Abstract
Background: The present study aims to evaluate the diagnostic accuracy between ultrasonography-guided fine-needle aspiration cytology (US-FNAC) and core needle biopsy (CNB) of axillary lymph nodes (ALNs) in patients with breast cancer through a meta-analysis and a diagnostic test accuracy (DTA) review. Methods: The present meta-analysis and DTA review included 67 eligible studies. The diagnostic accuracy of various preoperative assessments, including US-FNAC and CNB, was evaluated for ALNs assessments in patients with breast cancer. In addition, a subgroup analysis based on methods of cytologic preparation was performed. In the DTA review, the sensitivity, specificity, diagnostic odds ratio (OR) and area under the curve (AUC) on the summary receiver operating characteristic (SROC) curve were calculated. Results: The diagnostic accuracy of the preoperative assessments of ALNs was 0.850 (95% confidence interval (CI) 0.833–0.866) for patients with breast cancer. The diagnostic accuracy of CNB was significantly higher than that of US-FNAC (0.896, 95% CI 0.844–0.932 vs. 0.844, 95% CI 0.825–0.862; p = 0.044 in a meta-regression test). In the subgroup analysis based on cytologic preparation, the diagnosis accuracies were 0.860, 0.861 and 0.859 for the methods of conventional smear, liquid-based preparation and cell block, respectively. In the DTA review, CNB showed higher sensitivity than US-FNAC (0.849 vs. 0.760). However, there was no difference in specificity between US-FNAC and CNB (0.997 vs. 1.000). US-FNAC with liquid-based preparation and CNB showed the highest diagnostic OR and AUC on the SROC, respectively. Conclusion: Both US-FNAC and CNB are useful in preoperative assessments of ALNs in patients with breast cancer. Although the most sensitive test was found to be CNB in this study, there was no difference in specificity between various preoperative evaluations and the application of US-FNAC or CNB may be impacted by various factors.
Keywords: fine-needle aspiration cytology, core needle biopsy, axillary lymph node, meta-analysis, diagnostic test accuracy review
1. Introduction
In breast cancers, the assessment for axillary lymph node (ALN) is important in predicting the patient’s stage and prognosis and in determining treatment guidelines. According to the results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, in the case of clinical node-negative patients, ALN dissection is not performed according to the result of sentinel lymph node biopsy (SLNB) [1]. Preoperative assessments of ALNs in patients with breast cancer mainly include axillary ultrasound sonography (AUS) and/or ultrasonography-guided fine-needle aspiration cytology (US-FNAC) [2]. US-FNAC confirms whether metastatic ALN as suspicious ALNs during AUS. ALN dissection without SLNB is performed in patients with metastatic ALNs detected by US-FNAC. On the other hand, patients found negative using US-FNAC are subjected to the intraoperative SLNB. After AUS and US-FNAC, core needle biopsy (CNB) is recommended as the preoperative assessment. In daily practice, various methods are introduced for cytological preparation, such as conventional smear (CS), liquid-based preparation (LBP) and cell block [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. In pathological examinations, LBP, which has been widely applied to the screening of uterine cervical lesions, has gradually replaced CS. The diagnostic accuracy can differ between the methods of cytological preparation. However, confirmative information for a comparison of diagnostic accuracy between US-FNAC and CNB is lacking in terms of assessments of ALNs in patients with breast cancer.
In the present study, we investigated and elucidated the diagnostic accuracy of US-FNAC and CNB for ALN assessment in patients with breast cancer. The diagnostic accuracy between US-FNAC and CNB was compared through a meta-regression test. In addition, a diagnostic test accuracy (DTA) review was performed to obtain the pooled sensitivity and specificity, diagnostic odds ratio (OR) and area under the curve (AUC) on the summary receiver operating characteristic (SROC) curve.
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
Relevant articles were obtained by searching the PubMed database through 31 July 2020. We searched using the following keywords: “((Ultrasound OR US) OR (Ultrasound guided OR US guided) OR (sonography OR sonography guided)) AND ((FNA OR Fine needle aspiration) OR (CNB OR core needle biopsy)) AND (axillary lymph nodes OR axillary lymphadenopathy OR axillary staging) AND (Invasive breast cancer OR breast cancer OR breast carcinoma).” Review and non-English language articles were excluded in searching databases. The titles and the abstracts of all searched articles were screened for exclusion. Searched results were then reviewed and articles were included if the study investigated the axillary lymph nodes of breast cancers and there was information for the US-FNAC or CNB. Also, case reports were excluded. The PRISMA checklist is shown in the supplementary Table S1.
2.2. Data Extraction
Two individual authors extracted data from all eligible studies. Extracted data from each of the eligible studies included the following [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]: first author’s name, year of publication, study location, number of patients analyzed and the methods of preoperative assessment for ALNs. Besides, for the meta-analysis, we extracted all data associated with the diagnostic accuracy of US-FNAC and CNB in preoperative assessments for ALNs of breast cancers. Numbers of true positive, false positive, false negative and true negative of each method were investigated to obtain the sensitivity, specificity, diagnostic OR and the SROC curve.
2.3. Statistical Analysis
To obtain the diagnostic accuracy rate between the US-FNAC and CNB, we performed a meta-analysis using the Comprehensive Meta-Analysis software package 2.0 (Biostat, Englewood, NJ, USA). The diagnostic accuracy rate was evaluated by the concordance between preoperative assessments and histologic diagnosis. Because the eligible studies used various methods for ALNs and had a different number of patients, a random-effects model was more appropriate than a fixed-effects model. Heterogeneity between the eligible studies was checked using p statistics (p-value). In addition, comparisons between US-FNAC and CNB were performed through a meta-regression test. To evaluate publication bias, we conducted Begg’s funnel plot and Egger’s test. The results with p < 0.05 were considered statistically significant. If significant publication bias was found, the fail-safe N and trim-fill tests were additionally conducted to confirm the degree of publication bias. The results were considered statistically significant with p < 0.05.
For the DTA review, we used R software ver. 4.0.2 (R Studio, Boston, MA, USA). We calculated the pooled sensitivity and specificity, the diagnostic OR according to individual data, was collected from each eligible study in various categories of comparison. By plotting the ‘sensitivity’ and ‘1-specificity’ of each study, the SROC curve was constructed first and the curve fitting was performed through linear regression. As each dataset was heterogeneous, the accuracy data were pooled by fitting a SROC curve and measuring the value of the AUC. An AUC close to 1 means the test is strong and an AUC close to 0.5 means the test is considered inferior.
