Abstract
Postembolization syndrome (PES) is the most common side effect of vascular embolization of solid organs. The aim of this review was to determine the incidence of PES and its individual components after prostatic artery embolization (PAE). A systematic review with a pre-specified search strategy for PubMed, Embase, Web of Science and Cochrane Library was performed according to PRISMA guidelines. Studies in English regarding PAE in humans with 10 or more participants were eligible for inclusion. No restrictions on participant demographics or PAE technique were imposed. The search returned 378 references, of which 32 studies with a total of 2116 patients met the inclusion criteria. The results for overall PES frequency and individual PES components were presented as median (interquartile range, (IQR)). Overall median PES frequency was 25.5% (12.5–45.8). The two most frequent individual PES components were dysuria/urethral burning and local pain, with a median frequency of 21.7% (13.8–33.3) and 20% (5.4–29.4), respectively. Most outcome measures were characterized by a marked lack of uniformity and inconsistency in reporting across studies. Development of a uniform reporting system would help the clinicians recognize and treat PES accordingly.
Keywords: prostatic artery embolization, benign prostatic hyperplasia, postembolization syndrome
1. Introduction
Benign prostatic hyperplasia (BPH) is a frequent cause of lower urinary tract symptoms (LUTS) in men [1,2], with one fourth of men older than 70 years having moderate to severe LUTS that impair their quality of life (QOL) [3]. Prostatic artery embolization (PAE) is a new minimally invasive technique proven effective in reducing LUTS in BPH comparable to the preferred surgical treatment—the transurethral resection of the prostate (TURP) [4,5,6,7]. The most common side effect of vascular embolization of solid organs is a collection of inflammation- and tissue necrosis-related symptoms known as the postembolization syndrome (PES) [8,9,10]. The syndrome is characterized by influenza-like symptoms, pain and nausea and, in the case of PAE, dysuria and transient worsening of LUTS. Leukocytosis, leukopenia and/or elevation of C-reactive protein are also commonly seen [11]. The symptoms vary in their severity and duration and can, if pronounced, be mistaken for urosepsis. Consequently, a subset of patients may need admission to hospital for observation and symptomatic treatment, increasing the overall procedural costs. No uniform system for reporting PES exists, making its incidence fluctuate widely between studies. Moreover, trials investigating postoperative management plans or drugs to reduce PES do not exist, and PES is currently treated symptomatically with a combination of analgesics, antipyretics and antiemetics. No dedicated systematic reviews examining all components of PES after PAE have been published to date. Thus, there is a lack of deeper insight into incidence, grade and future management of PES after PAE. The aim of this study was to determine the incidence of PES and its components after PAE and subsequently assist the clinicians in correctly recognizing and treating the syndrome.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Review and Metanalysis (PRISMA) guidelines [12], and a published protocol with pre-specified inclusion criteria, outcomes and search strategy can be found in the PROSPERO database (PROSPERO ID: CRD42020164472) [13].
2.2. Information Sources and Search Strategy
PubMed, Embase, Web of Science and Cochrane Library were searched. The following search terms were applied: benign AND prostat* AND (hyperplasia OR hypertrophy OR enlargement OR obstruction) AND emboli?ation AND ALL= (side?effect* OR complication* OR adverse effect*). MeSH terms used were “Embolization, Therapeutic” and “Prostatic Hyperplasia”. The search terms were combined and conducted in appropriate combinations on 16 January 2020. A new search conducted on 1 June 2020 returned no new studies eligible for inclusion.
2.3. Eligibility Criteria and Study Selection
Studies regarding PAE in humans with 10 or more subjects were eligible for inclusion. Reviews, case reports, abstracts, supplements and conference papers as well as articles not published in English were excluded. No restrictions on publication dates were imposed. Two authors (P.S. and M.T.) reviewed abstracts. Full text of all included articles was obtained and read by the same two authors. Agreement was reached through consensus using Covidence Systematic Review software (Veritas Health Innovation, Melbourne, Australia) [14]. First author, publication year, study location, data collection period, study design, number of patients and outcome measures for all included articles were collected. Outcome measures were extracted in duplicate in a piloted data-extraction form.
2.4. Outcome Measures
The primary outcome measure was the overall percentage of PES in studies selected for the review. The secondary outcome measures were the overall percentages of each individual PES component. In the context of this review, PES was defined as one or more of the following components: fever, local (perineal, retroperitoneal, pelvic, perianal, urethral or retropubic) pain, nausea with or without vomiting, dysuria/urethral burning and transient worsening of LUTS. If an article reported separately more than one of the above PES components, and it was unclear if a single patient experienced more than one symptom, the component with the highest reported percentage was taken to represent the overall PES percentage in the study. In articles not reporting one or more of the above outcomes, that outcome is presumed not to have been recorded and not as having not occurred.
2.5. Risk of Bias
Risk of bias in randomized trials (RCTs) was assessed using the Cochrane Risk of Bias tool (RoB 2.0) [15]. Non-randomized trials were assessed for risk of bias using the Risk of Bias In Non-randomized Studies-of Interventions (ROBINS-I) tool [16]. Robvis online visualization tool was used to graphically present the risk of bias data [17].
2.6. Statistical Considerations
Due to study heterogeneity meta-analysis was not possible. The outcomes are presented as median (interquartile range, (IQR)).
