Abstract
Purpose
Programmed death ligand 1 (PD-L1) is widely used for predicting immune checkpoint inhibitors but has a limited effect on predicting clinical response. The aim of this study was to examine the prognostic value and PD-1 inhibitor therapeutic efficiency of SNX20 in lung adenocarcinoma.
Methods
We evaluated the mRNA and protein expression levels of SNX20 and PD-L1 and confirmed their predictive role in clinical response to anti-PD-1 therapy in 56 patients with advanced, refractory lung adenocarcinoma treated with PD-1 inhibitors. The expression of SNX family in different cancer types and the relationship between SNX20 and immune cells were evaluated in TCGA. The protein expression levels of SNX20, PD-L1 in 56 lung adenocarcinoma tissues were evaluated by immunohistochemistry.
Results
SNX20 mRNA expression has the strongest relationship with CD8a of the sorting nexin (SNX) family in lung adenocarcinoma and is strongly correlated with immune infiltration levels in 30 cancer types, especially in lung adenocarcinoma. A positive correlation between SNX20 and PD-L1 was found based on immunohistochemical data (Pearson’s r=0.3731 and p=0.0466). SNX20 and PD-L1 were also observed to have a significant positive correlation at the mRNA level. According to the receiver operating characteristic (ROC) curve, the best expression differentiation score of SNX20 and PD-L1 between responder versus non-responders in patients with lung adenocarcinoma using PD-1 inhibitors is 5. In univariate logistic regression analysis, both SNX20 (odds ratio [OR]=3.778, p=0.019) and PD-L1 (OR=5.727, p=0.004) expression levels are significant predictors of clinical response in the PD-1 inhibitor responder group, and SNX20 (OR=3.575, p=0.038) and PD-L1 (OR=5.484, p=0.007) are also predictors of the response to PD-1 inhibitors in the multivariate analysis. High SNX20/high PD-L1 expression group had longer overall survival than patients with high SNX20/low PD-L1 expression group or low SNX20/high PD-L1 expression group (p=0.013) and patients with low SNX20/low PD-L1 expression group (p=0.01).
Conclusion
SNX20 expression can be a promising predictor for therapeutic decision-making and treatment response assessment regarding PD-1 inhibitors, and special attention is required for the subgroup of patients with lung adenocarcinoma whose tumors express both high SNX20 and PD-L1.
Keywords: SNX20, PD-L1, immunotherapy, tumor microenvironment, lung adenocarcinoma
Introduction
In recent years, the use of PD-1/PD-L1 immune checkpoint inhibitors has offered new hope for the treatment of advanced non-small cell lung cancer (NSCLC).1 Currently, four PD-1/PD-L1 inhibitor-related immunotherapeutic drugs (nivolumab,2 pembrolizumab,3 atezolizumab,4 and durvalumab5) have been approved by the US FDA and the European Medicines Agency as the first- or second-line treatment for NSCLC, thus providing new and better options for patients with NSCLC. Although PD-1/PD-L1 inhibitors have resulted in major progress in the comprehensive treatment of NSCLC, the effectiveness of immunotherapy is low, at approximately 15–20%.6 The search for effective predictive markers of efficacy is a critical issue that must be urgently addressed in the field of NSCLC treatment.
It is generally believed that high PD-L1 expression can improve the response rate and clinical benefit of immunotherapy,7 but this cannot explain why some patients with low or no PD-L1 expression still benefit from immunotherapy. Clinical detection of PD-L1 protein levels has encountered some problems, such as the high heterogeneity of PD-L1 expression in tumors, and the dynamic changes of PD-L1 in treatment and disease development and progression.8 There are also inconsistencies in detection with different antibodies and platforms. Therefore, microsatellite instability (MSI-H),9 tumor mutation burden (TMB),10 tumor infiltrating lymphocytes (TIL),11 and POLD1 and POLE variants can further help guide PD-1 application.7,12 In summary, no good indicators are currently available to predict the efficacy of PD-1 monoclonal antibodies.
The sorting nexin (SNX) family is a class of functional proteins involved in protein sorting and transport.13 They are abnormally expressed in a variety of tumors and are associated with cancer development, metastasis, and disorders of the.14–17 We used the information in the cBioPortal for Cancer Genomics database (http://www.cbioportal.org/) to analyze the relationship between SNX family proteins, CD8 T cells, and lung adenocarcinoma, and found that SNX20 was most strongly correlated with CD8a. Relatively few studies have investigated SNX20. Studies have found that SNX20 acts as a sorting molecule that cycles PSGL-1 into endosomes.18 However, it remains unclear whether SNX20 is associated with PD-L1 and immunotherapy efficacy in advanced lung adenocarcinoma.
In this study, immunohistochemistry (IHC) and TCGA sequencing were used to determine the correlation between SNX20 and PD-L1 at the protein and mRNA expression levels. The aim was to elucidate the relationship between SNX20 and PD-L1 to allow prediction of the efficacy of PD-1 in the treatment of advanced lung adenocarcinoma.
