Abstract
Antioxidant compounds, including polyphenols, have therapeutic effects because of their anti-inflammatory, antihypertensive, antithrombotic and antiproliferative properties. They play important roles in protecting the cardiovascular and neurological systems, by having preventive or protective effects against free radicals produced by either normal or pathological metabolism in such systems. For instance, resveratrol, a well-known potent antioxidant, has a counteracting effect on the excess of reactive oxygen species (ROS) and has a number of therapeutic benefits, like anti-inflammatory, anti-cancer and cardioprotective activities. Based on previous work from our group, and on the most frequent OH substitutions of natural polyphenols, we designed two series of synthetically accessible bis-polyhydroxyphenyl derivatives, separated by amide or urea linkers. These compounds exhibit high antioxidant ability (oxygen radical absorbance capacity (ORAC) assay) and interesting radical scavenging activity (RSA) values (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and α,α-diphenyl-β-picrylhydrazyl (DPPH) tests). Some of the best polyphenols were evaluated in two biological systems, endothelial cells (in vitro) and whole aorta (ex vivo), highly susceptible for the deleterious effects of prooxidants under different inflammatory conditions, showing protection against oxidative stress induced by inflammatory stimuli relevant in cardiovascular diseases, i.e., Angiotensin II and IL-1β. Selected compounds also showed strong in vivo antioxidant properties when evaluated in the model organism Saccharomyces cerevisiae.
Keywords: antioxidants, ORAC, ABTS, DPPH, polyhydroxyphenyl amides, polyhydroxyphenyl ureas, NADPH oxidase, vasculoprotection, Saccharomyces cerevisiae
1. Introduction
In recent years, antioxidant compounds have become very popular because of their multiple benefits for health. Nutraceuticals are bioactive ingredients of fruits and vegetables, or products elaborated with natural substances, able to modulate metabolic processes. Among nutraceuticals, there are many antioxidant substances, like polyphenols [1,2,3]. There is an important number of compounds, biosynthesized by the secondary metabolism of plants, known as phytochemicals, which can be extracted from plants and have interesting therapeutic properties in the treatment of infectious diseases, cancer, hypercholesterolemia and immunological disorders [1,4]. Antioxidant compounds, in particular, polyphenols, have beneficial effects for human health, including, among others, anti-inflammatory, antihypertensive, antithrombotic and antiproliferative properties. They play important roles in protecting the cardiovascular and neurological systems, by having preventive or protective effects against the free radicals produced by normal metabolism in such systems [5,6,7,8,9]. As an example, resveratrol, a well-known potent antioxidant, has a counteracting effect to the excess of radical oxygen species (ROS) that is translated in a number of biological activities of therapeutic relevance, like anti-inflammatory and anti-cancer activities, as well as protection against cardiovascular diseases [10,11]. Antioxidants also have applications as additives in food and cosmetics for maintaining the quality of products and for extending their half-life [12,13].
Previous work from our group led us to the preparation of some polyhydroxy diphenylpropanones, which in addition to their activity as α7 nicotinic acetyl choline receptor positive allosteric modulation (nAChR, PAM), showed interesting antioxidant properties [14,15]. The two phenyl rings in these compounds are separated by a propanone linker. Since this connector could be susceptible to the action of natural nucleophiles from biomacromolecules, possibly leading to covalent complexes, we explored its change by a triazolyl ring, which excludes the possibility of covalent bonds with the linker. The resulting small collection of triazolyl polyphenols allowed us to study the influence of the number and position of OH groups on the antioxidant properties [16]. Although we obtained excellent antioxidants, the yield in the preparation of methoxythriazolyl intermediates was very low in many cases, limiting their future applications. Our interest on new antioxidant structures moved us to further explore the linker chain joining the two phenolic rings, looking for easy, effective reactions. As a result, a small collection of amide and urea polyhydroxyphenyl derivatives 1–18 was prepared and characterized for their antioxidant properties, using different methods, oxygen radical absorbance capacity (ORAC), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) assay (ABTS), and the α,α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging test. Compounds with high antioxidant ability and acceptable aqueous stability were selected for further studies. The potential biological activity as antioxidants was tested in two biological systems highly susceptible for the deleterious effects of prooxidants in different inflammatory conditions, endothelial cells and whole aorta. Selected compounds showed protection against oxidative stress induced by inflammatory stimuli important in cardiovascular diseases, i.e., Angiotensin II and Interleukin 1β (IL-1β). Compounds with different in vitro antioxidant profiles were chosen for studying the in vivo antioxidant properties in a model organism, Saccharomyces cerevisiae. All of them showed recovery of stressed yeast growth comparable to model antioxidants resveratrol and vitamin C.
2. Materials and Methods
2.1. Chemistry
General information, as well as the preparation and characterization of methoxy (OMe)-substituted intermediates (19 to 36) is described in the Supplementary Material document.
2.2. Preparation of Polyhydroxylated Amides (1 to 10)
General procedure for the deprotection of methoxy groups. To a previously cooled solution (0 °C) of the corresponding methoxy-substituted compound (1 equivalent) in dried CH2Cl2 (15 mL), a 1M solution of BBr3 in DCM (2 equivalents for each heteroatom) was slowly added under Ar atmosphere. After stirring 24–48 h at room temperature under Ar, monitoring the total disappearance of the methoxy groups by HPLC-MS, H2O was added to the reaction mixture. The solid precipitate, when formed, was separated by filtration and washed with H2O and CH2Cl2. When no precipitate was observed, the product was extracted with EtOAc. The organic extracts were washed with H2O and brine, dried over Na2SO4 and then evaporated to give the polyhydroxylated analogous compounds 1 to 10. The crude products were purified as indicated in each case.
2.2.1. N-(2,4-Dihydroxyphenyl)-2-(2′,5′-dihydroxyphenyl)acetamide (1)
Reddish solid, 41% yield. m.p.: 178–180 °C (Precipitated with Et2O). HPLC: tR = 6.06 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (300 MHz, DMSO-d6) δ: 3.51 (s, 2H, CH2), 6.15 (dd, 1H, J = 8.6, 2.5 Hz, 4′-H), 6.29 (d, 1H, J = 2.6 Hz, 6′-H), 6.48 (dd, 1H, J = 8.5, 2.9 Hz, 5-H), 6.59 (d, 1H, J = 2.9 Hz, 3-H), 6.63 (d, 1H, J = 8.5 Hz, 6-H), 7.47 (d, 1H, J = 8.6 Hz, 3′-H), 8.67 (s, 1H, NH), 9.01 (s, 1H, OH), 9.03 (s, 1H, OH), 9.08 (s, 1H, OH), 9.64 (s, 1H, OH) ppm. 13C-NMR (75 MHz, DMSO-d6) δ: 38.2 (CH2), 102.9 (C3), 105.6 (C5), 114.2 (C6′), 115.8 (C4′), 117.2 (C1), 118.3 (C3′), 122.5 (C1′), 122.8 (C6), 147.7 (C), 148.7 (C), 149.8 (C), 154.5 (C), 169.5 (CO) ppm. MS (ESI+): m/z 276.4 (M+H)+.
2.2.2. 2-(2′,4′-Dihydroxyphenyl)-N-(2,5-dihydroxyphenyl)acetamide (2)
Reddish solid, 44% yield, m.p.: 77–79 °C, (precipitated with Et2O). HPLC: tR = 2.50 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (300 MHz, DMSO-d6) δ: 1.91 (s, 2H, CH2), 6.19 (dd, 1H, J = 8.2, 2.3 Hz, 4′-H), 6.27 (dd, 1H, J = 8.5, 2.9 Hz, 5-H), 6.33 (d, 1H, J = 2.3 Hz, 6′-H), 6.58 (d, 1H, J = 8.5 Hz, 6-H), 6.93 (d, 1H, J = 8.2 Hz, 3′-H), 7.49, (d, 1H, J = 2.9 Hz, 3-H), 8.70 (br s, 1H, OH), 8.78 (s, 1H, NH) 9.03 (br s, 1H, OH), 9.19 (br s, 1H, OH), 9.61 (br s, 1H, OH) ppm. 13C-NMR (75 MHz, DMSO-d6) δ: 38.3 (CH2), 102.9 (C3), 106.4 (C6′), 107.3 (C5), 109.8 (C1), 112.4 (C4′), 115.4 (C3′), 127.1 (C1′), 131.4 (C6), 138.8 (C), 149.8 (C), 156.0 (C), 157.5 (C), 170.0 (CO) ppm. MS (ESI+): m/z 276.4 (M+H)+.
2.2.3. 2-(2′,5′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (3)
White lyophilized solid, purified by column chromatography, EtOAc-Hex (gradient from 1:2 to 3:1). Yield of 79%, m.p.: 185–187 °Cd. HPLC: tR = 2.17 min (5 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.46 (s, 2H, CH2), 6.45 (dd, 1H, J = 8.3, 2.9 Hz, 4′-H), 6.58 (d, 1H, J = 2.8 Hz, 6′-H), 6.59 (d, 1H, J = 8.6 Hz, 3′-H), 6.68 (d, 2H, J = 8.8 Hz, 3-H, 5-H), 7.37 (d, 1H, J = 8.6 Hz, 2-H,6-H), 8.63 (br s, 1H, OH), 8.84 (br s, 1H, OH), 9.16 (br s, 1H, NH), 9.80 (s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 38.0 (CH2), 113.9 (C4′), 115.0 (C3,C5), 115.6 (C3′), 117.1 (C6′), 120.9 (C2, C6), 123.2 (C1′), 130.9 (C1), 147.7 (C), 149.7 (C), 153.24 (C), 169.0 (CO) ppm. MS (ESI+): m/z 260.33 (M+H)+.
2.2.4. N-(2,5-Dihydroxyphenyl)-2-(2′,5′-dihydroxyphenyl)acetamide (4)
Reddish lyophilized solid, purified by column chromatography, EtOAc-Hex (gradient from 1:1 to 4:1). Yield of 79%, m.p.: 204–207 °Cd. HPLC: tR = 2.24 min (5 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.53 (s, 2H, CH2), 6.28 (dd, 1H, J = 8.6, 2.9 Hz, 4-H), 6.49 (dd, 1H, J = 8.6, 2.9 Hz, 4′-H), 6.60 (m, 2H, 3′-H,6′-H), 8.65 (d, 1H, J = 8.6 Hz, 3-H), 7.48 (d, 1H, J = 2.9 Hz, 6-H), 8.71 (s, 1H, OH), 8.73 (s, 1H, OH), 8.95 (s, 1H, NH), 0.03 (s, 1H, OH), 9.07 (br s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 39.8 (CH2), 107.6 (C6), 110.0 (C3), 114.4 (C4), 115.5 (C4′), 115.7 (C3′), 117.2 (6′), 122.5 (C1′), 127.0 (C1), 138.3 (C), 147.6 (C), 149.8 (C), 149.8 (C), 169.5 (CO) ppm. MS (ESI+): m/z 276.27 (M+H)+.