3. Results
3.1. Study Selection and Overview
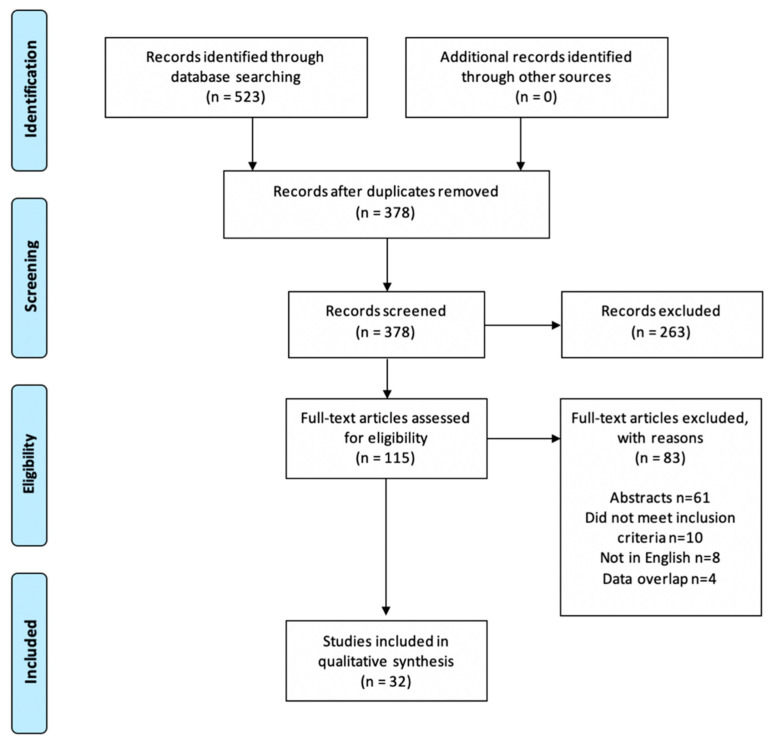
The database search returned 378 references with duplicates removed. A total of 263 articles were removed after reading the abstract. Of the remaining 115 studies assessed for full-text eligibility, 32 studies with a total of 2116 patients (ranging from 11–199) were selected for data extraction [5,6,7,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. A PRISMA flow diagram depicts the process of study selection (Figure 1).
Figure 1.
PRISMA flow diagram.
Seven of the included studies were RCTs [5,6,7,22,23,24,25], as presented in Table 1. Study characteristics of prospective and retrospective studies are presented in Table 2 and Table 3, respectively.
Table 1.
Study characteristics of randomized trials (RCTs).
| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention Group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Abt (2018) [7] | open-label RCT | Feb 2014–May 2017 | Switzerland | 48 | 65.7 | PAE with 250–400 µm Embozene® | TURP |
| Bilhim (2013) [22] | single-blind RCT | May 2011–Dec 2011 | Portugal | 80 | 63.9 | PAE with 80–180 µm or 180–300 µm particles | |
| Bilhim (2019) [23] | single-blind RCT | Nov 2017–Nov 2018 | Portugal | 84 | 67.3 cPAE; 65.8 bPAE | cPAE, bPAE (both with with 300–500 µm Embosphere®) | |
| Carnevale (2016) [6] | open-label RCT | Nov 2010–Dec 2012 | Brazil | 15 | 60.4 | PAE PErFecTED with 300–500 µm Embosphere® | original PAE and TURP |
| Gao (2014) [5] | open-label RCT | Jan 2007–Jan 2012 | China | 54 | 67.7 | PAE with 355–500 µm Ivalon® | TURP |
| Torres (2019) [24] | open-label RCT | Jul 2015–Dec 2016 | Portugal | 137 | 66.1 | PAE (3 groups: 100–300 µm, 300–500 µm, and 100–300 followed by 300–500 µm microspheres) | |
| Wang (2018) [25] | double-blind RCT | Jan 2010–Oct 2015 | China | 110 | 69.5 | PAE (2 groups: 50 µm followed by 100 µm and 100 µm spheres alone) |
bPAE, balloon-occlusion prostatic artery embolization; cPAE, conventional microcatheter prostatic artery embolization; PAE, prostatic artery embolization; PErFecTED, proximal embolization first then embolize distant; TURP, transurethral resection of the prostate.
Table 2.
Study characteristics of prospective studies.
| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention Group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Bagla (2014) [27] | prospective | Jan 2012–Mar 2013 | United States | 19 | 66.5 | PAE with 100–400 µm Embozene® | |
| Bilhim (2013) [29] | prospective | Mar 2009–Dec 2011 | Portugal | 122 | 65.8 bilateral PAE; 71.3 unilateral PAE | PAE with 100- and 200 µm particle sizes, unilateral vs. bilateral | |
| Brown (2018) [30] | prospective | Nov 2015–Feb 2017 | Australia | 51 | 67 | PAE with 250 µm Embozene® | |
| Carnevale (2013) [31] | prospective | Jun 2008–Nov 2011 | Brazil | 11 | 68.5 | PAE with 300–500 µm Embosphere® | |
| Franiel (2018) [32] | prospective | Jul 2014–Dec 2015 | Germany | 27 | 66 | PAE with 250 µm Embozene® | |
| Goncalves (2016) [33] | prospective | Aug 2011–Jun 2013 | Brazil | 30 | not mentioned | PAE with 100–300 or 300–500 µm Embosphere® | |
| Kenny (2019) [34] | prospective | Not mentioned | France | 20 | 75.3 | PAE with 300–500 µm Bead Block® in patients with indwelling catheters | |
| Kløw (2018) [35] | prospective | Dec 2015–Mar 2017 | Norway | 29 | 69 | PAE with 300–500 µm Embosphere® | |
| Kurbatov (2014) [18] | prospective | Jan 2009–Jan 2012 | Russia and Italy | 88 | 66.4 | PAE with 300–500 µm Embosphere® in prostates >80 cm3 | |
| Lindgren (2019) [36] | prospective | Jan 2015–Jun 2018 | Sweden | 37 | 73 | PAE with 300–500 µm Embosphere® | |
| Malling (2019) [21] | prospective | Jul 2017–Jul 2018 | Denmark | 11 | 75.2 | PAE PErFecTED with 300–500 µm Embosphere® | |
| Rampoldi (2017) [19] | prospective | Not mentioned | Italy | 41 | 77.9 | PAE PErFecTED with 300–500 µm Embosphere® in patients with indwelling catheters | Indwelling urinary catheter |
| Ray (2018) [39] | prospective | Jul 2014–Jan 2016 | United Kingdom | 199 | 66 | PAE | TURP |
| Russo (2015) [40] | prospective matched pair | Jan 2006–Jan 2014 | Italy | 80 | 67 | PAE with 300–500 µm Embosphere® | open prostatectomy |
| Salem (2018) [41] | prospective | Dec 2014–Jun 2017 | United States | 45 | 67 | PAE with 300–500 µm Embosphere® | |
| Wang (2016) [43] | prospective | Apr 2010–Dec 2013 | China | 115 | 72.5 (>80 cm3); 66 (50–80 cm3) | PAE with 100 µm particles in prostates >80 cm3 and 50–80 cm3 | |
| Wang (2016) [44] | prospective | Feb 2009–Apr 2014 | China | 158 | 82.5 (>75 yrs), 67.5 (<75 yrs) | PAE with 100 µm particles in men >75 years and <75 years | |
| Yu (2016) [45] | prospective | Jun 2015–Mar 2016 | Hong Kong SAR | 16 | 66 | PAE with 100–300 µm Embosphere® in patients with BPH and acute urinary retention | PAE with 100–300 µm Embosphere® n patients with BPH without urinary retention |
| Yu (2019) [46] | prospective | Jun 2015–Dec 2018 | Hong Kong SAR | 82 | 66 | PAE with 100–300 µm Embosphere® |
BPH, benign prostatic hyperplasia; PAE, prostatic artery embolization; PErFecTED, proximal embolization first then embolize distant; TURP, transurethral resection of the prostate.
