (2016) have shown that protein hydrolysates of rice, camel milk, and tomato by-products contain peptides with antioxidant and ACE-inhibitory activities, respectively [25,42,43,44]. Jonker et al. (2011) reported that the hydrolysis of casein and whey proteins leads to generation of antidiabetic peptides that these peptides reduced blood glucose in people with type 2 diabetes [45]. Based on a research conducted by Yang et al. (2012), the addition of peptides obtained from the hydrolysis of soybean protein to the diet of rats with type 1 diabetes potentiates insulinotropic actions and improves hepatic insulin sensitivity in diabetic rats [46].
According to our outcomes, the highest bioactivities (antioxidant activities, ACE, α-amylase-inhibitory, and α-glucosidase-inhibitory activities) of peptides were observed in the ratio of 2% enzyme Alcalase to the substrate and a hydrolysis time of 5 h (p < 0.05). Therefore, this sample was selected for further analysis in the next steps.
3.4. Fractionation of OSPH by SEC
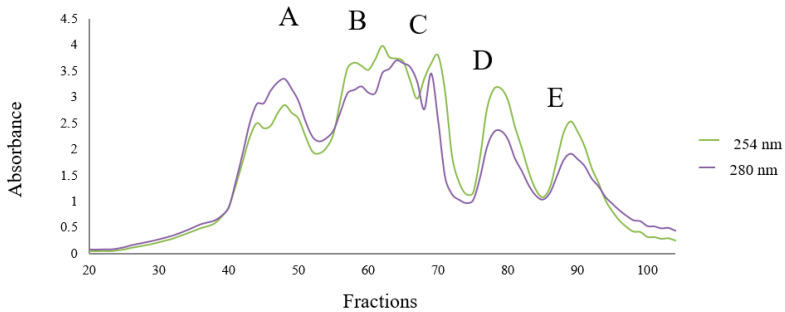
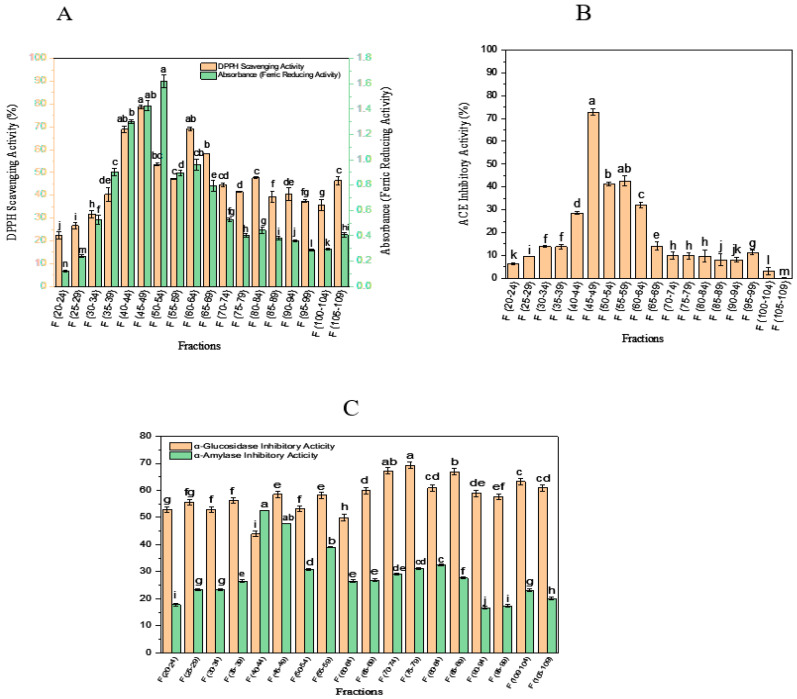
For investigating the effect of molecular mass distribution on different bioactive properties of OSPH, such as ACE-inhibitory activity, α-amylase- and α-glucosidase-inhibitory activity, and antioxidant activity, the hydrolysate was initially fractionated by using SEC, and the wavelengths of fractions were read at 254 and 280 nm. Results are illustrated in Figure 4.
Figure 4.
Size-exclusion chromatography (SEC) profile of OSPH (0.1 g/mL), using a Sephadex G-25 column.
OSPH showed five main peaks (Parts A, B, C, D, and E) in the molecular range of 200–70,000 Da. The first peak (Part A) includes the proteins or peptides having molecular mass between 13,000 and 70,000 Da. SEC profile also showed a peak with fragments that have low molecular weights around 1400–13,000 Da eluting after bacitracin oligopeptide standard (Part B). The next peaks (Parts C, D, and E) correspond to lower molecular weight fragments. As illustrated in Figure 5A,B the highest antioxidant activity (DPPH scavenging activity and ferric reducing power) was observed in fractions F(40–44), F(45–49), and F(50–54) with 68.86 ± 1.4%, 78.73 ± 0.6%, and 53.44 ± 0.5% in DPPH scavenging activity and 1.289 ± 0.01., 1.427 ± 0.03, and 1.619 ± 0.05 in ferric-reducing power, respectively. The highest ACE-inhibitory activity of OSPH (72.89 ± 1.3%) was reported in the fraction F(45–49) (Figure 5B). The fractions F(50–54) and F(55–59) also showed an ACE-inhibitory activity of 41.16 ± 0.8% and 42.65 ± 2.2%, respectively. Generally, peptides with low molecular weight display higher ACE-inhibitory activity [24,26]. However, the results of this research showed that the fractions F(45–49) expressed higher ACE-inhibitory activity in comparison with the fractions F(50–59), indicating that peptides with lower molecular mass do not necessarily show higher activities [25,27]. According to Figure 5C, the highest α-amylase-inhibitory activity of OSPH was seen in fractions F(40–44) and F(45–49), with an activity of 52.5 ± 0.006% and 47.7 ± 0.07%, respectively. The highest α-glucosidase-inhibitory activity was reported in fraction F(75–79). Based on these results, fraction F(40–44) and F(45–49) were selected for further purification by RP-HPLC.
Figure 5.
DPPH scavenging activity and ferric-reducing power (A), ACE-inhibitory activity (B), and α-amylase-inhibitory activity and α-glucosidase-inhibitory activity (C) in different fractions obtained from size-exclusion chromatography of OSPH. F(40–44) and F(45–49) were selected for further purification. Data are mean ± SD of three replications and the values with different letter for each activity are significantly different (p < 0.05).
