Abstract
Prognosis for patients with acute basilar artery occlusion (BAO) remains poor. Successful revascularization is a main predictor of favorable clinical outcomes after mechanical thrombectomy for BAO. However, even if mechanical thrombectomy is successful, some patients have a poor clinical outcome, including vegetative state and mortality. This study investigated the factors that are predictive of extremely poor clinical outcomes despite successful revascularization after mechanical thrombectomy for BAO.
We evaluated 35 consecutive patients who presented with acute ischemic stroke due to BAO and who were successfully treated with mechanical thrombectomy. A very poor outcome was defined as a modified Rankin Scale (mRS) score of 5 or 6 at 3 months after treatment. The associations between the clinical, imaging, procedural factors, and poor outcome were evaluated.
Using univariate analyses, there were significant differences in the preoperative National Institute of Health Stroke Scale (NIHSS) score (22.0 ± 9.0 vs. 30.5 ± 4.3, p <0.001), and infarct volume in brain stem (0.11 ± 0.19 cc vs. 2.55 ± 1.56 cc, p <0.001) between the control and very poor outcome groups. In receiver operating characteristic (ROC) curve analysis, the area under ROC curve of infarct volume in brain stem was 0.891 to predict very poor outcome.
Preoperative infarct volume in brain stem is strong predictor for very poor outcome. The infarct volume in brain stem is useful for deciding treatment indications.
Keywords: basilar artery occlusion, mechanical thrombectomy, very poor outcome
Introduction
Acute basilar artery occlusion (BAO) represents the most devastating type of ischemic stroke, which is associated with >90% mortality and high level of dependency among survivors without successful recanalization.1) In the modern stent retriever era, a series of randomized controlled trials demonstrating the effectiveness of mechanical thrombectomy for acute anterior circulation stroke have been reported.2–6) Patients with acute BAO were excluded from these clinical trials. Although a recent randomized controlled trial reported that there was no difference in favorable outcomes of patients receiving mechanical thrombectomy compared with those receiving standard medical therapy alone,7) mechanical thrombectomy is routinely performed because of that high recanalization rate. However, some patients have a very poor clinical outcome, that is to say, vegetative state and death, despite successful mechanical thrombectomy for BAO. Currently, the factors related to the very poor outcomes remain unknown after successful recanalization for BAO. Prevision of the very poor outcome before mechanical thrombectomy is greatly helpful for the surgeons and patients’ families to decide the required treatment. Therefore, this study aimed to investigate the predictors of very poor clinical outcomes despite successful revascularization after mechanical thrombectomy for acute BAO.
Patients and Methods
This retrospective study had been approved by Institutional Review Board of the hospital. We evaluated the data from 35 consecutive patients who presented with acute ischemic stroke due to BAOs and were successfully treated with mechanical thrombectomy at our hospital between July 2014 and November 2019. Indications for mechanical thrombectomy for acute basilar occlusion were the following: (1) acute occlusion of the basilar artery confirmed by magnetic resonance angiography (MRA), (2) the patients arrived at our hospital within 24 h of symptom onset, and (3) a National Institute of Health Stroke Scale (NIHSS) score of ≥6.
Clinical and neuroimaging characteristics, including patient age, sex, past history, NIHSS score on admission, stroke type, posterior circulation Acute Stroke Prognosis Early CT score (pc-ASPECTS),8) infarct volume in brain stem, and procedure details, were evaluated and analyzed. Stroke type was assessed according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification. Pc-ASPECTS, which measures the early ischemic changes in the posterior circulation, was evaluated in diffusion-weighted MRI (DWI) according to the method described by Tei et al.9) For measuring infarct volume in brain stem, the ABC/2 method was used, which was considered to be an effective method for the measurement of intraparenchymal hemorrhage and infarct volumes.10) In the ABC/2 method, the Synapse version 3.2.0 (FUJIFILM Medical System USA's Synapse PACS System, USA) was used. In the axial slices, A is defined as the largest diameter of the selected slice by human eye with largest infarct area and B is defined as the largest diameter perpendicular to the line above. Besides, C is the vertical diameter calculated by the number of slices. Finally, the following formula was used for calculation: 0.5 × A × B × C. The infarct area was defined as the high intensity area in DWI.