Table 3.
Study characteristics of retrospective studies.
| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Amouyal (2016) [20] | retrospective | Dec 2013–Jan 2015 | France | 32 | 65 | PAE PErFecTED with 300–500 µm Embosphere® | |
| Ayyagari (2019) [26] | retrospective | Apr 2013–Aug 2018 | United States | 93 | 76.0 end-hole; 72,8 balloon occlusion | end-hole vs. balloon occlusion PAE (both with 100–300 µm Embosphere®) | |
| Bhatia (2018) [28] | retrospective | Apr 2014–Oct 2017 | United States | 93 | 68.5 | PAE with 100–300 or 300–500 µm Embosphere® | |
| Pisco (2016) [37] | retrospective | Mar 2009–Sep 2014 | Portugal | 152 | 67.4 | PAE 100–200 µm PVA spheres, 300–500 µm Bead Block®, 300–500 µm Embosphere® or 400 µm Embozene® | |
| Qiu (2017) [38] | retrospective | Feb 2012–Mar 2015 | China | 17 | 75.53 | PAE with 90–180 µm Embosphere® | TURP |
| Tian (2019) [42] | retrospective | Feb 2014–Dec 2017 | China | 20 | 80.8 | PAE with 90–180 µm or 180–300 µm particles for control of gross haematuria in BPH |
BPH, benign prostatic hyperplasia; PAE, prostatic artery embolization; PErFecTED, proximal embolization first then embolize distant; TURP, transurethral resection of the prostate.
3.2. Outcome Measures
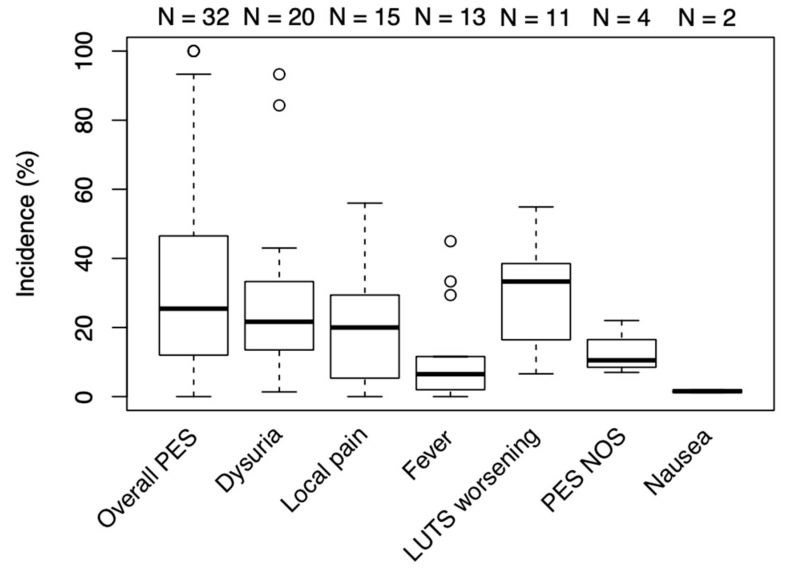
The most frequently reported symptom was dysuria or urethral burning, appearing in 20 of the 32 included studies. Local pain and fever were the second and third-most reported symptoms, present in 16 and 13 studies, respectively. The most infrequently mentioned symptom was nausea, appearing in just 2 studies. Four studies mentioned PES but did not provide their definition of the syndrome. Four studies had PES explicitly defined and recorded as a collection of symptoms, but no studies mentioned all the PES components as defined in outcome measures. Overlap between symptoms and patients was difficult to determine in studies where several PES components were mentioned independently. The overall median PES percentage was 25.5% (12.5–45.8), and median percentages of the individual PES components were as follows: 33.3% (16.5–38.5) for LUTS worsening, 21.7% (13.8–33.3) for dysuria/urethral burning, 20% (5.4–29.4) for local pain, 6.5% (2–11.6) for fever and 1.6% (1.3–18) for nausea and/or vomiting. The data with outliers is presented as a box plot in Figure 2.
Figure 2.
Median frequency of PES and its components. Box = 25th and 75th percentiles; bars = minimum and maximum values (1.5× IQR); bold line = median; N = number of studies included; outliers represented as circles. LUTS, lower urinary tract symptoms; PES, postembolization syndrome; NOS, not otherwise specified.
Two studies [6,20] reported the overall PES frequency to be 100%. The highest reported percentages for individual PES components were 93% for dysuria and/or urethral burning [33], 56% for local pain [7], 54% for LUTS worsening [30], 45% for fever [25] and 2% for nausea [30]. Symptoms with the most pronounced lack of uniformity in reporting were overall PES (ranging from 0% to 100%), urethral burning and/or dysuria (ranging from 1.35% to 93.3%) and fever (ranging from 0% to 45%). Remaining outcome measures had a more uniform distribution, with all data points within the 1.5× IQR from the first and third quartiles.