3. Results
3.1. Selection and Characteristics
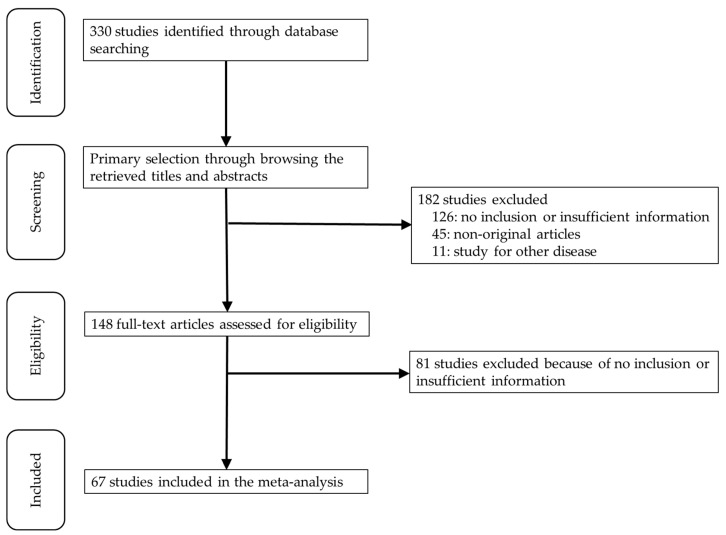
A total of 330 studies were searched and identified through database searching. Due to insufficient information on concordance rates and diagnostic accuracy, 207 studies were excluded. An additional 45 studies were excluded because they were non-original and 11 were excluded owing to study for other diseases. Finally, 67 studies were included in the present meta-analysis (Figure 1 and Table 1) and they provided data for 11,732 ALNs of breast cancers. Detailed information of eligible studies is shown in Table 1. Various techniques used for US-FNAC and CNB in eligible studies and were described.
Figure 1.
Flow chart of the searching strategy.
Table 1.
Main characteristics of the eligible studies.
| Reference | Location | Method | Number of Patients | Reference | Location | Method | Number of Patients | ||
|---|---|---|---|---|---|---|---|---|---|
| Accurate | Total | Accurate | Total | ||||||
| Abe 2009 [3] | USA | CNB (ND) | 78 | 88 | Koelliker 2008 [37] | Island | FNAC (LBP) | 60 | 72 |
| Ahn 2013 [4] | Korea | FNAC (CS) | 41 | 48 | Kramer 2016 [38] | Netherlands | FNAC (ND) | 430 | 543 |
| CNB-Stericut | 42 | 48 | Krishnamurthy 2002 [39] | USA | FNAC (CS) | 75 | 103 | ||
| Attieh 2019 [5] | Lebanon | FNAC (ND) | 89 | 101 | Kuenen 2003 [40] | Netherlands | FNAC (CS) | 103 | 134 |
| Barco 2017 [6] | Spain | FNAC (ND) | 320 | 390 | Leenders 2012 [41] | Netherlands | FNAC (ND) | 215 | 274 |
| Bedrosian 2003 [7] | USA | FNAC (ND) | 13 | 22 | Leenders 2013 [42] | Netherlands | FNAC (ND) | 363 | 530 |
| Bonnema 1997 [8] | Netherlands | FNAC (CS) | 71 | 81 | Liang 2017 [43] | China | FNAC (ND) | 237 | 263 |
| Boughey 2007 [9] | USA | FNAC (ND) | 60 | 76 | Machida 2013 [44] | Japan | FNAC (CS) | 33 | 41 |
| Breitbach 2019 [10] | Germany | FNAC (ND) | 46 | 60 | MacNeill 2011 [45] | UK | FNAC (ND) | 74 | 93 |
| CNB-BARD® | 10 | 10 | Marti 2012 [46] | USA | FNAC (CS) | 78 | 86 | ||
| Britton 2009 [11] | UK | CNB-BARD® | 91 | 116 | Maxwell 2016 [47] | UK | CNB-Achieve® | 33 | 37 |
| Bruzzone 2018 [12] | Italy | FNAC (ND) | 363 | 439 | Moorman 2015 [48] | Netherlands | FNAC (LBP) | 148 | 202 |
| Caretta-Weyer 2012 [13] | USA | CNB (ND) | 24 | 26 | Motomura 2001 [49] | Japan | FNAC (CS) | 25 | 29 |
| Castellano 2014 [14] | Italy | FNAC (CS) | 134 | 146 | Nakamura 2018 [50] | Japan | CNB-BARD® | 260 | 272 |
| Choi 2015 [15] | Korea | FNAC (CS) | 334 | 373 | FNAC (CS) | 650 | 744 | ||
| Ciatto 2007 [16] | Italy | FNAC (CS) | 337 | 418 | O’Leary 2012 [51] | Ireland | FNAC (CS) | 108 | 129 |
| Cools 2013 [17] | Canada | FNAC (ND) | 31 | 53 | Park 2011 [52] | Korea | FNAC (CS) | 293 | 382 |
| de Coninck 2016 [18] | Belgium | FNAC (CB) | 42 | 49 | Park 2013 [53] | Korea | FNAC (CS) | 127 | 145 |
| de Kanter 2006 [19] | Netherlands | FNAC (ND) | 113 | 161 | Podkrajsek 2005 [54] | Slovenia | FNAC (CS) | 39 | 44 |
| Devaraj 2011 [20] | UK | FNAC (ND) | 44 | 45 | Popli 2006 [55] | India | FNAC (CS) | 20 | 24 |
| Engohan 2011 [21] | Belgium | FNAC (CB) | 19 | 22 | Rao 2009 [56] | USA | FNAC (ND) | 18 | 22 |
| Fayyaz 2019 [22] | Pakistan | FNAC (CS) | 136 | 160 | CNB (ND) | 21 | 25 | ||
| Feng 2015 [23] | China | FNAC (LBP) | 1056 | 1152 | Rattay 2012 [57] | UK | FNAC (CS) | 49 | 56 |
| Fung 2014 [24] | USA | FNAC (LBP) | 106 | 130 | Rautiainen 2013 [58] | Finland | FNAC (CS) | 52 | 66 |
| García 2011 [25] | Spain | FNAC (CS) | 88 | 96 | CNB (ND) | 60 | 66 | ||
| Genta 2007 [26] | Italy | FNAC (CS) | 74 | 97 | Sapino 2003 [59] | Italy | FNAC (CS) | 79 | 85 |
| Gipponi 2016 [27] | Italy | FNAC (ND) | 329 | 400 | Schiettecatte 2011 [60] | Belgium | FNAC (LBP) | 48 | 58 |
| Hayes 2011 [28] | Ireland | FNAC (CS) | 131 | 161 | Swinson 2009 [61] | UK | FNAC (ND) | 87 | 96 |
| Hyun 2015 [29] | Korea | FNAC (CS) | 161 | 176 | Topal 2005 [62] | Turkey | CNB-BARD® | 36 | 39 |
| Imai 2018 [30] | Japan | FNAC (CS) | 140 | 162 | Tsai 2013 [63] | Taiwan | FNAC (ND) | 61 | 66 |
| Iwamoto 2019 [31] | Japan | FNAC (CS) | 140 | 174 | Usmani 2015 [64] | Kuwait | FNAC (LBP) | 47 | 53 |
| Jain 2008 [32] | USA | FNAC (CS) | 57 | 69 | Van Berckelaer 2016 [65] | Belgium | FNAC (LBP) | 291 | 317 |
| Jung 2010 [33] | Korea | FNAC (CS) | 37 | 39 | Van Wely 2013 [66] | Netherlands | FNAC (CS) | 179 | 198 |
| Kane 2019 [34] | Ireland | FNAC (ND) | 480 | 589 | Zhang 2018 [67] | China | FNAC (LBP) | 110 | 124 |
| Kim 2010 [35] | Korea | FNAC (CS) | 123 | 134 | Zhong 2018 [68] | China | FNAC (CS) | 120 | 126 |
| Kim 2016 [36] | Korea | FNAC (ND) | 24 | 32 | Zhu 2016 [69] | China | FNAC (CS) | 235 | 263 |
CNB, core needle biopsy; ND, no description; FNAC, fine-needle aspiration cytology; CS, conventional smear; CB, cell block; LBP, liquid-based preparation.