Generally, an Alcalase enzyme generates peptides with different range of molecular weights due to the site of cleavage imparted by the Alcalase enzyme [46]. As a Ser protease, Alcalase, contains Asn, His, and Ser amino acids in its cleavage site. The carboxyl group in Asn binds the nitrogen in the imidazole ring of His. Other nitrogen groups in His form some bonds with the proton in the hydroxyl group of Ser, to have a hydrogen bond and create a negative charge on the oxygen atom in Ser residue. The presence of an oxygen atom with a slight-negative charge makes it possible for the Alcalase enzyme to break down proteins and produce various peptides by nucleophilic attack on the amide bonds. The non-specific action of the Alcalase enzyme during the attack on amide bonds increases its efficiency in development of hydrolysis and the production of peptides with different chain lengths [47]. Several researchers have fractionated hydrolyzed proteins and peptides by using SEC and then studied the impacts of molecular size on biological activities of the fractions [25,48,49]. It has been reported that peptide size can affect the antioxidant activity and peptides with the molecular weight of less than 3000 Da reveal the highest antioxidant power [50]. Hence, the molecular weight below 3000 Da was suggested as the most efficient peptide size in antioxidant activity [24,25,47,51]. In addition to the size of peptides, the hydrophilic–hydrophobic balance of the peptides is also an influential element in the ACE-inhibitory activity [36]. The last fractions with the lowest molecular weight in SEC separation usually include free amino acids showing lower bioactivities than other peptides [24,47]. Many experiments have shown that peptides with antidiabetic activity have hydrophobic amino acids such as Val, Trp, Leu, Ile, Pro, Phe, and Cys in their sequences [42]. In addition, protein hydrolysates containing hydrophobic amino acids may be easily absorbed [51]. It has been reported that non-saccharide compounds impart their inhibitory activities through binding the enzyme active site via hydrophobic interactions [39].
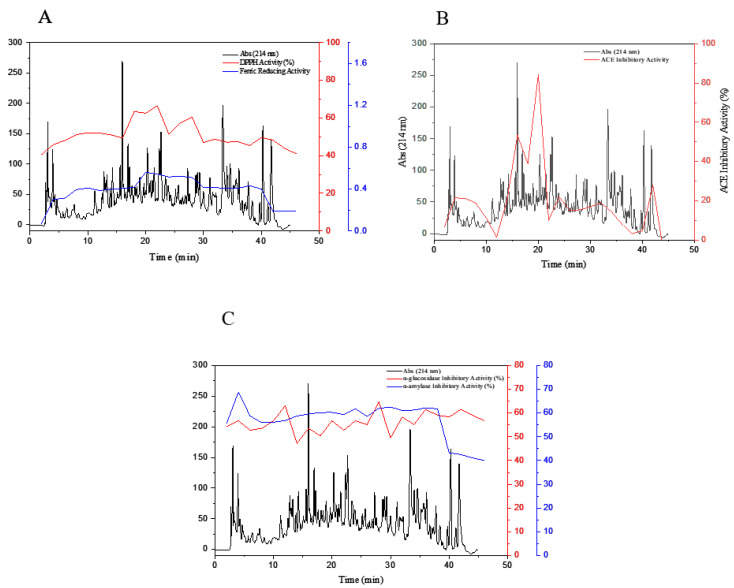
3.5. Purification of Peptide Fractions by RP-HPLC
RP-HPLC separation of the selected purified fractions F(40–44) and F(45–49) obtained from size-exclusion chromatography resulted in an elevated number of eluted peaks, as shown in Figure 6. The mentioned fractions were freeze-dried and assayed for their biological activities, including antioxidant, ACE-inhibitory activity, α-amylase-inhibitory activity, and α-glucosidase-inhibitory activity. In the DPPH scavenging activity assay, the fraction F22 expressed the highest antioxidant activity of 66.57% (Figure 6A). In the ferric-reducing power test, the fractions F20 and F22 showed the greatest activities, namely 0.560 and 0.544, respectively. The fraction F20 showed a maximum in ACE-inhibitory activity of 84.28% (see Figure 6B). Ultimately, the antidiabetic-potential tests revealed that F2 peptides fraction with 68.78% had the α-amylase-inhibitory activity, and F28 peptides fraction with 64.70% peptides fraction had maximum α-glucosidase-inhibitory activity (see Figure 6C). According to the results of this section, Fractions 19 to 20, obtained from RP-HPLC, were chosen for sequencing and peptide characterization.
Figure 6.
Reversed-phase high-performance liquid chromatography (RP-HPLC) separation of Fractions (40–44) and (45–49) obtained from SEC. The fractions were automatically collected and assayed for their DPPH scavenging activity and ferric-reducing power (A), ACE-inhibitory activity (B), and α-amylase-inhibitory activity, and α-glucosidase-inhibitory activity (C). Fractions 19 to 20, obtained from RP-HPLC, were selected for further sequencing and identification.
Generally, reversed-phase (RP) preparative HPLC is considered to be one of the best methods to isolate and purify peptides from hydrolyzed proteins, based on the hydrophobicity of the peptides [51]. It is clear that there was a relationship between possible increase of either DPPH or ferric-reducing power with relative increase in the hydrophobicity. Peptides with ACE-inhibitory activity need to connect to the active site of ACE or an inhibitor site placed on the ACE, adjusting the conformation of the protein [52]. Lassoued et al. (2016) declared that high hydrophilic peptides have less accessibility to the ACE active site [53].
3.6. Free Amino Acid Composition
The free amino acid composition of orange seed protein hydrolysate is shown in Table 1. As displayed in Table 1, the total amount of free amino acids of orange seed protein hydrolysate was 2.6657 mg/mL. The major free amino acids presented in OSPH were Arg, followed by Tyr, Trp, His, Phe, and Met. It is clear that there is not a high amount of free amino acids in OSPH (2.6657 mg/g of sample hydrolysate). Alcalase enzyme is an endonuclease that facilitates free amino acids formation in the hydrolyzed proteins [38,49]. Enzymatic hydrolysis in longer hydrolysis times and higher enzyme concentrations could simply result in a faster augmentation in the content of free amino acids. Generally, the estimation of free amino acids is frequently used as an evaluation of the intensity of hydrolysis [38].
Table 1.
