Abstract
Small heat shock proteins (sHSPs) are ubiquitous ATP-independent chaperones that play essential roles in response to cellular stresses and protein homeostasis. Investigations of sHSPs reveal that sHSPs are ubiquitously expressed in numerous types of tumors, and their expression is closely associated with cancer progression. sHSPs have been suggested to control a diverse range of cancer functions, including tumorigenesis, cell growth, apoptosis, metastasis, and chemoresistance, as well as regulation of cancer stem cell properties. Recent advances in the field indicate that some sHSPs have been validated as a powerful target in cancer therapy. In this review, we present and highlight current understanding, recent progress, and future challenges of sHSPs in cancer development and therapy.
Keywords: sHSPs, cancer, cancer stem cells, cancer therapy
1. Introduction
Although small heat shock proteins (sHSPs) were recognized as protein chaperones several decades ago, they have received considerably less attention for a long time than other heat shock proteins (HSPs). Small heat shock proteins are a class of the superfamily of HSPs with low molecular weight (12–43 kDa) that are ubiquitously expressed in all forms of life. Besides the molecular chaperone activity to prevent the formation of harmful protein aggregates in response to cellular stress, sHSPs are involved in diverse cellular functions such as protein degradation, stress tolerance, cell movement, cell death, cell differentiation, signal transduction, and cell development. As a consequence, sHSPs have important implications in physiological and pathological conditions. In recent years, growing evidence has shown that sHSPs play important roles in various types of cancer, and some sHSPs such as Hsp27 have been proposed to be therapeutic targets for cancer. Thus, understanding sHSPs’ functions and roles in human cancers and elucidating how their malfunction is linked mechanistically to cancers will help identify potential anticancer drug targets, develop effective chemotherapeutic drugs and other therapeutic strategies, and then improve cancer therapy. In this review, we summarize our current understanding of the role of sHSPs in cancers, as well as discuss anticancer drugs and current therapeutic strategies targeting sHSPs.
2. Small Heat Shock Proteins (sHSPs)
Small heat shock proteins are a ubiquitous family of ATP-independent molecular chaperones with low molecular mass. Molecular chaperones are proteins that assist other proteins in folding but are not components of these final structures [1,2]. They are classified by their molecular weight into the following families: Hsp110s, Hsp90s, Hsp70s, Hsp60s, Hsp40s, sHSPs, and CCT (TRiC) [3]. sHSPs exist in all kinds of life and play a key role in cellular stress responses.
Like all sHSPs, the primary structures of human sHSPs are characterized by a conserved, structured α-crystallin domain (ACD) flanked by a highly variable amino-terminal region (NTR) and a short flexible carboxy-terminal region (CTR) [4]. The ACD is thought to be essential for the dimerization and function of sHSPs [5]. The NTR is generally hydrophobic [6] and involved in oligomer formation of sHSPs and the interaction with substrate proteins [7]. The CTR is polar, flexible, and plays a key role in the stability and assembly of oligomers [8]. Under in vitro conditions, human sHSPs are often found to exist in a range of oligomeric states, although a few of them, such as HspB6, are reported to be stable and exist as dimers with chaperone activity [9,10]. Recently, HspB1 was reported to exist as phosphorylated monomers, which are from a progressive dissociation of HspB1 oligomers induced by palytoxin in MCF-7 cells and could play a protective role against palytoxin-induced cell death [11]. Similarly, another study showed that oligomer dissociation required only Ser90 phosphorylation of mammalian HspB1, while activation of thermoprotective activity required the phosphorylation of both Ser90 and Ser15 [12]. Further studies are required for understanding the role of phosphorylation of HspB1 in the interconversion of HspB1 ultrastructures between oligomeric/dimeric/monomeric forms and the regulation of its functions.
The human sHSPs contain 10 members, including HspB1 (Hsp27), HspB2 (myotonic dystrophy kinase-binding protein, MKBP), HspB3 (Hsp17), HspB4 (αA-crystallin), HspB5 (αB-crystallin), HspB6 (Hsp20), HspB7 (cardiovascular Hsp, cvHsp), HspB8 (Hsp22), HspB9 (cancer/testis antigen 51, CT51), and HspB10 (outer dense fiber protein 1, ODFP1). Some (HspB1, HspB5, HspB6, HspB8) are ubiquitously expressed in various tissues, while others are expressed in specific tissues (Table 1). In eukaryotes, the expression of sHSPs is under the control of the heat shock factor (HSF) transcription factors, which can initiate transcription of sHSPs genes and upregulate the expression of sHSPs in response to stress [13,14]. Moreover, sHSPs themselves are sensitive to their conditions, and their expression of proteins is coordinated by cellular conditions [15].
Table 1.
Human small heat shock proteins (sHSPs) and their expression 1.
| Protein Name | Alternative Names | Molecular Mass (kDa) | Tissue Expression |
|---|---|---|---|
| HspB1 | Hsp27, Hsp25, Hsp28 | 22.8 | Ubiquitous |
| HspB2 | MKBP | 20.2 | Cardiac and skeletal muscle |
| HspB3 | Hsp17 | 17.0 | Cardiac and skeletal muscle |
| HspB4 | αA-crystallin | 19.9 | Eye lens |
| HspB5 | αB-crystallin | 20.2 | Ubiquitous |
| HspB6 | Hsp20, p20 | 17.1 | Ubiquitous |
| HspB7 | cvHsp | 18.6 | Cardiac and skeletal muscle |
| HspB8 | Hsp22 | 21.6 | Ubiquitous |
| HspB9 | CT51 | 17.5 | Testis |
| HspB10 | ODF1 | 28.4 | Testis |
sHSPs are best known as molecular chaperones that bind various non-native (misfolded or unfolded) substrate proteins, prevent their uncontrolled aggregation, and facilitate the refolding of these proteins independently or by the cooperation of ATP-dependent chaperones. Usually, sHSPs do not exhibit refolding activities but capture the unfolded proteins and stabilize them in sHSP/substrate complexes. Refolding and release of bound substrates are under the help of other ATP-dependent chaperones [18,19,20]. In addition, sHSPs are involved in a growing number of other cellular functions, including cell protection, cellular signaling, cell differentiation, cell movement, cell apoptosis, and cell development [21,22]. Whether and how the chaperone and nonchaperone activities of sHSPs are related raise questions for the future. Pathologically, sHSPs have been reported to be linked to a number of diseases, such as cataracts, neurological disorders, myopathies, multiple sclerosis, aging, and cancers [21,22].
3. Functions of Small Heat Shock Proteins in Cancers
Although the original focus on the role of sHSPs revealed its chaperone functions, work over several decades has shed light on their important roles in cancers. Hsp27 (HspB1) was initially found to be expressed in meningiomas [23]. Increasing findings indicate that sHSPs are expressed in diverse malignancies and have been linked to several hallmark features of cancer, including tumorigenesis, cell growth, apoptosis, metastasis, and chemoresistance, as well as cancer stem cells.
3.1. Expression of Small Heat Shock Proteins in Cancers
Small heat shock proteins are ubiquitously expressed in numerous types of tumors, including head and neck (HspB1, HspB5) [23,24], breast (HspB1, HspB2, HspB5, HspB8) [25,26,27,28,29,30], cervical (HspB1) [31], colonrectal (HspB1, HspB5, HspB6, HspB9) [32,33,34,35], esophageal (HspB9) [35], gastric (HspB1, HspB5, HspB8) [36,37,38], larynx (HspB1, HspB5) [39,40], liver (HspB1, HspB5) [41,42], lung (HspB1, HspB5, HspB9) [35,43,44,45], oral (HspB5) [46], ovarian (HspB1, HspB8) [47,48], pancreatic (HspB4, HspB9) [35,49,50], prostate (HspB1) [51], renal (HspB1, HspB5, HspB7) [52,53,54], testis (HspB9) [35], cancers and glioblastoma (HspB1, HspB5) [55,56], and osteosarcoma (HspB5, HspB8) [57,58].
Increased levels of sHSP expression were identified in breast (HspB1, HspB2, HspB5) [25,26,27,28,29], cervical (HspB1) [31], colorectal (HspB1, HspB5) [32,33], gastric (HspB1, HspB5, HspB8) [36,37,38], glioblastoma (HspB1, HspB5) [55,56], larynx (HspB1, HspB5) [39,40], liver (HspB5) [42], lung (HspB1, HspB5) [43,45], oral (HspB5) [46], osteosarcoma (HspB5) [57], ovarian (HspB8) [48], prostate (HspB1) [51], renal (HspB1, HspB5) [52,53], and testis (HspB9) [35] cancers. Conversely, decreased expression of sHSPs was observed in colorectal (HspB6) [34], pancreatic (HspB4) [49,50], and renal (HspB7) [54] cancers. Additionally, sHSP expression is closely associated with the progression of cancers and poor clinical outcomes. We summarized the levels of sHSP expression in various cancers (Table 2).
Table 2.
sHSP expression in cancers.
| sHSPs (Alias) | Type of Cancers | Expression Status |
Functions | Reference |
|---|---|---|---|---|
| HspB1 (Hsp27) | Breast cancer | Overexpression | Oncogenic | [25,26] |
| Cervical cancer | Overexpression | [31] | ||
| Colorectal cancer | Overexpression | [32] | ||
| Gastric cancer | Overexpression | [36] | ||
| Glioblastoma | Overexpression | [55] | ||
| Larynx cancer | Overexpression | [39] | ||
| Lung cancer | Overexpression | [43] | ||
| Prostate cancer | Overexpression | [51] | ||
| Renal cancer | Overexpression | [52] | ||
| HspB2 (MKBP) | Breast cancer | Overexpression | Oncogenic | [27] |
| HspB3 (Hsp17) | No data | No data | No data | No data |
| HspB4 (αA-crystallin) | Pancreatic cancer | Underexpression | Tumor suppressive | [49,50] |
| HspB5 (αB-crystallin) | Breast cancer | Overexpression | Oncogenic | [28,29] |
| Colorectal cancer | Overexpression | [33] | ||
| Gastric cancer | Overexpression | [37] | ||
| Glioblastoma | Overexpression | [56] | ||
| Larynx cancer | Overexpression | [40] | ||
| Liver cancer | Overexpression | [42] | ||
| Lung cancer | Overexpression | [45] | ||
| Oral cancer | Overexpression | [46] | ||
| Osteosarcoma | Overexpression | [57] | ||
| Renal cancer | Overexpression | [53] | ||
| HspB6 (Hsp20) | Colorectal cancer | Underexpression | Tumor suppressive | [34] |
| HspB7 (cvHsp) | Renal cancer | Underexpression | Tumor suppressive | [54] |
| HspB8 (Hsp22) | Gastric cancer | Overexpression | Oncogenic | [38] |
| Ovarian cancer | Overexpression | [48] | ||
| HspB9 (CT51) | Testis cancer | Overexpression | Oncogenic | [35] |
| HspB10 (ODFP1) | No data | No data | No data | No data |
3.2. Tumorigenesis
Recent studies have indicated that sHSPs are related to the transformation into neoplastic cells. sHSPs can enhance or suppress tumorigenesis and cancer progression. Overexpression of HspB5 in human mammary epithelial cells (MECs) led to morphological, physiological transformation, and carcinomas formation in vivo [28]. Moreover, MECs with HspB5 overexpression showed neoplastic characteristics, including enhancing cell growth, migration and invasion, and inhibiting apoptosis. Conversely, HspB4, HspB6, and HspB7 are reported to suppress tumorigenesis in pancreatic, colorectal, and renal cancer, respectively [34,49,50,54]. In addition, HspB7 expression alteration was reported to be controlled by epigenetic regulation [54]. DNA hypermethylation is inversely correlated with the mRNA level of HspB7, and 5-aza-2′-deoxycytidine (5-Aza-dC) treatment elevated the expression of HspB7 [54]. These data suggested that methylation-dependent expression regulation of HspBs may be important for tumorigenesis of cancer cells. However, the mechanisms governing HspBs expression are not fully understood. Interestingly, HspB4 (αA-crystallin) and HspB5 (αB-crystallin) are two isoforms of α-crystallin, while existing evidence indicates that they may play different roles in tumorigenesis: HspB4 acts as a suppressor, and HspB5 acts as a promoter. Further investigation of the mechanism will clarify the divergent roles of sHSPs in tumorigenesis.