MR imaging was performed as soon as possible after admission using a 1.5-T scanner (Intera, Release 10; Philips Healthcare, Best, the Netherlands). The scanning sequences included MRA and (DWI). The scanning parameters were three-dimensional (3D) time of flight − MRA, time of repetition (TR)/echo time (TE) = 20 ms/26 ms, field of view (FOV) 200 mm × 200 mm, matrix 256 × 256, layer thickness = 1.2 mm; DWI (b = 0, 1000 s/mm2), single excitation spin echo, echo planar imaging (SEEPI) sequence, TR/TE = 6100 ms/86 ms, FOV 200 mm × 220 mm, matrix 128 × 128, layer thickness 2.5 mm.
Thrombolysis in cerebral infarction (TICI) scale was used to assess the revascularization status. Successful revascularization was defined as a modified TICI grade of 2b or 3.11) A symptomatic intracerebral hemorrhage (ICH) was defined as any ICH that caused neurological deterioration with an increase of ≥4 on the NIHSS.12) The clinical outcome was evaluated on the modified Rankin Scale (mRS) at 3 months after treatment. A very poor clinical outcome was defined as a mRS score of 5 or 6. Using a control group as the patients with mRS score ≤4, univariate analyses were performed.
Endovascular therapy was performed under local anesthesia in all patients. In principle, the target lesion is approached via a femoral artery approach. An 8Fr Roadmaster (Goodman, Aichi, Japan) was placed in the vertebral artery of either side. The techniques used for the subsequent mechanical thrombectomy were determined by each neurointerventionist (YT, MH, and KE). Two techniques were used, including the direct aspiration first-pass thrombectomy (ADAPT) technique with a 5MAX ACE reperfusion catheter (Penumbra, Oakland, CA, USA)13) and the stent-based thrombectomy technique with a Trevo Pro clot retriever (ProVue or XP; Stryker, Fremont, CA, USA). If thrombus removal was unsuccessful, both the Penumbra catheter and Trevo Pro clot retriever were used.
All neuroimaging data, including before, during, and after mechanical thrombectomy, were re-evaluated by a blinded neurosurgeon not knowing the clinical information.
Statistical Analysis
Statistical evaluations were performed using JMP 15.1.0 (SAS Institute Inc., NC, USA). For the univariate analyses, data were compared using Student’s t-tests for continuous variables or chi-squared tests for categorical variables. Receiver operating characteristic (ROC) curve analysis was drawn by plotting sensitivity against 1-specificity of optimal cutoff point of the pc-ASPECTS and the infarct volume in brain stem for predicting clinical outcome. Area under the ROC curve (AUC) was analyzed using the method described by DeLong et al.14) Statistical significance was set at P <0.05.
Results
Baseline patient characteristics
In all, 35 consecutive patients (26 men, 9 women; mean age 75.9 ± 10.6 years) with acute BAO were enrolled in the study. Of the 35 included patients, 19 patients (54.3%) had hypertension and 7 (20.0%) had diabetes mellitus. The mean NIHSS score at presentation was 25.1 (range, 8–34). The mean value of pc-ASPECTS was 7.1 (range, 4–10) and the mean infarct volume in the brain stem was 1.06 cc (range, 0–5.271). The mean time from symptom onset to femoral artery puncture and recanalization was 269 ± 170 min and 324 ± 168 min, respectively. The detailed baseline and clinical characteristics of the patients are shown in Table 1.
Table 1. Baseline characteristics of patients who underwent mechanical thrombectomy for acute basilar artery occlusion.