3.2. Comparison of Diagnostic Accuracy between Fine-Needle Aspiration Cytology and Core Needle Biopsy
The overall diagnostic accuracy for ALNs was 0.850 (95% confidence interval (CI) 0.833–0.866) (Table 2). The diagnostic accuracy of US-FNAC was 0.844 (95% CI 0.825–0.862). In subgroup analysis based on methods of cytological preparation, liquid-based preparation was slightly higher than CS and cell block. The diagnostic accuracy of CNB was 0.896 (95% CI 0.844–0.932). The diagnostic accuracy of CNB was significantly higher than that of US-FNAC (p = 0.044 in a meta-regression test).
Table 2.
Diagnostic accuracy of ultrasonography-guided fine-needle aspiration cytology and core needle biopsy in the axillary lymph node of breast cancers.
| Comparison | Number of Subsets |
Heterogeneity (p-Value) | Random Effect (95% CI) |
Egger’s Test (p-Value) | MRT * (p-Value) |
|---|---|---|---|---|---|
| Preoperative evaluation of ALNs | 72 | <0.001 | 0.850 (0.833, 0.866) | 0.005 | |
| Fine-needle aspiration cytology | 62 | <0.001 | 0.844 (0.825, 0.862) | 0.024 | 0.044 |
| CS | 32 | <0.001 | 0.860 (0.839, 0.879) | 0.029 | 0.145 |
| LBP | 8 | <0.001 | 0.861 (0.797, 0.908) | 0.460 | 0.332 |
| CB | 2 | 0.942 | 0.859 (0.758, 0.922) | - | 0.544 |
| Core needle biopsy | 10 | 0.002 | 0.896 (0.844, 0.932) | 0.344 |
CI, confidence interval; MAR, meta-regression test; ALNs, axillary lymph nodes; CS, conventional smear; LBC, liquid-based preparation; CB, cell block; *, compared to core needle biopsy subgroup in a meta-regression test.
3.3. Diagnostic Test Accuracy Review of Assessments for Axillary Lymph Nodes
Estimated sensitivities of US-FNAC and CNB were 0.760 (95% CI 0.723–0.794) and 0.849 (95% CI 0.776–0.901), respectively (Table 3). In subgroup analysis based on methods of cytological preparation, the sensitivity was the highest in conventional smear than LBP and cell block. Estimated specificities of US-FNAC and CNB were 0.997 (95% CI 0.990–0.999) and 1.000 (95% CI 0.002–1.000). There was no difference in diagnostic OR between US-FNAC and CNB (113.256, 95% CI 71.292–179.922 vs. 119.486, 95% CI 53.021–269.271). Diagnostic OR was the highest in liquid-based preparation compared to other methods. The AUC on SROC of CNB was higher than that of US-FNAC (0.951 vs. 0.922). Diagnostic accuracy of US-FNAC in ALN according to the results of axillary ultrasonography was summarized in Table S2.
Table 3.
Sensitivity, specificity, diagnostic odds ratio and area under curve of summary receiver operation characteristics curve of ultrasonography-guided fine-needle aspiration cytology and core needle biopsy in axillary lymph node of breast cancers.
| Comparison | Included Studies | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
Diagnostic OR (95% CI) |
AUC on SROC |
|---|---|---|---|---|---|
| Fine-needle aspiration cytology | 62 | 0.760 (0.723, 0.794) | 0.997 (0.990, 0.999) | 113.256 (71.292, 179, 922) | 0.922 |
| CS | 32 | 0.791 (0.750, 0.827) | 0.996 (0.982, 0.999) | 122.599 (68.009, 221.008) | 0.934 |
| LBP | 8 | 0.784 (0.717, 0.839) | 1.000 (0.000, 1.000) | 217.586 (49.755, 951.541) | 0.917 |
| CB | 2 | 0.643 (0.454, 0.796) | - | 72.146 (8.546, 609.058) | 0.934 |
| Core needle biopsy | 10 | 0.849 (0.776, 0.901) | 1.000 (0.002, 1.000) | 119.486 (53.021, 269.271) | 0.951 |
CI, confidence interval; OR, odds ratio; AUC, area under curve; SROC, summary receiver operating characteristic; CS, conventional smear; LBC, liquid-based preparation; CB, cell block.
4. Discussion
In preoperative assessments of breast cancer, US-FNAC or CNB is recommended for enlarged and suspect in a clinical trial and/or AUS ALNs. In patients found negative using US-FNAC or CNB, SLNB is performed through a frozen biopsy. To reduce unnecessary SLNB, lowering the false-negative rate in US-FNAC or CNB is necessary. If apparent data for preoperative US-FNAC and CNB of ALNs can be obtained, then important information can be further extracted from said data to reduce the false-negative rate. However, at present, there is limited detailed information in individual articles that can be obtained. To the best of our knowledge, the present study is the first meta-analysis and DTA review that compares US-FNAC and CNB for ALN assessment in patients with breast cancer.
Recommendations of preoperative assessment of ALNs can be differed based on AUS findings. If AUS is negative, SLNB without preoperative US-FNAC/CNB is recommended. In patients with suspicious ALNs in AUS, US-FNAC/CNB is recommended to define the preoperative staging. That is, in daily practice, US-FNAC/CNB is performed for only patients with suspicious ALN in AUS. If the LN is judged to be non-suspicious during the AUS, then it is possible that the US-FNAC or CNB did not perform appropriately. Therefore, such cases have no impact on the diagnostic accuracy of US-FNAC/CNB. In addition, when US-FNAC/CNB is performed for non-suspicious ALNs, these cases may be classified as true negatives. In AUS, ALNs are determined to be suspicious or not based on axillary nodal characteristics, such as LN size, cortical thickness, the ratio of long/short axis and a fatty hilum. However, ALNs can also be enlarged by benign conditions, such as hyperplasia and inflammation. If the strict criteria of AUS are applied, the sensitivity of US-FNAC/CNB may increase; however, this may result in an increase in the intraoperative SLNB rate. Therefore, a comparison of the diagnostic accuracy between suspicious and non-suspicious subgroups in AUS would be useful. In the present study, the diagnostic accuracy was significantly higher in the suspicious subgroup than in the non-suspicious subgroup (0.845 vs. 0.726; p = 0.048 in a meta-regression test; data not shown). The accuracy of AUS is also important for improving the diagnostic accuracy of preoperative assessments of ALNs.