2.2.5. N-(2,4-Dihydroxyphenyl)-2-(2′,4′-dihydroxyphenyl)acetamide (5)
White lyophilized solid, purified by column chromatography, EtOAc-Hex (gradient from 1:1 to 4:1). Yield of 94%, m.p.: 75–78 °Cd (MeOH). HPLC: tR = 2.24 min (5 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.43 (s, 2H, CH2), 6.13 (dd, 1H, J = 8.7, 2.6, 4′-H), 6.16 (dd, 1H, J = 8.1, 2.4, 4-H), 6.25 (d, 1H, J = 2.6 Hz, 6′-H), 6.29 (d, 1H, J = 2.4 Hz, 6-H), 6.90 (d, 1H, J = 8.2 Hz, 3′-H), 7.48 (d, 1H, J = 8.7 Hz, 3-H), 8.84 (br s, 1H, NH), 9.12 (br s, 2H, OH), 9.51 (br s, 2H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 37.8 (CH2), 102.6 (C3′), 102.9 (C3), 105. 7 (C5), 106.4 (C5′), 112.7 (C6), 118.5 (C6′), 122.2 (C1′), 131.3 (C1), 148.4 (C), 154.4 (C), 156.1 (C), 157.4 (C), 170.0 (CO) ppm. MS (ESI+): m/z 276.34 (M+H)+.
2.2.6. 2-(2′,5′-Dihydroxyphenyl)-N-(3,4-dihydroxyphenyl)acetamide (6)
Reddish lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:1 to 2:1), 56% yield, m.p.: 180 °Cd. HPLC: tR = 4.02 min (5 min gradient: 2 to 95% of A in B) 1H-NMR (400 MHz, DMSO-d6) δ: 3.34 (s, 2H, CH2), 6.45 (dd, 1H, J = 8.5, 2.9, 5′-H), 6.57 (d, 1H, J = 2.9, 6′-H), 6.59 (d, 1H, J = 8.5 Hz, 5-H), 6.62 (d, 1H, J = 8.5 Hz, 4′-H), 6.79 (dd, 1H, J = 8.5, 2.4 Hz, 6-H), 7.14 (d, 1H, J = 2.4 Hz, 2-H), 8.57 (s, 1H, OH), 8.62 (s, 1H, OH), 8.85 (s, 1H, NH), 8.92 (s, 1H, OH), 9.69 (s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 38.2 (CH2), 107.9 (C2), 110.4 (C6), 113.9 (C4′), 115.2 (C5), 115.6 (C3′), 117.1 (C6′), 123.2 (C1′), 131.3 (C1), 141.2 (C), 144.9 (C), 147.7 (C), 149.7 (C), 169.0 (CO) ppm. MS (ESI+): m/z 276.27 (M+H)+.
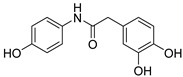
2.2.7. 2-(3′,4′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (7)
White lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:1 to 3:1), 75% yield, m.p.: 192–194 °C. HPLC: tR = 2.09 min (5 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.36 (s, 2H, CH2), 6.54 (dd, 1H, J = 8.0, 2.1, 6′-H), 6.64 (d, 1H, J = 8.0, 3′-H), 6.66 (d, 2H, J = 8.9 Hz, 3-H, 5-H), 6.72 (d, 1H, J = 2.1 Hz, 2′-H), 7.35 (d, 2H, J = 8.9 Hz, 2-H, 6-H), 8.70 (s, 1H, OH), 8.81 (br s, 1H, OH), 9.14 (s, 1H, NH), 9.76 (br s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 43.0 (CH2), 115.3 (C3, C5), 115.6 (C2′), 116.6 (C5′), 120.0 (C6′), 121.1 (C2, C6), 127.3 (C1′), 131.3 (C1), 144.2 (C), 145.3 (C), 153.5 (C), 169.2 (CO) ppm. MS (ESI+): m/z 260.33 (M+H)+.
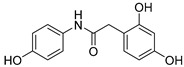
2.2.8. 2-(2′,4′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (8)
White lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:3 to 2:1), 80% yield, precipitated with Et2O (m.p.: 186–189 °C). HPLC: tR = 2.43 min (5 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.45 (s, 2H, CH2), 6.17 (dd, 1H, J = 8.2, 2.4, 5′-H), 6.27 (d, 1H, J = 2.4, 3′-H), 6.67 (d, 2H, J = 8.8 Hz, 3-H, 5-H), 6.88 (d, 1H, J = 8.2 Hz, 6′-H), 7.36 (d, 2H, J = 8.8 Hz, 2-H, 6-H), 9.07 (s, 1H, NH), 9.14 (s, 1H, OH), 9.39 (br s, 1H, OH), 9.69 (br s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 37.4 (CH2), 102.5 (C3), 106.1 (C5′), 113.1 (C1′), 115.0 (C3, C5), 120.9 (C2, C6), 131.0 (C-1), 131.1 (C6′), 153.2 (C), 156.1 (C), 157.1 (C), 169.6 (CO) ppm. MS (ESI+): m/z 260.33 (M+H)+.
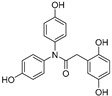
2.2.9. 2-(2′,5′-Dihydroxyphenyl)-N,N-bis(4-hydroxyphenyl)acetamide (9)
White lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:2 to 2:1), 72% yield, precipitated with Et2O, m.p.: 124–126 °C. HPLC: tR = 4.57 min (5 min gradient: 2 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.26 (s, 2H, CH2), 6.34 (dd, 1H, J = 8.5, 2.9, 4′-H), 6.27 (d, 1H, J = 2.9, 6′-H), 6.43 (d, 1H, J = 8.5 Hz, 3′-H), 6.62 (d, 2H, J = 8.1 Hz, 3-H, 5-H), 6.68 (d, 2H, J = 8.2 Hz, 3″-H, 5″-H), 6.98 (d, 2H, J = 8.2 Hz, 2″-H, 6″-H), 7.10 (d, 2H, J = 8.1 Hz, 2-H, 6-H), 8.51 (s, 1H, OH), 8.57 (s, 1H, OH), 9.33 (br s, 1H, OH), 9.57 (br s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 35.7 (CH2), 113.6 (C3″, C5″), 115.2 (C4′), 115.3 (C3, C5), 115.9 (C3′, C6′), 117.2 (C2″, C6″), 123.4 (C2, C6), 127.8 (C-1′), 129.4 (C1″), 134.9 (C1), 147.6 (C), 149.5 (C), 155.4 (C), 156.6 (C), 170.9 (CO) ppm. MS (ESI+): m/z 352.29 (M+H)+.
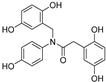
2.2.10. N-(2′,5′-Dihydroxybenzyl)-2-(2″,5″-dihydroxyphenyl)-N-(4-hydroxyphenyl) acetamide (10)
White lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:2 to 2:1), 81% yield, precipitated with Et2O, m.p.: 114–116 °C. HPLC: tR = 4.64 min (5 min gradient: 2 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 3.25 (s, 2H, CH2), 4.66 (s, 2H, NCH2), 6.41–6.46 (m, 3H, 4′H, 4″H, 6″H), 6.49 (d, 1H, J = 2.9, 6′-H), 6.52 (d, 1H, J = 8.2 Hz, 3′H), 6.54 (d, 1H, J = 8.5 Hz, 3″H), 6.74 (d, 2H, J = 8.7 Hz, 3H, 5H), 7.01 (d, 2H, J = 8.7 Hz, (d, 2H, J = 8.1 Hz, 2H, 6H), 8.56 (s, 1H, OH), 8.60 (s, 1H, OH), 8.68 (s, 1H, OH), 8.73 (br s, 1H, OH), 9.63 (br s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 35.0 (CH2), 48.2 (N-CH2), 113.8 (C4″), 114.6 (C6″), 115.4 (C3″), 115.8 (C3, C5), 115.9 (C4′), 117.4 (C3′, C6′), 123.1 (C1′), 123.7 (C1″), 129.1 (C2, C6), 133.7 (C1), 147.4 (C), 147.7 (C), 149.5 (C), 149.6 (C), 156.7 (C), 171.7 (CO) ppm. MS (ESI+): m/z 382.29 (M+H)+.
2.3. Synthesis of Polyhydroxylated Ureas (11 to 18)
Treatment with BBr3 of the corresponding methoxylated ureas, following the same procedure above described for the synthesis of amide derivatives 1 to 10, led to the polyhydroxylated ureas 11 to 18.
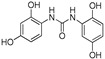
2.3.1. N-(2,4-Dihydroxyphenyl)-N′-(2′,5′-dihydroxyphenyl)urea (11)
Whitish lyophilized solid, 95% yield, precipitated with Et2O, m.p.: >155 °Cd. HPLC: tR = 5.97 min (15 min gradient: 2 to 40% of A in B). 1H-NMR (500 MHz, DMSO-d6) δ: 6.16 (dd, 1H, J = 8.6, 3.0 Hz, 4′-H), 6.18 (dd, 1H, J = 8.5, 2.9 Hz, 5-H), 6.31 (d, 1H, J = 2.7 Hz, 3-H), 6.58 (d, 1H, J = 8.5 Hz, 3′-H), 7.43 (d, 1H, J = 8.7 Hz, 6-H), 7.46 (d, 1H, J = 2.9 Hz, 6′-H), 8.45 (s, 1H, NH), 8.47 (s, 1H, NH), 8.59 (br s, 1H, OH), 8.93 (br s, 1H, OH), 9.00 (s, 1H, OH), 9.58 (s, 1H, OH) ppm. 13C-NMR (125 MHz, DMSO-d6) δ: 102.7 (C-3), 105.6 (C6′), 106.7 (C4′), 107.6 (C5), 115.0 (C3′), 119.0 (C1), 122.3 (C6), 128.6 (C1′), 138.4 (C), 148.5 (C), 149.9 (CO), 153.4 (C), 153.6 (C) ppm. MS (ESI+): m/z 277.3 (M+H)+.
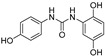
2.3.2. N-(2,5-Dihydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (12)
White prisms, 56% yield, m.p.: 180–182 °C (MeOH). HPLC: tR = 2.49 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 6.15 (dd, 1H, J = 8.4, 2.8 Hz, 4-H), 6.59 (d, 1H, J = 8.5 Hz, 3-H), 6.67 (d, 2H, J = 8.8 Hz, 3′-H, 5′-H), 7.20 (d, 2H, J = 8.8 Hz, 2′-H, 6′-H), 7.58 (d, 1H, J = 2.8 Hz, 6-H), 7.94 (d, 1H, NH), 8.60 (s, 1H, NH), 8.97 (s, H, OH), 9.03 (s, 1H, OH), 9.1 (s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 106.2 (C6), 107.3 (C4), 114.8 (C3), 115.3 (C3′, C5′), 120.1 (C2′, C6′), 128.7 (C1), 131.5 (C1′), 137.9 (C), 150.0 (C), 152.4 (CO), 152.7 (C), ppm. MS (ESI+): m/z 261.28 (M+H)+.
