Abstract
Bilirubin is a yellow endogenous derivate of the heme catabolism. Since the 1980s, it has been recognized as one of the most potent antioxidants in nature, able to counteract 10,000× higher intracellular concentrations of H2O2. In the recent years, not only bilirubin, but also its precursor biliverdin, and the enzymes involved in their productions (namely heme oxygenase and biliverdin reductase; altogether the “yellow players”—YPs) have been recognized playing a protective role in diseases characterized by a chronic prooxidant status. Based on that, there is an ongoing effort in inducing their activity as a therapeutic option. Nevertheless, the understanding of their specific contributions to pathological conditions of the central nervous system (CNS) and their role in these diseases are limited. In this review, we will focus on the most recent evidence linking the role of the YPs specifically to neurodegenerative and neurological conditions. Both the protective, as well as potentially worsening effects of the YP’s activity will be discussed.
Keywords: bilirubin, bilirubin oxidation products, biliverdin, heme, heme oxygenase, biliverdin reductase, yellow players, neurodegenerative diseases, central nervous system (CNS)
1. Introduction
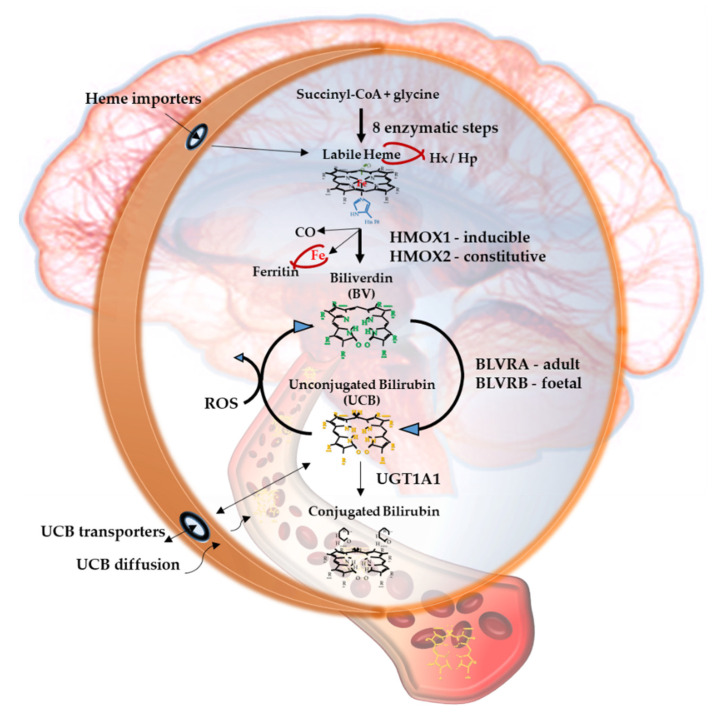
Bilirubin, the end product of the consecutive enzymatic activity of heme oxygenase (HMOX) and biliverdin reductase (BLVR) (Figure 1), is mostly known as a serum marker of hepatic diseases [1,2]. Bilirubin circulates in the blood in its unconjugated form (UCB, unconjugated bilirubin) bound to albumin, with a minimal portion being unbound (free bilirubin, Bf, about 0.1% in physiological conditions) [3], and is mainly produced from heme, originating from the senescent red blood cells in the spleen. UCB is highly hydrophobic and potentially toxic in high concentrations [4,5,6], and is conjugated in the liver with 1 or 2 molecules of glucuronic acid. The formed polar conjugated bilirubin (CB), after its further metabolism in the gut lumen, is easily discarded from the body though feces. Defects in hepatic conjugation will increase the UCB content in blood, with consequent rise of the Bf fraction in serum when UCB concentration exceed the capacity of its binding compounds [3]. Due to its lipophilic properties, Bf may diffuse across the cellular bilayer entering the cells. Based on this classic concept, the blood supply has been for a longtime considered the unique source of bilirubin content in the extrahepatic tissues, including the central nervous system (CNS) [7,8].
Figure 1.
The yellow players.
When entering cells, UCB may counteract 10,000× higher concentrations of H2O2, being one of the most potent antioxidants in nature [3,9]. For a long time the explanation of this incredible antioxidant ability has been based on the concept of the bilirubin-biliverdin redox cycle (Figure 1), where bilirubin is oxidized back to its precursor biliverdin (BV) by reactive oxygen species (ROS), and, in turn, BV is rapidly reduced by BLVR to bilirubin [10]. As a result, the antioxidant effects of UCB is amplified without increasing the cellular concentration of the pigment to a toxic level.
Figure 1 resumes the main steps of bilirubin metabolism, as well as the basis for its antioxidant capability. The concentration of systemic (blood) bilirubin derives from the transformation of the intracellular heme (the so-called labile heme) into biliverdin (BV), together with CO and Fe2+, by the action of heme oxygenase (HMOX) enzymes. BV is then converted into unconjugated bilirubin (UCB) by the enzyme biliverdin reductase (BLVR). Transported to the liver by blood, UCB hydrophobic and toxic in high concentrations, is then conjugated by the uridine diphospho-glucuronosyl transferase (UGT) 1A1 to conjugated bilirubin (CB), and eliminated from the body. Inside the cell, the powerful antioxidant action of UCB is due to its conversion back to BV during the scavenging of the cellular ROS. In this BV-bilirubin redox cycle, the protection is continuously renewed maintaining the intracellular physiological concentration of the pigments. Based on this traditional concept, the main source of labile heme (thus UCB) is the turnover of the senescent red blood cells in the spleen, and the intracellular concentration of UCB in extrahepatic tissues is believed to depend on blood supply. If true, it may account for even toxic supply of heme and UCB in case of stroke or CNS conditions compromising the blood-brain interfaces. Nevertheless, recent data suggest that extrahepatic cells may produce de novo UCB, starting from a pool of labile heme that might also be replenished from both an import, as well as an in situ (intracellular) synthesis. Added to the ubiquitarian on-demand induction of HMOX and BLVR under stressor stimuli, the YPs form a local homeostatic and defensive cellular system, that might act in synergy or independently from the systemic blood bilirubin, with hemopexin (Hx), haptoglobin (Hp), and ferritin preventing the generation of ROS by the chelating/binding of free hemoglobin and iron.
Based on the recent experimental as well as clinical data not only of UCB but also of the enzymes and precursors involved in its production seem to be importantly implemented in the pathogenesis of CNS’s disorders.
Both HMOX and BLVR possess multiple binding sites for transcription factors on the promoter region of the gene, making them able to react on demand to stressor stimuli, including those characterizing the diseases [11,12,13,14,15,16], pointing to an active role in the cellular defense. In line with this concept is their induction described in several pathological conditions [1,17].
Recently, different cell types (including neuronal cells), have been demonstrated in vitro to be able to produce de novo bilirubin from its precursors, increasing cellular resistance to damage [18,19,20]. In eels, UCB cellular production and storage (UCB bind to a protein named UnaG, belonging to the fatty acid-binding protein (FABP) family) have been suggested to provide a cellular homeostatic system able to face the oxidative challenge of the eel migration [21,22]. This has not only confirmed the idea of an active role of UCB in response to stress but has underlined the importance of the cellular UCB concentration in this process.
Finally, a correlation between UCB concentration, as well as HMOX1/BLVR activation, and the diseases have been described both in the experimental and clinical studies [1,17].
Considering quite a specific environment of the CNS-highly lipophilic, with high O2 consumption and a limited expression of antioxidant defense, making the brain highly susceptible to oxidative stress—the modulation of bilirubin and the YPs may be an intriguing therapeutic target.
The vast majority of our current knowledge on the role of the YPs derives from extra CNS diseases (such as cardiovascular diseases, metabolic syndrome, diabetes, etc.), while what this entails specifically for the CNS is still largely unknown.
In this work, we review the key knowledge and the most recent opinions about the potential effects of the YP on the onset and progression of the neurological conditions. We highlight the association of the YP with brain diseases and address the potential molecular mechanisms involved in both protection and damage of the CNS.
2. The Yellow Players (YP)
2.1. Heme
Heme is a cyclic tetrapyrrolic molecule belonging to a superfamily of the most conserved compounds in nature [3]. Heme forms a prosthetic group for a variety of hemoproteins, the most important being hemoglobin, myoglobin and cytochromes, and is implicated in multiple cellular functions including energy generation, oxygen transport, defense against increased oxidative stress, cell signaling as well as light-harvesting in higher plants, cyanobacteria and blue-green algae [3]. As usual, heme might be toxic when surpassing certain threshold concentrations, but may also exert potent protective effects [23], and this is true also for the CNS [12,24,25,26,27] (Table 1 and Table 2). In cultured neurons, heme accumulates intracellularly and can be even more neurotoxic than iron [28]. The heme metabolism in the brain seems to be impaired in neurodegenerative diseases as documented by elevated expression of HMOX1 in these pathologies ([29], see also below). Simultaneously, hereditary defects of the heme synthesis, cellular export, and import of heme as well as impairment of its incorporation into hemoproteins or heme degradation are associated with specific neurodegenerative disorders supporting the role of heme metabolism in the brain damage [24]. The role of heme in CNS pathologies is provided by studies on intracranial bleeding demonstrating neurotoxicity of free hemoglobin and its degradation products released during hemorrhage [30].
Table 1.
Association of the yellow players (YPs) with brain diseases.