Free amino acid composition of orange seed protein hydrolysate.
| Amino Acid Name | Concentration (mg/g of OSPH) ± SD |
|---|---|
| Asp | 0.1276 |
| Glu | 0.1434 |
| Ser | 0.1087 |
| Asn | 0.13 ± 0.0001 |
| Gly | 0.0742 |
| Gln | 0.1278 ± 0.0001 |
| β Ala | 0.0885 ± 0.0001 |
| His | 0.1616 ± 0.0001 |
| Thr | 0.1172 |
| Ala | 0.0871 ± 0.0003 |
| Arg | 0.1682 |
| Pro | 0.1103 |
| Tyr | 0.1665 |
| Val | 0.1158 |
| Met | 0.1439 |
| Ile | 0.1245 |
| Leu | 0.1305 |
| Phe | 0.153 |
| Trp | 0.1637 |
| Orn | 0.0812 |
| Lys | 0.1417 |
| Total | 2.6657 ± 0.001 |
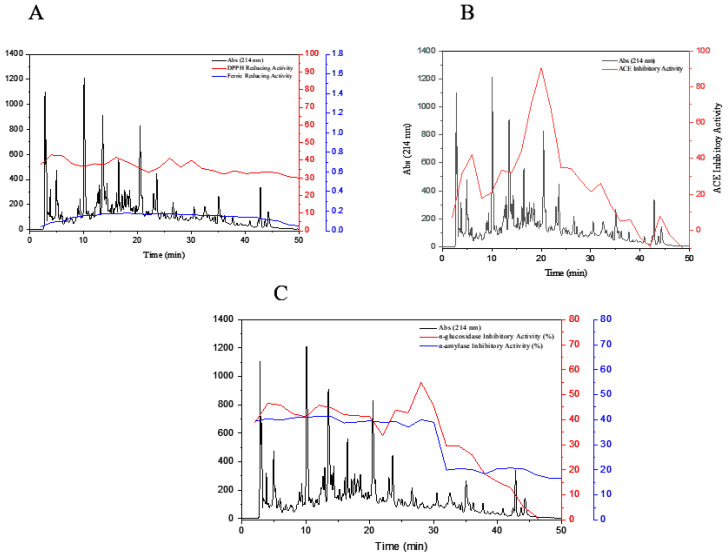
3.7. Influence of Simulated In Vitro Gastrointestinal Digestion on the Bioactivity of Peptides
Chromatograms of peptide fractions F(40–44) and F(45–49) and their potential bioactivity (antioxidant, ACE, α-amylase-inhibitory, and α-glucosidase-inhibitory activities) were compared before and after digestion. The results obtained after imitative gastrointestinal digestion on bioactivity of selected fractions are shown in Figure 7. Each fraction was analyzed by RP-HPLC after simulated gastrointestinal digestion, and then their respective bioactivities were investigated.
Figure 7.
Reversed-phase chromatographic separation of F(40–44) and F(45–49) from SEC and after in vitro gastrointestinal digestion. Fractions were automatically collected and assayed for their DPPH scavenging activity and ferric-reducing power (A), ACE-inhibitory activity (B), and α-amylase-inhibitory activity and α-glucosidase-inhibitory activity (C). Fractions 19 and 20 obtained from RP-HPLC were selected for sequencing and identification.
According to the results, after digestion, in F(19–20), DPPH scavenging activity decreased from 62.56 to 36.16% and ferric-reducing power also decreased from 0.560 to 0.172 (p < 0.05). A significant decrease in α-amylase-inhibitory activity (from 60.30 to 39.55%) and β-glucosidase-inhibitory activity (from 56.76 to 41.22%) was also observed. This decrease might be due to digestion of some active peptides (Figure 7A,C). However, the relative percentage of Part B in Figure 7 for F(19–20) increased from 84.28 to 90.52% in the ACE-inhibitory activity after digestion. This demonstrated that, during digestion, new peptides showing important ACE-inhibitory activity were generated as well. Generally, this result shows that fraction F20 was more resistant than other fractions against gastrointestinal digestion and, therefore, was selected for the next step.
Elimination of some peaks, changes in the time of withdrawal of some other peaks from the column after gastrointestinal digestion, and reduction of the following levels of a number of peaks in digested fractions indicated the digestive effect of proteases enzymes on changes in the structure of peptide chains. In addition, treatment in acidic conditions with pH = 2 and then increasing pH to 7 causes changes in peptide chains. The results implicated that the structure of F(19–20) of the peptide had less affected by gastrointestinal digestion than other fractions [54].
Meshginfar et al. (2018) reported that the net charge of peptide sequence influences its solubility and likely its digestion [54]. In another study, amaranth antioxidant peptides (antioxidant activity was investigated by the ABTS+ scavenging and the ORAC assays) and tomato antioxidant peptides (antioxidant activity was measured by DPPH scavenging activity) showed minimal changes in their bioactivities after in vitro gastrointestinal digestion [13,54].
3.8. Identification of Peptide in Most Active HPLC Fractions by MS/MS
Fractions 19 and 20, obtained from RP-HPLC, were chosen for characterization by nano LC–MS/MS analysis prior to and post simulated gastrointestinal digestion, and the corresponding results are shown in Table 2 and Table 3. Generally, 63 peptide sequences were identified in all samples before simulated digestion (Table 2). The size of the peptides in this study, before and after simulated gastrointestinal digestion, had 6–24 and 7–18 amino acids in length, respectively, whereas the analysis of poultry protein hydrolysate after in vitro gastrointestinal digestion on the ACE-inhibitory activity resulted in ACE-inhibitory peptides from five to nine amino acids in length [55].
Table 2.
Differential peptide sequences present in F(19–20) identified from OSPH (before gastrointestinal (GI) digestion), using NCBInr and UniProt database.