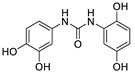
2.3.3. N-(2,5-Dihydroxyphenyl)- N′-(3′,4′-dihydroxyphenyl)urea (13)
White prisms, 68% yield, m.p.: 141–143 °C (Cl2CH2/MeOH). HPLC: tR = 1.73 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 6.1-6.2 (m, 2H, 4,6′-H), 6.31 (d, 1H, J = 2.6, 2′-H), 6.58 (d, 1H, J = 8.5 Hz, 3-H), 7.43 (d, 1H, J = 8.7 Hz, 5′-H), 7.46 (d, 1H, J = 2.9 Hz, 6-H), 8.44 (s, 1H, NH), 8.47 (s, 1H, OH), 8.59 (s, 1H, OH), 8.94 (s, 1H, OH), 9.01 (s, 1H, OH), 9.59 (s, 1H, NH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 102.8 (C6), 105.6 (C2′), 106.8 (C4), 107. 7 (C5′), 115.1 (C3), 119.0 (C6′), 122.4 (C1), 128.6 (C1′), 138.4 (C), 148.6 (C), 149.9 (C), 153,4 (C), 153.6 (CO) ppm. MS (ESI+): m/z 277.29 (M+H)+.
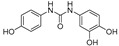
2.3.4. N-(3,4-Dihydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (14)
White prisms, 60% yield, m.p.: 184–187 °C (MeOH/Cl2CH2). HPLC: tR = 2.12 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 6.14 (dd, 1H, J = 8.7, 2.6 Hz, 6-H), 6.32 (d, 1H, J = 2.6 Hz, 2-H), 6.66 (d, 1H, J = 8.8 Hz, 3′-H, 5′-H), 7.18 (d, 1H, J = 8.8 Hz, 2′-H, 6′-H), 7.59 (d, 1H, J = 8.7 Hz, 5-H), 7.68 (d, 1H, OH), 8.72 (s, 1H, OH), 8.87 (s, 1H, NH), 8.99 (s, 1H, NH), 9.69 (s, 1H, OH) ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 102.5 (C2), 105.5 (C5), 115.2 (C3′,5′), 119.61 (C6), 120.0 (C2′, C6′), 120.6 (C1), 131.7 (C1′), 147.2 (C), 152.2 (C), 152.7 (CO), 153.2 (C) ppm. MS (ESI+): m/z 261.31 (M+H)+.
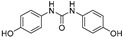
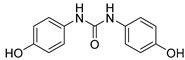
2.3.5. N,N′-bis(4-Hydroxyphenyl)urea (15)
White prisms, 84% yield, m.p.: 245 °Cd (MeOH; m.p. Lit [17] = 240 °Cd). HPLC: tR = 2.59 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 6.66 (d, 4H, J = 8.8, 3-H, 5-H), 7.18 (d, 4H, J = 8.8 Hz, 2-H, 6-H), 8.16 (s, 2H, NH), 8.99 (s, 2H, OH), ppm. 13C-NMR: (75 MHz, DMSO-d6) δ: 115.2 (C3, C5), 120.3 (C2, C6), 131.5 (C1), 152.3 (C4), 153.1 (CO), ppm. MS (ESI+): m/z 245.30 (M+H)+.
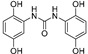
2.3.6. N,N′-bis(2,5-Dihydroxyphenyl)urea (16)
Lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:3 to 4:1), 20% yield, m.p.: 109 °Cd. HPLC: tR = 1.76 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (500 MHz, DMSO-d6) δ: 6.19 (dd, 2H, J = 8.5, 2.9 Hz, 4-H), 6.59 (d, 2H, J = 8.5 Hz, 3-H), 7.47 (d, 2H, J = 2.9 Hz, 6-H), 8.6 (s, 1H, NH), 8.74 (s, 2H, OH), 8.99 (s, 2H, OH) ppm. 13C-NMR: (125 MHz, DMSO-d6) δ: 107.2 (C6), 108.0 (C4), 115.1 (C3), 128.4 (C1), 138.7 (C2), 149.9 (C5), 153.0 (CO) ppm. MS (ESI+): m/z 277.39 (M+H)+.
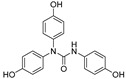
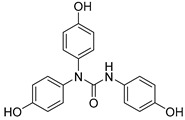
2.3.7. N,N-bis(4-Hydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (17)
Lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:2 to 3:1), 68% yield, m.p.: 186–187 °Cd. HPLC: tR = 7.15 min (10 min gradient: 2 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 6.60 (d, 2H, J = 8.9, 3′-H, 5′-H), 6.72 (d, 4H, J = 8.8, 3-H, 5-H), 7.05 (d, 4H, J = 8.7 Hz, 2-H, 6-H), 7.12 (d, 2H, J = 8.9 Hz, 2′-H, 6′-H), 7.41(s, 1H, NH), 9.02 (s, 1H, OH), 9.43 (s, 2H, OH) ppm. 13C-NMR: (75 MHz, CDCl3) δ: 114.7 (C3′, C5′), 115.7 (C3, C5), 122.3 (C2′, C6′), 128.7 (C2, C6), 131.2 (C), 134.9 (C), 152.8 (C), 154.6 (CO), 155.4 (C) ppm. MS (ESI+): m/z 337.32 (M+H)+.
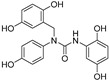
2.3.8. N-(2′,5′-Dihydroxybenzyl)- N′-(2″,5″-dihydroxiphenyl)-N-(4-hydroxyphenyl)urea (18)
White lyophilized solid, purified by column chromatography EtOAc-Hex (gradient from 1:3 to 4:1), 10% yield, m.p.: 114-115 °C (MeOH). HPLC: tR = 5.40 min (10 min gradient: 15 to 95% of A in B). 1H-NMR (400 MHz, DMSO-d6) δ: 4.67 (s, 2H, N-CH2), 6.14 (dd, 1H, J = 8.5, 2.8, 4″-H), 6.43–6.48 (m, 2H, 4′-H, 3′-H), 6.56 (d, 1H, J = 8.6 Hz, 3″-H), 6.59 (d, 1H, J = 2.8 Hz, 6′-H), 6.80 (d, 1H, J = 8.7 Hz, 3-H, 5-H), 7.05 (s, 1H, NH), 7.10 (d, 1H, J = 8.7 Hz, 2-H, 6-H), 7.57 (d, 1H, J = 2.8 Hz, 6″-H), 8.62 (br s, 1H, OH), 8.65 (br s, 1H, OH), 8.78 (br s, 1H, OH), 8.88 (br s, 1H, OH), 9.72 (br s, 1H, OH) ppm. 13C-NMR: (100 MHz, DMSO-d6) δ: 47.9 (N-CH2), 105.6 (C6″), 107.6 (C6′), 114.4 (C4″), 114.5 (C4′), 115.3 (C3″), 115. 9 (C3′), 116.5 (C3, C5), 124.7 (C), 128.2 (C), 129.5 (C2, C6), 132.0 (C), 137.5 (C), 147.2 (C), 149.7 (C), 150.0 (C), 154.4 (CO), 156.9 (C) ppm. MS (ESI+): m/z 383.25 (M+H)+.
2.4. Antioxidant Activity
2.4.1. Oxygen Radical Absorbance Capacity (ORAC) Experiment
The ORAC assay was performed following Ou et al. [18], as modified by Garcés-Rimón et al. [19]. All samples and reagents were dissolved in phosphate buffer (75 mM; pH 7.4). The reaction was performed in a final volume of 200 μL: 20 μL test samples or 20 μL 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) solutions (0.2–2 nM), 120 μL fluorescein solution (1.17 mM) and 60 μL 2,2′-azo-bis-(2-methylpropionamidine) dihydrochloride (AAPH) 1.3% solution (all from Sigma Aldrich, Alcobendas, Spain) were added to the wells of a black 96-well plate. The fluorescence was recorded at 40 °C every 55 s for 95 min using a fluorimeter (SpectraMax M2; Molecular Devices, California, USA), with excitation and emission wavelengths of 480 and 520 nm, respectively. All samples were tested in triplicate. ORAC values were expressed as μmol of Trolox equivalents (TE)/μmol of pure compound.
2.4.2. ABTS Experiment
The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assay was performed according to Re et al. [20] and modified by Oki et al. [21] for its use in microplates. Samples were diluted in methanol. An ABTS•+ stock solution was prepared by adding 44 μL of potassium persulfate (140 mmol/L) to a 2.5 mL ABTS•+ aqueous solution (7 mmol/L). The working solution of the radical ABTS+ was prepared by diluting the stock solution 1:75 (v/v) in a sodium phosphate buffer (5 mmol/L, pH 7.4) to obtain an absorbance value of 0.7±0.02 at 734 nm. Samples (30 μL) were added to 270 μL of the working solution of ABTS•+ in a microplate. Absorbance was measured at 734 nm and 30 °C for 20 min, every 5 min in a Synergy HT plate spectrophotometer (Biotek Instruments, Winoosky, VT, USA). A calibration curve was made with Trolox (20–250 μM). All samples were analyzed in triplicate. Results were expressed in μmol TE/μmol of pure compound, as for the ORAC assays.
2.4.3. DPPH Experiment
For the 2,2′-diphenylpicrylhydrazyl (DPPH) assay, antioxidant compounds were dissolved in ethanol with a stock concentration of 4 mM and stirred for 24 h. DPPH (Alfa Aesar) was also dissolved in ethanol at 0.127 mM. Different compound solutions were prepared by serial dilutions of each stock solution (4, 2, 1, 0.5, 0.25, 0.125, 0.063, 0.031, 0.015, and 0.007 M). Resveratrol was prepared using the same protocol and used as antioxidant control. Amounts of 80 µL of each solution and 80 µL of DPPH were added in a 96-well plate. The absorbance was measured at different times (10, 20 and 30 min and 1 and 2 h) by a Multi-Detection Microplate Reader Synergy HT (λMAX = 515 nm). Radical scavenging activity (RSA, %) was calculated using Equation (1)
| (1) |
where AEXTRACT and ADPPH correspond to the absorbance of DPPH with and without the extracts, respectively. Eight replicates were used for each compound and results were expressed as mean value ± standard deviation. The RSA half-maximal inhibitory concentration (IC50) values were calculated from the relationship curve of RSA versus concentrations by a non-linear fit using the software GraphPad Prism 7.
2.5. In Vitro Cellular Assays
Cell Viability
Cytotoxicity of selected antioxidant compounds was assessed using human dermal fibroblasts (HDF, Innoprot, P10856) and an AlamarBlue assay. Human fibroblasts were grown and maintained using Dulbecco’s Modified Eagle Medium high glucose (DMEM, Sigma Aldrich, D6171), supplemented with 10% Fetal Bovine Serum (FBS), 2% L-glutamine and 1% penicillin/streptomycin. Cells were incubated at 37 °C, 95% relative humidity and 5% CO2. Additionally, DMEM-high glucose, HEPES, no phenol red (Gibco, 2106329) was used to prepare the AlamarBlue solution. Stock solutions of the antioxidant compounds 3, 8, 15 and 17 were prepared in dimethyl sulfoxide (DMSO). Compound 7 was not soluble enough in DMSO to obtain a cell viability IC50 value. Serial dilutions of the antioxidant compounds were prepared using the previously mentioned stock solutions and DMEM. Final DMSO concentration was maintained lower than 1% v/v in the cell culture experiments. HDF were seeded at 9 × 104 cells/well in a 96-well plate and incubated for 24 h. The medium was replaced by the corresponding antioxidant solutions and incubated for an additional 24 h. Then, compounds were removed, cells were washed with Dulbecco’s Phosphate Buffered Saline (PBS, Sigma Aldrich) and treated with a 10% v/v AlamarBlue (Invitrogen) solution prepared in DMEM without phenol red. After 3 h of incubation, absorbance was monitored at 570 nm by a Multi-Detection Microplate Reader Synergy HT. Cells treated with DMEM without any antioxidant compound were used as the 100% viability control. Eight replicates were used for each antioxidant solution. IC50 values were obtained by a non-linear fit using the software GraphPad Prism 7.