| Yellow Players | Pathological Condition | Ref. |
|---|---|---|
| Heme | Essential for oxygen storage, neurogenesis, cell survival, differentiation, circadian rhythms regulation, cellular energy production, gene and micro RNA (miRNA) processing. | [12,24] |
| Accumulating in the brain in course of hemorrhage, traumatic brain injury, stroke, ischemia, and diseases with increased BBB permeability (such as Parkinson’s, Alzheimer’s, and Huntington’s disease). | [12] | |
| Neuroprotective against xenobiotic toxicity. | [25,26,27] | |
| Neuroprotective against ischemic, traumatic and neurodegenerative insults, by inducing neuroglobin. | [31] | |
| Protective in a pharmacological model of Huntington’s disease. | [32] | |
| Contributing to the progression of brain diseases (such as intracerebral/subarachnoid hemorrhage; neuropathic porphyria; Friedreich ataxia; posterior column ataxia, retinitis pigmentosa, hereditary sensory and autonomic neuropathy). | [12,24] | |
| Neurotoxic in brain hemorrhage. | [30] | |
| HMOX1/2 | ||
| HMOX1 | Protective in neurodegenerative and other neurological diseases (such as Alzheimer’s and Parkinson’s disease, ischemic brain injury). | [33,34] |
| Protective against glutamatergic/aspartatergic excitotoxicity. | [35,36] | |
| Protective against ethanol-induced neurotoxicity. | [37] | |
| Protective against mitochondrial toxicity. | [32,38] | |
| Protective in a pharmacological model of Huntington’s disease. | [32] | |
| Reduces the progression of neuropsychiatric syndrome. | [12] | |
| Protective against ROS via the production of bilirubin from heme. | [3] | |
| Depending on specific conditions, inducing apoptosis and cell cycle arrest (thus being protective), but also capable of increasing chemoresistance and worsening the diagnosis in CNS malignancies. | [39] | |
| Involved in neurodegeneration (such as cerebral infarction; Alzheimer’s and Parkinson’s disease, Down syndrome, schizophrenia; stroke and CNS trauma) and brain aging when excessively expressed. | [16,40] | |
| HMOX2 | Protective from cerebral ischemia-reperfusion injury; and traumatic brain injury. | [12,41,42,43] |
| Protective from oxidative stress-mediated brain injury, such as epileptic seizures | [44] | |
| Deletion of HMOX2 increases redox stress damage in epileptic seizures (protective). | [12] | |
| Iron | Neurotoxic and involved in neurodegenerative diseases when accumulating in the brain (almost all neurological conditions). | [12,45,46] |
| CO | Neuroprotective (in low concentrations) against vasospastic reaction accompanying subarachnoid bleeding. | [46] |
| Neuroprotective (in low concentrations) against toxic noxious substances. | [38] | |
| Neurotoxic in high concentrations. | [47] | |
| May impair auditory functions. | [48,49] | |
| May impair cognitive and olfactory functions as well as the neuroendocrine system. | [12] | |
| Biliverdin | Protective against cerebral infarction; cerebral ischemia-reperfusion. | [50,51] |
| Inducing brainstem auditory evoked potential abnormalities | [52]. | |
| Induces fetal toxicity. | [53,54] | |
| BLVRA/B | ||
| BLVRA | Protective in meningioma and glioma. | [55] |
| Ameliorating autoimmune encephalomyelitis (a model for multiple sclerosis). | [56] | |
| Involved in the pathogenesis of Alzheimer’s disease. | [29,57,58] | |
| Improving neurological function in germinal matrix hemorrhage (a disease of premature infants which could bring complications like developmental delay, mental retardation, hydrocephalus and cerebral palsy). | [59] | |
| BLVRB | Potentially protective during fetal life. | [53,54] |
| Biomarker for Alzheimer’s disease and ischemic stroke. | [60,61] | |
| Bilirubin | Neuroprotective in cellular and animal models of experimental autoimmune encephalomyelitis. | [44,62,63] |
| Protective in stroke and ischemia. | [64,65] | |
| Protective against ethanol-induced neurotoxicity. | [37] | |
| Protective against mitochondrial toxicity. | [38] | |
| Reducing tumor size and improving survival in glioma. | [63] | |
| Protective against neurotoxicity in Parkinson’s disease model. | [66] | |
| Protective in traumatic brain injury. | [67] | |
| Protective in asymptomatic intracranial atherosclerosis. | [68] | |
| Improving survival of grafted neural stem cells. | [69] | |
| Contributing to inflammation in intracerebral hemorrhage. | [70] | |
| Correlating negatively with the neuropsychiatric/neurodegenerative disorders (bipolar disorder, schizophrenia, schizoaffective disorder, Alzheimer’s disease, dementia, multiple sclerosis, cerebral infarction in adults.) | [1,2,71,72] | |
| Correlating with intraventricular hemorrhage, retinopathy and greater vision loss; hypoxic-ischemic encephalopathy; and neonatal encephalopathy due to hepatic injury in infants. | [72,73,74,75] | |
| Responsible for brain damage in severe neonatal hyperbilirubinemia (kernicterus spectrum disorder: KSD) and Crigler-Najjar type I syndrome. | [4,5,6,76] | |
| Bilirubin degradation products | ||
| Bilirubin photoisomers | Pro-inflammatory activities. | [77] |
| Biopyrrins | Increased urinary excretion in Parkinson’s disease. | [78] |
| Propentdyopents | Increased in cerebrospinal fluid in subarachnoid bleeding. | [79] |
| Z-BOX A/B | Increased in cerebrospinal fluid in subarachnoid bleeding. | [80] |
Abbreviations: BBB, blood brain barrier; BLVR, biliverdin reductase; Z-BOX A/B, Z isomer of bilirubin oxidation products type.A or B; CNS, central nervous system; CO, carbon monoxide; HMOX, heme oxygenase; KSD, kernicterus spectrum disorder; ROS, reactive oxygen species.
Table 2.
The YPs and their molecular targets.
| Yellow Players | Target | Effect | Ref |
|---|---|---|---|
| Heme | Generation of ROS/RNS | Vascular hypertension and vasoconstriction. In turn, the pro-oxidant milieu increases the oxidation of hemoglobin, enhancing heme release, protein carbonylation, lipids oxidation, MMP9 release, and tissue damage. |
[12,30,81] |
| Activation of TLR4 | Proinflammatory activity: neutrophil migration, secretion of IL8, TNFα (activating NF-κB); increased vascular permeability; edema. | [30,81] | |
| Nrf2/Bach1/Keap1 | Inhibiting the antioxidant response. | [24] | |
| Activation of PI3K/Akt | Reducing apoptosis, increasing the expression of antioxidant enzymes (SOD, and HMOX1). | [26] | |
| Binding to hemopexin (Hx) | Chelating heme. | [12,30] | |
| Binding to haptoglobin (Hp) | Chelating heme via binding of hemoglobin. If not sufficient: increased CNS hemorrhage, oxidative stress, impaired brain performance, and reduced neurological activity. Marker of BBB disruption. |
[12,30] | |
| Modulation of proteasome activity. | Impairing the activity of the ubiquitin-proteasome system; impairing the unfolded protein response (PERK/ATF6/IRE1a); and leading to the accumulation of unfolded proteins. | [24] | |
| Cofactor for cytochrome c and the mitochondrial electron transport chain (complexes II, III, IV) | Mitochondrial dysfunction, impairing ATP translocation into the cytoplasm; mitophagy and apoptosis. Impairing mitochondrial trafficking (especially relevant for neurons). | [24] | |
| Binding to Slo1 BK ion channel | Inhibiting the cellular excitability. | [12] | |
| Induction of neuroglobin expression | Reducing the apoptosis, cytochrome c, and mitochondrial dysfunction. | [25] | |
| Induction of ferritin | Chelating Fe. | [28] | |
| Inducing HMOX1 | Increasing cell survival and reducing redox stress. | [27] | |
| Decreasing lipid peroxidation, increasing the expression of the anti-apoptotic Bcl2, decreasing damage. | [46] | ||
| Possibly increasing Fe influx in mitochondria worsening the damage, increasing redox stress and inflammation. | [46] | ||
| HMOX1/2 | |||
| HMOX1 | Production of CO, BV and Fe. | Promoting proliferation trough synthesis of cGMP (maybe acting on CREB). | [18] |
| Increasing VEGF in astrocytes, leading to angiogenesis. Activating of the BDNF–TrkB–PI3K/Akt signaling with increased neuronal survival, and reduced inflammation. |
[67] | ||
| When overexpressed, increasing cholesterol synthesis and cellular efflux, with an increased presence of oxysterols (products of cholesterol oxidation). The same result is obtained by the addition of CO or iron to the culture, suggesting one or both the HMOX1 products as the real effectors. | [16,82] | ||
| Decreasing oxidative and nitrosative stress; increasing (restored) GSH and catalase activity; reducing the release of TNFα and IL1β; reducing (restored) the GSK3 activity. | [32] | ||
| Increasing Fe production and deposition into astroglial mitochondria, with cellular bioenergetics failure. Increasing DNA damage (8-OHdG), protein oxidation (carbonyls), and lipid peroxidation. Altering the mitochondria morphology and cellular distribution, with mitophagy and autophagy. Enhancing the conversion of catecholamines and catechol-estrogens to neurotoxic radicals, making neurons more sensitive to H2O2 and dopamine insult. |
[16] | ||
| Acutely induced after stimuli, mainly in glial cells (astrocytes, microglia). Acute up-regulation might be protective, while a chronic up-regulation may cause toxicity. Inducing Fe cell export. |
[33] | ||
| Decreasing the expression of NLRP1, possibly through the inhibition of ATF4, inhibiting the inflammasome, reducing the neuronal death by apoptosis, and improving functional recovery. | [83] | ||
| Increasing miRNA expression (miR16, 17 and 140) | Downregulating the mitochondrial functions (including ATP production; mitochondrial antioxidant enzymes level; intrinsic apoptotic pathway; enhancement of TNFα synthesis; up-regulation of MAPK signaling to compromise he oxidative phosphorylation). | [16] | |
| Migrating into nuclei | HMOX1 can migrate into nuclei and act as a transcription factor of the genes involved in the cellular antioxidant response, immunity and inflammation, autophagy, hypoxia, tumor resistance, etc. However, this mechanism has not been studied in CNS diseases so far. | [1] | |
| HMOX1/2 | Amyloid protein precursor binding to HMOX1/2 | Reduced HMOX1/2 activity, reduced UCB production, and increased cellular sensitivity to H2O2 and hemin toxicity. | [84] |
| HMOX2 | Generation of CO, BV and Fe2+. | Fostering cell survival (via UCB action) and proliferation (via cGMP signaling). | [18] |
| Basal production of UCB and CO in neurons. | CO: inducing cyclic guanylyl cyclase, in turn producing cGMP (possibly via ERK). | [33] | |
| Production of UCB and cGMP | Increasing neuroprotection toward redox stress. | [85] | |
| CO | Voltage-gated K+ channel | Modulating the cellular excitability. | [24] |
| Activation of guanylyl cyclase | Increasing cGMP, activating of cGMP protein kinase and p38 MAPK, preventing neurons degeneration, activating noradrenergic neurons, decreasing apoptosis, reducing inflammation. | [3,33,86] | |
| AMPK | Inhibiting AMPK activation, decreasing the toxicity of the Aβ. | [87,88] | |
| HIF1α | Activating the Ca channels, CAMK2B, AMPKα, increasing mitochondrial respiration. | [67] | |
| HMOX1 | Inducing HMOX1 (through Nrf2 signaling) | [67] | |
| miRNA | Increasing miR-140, 17, 16. Decreasing miR-297, 206, 187, 181a, 138, 29c, in turn reducing the mRNA levels of Ngfr, Vglut1, MAPK3, TNFα, and Sirt1, abnormally expressed in various central nervous system disorders. | [89] | |
| Iron | Generation of hydroxyl radicals. | Inducing a high rate of protein carbonylation, reducing SOD activity, increasing DNA damage, inhibiting the DNA repair system. Activating NF-κB and AP-1 with BBB disruption and worsening of damage. Reducing mitochondrial respiratory functions. |
[81] |
| Activation of NF-κB and induction of inflammation. | Inducting the glutamate excitotoxicity (release of glutamate) with increased BBB permeability, neuronal autophagy, neuronal atrophy and death. Releasing of MMP9, TNFα, IL1β, microgliosis. |
[81] | |
| miRNA | Increasing miR-140, 17, 16. Decreasing miR-297, 206, 187, 181a, 138, 29c, in turn reducing the mRNA levels of Ngfr, Vglut1, MAPK3, TNFα, and Sirt1, abnormally expressed in various CNS disorders. | [89] | |
| BV | Scavenging ROS | Lowering DNA damage (8-OHdG). | [51,90] |
| miR-204-5p, Ets1 | Lowering Th1 type response. | [51] | |
| NF-κB | Lowering NF-κB-DNA binding and pro-inflammatory factors transcription/production | [91,92] | |
| TLR4 | Inducing BLVR translocation into the nucleus, binding to TLR4 promoter, repressing the expression of TLR4, and leading to the inhibition of inflammatory cytokine production. | [93] | |
| JNK | Reducing the JNK activation, affecting JNK/AP-1 pathway, suppressing the transcription of TNFα and diminishing endothelial cell apoptosis. | [94] | |
| PI3K/Akt | Inducing the interaction of BLVRA with PI3K, activating Akt signaling, and increasing anti-inflammatory cytokine (IL10) production in macrophages. | [95] | |
| ROS, NRS formation | Preventing oxidative damage in rat brain microsomes | [50,90] | |
| Complement | Inhibiting C5aR gene and protein expression that is mediated by mTOR pathway accompanied by the reduction of pro-inflammatory cytokines (TNFα and IL6) gene expression (macrophages). | [93] | |