| Sequence | Protein | Bioactivity (Sequence Previously Described as Responsible for the Bioactivity) | MW |
|---|---|---|---|
| ATREQRQQQQRFQTQ | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Inhibition of prolyl endopeptidase (IHPFAQTQ) | 1933.3 |
| EQEQEFQGSGD | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | ACE-inhibitory (RKSGDPLGR) |
1253.36 |
| HNINDPSGA KHNINDPSGA |
Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Antimicrobial activities (GYVSGAVIEIPDEILDSAR) |
924.06 1052.51 |
| HNINDPSGAD KHNINDPSGAD |
Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Celiac toxic (GWFGGADWHA) |
1039.16 1166.53 |
| HNINDPSGADA | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Kinases inhibitor, (KKALRRQEAADAL) Antioxidative (LLPHHADADY) Antithrombotic, Antimicrobial (DNIADAVACAKRVVRDPQGIR) |
1110.25 |
| PSGADAYNPR | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Antioxidative (PRHVFYRWFLSNPRI) | 1052.25 |
| QESQQRSSESQSRSQDQHQKVR | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Antimicrobial (TKCFQWQRNMRKVRGPPVSCIKR) | 1167.35 |
| QQQQRFQTQ TREQRQQQQRFQTQ |
Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | ACE inhibitor (QTQSLVYP) |
1047.21 1903.93 |
| SSESQSRSQDQHQKVR FQSSKSQDQHQKVR |
Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Antimicrobial (AGRGKQGGKVRAKAKTRSSRA) ACE inhibitor (KVREGTTY) |
2644.05 1701.84 |
| LRHNIDKPSHAD | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Antimicrobial (LLPHHADADY) | 1191.41 |
| QDSQQQQSFQSS | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Neuropeptide (FSEFMRQYLVLSMQSSQ) | 1887.18 |
| SFQSSKSQDQHQKVR | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | ACE inhibitor (KVREGTTY) |
1862.21 |
| SQGGRSQGSQGSDDRRAGN SQGSQGSDDRRAGN |
Citrin OS = Citrus sinensis Q39627_CITSI Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI |
VLKMAGNSFQEN (Celiac Toxic coeliac toxic peptide) YLAGNQ, EVMAGNYLPG, EVMAGNLYPG (ACE inhibitor) |
1918.83 1433.59 |
| SQSQGGRSQGSQGSDDRRAGNL GSQGSDDGRGGNL SQGGRSQGSQGSDDGRGGNL SQSQGGRSQGSQGSDDGRGGNL |
Citrin OS = Citrus sinensis Q39627_CITSI | Antimicrobial (GLFDAIGNLLGGLGLG) Antiamnestic (PEP inhibitor) (RYDWWPYGNLFGGHTFISP) |
2247.01 1218.51 1918.83 2133.91 |
| VFPGCAETFQDSQQQQSFQSSKSQDQHQKVR | Uncharacterized protein OS = Penicillium digitatum tr|K9FZ18|K9FZ18_PEND1 | Antimicrobial (AGRGKQGGKVRAKAKTRSSRAGLQFPV) ACE inhibitor (KVREGTTY) |
3549.70 |
| VTDITREGKQQ | Citrin OS = Citrus sinensis tr|Q39627|Q39627_CITSI | Regulating (QQQKQQQQPSSQVS) | 1790.14 |
| GTQDHPHDDYAE | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Anti-inflammatory peptide (KGHYAERVG) ACE inhibitor (YAEERYPIL) |
1920.17 |
| GTQDHPHDDYAEAK | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Kinases inhibitor (VTCDILSVEAKGVKLG) | 1434.59 |
| AGDTHLGGED | Uncharacterized protein OS = Penicillium digitatum tr|K9FZ18|K9FZ18_PEND1 | Antithrombotic (EAGEDCDCGSPANPCCDAATCKLIPGAQCGEGLCCDQCSFIEEGTVCRIARGDDLDDYCNGRSAGCPRNPFH) | 2135.41 |
| DQGNRITPS | Uncharacterized protein OS = Penicillium digitatum tr|K9FZ18|K9FZ18_PEND1 | Antimicrobial (GIFSSRKCKTPSKTFKGICTRDSNCDTSCRYEGYPAGDCKGIRRRCMCSKPC) Neuropeptide (NKLASVYALTPSLRVG) TPSPR (Alpha-glucosidase inhibitor) |
2248.59 |
| SEGTEPIQSK GQKDAYVGDEAQSK |
Protein disulfide-isomerase OS = Citrus limon tr|V9HXG3|V9HXG3_CITLI | Antimicrobial (FVPYNPPRPGQSKPFPSFPGHGPFNPKIQWPYPLPNPGH) | 3585.3 |
| DYDKPVQQ | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Coeliac toxic peptide (QQPFVQQQQPFVQQ) | 1219.38 |
| GADDSADNKSSNAPTRTY | Uncharacterized protein OS = Citrus clementina tr|V4UJF9|V4UJF9_9ROSI | Erythropoietin receptor agonist peptide (YQRRPAIAINNPYVPRTYYANPAVVRPHAQIPQRQYLPNSHPPTVVRRPNLHPSF) | 1920.2 |
| GETGGPHPGYETR | Uncharacterized protein OS = Citrus clementina tr|V4SQX0|V4SQX0_9ROSI | Alpha amylase inhibitory (DETRL) | 2135.44 |
| IVPRKAASSEE | Uncharacterized protein OS = Citrus clementina tr|V4S6W0|V4S6W0_9ROSI | Antioxidative (RELEELNVPGEIVESLSSSEESITR) | 1274.56 |
| EVHNPATGE | Succinate-semialdehyde dehydrogenase OS = Citrus clementina tr|V4RMN0|V4RMN0_9ROSI | ACE inhibitor (VLSPPFTGE) | 1384.5 |
| QDQGPMVK | Uncharacterized protein OS = Citrus unshiu tr|A0A2H5NKL3|A0A2H5NKL3_CITUN | Neuropeptide (SPTISITAPIDVLRKTWEQERARKQMVKNREFLNSLN) Membrane-active peptides (MVKSKIGSWILVLFVAMWSDVGLCKKRPKP) |
1583.78 |
| GQMNEPPGAR | ATP synthase subunit beta (Fragment) OS =Poncirus trifoliata tr|Q9THW1|Q9THW1_PONTR | Antimicrobial, (GICACRRRFCPNSERFSGYCRVNGARYVRCCSRR)Opioid, (GICACRRRFCPNSERFSGYCRVNGARYVRCCSRR, YGGFTGARKSARKLANQ, FGGFTGARKSA) Neuropeptide (YGGFLGARKSARKLANQ) Antioxidative (QGAR) |
971.09 |
| FYLGGNPQPQLQ | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Neuropeptide (MPRVRSLFQEQEEPEPGMEEAGEMEQKQLQ) coeliac toxic peptide (LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF) |
987.16 |
| QQQRFQTQ | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | ACE inhibitor (QTQSLVYP) | 1075.28 |
| GSAKESGDKAEQGS | ABC transporter solute-binding protein OS = Streptomyces sp. tr|A0A3L8QSZ7|A0A3L8QSZ7_9ACTN | coeliac toxic peptide, (QQQQPSSQVSFQQPLQQYPLGQGSFRPSQQNPQA, QGSFRPSQQNPQAQ, QYPLGQGSFRPS, LGQGSFRPSQQN) ACE inhibitor, (YQGS) Antioxidative (AWEEREQGSR) |