Materials and Methods
Patients and Clinical Data Collection
A total of 56 patients with Stage IIIB and Stage IV advanced lung adenocarcinoma were enrolled; they were treated with PD-1 immune checkpoint inhibitor therapy and had pretreatment cancer tissues in the First Affiliated Hospital of Nanchang University from 2017 to 2020. The clinicopathological features of the patients with lung adenocarcinoma are described in Table 1. The overall survival time was calculated from the date of accepting anti-PD-1 therapy to the date of the final follow-up or death. Patients treated with PD-1 immune checkpoint inhibitors were classified into the responder group (complete response, partial response, or stable disease) or the non-responder group (progressing disease) according to iRECIST19 (modified RECIST 1.1 for immune-based therapeutics, iRECIST).
Table 1.
Demographic and Clinical Characteristics of Patients
| Variables | Number |
|---|---|
| Age, median (ranges) (years) | 62 (50–71) |
| Gender | |
| Male | 39 |
| Female | 17 |
| Smoking history | |
| Yes | 13 |
| NO | 43 |
| Clinical stage at diagnosis | |
| III | 8 |
| IV | 48 |
| Genetic alteration status | |
| EGFR-mutated | 6 |
| Wild type | 50 |
| Type of PD-1 blockade | |
| Nivolumab | 39 |
| Pembrolizumab | 17 |
| PD-L1 expression | |
| Low | 32 |
| High | 24 |
| SNX20 expression | |
| Low | 26 |
| High | 30 |
| Response to PD-1 blockade | |
| Responder | 29 |
| Non-responder | 27 |
Abbreviations: EGFR, epidermal growth factor receptor; PD-1, programmed death 1; PD-L1, programmed death ligand 1.
All the cancer tissues were collected after approval by the ethical committee of the First Affiliated Hospital of Nanchang University, and written informed consent was obtained from all participants or their appropriate surrogates. All patient specimens and clinical data involved in this study complied with the Declaration of Helsinki.
Immunohistochemistry (IHC) Staining and Scoring of SNX20 and PD-L1
Immunohistochemistry assays were performed and quantified as previously described.20 IHC staining was carried out using anti-SNX20 antibody (ab193191) at 1/100 dilution and SP263 (Ventana) assays to test PD-L1 expression for patients with lung adenocarcinoma. The proportion of positively stained cancer cells was graded as follows: 0 (0%), 1 (<10%), 2 (<50%), 3 (<75%), and 4 (≥75%). The staining intensity of the cancer cells was recorded as follows: 0 (no staining), 1 (weak, light yellow), 2 (moderate, yellow brown), and 3 (strong, brown). The staining index (SI) was calculated as the proportion of positively stained cells × the staining intensity, which were displayed as scores of 0, 1, 2, 3, 4, 6, 8, 9, and 12.21 The cutoff values for SNX20 and PD-L1 were determined by receiver operating curve (ROC) analysis. We enlisted the help of two senior pathologists to read the slides for H&E, SNX20, and PD-L1.
Analysis of the TCGA Data
We used the information in the cBioPortal22 for Cancer Genomics database (http://www.cbioportal.org/) to analyze the relationship between SNX family, immune cells, and lung adenocarcinoma. The online database Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/index.html)23 was used to further confirm the significantly correlated genes in cBioPortal. The mRNA levels of SNX20 were downloaded from the human protein atlas (https://www.proteinatlas.org/search/SNX20). TISIDB24 is a web portal for tumor and immune system interaction (http://cis.hku.hk/TISIDB/index.php). TIMER25 is a web resource for systematical evaluations of the clinical role of different immune cells in some cancer types (http://cistrome.dfci.harvard.edu/TIMER/) and was used to further confirm the results in TISIDB. We use OncoLnc (http://www.oncolnc.org/) to analyze the prognostic value of SNX20 mRNA in lung adenocarcinoma from the TCGA data.
Statistical Analysis
The correlation of gene expression was evaluated by Spearman correlation and statistical significance. Data were analyzed using the statistical software SPSS 18.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7.0. The probability of clinical benefit from a PD-1 immune checkpoint inhibitor-based on clinicopathologic variables was examined by univariate and multivariate logistic regression analyses. The cutoff values for SNX20 and PD-L1 were determined by receiver operating curve (ROC) analysis. All statistical tests were two-sided and differences were considered statistically significant at P<0.05.