3.3. Cell Growth, Death, and Tumor Development
sHSPs have been identified to play a pivotal role in cell proliferation and survival and cancer progression. HspB1 can enhance or inhibit cell proliferation and growth and tumor development. Studies have shown that overexpression of HspB1 promotes cell proliferation and growth of breast cancer cells in vitro [15]. However, HspB1 was also suggested as a suppressor for cell proliferation in human testis tumor cells [59]. HspB5 overexpression has been reported to promote tumor growth of xenografts derived from breast cancer cells [60]. Another sHSP protein, HspB8, has been recently identified to enhance cancer progression. The knockdown of HspB8 expression inhibited in-vitro cell proliferation of breast cancer cells [61], while HspB8 overexpression enhanced cell proliferation and growth of breast cancer [61]. Interestingly, studies have suggested that HspB8 might play an important role in estrogen response and breast cancer progression. HspB8 is regulated by estrogen and can augment cancer cell progression by estrogenic stimuli [62,63,64], suggesting HspB8 might be a target associated with ER-positive tumors. However, the molecular mechanism should be further explored.
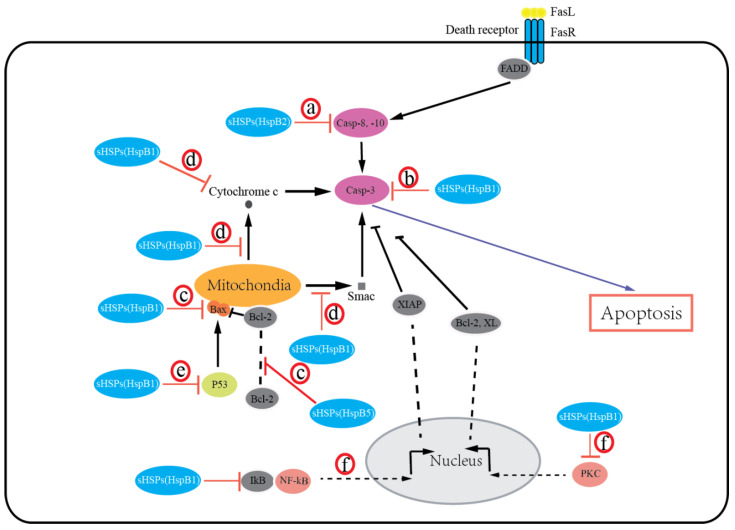
sHSPs protect cells from death by preventing the aggregation of denatured proteins and interacting with various components of the death-associated signaling pathways. Growing evidence suggests that most of the sHSPs (HspB1, HspB2, HspB5) possess an antiapoptotic activity and inhibit apoptotic cell death in various malignancies. The expression of HspB1, HspB2, and HspB5 is reported to be associated with resistance to apoptosis in human breast cancer cells [27,60] and oral verrucous carcinoma [65]. Overexpression of HspB1, HspB2, and HspB5 inhibits apoptosis in breast cancer cells [27,60,66]. Moreover, knockdown of HspB1 in-vitro induced apoptotic cell death, whereas HspB1 upregulation reduced apoptosis and enhanced tumorigenic potential in-vivo in glioblastoma multiforme [67]. Exceptionally, HspB6 was recently recognized to induce apoptosis in colorectal cancer [34]. Additionally, HspB8 is suggested to have either pro- or antiapoptotic effects in a context-dependent manner [68,69]. Several mechanisms for sHSPs regulating apoptosis by integrating with different signaling pathways have been suggested, including extrinsic and intrinsic apoptosis (Figure 1): (a) sHSPs (HspB2) inhibit the extrinsic apoptotic pathway by inactivating caspases-8, 10 [27]; (b) sHSPs (HspB1, HspB5) inhibit the activation of caspase-3 to block apoptosis [67,70,71]; (c) sHSPs (HspB1, HspB5) can regulate bax, bak, and other members of the Bcl-2 family or interact with Bcl-2 to sequester its translocation to mitochondria, thereby preventing the activation of the intrinsic apoptotic pathway [72,73]; (d) sHSPs (HspB1) block cytochrome c [74] and Smac [75] released from mitochondria or bind with cytochrome c released from mitochondria [76], then preventing the activation of caspase-9 and caspase-3; (e) sHSPs (HspB1, HspB5) inhibit the p53-dependent activation of the Bcl-2 family members, thus indirectly inhibiting its proapoptotic effect against apoptotic Bcl-2 proteins [77,78]; (f) sHSPs (HspB1) can also interact with protein kinase C (PKC) delta type [79] or nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor (IkB), alpha [80], thereby inhibiting caspase-3 activation. It is important to note that the phosphorylation states of sHSPs have been reported to regulate cancer cell death. Early studies have shown that both phosphorylated and unphosphorylated HspB1 inhibit apoptosis [77,78]. Recently, several studies have indicated that phosphorylation of HspB1 increases apoptosis in leukemia cells [70], and phosphorylation of both HspB1 and HspB5 increases apoptosis in breast cancer cells [73,81]. It is given that sHSPs undergo a number of post-translational modifications (PTMs) under physiologic and pathologic conditions (e.g., phosphorylation, acetylation, and glycosylation). In addition, these PTMs alter sHSP structure, then modulate apoptotic activity by interacting with different proteins and playing significant roles in regulating cell survival. However, our understanding of how these PTMs impact sHSPs’ apoptotic function is clearly incomplete. Thus, the significance and roles of sHSPs’ PTMs in cancer cells will need further investigation.
Figure 1.
Small heat shock proteins (sHSPs) regulate apoptosis in the extrinsic and intrinsic apoptotic pathways. sHSPs inhibit activation of caspase-8, -10 (a) or caspase-3 (b) to block apoptosis. sHSPs inhibit activation of the intrinsic apoptotic pathway by interacting with bax, bak, or other members of the Bcl-2 family (c), or by inhibiting cytochrome c and Smac release from mitochondria, or binding with cytochrome c (d), or by inhibiting the p53-dependent activation of the proapoptotic Bcl-2 family members (e). sHSPs also interact with PKC or IkB to inhibit caspase-3 activation (f).
In addition, sHSPs play cytoprotective roles against apoptosis induced by oxidative stress in cancer cells, and their high expression in cancer cells results in cancer cell radio- and chemoresistance. As a byproduct of oxygen metabolism, reactive oxygen species (ROS) induce oxidative stress, damage macromolecules such as proteins, lipids, and nucleic acids, and, therefore, trigger apoptotic pathways and result in cell death. Cells use the antioxidant system (e.g., antioxidant enzymes, GSH, and CoQ as internally synthesized antioxidants, and vitamin E and carotenoids as externally supplied antioxidants) to neutralize free radical cellular damage and maintain normal redox homeostasis. Unlike normal cells, many cancer cells develop efficient antioxidant systems to defend against oxidative damage caused by ROS to prevent cell death. Studies have shown that the expression of HspB1 can significantly decrease cellular the ROS level and upregulate the total glutathione level [82,83]. HspB1 may act in different ways to modulate cell intracellular redox status: (a) decreasing free radical production by reducing the activities of enzymes, including superoxide dismutase (SOD) [84,85] and catalase [85,86], or decreasing of intracellular iron to prevent its participation in free radical formation [87]; (b) scavenging free radicals by modulating the activity of antioxidant enzymes such as glucose 6-phosphate dehydrogenase (G6PDH) [88,89], glutathione reductase (GR) [90], and glutathione peroxidase (GPx) [85,90] to increase reduced glutathione (GSH) levels and downregulate ROS; (c) activating transcription factors, such as nuclear factor erythroid 2-related factor 2 (NRF2), thereby activating their target antioxidative enzymes [91]. Consistent with the abovementioned mechanisms, increased levels of sHSPs, together with multiple antioxidant molecules, including superoxide dismutase (MnSOD), thioredoxin reductase 2 (TXNRD2), glutathione (GSH), glutathione peroxidase (Gpx), were identified in thyroid tumors [92]. In fact, all antioxidants work cooperatively as a complex network to maintain optimal redox balance. The roles of sHSPs in redox regulation in different cancer cells and how sHSPs coordinate the antioxidant defense network to protect against oxidative stress-induced cell death will need further investigation. Furthermore, current reports indicate that HspB1 is associated with therapeutic resistance in colon [93], liver [94], and head and neck [95] cancer cells through the regulation of ROS levels; thus, targeting sHSPs could be a potent strategy to help overcome chemo/radioresistance in cancers.
3.4. Cell Migration/Invasion, Angiogenesis, and Tumor Metastasis
Consistent with sHSPs’ enhanced expression in many malignancies, increasing reports have demonstrated that sHSPs play important functional roles in cell migration, invasion, and angiogenesis. Initially, HspB1 was reported to promote the invasion of breast cancer cells [96] by upregulation of MMP-9 expression [97] but decrease the motility of breast cancer cells [96]. Subsequently, numerous studies have demonstrated that overexpression of HspB5 in breast cancer cells increased cell migration and invasion [28], and HspB1 silencing in colorectal [98], prostate [99], ovarian [100], and liver [101] cancers or head and neck squamous cell carcinoma [102], HspB8 silencing in breast cancer [61], or HspB5 silencing in colorectal [103] and renal [53] cancers inhibit migration and/or invasion of cancer cells. Moreover, a number of clinical data studies have shown that primary tumor expression of sHSPs is associated with aggressive tumor characteristics and tumor progression. For example, HspB1 mRNA and protein expression correlate with peritoneal metastasis and poor survival in ovarian cancer [104]. More recently, high serum HspB1 expression in ovarian cancer [105] and renal cancer [106] has been shown to be associated with tumor metastasis. Additionally, phosphorylated HspB1 is also reported to be associated with portal vein invasion in hepatocellular carcinoma [41] and lymph node metastasis in breast cancer [26]. Additionally, HspB5 expression correlates with the tumor–node–metastasis (TNM) stage and predicts poor survival in non-small-cell lung cancer [45]. In breast cancer, HspB5 expression is an independent predictor of brain metastasis and poor survival and can also predict brain metastasis as the first site of distant metastasis [107,108]. Although overwhelming data have indicated that sHsps can promote cell migration and invasion and tumor metastasis, a few notable exceptions have been reported. HspB1 mRNA and protein levels were lower in tumor tissue in a small study of thyroid carcinomas [109]. Additionally, a few studies showed that HspB5 expression was not significantly correlated with prognosis in head and neck carcinomas [110] and lung cancer [111]. Moreover, HspB4 has been reported to suppress pancreatic cancer cell migration [49,50]. Thus, it should be clarified whether these divergent and even contrasting results reflect differences in study design and/or tumor type. Although the mechanisms underlying sHSPs’ effects on cell migration/invasion have not been clearly defined, results have shown that sHSPs promote migration, invasion, and metastasis, likely by regulating MMP expression [97], by interacting with and regulating intermediate filament dynamics [98], or via epithelial–mesenchymal transition (EMT) [103]. Additionally, it is noteworthy that phosphorylation may be important for sHSPs’ effects on these actions [26,39,112], and understanding the roles and functions of sHSPs’ PTMs on cell migration/invasion will deepen our understanding of sHSPs’ roles in malignancy.