| BLVR | Inducing BLVR translocation into the nucleus. | [96] | |
| Histones | Possible inhibition of the histone synthesis. | [97] | |
| BLVRA | Akt gene | Modulating glycogen synthase kinase, and Tau protein deposition in the brain. | [98,99,100,101] |
| NF-κB | Direct binding, arresting the cell cycle. | [91] | |
| eNOS/NO/TLR4 pathway | Binding on the gene promoter, inhibition of transcription, reducing the inflammation. | [96] | |
| Improving hematoma resolution and neurological functions. | [59] | ||
| MAPK/PI3K | Maintaining the synaptic plasticity, memory consolidation, inducing the genes required for neuronal and synapse growth, maintenance and repair processes. | [29] | |
| MAPK/Akt | Inhibiting MAPK/Akt activation, reducing apoptosis, protecting the hippocampal neuronal cell from oxidative stress. | [102] | |
| BACE-1 protein | Reducing the BLVRA activation inducing the phosphorylation of BACE-1, promoting insulin resistance and increasing Aβ levels in the brain of an animal model of aging. | [103] | |
| MEK1-ERK1/2-Elk1 signaling | Transcriptional activation of stress-induced genes, including HMOX1. | [104] | |
| Insulin receptor | Inducing an early activation of IRS1 and improving brain insulin resistance. | [105] | |
| HMOX1 | Improving cellular antioxidant defense via HMOX1 induction. | [106] | |
| UCB | NF-κB | Direct binding, arresting the cell cycle. | [91] |
| Preventing NF-κB-DNA binding, suppressing T-cell activation. | [107] | ||
| Histone acetylation | Modulating histone 3 acetylation and the transcription of genes involved in brain development. | [108] | |
| ER | Inducing ER stress, inflammation and apoptosis | [109] | |
| Nrf2 | Activating the Nrf2 pathway, thus the antioxidant response. | [110] | |
| CREB | Increasing the phosphorylation of CREB possibly leading to BDNF production, boosting the survival and the repair processes in traumatic brain injury. | [67] | |
| AKT | Enhancing blood flow and regeneration in ischemic injury. | [67] | |
| HIF1α | Stabilizing HIF1α, activating Ca channels, CAMKβ, AMPKα, and increasing mitochondrial respiration. | [67] | |
| AhR | Modulating the transcription of genes coding for detoxification enzymes (CYP1A1, UGT1A1), acting on the cell cycle, MAPK cascade, Nrf2 pathway, and immune response. | [111,112] | |
| CAR | Involved in the disposal of exogenous and endogenous substances, and inhibition of gluconeogenesis. | [113] | |
| ApoD | A non-albumin carrier of bilirubin in human plasma, contributing to protection against oxidative stress, is highly expressed in the brain. | [113] | |
| Neurotrophic factor | Increasing the expression of BDNF in neurons and GDNF in glia, leading to reduction of neuronal loss in substantia nigra in animal model of Parkinson’s disease | [66] | |
| MRGPRX4 | Mediating the cholestatic itch in the primary sensory neurons, acting in host defense and immune reaction. | [113] | |
| Angiogenic and energy-sensing genes in astrocytes | Enhancing PGC1α and HIF1α production in astrocytes which plays a role in mitochondria biogenesis, reduction of inflammatory, and angiogenesis | [67] | |
| ROS/RNS generation | Inhibiting of NMDA excitotoxicity, preventing neuronal death. | [104,114] | |
| Affecting BBB permeability and preventing inflammatory cell invasion | [62] | ||
| Macrophages and T cells | Immuno-modulatory activity by reducing the expression of Fc receptor in macrophage and inhibiting T cell response. | [115] | |
| PKC/ICAM-1 signaling, | Contributing to neutrophil infiltration, early inflammation, and edema. Decreasing nitrate/nitrite formation, reduced perihematomal microgliosis. |
[70] | |
| Mrp1/ABCc1 | Upregulating and translocating its transporter (Mrp1/ABCc1) from the Golgi apparatus to the plasma membrane. | [116] | |
| Cellular and subcellular membranes | Altering membrane polarity and fluidity, the opening of the permeability transition pores, inducing cellular energy failure, activating the mitochondrial apoptotic pathway. | [117,118] | |
| P38MAPK-JNK1/2-NFkb | Inducting of inflammation, the release of TNFα, IL1β, reduction of the cellular viability | [119,120] | |
| NMDA receptors, glutamate | Inducing glutamate excitotoxicity, reducing the expression of the NMDA receptors, impairing long-term potentiation and long-term depression | [121,122,123] | |
| ERK-Akt-CREB | Scavenging ROS, decreasing neurotrophic factor availability. | [124] | |
| Mrp1/ABCc1 and Pgp/MDR1/ABCb1 | Modulating the expression of its transporters (Mrp1/ABCc1 and Pgp/MDR1/ABCb1) at the blood-brain interfaces | [125] | |
| DNA | Inducing ROS, which in turn leads to DNA damage, despite the activation of the DNA repair pathways. | [126] | |
| Cell cycle | Inducing cell cycle arrest | [127] | |
| UCB degradation products | Ca channels | Opening of the Ca channels and decreasing the conductance of the cerebral myocytes, inducing vasoconstriction. | [79] |
The present table is not intended to collate all the known targets and molecular mechanisms modulated by the YP, but it is restricted solely to the direct evidence of their effects in CNS biology. Abbreviations: Aβ, amyloid β; Akt, protein kinase B; ATF6, activating transcription factor 6; ATF4, activating transcription factor 4; ATP, adenosine triphosphate; AMPK, 5′ adenosine monophosphate activated protein kinase; AP-1, activator protein 1; AMPKα, 5′ AMP-activated protein kinase alpha; AhR, aryl receptor; ApoD, apolipoprotein D; Bcl2, B-cell lymphoma 2; BACE-1, β-site APP cleaving enzyme 1; Bach1, the transcription factor BTB and CNC homology 1;.BBB: blood brain barrier; BDNF, brain-derived neurotrophic factor; BLVR, biliverdin reductase; BV: biliverdin; C5aR, complement receptor 5a; CAMKβ, Ca-calmodulin-dependent protein kinases beta; CAMK2B, Ca-Calmodulin dependent protein kinase β; CAR, constitutive androstane receptor; cGMP, cyclic guanosine monophosphate; CNS, central nervous system; CO: carbon monoxide, CREB, cAMP responsive element binding; CYP, cytochromes P450; Elk1, ETS Like-1 protein; eNOS, endothelial nitric oxide synthase; ER stress, endoplasmic reticulum stress; ERK, extracellular signal-regulated kinases; GSH, glutathione; GSK3, glycogen synthase kinase 3β; GDNF, glial cell line-derived neurotrophic factor; H2O2, hydrogen peroxide; HIF1α, hypoxia-inducible factors; HMOX1, heme oxygenase 1 Hp, haptoglobin; Hx, hemopexin; IRS1, insulin receptor substrate-1; IRE1a, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α; IL: interleukin; MMP9: metalloproteinase 9; ICAM-1, intracellular adhesion molecule 1; JNK, c-Jun NH2-terminal kinase; Keap, Kelch-like ECH-associated protein 1; miRNA, micro RNA; MAPK, mitogen-activated protein kinase; MEK1, mitogen-activated protein kinase kinase; MRGPRX4, Mas-related G protein-coupled receptor X4; mTOR, mammalian target of rapamycin; Mrp1/ABCc1, multidrug resistance protein 1/ATP binding cassette protein c1; Ngfr, neuronal growth factor receptor; NLRP1, nod-like receptor protein 1; NO, nitric oxide; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor erythroid 2-related factor; NMDA, N-methyl-d-aspartic acid; PI3K: phosphatidylinositol 3-kinase; PERK: protein kinase RNA-like endoplasmic reticulum kinase; PGC-1α: peroxisome proliferators-activated receptor γ (PPARγ)-coactivator-1α; Pgp/MDR1/ABCb1, P-glycoprotein/multidrug resistance protein1/ATB binding cassette protein b1; PKC, protein kinase C; RNS, reactive nitrogen species; ROS: reactive oxygen species; Sirt1, Sirtun 1; Slo1 BK, Ca2+- and voltage-activated K+ channel; SOD, super oxide dismutase; TLR4, toll like receptor 4; TNFα, tumor necrosis factor alpha; TrkB, tropomyosin receptor kinase B; Th1, T helper cells; UCB, unconjugated bilirubin; UGT, uridine 5′-diphospho-glucuronosyltransferase; VEGF, vascular epithelial growth factor; Vglut1, vesicular glutamate transporters, 8-OHdG, 8-hydroxy-2′ deoxyguanosine (marker of DNA damage).
On the other hand, heme might be neuroprotective by reducing neuronal apoptosis, improving mitochondrial functions as shown in experimental animal studies. These effects might be mediated via HMOX1 induction or by increasing the expression of neuroglobin [25,26], the hemoprotein positively correlated with a beneficial outcome in several neurotoxic insults including ischemic and traumatic brain injuries and Alzheimer’s disease [31]. Neuroprotective effects of hemin against lead neurotoxicity, also mediated by increased expression of HMOX1, were reported also in another experimental study [27].
2.2. Heme Oxygenase (HMOX), Carbon Monoxide (CO) and Iron
As described above, heme is involved in the pathological processes of the brain. Under physiological conditions heme homeostasis is tightly regulated by HMOX enzymes. Two HMOX isoforms exist in the human body, the inducible HMOX1, and the HMOX2 isoenzyme constitutively expressed also in the CNS [15].
In addition to converting heme to BV, HMOX1 possess a wide spectrum of DNA-binding motifs on its promoter (e.g., CRE/Erg1—cAMP response element/early growth response, NF-kB, AP2, Hif, cJun/Fos, ATF, stRE—tress response element), making it able to rapidly modulating a plethora signaling pathway involved in adaptation to stress, proliferation, differentiation and cell survival, immunity, anti-oxidant response, as well as modulating the expression of HMOX itself [1].
In the CNS context, HMOX1 is generally viewed as neuroprotective and significant effort is being made to therapeutically induce HMOX1 to prevent various neuropsychiatric and neurodegenerative diseases, either via direct HMOX1 induction or by activating its transcription factor Nrf2 by therapeutics passing the blood-brain barrier (BBB) [33,128,129]. Based on mostly experimentally studies, HMOX1 was indeed proved to protect the brain in various neurotoxicity models such as acute glutamatergic and aspartatergic excitotoxicity [35,36], ethanol-induced neurotoxicity [37], glycolysis inhibition-induced neurotoxicity and toxicity against mitochondria in cerebellar granule neurons [38,130] as well as rat model [32] (Table 1). These data supporting the protective role of HMOX1 in neurotoxicity and neurodegeneration are in line with studies by Takahashi et al. demonstrating the inhibition of HMOX in neurons of a transgenic mice model of Alzheimer’s disease [84].
On the other hand, the exaggerated activity of HMOX1 may result in an overproduction of heme-derived carbon monoxide (CO) and especially iron, leading to increased astroglial stress accompanied with oxidative mitochondrial membrane damage, iron sequestration and mitophagy, as well as to gliopathy present in many aging-related neurodegenerative brain disorders [16] (Table 1). Excessive HMOX1 overexpression was reported to contribute to the pathological iron deposition and mitochondrial damage in aging-related neurodegenerative disorders [46,131] with all the pathological consequences associated with iron accumulation in the brain tissue [45], Similarly, although CO at low doses is neuroprotective by diminishing cerebral vasospasms in subarachnoid hemorrhage [129], and by protecting neurons from toxic noxious substances [38], CO at higher concentrations is certainly toxic [47,49] (Table 1).
Not only HMOX1, but also HMOX2 constitutively expressed in the CNS is implicated in the protection from various neurological disorders as demonstrated in the experimental models of cerebral ischemia-reperfusion injury [43,132] or oxidative stress-mediated hippocampal and neuronal toxicity (Table 1).
Altogether, the current knowledge suggests HMOX, and especially HMOX2, as part of a CNS cellular defensive machinery, and (particularly the inducible HMOX1) as an interesting pharmacological target for enhancing the brain adaptation to the pathological conditions. Nevertheless, and differently for the extra-CNS organs, special care of the side effects due to an excessive HMOX1 induction, must be taken into consideration (see BLVR section and Conclusion and perspective).
2.3. Biliverdin
BV, the greenish, water-soluble metabolite produced by the catalytic degradation of heme by HMOX [11,133], is probably the least studied product of this enzyme. Due to its rapid reduction to UCB by BLVR [134,135], BV is almost undetectable in serum and cells [90,136,137].
Nevertheless, experimental studies have demonstrated that BV administration to rats ameliorates brain damage by reducing oxidative stress, and decreasing DNA damage (Table 1 and Table 2) [50], and is a biomarker for oxidative stress in many neurodegenerative diseases (Figure 2) [138]. When administered in vivo, BV alleviates the pro-inflammatory response [51,91,92], playing a role in the progress of neurodegeneration [139], and inhibits the toll-like receptor (TLR) 4 signaling [93,96], a frequent contributor to neuronal death, BBB damage, edema, ischemic brain injury [140,141,142,143,144,145,146], and upregulated in microglia of Alzheimer’s disease patients [147,148,149]. The rapid conversion of BV to UCB still leaves open the question of which of the molecules (BV or UCB) is the more important effector.