Results
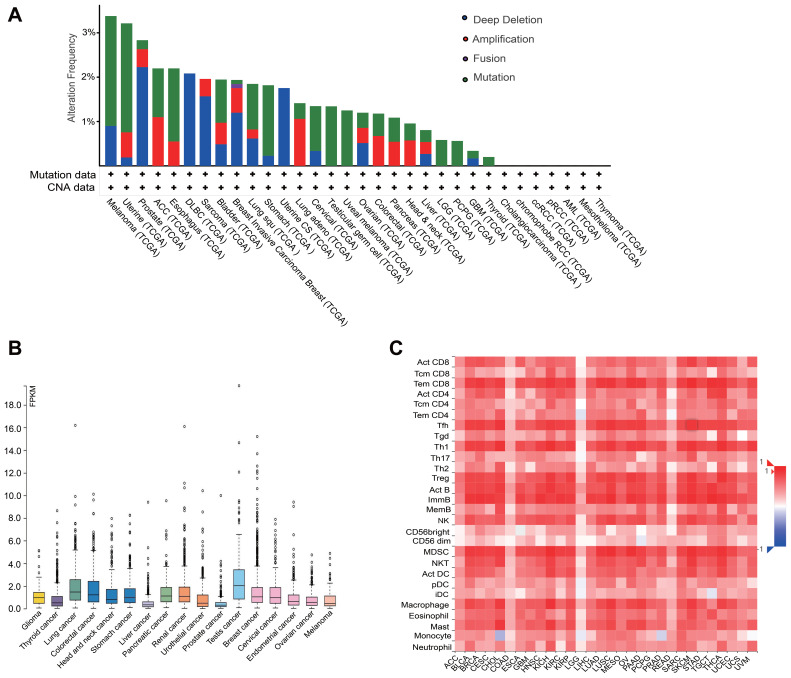
SNX20 Expression is Correlated with Immune Infiltration Level in Most Cancer Types
We used the information in the cBioPortal for Cancer Genomics database (http://www.cbioportal.org/) to analyze the relationship between SNX family and CD8 T cells, and found that SNX20 was most strongly correlated with CD8a in lung adenocarcinoma (Table 2). Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/index.html) was used to further confirm this result (Supplementary Table 1). Because little is known about SNX20 and human cancer, we first searched several available genomic and gene expression databases for SNX20 status. Our analysis of the TCGA genome database revealed that for the SNX20 gene there was no more than 3.5% change in amplification, mutation, deep deletion, and fusion in 25 types of cancer, with only 1.58% genomic change in lung adenocarcinoma (Figure 1A). Next, we examined SNX20 expression using the RNA-seq data of multiple cancers in TCGA. SNX20 mRNA had a lower expression in some cancer types, including lung cancer, colorectal, stomach, testis cancer (Figure 1B). For SNX20, mRNA was most strongly correlated with the CD8a marker in lung adenocarcinoma; we assessed the correlations of SNX20 expression with immune infiltration levels in 30 cancer types from TISIDB (Figure 1C). Interestingly, SNX20 mRNA expression, but not DNA copy number, methylation or mutation of SNX20, has significant correlations with infiltrating levels of CD8+ T cells, CD4+ T cells, B cells, NK cells, monocyte cells, and dendritic cells (DCs) in most cancer types.
Table 2.
Analyze the Relationship Between SNX Family and CD8 T Cells in Lung Adenocarcinoma (cBioPortal)
| Correlated Gene | Spearman's Correlation | p-value |
|---|---|---|
| SNX20 | 0.607 | 4.820E-52 |
| SNX10 | 0.481 | 1.550E-30 |
| SNX6 | 0.257 | 4.860E-09 |
| SNX33 | −0.25 | 1.250E-08 |
| SNX3 | 0.232 | 1.490E-07 |
| SNX11 | 0.214 | 1.260E-06 |
| SNX2 | 0.201 | 5.620E-06 |
| SNX32 | 0.165 | 1.950E-04 |
| SNX8 | 0.119 | 7.640E-03 |
| SNX27 | −0.109 | 1.440E-02 |
| SNX18 | 0.105 | 1.800E-02 |
| SNX13 | −0.0994 | 2.580E-02 |
| SNX29 | 0.0943 | 3.440E-02 |
| SNX31 | 0.0875 | 4.980E-02 |
| SNX17 | 0.861 | 5.360E-02 |
| SNX15 | 0.0807 | 7.070E-02 |
| SNX25 | −0.0793 | 7.560E-02 |
| SNX1 | −0.074 | 9.750E-02 |
| SNX14 | 0.069 | 0.122 |
| SNX19 | −0.0614 | 0.169 |
| SNX24 | 0.0567 | 0.205 |
| SNX16 | 0.0354 | 0.428 |
| SNX4 | 0.027 | 0.524 |
| SNX21 | 0.0255 | 0.569 |
| SNX5 | 0.0204 | 0.647 |
| SNX7 | 0.0199 | 0.656 |
Abbreviation: SNX, sorting nexin.
Figure 1.
SNX20 expression is correlate with immune infiltration level in most cancer types. (A) The change of SNX20 in amplification, mutation, deep deletion, and fusion in 25 types of cancer. (B) The RNA-seq data of SNX20 expression in multiple cancers. (C) The correlations of SNX20 expression with immune infiltration levels in 30 cancer types from TISIDB.
Abbreviations: SNX, sorting nexin; TISIDB, tumor and immune system interaction.