AUS is a basic and initial diagnostic tool used for patients with breast cancer [70,71]. Although the ability of AUS to provide high-quality images has gradually improved, the appearance of ALNs with normal-appearing morphology ranges between 26% and 52% [8,11,59,72,73,74,75]. The diagnostic accuracy of AUS can be affected by various factors, including the operator’s skill and experience and the ultrasound equipment used. The diagnostic accuracy of preoperative assessments can be improved through US-FNAC/CNB rather than the only US. In a previous study, the false-negative rate was approximately 90% for ALNs smaller than 5 mm. However, following the use of US-FNAC, the false-negative rate decreased to 9–41% [41,76,77]. In the current diagnostic algorithm, US-FNAC was recommended for suspicious ALNs detected during AUS. Lowering the false-negative rate of US and US-FNAC/CNB may be supported by improving the diagnostic accuracy and reducing inappropriate SLNB use.
If tumor cells are identified during US-FNAC or CNB, diagnosis is confirmed as metastatic ALNs. However, when the results of US-FNAC or CNB are negative, the possibility of a false negative by sampling error or overdiagnosis of AUS should be considered [78,79]. Because of these cases, the sensitivity can be lowered. In the pathological evaluation, the tumor foci can be classified into isolated tumor cells, micrometastasis or macrometastasis of ALNs. In preoperative US-FNAC, isolated tumor cells or micrometastasis may result in lower sensitivity compared to macrometastasis [55,74,80,81]. However, the correlation between the size of the metastatic foci and the false-negative rate is not clear. Retrospective confirmation of the diagnostic accuracy of US-FNAC/CNB is not easy because of the challenges presented by targeting and matching ALN-conducted US-FNAC/CNB. In addition, when ALNs with isolated tumor cells or micrometastasis are considered to be non-suspicious findings in AUS, these cases are classified as true negative or skipped US-FNAC/CNB. Thus, ALNs with isolated tumor cells or micrometastasis have no significant impact on the diagnostic accuracy of preoperative US-FNAC/CNB. As per the report of Kane et al. [34], macrometastasis and micrometastasis in the false-negative cases of FNAC were 69% and 31%, respectively. In a previous study, the false-negative rate was significantly correlated with the size of the suspicious ALNs [82]. Furthermore, US-FNAC with inadequate sampling may induce delayed treatments by having to resample [16,83]. On the contrary, the false-positive rates in US-FNAC have been shown to be 1.4–1.7% in previous studies [16,74], while false-positive cases have been shown to be caused by interpretation error during cytological examination [16,74].
US-FNAC has various advantages, including minimal invasiveness, safety, simplicity and low cost. In eligible studies, the sensitivity and specificity of US-FNAC ranged between 0.250 and 0.970 and 0.450 and 1.000, respectively, with a pooled sensitivity of 0.760 (95% CI 0.723–0.794). In daily practice, methods of cytological preparation include CS, LBP and cell block with pooled sensitivities 0.791, 0.784 and 0.643, respectively. The advantage of the cell block method is its ability to conduct ancillary tests. However, the sensitivity of the cell block was shown to be lower than that of the other cytological methods. In daily practice, cell block is additionally prepared with LBP for microscopic examination. Therefore, it would be reasonable to assume that the sensitivity of cell block is similar to the other cytological methods, CS and LBP. The effect of rapid-on site cytologic examination (ROSE) on the assessment of ALNs was investigated in previous studies [20,55]. However, there was no significant difference in the diagnostic accuracy between LBP with and without ROSE. O’Leary et al. reported that ROSE was helpful in assessments of ALNs [55]; however, the false-negative rate did not reduce after the application of ROSE [55]. Although ROSE can improve the sample adequacy, the improvement of diagnostic accuracy is not clear in assessments of ALNs. On the contrary, LBP is an automated method that can conduct ancillary tests, including genetic tests and immunocytochemistry. These ancillary tests may improve diagnostic accuracy; thus, ROSE may be more useful in CS, which is not reproducible.
In CNB, sensitivity and specificity ranged between 0.609 and 1.000 and 0.842 and 1.000, respectively, while the pooled specificities of US-FNAC and CNB were 0.997 and 1.000, respectively. There was no significant difference between the methodology of the preoperative assessments. Some studies reported an improved diagnostic accuracy of CNB compared to US-FNAC [84,85,86]. The sensitivity of CNB was higher than in US-FNAC (0.849 vs. 0.760). Indeed, in the present meta-analysis, the diagnostic accuracy was significantly higher in CNB than US-FNAC (0.896 vs. 0.844; p = 0.044 in a meta-regression test). However, we compared the diagnostic accuracy between CNB and each method of cytological preparation. Although a statistical significance between CNB and overall US-FNAC was found, there was no significant difference in the diagnostic accuracy between CNB and each method of cytological preparation.
However, this study has some limitations that need to be addressed. First, the needle gauge size and numbers of passage can be affected by the sample adequacy and diagnostic accuracy. However, a detailed analysis could not be performed due to insufficient information. Second, a detailed analysis for the causes of false-negative rates should be performed in a DTA review. Basically, US-FNAC and CNB have sampling errors; however, because of insufficient information in the eligible studies, detailed analyses could not be conducted. Third, in the previous study, CNB has disadvantages, such as bleeding and high cost, compared to US-FNAC [84]. The technical problem or adverse effect between US-FNAC and CNB will be needed in further studies. In addition, the impact of clinician’s skill on the adequacy of sampling may be more important rather than methodology of biopsy itself. However, this impact could not be evaluated due to insufficient information.
5. Conclusions
In conclusion, US-FNAC and CNB are useful diagnostic tools in preoperative assessments of suspicious ALNs in patients with breast cancer. The diagnostic accuracies of various US-FNAC methods are similar and the diagnostic accuracy of CNB is higher than that of US-FNAC.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/9/717/s1, Table S1. PRISMA Checklist; Table S2. Diagnostic accuracy of ultrasonography-guided fine-needle aspiration cytology in axillary lymph node of breast cancers according to the results of axillary ultrasonography.