Tumor angiogenesis, the process where tumors form new blood vessels from pre-existing ones, plays critical roles in tumor growth and metastasis [113]. HspB1 has been reported to be induced during tumor angiogenesis [114]. HspB5 was identified to promote tumor angiogenesis by modulating tubular morphogenesis and survival of endothelial cells [115]. Similarly, HspB1 has been reported to induce angiogenesis by increasing vascular endothelial growth factor (VEGF) [116]. Additionally, HspB1 expression was suggested to be associated with the expression and secretion of angiogenesis-associated proteins such as VEGF-A [117,118]. Consistent with this finding, HspB5 has been reported to regulate tumor angiogenesis by modulating VEGF-A [119]. Moreover, HspB1 has been identified as the target molecule of angiogenesis inhibitors [120]. Collectively, these data suggest that sHSPs have a proangiogenic effect and might be potential targets for antiangiogenic cancer therapy.
3.5. Chemoresistance
sHSPs are a group of molecular chaperones protecting cells by maintaining cellular homeostasis. HspB1’s murine homolog Hsp25 expression is induced significantly by treatment with chemotherapy drugs such as cisplatin and doxorubicin in Ehrlich ascites tumor (EAT) cells [121,122]. Additionally, the upregulation of HspB1 was also reported in cervical cancer cells and prostatic cancer cells by treatment with 17-allylamino-demethoxygeldanamycin (17-AAG) [123]. Moreover, HspB1 was significantly higher in geldanamycin-resistant A549 NSCLC cells [123]. Furthermore, HspB1 exhibited an alteration of its degree of phosphorylation when treatment with vinblastine, paclitaxel, and doxorubicin in breast cancer cells and serine 59 phosphorylation of HspB1 induced apoptosis of vinblastine-treated breast cancer cells [73], suggesting that specifically inducing the phosphorylation of HspB1 can improve therapeutic outcomes by circumventing the drug resistance of breast cancer. Artificial overexpression of HspB1 increased doxorubicin resistance of breast cancer cells [15], cisplatin and doxorubicin resistance of testis tumor cells [59], and 17-AAG resistance of cervical cancer cells [123]. Similarly, knockdown of HspB1 decreased doxorubicin resistance of breast cancer cells [15] and 17-AAG resistance of cervical cancer cells [123]. Collectively, these studies suggest that HspB1 has a significant role in chemotherapy drug resistance. Remarkably, it has been demonstrated that inhibition of Hsp90 is associated with the upregulation of HspB1. Hsp90 forms complexes with HSF1 and maintains HSF1 in a repressed, transcriptionally inactive form. Inhibitors or binding agents that target Hsp90 can disrupt Hsp90-HSF1 interaction, thereby freeing HSF-1 by dissociation from Hsp90. Activated HSF-1 is translocated into the nucleus, initiates transcription of previously silent Hsp genes, including HspB1, Hsp40, and Hsp70, and leads to the activation of a prosurvival process called the heat shock response, thus limiting the activity of Hsp90 inhibitors and contributing to drug resistance and toxicity. Studies show that a combination of downregulation of HspB1 by gene silencing or inhibitors and Hsp90 inhibitors could reduce drug resistance and enhance the efficacy of Hsp90-directed therapy [123,124]. However, other sHSPs’ roles in resistance to anticancer treatment are not clearly defined, and further in-depth investigations need to be followed to expand the roles of sHSPs in cancer therapy.
3.6. Cancer Stem Cells
Cancer stem cells (CSCs), also termed “tumor-initiating cells” (TICs) or “sphere-forming cells”, are a small subpopulation of stem-cell-like cancer cells that present the stem cell property of self-renewal, generating differentiated tumor cells and resistance to radiochemotherapy [125]. CSCs have been isolated from numerous solid and liquid tumors, including but not limited to leukemia, brain, head and neck, lung, breast, liver, stomach, colon, pancreatic, prostate, ovary, and bladder cancers and mesothelioma [126]. CSCs are believed to be responsible for cancer pathogenesis, namely, cancer initiation, recurrence, metastasis, and drug resistance, and are involved in the progression of human malignancies [127]. Thus, targeting CSCs and the elimination of CSCs have been considered as an emerging area in cancer therapy.
Current reports indicate that HspB1 plays a crucial role in regulating CSC-associated properties such as survival, stemness, high migration activity and invasiveness, and chemoresistance. Initially, HspB1 was reported to be associated with chemoresistance of lung cancer stem cells [128]. Then, HspB1 was identified to be related to CSC stemness by proteomics [129]. Indeed, several studies have shown that HspB1 is essential for CSC stemness in salivary adenoid cystic carcinoma [130], non-small-cell lung cancer [131], and breast cancer [132]. There are also reports linking HspB1 to the maintenance of CSC phenotypes in breast cancer stem cells [133] and gynecologic cancer stem cells [134]. Additionally, the contribution of HspB1 to cell migration, invasiveness, and EMT was found in several CSC-related studies, including breast cancer [133] and salivary adenoid cystic carcinoma [115]. Similarly, HspB1 was identified to regulate vasculogenic mimicry activity in CD24−CD44+ALDH+ breast cancer stem cells [135]. Furthermore, numerous reports have suggested that HspB1 is key for CSC survival and contributes to radio- and chemoresistance. For example, HspB1 is considered to be essential for the survival of CD133+ colon cancer stem cells [136]. Additionally, HspB1 (along with Hsp70) is upregulated in radioresistant CSC-like SP cells isolated from breast cancer cells [137]. In addition, HspB1 resistance to apoptosis, hyperthermia, and chemotherapeutic agents was reported in oral CSCs [138], lung CSCs [128,139], breast CSCs [132,140], and esophageal CSCs [141].
Importantly, it should be noted that HspB1 involvement in modulating CSC properties depends on the levels of its expression and activity. Though HspB1 can be expressed constitutively in mammalian cells, its expression is mainly coordinated by HSF1 (heat shock transcription factor 1), which is a stress-responsive transcriptional factor that induces the transcriptional activation and the expression of HSPs such as HspB1 and Hsp70 [13]. Under any proteotoxic stress (heat, hypoxia, energy starvation) stimuli, HSF1 is activated and translocated into the nucleus, where it activates HSP gene transcription. Additionally, HSF1 activation/inactivation mainly depends on its phosphorylation [142]. Indeed, phosphorylation of HSF1 at serine 326 was reported to be critical for the maintenance of gynecologic CSCs by induction of HspB1 [134]. Besides HspB1 expression regulated by HSF1, the inhibition of HspB1 by SMURF2 (SMAD ubiquitin regulatory factor 2) is reported to be involved in repressing the self-renewal capability of breast CSCs [132], indicating that ubiquitin-dependent protein degradation seems to play a role in modulating HspB1 expression and CSC properties. It is generally accepted that the oligomerization and cytoprotective activities of HspB1 are mainly regulated by phosphorylation. Recently, numerous studies have confirmed that HspB1 phosphorylation/dephosphorylation is a critical regulator in the formation/maintenance of CSC properties. HspB1 undergoes phosphorylation as the terminal substrate in the p38/MAPK pathway, and there are increasing reports linking this p38 MAPK/MAPKAPK2/HSP27 pathway to chemoresistance in lung CSCs [128,131] and oral CSCs [138], maintenance of CSC properties in lung CSCs [131], and EMT in renal [143] and lung CSCs [139]. Similarly, in colorectal CSCs, protein phosphatase PP2A was reported to dephosphorylate HspB1 and attenuate HspB1 effects by promoting CSC properties [136,144], indicating that the status of phosphorylated HspB1, mediated by kinase and phosphatase, is essential for HspB1 effects on CSC properties. However, the detailed regulatory mechanism of HspB1 phosphorylation that depends upon the temporal and spatial balance of kinase and phosphatase should be investigated further. Conversely, inactivation of p38 has been suggested to promote CSC properties in non-small-cell lung cancer cells [145]. In this case, phosphorylated HspB1 by the p38/MK2 pathway can interact with the stemness-related proteins such as SOX2, OCT4, NANOG, KLF4, and c-Myc, then promote their ubiquitination and degradation, suggesting that phosphorylated HspB1 may have different roles in various types of CSCs by interacting with the effector proteins.
Large numbers of publications have demonstrated that HspB1’s maintenance and modulation of CSC characteristic features are largely dependent on its interaction with client proteins. Evidence has shown that HspB1 can inhibit apoptosis by interacting with procaspase-9 and procaspase-3 to prevent activation of caspase-9 and caspase-3 [128]. The direct interaction between HspB1 and AKT in esophageal CSCs is suggested to be critical for the maintenance of CSC features such as a high metabolic rate [141]. Similarly, we report that the cancer stemness-related activities of Hsp90 are regulated by clusterin through Hsp90 direct interactions with its client protein, AKT, in gastric cancer stem cells [146]. In addition, HspB1 regulates the maintenance and EMT of breast CSCs by interacting with IkB, inducing its degradation, and activating NFkB [133]. Consequently, among HspB1’s interacting proteins, there are surely some proteins that contribute to CSC-driven cancer development or are responsible for the manifestation of certain properties of CSCs. However, HspB1’s interacting proteins and their implications and roles in the formation/maintenance of the CSC phenotype remain to be elucidated.
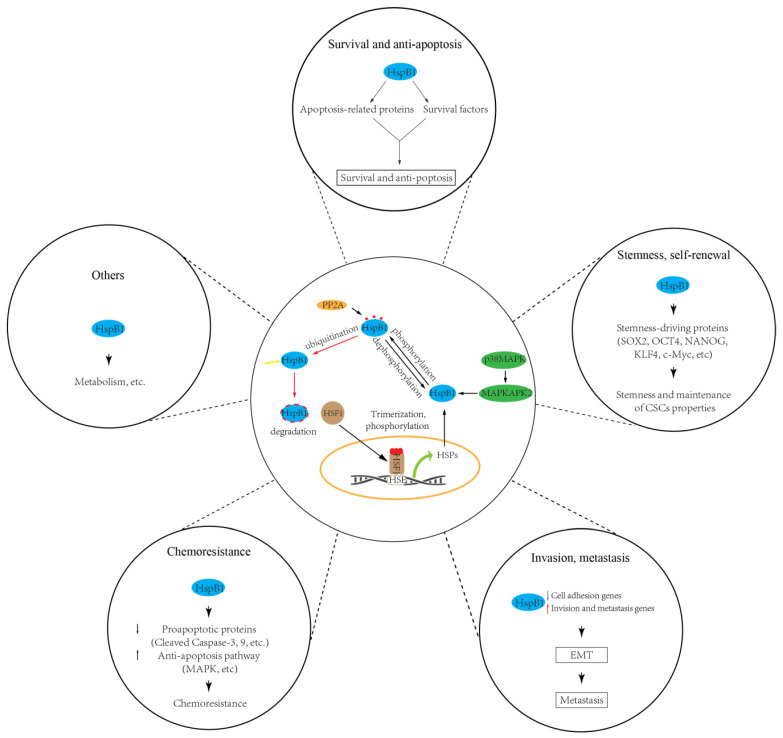
Taken together, these published data indicate that HspB1 is responsible for the maintenance/modulation of CSC properties such as the stemness and self-renewal of CSCs, their ability to promote EMT and metastasis, their resistance to apoptosis and radiochemotherapy, and their energy metabolism switching [147] (Figure 2). No doubt, HspB1 is one of the most promising targets for CSC-based cancer treatment. However, several points should be addressed for better understanding of sHsps’ roles in CSC maintenance and progression. First, the mechanisms leading to HspB1 expression alteration in CSCs are not fully understood. Up until now, HspB1 expression alteration in CSCs has been mainly focused on the transcriptional level; for example, the transcriptional factor HSF1. Other mechanisms, such as microRNA and epigenetic regulation governing HspB1 expression, remain elusive. Second, how do other PTMs’ roles in modulating CSC properties regulate HspB1 activity? Third, current publications are mainly focused on the roles of HspB1 in CSCs. How do other sHsps play a role in CSCs and the underlying mechanisms? Answering these questions will deepen our understanding of the role of sHsp proteins in CSCs and provide novel therapeutic strategies targeting sHsps in cancer treatment.