2.6. Reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase Activity Assay in Vascular Systems
The human microvascular endothelial cells line (HMEC-1) (ATCC®, Middlesex, UK; CRL-3243™) was used. Cells were cultured according to the manufacturer instructions with MCDB131 medium (Corning, NY, USA, Cat. No. 702564) supplemented with 10 ng/mL epidermal growth factor (Sigma-Aldrich, Alcobendas, Spain), 1 μg/mL hydrocortisone (Sigma-Aldrich), 10 mmol/L glutamine (Sigma-Aldrich), 10% fetal bovine serum (FBS, Sigma-Aldrich), 100 U/mL of penicillin and 100 µg/mL of streptomycin. At 80% confluence, cells were serum-deprived for 24 h before stimulation. HMEC were treated with AngII (1 nmol/L for 6 h; Sigma-Aldrich) in the absence or in the presence of the different compounds that were added 30 min before stimulation.
Aortic segments from three-month-old C57BL6/J mice were incubated with IL-1β (10 ng/mL, 6 h, Sigma Aldrich) in the presence and in the absence of the different compounds that were added 30 min before stimulation.
The O2 production generated by NADPH oxidase was determined by a chemiluminescence assay. Briefly, endothelial cells or aortic segments were homogenized in phosphate buffer (50 mmol/L KH2PO4, 1 mmol/L EGTA, 150 mmol/L sucrose, pH 7.4). The reaction was started by the addition of a lucigenin (5 μmol/L) and NADPH (100 μmol/L; Sigma-Aldrich) mixture to the protein sample in a final volume of 250 μL. Chemiluminescence was determined every 2.4 s for 3 min in a microtiter plate luminometer (Enspire Perkin Elmer). Basal activity in the absence of NADPH was subtracted from each reading and normalized to protein concentration. Data were normalized vs. control situation.
All data are expressed as mean values ± standard mean error and n represents the number of animals or different cell cultures. Statistical analysis was performed using GraphPad Prism Software (v7.04). Data distribution (by Shapiro–Wilk normality test) was used to choose the appropriate statistics test. Results were analyzed by the Mann–Whitney non-parametric or Student’s t-tests when appropriate (two-tailed) or one-way Anova or Kruskal–Wallis test when appropriate followed by Bonferroni’s post hoc test or uncorrected Dunn′s test, respectively.
2.7. In Vivo Antioxidant Capacity in Stressed Yeasts
2.7.1. Yeast Strain and Induction of Oxidative Stress
Saccharomyces cerevisiae haploid strain BY4741 was used as described previously [16,22]. Briefly, 20 μL of glycerol stock culture was revived in 5ml of YPD medium (1% yeast extract, 2% bacteriological peptone and 2% dextrose) at 28 °C, with shaking (210 rpm). Fifty microliters of 20-h-old culture was used to inoculate fresh 5ml YPD (control medium) or 5 mL of oxidative stress medium (YPOxD: YPOxD; 1% yeast extract, 2% bacteriological peptone, 1% dextrose and 3% H2O2). Stress (and control) was conducted during 16h at 28 °C, with shaking (220 rpm).
2.7.2. Measurement of Antioxidant Capacity
In vivo antioxidant capacity was determined as recovery of growth rate in stressed yeasts (yeast grown in YPOxD for 16h) challenged by compounds 8, 15 and 17 (dissolved in methanol at 2 mg/mL), and compared to Resveratrol and vitamin C (dissolved in methanol at 2 mg/mL) as positive controls at four different doses (2, 10, 20 and 40 μg of each pure compound) or compared to stressed cells directly, as previously described [16,23]. Growth rate was determined from growth kinetics using the BioScreen C plate-reader system (OY Growth Curves Ab Ldt., Helsinki, Finland). Stressed yeast cultures (16 h old) were used to inoculate 200 µl of fresh media to an initial OD595 of 0.05–0.07, being distributed in 100-well honeycomb plates (Oy Growth Curves) in triplicate per each condition (each different dose of each compound). Plates were then incubated at 28 °C for 72h, with shaking (High force), taking OD measurement each 15 min in the Bioscreen C. Maximum growth rate (μmax) and biomass production (carrying capacity) were determined in R using ‘Growthcurver’ package version 0.3 [24]. Growth curves were constructed in EXCEL, represented as mean ± SEM (standard error of mean). One-way ANOVA followed by Tukey’s multiple comparisons test was performed with PRISMA v 6.0 software to compare among maximum growth rate or carrying capacity between tested compounds, Resveratrol, Vitamin C or control yeasts (grown in YPD or in YPOxD). In addition, to measure survival after oxidative stress and antioxidant effectiveness of each compound, yeast cells from each challenge were serially diluted in 96-well plates, and each dilution was spotted (with a 8 × 6 stainless steel replica platter from SIGMA) in triplicate to determine viable colonies after 24–48 h at 28 °C. Survivors (mean ± SEM) were compared between treatments with one-way ANOVA, Fisher’s LSD test, with alpha at 0.05 for significance.
3. Results and Discussion
3.1. Preparation of Designed Polyphenols
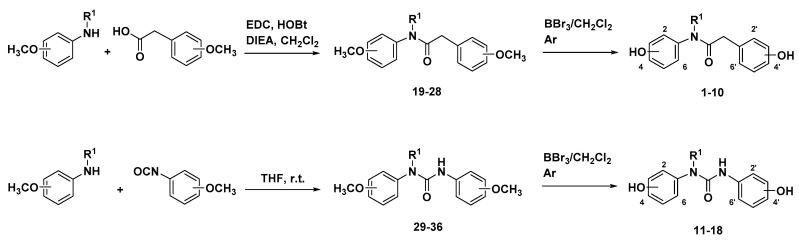
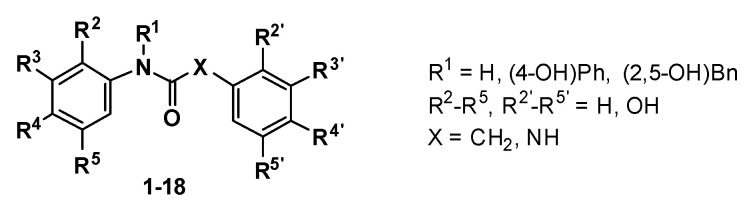
As indicated, two series of compounds were designed, both containing two polyphenol rings separated by a three-atoms linker, either containing an amide or a urea moiety. Combinations of 4-, 2,4-, 3,4-(catechol) and 2,5-OH were selected based on our previous results on the triazole series [16], and by their incidence in many polyphenols from natural sources. The synthesis of the amide and urea polyhydroxyphenyl derivatives 1 to 18 was performed following conventional methods, as depicted in Scheme 1.
Scheme 1.
Synthetic route to polyhydroxy amides 1 to 10, and polyhydroxy ureas 11 to 18.
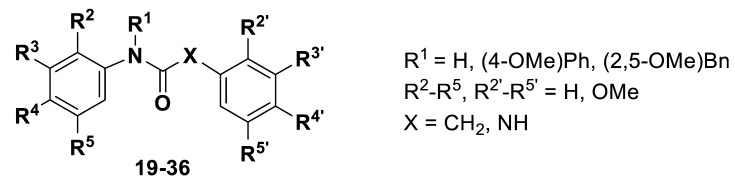
The methoxyphenyl amide derivatives 19 to 28 were prepared by reaction of the conveniently substituted aniline and the corresponding phenylacetic acid, in CH2Cl2, using 1-ethyl-3-(3-dimehylaminopropyl) carbodiimide (EDC.HCl) and 1-hydroxybenzotriazole (HOBt) as coupling agents, in the presence of N,N-diisopropylethylamine (DIEA) as a base. Further treatment of methoxy-substituted intermediates 19 to 28 with BBr3 led to the desired hydroxylated amides, compounds 1 to 10 (Scheme 1). On the other hand, the preparation of the ureido compounds 29–36 was carried out from the corresponding methoxyanilines and the methoxy-substituted isocyanates, by reaction at room temperature in tetrahydrofuran (THF) (Scheme 1). The final hydroxyl derivatives (11 to 18) were prepared from 29 to 36 also by demethylation reaction with BBr3. The synthesis proceeded very smoothly and most compounds were obtained in moderate-to-good yields with elevated grade of purity (Table 1 and Table 2). The acylation reaction was especially difficult in the case of the secondary bis-4-methoxyphenylamine to obtain methoxy amide 27, and during the urea formation of intermediate 36. Low yields in some demethylation reactions seem to be related to low stability of the final polyhydroxylated compounds (as explained later).
Table 1.
Structures and yield of synthetic polyhydroxy amides (1 to 10), and ureas (11 to 18) prepared.
| Compound | R1 | R2 | R3 | R4 | R5 | R2′ | R3′ | R4′ | R5′ | X | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | OH | H | OH | H | OH | H | H | OH | CH2 | 41 |
| 2 | H | OH | H | H | OH | OH | H | OH | H | CH2 | 44 |
| 3 | H | H | H | OH | H | OH | H | H | OH | CH2 | 79 |
| 4 | H | OH | H | H | OH | OH | H | H | OH | CH2 | 79 |
| 5 | H | OH | H | OH | H | OH | H | OH | H | CH2 | 94 |
| 6 | H | H | OH | OH | H | OH | H | H | OH | CH2 | 56 |
| 7 | H | H | H | OH | H | H | OH | OH | H | CH2 | 75 |
| 8 | H | H | H | OH | H | OH | H | OH | H | CH2 | 80 |
| 9 | (4-OH)Ph | H | H | OH | H | OH | H | H | OH | CH2 | 72 |
| 10 | (2,5-OH)Bn | H | H | OH | H | OH | H | H | OH | CH2 | 81 |
| 11 | H | OH | H | OH | H | OH | H | H | OH | NH | 95 |
| 12 | H | OH | H | H | OH | H | H | OH | H | NH | 56 |
| 13 | H | OH | H | H | OH | H | OH | OH | H | NH | 68 |
| 14 | H | H | OH | OH | H | H | H | OH | H | NH | 60 |
| 15 | H | H | H | OH | H | H | H | OH | H | NH | 84 |
| 16 | H | OH | H | H | OH | OH | H | H | OH | NH | 20 |
| 17 | (4-OH)Ph | H | H | OH | H | H | H | OH | H | NH | 68 |
| 18 | (2,5-OH)Bn | H | H | OH | H | OH | H | H | OH | NH | 10 |
Table 2.