992.16 |
| SQSRSQDQHQKVRQIRE | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Neuropeptide (SENFTPWAYIILNGEAPIIREVHYSPRL) | 1870.1 |
| HNIDKPSHAD | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | Antioxidative (LLPHHADADY) | 1495.76 |
| QSFQSSKSQDQHQKVR | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | ACE inhibitor (KVREGTTY, KVREGT) | 1357.59 |
| QDSQQQQSFQS | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | Antimicrobial (GLLSRLRDFLSDRGRRLGEKIERIGQKIKDLSEFFQS) | 1186.47 |
| QPAGKRGEGPPKRAGQVR | REVERSED Uncharacterized protein OS = Curtobacterium sp. RRRRRtr|A0A1E5MMN3|A0A1E5MMN3_9MICO | Celiac toxic (VQGQGHQPPQQPAQL, VQGQGIIQPQQPAQL) | 953.1 |
| QKVESEAGVT | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | Antioxidative (DSGVT) | 902.15 |
| VIGTVLAFALIASAESLF | Sulfate transporter OS = Streptomyces sp. tr|A0A3L8QBZ9|A0A3L8QBZ9_9ACTN | Antimicrobial (SLFSLIKAGAKFLGKNLLKQGAQYAACKVSKECG, SLFSLIKAGAKFLGKNLLKQGACYAACKASKQC) Neuropeptide (MPRVRSLFQEQEEPEPGMEEAGEMEQKQLQ) | 1056.3 |
| TAAEEVLQKAEAAP | CHAD domain-containing protein OS = Methylobacterium mesophilicum tr|M7YUM6|M7YUM6_9RHIZ | Antimicrobial (WNPFKELERAGQRVRDAVISAAPAVATVGQAAAIARG) | 1361.71 |
| SAEKGNLYQN | Uncharacterized protein OS = Citrus clementina tr|V4S993|V4S993_9ROSI | Regulating (EPFYQNVPD) | 1063.26 |
| NALEPR | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | Peptide stimulating insulin release Antioxidative (GFGSFLGKALKA ALKIGANALGGSPQQ) |
1350.55 |
| QNPANPVTLAQAIEGEPK | DNA-directed DNA polymerase OS = Methylobacterium mesophilicum tr|M7Y0I8|M7Y0I8_9RHIZ | ACE Inhibitor (EPKAIP, HSGIQSEPKAIP)Antioxidative (APIRMWYMYRKLTDMEPKPVA) | 2110.53 |
| SSDTIDNVKAK | Polyubiquitin OS = Avena fatua UBIQP_AVEFA | Antimicrobial (GCASRCKAKCAGRRCJGWASASFRGRCYCKCFRC) | 1133.32 |
| QSDVTTTS | Transcription factor MYB21 OS = Arabidopsis thaliana MYB21_ARATH | Antimicrobial (QLKTADLPAGRDETTSFVLV) Antioxidative (PIAAEVYEHTEGSTTSY) |
1918.29 |
| DESTGTIGKR | Fructose-bisphosphate aldolase, cytoplasmic isozyme 1 OS = Pisum sativum ALF1_PEA | Neuropeptide (KRQHPGKR) | 1310.46 |
| SAPKKEKSQGFLQ | Type II inositol polyphosphate 5-phosphatase 14 OS = Arabidopsis thaliana IP5PE_ARATH | ACE Inhibitor, DPP IV inhibitor (TPVVVPPFLQP, FLQP) | 1219.35 |
| FASTRRRQCPQ | Lignin-forming anionic peroxidase OS = Nicotiana sylvestris PERX_NICSY | Antimicrobial (CPQLQPQNPSQQQPQEQG) | 1889.41 |
| GSGAKPGTKPTKCT | Chaperone protein dnaJ A6, chloroplastic OS = Arabidopsis thaliana DNJA6_ARATH | Antimicrobial (RSVCRQIKICRRRGGCYYKCTNRPY) | 1047.27 |
| TNADKATTVS | Soluble starch synthase 3, chloroplastic/amyloplastic OS = Solanum tuberosum SSY3_SOLTU | Antimicrobial (GILDTLKQFAKGVGKDLVKGAAQGVLSTVSCKLAKTC, GIFSSRKCKTVSKTFRGICTRNANC) | 1822.44 |
| WTAEHSVNAALGQFE | Pyruvate, phosphate dikinase regulatory protein, chloroplastic OS = Zea mays PDRP1_MAIZE | Celiac Toxic (QQLPQFEEIRNL, GSVQPQQQLPQFEIR) | 1427.76 |
| GSEEPNVEEDS | FHA domain-containing protein DDL OS = Arabidopsis thaliana DDL_ARATH | Antimicrobial (YPGPQAKEDSEGPSQGPASREK) | 1123.33 |
Notes: Peptides are grouped and aligned according to their sequence. MW: molecular weight.
Table 3.
Differential peptide sequences present in F(19–20) identified from OSPH (after GI digestion), using NCBInr and UniProt database.
| Sequence | Protein | Bioactivity | MW |
|---|---|---|---|
| SQGSQGSDDGRGGNL | Citrin OS = Citrus sinensis Q39627_CITSI | PEP inhibitor (Antiamnestic), (RYDWWPYGNLFGGHTFISP) Antibacterial, (LLPIVGNLLKSLL, GLLDMVTGLLGNLG, GLFDAIGNLLGGLGLG)Antioxidative, (QLGNLGV)ACE inhibitor (EVMAGNLYPG) |
1219.38 |
| GSQGSDDGRGGNL | Citrin OS = Citrus sinensis Q39627_CITSI | PEP inhibitor (Antiamnestic), (RYDWWPYGNLFGGHTFISP)Antibacterial, (LLPIVGNLLKSLL, GLLDMVTGLLGNLG, GLFDAIGNLLGGLGLG) Antioxidative, (QLGNLGV)ACE inhibitor (EVMAGNLYPG) |
1434.62 |
| GSQGSDDGRGGNL | Citrin OS = Citrus sinensis Q39627_CITSI | PEP inhibitor (Antiamnestic), (RYDWWPYGNLFGGHTFISP)Antibacterial, (LLPIVGNLLKSLL, GLLDMVTGLLGNLG, GLFDAIGNLLGGLGLG)Antioxidative, (QLGNLGV)ACE inhibitor (EVMAGNLYPG) | 1219.38 |
| SQGSQGSDDRRAGN | Uncharacterized protein OS = Citrus clementina tr|V4U2L8|V4U2L8_9ROSI | Celiac Toxic, (VLKMAGNSFQEN) ACE inhibitor (YLAGNQ, EVMAGNYLPG, EVMAGNLYPG) |
1434.59 |
| IDLPQPQ | Serine/threonine-protein kinase TOR OS = Arabidopsis TOR_ARATH | ACE inhibitor, (QPQPLIYP) Potential coeliac toxic peptide, (PQNPSQQQPQEQVP) Celiac Toxic, (SQPQAFP) Antibacterial (PYPQPQPF) |
1219.38 |
| RLAIEEAISITTTLVAQY | Protein RETICULATA-RELATED 5, chloroplastic OS = Arabidopsis thaliana RER5_ARATH | Antibacterial, (NLLKQGAQYAACKVSKECG) ACE inhibitor (VLAQYK) |
1434.59 |
| SSCPVINVD | Uncharacterized GPI-anchored protein At1g61900 OS = Arabidopsis thaliana UGPI6_ARATH | Antibacterial, (CSCRTSSCRFGERL) Natriuretic (RSSCFGGRIDRIGAC) |
810.02 |
| RHSWMMN | Phosphoenolpyruvate carboxylase kinase 2 OS = Arabidopsis thaliana PPCK2_ARATH | Antibacterial, (VPMPKGRSSRGRRHS) Antioxidative (RHS) |
1992.57 |
Peptides have been grouped and aligned according to their sequence.