Author Contributions
Conceptualization, J.-S.P. and J.J.; methodology, S.G.L.; software, J.-S.P.; data curation, N.-Y.K.; writing—original draft preparation, J.-S.P. and J.J.; writing—review and editing, D.-W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Caudle A.S., Hunt K.K., Kuerer H.M., Meric-Bernstam F., Lucci A., Bedrosian I., Babiera G.V., Hwang R.F., Ross M.I., Feig B.W., et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: A practice-changing trial. Ann. Surg. Oncol. 2011;18:2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., Zackrisson S., Cardoso F. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015;26(Suppl. 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [Google Scholar]
- 3.Abe H., Schmidt R.A., Kulkarni K., Sennett C.A., Mueller J.S., Newstead G.M. Axillary lymph nodes suspicious for breast cancer metastasis: Sampling with US-guided 14-gauge core-needle biopsy--clinical experience in 100 patients. Radiology. 2009;250:41–49. doi: 10.1148/radiol.2493071483. [DOI] [PubMed] [Google Scholar]
- 4.Ahn H.S., Kim S.M., Jang M., La Yun B., Kim S.W., Kang E., Park S.Y., Moon W.K., Choi H.Y. Comparison of sonography with sonographically guided fine-needle aspiration biopsy and core-needle biopsy for initial axillary staging of breast cancer. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2013;32:2177–2184. doi: 10.7863/ultra.32.12.2177. [DOI] [PubMed] [Google Scholar]
- 5.Attieh M., Jamali F., Berjawi G., Saadeldine M., Boulos F. Shortcomings of ultrasound-guided fine needle aspiration in the axillary management of women with breast cancer. World J. Surg. Oncol. 2019;17:208. doi: 10.1186/s12957-019-1753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barco I., Chabrera C., García-Fernández A., Fraile M., González S., Canales L., Lain J.M., González C., Vidal M.C., Vallejo E., et al. Role of axillary ultrasound, magnetic resonance imaging, and ultrasound-guided fine-needle aspiration biopsy in the preoperative triage of breast cancer patients. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2017;19:704–710. doi: 10.1007/s12094-016-1589-7. [DOI] [PubMed] [Google Scholar]
- 7.Bedrosian I., Bedi D., Kuerer H.M., Fornage B.D., Harker L., Ross M.I., Ames F.C., Krishnamurthy S., Edeiken-Monroe B.S., Meric F., et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann. Surg. Oncol. 2003;10:1025–1030. doi: 10.1245/ASO.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Bonnema J., van Geel A.N., van Ooijen B., Mali S.P., Tjiam S.L., Henzen-Logmans S.C., Schmitz P.I., Wiggers T. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: New diagnostic method. World J. Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 9.Boughey J.C., Middleton L.P., Harker L., Garrett B., Fornage B., Hunt K.K., Babiera G.V., Dempsey P., Bedrosian I. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am. J. Surg. 2007;194:450–455. doi: 10.1016/j.amjsurg.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Breitbach G.P., Uhlmann J.H., Bohle R.M., Juhasz-Böss I., Linxweiler B., Takacs F.Z., Solomayer E.F., Juhasz-Böss S. Preoperative morphological diagnosis of axillary lymph nodes in a breast center consultation service: Evaluation of fine-needle aspiration and core biopsy techniques. Arch. Gynecol. Obstet. 2019;300:1659–1670. doi: 10.1007/s00404-019-05331-5. [DOI] [PubMed] [Google Scholar]
- 11.Britton P.D., Goud A., Godward S., Barter S., Freeman A., Gaskarth M., Rajan P., Sinnatamby R., Slattery J., Provenzano E., et al. Use of ultrasound-guided axillary node core biopsy in staging of early breast cancer. Eur. Radiol. 2009;19:561–569. doi: 10.1007/s00330-008-1177-5. [DOI] [PubMed] [Google Scholar]
- 12.Bruzzone M., Saro F., Bruno S., Celiento T., Mazzarella G., Lanata S., Aquilano M.C., Parmigiani G., Pollone M., Gandolfo F., et al. Synergy of cytological methods in the pathological staging of breast cancer: Axillary fine-needle aspiration and intraoperative scrape cytology of the sentinel lymph node. Diagn. Cytopathol. 2018;46:919–926. doi: 10.1002/dc.23995. [DOI] [PubMed] [Google Scholar]
- 13.Caretta-Weyer H., Sisney G.A., Beckman C., Burnside E.S., Salkowsi L.R., Strigel R.M., Wilke L.G., Neuman H.B. Impact of axillary ultrasound and core needle biopsy on the utility of intraoperative frozen section analysis and treatment decision making in women with invasive breast cancer. Am. J. Surg. 2012;204:308–314. doi: 10.1016/j.amjsurg.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Castellano I., Deambrogio C., Muscarà F., Chiusa L., Mariscotti G., Bussone R., Gazzetta G., Macrì L., Cassoni P., Sapino A. Efficiency of a preoperative axillary ultrasound and fine-needle aspiration cytology to detect patients with extensive axillary lymph node involvement. PLoS ONE. 2014;9:e106640. doi: 10.1371/journal.pone.0106640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J.S., Han K.H., Kim E.K., Moon H.J., Yoon J.H., Kim M.J. Fine-needle aspirate CYFRA 21-1, an innovative new marker for diagnosis of axillary lymph node metastasis in breast cancer patients. Medicine. 2015;94:e811. doi: 10.1097/MD.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciatto S., Brancato B., Risso G., Ambrogetti D., Bulgaresi P., Maddau C., Turco P., Houssami N. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res. Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]
- 17.Cools-Lartigue J., Sinclair A., Trabulsi N., Meguerditchian A., Mesurolle B., Fuhrer R., Meterissian S. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: Predictors of accuracy and future implications. Ann. Surg. Oncol. 2013;20:819–827. doi: 10.1245/s10434-012-2609-7. [DOI] [PubMed] [Google Scholar]
- 18.De Coninck C., Noël J.C., Boutemy R., Simon P. Preoperative axillary lymph node staging by ultrasound-guided cytology using a four-level sonographic score. BMC Med. Imaging. 2016;16:13. doi: 10.1186/s12880-016-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Kanter A.Y., Menke-Pluijmers M.B., Henzen-Logmans S.C., van Geel A.N., van Eijck C.J., Wiggers T., Eggermont A.M. Reasons for failure to identify positive sentinel nodes in breast cancer patients with significant nodal involvement. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2006;32:498–501. doi: 10.1016/j.ejso.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj S., Iqbal M., Donnelly J., Corder A.P. Axillary ultrasound in invasive breast cancer: experience of our surgeons. Breast J. 2011;17:191–195. doi: 10.1111/j.1524-4741.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- 21.Engohan-Aloghe C., Hottat N., Noël J.C. Accuracy of lymph nodes cell block preparation according to ultrasound features in preoperative staging of breast cancer. Diagn. Cytopathol. 2010;38:5–8. doi: 10.1002/dc.21153. [DOI] [PubMed] [Google Scholar]