Structures and yield of OMe precursors prepared, amides 19 to 28 and ureas 29 to 36.
| Compound | R1 | R2 | R3 | R4 | R5 | R2′ | R3′ | R4′ | R5′ | X | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | H | OMe | H | OMe | H | OMe | H | H | OMe | CH2 | 81 |
| 20 | H | OMe | H | H | OMe | OMe | H | OMe | H | CH2 | 77 |
| 21 | H | H | H | OMe | H | OMe | H | H | OMe | CH2 | 50 |
| 22 | H | OMe | H | H | OMe | OMe | H | H | OMe | CH2 | 65 |
| 23 | H | OMe | H | OMe | H | OMe | H | OMe | H | CH2 | 74 |
| 24 | H | H | OMe | OMe | H | OMe | H | H | OMe | CH2 | 73 |
| 25 | H | H | H | OMe | H | H | OMe | OMe | H | CH2 | 81 |
| 26 | H | H | H | OMe | H | OMe | H | OMe | H | CH2 | 76 |
| 27 | (4-OMe)Ph | H | H | OMe | H | OMe | H | H | OMe | CH2 | 28 |
| 28 | (2,5-OMe)Bn | H | H | OMe | H | OMe | H | H | OMe | CH2 | 74 |
| 29 | H | OMe | H | OMe | H | OMe | H | H | OMe | NH | 55 |
| 30 | H | OMe | H | H | OMe | H | H | OMe | H | NH | 85 |
| 31 | H | OMe | H | H | OMe | H | OMe | OMe | H | NH | 69 |
| 32 | H | H | OMe | OMe | H | H | H | OMe | H | NH | 42 |
| 33 | H | H | H | OMe | H | H | H | OMe | H | NH | 80 |
| 34 | H | OMe | H | H | OMe | OMe | H | H | OMe | NH | 84 |
| 35 | (4-OMe)Ph | H | H | OMe | H | H | H | OMe | H | NH | 63 |
| 36 | (2,5-OMe)Bn | H | H | OMe | H | OMe | H | H | OMe | NH | 17 |
3.2. Antioxidant Characterization of the New Polyphenols Collection
The antioxidant capacity of the small collection of new polyphenols (Table 1) was firstly evaluated in an Oxygen Radical Absorbance Capacity (ORAC) assay (Table 3). All compounds exhibited strong radical scavenging activities; 14 out of the 18 compounds tested, showed values even higher than those found for resveratrol (own data), a well-known natural antioxidant used as control. Compared to resveratrol (TE 8 µmol trolox/µmol, TE: Trolox Equivalents), six of the compounds show 2–3-fold higher antioxidant capacity. Thus, some of the results found were quite outstanding, as in the case of compounds 9 and 10 that showed values above 25 TE per sample, and 3, 7, 8 and 17 with >19 TE. Compounds 1, 4, 12, 16, and 18 still display higher antioxidant activity than resveratrol, while derivatives 2, 6, 13 and 15 are comparable to the control, and only polyphenols 5 and 11 have lower antioxidant ability compared to resveratrol (Table 3).
Table 3.
Antioxidant activity (oxygen radical absorbance capacity (ORAC)) and chemical stability of compounds 1 to 18.
| Compound | Chemical Structure | ORAC a | Aqueous Stability b(%) |
|---|---|---|---|
| 1 |
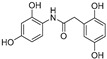
|
11.0 ± 0.4 | 2 |
| 2 |
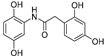
|
8.1 ± 0.1 | - |
| 3 |
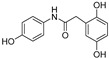
|
19.2 ± 0.2 | 77 |
| 4 |
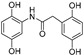
|
14.4 ± 0.2 | 0 |
| 5 |
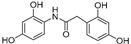
|
5.2 ± 0.4 | - |
| 6 |
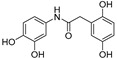
|
9.4 ± 0.4 | 0 |
| 7 |
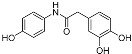
|
19.2 ± 0.4 | 90 |
| 8 |
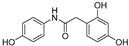
|
19.3 ± 0.5 | 98 |
| 9 |
|
29.5 ± 0.5 | 0 |
| 10 |
|
27.6 ± 0.5 | 0 |
| 11 |
|
6.6 ± 0.1 | - |
| 12 |
|
12.5 ± 0.7 | 0 |
| 13 |
|
7.8 ± 0.3 | - |
| 14 |
|
9.6 ± 0.1 | 0 |
| 15 |
|
8.9 ± 0.4 | 90 |
| 16 |
|
11.9 ± 0.4 | 0 |
| 17 |
|
19.4 ± 0.7 | 93 |
| 18 |
|
15.3 ± 0.7 | 0 |
| Resveratrol |
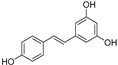
|
8.1 ± 1.17 | - |
a µmol of trolox/µmol of pure compound. b Percentage of remaining pure compound (measured by HPLC) after two months at room temperature in aqueous solution (+20% acetonitrile).
Going into more detail through the data from the ORAC assay, and taking into consideration the chemical structure, we firstly focused on the compounds having only two phenyl rings in their structure. In general, it can be said that amide derivatives showed higher TE values than ureas. Comparing compounds with the same pattern of hydroxyl substituents, TE values showed by amides 1, 3, 7 and 8, are higher than those of their corresponding urea derivatives 11, 12, 13 and 14. In the case of 8 and 14, the value was the double for the former. Besides, for the three best amides, 3, 7 and 8, it is remarkable that all of them have a p-monohydroxyphenyl ring at the N-amide atom, while the benzyl ring has two hydroxyl groups in 2,5-, 2,4- or 3,4- positions, respectively (Table 3).
On the other hand, both in the case of amide and urea derivatives, the antioxidant capacity was increased by incorporation of a third phenolic ring at N position, like in 9, 10, 17 and 18 compounds. A 4-hydroxyphenyl substitution (9 and 17) led to slightly better TE values than a 2,5-dihydroxybenzyl moiety (compounds 10 and 18).
ORAC values for most of the prepared compounds were also higher than those found for other natural antioxidants, like quercetin, hydrotyrosol, D-catechin and (-)-epicatechin (Table S1).
Considering the high tendency to oxidation of polyphenols, and before further characterization of this series of compounds, we decided to check the stability in aqueous media of all polyphenol derivatives showing higher antioxidant capacity than resveratrol (Table 3). To facilitate the solubility, compounds were dissolved in a mixture of H2O/acetonitrile (80:20%) and maintained at room temperature. The solutions were checked by HPLC-MS at regular intervals of time for 60 days. Only amides 3, 7, 8 and ureas 15 and 17 proved to be sufficiently stable after two months. The rest of the compounds suffered progressive degradation, with complete disappearance from the solution after 10 or 20 days. The most unstable polyphenol derivatives were 4 and 10 (Figure S1), having both two p-dihydroxy substitutions in the phenyl rings, which points to the rapid and easy oxidation to the p-hydroquinone. From the four compounds with three phenyl rings, only one was stable in aqueous solution (17).
After checking the chemical stability, only the five more stable compounds (3, 7, 8, 15 and 17) were selected to complete their characterization as antioxidants. For this purpose, we used ABTS and DPPH assays, two spectrophotometric methods normally applied to show the radical scavenging ability of antioxidant compounds (Table 4). First, the compounds were evaluated in the ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) assay [20]. In the presence of oxidant agents, ABTS form a stable radical cation (ABTS•+) chromophore, with an absorbance band at 734 nm. The presence of this radical cation is reduced by hydrogen-donating compounds, like polyphenols, producing a discoloration of the solution proportional to the amount of antioxidant. In this assay, four out of five compounds showed TE values higher than resveratrol (Table 4), with amide derivative 7 as the best radical scavenger (nearly fourfold higher than control). However, urea 15, which has already the lowest ORAC value among the selected compounds, was worse than resveratrol in this assay. The radical scavenging ability of compounds 7 and 8 is higher and comparable, respectively, to those of quercetin and (-)-epicatechin natural products in this ABTS assay (Table S1).
Table 4.
Antioxidant profile of selected stable compounds.
| Comp | Chemical Structure | ORAC a | ABTS a | DPPH PSA IC50 (µM) b | |
|---|---|---|---|---|---|
| 10 min | 2 h | ||||
| 3 |
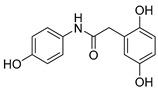
|
19.2 ± 0.2 | 3.27 ± 0.1 | 8.30 | 6.77 |
| 7 |
|
19.2 ± 0. | 8.12 ± 0.09 | 24.10 | 23.30 |
| 8 |
|
19.3 ± 0.5 | 5.00 ± 0.09 | 150.90 | 64.20 |
| 15 |
|
8.9 ± 0.4 | 1.17 ± 0.01 | ND c | ND c |
| 17 |
|
19.4 ± 0.7 | 3.57 ± 0.00 | 17.92 | 9.60 |
| Resveratrol |
|
8.08 ± 1.2 | 2.13 ± 0.02 | 66.22 | 19.48 |
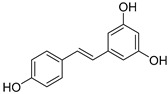
a µmol of trolox/µmol of pure compound. b The half-maximal inhibitory concentration (IC50) values for the α,α-diphenyl-β-picrylhydrazyl (DPPH) assay correspond to the amount of compound needed to reach 50% of radical scavenging activity (RSA) after 10 min and 2 h of addition. c ND: not determined.
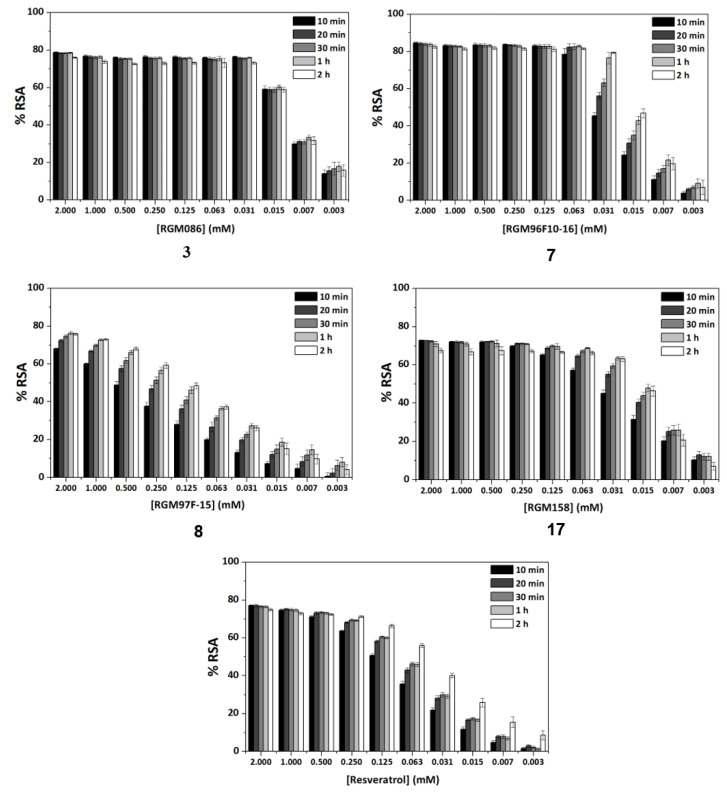
Another frequently used method for antioxidant determination is the DPPH assay, based on the capacity of the 2,2-diphenyl-1-picryl-hydrazyl, DPPH free radical to be reduced in the presence of an antioxidant [25]. According to the previous results, only four compounds, three amides, 3, 7 and 8, and the urea derivative 17 were evaluated in this assay, always using resveratrol as control. The antioxidant capacity was measured at different concentrations in a progressive descending order, going from 2 to 0.003 mM, and additionally, at an interval of time for each concentration (five measures from 10 min to 2 h). For all of them, a concentration dependent behavior was observed (Figure 1). Comparing the maximum antioxidant capacities observed at the highest concentration (2 mM), at the beginning of the experiment (10 min time) the four compounds showed RSA percentage values comparable to the control, resveratrol (77%). Compounds 3 and 17, reached about the same values as control, 78% and 72%, respectively, 8 showed a slightly lower percentage of 67%, and amide 7 proved to have a slightly higher capacity than resveratrol, 82% (Figure 1). As for the potency, the IC50 values shown in Table 4 indicate that the best compound in this assay is amide 3, followed by urea 17 and amide 7. Compared to resveratrol, the RSA capacity after 10 min was 8-, 2.8- and 3.7-fold higher for 3, 7 and 17, respectively.