With regard to the amino acid composition, His, Pro, Ser, Asp, and Glu were the main amino acids existing in the peptide sequences with antioxidant activity investigated in OSPH. On the other hand, the amino acids His, Pro, Ser, Glu, and Tyr and the amino acids Pro, Ser, Asp, and Glu were the most important amino acids present in the peptides with ACE-inhibitory and α-amylase- and α-glucosidase-inhibitory activities, respectively; this results are similar to those of the study conducted by Maqsoudlou et al. (2018), Meshginfar et al. (2018), who reported that these amino acids and other hydrophobic amino acids were the principal amino acids in the peptide sequences of pollen and tomato protein hydrolysate, respectively [49,54]. Mora et al. (2015) claimed that the ACE-inhibitory peptides contain Lys, Pro, or aromatic residues preferably in the three spots nearest to the C-terminal section [28]. Regarding the amino acid mixture in the bioactive peptide sequences in OSPH, after gastrointestinal digestion process, His, Pro, Ser, Asp, Glu, and Tyr were the leading amino acids existing in the peptides.
Bioactivity of hydrolyzed proteins and peptides depends on the hydrolysis situations, protein of origin, degree of hydrolysis, molecular weight, and amino acid sequences [47]. Identified peptides from OSPH in this research included peptides with molecular weights, between 900 and 3000 and between 900 and 2000 Da, before and after gastrointestinal digestion, respectively. Moayedi et al. (2016), Maqsoudlou et al. (2018), and Lassoued et al. (2015) recorded the same sizes for the ACE-inhibitory and antioxidant peptides [25,49]. Salema et al. (2018) announced that the peptides identified from protein hydrolysates from Octopus vulgaris by nano-liquid chromatography and mass spectrometry in tandem after RP-HPLC separation, with the highest antidiabetic and anti-hyperlipidemic effects, were composed of molecular weights around 400–2500 Da, and the majority of them included many hydrophobic amino acid residues [56]. Liu et al. (2014) investigated the antioxidant activity of peptides from porcine plasma protein hydrolysate prepared by Alcalase enzyme and showed that fractions with molecular weight less than 3 kDa exhibited the greatest DPPH scavenging activity and ferric-reducing power [57]. Elimination of some peaks, changes in exit time of some other peaks from the column, and reduction of the following levels of some peaks indicated the digestion effect of proteases in the structural change of peptide chains. Treatment in acidic conditions also reduces some peaks in high-molecular-weight peptide chains in the chromatogram chart, and peptide sequences appear at about 1 kDa and less [54]. The biological activity of peptides has been defined to be highly dependent on the amino acids composing the sequence, the hydrophobicity and molecular weight of the molecule, and the length of amino acids. Some bioactive peptides are resistant to the action of proteases, so they can remain intact after ingestion and further gastrointestinal digestion and bloodstream transport to the target sites [54,55].
Thus, the impacts of molecular weight and amino acid mixture on the antioxidant, α-amylase inhibitory- and α-glucosidase-inhibitory activities, and ACE-inhibitory activity showed that both of these two elements play a vital role in the bioactivity of peptides generated during protein hydrolysis. The fractions 19 to 20 obtained from RP-HPLC were chosen to be identified, and a total of 63 peptide sequences were recognized in all samples. According to results obtained after imitative gastrointestinal digestion, DPPH scavenging activity, ferric-reducing power, α-amylase-inhibitory activity, and α-glucosidase-inhibitory activity decreased after digestion, and ACE-inhibitory activity increased. These results showed significant differences in any of the biological activities studied after digestion (p < 0.05). Thus, orange seed proteins could be employed as a new protein source for the manufacturing of peptides with antioxidant ability, ACE-inhibitory activity, and α-amylase-inhibitory and α-glucosidase-inhibitory activities with relative resistance to gastrointestinal digestion. These peptides could be applied in different food formulations and protein-based food supplements, to improve human health.
4. Conclusions
This study was conducted to evaluate antioxidant, ACE-inhibitory activities and antidiabetic capacity, as well as to study the peptides stability of hydrolyzed orange seed proteins by Alcalase enzyme. Our results indicated that the maximum biological activities, especially antioxidant and ACE-inhibitory activities, of the hydrolyzed orange seed proteins were obtained at hydrolysis time of 5 h and the enzyme to substrate ratio of 0.02. Based on the SEC and RP-HPLC tests, we observed that both content and molecular weight of the amino acids play a very important role in the biological activity of the obtained peptides. The peptides fractions (19–20) derived from RP-HPLC were selected to sequence and identify peptides. The molecular weight of the peptides before and after simulated gastrointestinal digestion was 900–3000 and 900–2000 Da, respectively. Elimination of some peaks, changes in the time of withdrawal of some other peaks from the column after gastrointestinal digestion, and reduction of the following levels of a number of peaks in digested fractions indicated the digestive effect of proteases enzymes on changes in the structure of peptide chains. It seems that orange seeds can be used to produce beneficial bioactive peptides that are resistant to digestive enzymes. Therefore, Alcalase hydrolyzed orange seed proteins can be suggested as health beneficial product to reduce blood pressure and diabetes management. Future studies especially randomized clinical trials are warranted to obtain a precise conclusion about health beneficial effects of orange seed proteins hydrolysate.