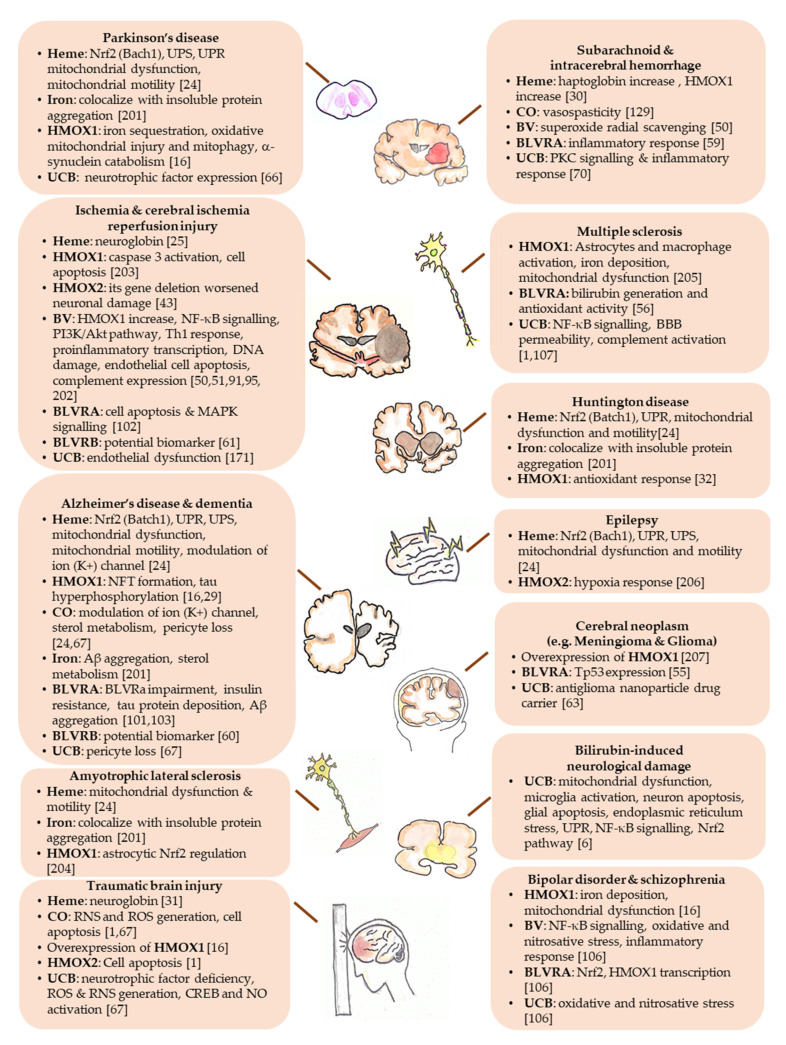
Figure 2.
The known and the putative molecular targets of the YPs on the CNS diseases.
The specific contribution of BV has been thoroughly investigated in in vitro (chemical) studies where BV has been demonstrated to scavenge NO radicals [150], and inhibit lipid peroxidation with a 2-fold higher efficacy compared to α-tocopherol [90]. This data has been supported in vivo studies with BLVRA deficient mice as well as in the cell lines in which the BLVRA was silenced [151].
Altogether, the data indicate that the protection observed both in cellular systems as well as in vivo, might be a combination of a direct antioxidant effect of BV and its conversion into bilirubin.
On the other hand, BV administration in jaundiced Gunn rats has been shown to induce abnormalities in the brainstem auditory evoked potential comparable with those observed in human newborn hyperbilirubinemia (Table 1). In this study, BV administration was followed by an increase in plasma bilirubin level, the real effector of the brain damage [52].
2.4. Biliverdin Reductase (BLVR)
Two isoforms of BLVR (A and B) reduce BV to UCB, and both possess kinase activity.
BLVRB is highly expressed in the early fetal stages and reduces the fetal BV IXβ, whose accumulation, together with the ferric ion derived from the heme cleavage, may leads to toxicity to the developing fetus [53,54] (Table 1). Despite detectable in the adult tissues, the role in adults has not been deciphered. Nevertheless its detection in serum has been suggested as a potential biomarker for early diagnosis of Alzheimer’s disease [60], intra-plaque hemorrhage in atherosclerosis and carotid atherosclerosis, common causes of cerebral thromboembolism or ischemic stroke [61].
BLVRA has been much more investigated. Its expression increases later in gestation [152] and is ubiquitously expressed in the adult [153], with maximal levels in the brain and lungs.
BLVR may be found both in the cytoplasm and in the nucleus. In the cytoplasm, apart from reducing BV to bilirubin IXα, it may be a substrate for the insulin receptor tyrosine kinase (IRK), and acting as a kinase on itself, as well as on several signaling pathway with important adaptive/defensive functions (e.g.,—anti-oxidant, inflammatory and hypoxia response, detoxification, apoptosis, carcinogenesis; response to insulin. For details see [1], in addition to Table 2 in this review). BLVRA may also translocate into the nucleus transporting heme and ERK (extracellular signal-regulated kinases) and act as a transcription factor binding directly to ARE (antioxidant responsive elements )/AP1-2 (activating protein), and ATF2 (activating transcription factor)/CRE (cAMP response element) DNA sequences (present also on the promoter region of HMOX1), or acting in complex with ERK/Elk (ETS domain-containing protein) or Nrf2 (nuclear factor (erythroid-derived 2)-like 2)/ARE (antioxidant responsive elements) [1,154,155,156] (Table 2). Altogether, BLVR possesses the potential for modulating a wide number of biological function in the cells, including the self-regulation of the YPs, through an impressive array of signaling pathway [1].
As a transcription factor, BLVRA binds to NF-κB, arresting the cell cycle [91]. As a consequence, BLVRA is downregulated in brain tumors, particularly meningiomas and gliomas, where a correlation between the enzyme expression and the anti-oxidant status has been found [55]. BLVRA deficiency has a role also in the maintenance of the endothelial phenotype controlled by HMOX and iron homeostasis control, with potential implications for the BBB integrity during diseases [157,158,159,160]. Deregulation of the BLVRA activity is a common feature of Alzheimer’s disease, at least in the most advanced stages, with BLVRA inhibition enhancing Tau phosphorylation and deposition in the brain [98,99,100,101] (Table 1 and Table 2). The suggested explanation for the BLVRA enzymatic inactivation lies in the excessive oxidative and nitrosative stress ongoing the disease, damaging the enzymatic functions [57,58,161], a phenomenon common in most of the neurological conditions.
Notably, BLVRA is also a member of the insulin receptor substrate family [162], modulating the glucose uptake [105,154,163] (Table 2), with insulin resistance frequently observed in Alzheimer’s disease [164,165,166]. The role of BLVRA in insulin resistance and disease progression, has been better unraveled in animals models, where the reduced BLVRA activity, the brain insulin resistance, and the disease severity, have been improved by intranasal insulin administration, the effect not occurring in the BLVRA knock-out animals [167].
Vice versa, BLVR intracranial administration in rats ameliorates the outcome of autoimmune encephalomyelitis (a model for multiple sclerosis). The efficacy has been explained by the multifactorial functions of bilirubin (anti-complement, inhibiting the antibody-dependent lymphocytes cell-mediated cytotoxicity, in addition to its antioxidant action (Table 2)).
Collectively, BLVRA induction seems always beneficial to CNS, while its enzymatic inactivation looks detrimental, possibly by reducing the final concentration of UCB inside the cell. Convincing experimental demonstrations of the role of BLVR are still required to unravel the importance of this YP per se, and the side effects linked with a hyper-activation of HMOX1.
2.5. Unconjugated Bilirubin (UCB)
UCB is considered a powerful anti-oxidant molecule [9,10], with its chemical characteristic contributing to the physiological implications. Bilirubin contains an extended system of conjugated double bonds and a pair reactive hydrogen atom that is involved in antioxidant activity via H-donation to an incipient radical [168]. Owing to its hydrophobic nature, bilirubin mostly accounts for the preferential scavenging of lipophilic radicals that can attack lipid membranes, with the GSH/GSSG system more active on the cytosolic protection [169].
Unlike BV that has a double bond between the inner pyrrole rings, UCB contains a single bond. This UCB electrophilic characteristic accounts for its ability to react with thiol compounds characteristic of many transcription nuclear factors [133]. Thus, UCB may modulate key signalling pathways [107,112,113] (Table 2).
Among the biological functions, UCB scavenges not only ROS [62], but also RNS (reactive nitrogen species) [90,150], with reduction of the superoxide production [114]), and inhibition of the glutamate excitotoxicity [170] (Table 2). Besides, UCB is a known multi-target anti-inflammatory molecule with the pro-inflammatory processes ever noticed in CNS diseases and co-responsible for the neurological damage [107,171] (Table 2).
These properties explain why bilirubin might play a key role in reducing neuronal damage in CNS pathologies (Table 1) [42,62,63,64,65]. Nanoparticle-delivered UCB [172] into the brain reduced the tumor size and improved the survival in a mice model of glioma [63].
An interesting correlation between the serum bilirubin and the neurological conditions is emerging. Increasing clinical observations indicate a lower serum bilirubin concentrations during oxygen radical associated and inflammatory neurological conditions of the adult life (Table 1), with both a correlation with the diagnosis and the prognosis [1,2,171]. As reviewed by Fujiwara et Al. [75], similar data are present also in neonates [72,73,74], where a close association between the plasma bilirubin concentration and the plasma antioxidant capacity has been reported [75], with icteric neonates showing a favourable plasma antioxidant capacity, that phototherapy worsened [173]. After more than a century, this supports the speculation that the production of UCB from BV, an unnecessary energy-consuming reaction, is motivated by the benefits of having higher antioxidant defense. Altogether, these data suggest that lower serum bilirubin concentrations harm the systemic antioxidant defence system, possibly starting or enhancing the progression of oxidative stress-mediated neurological diseases. The real contribution of the serum bilirubin level vs. the in situ (CNS) activity of the UCB players must be further explored.
The complexity in interpreting the interplay between the liver (as the main controller of the systemic UCB level), the brain (neurological diseases) and the YPs is also present in the non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome. NALFD is a pandemic condition involving also the pediatric population [174,175], and regarded as one of the newest risk factors for neurological diseases [176,177], with the life style and the diet regimen being key factors in the CNS pathology progression [178,179,180]. The liver and brain appear to be inter-connected at various levels (so-called liver brain axis): (1) A negative correlation between serum bilirubin concentrations and NAFLD stage has been reported [75,181,182,183,184]; (2) the modulation of HMOX1/CO/iron, in turn acting on sirtuin1 (Sirt1—see Table 2), a histone deacetylase controlling the adaptive mechanism to disease and the bilirubin transport in both organs [89,185,186,187] has been also demonstrated; and (3), the liver and brain may be connected by insulin resistance [183], a feature of the metabolic syndrome whose CNS consequences have been discussed in Section 2.4.
On the other side, UCB in high concentrations such in severe neonatal hyperbilirubinemia may cause neurological sequelae with temporary or permanent auditory dysfunctions, cognitive and motor impairment or even death [4] due to its prooxidant, proinflammatory and proapoptotic activities as well as alteration of the epigenetic control of postnatal brain development [6,108]. At the toxic level, no doubts exist that the UCB content in the CNS is due to the pigment entering from the blood (Table 2).