| All (n = 35) |
Control (n = 22) |
Very poor (n = 13) |
p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean ± SD | 75.9 ± 10.6 | 77.9 ± 10.5 | 72.7 ± 10.0 | 0.172 |
| Sex, male | 26 (74.3%) | 16 (72.7%) | 10 (76.9%) | 0.900 |
| Vascular risk factors | ||||
| Hypertension | 19 (54.3%) | 13 (59.1%) | 6 (46.2%) | 0.696 |
| Diabetes | 7 (20.0%) | 5 (22.7%) | 2 (15.4%) | 0.930 |
| Dyslipidemia | 6 (17.1%) | 5 (17.4%) | 1 (7.7%) | 0.499 |
| Smoking | 11 (31.4%) | 8 (36.4%) | 3 (23.1%) | 0.659 |
| Clinical presentation | ||||
| Preoperative NIHSS score, mean ± SD | 25.1± 8.6 | 22.0 ± 9.0 | 30.5 ± 4.3 | <0.001 |
| Pc-ASPECTS, mean ± SD | 7.1 ± 1.2 | 7.3 ± 1.3 | 6.8 ± 0.8 | 0.071 |
| Infarct volume in brain stem (cc), mean ± SD | 1.02 ± 1.52 | 0.11 ± 0.19 | 2.55 ± 1.56 | <0.001 |
| Etiology | ||||
| Cardioembolism | 21 (34.8%) | 15 (68.2%) | 6 (46.2%) | 0.353 |
| Large-artery atherosclerosis | 13 (59.4%) | 7 (31.8%) | 6 (46.2%) | |
| Others | 1 (2.8%) | 0 | 1 (7.7%) | |
Pc-ASPECTS: posterior circulation of Alberta Stroke Program Early Computed Tomography Score, NIHSS: National Institutes of Health Stroke Scale, SD: standard deviation.
Procedural results
Detailed procedural results are shown in Table 2. In 8 of the 35 patients (22.9%), tissue plasminogen activator was intravenously injected. Only three patients (8.6%) were treated with a combination of the ADAPT and stent retriever techniques. In 15 patients (42.9%), a TICI 3 classification was achieved. Post-treatment CT images revealed ICHs in six patients (17.1%), whereas three patients (8.6%) suffered from symptomatic ICHs.
Table 2. Angiographic and clinical outcomes of patients who underwent mechanical thrombectomy for acute basilar artery occlusion in our hospital.
| All (n = 35) |
Control (n = 22) |
Very poor (n = 13) |
p value | |
|---|---|---|---|---|
| IV-tPA | 8 (22.9%) | 6 (27.3%) | 2 (15.4%) | 0.695 |
| Time course | ||||
| Time from onset to puncture, (min, mean ± SD) | 269 ± 170 | 243 ± 128 | 300 ± 205 | 0.137 |
| Time from puncture to revascularization (min, mean ± SD) | 56 ± 45 | 58 ± 46 | 51 ± 42 | 0.659 |
| Time from onset to revascularization (min, mean ± SD) | 324 ± 168 | 301 ± 126 | 351 ± 179 | 0.198 |
| Device | ||||
| Penumbra reperfusion system only | 23 (65.7%) | 14 (63.7%) | 9 (69.6%) | 0.499 |
| Stent retriever only | 9 (25.7%) | 7 (31.8%) | 2 (43.5%) | 0.742 |
| Multiple devices | 3 (8.6%) | 1 (4.5%) | 2 (15.4%) | 0.630 |
| Angiographic outcome | ||||
| TICI 3 | 15 (42.9%) | 12 (54.5%) | 3 (23.1%) | 0.143 |
| Complication | ||||
| Symptomatic ICH | 3 (8.6%) | 1 (4.5%) | 2 (15.4%) | 0.630 |
ICH: intracerebral hemorrhage, IV-tPA: intravenous tissue-type plasminogen activator, SD: standard deviation, TICI: thrombolysis in cerebral infarction.
Clinical outcomes
At 3 months after treatment, 13 patients (37.1%) were functionally independent (mRS score of 0–2), 7 (20.0%) had moderate disability (mRS score of 3), 2 (5.7%) were severely disabled (mRS score of 4), 8 (22.9%) were in a vegetative state, and 5 (14.3%) were deceased. On the basis of these results, these 13 (37.1%) patients were placed in the very poor outcome group.
Prognostic factors
As shown in Tables 1 and 2, the univariate analyses revealed significant differences in the preoperative NIHSS score (22.0 ± 9.0 vs. 30.5 ± 4.3, p <0.001), and infarct volume in brain stem (0.11 ± 0.19 cc vs. 2.55 ± 1.56 cc, p <0.001) between the control and very poor outcome groups. There was no significant difference in pc-ASPECTS between the two groups (7.3 ± 1.3 vs 6.8 ± 0.8, p = 0.073). The AUC, which approximated the chance that the infarct volume in brain stem correctly predicted a very poor outcome, was 0.89. According to the ROC, the optimal cutoff value to predict a very poor outcome was 0.7 cc for the infarct volume in brain stem (sensitivity = 84.6, specificity = 100).