SNX20 Expression is Correlated with Immune Infiltration Level in Lung Adenocarcinoma and Predicts Good Prognosis
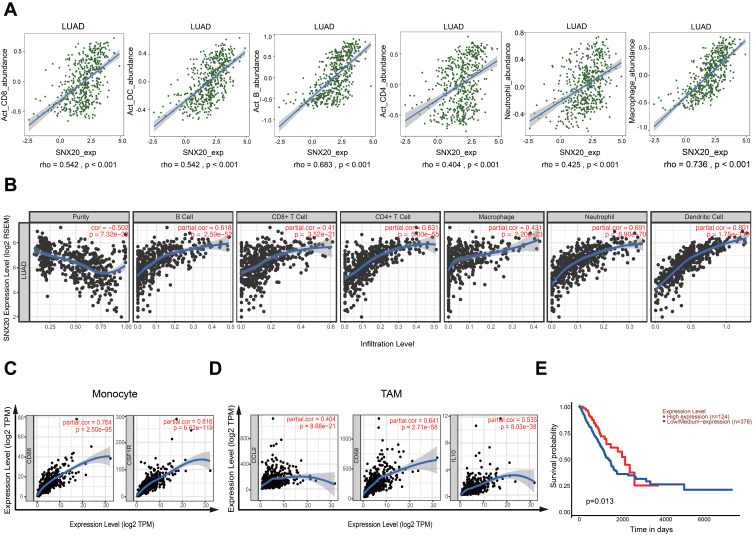
To better understand the detailed immune infiltration relationship between SNX20 and lung adenocarcinoma, we noted that the SNX20 expression level has significant positive correlations with infiltrating levels of activated CD8+ T cells (r=0.542, p<0.001), activated DCs (r=0.542, p<0.001), activated B cells (r=0.683, p<0.001), activated CD4+ T cells (r=0.404, p<0.001), neutrophils (r=0.425, p<0.001), and macrophage cells (r=0.736, p<0.001) in the TISIDB database (Figure 2A). To confirm this result, we focused on the correlations between SNX20 and immune infiltration level in the TIMER databases. We found that SNX20 has a significant correlation with tumor purity in lung adenocarcinoma. Moreover, the SNX20 expression level has significant positive correlations with infiltrating levels of activated B cells (r = 0.618, p<0.001), CD8+ T cells (r = 0.410, p<0.001), CD4+ T cells (r = 0.631, p<0.001), macrophage cells (r = 0.431, p<0.001), neutrophils (r = 0.691, p<0.001), and DCs (r = 0.801, p<0.001) (Figure 2B). Interestingly, we found that the expression levels of most marker sets of monocytes and tumor-associated macrophages (TAMs) have strong correlations with SNX20 (Figure 2C and D). To further examine the prognostic potential of SNX20 in non-small cell lung cancer (NSCLC), we noted that high SNX20 expression predicted a good prognosis for patients with lung adenocarcinoma (Figure 2E), but not for patients with lung squamous cell carcinoma (Supplementary Figure 1). These data suggest that high SNX20 expression indicated immune cell infiltration in patients with lung adenocarcinoma and a better prognosis.
Figure 2.
SNX20 expression is correlate with immune infiltration level in lung adenocarcinoma and predicts good prognosis. (A) The correlation of SNX20 and immune infiltration in lung adenocarcinoma in the TISIDB database. (B) The correlations between SNX20 and immune infiltration level in the TIMER databases. (C and D) The correlation between the expression of SNX20 and sets gene marker of monocytes (CD86, CSF1R) and TAMs (CCL2, CD68, IL10) in TIMER database. (E) High SNX20 expression predicts a good prognosis for patients with lung adenocarcinoma in TCGA.
Abbreviations: CCL2, C-C motif chemokine ligand 2; CD68, cluster of differentiation 68; CD86, cluster of differentiation 86; CSF1R, colony stimulating factor 1 receptor; IL10, interleukin 10; SNX, sorting nexin; TAMs, tumor-associated macrophages; TCGA, The Cancer Genome Atlas; TIMER, Tumor Immune Estimation Resource; TISIDB, tumor and immune system interaction.
Patient Demographics
The demographic data of patients included in this study are shown in Table 1. All samples were pulmonary specimens. There were 17 patients (30.36%) receiving pembrolizumab and 39 patients (69.64%) receiving nivolumab. In our study, all patients received PD-1 blockade therapy as a second-or-higher line of treatment because they were refractory to chemotherapy, radiotherapy, and targeted therapy. Of these, 29 patients (51.79%) responded to PD-1 inhibitors and 27 patients (48.21%) did not. There were also 6 EGFR-mutated patients who were treated with tyrosine kinase inhibitor therapy before they received PD-1 inhibitors.
Correlation Between SNX20 and PD-L1
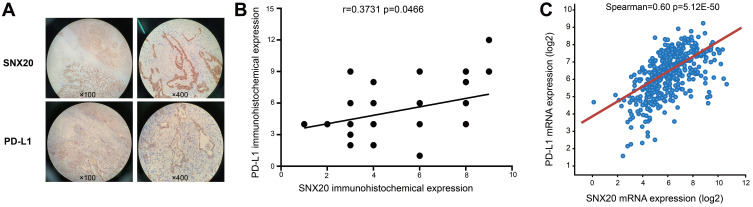
To better understand the relationship between SNX20 and immune infiltration, we examined the correlation between SNX20 and the protein- and mRNA expression of PD-L1 by using immunohistochemistry (IHC) and TCGA sequencing. We observed that the expression of SNX20 was positively correlated with protein levels of PD-L1 (r = 0.3731, p<0.0466) (Figure 3B). Higher levels of SNX20 protein expression were detected (30/56; 53.57%) (Table 1), and SNX20 was distributed in the cytoplasm (Figure 3A, Supplementary Figure 2). Higher levels of PD-L1 protein expression were detected (24/56; 42.86%) (Table 1), and PD-L1 was distributed in the cell membrane and cytoplasm (Figure 3A, Supplementary Figure 2). We used the information in the cBioPortal for Cancer Genomics database to analyze the relationship between SNX20 and PD-L1, and found that SNX20 was most strongly correlated with PD-L1 in lung adenocarcinoma (n=510, r = 0.6, p<0.001) (Figure 3C). These data suggest that SNX20 was positively correlated with PD-L1 in lung adenocarcinoma.