2.6. UCB Degradation Products
Apart from the main heme catabolic pathway comprising in the reduction of double bonds within the UCB molecule and resulting in the production of a series of products known as urobilinoids [188], UCB, under conditions of increased oxidative stress or upon exposure to light, can be oxidized to several UCB oxidation products. Although these include also BV produced in the so-called bilirubin/biliverdin redox cycle scavenging the overproduction of ROS [189] (Figure 1), UCB is easily (photo)oxidized into many oxidation products with biological importance [190]. These bilirubin oxidation derivatives include tetra-, tri-, di-, and mono-pyrrolic bilirubin oxidation products. Probably most clinically important are tetrapyrrolic bilirubin photo-isomers formed during phototherapy of severe unconjugated hyperbilirubinemia. However, no solid data exists whether the bilirubin photo-isomers might have the potential to affect pathologic processes of the brain tissue. Nevertheless, bilirubin photo-isomers might have neuro-inflammatory effects, as shown in vitro [77] (Table 1). Although proinflammatory cytokines and chemokines have generally been considered deleterious for the CNS and is involved in neurodegeneration [191,192], these cytokines, apart from being mediators of damage, might also have beneficial functions, serving as trophic and/or neuroprotective agents (for review see [193]). For instance, the beneficial role of IL-6 in neuroregeneration [194], as well as increased proliferation of neural progenitor cells upon exposure to TNFα treatment [195] have been reported. More data are necessary to identify the exact roles of bilirubin photo-isomers in the biology of the cells of the CNS, compared to proved deleterious (proinflammatory) effects of high concentrations of UCB [196,197].
Biopyrrins, tripyrrolic compounds representing clinically relevant markers of increased oxidative stress, comprise another group of bilirubin oxidation products [198,199]. Although their increased urinary outputs have been reported in many human pathologies associated with increased oxidative stress, their role in brain biology or bilirubin phototherapy is unexplored and deserves further investigation. The only clinical evidence on the possible role of biopyrrins in the brain pathology is the report by Chinese researchers demonstrating increased urinary excretion of biopyrrins in patients with Parkinson’s disease [78] (Table 1).
Much more is known about dipyrrolic propentdyopents and monopyrrolic bilirubin oxidation products (Z-BOX A and B) which recently have been demonstrated to have potential clinical impact, especially in the pathogenesis of brain damage during subarachnoid hemorrhage [79,80] (Table 1 and Table 2). Again, further studies are desperately needed to reveal all the biological roles of these bilirubin oxidation products. The recently reported analytical method for the simultaneous determination of major bilirubin photooxidation products [200] will be instrumental.
As discussed in the text, the YPs have been demonstrated to be involved in the pathogenesis and/or in the protection in neurodegenerative diseases and other CNS diseases. This figure highlights the potential molecular targets of each one of the YPs in the specific CNS diseases, based on the available literature (see References in Figure 2), resuming and connecting the text to Table 1 and Table 2. The YPs: Aβ, amyloid β; BV, biliverdin; BVR, biliverdin reductase; CO, carbon monoxide; CREB, cAMP-responsive element-binding; HMOX, heme oxygenase; NFT, neurofibrillary tangles; Nrf2, nuclear factor erythroid 2–related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; ROS, reactive oxygen species; RNS, reactive nitrogen species; UCB, unconjugated bilirubin; UPR, unfolded protein response; UPS, ubiquitin-proteasome system; Tp53, human p53 tumor protein.(For details see [201,202,203,204,205,206,207]).
3. Conclusions
Heme, UCB, BV, BVLR and HMOX, are the components of a complex cellular system. In this review, we addressed the role of each YPs on brain heath, discussing both beneficial and detrimental effects. Recent experimental and clinical studies have demonstrated their role and importance in development and progression of various neurological conditions. Future detailed and controlled studies are needed to explore precise role of all the YPs in pathogenesis of these diseases, and how to modulate the YPs in a balanced fashion to prevent or improve their course.
Author Contributions
Conceptualization, L.V., S.J., C.T. and S.G.; writing—original draft preparation, L.V., S.J., C.T. and S.G.; writing—review and editing, L.V., C.T. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Italian Liver Foundation—ONLUS (S.J., C.T., and S.G.), the Indonesia Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan, LPDP) from the Ministry of Finance of Indonesia (S.J.); and grants NV18-07-00342 and RVO-VFN64165/2020 from the Czech Ministry of Health (L.V.) for supporting the Authors in the preparation of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gazzin S., Vitek L., Watchko J., Shapiro S.M., Tiribelli C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016;22:758–768. doi: 10.1016/j.molmed.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Gazzin S., Masutti F., Vítek L., Tiribelli C. The molecular basis of jaundice: An old symptom revisited. Liver Int. 2016;37:1094–1102. doi: 10.1111/liv.13351. [DOI] [PubMed] [Google Scholar]
- 3.Vítek L., Ostrow J.D. Bilirubin Chemistry and Metabolism; Harmful and Protective Aspects. [(accessed on 27 July 2020)]; doi: 10.2174/138161209789058237. Available online: https://www.eurekaselect.com/69920/article. [DOI] [PubMed]
- 4.Le Pichon J.-B., Riordan S.M., Watchko J., Shapiro S.M. The Neurological Sequelae of Neonatal Hyperbilirubinemia: Definitions, Diagnosis and Treatment of the Kernicterus Spectrum Disorders (KSDs) Curr. Pediatr. Rev. 2017;13:199–209. doi: 10.2174/1573396313666170815100214. [DOI] [PubMed] [Google Scholar]
- 5.Strauss K.A., Robinson D.L., Vreman H.J., Puffenberger E.G., Hart G., Morton D.H. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur. J. Pediatr. 2006;165:306–319. doi: 10.1007/s00431-005-0055-2. [DOI] [PubMed] [Google Scholar]
- 6.Watchko J.F., Tiribelli C. Bilirubin-Induced Neurologic Damage—Mechanisms and Management Approaches. N. Engl. J. Med. 2013;369:2021–2030. doi: 10.1056/NEJMra1308124. [DOI] [PubMed] [Google Scholar]
- 7.Diamond I.D., Schmid R.S. Experimental bilirubin encephalopathy. The mode of entry of bilirubin-14C into the central nervous system. J. Clin. Investig. 1966;45:678–689. doi: 10.1172/JCI105383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wennberg R.P., Ahlfors C.E., Bhutani V.K., Johnson L.H., Shapiro S.M. Toward Understanding Kernicterus: A Challenge to Improve the Management of Jaundiced Newborns. Pediatrics. 2006;117:474–485. doi: 10.1542/peds.2005-0395. [DOI] [PubMed] [Google Scholar]
- 9.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 10.Baranano D.E., Rao M., Ferris C.D., Snyder S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham N.G., Kappas A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 12.Gozzelino R. The Pathophysiology of Heme in the Brain. [(accessed on 27 July 2020)]; Available online: https://www.eurekaselect.com/135089/article.
- 13.Maines M.D. New Insights into Biliverdin Reductase Functions: Linking Heme Metabolism to Cell Signaling. Physiology. 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 14.Nitti M., Piras S., Brondolo L., Marinari U.M., Pronzato M.A., Furfaro A.L. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int. J. Mol. Sci. 2018;19:2260. doi: 10.3390/ijms19082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryter S.W., Alam J., Choi A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 16.Schipper H.M., Song W., Tavitian A., Cressatti M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019;172:40–70. doi: 10.1016/j.pneurobio.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K.-H., Wallner M., Mölzer C., Gazzin S., Bulmer A.C., Tiribelli C., Vitek L. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin. Sci. 2015;129:1–25. doi: 10.1042/CS20140566. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Tu Y., Moon C., Nagata E., Ronnett G.V. Heme oxygenase-1 and heme oxygenase-2 have distinct roles in the proliferation and survival of olfactory receptor neurons mediated by cGMP and bilirubin, respectively. J. Neurochem. 2003;85:1247–1261. doi: 10.1046/j.1471-4159.2003.01776.x. [DOI] [PubMed] [Google Scholar]
- 19.Park J.-S., Nam E., Lee H.-K., Lim M.H., Rhee H.-W. In Cellulo Mapping of Subcellular Localized Bilirubin. ACS Chem. Biol. 2016;11:2177–2185. doi: 10.1021/acschembio.6b00017. [DOI] [PubMed] [Google Scholar]
- 20.Takeda T., Mu A., Tai T.T., Kitajima S., Taketani S. Continuous de novo biosynthesis of haem and its rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci. Rep. 2015;5:10488. doi: 10.1038/srep10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funahashi A., Komatsu M., Furukawa T., Yoshizono Y., Yoshizono H., Orikawa Y., Takumi S., Shiozaki K., Hayashi S., Kaminishi Y., et al. Eel green fluorescent protein is associated with resistance to oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016;181–182:35–39. doi: 10.1016/j.cbpc.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai A., Ando R., Miyatake H., Greimel P., Kobayashi T., Hirabayashi Y., Shimogori T., Miyawaki A. A Bilirubin-Inducible Fluorescent Protein from Eel Muscle. Cell. 2013;153:1602–1611. doi: 10.1016/j.cell.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Vítek L., Schwertner H.A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv. Clin. Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- 24.Chiabrando D., Fiorito V., Petrillo S., Tolosano E. Unraveling the Role of Heme in Neurodegeneration. Front. Neurosci. 2018;12:712. doi: 10.3389/fnins.2018.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F., Shan Y., Tang Z., Wu X., Bi C., Zhang Y., Gao Y., Liu H. The Neuroprotective Effect of Hemin and the Related Mechanism in Sevoflurane Exposed Neonatal Rats. Front. Neurosci. 2019;13:537. doi: 10.3389/fnins.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F., Zhang Y., Tang Z., Shan Y., Wu X., Liu H. Hemin treatment protects neonatal rats from sevoflurane-induced neurotoxicity via the phosphoinositide 3-kinase/Akt pathway. Life Sci. 2020;242:117151. doi: 10.1016/j.lfs.2019.117151. [DOI] [PubMed] [Google Scholar]
- 27.Ye F., Li X., Liu Y., Chang W., Liu W., Yuan J., Chen J. Hemin provides protection against lead neurotoxicity through heme oxygenase 1/carbon monoxide activation. J. Appl. Toxicol. 2018;38:1353–1364. doi: 10.1002/jat.3646. [DOI] [PubMed] [Google Scholar]
- 28.Dang T.N., Robinson S.R., Dringen R., Bishop G.M. Uptake, metabolism and toxicity of hemin in cultured neurons. Neurochem. Int. 2011;58:804–811. doi: 10.1016/j.neuint.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Barone E., Di Domenico F., Mancuso C., Butterfield D.A. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: It’s time for reconciliation. Neurobiol. Dis. 2014;62:144–159. doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulters D., Gaastra B., Zolnourian A., Alexander S., Ren D., Blackburn S.L., Borsody M., Doré S., Galea J., Iihara K., et al. Haemoglobin scavenging in intracranial bleeding: Biology and clinical implications. Nat. Rev. Neurol. 2018;14:416–432. doi: 10.1038/s41582-018-0020-0. [DOI] [PubMed] [Google Scholar]
- 31.Van Acker Z.P., Luyckx E., Dewilde S. Neuroglobin Expression in the Brain: A Story of Tissue Homeostasis Preservation. Mol. Neurobiol. 2019;56:2101–2122. doi: 10.1007/s12035-018-1212-8. [DOI] [PubMed] [Google Scholar]
- 32.Khan A., Jamwal S., Bijjem K.R.V., Prakash A., Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287:66–77. doi: 10.1016/j.neuroscience.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Chen J. Heme oxygenase in neuroprotection: From mechanisms to therapeutic implications. Rev. Neurosci. 2014;25:269–280. doi: 10.1515/revneuro-2013-0046. [DOI] [PubMed] [Google Scholar]