Figure 2.
sHSPs (HspB1) regulate cancer stem cell (CSC) properties, including survival, stemness, invasiveness, chemoresistance, and others. HspB1 involvement in modulating CSC properties depends on the levels of its expression and activity. HspB1 can be upregulated by transcription factor HSF1, while its activity can be modulated by phosphorylation (e.g., by p38/MAPK pathway) and dephosphorylation (e.g., by PP2A).
4. Small Heat Shock Proteins in Cancer Therapy
4.1. Anticancer Drugs Targeting sHSPs
As a majority of clinical and preclinical findings indicate sHSPs as a promising therapeutic target in cancer, a number of drugs or inhibitors have been reported and utilized to interrogate sHSPs’ roles in cancer. The reports are mainly focused on HspB1 as a molecular target for cancer therapy. Hence, in the present subsection, the drugs targeting HspB1 are analyzed in more detail. Although several drugs or compounds for other sHSPs in cancer therapy have been described, other sHSPs are omitted here because no selective inhibitors targeting these sHSPs have been reported.
Here, we summarize the drugs aimed at reducing HspB1 expression or inhibiting its actions in cancer therapy (Table 3). Small molecule inhibitors (RP101, quercetin, J2, ovatodiolide, and methyl antcinate) bind to the HspB1 protein and inhibit its function. Another strategy utilizes peptide aptamers (PA11, PA50) that bind directly to the protein and inhibit its oligomerization or dimerization. Moreover, the third approach is antisense oligonucleotide (OGX-427), which targets HspB1 mRNA and prevents translation of the protein.
Table 3.
Summary of reported cancer drugs targeting HspB1.
| Types | Names | Mechanism | Binding Sites | Reference |
|---|---|---|---|---|
| Small Molecules | RP101 | Binds to HspB1 protein and inhibits HspB1 function |
Phe29 and Phe33 | [148,149,150,151,152] |
| quercetin | No data available | [132,138,153,154,155,156,157,158,159,160] | ||
| J2 | Cysteine thiol group | [161] | ||
| ovatodiolide | No data available | [162] | ||
| methyl antcinate | No data available | [163] | ||
| Peptide Aptamers | PA11 | Binds to HspB1 protein and inhibits its oligomerization or dimerization | No data available | [164,165,166] |
| PA50 | No data available | |||
| Antisence Oligonucleotide | OGX-427 | Binds to HspB1 mRNA and prevents translation of the protein | No data available | [167,168,169,170,171] |
Several small molecule inhibitors targeting HspB1 are currently under development: RP101, quercetin, J2, ovatodiolide, and methyl antcinate. RP101 (also known as bromovinyldeoxyuridine, BVDU, or brivudine) is a nucleoside that inhibits HspB1 function via binding with Phe29 and Phe33 of HspB1. RP101 functions as a chemo-sensitizing agent that inhibits the resistance and potentiates the effects of many chemotherapeutic drugs including mitomycin [148,149], gemcitabine [149,150], cisplatin [149,150], and cyclophosphamide [149]. In clinical studies, RP101 [149,150] or RP101 with gemcitabine [150,151] increased the overall survival rate of pancreatic cancer patients. However, overdosing of RP101 caused increased toxic side effects of gemcitabine in some patients [149], and new second-generation candidates of RP101 have been identified and are being developed for further evaluation [152]. Quercetin is a plant-derived bioflavonoid with anticancer properties [153]. It suppresses the HSF1-dependent induction of the Hsps and shows antitumor effects in gastric, oral, lymphomas, prostate, colorectal, breast, pancreatic, liver, and lung cancer cell lines and various cancer stem cells [154,155,156,157]. Quercetin can act as a chemo-sensitizer, and it enhances the antitumor effects of first-line chemotherapeutic drugs such as doxorubicin, gemcitabine, 5-fluorouracil, and cisplatin [158,159]. Interestingly, besides its inhibitory effect on HspB1 expression, quercetin can suppress HspB1 activity by impairing its phosphorylation in CSCs [138]. Despite studies showing that quercetin can be a suitable agent for cancer treatment, there are no ongoing anticancer trials for quercetin. J2, a synthetic chromone compound, can induce the crosslinking of HspB1 protein and form HspB1 abnormal dimerization, thereby inhibiting its functions [160]. Recently, ovatodiolide [132] and methyl antcinate [161], two plant-derived compounds, have been reported to decrease HspB1 protein expression in breast CSCs and inhibit CSCs. It has to be elucidated whether these compounds are clinically applicable against breast cancer.
The second approach to targeting HspB1 is the use of specific peptides, which are called peptide aptamers, to bind the protein and inhibit the functions of HspB1. Peptide aptamers are short peptides that are designed to bind to specific protein domains and disrupt the protein function. Recent research showed that two peptide aptamers, PA11 and PA50, can specifically bind to HspB1, inhibiting HspB1 dimerization or oligomerization, thereby negatively modulating the functions of HspB1 [162]. These peptide aptamers are reported to show antitumor effects in vitro [162] and in vivo [163]. Similar to the small molecule inhibitors of HspB1, a peptide aptamer is always more effective when used with other anticancer drugs than when used alone. More efforts are needed to promote the preclinical and clinical application of peptide aptamers and provide a potential application to cancer therapy.
The third approach utilizes antisense oligonucleotide (ASO) targeting HspB1 mRNA, and OGX-427, which prevents the expression of HspB1 protein. OGX-427 reduced xenograft tumor growth when used in combination with chloroquine [164] or gemcitabine, respectively [165], compared to treatment with the drug alone. The Phase I study of dose-escalation OGX-427 in prostate, bladder, breast, and lung cancers showed that OGX-427 was well tolerated at a high dose (1000 mg), and it can decrease tumor marker expression and the number of circulating tumor cells (CTCs) in patients with prostate and ovarian cancers [166]. In a Phase II trial for castrate-resistant prostate cancer (CRPC), 71% of patients treated with OGX-427 and prednisone were progression-free at 12 weeks, compared to 40% of patients treated with prednisone alone [167]. However, in another Phase II trial for metastatic non-small-cell lung cancer (NSCLC), the addition of OGX-427 to the carboplatin–pemetrexed regimen did not improve outcomes and the efficacy of first-line chemotherapy for patients [168]. More clinical studies are needed to evaluate the efficacy and side effects of OGX-427 as a combinational clinical therapy in the treatment of different cancer patients.
4.2. sHSPs-Based Cancer Therapy
Other than a molecular target for cancer therapy, sHsps have been reported to be used as a multifunctional scaffold for the targeted therapeutic and imaging systems in cancers. The naturally occurring small heat shock protein 16.5, which originates from Methanocaldococcus jannaschii, is reported to form a cage-like structure to act as multifunctional biomaterials. The genetically and chemically modified Hsp16.5 cages, Cy5.5-HspDEVD-BHQ3, were developed for imaging caspase activity in vitro and in vivo [169]. Thus, these sHsp cages may provide efficient imaging agent carriers to monitor the therapeutic evaluation by imaging caspase activity within tumors. Similarly, Hsp16.5-based nanocages, conjugated with gadolinium (III)-chelated agents and iRGD peptides, were developed for the diagnosis of pancreatic cancers by magnetic resonance imaging (MRI) [170]. It showed that sHsps have great potential in the diagnosis of cancers as a carrier to construct a specific and sensitive MRI contrast agent. Moreover, Hsp16.5-based cages carrying doxorubicin (an anticancer agent) were tested in various cancer cell lines [171] and could provide a useful drug delivery system in cancer therapy. As sHsps cages have good biocompatibility, biodegradability, and easy fabrication, they may be promising as biomedical materials for drug or imaging agent delivery in cancer therapy and other biomedical applications.
5. Conclusions and Future Perspectives
In summary, sHSPs are crucial to the regulation of the tumorigenesis, development, metastasis, and chemoresistance of cancers, as well as cancer stem cells, and they may act as an oncogene or a tumor suppressor in a context-dependent manner in cancer progression. In addition, biological and clinical data strengthen the idea that sHSPs are closely associated with the prognosis and progression of cancers. Moreover, sHSP-targeted drugs or inhibitors and therapeutic and imaging strategies in cancers have been developed and show great potential in cancer treatment. Considerable progress has been made in recent years in understanding the functions and mechanisms of sHSPs in cancers. However, many central aspects are still in need of further clarification. First, in various types of cancers, the precise roles of sHSPs and the detailed underlying mechanisms in cancer progression should be investigated. How do sHSPs regulate cancer progression in a context-dependent manner? Second, the precise mechanisms of the regulation of sHSP expression and activity in cancer progression need to be determined. Third, the cellular substrate molecules for different sHSPs and the dynamic interaction between sHSPs and these molecules in different types of cancers remain to be elucidated more in detail. Moreover, the discovery of therapeutic inhibitors or drugs targeted to different sHSPs has so far remained largely unexplored. As the structural complexity of Hsp27 and its dynamic interactions with substracts challenge the discovery of therapeutic inhibitors and drugs, potent and selective inhibitors of Hsp27 are still missing. In addition, most inhibitors and drugs have been developed for Hsp27; however, drugs targeting other sHsps remain largely open issues. Furthermore, recent studies have suggested combinations of sHSP inhibitors and other chemotherapy agents, and these combinations are promising in drug-resistant cancer treatment. Researchers should pay more attention to this direction when investigating potential new cancer therapies.