Figure 3.
Correlation between SNX20 and PD-L1. (A) The distribution of SNX20 and PD-L1 in lung adenocarcinoma tissues. (B) The expression of SNX20 is positively correlated with protein levels of PD-L1 in lung adenocarcinoma. (C) The mRNA level of SNX20 expression is positively correlated with mRNA levels of PD-L1 in lung adenocarcinoma.
Abbreviations: PD-L1, programmed death ligand 1; SNX20, sorting nexin 20.
Associations Between SNX20, PD-L1, Clinicopathologic Parameters, and Response to PD-1 Immune Checkpoint Inhibitor
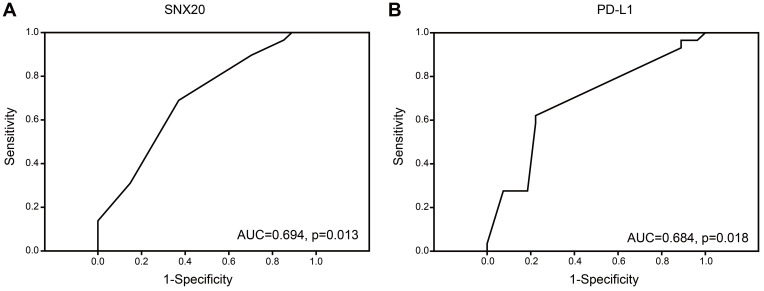
According to the receiver operating characteristic (ROC) curve, the best expression differentiation score of SNX20 and PD-L1 between responder versus non-responder in patients with lung adenocarcinoma using PD-1 inhibitors is 5, corresponding to the maximum joint sensitivity and specificity on the ROC curve (SNX20: 69% sensitivity and 63% specificity, PD-L1: 62.1% sensitivity and 77.8% specificity). Also, the area under the curve (AUC) for SNX20 and PD-L1 was 0.694 and 0.684, respectively (Figure 4A and B). Univariate and multivariate logistic regression analysis was conducted to examine the clinicopathologic factors predicting clinical response to PD-1 blockade therapy. Univariate analysis revealed that SNX20 (p=0.019, odds ratio [OR]=3.778) and PD-L1 (p=0.004, OR=5.727) were found to be predictors of the response to PD-1 blockade therapy (Table 3). Multivariate analysis revealed that SNX20 (p=0.038, OR=3.575) and PD-L1 (p =0.007, OR=5.484) were also found to be predictors of the response to PD-1 blockade therapy (Table 3).
Figure 4.
Association between SNX20, PD-L1, clinicopathologic parameters, and response to PD-1 immune checkpoint inhibitor. (A and B) The ROC of SNX20 and PD-L1 between responder versus non-responder.
Abbreviations: ROC, receiver operating characteristic; PD-1, programmed death 1; PD-L1, programmed death ligand 1; SNX20, sorting nexin 20.
Table 3.
Univariate and Multivariate Logistic Regression Analysis for Predicting Clinical Response to PD-1 Blockade
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (≥65 years vs.<65 years) | 0.655 | 0.228–1.885 | 0.433 | |||
| Sex (male vs female) | 1.312 | 0.419–4.111 | 0.641 | |||
| Smoking history (+ vs –) | 1.114 | 0.321–3.862 | 0.865 | |||
| Presence of EGFR mutation (+ vs -) | 0.694 | 0.115–4.211 | 0.692 | |||
| Type of PD-1 blockade | 0.665 | 0.21–2.104 | 0.488 | |||
| (Nivolumab vs Pembrolizumab) | ||||||
| PD-L1 (≥ 4.5 vs.<4.5) | 5.727 | 1.765–18.587 | 0.004 | 5.484 | 1.601–18.071 | 0.007 |
| SNX20 (≥5 vs <5) | 3.778 | 1.247–11.447 | 0.019 | 3.575 | 1.075–11.893 | 0.038 |
Abbreviations: CI, confidence interval; EGFR, epidermal growth factor receptor; OR, odds ratio; PD-1, programmed death 1; PD-L1, programmed death ligand 1; SNX, sorting nexin.