- 34.Jazwa J.A., Cuadrado C.A. Targeting Heme Oxygenase-1 for Neuroprotection and Neuroinflammation in Neurodegenerative Diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A.S., Zhuang H., Doré S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Colín-González A.L., Orozco-Ibarra M., Chánez-Cárdenas M.E., Rangel-López E., Santamaría A., Pedraza-Chaverri J., Barrera-Oviedo D., Maldonado P.D. Heme oxygenase-1 (HO-1) upregulation delays morphological and oxidative damage induced in an excitotoxic/pro-oxidant model in the rat striatum. Neuroscience. 2013;231:91–101. doi: 10.1016/j.neuroscience.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Ku B.M., Joo Y., Mun J., Roh G.S., Kang S.S., Cho G.J., Choi W.S., Kim H.J. Heme oxygenase protects hippocampal neurons from ethanol-induced neurotoxicity. Neurosci. Lett. 2006;405:168–171. doi: 10.1016/j.neulet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 38.Orozco-Ibarra M., Estrada-Sánchez A.M., Massieu L., Pedraza-Chaverrí J. Heme oxygenase-1 induction prevents neuronal damage triggered during mitochondrial inhibition: Role of CO and bilirubin. Int. J. Biochem. Cell Biol. 2009;41:1304–1314. doi: 10.1016/j.biocel.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Sferrazzo G., Di Rosa M., Barone E., Li Volti G., Musso N., Tibullo D., Barbagallo I. Heme Oxygenase-1 in Central Nervous System Malignancies. J. Clin. Med. 2020;9:1562. doi: 10.3390/jcm9051562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barone E., Di Domenico F., Sultana R., Coccia R., Mancuso C., Perluigi M., Butterfield D.A. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang E.F., Wong R.J., Vreman H.J., Igarashi T., Galo E., Sharp F.R., Stevenson D.K., Noble-Haeusslein L.J. Heme Oxygenase-2 Protects against Lipid Peroxidation-Mediated Cell Loss and Impaired Motor Recovery after Traumatic Brain Injury. J. Neurosci. 2003;23:3689–3696. doi: 10.1523/JNEUROSCI.23-09-03689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doré S., Snyder S.H. Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures. Ann. N. Y. Acad. Sci. 1999;890:167–172. doi: 10.1111/j.1749-6632.1999.tb07991.x. [DOI] [PubMed] [Google Scholar]
- 43.Doré S., Goto S., Sampei K., Blackshaw S., Hester L.D., Ingi T., Sawa A., Traystman R.J., Koehler R.C., Snyder S.H. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99:587–592. doi: 10.1016/S0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 44.Doré S., Takahashi M., Ferris C.D., Hester L.D., Guastella D., Snyder S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrade V.M., Aschner M., Marreilha dos Santos A.P. Neurotoxicity of Metal Mixtures. In: Aschner M., Costa L.G., editors. Neurotoxicity of Metals. Springer International Publishing; Cham, Switzerland: 2017. pp. 227–265. Advances in Neurobiology. [Google Scholar]
- 46.Schipper H.M. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J., Piantadosi C.A. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J. Clin. Investig. 1992;90:1193–1199. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockard-Sullivan J.E., Korsak R.A., Webber D.S., Edmond J. Mild carbon monoxide exposure and auditory function in the developing rat. J. Neurosci. Res. 2003;74:644–654. doi: 10.1002/jnr.10808. [DOI] [PubMed] [Google Scholar]
- 49.Webber D.S., Korsak R.A., Sininger L.K., Sampogna S.L., Edmond J. Mild carbon monoxide exposure impairs the developing auditory system of the rat. J. Neurosci. Res. 2003;74:655–665. doi: 10.1002/jnr.10809. [DOI] [PubMed] [Google Scholar]
- 50.Deguchi K., Hayashi T., Nagotani S., Sehara Y., Zhang H., Tsuchiya A., Ohta Y., Tomiyama K., Morimoto N., Miyazaki M., et al. Reduction of cerebral infarction in rats by biliverdin associated with amelioration of oxidative stress. Brain Res. 2008;1188:1–8. doi: 10.1016/j.brainres.2007.07.104. [DOI] [PubMed] [Google Scholar]
- 51.Zou Z.-Y., Liu J., Chang C., Li J.-J., Luo J., Jin Y., Ma Z., Wang T.-H., Shao J.-L. Biliverdin administration regulates the microRNA-mRNA expressional network associated with neuroprotection in cerebral ischemia reperfusion injury in rats. Int. J. Mol. Med. 2019;43:1356–1372. doi: 10.3892/ijmm.2019.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice A.C., Shapiro S.M. Biliverdin-induced brainstem auditory evoked potential abnormalities in the jaundiced Gunn rat. Brain Res. 2006;1107:215–221. doi: 10.1016/j.brainres.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham O., Gore M.G., Mantle T.J. Initial-rate kinetics of the flavin reductase reaction catalysed by human biliverdin-IXbeta reductase (BVR-B) Biochem. J. 2000;345:393–399. doi: 10.1042/bj3450393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shalloe F., Elliott G., Ennis O., Mantle T.J. Evidence that biliverdin-IXβ reductase and flavin reductase are identical. Biochem. J. 1996;316:385–387. doi: 10.1042/bj3160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atukeren P., Oner S., Baran O., Kemerdere R., Eren B., Cakatay U., Tanriverdi T. Oxidant and anti-oxidant status in common brain tumors: Correlation to TP53 and human biliverdin reductase. Clin. Neurol. Neurosurg. 2017;158:72–76. doi: 10.1016/j.clineuro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y., Liu J., Tetzlaff W., Paty D.W., Cynader M.S. Biliverdin reductase, a major physiologic cytoprotectant, suppresses experimental autoimmune encephalomyelitis. Free Radic. Biol. Med. 2006;40:960–967. doi: 10.1016/j.freeradbiomed.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Barone E., Di Domenico F., Cenini G., Sultana R., Cini C., Preziosi P., Perluigi M., Mancuso C., Butterfield D.A. Biliverdin reductase--a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim. Biophys. Acta. 2011;1812:480–487. doi: 10.1016/j.bbadis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Domenico F., Barone E., Mancuso C., Perluigi M., Cocciolo A., Mecocci P., Butterfield D.A., Coccia R. HO-1/BVR-a system analysis in plasma from probable Alzheimer’s disease and mild cognitive impairment subjects: A potential biochemical marker for the prediction of the disease. J. Alzheimers Dis. 2012;32:277–289. doi: 10.3233/JAD-2012-121045. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Ding Y., Lu T., Zhang Y., Xu N., Yu L., McBride D.W., Flores J.J., Tang J., Zhang J.H. Bliverdin reductase-A improves neurological function in a germinal matrix hemorrhage rat model. Neurobiol. Dis. 2018;110:122–132. doi: 10.1016/j.nbd.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller C., Zhou W., VanMeter A., Heiby M., Magaki S., Ross M.M., Espina V., Schrag M., Dickson C., Liotta L.A., et al. The Heme Degradation Pathway is a Promising Serum Biomarker Source for the Early Detection of Alzheimer’s Disease. J. Alzheimer’s Dis. 2010;19:1081–1091. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matic L.P., Jesus Iglesias M., Vesterlund M., Lengquist M., Hong M.-G., Saieed S., Sanchez-Rivera L., Berg M., Razuvaev A., Kronqvist M., et al. Novel Multiomics Profiling of Human Carotid Atherosclerotic Plaques and Plasma Reveals Biliverdin Reductase B as a Marker of Intraplaque Hemorrhage. JACC Basic Transl. Sci. 2018;3:464–480. doi: 10.1016/j.jacbts.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Zhu B., Wang X., Luo L., Li P., Paty D.W., Cynader M.S. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: Implications for the role of oxidative stress in the development of multiple sclerosis. J. Neuroimmunol. 2003;139:27–35. doi: 10.1016/S0165-5728(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 63.Yu M., Su D., Yang Y., Qin L., Hu C., Liu R., Zhou Y., Yang C., Yang X., Wang G., et al. D-T7 Peptide-Modified PEGylated Bilirubin Nanoparticles Loaded with Cediranib and Paclitaxel for Antiangiogenesis and Chemotherapy of Glioma. ACS Appl. Mater. Interfaces. 2019;11:176–186. doi: 10.1021/acsami.8b16219. [DOI] [PubMed] [Google Scholar]
- 64.Oda E., Kawai R. A possible cross-sectional association of serum total bilirubin with coronary heart disease and stroke in a Japanese health screening population. Heart Vessels. 2012;27:29–36. doi: 10.1007/s00380-011-0123-7. [DOI] [PubMed] [Google Scholar]
- 65.Thakkar M., Edelenbos J., Doré S. Bilirubin and Ischemic Stroke: Rendering the Current Paradigm to Better Understand the Protective Effects of Bilirubin. Mol. Neurobiol. 2019;56:5483–5496. doi: 10.1007/s12035-018-1440-y. [DOI] [PubMed] [Google Scholar]
- 66.Hung S.-Y., Liou H.-C., Kang K.-H., Wu R.-M., Wen C.-C., Fu W.-M. Over-expression of Heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol. Pharmacol. 2008;74:1564–1575. doi: 10.1124/mol.108.048611. [DOI] [PubMed] [Google Scholar]
- 67.Lee H., Choi Y.K. Regenerative Effects of Heme Oxygenase Metabolites on Neuroinflammatory Diseases. Int. J. Mol. Sci. 2019;20:78. doi: 10.3390/ijms20010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong K., Wang X., Ma X., Ji X., Sang S., Shao S., Zhao Y., Xiang Y., Li J., Wang G., et al. Association between serum bilirubin and asymptomatic intracranial atherosclerosis: Results from a population-based study. Neurol. Sci. 2020;41:1531–1538. doi: 10.1007/s10072-020-04268-x. [DOI] [PubMed] [Google Scholar]
- 69.Yang F.-C., Riordan S.M., Winter M., Gan L., Smith P.G., Vivian J.L., Shapiro S.M., Stanford J.A. Fate of Neural Progenitor Cells Transplanted into Jaundiced and Nonjaundiced Rat Brains. Cell Transpl. 2017;26:605–611. doi: 10.3727/096368917X694840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loftspring M.C., Johnson H.L., Feng R., Johnson A.J., Clark J.F. Unconjugated Bilirubin Contributes to Early Inflammation and Edema after Intracerebral Hemorrhage. J. Cereb. Blood Flow Metab. 2010;31:1133–1142. doi: 10.1038/jcbfm.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marques J.G., Pedro I., Ouakinin S. Unconjugated bilirubin and acute psychosis: A five years retrospective observational and controlled study in patients with schizophrenia, schizoaffective and bipolar disorders. Int. J. Psychiatry Clin. Pract. 2019;23:281–285. doi: 10.1080/13651501.2019.1638940. [DOI] [PubMed] [Google Scholar]
- 72.Bin-Nun A., Mimouni F.B., Kasirer Y., Schors I., Schimmel M.S., Kaplan M., Hammerman C. Might Bilirubin Serve as a Natural Antioxidant in Response to Neonatal Encephalopathy? Am. J. Perinatol. 2018;35:1107–1112. doi: 10.1055/s-0038-1641746. [DOI] [PubMed] [Google Scholar]
- 73.Dani C., Poggi C., Fancelli C., Pratesi S. Changes in bilirubin in infants with hypoxic–ischemic encephalopathy. Eur. J. Pediatr. 2018;177:1795–1801. doi: 10.1007/s00431-018-3245-4. [DOI] [PubMed] [Google Scholar]
- 74.Fereshtehnejad S.M., Poorsattar Bejeh Mir K., Poorsattar Bejeh Mir A., Mohagheghi P. Evaluation of the possible antioxidative role of bilirubin protecting from free radical related illnesses in neonates. Acta Med. Iran. 2012;50:153–163. [PubMed] [Google Scholar]
- 75.Fujiwara R., Haag M., Schaeffeler E., Nies A.T., Zanger U.M., Schwab M. Systemic regulation of bilirubin homeostasis: Potential benefits of hyperbilirubinemia. Hepatology. 2018;67:1609–1619. doi: 10.1002/hep.29599. [DOI] [PubMed] [Google Scholar]
- 76.Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: Role of glial cells and inflammation. Front. Pharmacol. 2012;3:88. doi: 10.3389/fphar.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jašprová J., Dal Ben M., Hurný D., Hwang S., Žížalová K., Kotek J., Wong R.J., Stevenson D.K., Gazzin S., Tiribelli C., et al. Neuro-inflammatory effects of photodegradative products of bilirubin. Sci. Rep. 2018;8:7444. doi: 10.1038/s41598-018-25684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luan H., Liu L.-F., Tang Z., Mok V.C.T., Li M., Cai Z. Elevated excretion of biopyrrin as a new marker for idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21:1371–1372. doi: 10.1016/j.parkreldis.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Alexander J., Marcel R., Niklas L., Andreas S.R., Diana F., Karl-Heinz H., Anna S., Marvin R., Milena G., Charline S., et al. Propentdyopents as Heme Degradation Intermediates Constrict Mouse Cerebral Arterioles and Are Present in the Cerebrospinal Fluid of Patients With Subarachnoid Hemorrhage. Circ. Res. 2019;124:e101–e114. doi: 10.1161/CIRCRESAHA.118.314160. [DOI] [PubMed] [Google Scholar]
- 80.Clark J.F., Loftspring M., Wurster W.L., Pyne-Geithman G.J. Chemical and biochemical oxidations in spinal fluid after subarachnoid hemorrhage. Front. Biosci. 2008;13:1806–1812. doi: 10.2741/2801. [DOI] [PubMed] [Google Scholar]
- 81.Righy C., Bozza M.T., Oliveira M.F., Bozza F.A. Molecular, Cellular and Clinical Aspects of Intracerebral Hemorrhage: Are the Enemies Within? Curr. Neuropharmacol. 2016;14:392–402. doi: 10.2174/1570159X14666151230110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaya J., Song W., Khatib S., Geng G., Schipper H.M. Effects of heme oxygenase-1 expression on sterol homeostasis in rat astroglia. Free Radic. Biol. Med. 2007;42:864–871. doi: 10.1016/j.freeradbiomed.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 83.Lin W.-P., Xiong G.-P., Lin Q., Chen X.-W., Zhang L.-Q., Shi J.-X., Ke Q.-F., Lin J.-H. Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J. Neuroinflamm. 2016;13:52. doi: 10.1186/s12974-016-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi M., Doré S., Ferris C.D., Tomita T., Sawa A., Wolosker H., Borchelt D.R., Iwatsubo T., Kim S.-H., Thinakaran G., et al. Amyloid Precursor Proteins Inhibit Heme Oxygenase Activity and Augment Neurotoxicity in Alzheimer’s Disease. Neuron. 2000;28:461–473. doi: 10.1016/S0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 85.Chen J., Tu Y., Connolly E.C., Ronnett G.V. Heme oxygenase-2 protects against glutathione depletion-induced neuronal apoptosis mediated by bilirubin and cyclic GMP. Curr. Neurovasc. Res. 2005;2:121–131. doi: 10.2174/1567202053586767. [DOI] [PubMed] [Google Scholar]