Abbreviations
| sHSPs | Small Heat Shock Proteins |
| HSPs | Heat shock proteins |
| ACD | α-crystallin domain |
| NTR | Amino-terminal region |
| CTR | Carboxy-terminal region |
| MKBP | Myotonic dystrophy kinase-binding protein |
| cvHsp | Cardiovascular Hsp |
| CT51 | Cancer/testis antigen 51 |
| ODFP1 | Outer dense fiber protein 1 |
| HSF | Heat shock factor |
| MECs | Mammary epithelial cells |
| 5-Aza-dC | 5-aza-2′-deoxycytidine |
| PKC | Protein kinase C |
| IkB | Kappa light polypeptide gene enhancer in B-cells inhibitor |
| PTMs | Post-translational modifications |
| TNM | Tumor-node-metastasis |
| EMT | Epithelial-mesenchymal transition |
| EAT | Ehrlich ascites tumor |
| 17-AAG | 17-allylamino-demethoxygeldanamycin |
| CSCs | Cancer stem cells |
| TICs | Tumor-initiating cells |
| HSF1 | Heat shock transcription factor 1 |
| SMURF2 | SMAD ubiquitin regulatory factor 2 |
| ASO | Antisense oligonucleotide |
| CTCs | Circulating tumor cells |
| CRPC | Castrate resistant prostate cancer |
| NSCLC | Non-small cell lung cancer |
| MRI | Magnetic resonance imaging |
Author Contributions
J.X. and L.F. wrote the manuscript with contributions of Y.L. and X.T.; J.X. and L.F. planed and designed figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (2020A1515010989, 2018A030310586), the Medical Scientific Research Foundation of Guangdong Province (A2019405), the National Natural Science Foundation of China (81772957), the Guangdong Provincial Science and Technology Program (No. 2019B030301009), and the SZU Top Ranking Project (86000000210).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Freilich R., Arhar T., Abrams J.L., Gestwicki J.E. Protein-protein interactions in the molecular chaperone network. Acc. Chem. Res. 2018;51:940–949. doi: 10.1021/acs.accounts.8b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderwood S.K., Repasky E.A., Neckers L., Hightower L.E. The IXth CSSI international symposium on heat shock proteins in biology and medicine: Stress responses in health and disease, Alexandria old town, Alexandria, Virginia, 10–13 November 2018. Cell Stress Chaperones. 2019;24:1–6. doi: 10.1007/s12192-018-00966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shevtsov M., Balogi Z., Khachatryan W., Gao H., Vígh L., Multhoff G. Membrane-associated heat shock proteins in oncology: From basic research to new theranostic targets. Cells. 2020;9:1263. doi: 10.3390/cells9051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janowska M.K., Baughman H.E.R., Woods C.N., Klevit R.E. Mechanisms of small heat shock proteins. Cold Spring Harb. Perspect. Biol. 2019;11:a034025. doi: 10.1101/cshperspect.a034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabbaghizadeh A., Tanguay R.M. Structural and functional properties of proteins interacting with small heat shock proteins. Cell Stress Chaperones. 2020;25:629–637. doi: 10.1007/s12192-020-01097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klevit R.E. Peeking from behind the veil of enigma: Emerging insights on small heat shock protein structure and function. Cell Stress Chaperones. 2020;25:573–580. doi: 10.1007/s12192-020-01092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haslbeck M., Weinkauf S., Buchner J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019;294:2121–2132. doi: 10.1074/jbc.REV118.002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbecq S.P., Rosenbaum J.C., Klevit R.E. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry. 2015;54:4276–4284. doi: 10.1021/acs.biochem.5b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks S.D., Baranova E.V., Heirbaut M., Beelen S., Shkumatov A.V., Gusev N.B., Strelkov S.V. Molecular structure and dynamics of the dimeric human small heat shock protein HSPB6. J. Struct. Biol. 2014;185:342–354. doi: 10.1016/j.jsb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Sluchanko N.N., Beelen S., Kulikova A.A., Weeks S.D., Antson A.A., Gusev N.B., Strelkov S.V. Structural basis for the interaction of a human small heat shock protein with the 14-3-3 universal signaling regulator. Structure. 2017;25:305–316. doi: 10.1016/j.str.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berni C., Bellocci M., Sala G.L., Rossini G.P. Palytoxin induces dissociation of HSP 27 oligomers through a p38 protein kinase pathway. Chem. Res. Toxicol. 2015;28:752–764. doi: 10.1021/tx500511q. [DOI] [PubMed] [Google Scholar]
- 12.Thériault J.R., Lambert H., Chávez-Zobel A.T., Charest G., Lavigne P., Landry J. Essential role of the NH2-terminal WD/EPF motif in the phosphorylation-activated protective function of mammalian Hsp27. J. Biol. Chem. 2004;279:23463–23471. doi: 10.1074/jbc.M402325200. [DOI] [PubMed] [Google Scholar]
- 13.Prince T.L., Lang B.J., Guerrero-Gimenez M.E., Fernandez-Muñoz J.M., Ackerman A., Calderwood S.K. HSF1: Primary factor in molecular chaperone expression and a major contributor to cancer morbidity. Cells. 2020;9:1046. doi: 10.3390/cells9041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilaboa N., Boré A., Martin-Saavedra F., Bayford M., Winfield N., Firth-Clark S., Kirton S.B., Voellmy R. New inhibitor targeting human transcription factor HSF1: Effects on the heat shock response and tumor cell survival. Nucleic Acids Res. 2017;45:5797–5817. doi: 10.1093/nar/gkx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treweek T.M., Meehan S., Ecroyd H., Carver J.A. Small heat-shock proteins: Important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 2015;72:429–451. doi: 10.1007/s00018-014-1754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampinga H.H., Hageman J., Vos M.J., Kubota H., Tanguay R.M., Bruford E.A., Cheetham M.E., Chen B., Hightower L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mymrikov E.V., Daake M., Richter B., Haslbeck M., Buchner J. The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem. 2017;292:672–684. doi: 10.1074/jbc.M116.760413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungelenk S., Moayed F., Ho C.T., Grousl T., Scharf A., Mashaghi A., Tans S., Mayer M.P., Mogk A., Bukau B. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 2016;7:13673. doi: 10.1038/ncomms13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwirowski S., Kłosowska A., Obuchowski I., Nillegoda N.B., Piróg A., Ziętkiewicz S., Bukau B., Mogk A., Liberek K. Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding. EMBO J. 2017;36:783–796. doi: 10.15252/embj.201593378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nillegoda N.B., Wentink A.S., Bukau B. Protein disaggregation in multicellular organisms. Trends BioChem. Sci. 2018;43:285–300. doi: 10.1016/j.tibs.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Bakthisaran R., Tangirala R., Rao C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Carra S., Alberti S., Arrigo P.A., Benesch J.L., Benjamin I.J., Boelens W., Bartelt-Kirbach B., Brundel B.J.J.M., Buchner J., Bukau B., et al. The growing world of small heat shock proteins: From structure to functions. Cell Stress Chaperones. 2017;22:601–611. doi: 10.1007/s12192-017-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama N., Iwaki T., Goldman J.E., Tateishi J., Fukui M. Small heat-shock protein is expressed in meningiomas and in granulofilamentous inclusion bodies. Acta Neuropathol. 1993;85:248–255. doi: 10.1007/BF00227718. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama A., Steiger R., Frohli E., Schafer R., von Deimling A., Wiestler O., Klemenz R. Expression of alpha B-crystallin in human brain tumors. Int. J. Cancer. 1993;55:760–764. doi: 10.1002/ijc.2910550511. [DOI] [PubMed] [Google Scholar]
- 25.Conroy S.E., Sasieni P.D., Amin V., Wang D.Y., Smith P., Fentiman I.S., Latchman D.S. Antibodies to heat-shock protein 27 are associated with improved survival in patients with breast cancer. Br. J. Cancer. 1998;77:1875–1879. doi: 10.1038/bjc.1998.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaigorodova E.V., Zavyalova M.V., Bogatyuk M.V., Tarabanovskaya N.A., Slonimskaya E.M., Perelmuter V.M. Relationship between the expression of phosphorylated heat shock protein beta-1 with lymph node metastases of breast cancer. Cancer Biomark. 2015;15:143–150. doi: 10.3233/CBM-140446. [DOI] [PubMed] [Google Scholar]
- 27.Oshita S.E., Chen F., Kwan T., Yehiely F., Cryns V.L. The small heat shock protein HspB2 is a novel anti-apoptotic protein that inhibits apical caspase activation in the extrinsic apoptotic pathway. Breast Cancer Res. Treat. 2010;124:307–315. doi: 10.1007/s10549-010-0735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyano J.V., Evans J.R., Chen F., Lu M., Werner M.E., Yehiely F., Diaz L.K., Turbin D., Karaca G., Wiley E., et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J. Clin. Investig. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsang J.Y., Lai M.W., Wong K.H., Chan S.K., Lam C.C., Tsang A.K., Yu A.M., Tan P.H., Tse G.M. AlphaB-crystallin is a useful marker for triple negative and basal breast cancers. Histopathology. 2012;61:378–386. doi: 10.1111/j.1365-2559.2012.04234.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun X., Fontaine J.M., Bartl I., Behnam B., Welsh M.J., Benndorf R. Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor-positive breast cancer cells. Cell Stress Chaperones. 2007;12:307–319. doi: 10.1379/CSC-276.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tozawa-Ono A., Yoshida A., Yokomachi N., Handa R., Koizumi H., Kiguchi K., Ishizuka B., Suzuki N. Heat shock protein 27 and p16 immunohistochemistry in cervical intraepithelial neoplasia and squamous cell carcinoma. Hum. Cell. 2012;25:24–28. doi: 10.1007/s13577-011-0040-1. [DOI] [PubMed] [Google Scholar]
- 32.Yu Z., Zhi J., Peng X., Zhong X., Xu A. Clinical significance of HSP27 expression in colorectal cancer. Mol. Med. Rep. 2010;3:953–958. doi: 10.3892/mmr.2010.372. [DOI] [PubMed] [Google Scholar]
- 33.Li Q., Wang Y., Lai Y., Xu P., Yang Z. HspB5 correlates with poor prognosis in colorectal cancer and prompts epithelial-mesenchymal transition through ERK signaling. PLoS ONE. 2017;12:e0182588. doi: 10.1371/journal.pone.0182588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju Y.T., Kwag S.J., Park H.J., Jung E.J., Jeong C.Y., Jeong S.H., Lee Y.J., Choi S.K., Kang K.R., Hah Y.S., et al. Decreased expression of heat shock protein 20 in colorectal cancer and its implication in tumorigenesis. J. Cell. Biochem. 2015;116:277–286. doi: 10.1002/jcb.24966. [DOI] [PubMed] [Google Scholar]
- 35.de Wit N.J., Verschuure P., Kappé G., King S.M., de Jong W.W., van Muijen G.N.P., Boelens W.C. Testis-specific human small heat shock protein HSPB9 is a cancer/testis antigen, and potentially interacts with the dynein subunit TCTEL1. Eur. J. Cell Biol. 2004;83:337–345. doi: 10.1078/0171-9335-00396. [DOI] [PubMed] [Google Scholar]
- 36.Huang Q., Ye J., Huang Q., Chen W., Wang L., Lin W., Lin J., Lin X. Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin. Chem. Lab. Med. 2010;48:263–269. doi: 10.1515/CCLM.2010.043. [DOI] [PubMed] [Google Scholar]
- 37.Chen D., Cao G., Qiao C., Liu G., Zhou H., Liu Q. Alpha B-crystallin promotes the invasion and metastasis of gastric cancer via NF-κB-induced epithelial-mesenchymal transition. J. Cell. Mol. Med. 2018;22:3215–3222. doi: 10.1111/jcmm.13602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Li X.S., Xu Q., Fu X.Y., Luo W.S. Heat shock protein 22 overexpression is associated with the progression and prognosis in gastric cancer. J. Cancer Res. Clin. Oncol. 2014;140:1305–1313. doi: 10.1007/s00432-014-1698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaigorodova E.V., Zavyalova M.V., Bychkov V.A., Perelmuter V.M., Choynzonov E.L. Functional state of the Hsp27 chaperone as a molecular marker of an unfavorable course of larynx cancer. Cancer Biomark. 2016;17:145–153. doi: 10.3233/CBM-160625. [DOI] [PubMed] [Google Scholar]