- 22.Fayyaz M.B., Niazi I.K. Diagnostic accuracy of us-fnac of axillary lymph nodes in patients with primary breast cancer using sentinel lymph node biopsy as standard reference. J. Ayub Med. Coll. Abbottabad JAMC. 2019;31:242–247. [PubMed] [Google Scholar]
- 23.Feng Y., Huang R., He Y., Lu A., Fan Z., Fan T., Qi M., Wang X., Cao W., Wang X., et al. Efficacy of physical examination, ultrasound, and ultrasound combined with fine-needle aspiration for axilla staging of primary breast cancer. Breast Cancer Res. Treat. 2015;149:761–765. doi: 10.1007/s10549-015-3280-z. [DOI] [PubMed] [Google Scholar]
- 24.Fung A.D., Collins J.A., Campassi C., Ioffe O.B., Staats P.N. Performance characteristics of ultrasound-guided fine-needle aspiration of axillary lymph nodes for metastatic breast cancer employing rapid on-site evaluation of adequacy: Analysis of 136 cases and review of the literature. Cancer Cytopathol. 2014;122:282–291. doi: 10.1002/cncy.21384. [DOI] [PubMed] [Google Scholar]
- 25.García Fernández A., Fraile M., Giménez N., Reñe A., Torras M., Canales L., Torres J., Barco I., González S., Veloso E., et al. Use of axillary ultrasound, ultrasound-fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med. Biol. 2011;37:16–22. doi: 10.1016/j.ultrasmedbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Genta F., Zanon E., Camanni M., Deltetto F., Drogo M., Gallo R., Gilardi C. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J. Surg. 2007;31:1155–1163. doi: 10.1007/s00268-007-9009-3. [DOI] [PubMed] [Google Scholar]
- 27.Gipponi M., Fregatti P., Garlaschi A., Murelli F., Margarino C., Depaoli F., Baccini P., Gallo M., Friedman D. Axillary ultrasound and Fine-Needle Aspiration Cytology in the preoperative staging of axillary node metastasis in breast cancer patients. Breast (Edinb. Scotl.) 2016;30:146–150. doi: 10.1016/j.breast.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Hayes B.D., Feeley L., Quinn C.M., Kennedy M.M., O’Doherty A., Flanagan F., O’Connell A.M. Axillary fine needle aspiration cytology for pre-operative staging of patients with screen-detected invasive breast carcinoma. J. Clin. Pathol. 2011;64:338–342. doi: 10.1136/jcp.2010.084772. [DOI] [PubMed] [Google Scholar]
- 29.Hyun S.J., Kim E.K., Yoon J.H., Moon H.J., Kim M.J. Adding MRI to ultrasound and ultrasound-guided fine-needle aspiration reduces the false-negative rate of axillary lymph node metastasis diagnosis in breast cancer patients. Clin. Radiol. 2015;70:716–722. doi: 10.1016/j.crad.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Imai N., Kitayama M., Shibahara A., Bessho Y., Shibusawa M., Noro A., Inakami K., Hanamura N., Imai H., Ogawa T. Strategy for the accurate preoperative evaluation of the number of metastatic axillary lymph nodes in breast cancer. Asian J. Surg. 2019;42:228–234. doi: 10.1016/j.asjsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto N., Aruga T., Horiguchi S., Asami H., Saita C., Onishi M., Goto R., Ishiba T., Honda Y., Miyamoto H., et al. Ultrasound-guided fine-needle aspiration of axillary lymph nodes in breast cancer: Diagnostic accuracy and role in surgical management. Diagn. Cytopathol. 2019;47:788–792. doi: 10.1002/dc.24203. [DOI] [PubMed] [Google Scholar]
- 32.Jain A., Haisfield-Wolfe M.E., Lange J., Ahuja N., Khouri N., Tsangaris T., Zhang Z., Balch C., Jacobs L.K. The role of ultrasound-guided fine-needle aspiration of axillary nodes in the staging of breast cancer. Ann. Surg. Oncol. 2008;15:462–471. doi: 10.1245/s10434-007-9623-1. [DOI] [PubMed] [Google Scholar]
- 33.Jung J., Park H., Park J., Kim H. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J. Surg. 2010;80:271–275. doi: 10.1111/j.1445-2197.2009.05090.x. [DOI] [PubMed] [Google Scholar]
- 34.Kane G., Fleming C., Heneghan H., McCartan D., James P., Trueick R., Harrington L., Nally F., Quinn C., O’Doherty A., et al. False-negative rate of ultrasound-guided fine-needle aspiration cytology for identifying axillary lymph node metastasis in breast cancer patients. Breast J. 2019;25:848–852. doi: 10.1111/tbj.13402. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.J., Park B.W., Lim J.B., Kim H.S., Kwak J.Y., Kim S.J., Park S.H., Sohn Y.M., Moon H.J., Kim E.K. Axillary lymph node metastasis: CA-15-3 and carcinoembryonic antigen concentrations in fine-needle aspirates for preoperative diagnosis in patients with breast cancer. Radiology. 2010;254:691–697. doi: 10.1148/radiol.09091031. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.Y., Kim E.K., Moon H.J., Yoon J.H., Kim M.J. Is pre-operative axillary staging with ultrasound and ultrasound-guided fine-needle aspiration reliable in invasive lobular carcinoma of the breast? Ultrasound Med. Biol. 2016;42:1263–1272. doi: 10.1016/j.ultrasmedbio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Koelliker S.L., Chung M.A., Mainiero M.B., Steinhoff M.M., Cady B. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer–correlation with primary tumor size. Radiology. 2008;246:81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]
- 38.Kramer G.M., Leenders M.W., Schijf L.J., Go H.L., van der Ploeg T., van den Tol M.P., Schreurs W.H. Is ultrasound-guided fine-needle aspiration cytology of adequate value in detecting breast cancer patients with three or more positive axillary lymph nodes? Breast Cancer Res. Treat. 2016;156:271–278. doi: 10.1007/s10549-016-3755-6. [DOI] [PubMed] [Google Scholar]
- 39.Krishnamurthy S., Sneige N., Bedi D.G., Edieken B.S., Fornage B.D., Kuerer H.M., Singletary S.E., Hunt K.K. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 40.Kuenen-Boumeester V., Menke-Pluymers M., de Kanter A.Y., Obdeijn I.M., Urich D., Van Der Kwast T.H. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur. J. Cancer (Oxf. Engl. 1990) 2003;39:170–174. doi: 10.1016/S0959-8049(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 41.Leenders M.W., Broeders M., Croese C., Richir M.C., Go H.L., Langenhorst B.L., Meijer S., Schreurs W.H. Ultrasound and fine needle aspiration cytology of axillary lymph nodes in breast cancer. to do or not to do? Breast (Edinb. Scotl.) 2012;21:578–583. doi: 10.1016/j.breast.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Leenders M., Richir M., Broeders M., Moormann G., Mollema R., Lopes Cardozo A., Meijer S., Schreurs H. Axillary staging by ultrasound-guided fine-needle aspiration cytology in breast cancer patients. still up to date? Breast J. 2013;19:637–642. doi: 10.1111/tbj.12176. [DOI] [PubMed] [Google Scholar]
- 43.Liang Y., Chen X., Zhan W., Garfield D.H., Wu J., Huang O., Li Y., Zhu L., Chen W., Shen K. Can clinically node-negative breast cancer patients with suspicious axillary lymph nodes at ultrasound but negative fine-needle aspiration be approached as having node-negative disease? Ann. Surg. Oncol. 2017;24:1874–1880. doi: 10.1245/s10434-017-5798-2. [DOI] [PubMed] [Google Scholar]