- 86.Cuadrado A., Rojo A.I. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr. Pharm. Des. 2008;14:429–442. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- 87.Hettiarachchi N., Dallas M., Al-Owais M., Griffiths H., Hooper N., Scragg J., Boyle J., Peers C. Heme oxygenase-1 protects against Alzheimer’s amyloid-β(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis. 2014;5:e1569. doi: 10.1038/cddis.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hettiarachchi N.T., Boyle J.P., Dallas M.L., Al-Owais M.M., Scragg J.L., Peers C. Heme oxygenase-1 derived carbon monoxide suppresses Aβ1-42 toxicity in astrocytes. Cell Death Dis. 2017;8:e2884. doi: 10.1038/cddis.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin S.-H., Song W., Cressatti M., Zukor H., Wang E., Schipper H.M. Heme oxygenase-1 modulates microRNA expression in cultured astroglia: Implications for chronic brain disorders. Glia. 2015;63:1270–1284. doi: 10.1002/glia.22823. [DOI] [PubMed] [Google Scholar]
- 90.Mancuso C., Barone E., Guido P., Miceli F., Di Domenico F., Perluigi M., Santangelo R., Preziosi P. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci. Lett. 2012;518:101–105. doi: 10.1016/j.neulet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 91.Gibbs P.E.M., Maines M.D. Biliverdin inhibits activation of NF-κB: Reversal of inhibition by human biliverdin reductase. Int. J. Cancer. 2007;121:2567–2574. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- 92.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bisht K., Wegiel B., Tampe J., Neubauer O., Wagner K.-H., Otterbein L.E., Bulmer A.C. Biliverdin modulates the expression of C5aR in response to endotoxin in part via mTOR signaling. Biochem. Biophys. Res. Commun. 2014;449:94–99. doi: 10.1016/j.bbrc.2014.04.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakao A., Murase N., Ho C., Toyokawa H., Billiar T.R., Kanno S. Biliverdin Administration Prevents the Formation of Intimal Hyperplasia Induced by Vascular Injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 95.Wegiel B., Baty C.J., Gallo D., Csizmadia E., Scott J.R., Akhavan A., Chin B.Y., Kaczmarek E., Alam J., Bach F.H., et al. Cell Surface Biliverdin Reductase Mediates Biliverdin-induced Anti-inflammatory Effects via Phosphatidylinositol 3-Kinase and Akt. J. Biol. Chem. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wegiel B., Gallo D., Csizmadia E., Roger T., Kaczmarek E., Harris C., Zuckerbraun B.S., Otterbein L.E. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proc. Natl. Acad. Sci. USA. 2011;108:18849–18854. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gurba P.E., Zand R. Bilirubin binding to myelin basic protein, histones and its inhibition in vitro of cerebellar protein synthesis. Biochem. Biophys. Res. Commun. 1974;58:1142–1147. doi: 10.1016/S0006-291X(74)80262-7. [DOI] [PubMed] [Google Scholar]
- 98.Pei J.J., Braak E. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Medina M., Garrido J.J., Wandosell F.G. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front. Mol. Neurosci. 2011;4:24. doi: 10.3389/fnmol.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miralem T., Lerner-Marmarosh N., Gibbs P.E.M., Jenkins J.L., Heimiller C., Maines M.D. Interaction of human biliverdin reductase with Akt/protein kinase B and phosphatidylinositol-dependent kinase 1 regulates glycogen synthase kinase 3 activity: A novel mechanism of Akt activation. FASEB J. 2016;30:2926–2944. doi: 10.1096/fj.201600330RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma N., Tramutola A., Lanzillotta C., Arena A., Blarzino C., Cassano T., Butterfield D.A., Di Domenico F., Perluigi M., Barone E. Loss of biliverdin reductase-A favors Tau hyper-phosphorylation in Alzheimer’s disease. Neurobiol. Dis. 2019;125:176–189. doi: 10.1016/j.nbd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 102.Kim S.J., Shin M.J., Kim D.W., Yeo H.J., Yeo E.J., Choi Y.J., Sohn E.J., Han K.H., Park J., Lee K.W., et al. Tat-Biliverdin Reductase A Exerts a Protective Role in Oxidative Stress-Induced Hippocampal Neuronal Cell Damage by Regulating the Apoptosis and MAPK Signaling. Int. J. Mol. Sci. 2020;21:2672. doi: 10.3390/ijms21082672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Triani F., Tramutola A., Di Domenico F., Sharma N., Butterfield D.A., Head E., Perluigi M., Barone E. Biliverdin reductase-A impairment links brain insulin resistance with increased Aβ production in an animal model of aging: Implications for Alzheimer disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018;1864:3181–3194. doi: 10.1016/j.bbadis.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Mancuso C. Bilirubin and brain: A pharmacological approach. Neuropharmacology. 2017;118:113–123. doi: 10.1016/j.neuropharm.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 105.Barone E., Di Domenico F., Cassano T., Arena A., Tramutola A., Lavecchia M.A., Coccia R., Butterfield D.A., Perluigi M. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: A new paradigm. Free Radic. Biol. Med. 2016;91:127–142. doi: 10.1016/j.freeradbiomed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Morris G., Puri B.K., Walker A.J., Berk M., Walder K., Bortolasci C.C., Marx W., Carvalho A.F., Maes M. The compensatory antioxidant response system with a focus on neuroprogressive disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;95:109708. doi: 10.1016/j.pnpbp.2019.109708. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y., Li P., Lu J., Xiong W., Oger J., Tetzlaff W., Cynader M. Bilirubin Possesses Powerful Immunomodulatory Activity and Suppresses Experimental Autoimmune Encephalomyelitis. J. Immunol. 2008;181:1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 108.Vianello E., Zampieri S., Marcuzzo T., Tordini F., Bottin C., Dardis A., Zanconati F., Tiribelli C., Gazzin S. Histone acetylation as a new mechanism for bilirubin-induced encephalopathy in the Gunn rat. Sci. Rep. 2018;8:13690. doi: 10.1038/s41598-018-32106-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qaisiya M., Brischetto C., Jašprová J., Vitek L., Tiribelli C., Bellarosa C. Bilirubin-induced ER stress contributes to the inflammatory response and apoptosis in neuronal cells. Arch. Toxicol. 2017;91:1847–1858. doi: 10.1007/s00204-016-1835-3. [DOI] [PubMed] [Google Scholar]
- 110.Qaisiya M., Coda Zabetta C.D., Bellarosa C., Tiribelli C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell. Signal. 2014;26:512–520. doi: 10.1016/j.cellsig.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen N.T., Hanieh H., Nakahama T., Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int. Immunol. 2013;25:335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 112.Phelan D., Winter G.M., Rogers W.J., Lam J.C., Denison M.S. Activation of the Ah Receptor Signal Transduction Pathway by Bilirubin and Biliverdin. Arch. Biochem. Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 113.Vítek V.L. Bilirubin as a signaling molecule. Med. Res. Rev. 2020;40:1335–1351. doi: 10.1002/med.21660. [DOI] [PubMed] [Google Scholar]
- 114.Datla Srinivasa R., Dusting Gregory J., Mori Trevor A., Taylor Caroline J., Croft Kevin D. Jiang Fan Induction of Heme Oxygenase-1 In Vivo Suppresses NADPH Oxidase–Derived Oxidative Stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- 115.Peng F., Deng X., Yu Y., Chen X., Shen L., Zhong X., Qiu W., Jiang Y., Zhang J., Hu X. Serum bilirubin concentrations and multiple sclerosis. J. Clin. Neurosci. 2011;18:1355–1359. doi: 10.1016/j.jocn.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 116.Gennuso F., Fernetti C., Tirolo C., Testa N., L’Episcopo F., Caniglia S., Morale M.C., Ostrow J.D., Pascolo L., Tiribelli C., et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1) Proc. Natl. Acad. Sci. USA. 2004;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodrigues C.M.P., Solá S., Brito M.A., Brites D., Moura J.J.G. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J. Hepatol. 2002;36:335–341. doi: 10.1016/S0168-8278(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 118.Rodrigues C.M.P., Solá S., Brites D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology. 2002;35:1186–1195. doi: 10.1053/jhep.2002.32967. [DOI] [PubMed] [Google Scholar]
- 119.Fernandes A., Falcão A.S., Silva R.F.M., Gordo A.C., Gama M.J., Brito M.A., Brites D. Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin. J. Neurochem. 2006;96:1667–1679. doi: 10.1111/j.1471-4159.2006.03680.x. [DOI] [PubMed] [Google Scholar]
- 120.Fernandes A., Falcão A.S., Silva R.F.M., Brito M.A., Brites D. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur. J. Neurosci. 2007;25:1058–1068. doi: 10.1111/j.1460-9568.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 121.Chang F.-Y., Lee C.-C., Huang C.-C., Hsu K.-S. Unconjugated Bilirubin Exposure Impairs Hippocampal Long-Term Synaptic Plasticity. PLoS ONE. 2009;4:e5876. doi: 10.1371/journal.pone.0005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grojean S., Koziel V., Vert P., Daval J.L. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp. Neurol. 2000;166:334–341. doi: 10.1006/exnr.2000.7518. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L., Liu W., Tanswell A.K., Luo X. The Effects of Bilirubin on Evoked Potentials and Long-Term Potentiation in Rat Hippocampus In Vivo. Pediatric Res. 2003;53:939–944. doi: 10.1203/01.PDR.0000061563.63230.86. [DOI] [PubMed] [Google Scholar]
- 124.Mancuso C., Capone C., Ranieri S.C., Fusco S., Calabrese V., Eboli M.L., Preziosi P., Galeotti T., Pani G. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J. Neurosci. Res. 2008;86:2235–2249. doi: 10.1002/jnr.21665. [DOI] [PubMed] [Google Scholar]
- 125.Gazzin S., Berengeno A.L., Strazielle N., Fazzari F., Raseni A., Ostrow J.D., Wennberg R., Ghersi-Egea J.-F., Tiribelli C. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by Bilirubin at the Blood-CSF and Blood-Brain Barriers in the Gunn Rat. PLoS ONE. 2011;6:e16165. doi: 10.1371/journal.pone.0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rawat V., Bortolussi G., Gazzin S., Tiribelli C., Muro A.F. Bilirubin-Induced Oxidative Stress Leads to DNA Damage in the Cerebellum of Hyperbilirubinemic Neonatal Mice and Activates DNA Double-Strand Break Repair Pathways in Human Cells. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/1801243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Robert M.C., Furlan G., Rosso N., Gambaro S.E., Apitsionak F., Vianello E., Tiribelli C., Gazzin S. Alterations in the Cell Cycle in the Cerebellum of Hyperbilirubinemic Gunn Rat: A Possible Link with Apoptosis? PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0079073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neis V.B., Rosa P.B., Moretti M., Rodrigues A.L.S. Involvement of Heme Oxygenase-1 in Neuropsychiatric and Neurodegenerative Diseases. Curr. Pharm. Des. 2018;24:2283–2302. doi: 10.2174/1381612824666180717160623. [DOI] [PubMed] [Google Scholar]
- 129.Schipper H.M. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 130.González-Reyes S., Orozco-Ibarra M., Guzmán-Beltrán S., Molina-Jijón E., Massieu L., Pedraza-Chaverri J. Neuroprotective role of heme-oxygenase 1 against iodoacetate-induced toxicity in rat cerebellar granule neurons: Role of bilirubin. Free Radic. Res. 2009;43:214–223. doi: 10.1080/10715760802676670. [DOI] [PubMed] [Google Scholar]
- 131.Schipper H.M. Heme oxygenase-1: Role in brain aging and neurodegeneration. Exp. Gerontol. 2000;35:821–830. doi: 10.1016/S0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 132.Doré S., Sampei K., Goto S., Alkayed N.J., Guastella D., Blackshaw S., Gallagher M., Traystman R.J., Hurn P.D., Koehler R.C., et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol. Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 133.Nam J., Lee Y., Yang Y., Jeong S., Kim W., Yoo J.-W., Moon J.-O., Lee C., Chung H.Y., Kim M.-S., et al. Is it worth expending energy to convert biliverdin into bilirubin? Free Radic. Biol. Med. 2018;124:232–240. doi: 10.1016/j.freeradbiomed.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Mancuso C., Barone E. The Heme Oxygenase/Biliverdin Reductase Pathway in Drug Research and Development. [(accessed on 27 July 2020)]; Available online: https://www.eurekaselect.com/70167/article.