- 40.Mao Y., Zhang D.W., Lin H., Xiong L., Liu Y., Li Q.D., Ma J., Cao Q., Chen R.J., Zhu J., et al. Alpha B-crystallin is a new prognostic markerfor laryngeal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2012;31:101. doi: 10.1186/1756-9966-31-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eto D., Hisaka T., Horiuchi H., Uchida S., Ishikawa H., Kawashima Y., Kinugasa T., Nakashima O., Yano H., Okuda K., et al. Expression of HSP27 in Hepatocellular Carcinoma. Anticancer Res. 2016;36:3775–3779. [PubMed] [Google Scholar]
- 42.Tang Q., Liu Y.F., Zhu X.J., Li Y.H., Zhu J., Zhang J.P., Feng Z.Q., Guan X.H. Expression and prognostic significance of the alpha B-crystallin gene in human hepatocellular carcinoma. Hum. Pathol. 2009;40:300–305. doi: 10.1016/j.humpath.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Sheng B., Qi C., Liu B., Lin Y., Fu T., Zeng Q. Increased HSP27 correlates with malignant biological behavior of non-small cell lung cancer and predicts patient’s survival. Sci. Rep. 2017;7:13807. doi: 10.1038/s41598-017-13956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherneva R., Petrov D., Georgiev O., Slavova Y., Toncheva D., Stamenova M., Trifonova N. Expression profile of the small heat-shock protein alpha-B-crystallin in operated-on non-small-cell lung cancer patients: Clinical implication. Eur. J. Cardio-Thaorac. Surg. 2010;37:44–50. doi: 10.1016/j.ejcts.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 45.Qin H., Ni Y., Tong J., Zhao J., Zhou X., Cai W., Liang J., Yao X. Elevated expression of CRYAB predicts unfavorable prognosis in non-small cell lung cancer. Med. Oncol. 2014;31:142. doi: 10.1007/s12032-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 46.Quan H.Z., Tang Z.G., Zhao L.L., Yao Z.G., Wang B.S., Xie S. Study of αB-crystallin and its possible role of anti-apoptosis in oral verrucous carcinoma. Shanghai Kou Qiang Yi Xue. 2012;21:432–436. [PubMed] [Google Scholar]
- 47.Arts H.J., Hollema H., Lemstra W., Willemse P.H., De Vries E.G., Kampinga H.H., Van der Zee A.G. Heat-shock-protein-27 (Hsp27) expression in ovarian carcinoma: Relation in response to chemotherapy and prognosis. Int. J. Cancer. 1999;84:234–238. doi: 10.1002/(SICI)1097-0215(19990621)84:3<234::AID-IJC6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki M., Matsushima-Nishiwaki R., Kuroyanagi G., Suzuki N., Takamatsu R., Furui T., Yoshimi N., Kozawa O., Morishige K.I. Regulation by heat shock protein 22 (HSPB8) of transforming growth factor-α-induced ovary cancer cell migration. Arch. Biochem. Biophys. 2015;571:40–49. doi: 10.1016/j.abb.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Deng M., Chen P.C., Xie S., Zhao J., Gong L., Liu J., Zhang L., Sun S., Liu J., Ma H., et al. The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim. Biophys. Acta. 2010;1802:621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Luo Z., Zhang L., Wang L., Nie Q., Wang Z.F., Huang Z., Hu X., Gong L., Arrigo A.P., et al. The small heat shock protein αA-crystallin negatively regulates pancreatic tumorigenesis. Oncotarget. 2016;7:65808–65824. doi: 10.18632/oncotarget.11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornford P.A., Dodson A.R., Parsons K.F., Desmond A.D., Woolfenden A., Fordham M., Neoptolemos J.P., Ke Y., Foster C.S. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 52.Wu P.S., Chang Y.H., Pan C.C. High expression of heat shock proteins and heat shock factor-1 distinguishes an aggressive subset of clear cell renal cell carcinoma. Histopathology. 2017;71:711–718. doi: 10.1111/his.13284. [DOI] [PubMed] [Google Scholar]
- 53.Ho P.Y., Chueh S.C., Chiou S.H., Wang S.M., Lin W.C., Lee I.L., Yang H.Y., Peng H.C., Lai M.K. αB-crystallin in clear cell renal cell carcinoma: Tumor progression and prognostic significance. Ulturol Oncol. 2013;31:1367–1377. doi: 10.1016/j.urolonc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Lin J., Deng Z., Tanikawa C., Shuin T., Miki T., Matsuda K., Nakamura Y. Downregulation of the tumor suppressor HSPB7, involved in the p53 pathway, in renal cell carcinoma by hypermethylation. Int. J. Oncol. 2014;44:1490–1498. doi: 10.3892/ijo.2014.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimenez M., Marie S.K., Oba-Shinjo S., Uno M., Izumi C., Oliveira J.B., Rosa J.C. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer. 2015;15:481. doi: 10.1186/s12885-015-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stegh A.H., Kesari S., Mahoney J.E., Jenq H.T., Forloney K.L., Protopopov A., Louis D.N., Chin L., DePinho R.A. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc. Natl. Acad. Sci. USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Q.M., Luo J., Wu K., Yin M., Gu Y.R., Cheng X.G. High level of αB-crystallin contributes to the progression of osteosarcoma. Oncotarget. 2016;7:9007–9016. doi: 10.18632/oncotarget.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pawłowski K.M., Majewska A., Szyszko K., Dolka I., Motyl T., Król M. Gene expression pattern in canine mammary osteosarcoma. Pol. J. Vet. Sci. 2011;14:11–20. doi: 10.2478/v10181-011-0002-2. [DOI] [PubMed] [Google Scholar]
- 59.Arneaud S.L., Douglas P.M. The stress response paradox: Fighting degeneration at the cost of cancer. FEBS J. 2016;283:4047–4055. doi: 10.1111/febs.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carra S., Alberti S., Benesch J.L.P., Boelens W., Buchner J., Carver J.A., Cecconi C., Ecroyd H., Gusev N., Hightower L.E., et al. Small heat shock proteins: Multifaceted proteins with important implications for life. Cell Stress Chaperones. 2019;24:295–308. doi: 10.1007/s12192-019-00979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piccolella M., Crippa V., Cristofani R., Rusmini P., Galbiati M., Cicardi M.E., Meroni M., Ferri N., Morelli F.F., Carra S., et al. The small heat shock protein B8 (HSPB8) modulates proliferation and migration of breast cancer cells. Oncotarget. 2017;8:10400–10415. doi: 10.18632/oncotarget.14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charpentier A.H., Bednarek A.K., Daniel R.L., Hawkins K.A., Laflin K.J., Gaddis S., MacLeod M.C., Aldaz C.M. Effects of estrogen on global gene expression: Identification of novel targets of estrogen action. Cancer Res. 2000;60:5977–5983. [PubMed] [Google Scholar]
- 63.Drabovich A.P., Pavlou M.P., Schiza C., Diamandis E.P. Dynamics of protein expression reveals primary targets and secondary messengers of estrogen receptor alpha signaling in MCF-7 breast cancer cells. Mol. Cell. Proteom. 2016;15:2093–2107. doi: 10.1074/mcp.M115.057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vydra N., Janus P., Toma-Jonik A., Stokowy T., Mrowiec K., Korfanty J., Długajczyk A., Wojtaś B., Gielniewski B., Widłak W. 17β-estradiol activates HSF1 via MAPK signaling in ERα-positive breast cancer cells. Cancers. 2019;11:1533. doi: 10.3390/cancers11101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hosseinpour S., Mashhadiabbas F., Ahsaie M.G. Diagnostic biomarkers in oral verrucous carcinoma: A systematic review. Pathol. Oncol. Res. 2017;23:19–32. doi: 10.1007/s12253-016-0150-x. [DOI] [PubMed] [Google Scholar]
- 66.Choi S.K., Kam H., Kim K.Y., Park S.I., Lee Y.S. Targeting heat shock protein 27 in cancer: A druggable target for cancer treatment? Cancers. 2019;11:1195. doi: 10.3390/cancers11081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Ommeren R., Staudt M.D., Xu H., Hebb M.O. Advances in HSP27 and HSP90-targeting strategies for glioblastoma. J. Neurooncol. 2016;127:209–219. doi: 10.1007/s11060-016-2070-8. [DOI] [PubMed] [Google Scholar]
- 68.Gober M.D., Smith C.C., Ueda K., Toretsky J.A., Aurelian L. Forced expression of the H11 heat shock protein can be regulated by DNA methylation and trigger apoptosis in human cells. J. Biol. Chem. 2003;278:37600–37609. doi: 10.1074/jbc.M303834200. [DOI] [PubMed] [Google Scholar]
- 69.Li F., Xiao H., Hu Z., Zhou F., Yang B. Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur. J. Cell Biol. 2018;97:216–229. doi: 10.1016/j.ejcb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Singh M.K., Sharma B., Tiwari P.K. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017;69:149–154. doi: 10.1016/j.jtherbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Kamradt M.C., Chen F., Cryns V.L. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 72.Konda J.D., Olivero M., Musiani D., Lamba S., Di Renzo M.F. Heat-shock protein 27 (HSP27, HSPB1) is synthetic lethal to cells with oncogenic activation of MET, EGFR and BRAF. Mol. Oncol. 2017;11:599–611. doi: 10.1002/1878-0261.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Launay N., Tarze A., Vicart P., Lilienbaum A. Serine 59 phosphorylation of {alpha}B-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells. J. Biol. Chem. 2010;285:37324–37332. doi: 10.1074/jbc.M110.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garrido C., Brunet M., Didelot C., Zermati Y., Schmitt E., Kroemer G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 75.Arrigo A.P., Simon S., Gibert B., Kretz-Remy C., Nivon M., Czekalla A., Guillet D., Moulin M., Diaz-Latoud C., Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 76.Garrido C., Bruey J.M., Fromentin A., Hammann A., Arrigo A.P., Solary E. HSP27 inhibits cytochromec-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 77.O’Callaghan-Sunol C., Gabai V.L., Sherman M.Y. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 78.Liu S., Yan B., Lai W., Chen L., Xiao D., Xi S., Jiang Y., Dong X., An J., Chen X., et al. As a novel p53 direct target, bidirectional gene HspB2/αB-crystallin regulates the ROS level and Warburg effect. Biochim. Biophys. Acta. 2014;1839:592–603. doi: 10.1016/j.bbagrm.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Kim E.H., Lee H.J., Lee D.H., Bae S., Soh J.W., Jeoung D., Kim J., Cho C.K., Lee Y.J., Lee Y.S. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res. 2007;67:6333–6341. doi: 10.1158/0008-5472.CAN-06-4344. [DOI] [PubMed] [Google Scholar]
- 80.Parcellier A., Schmitt E., Gurbuxani S., Seigneurin-Berny D., Pance A., Chantome A., Plenchette S., Khochbin S., Solary E., Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujita R., Ounzain S., Wang A.C., Heads R.J., Budhram-Mahadeo V.S. Hsp-27 induction requiRes. POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localisation following drug treatment. Cell Stress Chaperones. 2011;16:427–439. doi: 10.1007/s12192-011-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huot J., Houle F., Spitz D.R., Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 83.Garrido C., Ottavi P., Fromentin A., Hammann A., Arrigo A.P., Chauffert B., Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res. 1997;57:2661–2667. [PubMed] [Google Scholar]
- 84.Krishnan J., Lemmens R., Robberecht W., Van Den Bosch L. Role of heat shock response and Hsp27 in mutant SOD1-dependent cell death. Exp. Neurol. 2006;200:301–310. doi: 10.1016/j.expneurol.2006.02.135. [DOI] [PubMed] [Google Scholar]
- 85.Lee J., Lim K.T. Inhibitory effect of SJSZ glycoprotein (38 kDa) on expression of heat shock protein 27 and 70 in chromium (VI)-treated hepatocytes. Mol. Cell. Biochem. 2012;359:45–57. doi: 10.1007/s11010-011-0998-8. [DOI] [PubMed] [Google Scholar]
- 86.Lee J., Lim K.T. Activity of tumor necrosis factor-α blocked by phytoglycoprotein (38 kDa) at initiation stage in N-nitrosodiethylamine-induced ICR mice. Mol. Cell. Biochem. 2012;362:177–186. doi: 10.1007/s11010-011-1140-7. [DOI] [PubMed] [Google Scholar]