Discussion
The result of this study showed that the infarct volume in brain stem is a predictor for the very poor clinical outcome of patients with acute BAO. The volume of the brain stem is small, whereas its function is very strong. Most importantly, all patients whose infarct volume in brain stem was >0.7 cc were in the very poor outcome group. The above volume indicates that the diameter is approximately 1.12 cm if the infarct is a round circle.
The brain stem can be divided into the medulla, pons, and the midbrain. It is an important pathway of the brain, cerebellum, and spinal cord; therefore, a small infarction can lead to very serious clinical consequences. Several studies evaluated the patient’s prognosis in the acute BAO.15–17) With regard to imaging studies, whether pc-ASPECTS on DWI is a predictor of clinical outcome after mechanical thrombectomy to BAO or not is controversial. Mourand et al. reported pc-ASPECTS score ≥7 was not statistically relevant for predicting good outcome.18) Besides, Yang et al.19) reported that a pc-ASPECTS score ≥6 did not predict favorable outcome. In contrast, Yoon et al.20) reported that pc-ASPECTS was an independent predictor of clinical outcome of acute BAO. In our study, pc-ASPECTS did not differ statistically between the very poor outcome and control groups. Although the difference of borderline of mRS was contributed to this consistency; the validation method of pc-ASPECTS itself is flawed. Pc-ASPECTS allots posterior circulation 10 points.8) One point each is subtracted for hypoattenuation in the left or right thalamus, cerebellum, or posterior cerebral artery territory, respectively, and two points each are subtracted for hypoattenuation in any part of the midbrain or pons.19) That is to say, the infarct volume in brain stem does not directly contribute to pc-ASPECTS. For instance, as shown in Fig. 1, the score of pc-ASPECTS was 8, although, almost all part of pons suffered infarction. On the other hand, the score of pc-ASPECTS of patients in Fig. 2 was 7 although small part of pons and left cerebellum suffered infarction. The cerebellum, thalamus, and temporo-occipital lobe lesions are less involved in functional prognoses than those in the brain stem. Mourand et al.18) reported that the brain stem score was an independent predictor of clinical outcome after mechanical thrombectomy for BAO, which is a semiquantitative DWI score of the brain stem. They also emphasized the importance of brain stem lesions in acute BAO, which was consistent with this study. Calculation of the infarct volume in the brain stem may help to identify patients who could benefit from recanalization therapy. As the measuring method is remarkably simple, it is possible to calculate the volume of a lesion in brain stem in an emergency.
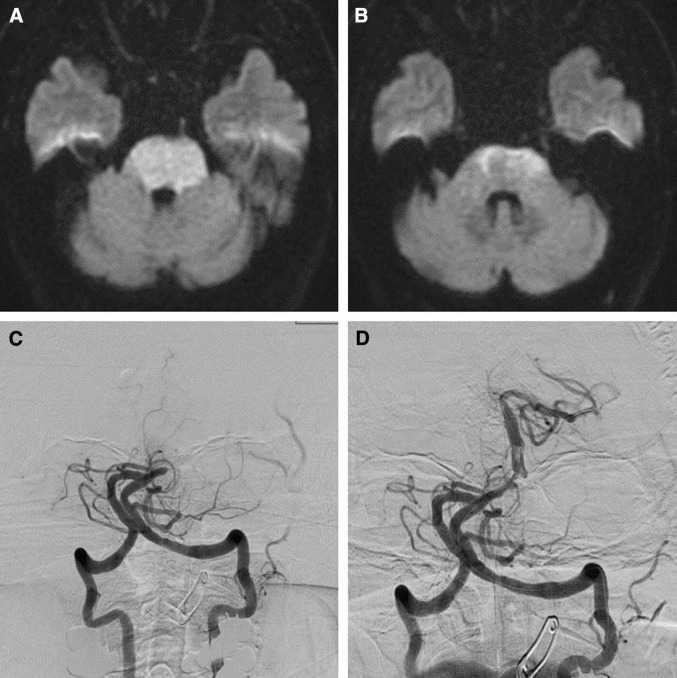
Fig. 1.