Figure 1.
Study of the radical scavenging activity (RSA) capacity showed by compounds 3, 7, 8 and 17 and control (resveratrol) low in the DPPH assay. Variations with sample concentration and time.
Looking at how the RSA capacity of compounds is modified with time (Figure 1), resveratrol (right graph) maintained the RSA capacity at concentrations above 0.5 mM. However, at lower concentrations the RSA percentages clearly diminished as a function of concentration, but increased with time (RSA 2h > RSA 10 min). In our case, compound 8, shows this time-dependent behavior already from high concentrations (Figure S2). However, compounds 3, 7 and 17 maintained the RSA ability nearly at the same percentage levels, independently on time, until quite low concentrations, 0.031, 0.063 and 0.25 mM, respectively, which is a remarkable difference with resveratrol (Figure 1). This behavior of 3, 7 and 17, will allow the use of low concentrations of sample for achieving a high effect. On the other hand, like for resveratrol, and with the exception of compound 3, at the low concentration of 0.031 mM for 7 and 17, the RSA ability increased with time (Figure 1).
Compound 3, at a concentration of 0.031 mM, still shows a remarkably high RSA (78%), which is maintained with time, from 10 min to 2 h. This time-independent behavior is observed even at lower concentrations (0.015–0.003 M), which can be an advantage for those applications that require an immediate antioxidant effect.
3.3. Biological Evaluation of Synthetic Polyphenols
3.3.1. Cell Viability
To evaluate the potential cytotoxicity of the selected compounds, an AlamarBlue assay was performed and the cell viability IC50 values were extrapolated using a non-linear fit. Compound 15 crystallized when in contact with DMEM and did not allow a correct absorbance measurement in the microplate reader. For compounds 3, 8, and 17, IC50 values were 0.246, 1.411 and 1.789 mM, respectively (Table S3, Figure S2). Compounds 17 and 8 showed a similar IC50, both of them over 1.4 mM, while 3 was slightly more cytotoxic, having an IC50 value about 5–7-fold lower (0.24 mM). Nonetheless, considering the high antioxidant capacity shown by compounds 3, 8 and 17 with RSA IC50 values in the 10 min DPPH assay of 8.30, 150.90 and 17.92 µM, respectively, the concentrations needed to produce 50% of cytotoxicity are at least one order of magnitude higher than those required to reduce 50% RSA for amides 3 and 8, and two orders greater in the case of urea 17. Compound 7 did not show any significant cytotoxic effects at the range of concentrations where the compound was soluble in DMSO (up to 6 mM). In general, a non-cytotoxic behavior can be assured in a range of concentrations where the antioxidant compounds exhibit RSA potential. Therefore, the compounds were progressed to further, parallel biological characterization in different systems.
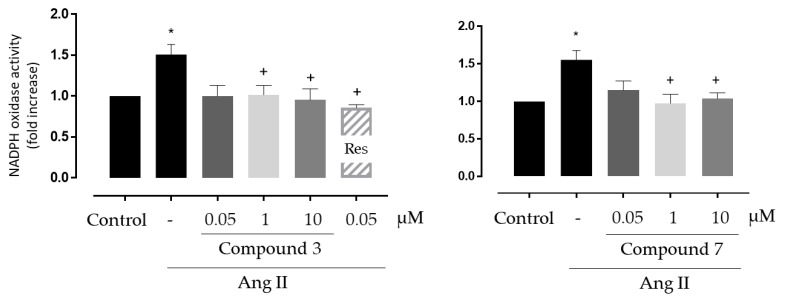
3.3.2. Antioxidant Activity in Vascular Systems
First, we tested the ability of two of the best antioxidant compounds, 3 and 7, on NADPH oxidase activity in human endothelial cells stimulated with Angiotensin II, one of the most important mediators of oxidative stress and vascular damage [26]. NADPH oxidase enzymes are the major producers of ROS at the vascular level and one of the protective effect of polyphenols versus the oxidative stress is mediated by the inhibition of the expression and activity of the NADPH oxidase complex [5]. Compared to the control antioxidant resveratrol, compound 3 and 7 showed high ORAC (>19 vs. 8 TE) and ABTS (3.2 and 8.1 vs. 2.1 TE, respectively) figures, and better and comparable DPPH values, respectively (Table 4).
As shown in Figure 2, compounds 3 and 7 were able to protect the cells against the oxidative stress promoted by Angiotensin II, decreasing NADPH oxidase activity levels in the same order as resveratrol (used as control). No remarkable differences were observed between the activities of both compounds.
Figure 2.
Effect of compounds 3 and 7 on Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase activity induced by Angiotensin II (Ang II) in cultured human microvascular endothelial cells. * p < 0.05 vs. control, + p < 0.05 vs. Ang II. Res: resveratrol (dashed bars) was used as positive control. N = 8 for compound 3 and n = 7 for compound 7.
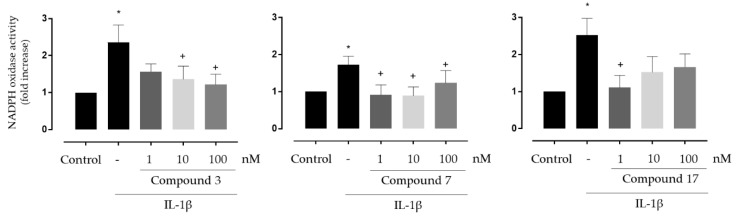
To further confirm the beneficial effects of these compounds in oxidative stress models at the vascular level, their antioxidant capacity was evaluated in an ex vivo whole organ system. Thus, we tested the effects of compounds 3 and, 7 on NADPH oxidase activity in mice aorta stimulated ex vivo with IL-1β, another inducer of vascular NADPH oxidase expression and activity in vascular cells [27]. Additionally, considering the good results obtained for 17 in the ORAC, ABTS and DPPH antioxidant assays, and its low toxicity, we decided to extend the study to this compound in the aorta experiment. As shown in Figure 3, IL-1β increased NADPH oxidase activity under ex vivo conditions, whereas all the three tested compounds showed significant inhibition of the IL-1β effects.
Figure 3.
Effect of compounds 3, 7 and 17 on NADPH oxidase activity induced by IL-1β in segments of aorta from C57BL6/J mice. * p < 0.05 vs. control, + p < 0.05 vs. IL-1β. n = 9 for compound 3, n = 8 for compound 7 and n = 7 for compound 17.
Altogether, our results demonstrate in vitro and ex vivo antioxidant capacity of the selected compounds and suggest that they are versatile antioxidants in response to a variety of stimuli (i.e., Ang II and IL-1β) important in cardiovascular disease at the clinical level. Eventually, these compounds might be used in the treatment of vascular alterations such as endothelial dysfunction, vascular smooth muscle cells proliferation and migration, extracellular matrix remodeling, or inflammation which depend on oxidative stress milieu and that are characteristic of a number of vascular diseases including hypertension, obesity, atherosclerosis or abdominal aortic aneurysms.
3.3.3. In Vivo Antioxidant Activity of Selected Compounds in Yeasts
Bioavailability, in vivo reactivity and stability, and tissue differential storage are compound properties not associated with in vitro antioxidant assays, increasing the difficulties of correlating between in vitro and in vivo transfer of activities. Therefore, we required in vivo evidence supporting the in vitro-based antioxidant activity of our compounds. In this respect, Saccharomyces cerevisiae is a model organism used to investigate oxidative stress and the effect of nutraceuticals in resistance to oxidative stress [16,28,29,30], among many other biological processes linked to human health. Actually, the yeast S. cerevisiae had become a model for the study of effects of nutraceuticals and its transfer to humans due to the new EU regulation (EC 1924/2006), because it is easy to handle, millions of cells can be studied in a single-small culture, and its genetics, biochemistry and physiology are transferrable. Indeed, yeast and humans share one in four genes as orthologues, both in sequence and in function (as reviewed in [31,32,33]). Moreover, several studies have characterized the interaction of L-ascorbic acid (vitamin C) or resveratrol, two compounds with a variety of antioxidant properties, with endogenous cellular antioxidative defense systems in S. cerevisiae yeast strains, which will aid in the comparison of results [32,34].
In this study, we have performed experiments with S. cerevisiae strain BY4741 to assess the putative in vivo antioxidant effects of compounds 8, 15 and 17, by assessing the growth recovery ability of yeast cells subjected to oxidative stress for at least 16h, as described previously [16]. These three compounds were selected by their differential behavior in the three in vitro antioxidant experiments. Compounds 8 and 17 show higher ORAC activity than resveratrol (used as control), while 15 is comparable to the natural product (Table 4). In the ABTS assay, urea 15 shows a lowest value than resveratrol, while the other two polyphenolic derivatives have higher radical scavenging capacities. Finally, among all selected compounds, 8 displays the shorter DPPH value.
DMSO affects oxidative stress-induced cytotoxicity, inhibits methionine sulfoxide reductase A, and reduces cell viability and survival, depending on the concentration used (as reviewed in [35], and references herein). However, methanol only affects yeast growth, by reducing its growth rate, due to the inhibition of nutrients uptake, when used at more than 10% concentration [36]. Therefore, we select methanol (up to 0.1%) as solvent for compounds in our study of determination of in vivo antioxidant and oxidant-resistance properties of the selected polyphenols. This study was performed as previously described; BY4741 yeast cells were exposed to H2O2 as oxidative agent for 16h, and then cultures were challenged by different doses to ascertain changes in growth curve parameters and in yeast cell survivorship [16,30]. Growth ability was assessed with Bioscreen c, on which H2O2-stressed cells were challenged by each compound 8, 15 and 17, along with Resveratrol and vitamin C as control antioxidants, at three different doses (2, 20 and 40 μg). Plates also contained several types of controls (un-inoculated media, un-inoculated media with methanol, completely stressed cells in YPOxD and un-stressed cells cultured in YPD medium), all at least in triplicate. From the visual analysis of growth curve shape, tested compounds were enhancing growth recovery as compared with completely stressed cells (OxDinOxD), similarly as resveratrol or vitamin C, the two control antioxidants used (see an example in Figure S3). However, nearly none of the tested doses achieved a complete recovery similar to non-oxidative stressed cells (YPD (CTRL)).