Author Contributions
S.N.M., investigation and writing—original draft; L.M., conceptualization, project administration, writing—review and editing, and funding; M-C.A., conceptualization, project administration, writing—review and editing, and funding; A.S.M., conceptualization, project administration, writing—review and editing, and funding; M.G., writing—review and editing; G.H., resources, review, and editing; F.T., conceptualization, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Grant AGL2017-89381-R and FEDER funds from the Spanish Ministry of Economy, Industry and Competitiveness, and Ramón y Cajal postdoctoral contract by L.M. are acknowledged. This work was also supported by Intramural project from CSIC number 201870E006.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Danquah M.K., Agyei D. Pharmaceutical applications of bioactive peptides. OA Biotechnol. 2012;1:1–7. doi: 10.13172/2052-0069-1-2-294. [DOI] [Google Scholar]
- 2.Korhonen H., Pihlanto A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- 3.Garcia R.A., Pyle D.J., Piazza G.J., Wen Z. Hydrolysis of animal protein meals for improved utility in non-feed applications. ASABE. 2011;27:269–275. [Google Scholar]
- 4.Zambrowicz A., Timmer M., Polanowski A., Lubec G., Trziszka T. Manufacturing of peptides exhibiting biological activity. Amino Acids. 2013;44:315–320. doi: 10.1007/s00726-012-1379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taha S.F., Mohamed S.S., Wagdy M.S., Mohamed F.G. Antioxidant and antimicrobial activities of enzymatic hydrolysis products from sunflower protein isolate. World Appl. Sci. J. 2013;21:651–658. [Google Scholar]
- 6.Cheng X., Tang X., Wang Q., Mao X.Y. Antibacterial effect and hydrophobicity of yak κ-casein hydrolysate and its fractions. Int. Dairy J. 2013;31:16–111. doi: 10.1016/j.idairyj.2012.12.004. [DOI] [Google Scholar]
- 7.Chakrabarti S., Jahandideh F., Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed. Res. Int. 2014;5:1–11. doi: 10.1155/2014/608979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szwajkowska M., Wolanciuk A., Barłowska J., Król J., Litwinczuk Z. Bovine milk proteins as the source of bioactive peptides influencing the consumers’ immune system—A review. Food Sci. Tech. 2011;7:120–125. [Google Scholar]
- 9.Wiriyaphan C., Chitsomboon B., Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012;132:104–111. doi: 10.1016/j.foodchem.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Wouters A.G.B., Rombouts I., Fierens E., Brijs K., Delcour J.A. Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. CRFSFS. 2016;15:786–800. doi: 10.1111/1541-4337.12209. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S., Singh R., Rana S. Bioactive Peptides: A Review. Int. J. Bioautom. 2011;15:223–250. [Google Scholar]
- 12.Weiguang S., Xiangzhen K., Yufei H., Xingfei L., Caimeng Z., Yeming C. Antioxidant and antibacterial activity and in vitro digestion stability of cottonseed protein hydrolysates. LWT Food Sci. Technol. 2020;118:10–18. [Google Scholar]
- 13.Delgado M.C.O., Tironi V.A., Añón M.C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT Food Sci. Technol. 2011;44:1752–1760. doi: 10.1016/j.lwt.2011.04.002. [DOI] [Google Scholar]
- 14.Gul Akıllıoglu H.G., Karakaya S. Effects of heat treatment and in vitro digestion on the Angiotensin converting enzyme inhibitory activity of some legume species. Eur. Food Res. Technol. 2009;229:915–921. doi: 10.1007/s00217-009-1133-x. [DOI] [Google Scholar]
- 15.Mazloomi S.N., Mahoonak A.S., Ghorbani M., Houshmand G. Physicochemical properties of chitosan-coated nanoliposome loaded with orange seed protein hydrolysate. J. Food Eng. 2020;280:109976. doi: 10.1016/j.jfoodeng.2020.109976. [DOI] [Google Scholar]
- 16.Mohamed B., El-Shenawi M. Functional properties and In-vitro digestibility of bitter orange (Citrus aurantium) seed flour. MRJASSS. 2013;1:042–047. [Google Scholar]
- 17.Horax R., Hettiarachchy N., Over K., Chen P., Gbur E. Extraction, fractionation and characterization of Bitter Melon seed proteins. J. Agric. Food Chem. 2010;58:1892–1897. doi: 10.1021/jf902903s. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka T., Kawashima T., Nakamura T., Kanamaru Y., Yabe T. Isolation and characterization of proteases that hydrolyze royal jelly proteins from queen bee larvae of the honeybee, Apis mellifera. Apidologie. 2012;43:685–697. doi: 10.1007/s13592-012-0143-z. [DOI] [Google Scholar]
- 19.Kaewka K., Therakulkait C.R., Cadwallader K. Effect of preparation conditions on composition and sensory aroma characteristics of acid hydrolyzed rice bran protein concentrate. J. Cereal Sci. 2009;50:56–60. doi: 10.1016/j.jcs.2009.02.006. [DOI] [Google Scholar]
- 20.Bersuder P., Hole M., Smith G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- 21.Bougatef A., Hajji M., Balti R. Antioxidant and free radical—Scavenging activities of smoth hound muscle protein hydrolysates obtained by gastro intestinal proteases. Food Chem. 2010;15:1198–1255. [Google Scholar]
- 22.Sentandreu M., Toldra F. A Fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat. Protoc. 2006;1:2423–2427. doi: 10.1038/nprot.2006.349. [DOI] [PubMed] [Google Scholar]
- 23.Lassoued I., Mora L., Barkia A., Aristoy M.C., Nasri M., Toldra F. Bioactive peptides identified in thornback ray skin’s gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. J. Proteom. 2015;128:8–17. doi: 10.1016/j.jprot.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Jemil I., Abdelhedi O., Mora L., Nasri R., Aristoy M.-C., Jridi M., Hajji M., Toldrá F., Nasri M. Peptidomic analysis of bioactive peptides in zebra blenny (Salaria basilisca) muscle protein hydrolysate exhibiting antimicrobial activity obtained by fermentation with Bacillus mojavensis A21. Process Biochem. 2016;51:2186–2197. doi: 10.1016/j.procbio.2016.08.021. [DOI] [Google Scholar]
- 25.Moayedi A., Mora L., Aristoy C.M., Hashemi M., Safari M., Toldrá M. ACE inhibitory and antioxidant activities of peptide fragments obtained from tomato processing by-products fermented usingbacillus subtilis: Effect of amino acid composition and peptides molecular mass distribution. Appl. Biochem. Biotechnol. 2016;16:18–35. doi: 10.1007/s12010-016-2198-1. [DOI] [PubMed] [Google Scholar]
- 26.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Brodkorb A. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 27.Flores M., Aristoy M.C., Spanier A.M., Toldrá F. Non-volatile components effects on quality of “Serrano” dry-cured ham as related to processing time. Food Sci. 1997;62:1235–1239. doi: 10.1111/j.1365-2621.1997.tb12252.x. [DOI] [Google Scholar]
- 28.Mora L., Escudero E., Aristoy M.C., Toldrá F. A peptidomic approach to study the contribution of added casein proteins to the peptide profile in Spanish dryfermented sausages. Int. J. Food Microbiol. 2015;212:41–48. doi: 10.1016/j.ijfoodmicro.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Mora L., Aristoy M.C., Toldra F. Bioactive peptides in foods. Encycl. Food Health. 2016;35:395–400. [Google Scholar]
