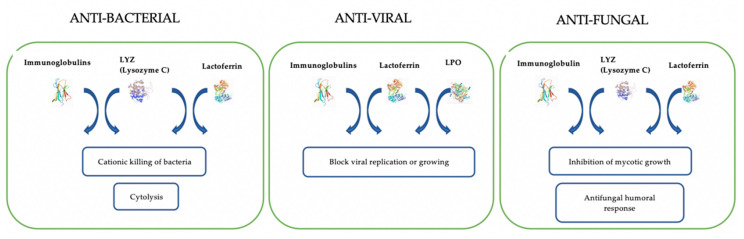
Figure 1.
Molecular mechanism of anti-microbial activity of donkey milk (DM) proteins (protein structure is a reference to UniProtKB [55] and the Protein Data Bank—PDB [56] (LYS: Lysozyme C: (P11375); Lactoferrin (A0A3Q9HG40); Immunoglobulin (Q861S3); LPO—Lactoperoxidase (P80025))). The anti-bacterial activity of DM is mainly due to lysozyme, lactoferrin, and immunoglobulins (IgG, IgA, IgM) through two molecular mechanisms: cytolysis and the cationic killing of bacteria (LYS could act synergistically with lactoferrin, and immunoglobulins). The anti-viral capability of DM was related to synergetic action between LYS, LPO, immunoglobulins, and low abundant–low molecular weight protein fraction (<30,000 Da) through blocking viral replication or growing by binding to host cells and/or direct interaction with the viruses. The anti-fungal activity is due to the antifungal capacity of DM’s proteins (lactoferrin, LYS, and immunoglobulins) within the inhibition of mycotic growth and protective cell reactions triggered in response to the fungi presence; (the arrows represent the synergistic activity of proteins).