3.3. Risk of Bias
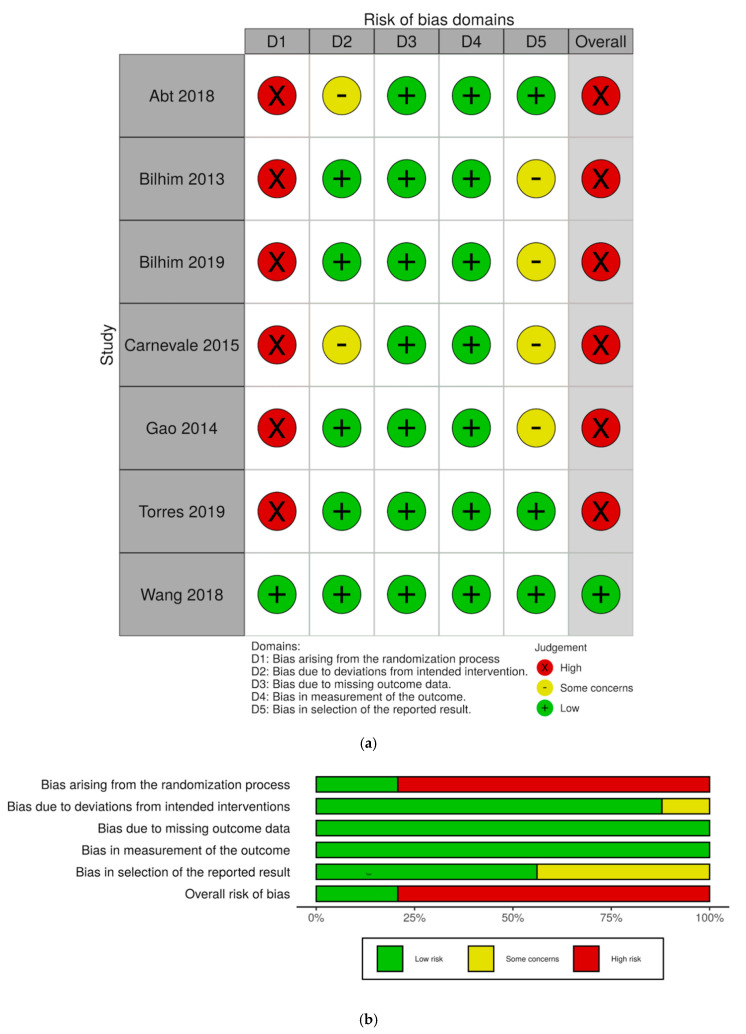
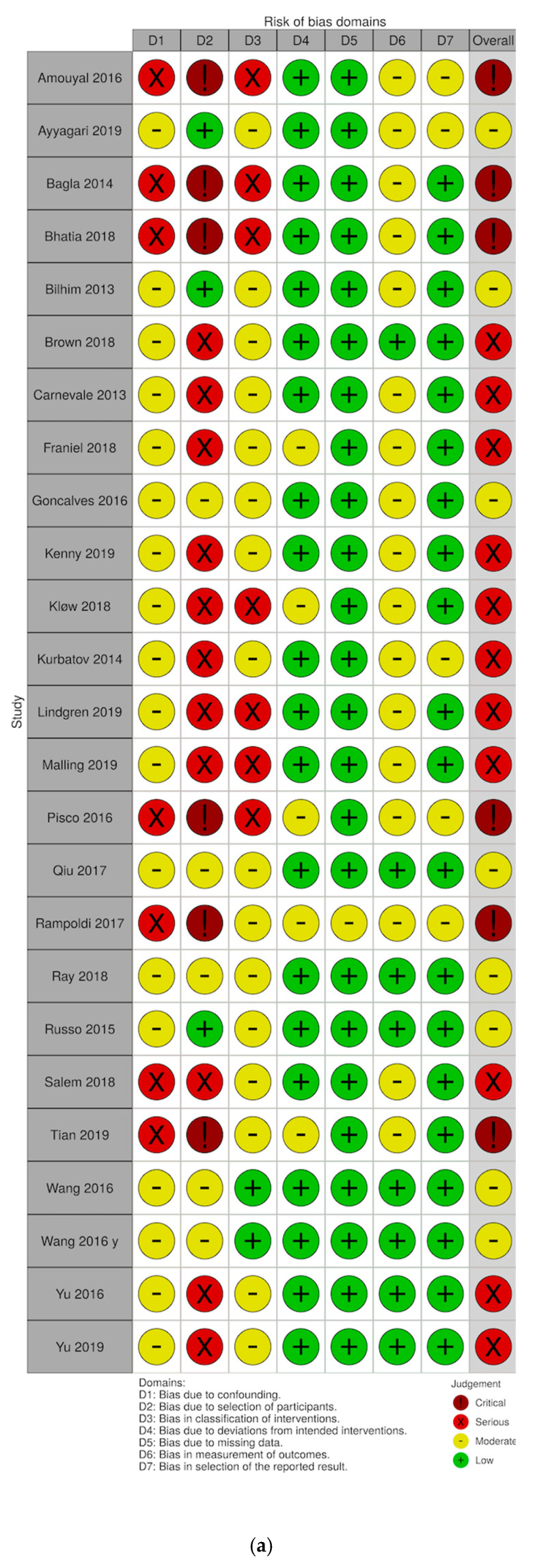
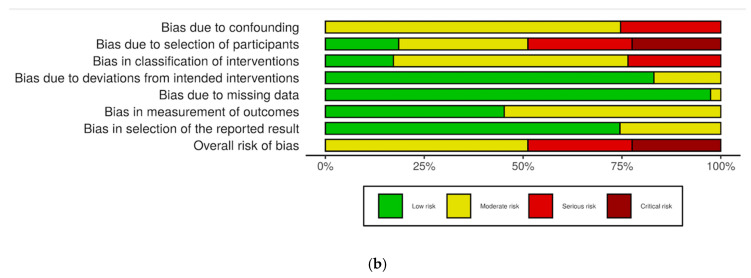
Risk of bias assessments and judgement distribution within each domain for the randomized studies are visualized as “traffic-light” and weighted bar plots using the robvis tool [17] (Figure 3a,b). The most common risk of bias in the RCTs was bias due to randomization process, making all but one study at a high risk of bias. Likewise, most of the non-randomized studies were assessed to be at either moderate or high risk of bias, owing to their retrospective design and lack of control groups (Figure A1).