Prognostic Significance of SNX20, PD-L1, and Clinicopathologic Parameters
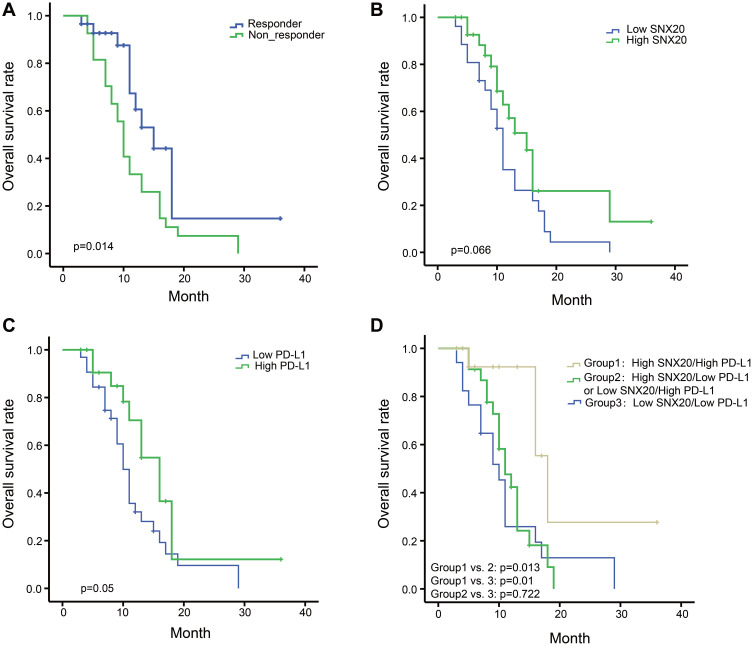
Responders to PD-1 inhibitors had a significant better overall survival compared with non-responders to PD-1 inhibitors (p=0.014) (Figure 5A). Patients with high SNX20 or high PD-L1 protein expression had better overall survival than patients with low SNX20 or low PDL-1 protein expression with borderline statistical significance (p=0.066 and p=0.05, respectively) (Figure 5B and C). However, the high SNX20/high PD-L1 group had longer overall survival than patients with high SNX20/low PD-L1 expression or low SNX20/high PD-L1 expression (p=0.013) and patients with low SNX20/low PD-L1 expression (p=0.01) (Figure 5D).
Figure 5.
Prognostic significance of SNX20, PD-L1, and clinicopathologic parameters. (A) The overall survival curve of responders to PD-1 inhibitors compared with non-responders to PD-1 inhibitors. (B) The overall survival curve of high expression of SNX20 compared with low expression of SNX20 to PD-1 inhibitors. (C) The overall survival curve of high expression of PD-L1 compare with low expression of PD-L1 to PD-1 inhibitors. (D) Effects of SNX20 and PD-L1 co-expression on overall survival in patients with PD-1 inhibitors.
Abbreviations: PD-1, programmed death 1; PD-L1, programmed death ligand 1; SNX20, sorting nexin 20.
Discussion
Three new findings were obtained in this study: (1) High SNX20 expression indicated immune cell infiltration in patients with lung adenocarcinoma and better prognosis; (2) SNX20 and PD-L1 protein expression were positively correlated and can be used to predict the efficacy of PD-1 monoclonal antibody therapy; (3) High expression of SNX20 and PD-L1 is an independent predictor of PD-1 treatment efficacy for advanced lung adenocarcinoma.
Endogenous PD-L1 expression is regulated by a variety of mechanisms, such as amplification of PD-L1 DNA levels,26 abnormal miRNA expression,27 abnormalities in p53,28 c-Myc,29 HIF-1α,30 STATs, NF-κB,31 Hippo,32 and other signaling pathways, as well as ubiquitination,33 glycosylation,34 and other post-translational modifications (PTMs) of PD-L1 protein. PD-L1 expression is dynamic and may disappear or abnormally increase under various stress conditions. Tumors stimulate the release of IFN-γ by macrophages and lymphocytes, thereby inducing the expression of PD-L1 and exerting a suppressive effect on the immune system.35 Therefore, owing to tumor heterogeneity and dynamic changes in PD-L1, this provides some explanation as to why PD-1 treatment is effective in PD-L1 expression-negative NSCLC. Thus, the discovery of new molecules to support the use of PD-L1 in predicting the efficacy of PD-1 for lung cancer immunotherapy is of great interest.
The most commonly studied members of the SNX family include SNX1, 2, 4, 9, 18, 30, 32, and 33. They are known to participate in various essential cell activities, such as protein endocytosis, sorting, transport, degradation, and signaling.13 The SNX family is closely associated with cancer. For example, SNX1 participates in the process of EGFR drug resistance in lung cancer by sorting EGFR into lysosomes for degradation.36 In addition, SNX10 expression is decreased in colorectal cancers, which promotes tumor formation and proliferation.37 Moreover, in response to inflammatory stimuli, SNX10 acts as a novel regulator of M2 polarization in macrophages and plays a regulatory role in colitis.14 Polymorphism of the SNX20 gene has been linked to inflammatory bowel disease in African Americans.38 Through analysis of cBioPortal data, we found that SNX20 was strongly correlated with CD8a (a CD8 T cell marker). Furthermore, Tumor Immune Estimation Resource (TIMER) and TISIDB analyses of the relationship between SNX20 expression and the immune microenvironment in cancer showed that SNX20 expression was positively correlated with immune infiltration in various tumors. Further analysis found that SNX20 was positively correlated with immune infiltration in lung adenocarcinoma. High SNX20 mRNA expression suggested a long overall survival (OS) time in patients with lung adenocarcinoma, whereas there was no predictive effect in lung squamous cell carcinoma.