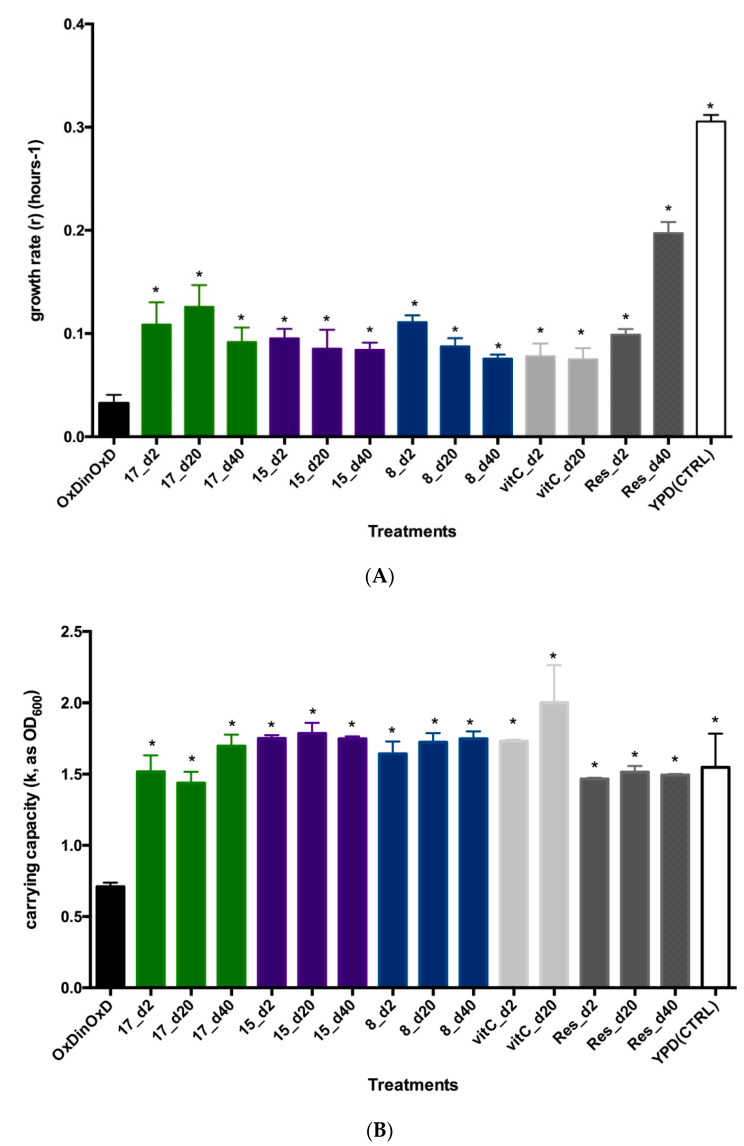
To ascertain if this observed recovery of growth curve shape is statistically significant, two growth parameters were assessed: growth rate (r) and carrying capacity (k). Optical values (OD) were analyzed with Growthcurver R package, and obtained parameters r and k were compared with yeast cells under complete oxidative stress (OxDinOxD) or with completely unstressed cells (YPD (CTRL)). Growth rate (r) was significantly higher than the values obtained for completely stressed cells, indicating a recovery of biological functions in the yeast, but not the complete return to normality. As the unstressed yeasts, all tested compounds-treated yeast cells showed a highly significant higher growth rate (r = 0.3056 ± 0.0063 h−1; F(22,46) = 31.43; p < 0.0001). The comparison of growth rate (r) between treatments (Figure 4A) indicates that apparently the best performing antioxidant is compound 17 at each single dose tested, whereas compounds 15 and 8, showed a dose-related antioxidant effect, with the best growth rate values at a 2-μg dose. None of the tested compounds nor control antioxidants, vitamin C or resveratrol, recover completely growth rate, as mean values were significantly different from unstressed yeast (YPD(CTRL)). This recovery of growth rate is similar to that obtained with other synthetic [16] or food-derived phenolic compounds like onion skin extracts or cocoa polyphenols [30,37] or elements like selenium [38]. When comparing the carrying capacity (k) (Figure 4B), all three compounds at each single dose tested were able to recover the carrying capacity of yeast cells similarly as control antioxidants, and even to the same levels of unstressed yeast cells. In this case, compound 15, at a low 2 μg dose, seems slightly better than the other two polyphenols and resveratrol, and comparable to vitamin C. In general, measured growth rate and carrying capacity are quite similar for the three new polyphenols. Therefore, we validate the S. cerevisiae as a good model for measuring antioxidant activity in vivo.
Figure 4.
Saccharomyces cerevisiae growth parameters comparisons. (A) Comparison of growth rate (r) as mean ± SEM (h−1) of S. cerevisiae subjected to oxidative damage (grown in YPOxD medium) and challenged by different doses of compounds 17, 15 and 8, or by the antioxidants vitamin C (vitC) or resveratrol (Res). Comparisons have been made against completely stressed cells (OxDinOxD). To account for the oxidative damage, control lines (YPD(CTRL)) were also included, and consisted in S. cerevisiae cells grown in YPD (normal) medium, on which yeast cells can grow at maximum speed. Notice that oxidative stress reduces highly the growth rate, which is significantly recovered (*) after being challenged by compounds 17, 15 or 8, in a similar way as the control antioxidant resveratrol. (B) Comparison of carrying capacity (k) as mean ± SEM, after oxidative stress in S. cerevisiae. As above, yeast cultures responded positively recovering carrying capacity after being challenged by different doses of compounds 17, 15 and 8. In this case, carrying capacity was similarly recovered to unstressed yeast cells (YPD(CTRL)). Asterisks denote statistical significance with alpha 0.05.
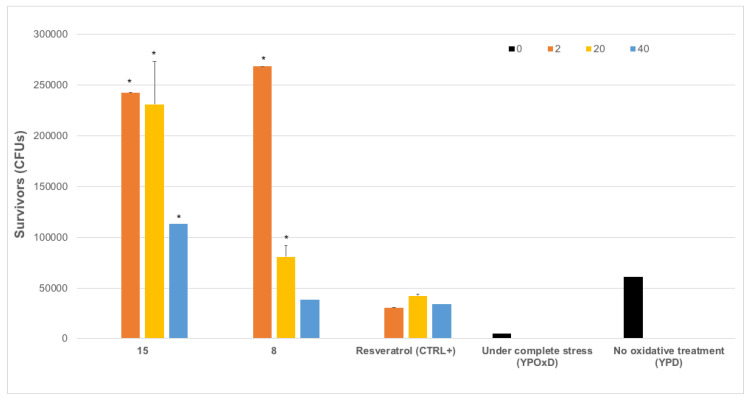
This carrying capacity also reflects the ability of yeast cells to respond to oxidative damage by producing viable offspring or survivors, which sometimes is called stress tolerance assays. These studies represent the capacity of the cells to counteract and resist the oxidative damage, representing cell survival [30,31,36,38,39,40,41,42]. Even showing statistical differences between applied doses, and being significantly different from completely stressed yeasts (OxDinOxD) and unstressed ones (YPD), our results indicate that tested compounds enhance biological functions enabling the complete recovery of cell biochemistry and metabolism Indeed, tested compounds counteract the oxidative damage induced by the used H2O2 medium and increase significantly the oxidative protection rate when compared to resveratrol (Figure 5). Considering how this effect is possible, we do not yet know about the exact molecular mechanisms behind this antioxidant activity.
Figure 5.
Saccharomyces cerevisiae survivors (mean CFUs (colony forming units) ± SEM) to oxidative stress after treatment with compounds 15 and 8. Yeast cells were subjected to oxidative stress by growing in YPOxD medium, after 16h, cells were subjected to treatment with different doses (2, 10, 20 or 40 μg, check color code in legend) in triplicate, and after 60 h of growth monitorization by OD600 measurement in Bioscreen c, cells were serially diluted in 10% glycerol and plated in YPD medium plates, to determine number of CFUs per ml. Stressed yeasts without antioxidant treatment (under complete stress (YPOxD)), non-stressed yeasts (no oxidative treatment (YPD)) and stressed yeasts treated with resveratrol (resveratrol (CTRL+) were also included. Statistical significance (F-test with p-value lower than 0.05) was assessed first between YPOxD and YPD yeast survivors; and then between YPOxD stressed and antioxidant-treated, followed by comparison between control antioxidant (Resveratrol) and tested compounds at each dose. Asterisk indicates statistically significant comparisons (alpha = 0.05).
The obtained data supported the recovery of growth rate, whole metabolic capability and reproductive physiology by the selected synthetic polyphenols. However, the data also indicate that S. cerevisiae endogenous cellular antioxidative defense systems are enhanced at a similar or even better rate than those measured for well-known antioxidants resveratrol and vitamin C (Figure 5) [34,38].
4. Final Remarks and Conclusions
Polyphenols are widely distributed in nature (tea, coffee, chocolate, fruits, legumes, etc.), they function as antioxidants and protect us from free radical damage in several chronic diseases (cancer, diabetes, cardiovascular and neurodegenerative diseases). Among different applications, such as oil, food and cosmetic preservatives, synthetic polyphenols could also be of interest in the search for new bioactive agents. Following our previous work on antioxidant triazolyl polyphenols, and inspired by the OH substitutions in natural products, we designed a small collection of amide and urea polyhydroxyphenyl analogues. Synthetically more accessible than the triazolyl derivatives, compounds 1–18 were first analyzed by their antioxidant capacity in ORAC assays, with most compounds comparing favorably to resveratrol (6.6–27.6 TE versus 8.1 TE). Measurement of the aqueous stability allowed the identification of polyphenol derivatives prone to oxidization to the corresponding quinones, which were removed from further characterization to avoid misinterpretations. The most stable polyphenols (3, 7, 8, 15 and 17) were selected for further characterization, using ABTS and DPPH experiments, where most derivatives showed comparable and even higher radical scavenging abilities than the natural product resveratrol, except for 15. Selected compounds were further evaluated in different biological systems, namely endothelial cells, whole aorta and/or yeast. Compounds 3 and 7 were able to protect cultured human microvascular endothelial cells against oxidative stress promoted by Angiotensin II, decreasing the levels of NADPH activity. In addition, these polyphenols and urea 17 inhibited the effects of IL-1β-induced NADPH oxidase activity ex vivo, at 1nM concentration or higher, confirming their beneficial effects in oxidative stress models at the vascular level. To have in vivo evidence supporting the in vitro-based antioxidant activity of our compounds, a Saccharomyces cerevisiae model organism was used to investigate the effect of selected synthetic polyphenols with different in vitro profiles (8, 15 and 17). All investigated amide and urea derivatives displayed similar in vivo antioxidant properties in this model, with recovery of growth rate and of whole metabolic capability similar or even better than model antioxidants (resveratrol and vitamin C). Polyphenol 17 is the most balanced compound in the series, because of the good in vitro, ex vivo and in vivo antioxidant properties, and its low toxicity. The synthetic polyphenols described here could have future applications in the treatment of aging and many clinical conditions characterized by high oxidative–stress levels, such as cancer, cardiovascular diseases, chronic obstructive pulmonary disease, chronic kidney illness, and different neurodegenerative processes. Confirmation of other therapeutic uses will require additional experiments on different models.