- 87.Chen H., Zheng C., Zhang Y., Chang Y.Z., Qian Z.M., Shen X. Heat shock protein 27 downregulates the transferrin receptor 1-mediated iron uptake. Int. J. Biochem. Cell Biol. 2006;38:1402–1416. doi: 10.1016/j.biocel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Préville X., Salvemini F., Giraud S., Chaufour S., Paul C., Stepien G., Ursini M.V., Arrigo A.P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999;247:61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto Y., Hosoda K., Imahori T., Tanaka J., Matsuo K., Nakai T., Irino Y., Shinohara M., Sato N., Sasayama T., et al. Pentose phosphate pathway activation via HSP27 phosphorylation by ATM kinase: A putative endogenous antioxidant defense mechanism during cerebral ischemia-reperfusion. Brain Res. 2018;1687:82–94. doi: 10.1016/j.brainres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Ryazantseva N.V., Stepovaya E.A., Nosareva O.L., Konovalova E.V., Orlov D.S., Naumova A.I., Didenko S.A., Vesnina O.N., Shakhristova E.V., Zima A.P., et al. Role of heat shock protein 27 in regulation of glutathione system and apoptosis of Jurkat tumor cells and blood lymphocytes. Bull. Exp. Biol. Med. 2015;158:377–379. doi: 10.1007/s10517-015-2766-3. [DOI] [PubMed] [Google Scholar]
- 91.Hudecova S., Markova J., Simko V., Csaderova L., Stracina T., Sirova M., Fojtu M., Svastova E., Gronesova P., Pastorek M., et al. Sulforaphane-induced apoptosis involves the type 1 IP3 receptor. Oncotarget. 2016;7:61403–61418. doi: 10.18632/oncotarget.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S., Yang S., Vlantis A.C., Liu S.Y., Ng E.K., Chan A.B., Wu J., Du J., Wei W., Liu X., et al. Expression of Antioxidant Molecules and Heat Shock Protein 27 in Thyroid Tumors. J. Cell. Biochem. 2016;117:2473–2481. doi: 10.1002/jcb.25539. [DOI] [PubMed] [Google Scholar]
- 93.Liang H.H., Huang C.Y., Chou C.W., Makondi P.T., Huang M.T., Wei P.L., Chang Y.J. Heat shock protein 27 influences the anti-cancer effect of curcumin in colon cancer cells through ROS production and autophagy activation. Life Sci. 2018;209:43–51. doi: 10.1016/j.lfs.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen Ho-Bouldoires T.H., Clapéron A., Mergey M., Wendum D., Desbois-Mouthon C., Tahraoui S., Fartoux L., Chettouh H., Merabtene F., Scatton O., et al. Mitogen-activated protein kinase-activated protein kinase 2 mediates resistance to hydrogen peroxide-induced oxidative stress in human hepatobiliary cancer cells. Free Radic. Biol. Med. 2015;89:34–46. doi: 10.1016/j.freeradbiomed.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 95.Kim J., Lim H., Kim S., Cho H., Kim Y., Li X., Choi H., Kim O. Effects of HSP27 downregulation on PDT resistance through PDT-induced autophagy in head and neck cancer cells. Oncol. Rep. 2016;35:2237–2245. doi: 10.3892/or.2016.4597. [DOI] [PubMed] [Google Scholar]
- 96.Lemieux P., Oesterreich S., Lawrence J.A., Steeg P.S., Hilsenbeck S.G., Harvey J.M., Fuqua S.A. The small heat shock protein hsp27 increases invasiveness but decreases motility of breast cancer cells. Invasion Metastasis. 1997;17:113–123. [PubMed] [Google Scholar]
- 97.Hansen R.K., Parra I., Hilsenbeck S.G., Himelstein B., Fuqua S.A. Hsp27-induced MMP-9 expression is influenced by the Src tyrosine protein kinase yes. Biochem. Biophys. Res. Commun. 2001;282:186–193. doi: 10.1006/bbrc.2001.4548. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y., Lu Z., Wang N., Feng J., Zhang J., Luan L., Zhao W., Zeng X. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med. 2018;50:1–17. doi: 10.1038/s12276-018-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Azad A.A., Zoubeidi A., Gleave M.E., Chi K.N. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat. Rev. Urol. 2015;12:26–36. doi: 10.1038/nrurol.2014.320. [DOI] [PubMed] [Google Scholar]
- 100.Pavan S., Musiani D., Torchiaro E., Migliardi G., Gai M., Di Cunto F., Erriquez J., Olivero M., Di Renzo M.F. HSP27 is required for invasion and metastasis triggered by hepatocyte growth factor. Int. J. Cancer. 2014;134:1289–1299. doi: 10.1002/ijc.28464. [DOI] [PubMed] [Google Scholar]
- 101.Ge H., Du J., Xu J., Meng X., Tian J., Yang J., Liang H. SUMOylation of HSP27 by small ubiquitin-like modifier 2/3 promotes proliferation and invasion of hepatocellular carcinoma cells. Cancer Biol. Ther. 2017;18:552–559. doi: 10.1080/15384047.2017.1345382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu Z., Xu X., Yu Y., Graham M., Prince M.E., Carey T.E., Sun D. Silencing heat shock protein 27 decreases metastatic behavior of human head and neck squamous cell cancer cells in vitro. Mol. Pharm. 2010;7:1283–1290. doi: 10.1021/mp100073s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi C., Yang X., Bu X., Hou N., Chen P. Alpha B-crystallin promotes the invasion and metastasis of colorectal cancer via epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017;489:369–374. doi: 10.1016/j.bbrc.2017.05.070. [DOI] [PubMed] [Google Scholar]
- 104.Hoter A., Naim H.Y. Heat shock proteins and ovarian cancer: Important roles and therapeutic opportunities. Cancers. 2019;11:1389. doi: 10.3390/cancers11091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao M., Ding J.X., Zeng K., Zhao J., Shen F., Yin Y.X., Chen Q. Heat shock protein 27: A potential biomarker of peritoneal metastasis in epithelial ovarian cancer? Tumour Biol. 2014;35:1051–1056. doi: 10.1007/s13277-013-1139-7. [DOI] [PubMed] [Google Scholar]
- 106.White N.M., Masui O., Desouza L.V., Krakovska O., Metias S., Romaschin A.D., Honey R.J., Stewart R., Pace K., Lee J., et al. Quantitative proteomic analysis reveals potential diagnostic markers and pathways involved in pathogenesis of renal cell carcinoma. Oncotarget. 2014;5:506–518. doi: 10.18632/oncotarget.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voduc K.D., Nielsen T.O., Perou C.M., Harrell J.C., Fan C., Kennecke H., Minn A.J., Cryns V.L., Cheang M.C.U. αB-crystallin Expression in Breast Cancer is Associated with Brain Metastasis. NPJ Breast Cancer. 2015;1:15014. doi: 10.1038/npjbcancer.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malin D., Strekalova E., Petrovic V., Deal A.M., Al Ahmad A., Adamo B., Miller C.R., Ugolkov A., Livasy C., Fritchie K., et al. αB-crystallin: A novel regulator of breast cancer metastasis to the brain. Clin. Cancer Res. 2014;20:56–67. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mineva I., Gartner W., Hauser P., Kainz A., Loffler M., Wolf G., Oberbauer R., Weissel M., Wagner L. Differential expression of alphaB-crystallin and Hsp27-1 in anaplastic thyroid carcinomas because of tumor-specific alphaB-crystallin gene (CRYAB) silencing. Cell Stress Chaperones. 2005;10:171–184. doi: 10.1379/CSC-107R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yilmaz M., Karatas O.F., Yuceturk B., Dag H., Yener M., Ozen M. Alpha-B-crystallin expression in human laryngeal squamous cell carcinoma tissues. Head Neck. 2015;37:1344–1348. doi: 10.1002/hed.23746. [DOI] [PubMed] [Google Scholar]
- 111.Campbell-Lloyd A.J., Mundy J., Deva R., Lampe G., Hawley C., Boyle G., Griffin R., Thompson C., Shah P. Is alpha-B crystallin an independent marker for prognosis in lung cancer? Heart Lung Circ. 2013;22:759–766. doi: 10.1016/j.hlc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Matsushima-Nishiwaki R., Toyoda H., Nagasawa T., Yasuda E., Chiba N., Okuda S., Maeda A., Kaneoka Y., Kumada T., Kozawa O. Phosphorylated heat shock protein 20 (HSPB6) regulates transforming growth factor-α-induced migration and invasion of hepatocellular carcinoma cells. PLoS ONE. 2016;11:e0151907. doi: 10.1371/journal.pone.0151907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ronca R., Benkheil M., Mitola S., Struyf S., Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev. 2017;37:1231–1274. doi: 10.1002/med.21452. [DOI] [PubMed] [Google Scholar]
- 114.Wartenberg M., Dönmez F., Ling F.C., Acker H., Hescheler J., Sauer H. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. FASEB J. 2001;15:995–1005. doi: 10.1096/fj.00-0350com. [DOI] [PubMed] [Google Scholar]
- 115.Dimberg A., Rylova S., Dieterich L.C., Olsson A.K., Schiller P., Wikner C., Bohman S., Botling J., Lukinius A., Wawrousek E.F., et al. alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood. 2008;111:2015–2023. doi: 10.1182/blood-2007-04-087841. [DOI] [PubMed] [Google Scholar]
- 116.Thuringer D., Jego G., Wettstein G., Terrier O., Cronier L., Yousfi N., Hébrard S., Bouchot A., Hazoumé A., Joly A.L., et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- 117.Straume O., Shimamura T., Lampa M.J., Carretero J., Øyan A.M., Jia D., Borgman C.L., Soucheray M., Downing S.R., Short S.M., et al. Suppression of heat shock protein 27 induces long-term dormancy in human breast cancer. Proc. Natl. Acad. Sci. USA. 2012;109:8699–8704. doi: 10.1073/pnas.1017909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen X., Dong X.S., Gao H.Y., Jiang Y.F., Jin Y.L., Chang Y.Y., Chen L.Y., Wang J.H. Suppression of HSP27 increases the anti-tumor effects of quercetin in human leukemia U937 cells. Mol. Med. Rep. 2016;13:689–696. doi: 10.3892/mmr.2015.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kase S., He S., Sonoda S., Kitamura M., Spee C., Wawrousek E., Ryan S.J., Kannan R., Hinton D.R. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keezer S.M., Ivie S.E., Krutzsch H.C., Tandle A., Libutti S.K., Roberts D.D. Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer Res. 2003;63:6405–6412. [PubMed] [Google Scholar]
- 121.Bielka H., Hoinkis G., Oesterreich S., Stahl J., Benndorf R. Induction of the small stress protein, hsp25, in Ehrlich ascites carcinoma cells by anticancer drugs. FEBS Lett. 1994;343:165–167. doi: 10.1016/0014-5793(94)80311-0. [DOI] [PubMed] [Google Scholar]
- 122.Gotthardt R., Neininger A., Gaestel M. The anti-cancer drug cisplatin induces H25 in Ehrlich ascites tumor cells by a mechanism different from transcriptional stimulation influencing predominantly H25 translation. Int. J. Cancer. 1996;66:790–795. doi: 10.1002/(SICI)1097-0215(19960611)66:6<790::AID-IJC14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 123.McCollum A.K., Teneyck C.J., Sauer B.M., Toft D.O., Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res. 2006;66:10967–10975. doi: 10.1158/0008-5472.CAN-06-1629. [DOI] [PubMed] [Google Scholar]
- 124.Lee C.H., Hong H.M., Chang Y.Y., Chang W.W. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie. 2012;94:1382–1389. doi: 10.1016/j.biochi.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 125.Yadav U.P., Singh T., Kumar P., Sharma P., Kaur H., Sharma S., Singh S., Kumar S., Mehta K. Metabolic adaptations in cancer stem cells. Front Oncol. 2020;10:1010. doi: 10.3389/fonc.2020.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saygin C., Matei D., Majeti R., Reizes O., Lathia J.D. Targeting cancer stemness in the clinic: From hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 127.Pützer B.M., Solanki M., Herchenröder O. Advances in cancer stem cell targeting: How to strike the evil at its root. Adv. Drug Deliv. Rev. 2017;120:89–107. doi: 10.1016/j.addr.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 128.Hsu H.S., Lin J.H., Huang W.C., Hsu T.W., Su K., Chiou S.H., Tsai Y.T., Hung S. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2010;117:1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- 129.Li G., Zhao F., Cui Y. Proteomics using mammospheres as a model system to identify proteins deregulated in breast cancer stem cells. Curr. Mol. Med. 2013;13:459–463. [PubMed] [Google Scholar]