To further understand the relationship between SNX20 and immune infiltration, we examined the correlation between SNX20 and the protein and mRNA expression of PD-L1 by using IHC and TCGA sequencing. The results showed a positive correlation for both protein and mRNA expression, suggesting that SNX20 may exert its action by stabilizing PD-L1; however, the specific mechanism needs to be investigated further. For example, studies have found that SNX16 regulates the transport of the cell membrane protein E-cadherin through a cyclic pathway and that SNX16 overexpression can delay E-cadherin degradation.39 We expect that SNX20 may also regulate PD-L1 through a similar mechanism.
Multiple clinical trials have shown that PD-L1 expression is related to the efficacy of PD-1 inhibitor therapy in advanced lung adenocarcinoma.40 We found that patients with high PD-L1 expression have a higher objective response rate and OS than patients with low expression. Given the positive correlation between SNX20 and PD-L1, we found for the first time that patients with high SNX20 expression had a better objective response rate and OS than patients with low expression. The high SNX20/high PD-L1 group had better prediction of PD-1 therapeutic efficacy and OS than the high SNX20/low PD-L1, low SNX20/high PD-L1, and low SNX20/low PD-L1 groups. Univariate and multivariate analysis showed that high SNX20/high PD-L1 was an independent predictor of PD-1 treatment efficacy for advanced lung adenocarcinoma. However, considering that tumor mutation burden (TMB) testing is expensive in China and has not yet been included in medical insurance, it is not widely available. The combined testing of SNX20/PD-L1 can thus further compensate for the insufficiency of only testing for the PD-L1 protein and thus improve the efficacy of immunotherapy.
This study has some limitations: (1) this was a retrospective study and included only a small number of cases, and thus may contain bias. (2) the samples were postoperative specimens, bronchoscopic biopsy specimens, and CT-guided lung biopsy specimens; as such, the sample processing and quality control lacked uniformity, which affected the analysis of SNX20 and PD-L1 slides. To minimize error, we took sections at the same time and enlisted the help of two senior pathologists to read the slides. (3) SNX20 and PD-L1 testing was not conducted or validated at multiple cancer centers. (4) During univariate and multivariate analysis, tumor mutation burden, tumor-infiltrating lymphocytes, and other indices were not included in the analysis. (5) Cell and animal experiments to support how SNX20 stabilizes the expression of PD-L1 are still lacking and must be conducted in the future.
Conclusion
In conclusion, the present study found that SNX20 was associated with immune infiltration in lung adenocarcinoma and other cancers, and that there was a positive correlation between SNX20 and PD-L1 expression. High SNX20/high PD-L1 group was a better predictor of PD-1 treatment efficacy and OS compared with that of other groups. Univariate and multivariate analysis showed that high expression of both SNX20 and PD-L1 was an independent predictor of PD-1 treatment efficacy in advanced lung adenocarcinoma. Combined SNX20 and PD-L1 testing is an effective predictor of therapeutic efficacy in driver gene-negative advanced lung adenocarcinoma.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant numbers: 81760432, 81860427, 81660402, and 81660405), the Jiangxi Province Talent 555 Project (grant number: 20152ACB20024), the National Natural Science Foundation of Jiangxi Province (grant numbers: 20152ACB20024, and 20151BBG70228), the Jiangxi Province General Project (grant number: 20164BCD40097, 20171BBG70121 and 2017BBH80027), the Youth Scientific Funds-Youth Fund Project (grant number: 20171BAB215041, 2018ACB21037), the Jiangxi Province Education Fund Project (grant number: 700653002), the department of health of Jiangxi Province Project (grant number: 20181041), and the Jiangxi Province Postgraduate Special Innovation Fund (grant number: YC2019-B034).
Abbreviations
PD-L1, Programmed death ligand 1; SNX, Sorting nexin; ROC, Receiver operating characteristic; OR, Odds ratio; PD-1, Programmed death 1; NSCLC, Non-small cell lung cancer; MSI-H, Microsatellite instability; TMB, Tumor mutation burden; TIL, Tumor infiltrating lymphocytes; IHC, Immunohistochemistry; DCs, Dendritic cells; TAMs, Tumor-associated macrophages; AUC, The area under the curve; GEPIA, Gene Expression Profiling Interactive Analysis; PTMs, Post-translational modifications; TIMER, Tumor Immune Estimation Resource; OS, Overall survival; TCGA, The Cancer Genome Atlas; TISIDB, Tumor and Immune System Interaction; SI, Staining index; ROC, Receiver operating curve.