- 30.Minkiewicz P., Iwaniak A., Darewicz M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019;20:5978. doi: 10.3390/ijms20235978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerar F., Guimas L., Binet A. Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catal. 2002;19:489–498. doi: 10.1016/S1381-1177(02)00203-5. [DOI] [Google Scholar]
- 32.Jamdar S.N., Rajalakshmi V., Pednekar M.D., Juan F., Yardi V., Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- 33.Je J.Y., Lee M.H., Lee K.H., Ahn C.B. Antioxidant and hypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. 2009;42:1266–1272. doi: 10.1016/j.foodres.2009.06.013. [DOI] [Google Scholar]
- 34.Ren J., Zhao M., Shi J., Wang J., Jiang Y., Cui C., Xue S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108:727–736. doi: 10.1016/j.foodchem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Power O., Jakeman P., FitzGerald R. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milkderived antioxidative peptides. Amino Acids. 2013;44:797–820. doi: 10.1007/s00726-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 36.Coscueta R.F., Amorim M.M., Voss G.B., Nerli B.B., Picó G.A., Pintado M.A. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chem. 2016;198:36–44. doi: 10.1016/j.foodchem.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 37.Ktari N., Nasri R., Mnafgui K., Hamden K., Belguith O., Boudaouara T., Nasri M. Antioxidative and ACE inhibitory activities of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Process Biochem. 2014;5113:73–77. doi: 10.1016/j.procbio.2014.01.032. [DOI] [Google Scholar]
- 38.Ambigaipalan P., Al-Khalifa A.S., Shahidi F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods. 2015;18:1125–1137. doi: 10.1016/j.jff.2015.01.021. [DOI] [Google Scholar]
- 39.Shahidi F., Zhong Y. Bioactive peptides. J. AOAC Int. 2008;91:914–931. doi: 10.1093/jaoac/91.4.914. [DOI] [PubMed] [Google Scholar]
- 40.Lacroix I.M.E., Li-Chan E.C.Y. Inhibition of Dipeptidyl Peptidase (DPP)-IV and α-Glucosidase Activities by Pepsin-Treated Whey Proteins. J. Agric. Food Chem. 2013;61:7500–7506. doi: 10.1021/jf401000s. [DOI] [PubMed] [Google Scholar]
- 41.Siow H.L., Lim T.S., Gan C.Y. Development of a workflow for screening and identification of a-amylase inhibitory peptides from food source using an integrated Bioinformatics-phage display approach: Case study—Cumin seed. Food Chem. 2017;214:67–76. doi: 10.1016/j.foodchem.2016.07.069. [DOI] [PubMed] [Google Scholar]
- 42.Meinert L., de Lichtenberg Broge E.H., Bejerholm C., Jensen K. Application of hydrolyzed proteins of animal origin in processed meat. Food Sci. Nutr. 2015;4:290–297. doi: 10.1002/fsn3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selamassakul O., Laohakunjit N., Kerdchoechuen O., Yang L., Maier C. Isolation and characterisation of antioxidative peptides from bromelain-hydrolysed brown rice protein by proteomic technique. Process Biochem. 2018;17:359–391. doi: 10.1016/j.procbio.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soleymanzadeh N., Mirdamadi S., Mirzaei M., Kianirad M. Novel β-casein derived antioxidant and ACE-inhibitory active peptide from camel milk fermented by Leuconostoc lactis PTCC1899: Identification and molecular docking. Int. Dairy J. 2019;19:12–46. doi: 10.1016/j.idairyj.2019.05.012. [DOI] [Google Scholar]
- 45.Jonker J.T., Wijngaarden M.A., Kloek J., Groeneveld Y., Gerhardt C., Brand R., Kies A.K., Romijn J.A., Smit J.W.A. Effects of low doses of casein hydrolysate on postchallenge glucose and insulin levels. Eur. J. Intern. Med. 2011;22:245–248. doi: 10.1016/j.ejim.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Yang H.J., Kwon D.Y., Kim M.J., Kang S., Park S. Meju, unsalted soybeans fermented with Bacillus subtilis and Aspergilus oryzae, potentiates insulinotropic actions and improves hepatic insulin sensitivity in diabetic rats. Nutr. J. 2012;9:37–45. doi: 10.1186/1743-7075-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva C.M., Fonseca R.A.D.S., Prentice C. Comparing the hydrolysis degree of industrialization byproducts of Withemouth croaker (Micropogonias furnieri) using microbial enzymes. Int. Food Res. J. 2014;21:1757–1761. [Google Scholar]
- 48.Oveisipour M., Abedian A.M., Motamedzadegan A., Rasco B., Safari R., Shahiri H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from the Persian sturgeon (Acipenser persicus) viscera. Food Chem. 2009;115:238–242. doi: 10.1016/j.foodchem.2008.12.013. [DOI] [Google Scholar]
- 49.Maqsoudlou A., Sadeghi Mahoonak A., Mora L., Mohebodini H., Toldrá F., Ghorbani M. Peptide identification in alcalase hydrolysated pollen and comparison of its bioactivity with royal jelly. Food Res. Int. 2018;88:963–974. doi: 10.1016/j.foodres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.Y., Je J.Y., Kim S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007;18:31–38. doi: 10.1016/j.jnutbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Yang B., Yang H., Li J., Li Z., Jiang Y. Amino acid composition, molecular weight distribution and antioxidant activity of protein hydrolysates of soy sauce lees. Food Chem. 2010;124:551–555. doi: 10.1016/j.foodchem.2010.06.069. [DOI] [Google Scholar]
- 52.Ruiz J., Dávila-Ortíz G., Chel-Guerrero L., Betancur-Ancona D. Angiotensin iconverting enzyme inhibitory and antioxidant peptide fractions from hard-to-cook bean enzymatic hydrolysates. J. Food Biochem. 2013;37:26–35. doi: 10.1111/j.1745-4514.2011.00594.x. [DOI] [Google Scholar]
- 53.Lassoued I., Mora L., Barkia A., Aristoy M.C., Nasri M., Toldrá F. Angiotensin I-converting enzyme inhibitory peptides FQPSF and LKYPI identified in Bacillus subtilisA26 hydrolysate of thornback ray muscle. Int. J. Food Sci. Technol. 2016;51:1604–1615. doi: 10.1111/ijfs.13130. [DOI] [Google Scholar]
- 54.Meshginfar N., Sadeghi Mahoonak A., Hosseinian F., Ghorbani M., Tsopmo A. Production of antioxidant peptide fractions from a by-product of tomato processing: Mass spectrometry identification of peptides and stability to gastrointestinal digestion. Int. J. Food Sci. Technol. 2018;18:274–287. doi: 10.1007/s13197-018-3274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torkova A., Kononikhin A., Bugrova A., Khotchenkov V., Tsentalovich M., Medvedeva U. Effect of in vitro gastrointestinal digestion on bioactivity of poultry protein hydrolysate. CRNFSJ. 2016;4:77–86. [Google Scholar]
- 56.Salema R.B., Ktaria N., Bkhairiaa I., Nasria R., Morab L., Kallelc R., Hamdid S., Jamoussid K., Boudaouarac T., El-Fekie A., et al. In vitro and in vivo anti-diabetic and anti-hyperlipidemic effects of protein hydrolysates from Octopus vulgaris in alloxanic rats. Food Res. 2018;106:952–963. doi: 10.1016/j.foodres.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 57.Liu R., Zheng W., Li J., Wang L., Wu H., Wang X., Shi L. Rapid identification of bioactive peptides with antioxidant activity from the enzymatic hydrolysate of Mactra veneriformis by UHPLC–Q-TOF mass spectrometry. Food Chem. 2014;167:484–489. doi: 10.1016/j.foodchem.2014.06.113. [DOI] [PubMed] [Google Scholar]