Figure 3.
RoB2 assessments of RCTs. (a) “Traffic-light” illustration of risk of bias in individual studies. (b) Weighted bar plots depicting risk of bias judgement distributions within each domain.
4. Discussion
This systematic review is the first dedicated review investigating the overall incidence and individual components of PES after PAE. From the data of 32 studies with a total of 2116 patients, we have demonstrated an overall median PES incidence of 25.5% with a pronounced lack of uniformity in reporting between studies.
PAE is a procedure that can reduce LUTS in men with BPH, demonstrated to be safe and rarely associated with severe complications, such as non-target embolization. PES is a well-known side effect of endovascular arterial embolization in other organs or tumors. However, PES is often overlooked when reporting the possible side effects to PAE, and no consensus exists on whether it is an expected side effect to PAE or a complication to the procedure, even though PES may temporarily impair quality of life and lead to secondary hospital admissions for pain and/or fever management. This review has underlined that PES is indeed very common. Surprisingly, no uniform reporting of PES exists, which raises concerns about its true frequency following PAE.
Several studies have addressed the pathogenesis and incidence of PES in other organs [10,11]. In PAE, Moreira et al. [8] were one of the first to describe PES as the most common side-effect of PAE. The symptoms of PES are typically followed by leucopenia, leukocytosis and/or elevation of C-reactive protein (CRP) [9,10,11], which suggests that systemic manifestations of PES (fever, nausea, malaise) could be regarded as components of the systemic inflammatory response syndrome (SIRS) [47]. This is most likely caused by prostate tissue hypoxia and cell death mediated release of tissue breakdown products, inflammatory mediators (interleukin-6, tumor necrosis factor α, and others) and vasoactive substances [11]. Similarly, periprostatic and prostatic inflammatory response is probably responsible for observed local PES components (local pain, dysuria and LUTS worsening) [8]. The prostate is innervated with an abundant nervous complex that ultimately ends in the corpora cavernosa. Most nerves are noradrenergic fibers that via alfa-1-adrenoreceptors cause smooth muscle contraction. It is likely that ischemia and necrosis activate nervous innervation and lead to frequent urination and urgency. The release of inflammatory mediators may be responsible for the pain observed by men with PES. It is well-known from bacterial and non-bacterial prostatitis that inflammation of the prostate results in diffuse pain in the pelvis area, tip of the penis and dysuria. It is striking that the severity of PES varies widely between patients. Wang et al. [43] showed that large size prostates (>80 cm3) had a statistically significant increase in risk for urethral burning compared to smaller prostates (16.7% vs 10.2% for urethral burning, respectively), suggesting a proportional relationship between prostate size and symptom severity.
Reported incidence of PES in other anatomical sites varies from 40% in uterine artery embolization [11] to 89% in renal angiomyolipoma embolization [10]. Empirical observations from our own group of men undergoing PAE suggest that PES occurs in up to 90% with a varying degree of severity ranging from admission to hospital to only mild discomfort 2–3 days after intervention. In contrast, the median overall PES incidence in this review was only 25.5%. The incidence ranged from 0% in studies by Kurbatov et al. [18] and Yu et al. [45] to a 100% in an RCT conducted by Carnevale et al. [6] and a study by Amouyal et al. [20]. This underreporting of PES symptoms in some studies can partially be explained by a stance held by some authors that PES symptoms are not to be regarded as complications but as expected neglectable side-effects to PAE and are consequently not mentioned in publications [48]. Additionally, the overall PES incidence in this review probably underestimated the true overall figure due to unclear overlap between patients and symptoms in 19 of the 32 studies, resulting in an inability to combine different individual PES components.
PES is a self-limiting condition that is treated symptomatically with a combination of analgesics, antiemetics and antipyretics. However, PES can be so severe that patients experience high fever, shivers, dysuria and urgency mimicking a septicemia from the urinary tract. As shown by Ganguli et al. [11] in uterine artery embolization, leukocytosis is frequent after solid organ embolization, further complicating the discerption of PES from infection. In this review, the incidence of urinary tract infections (UTIs) requiring antibiotic treatment as reported by 20 studies was 2.7% (SD 3.7). Seven studies recorded no UTIs and the highest UTI percentage of 13.8% was reported in a study by Kløw et al. [35]. Currently, antibiotic prophylaxis covering Gram-negative rods is routinely administered prior to PAE in most centers, even though no randomized trials evaluating its efficacy exist to date. A study by Cochran et al. [49] regarding percutaneous nephrostomy tube placement found no significant difference in urosepsis rates in low-risk group with and without antibiotic prophylaxis, though reservations for small sample size had to be made. However, the same trial showed a significant decrease in urosepsis rates (from 50% to 9%) with antibiotic prophylaxis in high-risk group (advanced age, diabetes, bladder dysfunction, indwelling catheter, earlier manipulation, urointestinal anastomosis, bacteriuria and stones). This might suggest a more individual approach is needed in the future, especially in low-risk patients without significant comorbidities.
Following the inflammation hypothesis, prophylactic corticosteroids were used and proven successful in reducing the incidence, severity and duration of PES after renal angiomyolipoma ablation [10], endovascular abdominal aortic repair (EVAR) [50] and transcatheter arterial chemoembolization (TACE) of the liver [51]. The last two studies were conducted as double-blind randomized placebo-controlled trials with a low risk of bias, providing good evidence quality for corticosteroid usage. Administration of a single-dose perioperative corticosteroid was not associated with any significant side-effects in a meta-analysis of RCTs by De Oliveira et al. [52]. No similar studies were conducted concerning PES after PAE, and symptomatic therapy is still the mainstay treatment.