Ethics Approval and Consent to Participate
All procedures followed in this study were investigated by Medical Research Ethics Committee of The First Affiliated Hospital of Nanchang University. This project conforms to the ethics requirements. Informed consent for it was obtained from all patients for their being included in the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. First authors: Linwei Fan and Li Li contributed equally to this work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. doi: 10.1634/theoncologist.2015-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pai-Scherf L, Blumenthal GM, Li H, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. 2017;22(11):1392–1399. doi: 10.1634/theoncologist.2017-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 6.Shukuya T, Carbone DP. Predictive markers for the efficacy of anti-PD-1/PD-L1 antibodies in lung cancer. J Thorac Oncol. 2016;11(7):976–988. doi: 10.1016/j.jtho.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2(9):1217–1222. doi: 10.1001/jamaoncol.2016.0639 [DOI] [PubMed] [Google Scholar]
- 8.Buttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867–3876. doi: 10.1200/JCO.2017.74.7642 [DOI] [PubMed] [Google Scholar]
- 9.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–820. doi: 10.1158/1078-0432.CCR-15-1678 [DOI] [PubMed] [Google Scholar]
- 10.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer. 2018;25(1):34–42. doi: 10.1007/s12282-017-0781-0 [DOI] [PubMed] [Google Scholar]
- 12.Gadducci A, Guerrieri ME. Immune checkpoint inhibitors in gynecological cancers: update of literature and perspectives of clinical research. Anticancer Res. 2017;37(11):5955–5965. [DOI] [PubMed] [Google Scholar]
- 13.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9(7):574–582. doi: 10.1038/nrm2427 [DOI] [PubMed] [Google Scholar]
- 14.You Y, Zhou C, Li D, et al. Sorting nexin 10 acting as a novel regulator of macrophage polarization mediates inflammatory response in experimental mouse colitis. Sci Rep. 2016;6:20630. doi: 10.1038/srep20630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendris N, Williams KC, Reis CR, et al. SNX9 promotes metastasis by enhancing cancer cell invasion via differential regulation of RhoGTPases. Mol Biol Cell. 2016;27(9):1409–1419. doi: 10.1091/mbc.E16-02-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Chen S, Hang W, Huang H, Ma H. MiR-95 induces proliferation and chemo- or radioresistance through directly targeting sorting nexin1 (SNX1) in non-small cell lung cancer. Biomed Pharmacother. 2014;68(5):589–595. doi: 10.1016/j.biopha.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Huang T, Jiang Z, et al. Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway. Oncogene. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Schaff UY, Shih HH, Lorenz M, et al. SLIC-1/sorting nexin 20: a novel sorting nexin that directs subcellular distribution of PSGL-1. Eur J Immunol. 2008;38(2):550–564. doi: 10.1002/eji.200737777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Lei W, Xiang X, et al. Cullin 4A (CUL4A), a direct target of miR-9 and miR-137, promotes gastric cancer proliferation and invasion by regulating the Hippo signaling pathway. Oncotarget. 2016;7(9):10037–10050. doi: 10.18632/oncotarget.7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrichs K, Gluba S, Eidtmann H, Jonat W. Overexpression of p53 and prognosis in breast cancer. Cancer. 1993;72(12):3641–3647. doi: [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ru B, Wong CN, Tong Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi: 10.1093/bioinformatics/btz210 [DOI] [PubMed] [Google Scholar]
- 25.Li T, Fan J, Wang B, et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George J, Saito M, Tsuta K, et al. Genomic amplification of CD274 (PD-L1) in small-cell lung cancer. Clin Cancer Res. 2017;23(5):1220–1226. doi: 10.1158/1078-0432.CCR-16-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Lin W, Tang X, et al. The roles of microRNAs in regulating the expression of PD-1/PD-L1 immune checkpoint. Int J Mol Sci. 2017;18(12). doi: 10.3390/ijms18122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra P, Petat A, Maury JM, et al. Programmed cell death-ligand 1 (PD-L1) expression is associated with RAS/TP53 mutations in lung adenocarcinoma. Lung Cancer. 2018;118:62–68. doi: 10.1016/j.lungcan.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 29.Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi: 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bi XW, Wang H, Zhang WW, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BS, Park DI, Lee DH, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491(2):493–499. doi: 10.1016/j.bbrc.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Meng X, Liu X, Guo X, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564(7734):130–135. doi: 10.1038/s41586-018-0756-0 [DOI] [PubMed] [Google Scholar]
- 34.Li CW, Lim SO, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi: 10.1038/bjc.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura Y, Takiguchi S, Yoshioka K, Nakabeppu Y, Itoh K. Silencing of SNX1 by siRNA stimulates the ligand-induced endocytosis of EGFR and increases EGFR phosphorylation in gefitinib-resistant human lung cancer cell lines. Int J Oncol. 2012;41(4):1520–1530. doi: 10.3892/ijo.2012.1578 [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Yang Z, Bao W, et al. SNX10 (sorting nexin 10) inhibits colorectal cancer initiation and progression by controlling autophagic degradation of SRC. Autophagy. 2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brant SR, Okou DT, Simpson CL, et al. Genome-wide association study identifies African-specific susceptibility Loci in African Americans with inflammatory bowel disease. Gastroenterology. 2017;152(1):206–217 e202. doi: 10.1053/j.gastro.2016.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Zhang L, Ye Y, et al. SNX16 regulates the recycling of E-cadherin through a unique mechanism of coordinated membrane and cargo binding. Structure. 2017;25(8):1251–1263 e1255. doi: 10.1016/j.str.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 40.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983 [DOI] [PubMed] [Google Scholar]