Prethrombectomy DWI (A and B) of a 72-year-old man with tetraplegia in a coma. The preoperative NIHSS score was 32. The DWI shows extensive bilateral lesions in the pons. The posterior circulation Acute Stroke Prognosis Early CT score was 8 and the infarct volume in the brain stem was 5.271 cc. DSA shows a mid-basilar occlusion (C) successfully recanalized after mechanical thrombectomy (TICI 2b). Time from symptom onset to recanalization was 236 min. The mRS was 5 at 3 months after mechanical thrombectomy. DSA: digital subtraction angiography, DWI: diffusion-weighed imaging, mRS: modified Rankin Scale, NIHSS: National Institute of Health Stroke Scale, TICI: thrombolysis in cerebral infarction.
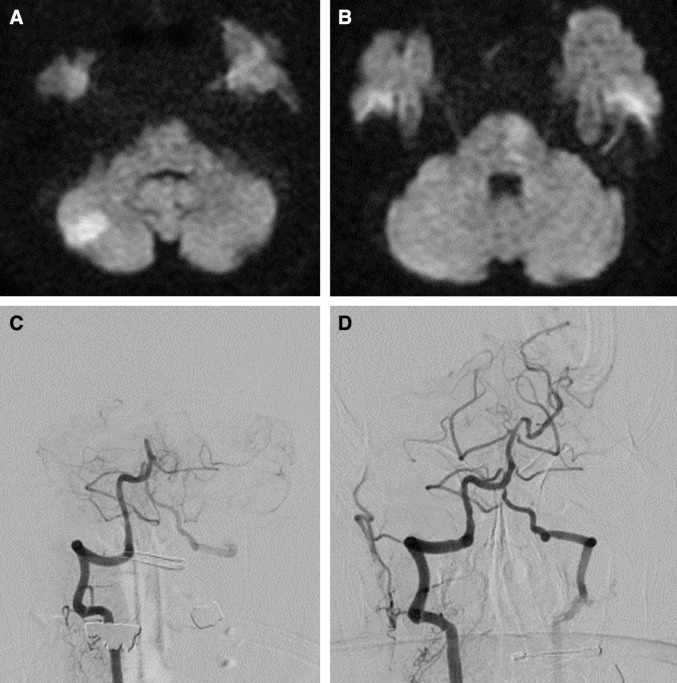
Fig. 2.
Prethrombectomy DWI (A and B) of an 88-year-old man with tetraplegia in a coma. The preoperative NIHSS score was 31. The DWI shows unilateral lesions in the left pons and the right cerebellum. The posterior circulation Acute Stroke Prognosis Early CT score was 7 and the infarct volume in brain stem was 0.23 cc. DSA shows a mid-basilar occlusion (C) successfully recanalized after mechanical thrombectomy (TICI 3). Time from symptom onset to recanalization was 132 minutes. The mRS was 1 at 3 months after mechanical thrombectomy. DSA: digital subtraction angiography, DWI: diffusion-weighed imaging, mRS: modified Rankin Scale, NIHSS: National Institute of Health Stroke Scale, TICI: thrombolysis in cerebral infarction.
As mentioned above, acute BAO is associated with >90% mortality and high level of dependency among survivors without successful recanalization. Given such a devastating natural course, a certain level of permanent damage including hemiparesis and aphsia (mRS 3 or 4) might be acceptable after treatment for BAO. However, the patients with mRS 5 always need care and most of them also have communication difficulty. The patients’ family might not desire that outcome. Given the fact that all the patients whose infarct volume in brain stem was >0.7 cc fell in the very poor outcome group; patients in this group should not be considered for mechanical thrombectomy. Therefore, calculating the infarct volume in brain stem and not the pc-ASPECTS score is highly useful for deciding whether mechanical thrombectomy should be performed or not.