We believe that raised awareness and uniform reporting of incidence and symptoms of PES would help the clinicians recognize the syndrome correctly, avoiding unnecessary antibiotics treatment and hospital admission. Patient information on the symptoms of PES is also crucial to optimize care. We suggest that the presence of dysuria, urgency, frequent urination, nausea, fever, pelvis or prostate pain, urine retention or overall worsening of LUTS during the first 7 days following PAE be regarded and reported as PES no matter if they occur individually or together. This would greatly improve the transparency and uniformity of reporting in future publications. Moreover, we urge the PAE community to address PES in interventional trials in order to reduce the incidence and/or duration of PES following PAE.
This systematic review is limited by heterogeneity in patient inclusion and exclusion criteria across studies as well as the use of different embolization techniques and material. This resulted in a heterogeneous group of studies with no possibility for meta-analysis. Additionally, overall PES frequency was probably underestimated due to underreporting as well as difficulties in calculating overall PES frequency from individual PES components.
5. Conclusions
PES is the most frequent adverse event following PAE. This systematic review showed a lack of uniformity in reporting the symptoms of PES after PAE. We urge the PAE community to define the criteria for PES to improve transparency and help the clinicians recognize and treat the symptoms accordingly. Further studies to reduce PES after PAE are also warranted.
Appendix A
Figure A1.
Risk of bias in non-randomized studies-of interventions (ROBINS-I) assessments of non-RCTs. (a) “Traffic-light” illustration of risk of bias in individual studies. (b) Weighted bar plots depicting risk of bias judgement distributions within each domain.
Author Contributions
Conceptualization, P.S., M.T., L.L and M.A.R.; methodology, P.S, M.T, L.L. and M.A.R.; writing—original draft preparation, P.S.; writing—review and editing, P.S., L.L., M.A.R., M.B.N. and H.V.S.; supervision, L.L. and M.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Roehrborn C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005;7(Suppl. S9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- 2.Thorpe A., Neal D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–1367. doi: 10.1016/S0140-6736(03)13073-5. [DOI] [PubMed] [Google Scholar]
- 3.Irwin D.E., Kopp Z.S., Agatep B., Milsom I., Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction: Worldwide prevalence of luts. BJU Int. 2011;108:1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 4.Malling B., Røder M.A., Brasso K., Forman J., Taudorf M., Lönn L. Prostate artery embolisation for benign prostatic hyperplasia: A systematic review and meta-analysis. Eur. Radiol. 2019;29:287–298. doi: 10.1007/s00330-018-5564-2. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y., Huang Y., Zhang R., Yang Y., Zhang Q., Hou M., Wang Y. Benign Prostatic Hyperplasia: Prostatic Arterial Embolization versus Transurethral Resection of the Prostate—A Prospective, Randomized, and Controlled Clinical Trial. Radiology. 2014;270:920–928. doi: 10.1148/radiol.13122803. [DOI] [PubMed] [Google Scholar]
- 6.Carnevale F.C., Iscaife A., Yoshinaga E.M., Moreira A.M., Antunes A.A., Srougi M. Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis. Cardiovasc. Interv. Radiol. 2016;39:44–52. doi: 10.1007/s00270-015-1202-4. [DOI] [PubMed] [Google Scholar]
- 7.Abt D., Hechelhammer L., Müllhaupt G., Markart S., Güsewell S., Kessler T.M., Schmid H.-P., Engeler D.S., Mordasini L. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: Randomised, open label, non-inferiority trial. BMJ. 2018;361:k2338. doi: 10.1136/bmj.k2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira A.M., de Assis A.M., Carnevale F.C., Antunes A.A., Srougi M., Cerri G.G. A Review of Adverse Events Related to Prostatic Artery Embolization for Treatment of Bladder Outlet Obstruction Due to BPH. Cardiovasc. Interv. Radiol. 2017;40:1490–1500. doi: 10.1007/s00270-017-1765-3. [DOI] [PubMed] [Google Scholar]
- 9.Leung D.A., Goin J.E., Sickles C., Raskay B.J., Soulen M.C. Determinants of postembolization syndrome after hepatic chemoembolization. J. Vasc. Interv. Radiol. 2001;12:321–326. doi: 10.1016/S1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 10.Bissler J.J., Racadio J., Donnelly L.F., Johnson N.D. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am. J. Kidney Dis. 2002;39:966–971. doi: 10.1053/ajkd.2002.32770. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli S., Faintuch S., Salazar G.M., Rabkin D.J. Postembolization Syndrome: Changes in White Blood Cell Counts Immediately after Uterine Artery Embolization. J. Vasc. Interv. Radiol. 2008;19:443–445. doi: 10.1016/j.jvir.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svarc Petra Components and Incidence of the Postembolization Syndrome after Prostatic Artery Embolization for Benign Prostatic Hyperplasia: A Systematic Review. [(accessed on 1 August 2020)]; Available online: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=164472.
- 14.Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. [(accessed on 20 January 2020)]; Available online: https://www.covidence.org.