- 44.Machida Y., Kubota K., Katayama T., Toriihara A., Shibuya H. Diagnostic performance of fluorodeoxyglucose-positron emission tomography/computed tomography combined with ultrasonography-guided fine needle aspiration cytology for identifying axillary lymph node status in patients with breast cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2013;39:26–30. doi: 10.1016/j.ejso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 45.MacNeill M., Arnott I., Thomas J. Fine needle aspiration cytology is a valuable adjunct to axillary ultrasound in the preoperative staging of breast cancer. J. Clin. Pathol. 2011;64:42–46. doi: 10.1136/jcp.2010.083063. [DOI] [PubMed] [Google Scholar]
- 46.Marti J.L., Ayo D., Levine P., Hernandez O., Rescigno J., Axelrod D.M. Nonimage-guided fine needle aspiration biopsy of palpable axillary lymph nodes in breast cancer patients. Breast J. 2012;18:3–7. doi: 10.1111/j.1524-4741.2011.01180.x. [DOI] [PubMed] [Google Scholar]
- 47.Maxwell A.J., Bundred N.J., Harvey J., Hunt R., Morris J., Lim Y.Y. A randomised pilot study comparing 13 G vacuum-assisted biopsy and conventional 14 G core needle biopsy of axillary lymph nodes in women with breast cancer. Clin. Radiol. 2016;71:551–557. doi: 10.1016/j.crad.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Moorman A.M., Bourez R.L., de Leeuw D.M., Kouwenhoven E.A. Pre-operative Ultrasonographic evaluation of axillary lymph nodes in breast cancer patients: For which group still of additional value and in which group cause for special attention? Ultrasound Med. Biol. 2015;41:2842–2848. doi: 10.1016/j.ultrasmedbio.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Motomura K., Inaji H., Komoike Y., Kasugai T., Nagumo S., Hasegawa Y., Noguchi S., Koyama H. Gamma probe and ultrasonographically-guided fine-needle aspiration biopsy of sentinel lymph nodes in breast cancer patients. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2001;27:141–145. doi: 10.1053/ejso.2000.1059. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura R., Yamamoto N., Miyaki T., Itami M., Shina N., Ohtsuka M. Impact of sentinel lymph node biopsy by ultrasound-guided core needle biopsy for patients with suspicious node positive breast cancer. Breast Cancer (Tokyo Jpn.) 2018;25:86–93. doi: 10.1007/s12282-017-0795-7. [DOI] [PubMed] [Google Scholar]
- 51.O’Leary D.P., O’Brien O., Relihan N., McCarthy J., Ryan M., Barry J., Kelly L.M., Redmond H.P. Rapid on-site evaluation of axillary fine-needle aspiration cytology in breast cancer. Br. J. Surg. 2012;99:807–812. doi: 10.1002/bjs.8738. [DOI] [PubMed] [Google Scholar]
- 52.Park S.H., Kim M.J., Park B.W., Moon H.J., Kwak J.Y., Kim E.K. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann. Surg. Oncol. 2011;18:738–744. doi: 10.1245/s10434-010-1347-y. [DOI] [PubMed] [Google Scholar]
- 53.Park S.H., Kim E.K., Park B.W., Kim S.I., Moon H.J., Kim M.J. False negative results in axillary lymph nodes by ultrasonography and ultrasonography-guided fine-needle aspiration in patients with invasive ductal carcinoma. Ultraschall der Med. (Stuttg. Ger. 1980) 2013;34:559–567. doi: 10.1055/s-0032-1313113. [DOI] [PubMed] [Google Scholar]
- 54.Podkrajsek M., Music M.M., Kadivec M., Zgajnar J., Besic N., Pogacnik A., Hocevar M. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur. Radiol. 2005;15:1044–1050. doi: 10.1007/s00330-004-2545-4. [DOI] [PubMed] [Google Scholar]
- 55.Popli M.B., Sahoo M., Mehrotra N., Choudhury M., Kumar A., Pathania O.P., Thomas S. Preoperative ultrasound-guided fine-needle aspiration cytology for axillary staging in breast carcinoma. Australas. Radiol. 2006;50:122–126. doi: 10.1111/j.1440-1673.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 56.Rao R., Lilley L., Andrews V., Radford L., Ulissey M. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann. Surg. Oncol. 2009;16:1170–1175. doi: 10.1245/s10434-009-0421-9. [DOI] [PubMed] [Google Scholar]
- 57.Rattay T., Muttalib M., Khalifa E., Duncan A., Parker S.J. Clinical utility of routine pre-operative axillary ultrasound and fine needle aspiration cytology in patient selection for sentinel lymph node biopsy. Breast (Edinb. Scotl.) 2012;21:210–214. doi: 10.1016/j.breast.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Rautiainen S., Masarwah A., Sudah M., Sutela A., Pelkonen O., Joukainen S., Sironen R., Kärjä V., Vanninen R. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: Comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology. 2013;269:54–60. doi: 10.1148/radiol.13122637. [DOI] [PubMed] [Google Scholar]
- 59.Sapino A., Cassoni P., Zanon E., Fraire F., Croce S., Coluccia C., Donadio M., Bussolati G. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: Role in breast cancer management. Br. J. Cancer. 2003;88:702–706. doi: 10.1038/sj.bjc.6600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiettecatte A., Bourgain C., Breucq C., Buls N., De Wilde V., de Mey J. Initial axillary staging of breast cancer using ultrasound-guided fine needle aspiration: A liquid-based cytology study. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2011;22:30–35. doi: 10.1111/j.1365-2303.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- 61.Swinson C., Ravichandran D., Nayagam M., Allen S. Ultrasound and fine needle aspiration cytology of the axilla in the pre-operative identification of axillary nodal involvement in breast cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2009;35:1152–1157. doi: 10.1016/j.ejso.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Topal U., Punar S., Taşdelen I., Adim S.B. Role of ultrasound-guided core needle biopsy of axillary lymph nodes in the initial staging of breast carcinoma. Eur. J. Radiol. 2005;56:382–385. doi: 10.1016/j.ejrad.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Tsai W.C., Lin C.K., Wei H.K., Yu B.L., Hung C.F., Cheng S.H., Chen C.M. Sonographic elastography improves the sensitivity and specificity of axilla sampling in breast cancer: A prospective study. Ultrasound Med. Biol. 2013;39:941–949. doi: 10.1016/j.ultrasmedbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Usmani S., Ahmed N., Al Saleh N., abu Huda F., Amanguno H.G., Amir T., al Kandari F. The clinical utility of combining pre-operative axillary ultrasonography and fine needle aspiration cytology with radionuclide guided sentinel lymph node biopsy in breast cancer patients with palpable axillary lymph nodes. Eur. J. Radiol. 2015;84:2515–2520. doi: 10.1016/j.ejrad.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Van Berckelaer C., Huizing M., Van Goethem M., Vervaecke A., Papadimitriou K., Verslegers I., Trinh B.X., Van Dam P., Altintas S., Van den Wyngaert T., et al. Preoperative ultrasound staging of the axilla make’s peroperative examination of the sentinel node redundant in breast cancer: saving tissue, time and money. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;206:164–171. doi: 10.1016/j.ejogrb.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Van Wely B.J., de Wilt J.H., Schout P.J., Kooistra B., Wauters C.A., Venderinck D., Strobbe L.J. Ultrasound-guided fine-needle aspiration of suspicious nodes in breast cancer patients; selecting patients with extensive nodal involvement. Breast Cancer Res. Treat. 2013;140:113–118. doi: 10.1007/s10549-013-2624-9. [DOI] [PubMed] [Google Scholar]