- 130.Chen W., Ren X., Wu J., Gao X., Cen X., Wang S., Sheng S., Chen Q., Tang Y.J., Liang X.H., et al. HSP27 associates with epithelial–mesenchymal transition, stemness and radioresistance of salivary adenoid cystic carcinoma. J. Cell. Mol. Med. 2018;22:2283–2298. doi: 10.1111/jcmm.13510. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Deng K., Liu L., Tan X., Zhang Z., Li J., Ou Y., Wang X., Yang S., Xiang R., Sun P. WIP1 promotes cancer stem cell properties by inhibiting p38 MAPK in NSCLC. Signal Transduct. Target. Ther. 2020;5:36. doi: 10.1038/s41392-020-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu K.T., Wang B.Y., Chi W.Y., Chang-Chien J., Yang J.J., Lee H.T., Tzeng Y.M., Chang W.W. Ovatodiolide inhibits breast cancer stem/progenitor cells through SMURF2-mediated downregulation of Hsp27. Toxins. 2016;8:127. doi: 10.3390/toxins8050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei L., Liu T.T., Wang H.H., Hong H.M., Yu A.L., Feng H.P., Chang W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011;13:R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yasuda K., Hirohashi Y., Mariya T., Murai A., Tabuchi Y., Kuroda T., Kusumoto H., Takaya A., Yamamoto E., Kubo T., et al. Phosphorylation of HSF1 at serine 326 residue is related to the maintenance of gynecologic cancer stem cells through expression of HSP27. Oncotarget. 2017;8:31540–31553. doi: 10.18632/oncotarget.16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee C.H., Wu Y., Hsieh H.C., Yu Y., Yu A.L., Chang W.W. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie. 2014;104:117–126. doi: 10.1016/j.biochi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 136.Lin S.P., Lee Y.T., Yang S.H., Miller S.A., Chiou S.H., Hung M.C., Hung S. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013;328:226–234. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 137.Matchuk O.N., Zamulaeva I.A. High level of radiation-induced heat shock protein with a molecular weight of 27 and 70 kDa is the hallmark of radioresistant SP cells of MCF-7 breast cancer culture. Radiats. Biol. Radioecol. 2016;56:382–388. [PubMed] [Google Scholar]
- 138.Chen S.F., Nieh S., Jao S.W., Liu C.L., Wu C.H., Chang Y.C., Yang C.Y., Lin Y.S. Quercetin suppresses drug-resistant spheres via the p38 MAPK–Hsp27 apoptotic pathway in oral cancer cells. PLoS ONE. 2012;7:e49275. doi: 10.1371/journal.pone.0049275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C.L., Chen S.F., Wu M.Z., Jao S.W., Lin Y.S., Yang C.Y., Lee T.Y., Wen L.W., Lan G.L., Nieh S. The molecular and clinical verification of therapeutic resistance via the p38 MAPK–Hsp27 axis in lung cancer. Oncotarget. 2016;7:14279–14290. doi: 10.18632/oncotarget.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mu C., Wu X., Zhou X., Wolfram J., Shen J., Zhang D., Mai J., Xia X., Holder A.M., Ferrari M., et al. Chemotherapy sensitizes therapy-resistant cells to mild hyperthermia by suppressing heat shock protein 27 expression in triple-negative breast cancer. Clin. Cancer Res. 2018;24:4900–4912. doi: 10.1158/1078-0432.CCR-17-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu C.C., Chou K., Hsu J., Lin J., Hsu T., Yen D.H., Hung S., Hsu H. High metabolic rate and stem cell characteristics of esophageal cancer stem-like cells depend on the Hsp27–AKT–HK2 pathway. Int. J. Cancer. 2019;145:2144–2156. doi: 10.1002/ijc.32301. [DOI] [PubMed] [Google Scholar]
- 142.Naidu S.D., Dinkova-Kostova A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017;284:1606–1627. doi: 10.1111/febs.13999. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y., Qian J., Li X., Chen W., Xu A., Zhao K., Hua Y., Huang Z., Zhang J., Liang C., et al. Long noncoding RNA BX357664 regulates cell proliferation and epithelial-to-mesenchymal transition via inhibition of TGF-β1/p38/HSP27 signaling in renal cell carcinoma. Oncotarget. 2016;7:81410–81422. doi: 10.18632/oncotarget.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin S.P., Lee Y.T., Wang J.Y., Miller S.A., Chiou S.H., Hung M.C., Hung S.C. Survival of cancer stem cells under hypoxia and serum depletion via decrease in PP2A activity and activation of p38-MAPKAPK2-Hsp27. PLoS ONE. 2012;7:e49605. doi: 10.1371/journal.pone.0049605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fang Y., Wang J., Wang G., Zhou C., Wang P., Zhao S., Zhao S., Huang S., Su W., Jiang P., et al. Inactivation of p38 MAPK contributes to stem cell-like properties of non-small cell lung cancer. Oncotarget. 2017;8:26702–26717. doi: 10.18632/oncotarget.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiong J., Wang S., Chen T., Shu X., Mo X., Chang G., Chen J.J., Li C., Luo H., Lee J.D. Verteporfin blocks Clusterin which is required for survival of gastric cancer stem cell by modulating HSP90 function. Int. J. Biol. Sci. 2019;15:312–324. doi: 10.7150/ijbs.29135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kabakov A., Yakimova A., Matchuk O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells. 2020;9:892. doi: 10.3390/cells9040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fahrig R., Heinrich J.C., Nickel B., Wilfert F., Leisser C., Krupitza G., Praha C., Sonntag D., Fiedler B., Scherthan H., et al. Inhibition of induced chemoresistance by cotreatment with (E)-5-(2-bromovinyl)-2′-deoxyuridine (RP101) Cancer Res. 2003;63:5745–5753. [PubMed] [Google Scholar]
- 149.Heinrich J.C., Tuukkanen A., Schroeder M., Fahrig T., Fahrig R. RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J. Cancer Res. Clin. Oncol. 2011;137:1349–1361. doi: 10.1007/s00432-011-1005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fahrig R., Quietzsch D., Heinrich J.C., Heinemann V., Boeck S., Schmid R.M., Praha C., Liebert A., Sonntag D., Krupitza G., et al. RP101 improves the efficacy of chemotherapy in pancreas carcinoma cell lines and pancreatic cancer patients. Anticancer Drugs. 2006;17:1045–1056. doi: 10.1097/01.cad.0000231472.92406.d2. [DOI] [PubMed] [Google Scholar]
- 151.Hidalgo M. Phase II Study to Evaluate Efficacy and Safety of RP101 in Combination With Gemcitabine (RP101) [(accessed on 10 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00550004?term=NCT00550004.
- 152.Fahrig R., Kutz K., Heinrich J.C., Fahrig T., Koch A. 2nd Generation Product Candidates. [(accessed on 10 August 2020)]; Available online: https://resprotect.de/2nd-Generation-Product-Candidates/2nd-Generation-Product-Candidates.html.
- 153.Reyes-Farias M., Carrasco-Pozo C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019;20:3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vafadar A., Shabaninejad Z., Movahedpour A., Fallahi F., Taghavipour M., Ghasemi Y., Akbari M., Shafiee A., Hajighadimi S., Moradizarmehri S., et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell BioSci. 2020;10:32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Erdogan S., Turkekul K., Dibirdik I., Doganlar O., Doganlar Z.B., Bilir A., Oktem G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018;107:793–805. doi: 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 156.Wang R., Yang L., Li S., Ye D., Yang L., Liu Q., Zhao Z., Cai Q., Tan J., Li X. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), Mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM) Med. Sci. Monit. 2018;24:412–420. doi: 10.12659/MSM.908022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cao C., Sun L., Mo W., Sun L., Luo J., Yang Z., Ran Y. Quercetin mediates β-catenin in pancreatic cancer stem-like cells. Pancreas. 2015;44:1334–1339. doi: 10.1097/MPA.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 158.Staedler D., Idrizi E., Kenzaoui B.H., Juillerat-Jeanneret L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011;68:1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 159.Xavier C.P., Lima C.F., Rohde M., Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011;68:1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 160.Choi B., Choi S.K., Park Y.N., Kwak S.Y., Lee H.J., Kwon Y., Na Y., Lee Y.S. Sensitization of lung cancer cells by altered dimerization of HSP27. Oncotarget. 2017;8:105372–105382. doi: 10.18632/oncotarget.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Peng C.Y., Fong P.C., Yu C.C., Tsai W.C., Tzeng Y.M., Chang W.W. Methyl antcinate a suppresses the population of cancer stem-like cells in MCF7 human breast cancer cell line. Molecules. 2013;18:2539–2548. doi: 10.3390/molecules18032539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gibert B., Hadchity E., Czekalla A., Aloy M.T., Colas P., Rodriguez-Lafrasse C., Arrigo A.P., Diaz-Latoud C. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene. 2011;34:3672–3681. doi: 10.1038/onc.2011.73. [DOI] [PubMed] [Google Scholar]
- 163.Gibert B., Simon S., Dimitrova V., Diaz-Latoud C., Arrigo A.P. Peptide aptamers: Tools to negatively or positively modulate HSPB1(27) function. Philos. Trans. R. Soc. B. 2013;368:0120075. doi: 10.1098/rstb.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumano M., Furukawa J., Shiota M., Zardan A., Zhang F., Beraldi E., Wiedmann R.M., Fazli L., Zoubeidi A., Gleave M.E. Cotargeting stress-activated Hsp27 and autophagy as a combinatorial strategy to amplify endoplasmic reticular stress in prostate cancer. Mol. Cancer Ther. 2012;11:1661–1671. doi: 10.1158/1535-7163.MCT-12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lelj-Garolla B., Kumano M., Beraldi E., Nappi L., Rocchi P., Ionescu D.N., Fazli L., Zoubeidi A., Gleave M.E. Hsp27 Inhibition with OGX-427 sensitizes non-small cell lung cancer cells to erlotinib and chemotherapy. Mol. Cancer Ther. 2015;14:1107–1116. doi: 10.1158/1535-7163.MCT-14-0866. [DOI] [PubMed] [Google Scholar]
- 166.Chi K.N., Yu E.Y., Jacobs C., Bazov J., Kollmannsberger C., Higano C.S., Mukherjee S.D., Gleave M.E., Stewart P.S., Hotte S.J. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann. Oncol. 2016;27:1116–1122. doi: 10.1093/annonc/mdw068. [DOI] [PubMed] [Google Scholar]
- 167.Chi K.N., Hotte S.J., Ellard S., Gingerich J.R., Joshua A.M., Kollmannsberger C.K., Yu E.Y., Gleave M.E. A randomized phase II study of OGX-427 plus prednisone versus prednisone alone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2012;30:121. doi: 10.1200/jco.2012.30.5_suppl.121. [DOI] [Google Scholar]
- 168.Spigel D.R., Shipley D.L., Waterhouse D.M., Jones S.F., Ward P.J., Shih K.C., Hemphill B., McCleod M., Whorf R.C., Page R.D., et al. A randomized, double-blinded, phase II trial of carboplatin and pemetrexed with or without Apatorsen (OGX-427) in patients with previously untreated stage IV non-squamous-non-small-cell lung cancer: The SPRUCE Trial. Oncologist. 2019;24:e1409–e1416. doi: 10.1634/theoncologist.2018-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Choi S.H., Kwon I.C., Hwang K.Y., Kim I.S., Ahn H.J. Small heat shock protein as a multifunctional scaffold: Integrated tumor targeting and caspase imaging within a single cage. Biomacromolecules. 2011;12:3099–3106. doi: 10.1021/bm200743g. [DOI] [PubMed] [Google Scholar]
- 170.Kawano T., Murata M., Kang J.H., Piao J.S., Narahara S., Hyodo F., Hamano N., Guo J., Oguri S., Ohuchida K., et al. Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage-based targeted contrast agent. Biomaterials. 2018;152:37–46. doi: 10.1016/j.biomaterials.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 171.Toita R., Murata M., Abe K., Narahara S., Piao J.S., Kang J.H., Ohuchida K., Hashizume M. Biological evaluation of protein nanocapsules containing doxorubicin. Int. J. Nanomed. 2013;8:1989–1999. doi: 10.2147/IJN.S40239. [DOI] [PMC free article] [PubMed] [Google Scholar]