- 15.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualisation (robvis): An R package and Shiny web app for visualising risk-of-bias assessments. Res. Synth. Methods. 2020;2020:1–7. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 18.Kurbatov D., Russo G.I., Lepetukhin A., Dubsky S., Sitkin I., Morgia G., Rozhivanov R., Cimino S., Sansalone S. Prostatic Artery Embolization for Prostate Volume Greater Than 80 cm3: Results From a Single-center Prospective Study. Urology. 2014;84:400–404. doi: 10.1016/j.urology.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Rampoldi A., Barbosa F., Secco S., Migliorisi C., Galfano A., Prestini G., Harward S.H., Di Trapani D., Brambillasca P.M., Ruggero V., et al. Prostatic Artery Embolization as an Alternative to Indwelling Bladder Catheterization to Manage Benign Prostatic Hyperplasia in Poor Surgical Candidates. Cardiovasc. Interv. Radiol. 2017;40:530–536. doi: 10.1007/s00270-017-1582-8. [DOI] [PubMed] [Google Scholar]
- 20.Amouyal G., Thiounn N., Pellerin O., Yen-Ting L., Del Giudice C., Dean C., Pereira H., Chatellier G., Sapoval M. Clinical Results After Prostatic Artery Embolization Using the PErFecTED Technique: A Single-Center Study. Cardiovasc. Interv. Radiol. 2016;39:367–375. doi: 10.1007/s00270-015-1267-0. [DOI] [PubMed] [Google Scholar]
- 21.Malling; Lönn; Jensen; Lindh; Frevert; Brasso; Røder Prostate Artery Embolization for Lower Urinary Tract Symptoms in Men Unfit for Surgery. Diagnostics. 2019;9:46. doi: 10.3390/diagnostics9020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilhim T., Pisco J., Campos Pinheiro L., Rio Tinto H., Fernandes L., Pereira J.A., Duarte M., Oliveira A.G. Does Polyvinyl Alcohol Particle Size Change the Outcome of Prostatic Arterial Embolization for Benign Prostatic Hyperplasia? Results from a Single-Center Randomized Prospective Study. J. Vasc. Interv. Radiol. 2013;24:1595–1602. doi: 10.1016/j.jvir.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Bilhim T., Costa N.V., Torres D., Pisco J., Carmo S., Oliveira A.G. Randomized Clinical Trial of Balloon Occlusion versus Conventional Microcatheter Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019;30:1798–1806. doi: 10.1016/j.jvir.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Torres D., Costa N.V., Pisco J., Pinheiro L.C., Oliveira A.G., Bilhim T. Prostatic Artery Embolization for Benign Prostatic Hyperplasia: Prospective Randomized Trial of 100–300 μm versus 300–500 μm versus 100- to 300-μm + 300- to 500-μm Embospheres. J. Vasc. Interv. Radiol. 2019;30:638–644. doi: 10.1016/j.jvir.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Wang M.Q., Zhang J.L., Xin H.N., Yuan K., Yan J., Wang Y., Zhang G.D., Fu J.X. Comparison of Clinical Outcomes of Prostatic Artery Embolization with 50-μm Plus 100-μm Polyvinyl Alcohol (PVA) Particles versus 100-μm PVA Particles Alone: A Prospective Randomized Trial. J. Vasc. Interv. Radiol. 2018;29:1694–1702. doi: 10.1016/j.jvir.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Ayyagari R., Powell T., Staib L., Chapiro J., Schoenberger S., Devito R., Pollak J. Case-Control Comparison of Conventional End-Hole versus Balloon-Occlusion Microcatheter Prostatic Artery Embolization for Treatment of Symptomatic Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019;30:1459–1470. doi: 10.1016/j.jvir.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Bagla S., Martin C.P., van Breda A., Sheridan M.J., Sterling K.M., Papadouris D., Rholl K.S., Smirniotopoulos J.B., van Breda A. Early Results from a United States Trial of Prostatic Artery Embolization in the Treatment of Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2014;25:47–52. doi: 10.1016/j.jvir.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S., Sinha V.K., Harward S., Gomez C., Kava B.R., Parekh D.J. Prostate Artery Embolization in Patients with Prostate Volumes of 80 mL or More: A Single-Institution Retrospective Experience of 93 Patients. J. Vasc. Interv. Radiol. 2018;29:1392–1398. doi: 10.1016/j.jvir.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Bilhim T., Pisco J., Rio Tinto H., Fernandes L., Campos Pinheiro L., Duarte M., Pereira J.A., Oliveira A.G., O’Neill J. Unilateral Versus Bilateral Prostatic Arterial Embolization for Lower Urinary Tract Symptoms in Patients with Prostate Enlargement. Cardiovasc. Interv. Radiol. 2013;36:403–411. doi: 10.1007/s00270-012-0528-4. [DOI] [PubMed] [Google Scholar]
- 30.Brown N., Walker D., McBean R., Pokorny M., Kua B., Gianduzzo T., Dunglison N., Esler R., Yaxley J. Prostate artery Embolisation Assessment of Safety and feasibilitY (P-EASY): A potential alternative to long-term medical therapy for benign prostate hyperplasia. BJU Int. 2018;122:27–34. doi: 10.1111/bju.14504. [DOI] [PubMed] [Google Scholar]
- 31.Carnevale F.C., da Motta-Leal-Filho J.M., Antunes A.A., Baroni R.H., Marcelino A.S.Z., Cerri L.M.O., Yoshinaga E.M., Cerri G.G., Srougi M. Quality of Life and Clinical Symptom Improvement Support Prostatic Artery Embolization for Patients with Acute Urinary Retention Caused by Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2013;24:535–542. doi: 10.1016/j.jvir.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Franiel T., Aschenbach R., Trupp S., Lehmann T., von Rundstedt F.-C., Grimm M.-O., Teichgräber U. Prostatic Artery Embolization with 250-μm Spherical Polyzene-Coated Hydrogel Microspheres for Lower Urinary Tract Symptoms with Follow-up MR Imaging. J. Vasc. Interv. Radiol. 2018;29:1127–1137. doi: 10.1016/j.jvir.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Gonçalves O.M., Carnevale F.C., Moreira A.M., Antunes A.A., Rodrigues V.C., Srougi M. Comparative Study Using 100–300 Versus 300–500 μm Microspheres for Symptomatic Patients Due to Enlarged-BPH Prostates. Cardiovasc. Interv. Radiol. 2016;39:1372–1378. doi: 10.1007/s00270-016-1443-x. [DOI] [PubMed] [Google Scholar]