- 67.Zhang F., Zhang J., Meng Q.X., Zhang X. Ultrasound combined with fine needle aspiration cytology for the assessment of axillary lymph nodes in patients with early stage breast cancer. Medicine. 2018;97:e9855. doi: 10.1097/MD.0000000000009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong J., Sun D.S., Wei W., Liu X., Liu J., Wu X., Zhang Y., Luo H., Li Y. Contrast-enhanced ultrasound-guided fine-needle aspiration for sentinel lymph node biopsy in early-stage breast cancer. Ultrasound Med. Biol. 2018;44:1371–1378. doi: 10.1016/j.ultrasmedbio.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Y., Zhou W., Zhou J.Q., Fei X.C., Ye T.J., Huang O., Chen X.S., Zhan W.W. Axillary staging of early-stage invasive breast cancer by ultrasound-guided fine-needle aspiration cytology: Which ultrasound criteria for classifying abnormal lymph nodes should be adopted in the Post-ACOSOG Z0011 trial era? J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016;35:885–893. doi: 10.7863/ultra.15.06019. [DOI] [PubMed] [Google Scholar]
- 70.Ewing D.E., Layfield L.J., Joshi C.L., Travis M.D. Determinants of false-negative fine-needle aspirates of axillary lymph nodes in women with breast cancer: Lymph node size, cortical thickness and hilar fat retention. Acta Cytol. 2015;59:311–314. doi: 10.1159/000440797. [DOI] [PubMed] [Google Scholar]
- 71.Deurloo E.E., Tanis P.J., Gilhuijs K.G., Muller S.H., Kröger R., Peterse J.L., Rutgers E.J., Valdés Olmos R., Schultze Kool L.J. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur. J. Cancer (Oxf. Engl. 1990) 2003;39:1068–1073. doi: 10.1016/S0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 72.Ertan K., Linsler C., di Liberto A., Ong M.F., Solomayer E., Endrikat J. Axillary ultrasound for breast cancer staging: An attempt to identify clinical/histopathological factors impacting diagnostic performance. Breast Cancer Basic Clin. Res. 2013;7:35–40. doi: 10.4137/BCBCR.S11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damera A., Evans A.J., Cornford E.J., Wilson A.R., Burrell H.C., James J.J., Pinder S.E., Ellis I.O., Lee A.H., Macmillan R.D. Diagnosis of axillary nodal metastases by ultrasound-guided core biopsy in primary operable breast cancer. Br. J. Cancer. 2003;89:1310–1313. doi: 10.1038/sj.bjc.6601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Kanter A.Y., van Eijck C.H., van Geel A.N., Kruijt R.H., Henzen S.C., Paul M.A., Eggermont A.M., Wiggers T. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br. J. Surg. 1999;86:1459–1462. doi: 10.1046/j.1365-2168.1999.01243.x. [DOI] [PubMed] [Google Scholar]
- 75.Van Rijk M.C., Deurloo E.E., Nieweg O.E., Gilhuijs K.G., Peterse J.L., Rutgers E.J., Kröger R., Kroon B.B. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann. Surg. Oncol. 2006;13:31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Vassallo P., Wernecke K., Roos N., Peters P.E. Differentiation of benign from malignant superficial lymphadenopathy: The role of high-resolution US. Radiology. 1992;183:215–220. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 77.Del Bianco P., Zavagno G., Burelli P., Scalco G., Barutta L., Carraro P., Pietrarota P., Meneghini G., Morbin T., Tacchetti G., et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: Results of the sentinella-GIVOM Italian randomised clinical trial. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2008;34:508–513. doi: 10.1016/j.ejso.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Stachs A., Göde K., Hartmann S., Stengel B., Nierling U., Dieterich M., Reimer T., Gerber B. Accuracy of axillary ultrasound in preoperative nodal staging of breast cancer-size of metastases as limiting factor. SpringerPlus. 2013;2:350. doi: 10.1186/2193-1801-2-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akıncı M., Bulut S.P., Erözgen F., Gürbüzel M., Gülşen G., Kocakuşak A., Gülen M., Kaplan R. Predictive value of fine needle aspiration biopsy of axillary lymph nodes in preoperative breast cancer staging. Ulus. Cerrahi Derg. 2016;32:191–196. doi: 10.5152/ucd.2015.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilani S.M., Fathallah L., Al-Khafaji B.M. Preoperative fine needle aspiration of axillary lymph nodes in breast cancer: Clinical utility, diagnostic accuracy and potential pitfalls. Acta Cytol. 2014;58:248–254. doi: 10.1159/000362682. [DOI] [PubMed] [Google Scholar]
- 81.Mustonen P., Farin P., Kosunen O. Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann. Chir. Gynaecol. 1990;79:15–18. [PubMed] [Google Scholar]
- 82.Yang W.T., Ahuja A., Tang A., Suen M., King W., Metreweli C. High resolution sonographic detection of axillary lymph node metastases in breast cancer. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 1996;15:241–246. doi: 10.7863/jum.1996.15.3.241. [DOI] [PubMed] [Google Scholar]
- 83.Luparia A., Campanino P., Cotti R., Lucarelli D., Durando M., Mariscotti G., Gandini G. Role of axillary ultrasound in the preoperative diagnosis of lymph node metastases in patients affected by breast carcinoma. Radiol. Med. 2010;115:225–237. doi: 10.1007/s11547-009-0465-8. [DOI] [PubMed] [Google Scholar]
- 84.Balasubramanian I., Fleming C.A., Corrigan M.A., Redmond H.P., Kerin M.J., Lowery A.J. Meta-analysis of the diagnostic accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph node metastasis. Br. J. Surg. 2018;105:1244–1253. doi: 10.1002/bjs.10920. [DOI] [PubMed] [Google Scholar]
- 85.Topps A.R., Barr S.P., Pikoulas P., Pritchard S.A., Maxwell A.J. Pre-operative axillary ultrasound-guided needle sampling in breast cancer: Comparing the sensitivity of fine needle aspiration cytology and core needle biopsy. Ann. Surg. Oncol. 2018;25:148–153. doi: 10.1245/s10434-017-6090-1. [DOI] [PubMed] [Google Scholar]
- 86.Vidya R., Iqbal F.M., Bickley B. Pre-operative axillary staging: Should core biopsy be preferred to fine needle aspiration cytology? Ecancermedicalscience. 2017;11:724. doi: 10.3332/ecancer.2017.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.