Acknowledgments
We thank Daniel Toledo and Jessy Medina for technical assistance with purification of compounds and stability studies. We also thank Víctor Gutiérrez for his help with some experiments.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/9/787/s1, Chemical description of MeO analogues, Figure S1: HPLC-MS study of aqueous stability of compounds in aqueous solution, Table S1: Antioxidant evaluation of several natural products, Table S2: Theoretical Log P values and aqueous thermodynamic solubility measured for selected compounds, Table S3: Variations with sample concentration and time, Figure S2: Cytotoxicity, IC50 values for selected compounds and non-linear fits, Figure S3: In vivo growth ability of selected compounds in yeast.
Author Contributions
Conceptualization, R.G.-M.; synthesis, M.J.P.d.V and R.G.-M.; ORAC and ABTS assays, S.M.-F.; DPPH, G.M.P.-Q., and B.V.-L.; vascular assays, M.G.-A.; in vivo yeast assay, B.S.-M.; supervision, A.M.B., M.R.A., M.M., R.G.-M.; writing, original draft preparation, contributions by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Spanish Ministerio de Ciencia, Innovación y Universidades (MICIU-FEDER) grant number RTI2018-097189-B-C22 to RGM, SAF2016-80305P to AMB, and CSIC grant number 2019E030 to RGM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nicoletti M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012;63:2–6. doi: 10.3109/09637486.2011.628012. [DOI] [PubMed] [Google Scholar]
- 2.Piccolella S., Crescente G., Candela L., Pacifico S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019;175:112774. doi: 10.1016/j.jpba.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R., Padwad Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci. Technol. 2020;98:41–52. doi: 10.1016/j.tifs.2020.02.004. [DOI] [Google Scholar]
- 4.Thomford N.E., Senthebane D.A., Rowe A., Munro D., Seele P., Maroyi A., Dzobo K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018;19:1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serino A., Salazar G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients. 2019;11:53. doi: 10.3390/nu11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss J.W.E., Williams J.O., Ramji D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1562–1572. doi: 10.1016/j.bbadis.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasines-Perea Z., Teissedre P.-L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules. 2017;22:68. doi: 10.3390/molecules22010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajadimajd S., Bahramsoltani R., Iranpanah A., Kumar Patra J., Das G., Gouda S., Rahimi R., Rezaeiamiri E., Cao H., Giampieri F., et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2020;151:104584. doi: 10.1016/j.phrs.2019.104584. [DOI] [PubMed] [Google Scholar]
- 9.Xing L., Zhang H., Qi R., Tsao R., Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019;67:1029–1043. doi: 10.1021/acs.jafc.8b06146. [DOI] [PubMed] [Google Scholar]
- 10.Galiniak S., Aebisher D., Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019;66:13–21. doi: 10.18388/abp.2018_2749. [DOI] [PubMed] [Google Scholar]
- 11.Dyck G., Raj P., Zieroth S., Dyck J., Ezekowitz J. The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. Int. J. Mol. Sci. 2019;20:904. doi: 10.3390/ijms20040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martillanes S., Rocha-Pimienta J., Cabrera-Bañegil M., Martín-Vertedor D., Delgado-Adámez J. Phenolic Compounds—Biological Activity. InTech; London, UK: 2017. Application of phenolic compounds for food preservation: Food additive and active packaging. [Google Scholar]
- 13.Michalak I., Chojnacka K., Saeid A. Plant growth biostimulants, dietary feed supplements and cosmetics formulated with supercritical CO2 algal extracts. Molecules. 2017;22:66. doi: 10.3390/molecules22010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criado M., Balsera B., Mulet J., Sala S., Sala F., de la Torre-Martínez R., Fernández-Carvajal A., Ferrer-Montiel A., Moreno-Fernández S., Miguel M., et al. 1,3-diphenylpropan-1-ones as allosteric modulators of α7 nACh receptors with analgesic and antioxidant properties. Future Med. Chem. 2016;8:731–749. doi: 10.4155/fmc-2015-0001. [DOI] [PubMed] [Google Scholar]
- 15.Pérez De Vega M.J., Fernandez-Mendivil C., De La Torre Martínez R., González-Rodríguez S., Mullet J., Sala F., Sala S., Criado M., Moreno-Fernández S., Miguel M., et al. 1- (2′, 5′-Dihydroxyphenyl)-3- (2-fluoro-4-hydroxyphenyl)-1-propanone (RGM079): A positive allosteric modulator of α7 nicotinic receptors with analgesic and neuroprotective activity. ACS Chem. Neurosci. 2019;10:3900–3909. doi: 10.1021/acschemneuro.9b00364. [DOI] [PubMed] [Google Scholar]
- 16.Bonache M.A., Moreno-Fernández S., Miguel M., Sabater-Muñoz B., González-Muñiz R. Small library of triazolyl polyphenols correlating antioxidant activity and stability with number and position of hydroxyl groups. ACS Comb. Sci. 2018;20:694–699. doi: 10.1021/acscombsci.8b00118. [DOI] [PubMed] [Google Scholar]
- 17.Franz R.A., Applegath F., Morriss F.V., Baiocchi F., Bolze C. A New synthesis of ureas. III. the reaction of aromatic amines with carbon monoxide and sulfur. J. Org. Chem. 1961;26:3309–3312. doi: 10.1021/jo01067a067. [DOI] [Google Scholar]
- 18.Ou B., Hampsch-Woodill M., Prior R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 19.Garcés-Rimón M., López-Expósito I., López-Fandiño R., Miguel M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur. Food Res. Technol. 2016;242:61–69. doi: 10.1007/s00217-015-2518-7. [DOI] [Google Scholar]
- 20.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Oki T., Nagai S., Yoshinaga M., Nishiba Y., Suda I. Contribution of BETA.-carotene to radical scavenging capacity varies among orange-fleshed sweet potato cultivars. Food Sci. Technol. Res. 2006;12:156–160. doi: 10.3136/fstr.12.156. [DOI] [Google Scholar]
- 22.Mattenberger F., Sabater-Muñoz B., Hallsworth J.E., Fares M.A. Glycerol stress in Saccharomyces cerevisiae: Cellular responses and evolved adaptations. Environ. Microbiol. 2017;19:990–1007. doi: 10.1111/1462-2920.13603. [DOI] [PubMed] [Google Scholar]
- 23.Mattenberger F., Sabater-Muñoz B., Toft C., Fares M.A. The phenotypic plasticity of duplicated genes in Saccharomyces cerevisiae and the origin of adaptations. G3 Genes Genomes Genet. 2017;7:63–75. doi: 10.1534/g3.116.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprouffske K., Wagner A. Growthcurver: An R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform. 2016;17:172. doi: 10.1186/s12859-016-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 26.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 27.Aguado A., Fischer T., Rodríguez C., Manea A., Martínez-González J., Touyz R.M., Hernanz R., Alonso M.J., Dixon D.A., Briones A.M., et al. Hu antigen R is required for NOX-1 but not NOX-4 regulation by inflammatory stimuli in vascular smooth muscle cells. Hypertens. Res. 2016;34:253–265. doi: 10.1097/HJH.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrero M., Mendiola J.A., Cifuentes A., Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Korczyński M., Witkowska Z., Opaliński S., Świniarska M., Dobrzański Z. Marine Algae Extracts. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2015. Algae extract as a potential feed additive. [Google Scholar]
- 30.Martorell P., Forment J.V., De Llanos R., Montón F., Llopis S., González N., Genovés S., Cienfuegos E., Monzó H., Ramón D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J. Agric. Food Chem. 2011;59:2077–2085. doi: 10.1021/jf104217g. [DOI] [PubMed] [Google Scholar]
- 31.De La Torre-Ruiz M., Pujol N., Sundaran V. Coping with oxidative stress. The yeast model. Curr. Drug Targets. 2015;16:2–12. doi: 10.2174/1389450115666141020160105. [DOI] [PubMed] [Google Scholar]
- 32.Jamieson D.J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Lushchak V.I. Budding yeast Saccharomyces cerevisiae as a model to study oxidative modification of proteins in eukaryotes. Acta Biochim. Pol. 2006;53:679–684. doi: 10.18388/abp.2006_3295. [DOI] [PubMed] [Google Scholar]
- 34.Saffi J., Sonego L., Varela Q.D., Salvador M. Antioxidant activity of L-ascorbic acid in wild-type and superoxide dismutase deficient strains of Saccharomyces cerevisiae. Redox Rep. 2006;11:179–184. doi: 10.1179/135100006X116691. [DOI] [PubMed] [Google Scholar]
- 35.Sadowska-Bartosz I., Paczka A., Moloń M., Bartosz G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13:820–830. doi: 10.1111/1567-1364.12091. [DOI] [PubMed] [Google Scholar]
- 36.Antoce O.A., Antoce V., Takahashi K., Yoshizako F. Quantitative study of yeast growth in the presence of added ethanol and methanol using a calorimetric approach. Biosci. Biotechnol. Biochem. 1997;61:664–669. doi: 10.1271/bbb.61.664. [DOI] [PubMed] [Google Scholar]
- 37.Piechowiak T., Balawejder M. Onion skin extract as a protective agent against oxidative stress in Saccharomyces cerevisiae induced by cadmium. J. Food Biochem. 2019;43:e12872. doi: 10.1111/jfbc.12872. [DOI] [PubMed] [Google Scholar]
- 38.Kieliszek M., Błażejak S., Bzducha-Wróbel A., Kot A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019;46:1797–1808. doi: 10.1007/s11033-019-04630-z. [DOI] [PubMed] [Google Scholar]
- 39.Poljak A., Dawes I.W., Ingelse B.A., Duncan M.W., Smythe G.A., Grant C.M. Oxidative damage to proteins in yeast cells exposed to adaptive levels of H2O2. Redox Rep. 2003;8:371–377. doi: 10.1179/135100003225003401. [DOI] [PubMed] [Google Scholar]
- 40.Sha W., Martins A.M., Laubenbacher R., Mendes P., Shulaev V. The genome-wide early temporal response of Saccharomyces cerevisiae to oxidative stress induced by cumene hydroperoxide. PLoS ONE. 2013;8:e74939. doi: 10.1371/journal.pone.0074939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao L., Tang Y., Quan Z., Zhang Z., Oliver S.G., Zhang N. Chronological lifespan in yeast is dependent on the accumulation of storage carbohydrates mediated by Yak 1, Mck 1 and Rim 15 kinases. PLoS Genet. 2016;12:e1006458. doi: 10.1371/journal.pgen.1006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim I.-S., Kim Y.-S., Kim Y.-H., Park A.-K., Kim H.-W., Lee J.-H., Yoon H.-S. Potential application of the Oryza sativa monodehydroascorbate reductase gene (OsMDHAR) to improve the stress tolerance and fermentative capacity of Saccharomyces cerevisiae. PLoS ONE. 2016;11:e0158841. doi: 10.1371/journal.pone.0158841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.