- 34.Kenny A.G., Pellerin O., Amouyal G., Desgranchamps F., Méria P., De Gouvello A., Dariane C., Déan C., Pereira H., Thiounn N., et al. Prostate Artery Embolization in Patients With Acute Urinary Retention. Am. J. Med. 2019;132:e786–e790. doi: 10.1016/j.amjmed.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Kløw N.E., Grøtta O.J., Bay D., Sandbæk G., Johansen T.E.B., Hagen T., Baco E. Outcome after prostatic artery embolization in patients with symptomatic benign prostatic hyperplasia. Acta Radiol. 2019;60:1175–1180. doi: 10.1177/0284185118813709. [DOI] [PubMed] [Google Scholar]
- 36.Lindgren H., Bläckberg M. Introduction of prostate artery embolization (PAE) in Sweden. Scand. J. Urol. 2019;53:151–155. doi: 10.1080/21681805.2019.1610494. [DOI] [PubMed] [Google Scholar]
- 37.Pisco J., Bilhim T., Pinheiro L.C., Fernandes L., Pereira J., Costa N.V., Duarte M., Oliveira A.G. Prostate Embolization as an Alternative to Open Surgery in Patients with Large Prostate and Moderate to Severe Lower Urinary Tract Symptoms. J. Vasc. Interv. Radiol. 2016;27:700–708. doi: 10.1016/j.jvir.2016.01.138. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Z., Zhang C., Wang X., Cheng K., Liang X., Wang D., Hou S. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: A single-center retrospective study. Videosurgery Other Miniinvasive Tech. 2017;4:409–416. doi: 10.5114/wiitm.2017.72324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray A.F., Powell J., Speakman M.J., Longford N.T., DasGupta R., Bryant T., Modi S., Dyer J., Harris M., Carolan-Rees G., et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: An observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study) BJU Int. 2018;122:270–282. doi: 10.1111/bju.14249. [DOI] [PubMed] [Google Scholar]
- 40.Russo G.I., Kurbatov D., Sansalone S., Lepetukhin A., Dubsky S., Sitkin I., Salamone C., Fiorino L., Rozhivanov R., Cimino S., et al. Prostatic Arterial Embolization vs Open Prostatectomy: A 1-Year Matched-pair Analysis of Functional Outcomes and Morbidities. Urology. 2015;86:343–348. doi: 10.1016/j.urology.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 41.Salem R., Hairston J., Hohlastos E., Riaz A., Kallini J., Gabr A., Ali R., Jenkins K., Karp J., Desai K., et al. Prostate Artery Embolization for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Results From a Prospective FDA-Approved Investigational Device Exemption Study. Urology. 2018;120:205–210. doi: 10.1016/j.urology.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Tian W., Zhou C., Leng B., Shi H., Liu S. Prostatic Artery Embolization for Control of Gross Hematuria in Patients with Benign Prostatic Hyperplasia: A Single-Center Retrospective Study in 20 Patients. J. Vasc. Interv. Radiol. 2019;30:661–667. doi: 10.1016/j.jvir.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Wang M., Guo L., Duan F., Yuan K., Zhang G., Li K., Yan J., Wang Y., Kang H. Prostatic arterial embolization for the treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia: A comparative study of medium- and large-volume prostates. BJU Int. 2016;117:155–164. doi: 10.1111/bju.13147. [DOI] [PubMed] [Google Scholar]
- 44.Wang M.Q., Wang Y., Yan J.Y., Yuan K., Zhang G.D., Duan F., Li K. Prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia in men ≥75 years: A prospective single-center study. World J. Urol. 2016;34:1275–1283. doi: 10.1007/s00345-016-1771-0. [DOI] [PubMed] [Google Scholar]
- 45.Yu S.C.H., Cho C.C.M., Hung E.H.Y., Chiu P.K.F., Yee C.H., Ng C.F. Prostate Artery Embolization for Complete Urinary Outflow Obstruction Due to Benign Prostatic Hypertrophy. Cardiovasc. Interv. Radiol. 2017;40:33–40. doi: 10.1007/s00270-016-1502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu S.C.H., Cho C.C.M., Hung E.H.Y., Zou J., Yuen B.T.Y., Shi L., Chiu P.K.F., Yee S.C.H., Ng A.C.F. Thickness-to-Height Ratio of Intravesical Prostatic Protrusion Predicts the Clinical Outcome and Morbidity of Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019;30:1807–1816. doi: 10.1016/j.jvir.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Marik P.E., Taeb A.M. SIRS, qSOFA and new sepsis definition. J. Thorac. Dis. 2017;9:943–945. doi: 10.21037/jtd.2017.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Assis A.M., Moreira A.M., de Paula Rodrigues V.C., Yoshinaga E.M., Antunes A.A., Harward S.H., Srougi M., Carnevale F.C. Prostatic Artery Embolization for Treatment of Benign Prostatic Hyperplasia in Patients with Prostates > 90 g: A Prospective Single-Center Study. J. Vasc. Interv. Radiol. 2015;26:87–93. doi: 10.1016/j.jvir.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Cochran S.T., Barbaric Z.L., Lee J.J., Kashfian P. Percutaneous nephrostomy tube placement: An outpatient procedure? Radiology. 1991;179:843–847. doi: 10.1148/radiology.179.3.2028003. [DOI] [PubMed] [Google Scholar]
- 50.de la Motte L., Kehlet H., Vogt K., Nielsen C.H., Groenvall J.B., Nielsen H.B., Andersen A., Schroeder T.V., Lönn L. Preoperative Methylprednisolone Enhances Recovery After Endovascular Aortic Repair: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Ann. Surg. 2014;260:540–549. doi: 10.1097/SLA.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 51.Ogasawara S., Chiba T., Ooka Y., Kanogawa N., Motoyama T., Suzuki E., Tawada A., Nagai K., Nakagawa T., Sugawara T., et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575–585. doi: 10.1002/hep.29403. [DOI] [PubMed] [Google Scholar]
- 52.De Oliveira G.S., Almeida M.D., Benzon H.T., McCarthy R.J. Perioperative Single Dose Systemic Dexamethasone for Postoperative Pain: A Meta-analysis of Randomized Controlled Trials. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]