This study has some limitations. First, it was only performed at a single institution and the sample size was small. Second, as mentioned above, this was a retrospective and noncontrolled study with a bias in patient selection. Lastly, we used the ABC/2 method for measuring infarct volume in brain stem. Although this method has the advantage on easily and quickly, the work-out volume is not real value. Further prospective studies using the software that can calculate infarct volume automatically will be required to determine the cutoff infarct volume in brain stem.
Conclusions
Our findings show that infarct volume in brain stem is useful in predicting very poor outcome in acute BAO treated by mechanical thrombectomy and may simplify the decision-making algorithm. Patients with a high infarct volume in the brain stem are extremely likely to show very poor outcome after mechanical thrombectomy, whereas the pc-ASPECTS score is high. Further studies are required with a large number case series and randomized clinical trial using the software that can calculate infarct volume automatically validate whether infarct volume in brain stem is a reliable predictor for clinical outcome.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflicts of interest to declare.
References
- 1).Brandt T, von Kummer R, Müller-Küppers M, Hacke W: Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke 27: 875–881, 1996 [DOI] [PubMed] [Google Scholar]
- 2).Berkhemer OA, Fransen PS, Beumer D, et al. : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11–20, 2015 [DOI] [PubMed] [Google Scholar]
- 3).Goyal M, Demchuk AM, Menon BK, et al. : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019–1030, 2015 [DOI] [PubMed] [Google Scholar]
- 4).Saver JL, Goyal M, Bonafe A, et al. : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285–2295, 2015 [DOI] [PubMed] [Google Scholar]
- 5).Campbell BC, Mitchell PJ, Kleinig TJ, et al. : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009–1018, 2015 [DOI] [PubMed] [Google Scholar]
- 6).Jovin TG, Chamorro A, Cobo E, et al. : Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296–2306, 2015 [DOI] [PubMed] [Google Scholar]
- 7).Liu X, Dai Q, Ye R: Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 19: 115–122, 2020 [DOI] [PubMed] [Google Scholar]
- 8).Puetz V, Sylaja PN, Coutts SB, et al. : Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 39: 2485–2490, 2008 [DOI] [PubMed] [Google Scholar]
- 9).Tei H, Uchiyama S, Usui T, Ohara K: Posterior circulation ASPECTS on diffusion-weighted MRI can be a powerful marker for predicting functional outcome. J Neurol 257: 767–773, 2010 [DOI] [PubMed] [Google Scholar]
- 10).Kothari RU, Brott T, Broderick JP, et al. : The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27: 1304–1305, 1996 [DOI] [PubMed] [Google Scholar]
- 11).Zaidat OO, Yoo AJ, Khatri P, et al. : Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44: 2650–2663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Trouillas P, von Kummer R: Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke 37: 556–561, 2016 [DOI] [PubMed] [Google Scholar]
- 13).Turk AS, Spiotta A, Frei D, et al. : Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 6: 231–237, 2011 [DOI] [PubMed] [Google Scholar]
- 14).DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 15).Vergouwen MD, Algra A, Pfefferkorn T, et al. : Time is brain(stem) in basilar artery occlusion. Stroke 43: 3003–3006, 2012 [DOI] [PubMed] [Google Scholar]
- 16).Carneiro AA, Rodrigues JT, Pereira JP, Alves JV, Xavier JA: Mechanical thrombectomy in patients with acute basilar occlusion using stent retrievers. Interv Neuroradiol 21: 710–714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Gory B, Mazighi M, Labreuche J, et al. : Predictors for mortality after mechanical thrombectomy of acute basilar artery occlusion. Cerebrovasc Dis 45: 61–67, 2018 [DOI] [PubMed] [Google Scholar]
- 18).Mourand I, Machi P, Nogué E, et al. : Diffusion-weighted imaging score of the brain stem: a predictor of outcome in acute basilar artery occlusion treated with the Solitaire FR device. AJNR Am J Neuroradiol 35: 1117–1123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Yang H, Ma N, Liu L, et al. : Early diffusion-weighted imaging brain stem score for acute basilar artery occlusion treated with mechanical thrombectomy. J Stroke Cerebrovasc Dis 27: 2822–2828, 2018 [DOI] [PubMed] [Google Scholar]
- 20).Yoon W, Kim SK, Heo TW, Baek BH, Lee YY, Kang HK: Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 46: 2972–2975, 2015 [DOI] [PubMed] [Google Scholar]