- 135.Maines M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 136.McDonagh A.F., Palma L.A., Schmid R. Reduction of biliverdin and placental transfer of bilirubin and biliverdin in the pregnant guinea pig. Biochem. J. 1981;194:273–282. doi: 10.1042/bj1940273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Itoh S., Kondo M., Imai T., Kusaka T., Isobe K., Onishi S. Relationships between serum (ZZ)-bilirubin, its subfractions and biliverdin concentrations in infants at 1-month check-ups. Ann. Clin. Biochem. 2001;38:323–328. doi: 10.1258/0004563011900821. [DOI] [PubMed] [Google Scholar]
- 138.Niedzielska E., Smaga I., Gawlik M., Moniczewski A., Stankowicz P., Pera J., Filip M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016;53:4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei-Wei C., Zhang X., Wen-Juan H. Role of neuroinflammation in neurodegenerative diseases (Review) Mol. Med. Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Azam S., Jakaria M., Kim I.-S., Kim J., Haque M.E., Choi D.-K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao C.-X., Yang Q.-W., Lv F.-L., Cui J., Fu H.-B., Wang J.-Z. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem. Biophys. Res. Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 142.Lotz M., Ebert S., Esselmann H., Iliev A.I., Prinz M., Wiazewicz N., Wiltfang J., Gerber J., Nau R. Amyloid beta peptide 1–40 enhances the action of Toll-like receptor-2 and -4 agonists but antagonizes Toll-like receptor-9-induced inflammation in primary mouse microglial cell cultures. J. Neurochem. 2005;94:289–298. doi: 10.1111/j.1471-4159.2005.03188.x. [DOI] [PubMed] [Google Scholar]
- 143.Mellanby R.J., Cambrook H., Turner D.G., O’Connor R.A., Leech M.D., Kurschus F.C., MacDonald A.S., Arnold B., Anderton S.M. TLR-4 ligation of dendritic cells is sufficient to drive pathogenic T cell function in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2012;9:248. doi: 10.1186/1742-2094-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Minoretti P., Gazzaruso C., Vito C.D., Emanuele E., Bianchi M., Coen E., Reino M., Geroldi D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neurosci. Lett. 2006;391:147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 145.Noelker C., Morel L., Lescot T., Osterloh A., Alvarez-Fischer D., Breloer M., Henze C., Depboylu C., Skrzydelski D., Michel P.P., et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walter S., Letiembre M., Liu Y., Heine H., Penke B., Hao W., Bode B., Manietta N., Walter J., Schulz-Schüffer W., et al. Role of the Toll-Like Receptor 4 in Neuroinflammation in Alzheimer’s Disease. CPB. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 147.Ager R.R., Fonseca M.I., Chu S.-H., Sanderson S.D., Taylor S.M., Woodruff T.M., Tenner A.J. Microglial C5aR (CD88) expression correlates with amyloid-β deposition in murine models of Alzheimer’s disease. J. Neurochem. 2010;113:389–401. doi: 10.1111/j.1471-4159.2010.06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.An X., Xi W., Gu C., Huang X. Complement protein C5a enhances the β-amyloid-induced neuro-inflammatory response in microglia in Alzheimer’s disease. Med. Sci. (Paris) 2018;34:116–120. doi: 10.1051/medsci/201834f120. [DOI] [PubMed] [Google Scholar]
- 149.Nizami S., Hall-Roberts H., Warrier S., Cowley S.A., Daniel E.D. Microglial inflammation and phagocytosis in Alzheimer’s disease: Potential therapeutic targets. Br. J. Pharmacol. 2019;176:3515–3532. doi: 10.1111/bph.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaur H., Hughes M.N., Green C.J., Naughton P., Foresti R., Motterlini R. Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett. 2003;543:113–119. doi: 10.1016/S0014-5793(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 151.Gonzalez-Sanchez E., Perez M.J., Nytofte N.S., Briz O., Monte M.J., Lozano E., Serrano M.A., Marin J.J.G. Protective role of biliverdin against bile acid-induced oxidative stress in liver cells. Free Radic. Biol. Med. 2016;97:466–477. doi: 10.1016/j.freeradbiomed.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 152.Blumenthal S.G., Stucker T., Rasmussen R.D., Ikeda R.M., Ruebner B.H., Bergstrom D.E., Hanson F.W. Changes in bilirubins in human prenatal development. Biochem. J. 1980;186:693–700. doi: 10.1042/bj1860693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Komuro A., Tobe T., Nakano Y., Yamaguchi T., Tomita M. Cloning and characterization of the cDNA encoding human biliverdin-IX alpha reductase. Biochim. Biophys. Acta. 1996;1309:89–99. doi: 10.1016/S0167-4781(96)00099-1. [DOI] [PubMed] [Google Scholar]
- 154.Kapitulnik J., Maines M.D. Pleiotropic functions of biliverdin reductase: Cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 155.O’Brien L., Hosick P.A., John K., Stec D.E., Hinds T.D. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015;26:212–220. doi: 10.1016/j.tem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tudor C., Lerner-Marmarosh N., Engelborghs Y., Gibbs P.E.M., Maines M.D. Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem. J. 2008;413:405–416. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Derada Troletti C., de Goede P., Kamermans A., de Vries H.E. Molecular alterations of the blood–brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016;1862:452–460. doi: 10.1016/j.bbadis.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 158.Galaris D., Pantopoulos K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 159.Loboda A., Jazwa A., Grochot-Przeczek A., Rutkowski A.J., Cisowski J., Agarwal A., Jozkowicz A., Dulak J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 160.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barone E., Di Domenico F., Cenini G., Sultana R., Coccia R., Preziosi P., Perluigi M., Mancuso C., Butterfield D.A. Oxidative and Nitrosative Modifications of Biliverdin Reductase-A in the Brain of Subjects with Alzheimer’s Disease and Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2011;25:623–633. doi: 10.3233/JAD-2011-110092. [DOI] [PubMed] [Google Scholar]
- 162.Lerner-Marmarosh N., Shen J., Torno M.D., Kravets A., Hu Z., Maines M.D. Human biliverdin reductase: A member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc. Natl. Acad. Sci. USA. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gibbs P.E.M., Lerner-Marmarosh N., Poulin A., Farah E., Maines M.D. Human biliverdin reductase-based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. FASEB J. 2014;28:2478–2491. doi: 10.1096/fj.13-247015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rivera E.J., Goldin A., Fulmer N., Tavares R., Wands J.R., de la Monte S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005;8:247–268. doi: 10.3233/JAD-2005-8304. [DOI] [PubMed] [Google Scholar]
- 165.Steen E., Terry B.M., Rivera E.J., Cannon J.L., Neely T.R., Tavares R., Xu X.J., Wands J.R., de la Monte S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J. Alzheimers Dis. 2005;7:63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 166.Talbot K., Wang H.-Y., Kazi H., Han L.-Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barone E., Tramutola A., Triani F., Calcagnini S., Di Domenico F., Ripoli C., Gaetani S., Grassi C., Butterfield D.A., Cassano T., et al. Biliverdin Reductase-A Mediates the Beneficial Effects of Intranasal Insulin in Alzheimer Disease. Mol. Neurobiol. 2019;56:2922–2943. doi: 10.1007/s12035-018-1231-5. [DOI] [PubMed] [Google Scholar]
- 168.Stocker R. Antioxidant Activities of Bile Pigments. Antioxid. Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 169.Sedlak T.W., Saleh M., Higginson D.S., Paul B.D., Juluri K.R., Snyder S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vasavda C., Kothari R., Malla A.P., Tokhunts R., Lin A., Ji M., Ricco C., Xu R., Saavedra H.G., Sbodio J.I., et al. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019;26:1450–1460.e7. doi: 10.1016/j.chembiol.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jayanti S., Moretti R., Tiribelli C., Gazzin S. Bilirubin and inflammation in neurodegenerative and other neurological diseases. Neuroimmunol. Neuroinflamm. 2020;7:92–108. doi: 10.20517/2347-8659.2019.14. [DOI] [Google Scholar]
- 172.Lee Y., Lee S., Lee D.Y., Yu B., Miao W., Jon S. Multistimuli-Responsive Bilirubin Nanoparticles for Anticancer Therapy. Angew. Chem. Int. Ed. 2016;55:10676–10680. doi: 10.1002/anie.201604858. [DOI] [PubMed] [Google Scholar]
- 173.Aycicek A., Erel O. Total oxidant/antioxidant status in jaundiced newborns before and after phototherapy. J. Pediatr. 2007;83:319–322. doi: 10.2223/JPED.1645. [DOI] [PubMed] [Google Scholar]
- 174.Araújo A.R., Rosso N., Bedogni G., Tiribelli C., Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38(Suppl. 1):47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 175.Bush H., Golabi P., Younossi Z.M. Pediatric Non-Alcoholic Fatty Liver Disease. Children (Basel) 2017;4:48. doi: 10.3390/children4060048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moretti R., Caruso P., Gazzin S. Non-alcoholic fatty liver disease and neurological defects. Ann. Hepatol. 2019;18:563–570. doi: 10.1016/j.aohep.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 177.Lombardi R., Fargion S., Fracanzani A.L. Brain involvement in non-alcoholic fatty liver disease (NAFLD): A systematic review. Dig. Liver Dis. 2019;51:1214–1222. doi: 10.1016/j.dld.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 178.Satoh A., Brace C.S., Ben-Josef G., West T., Wozniak D.F., Holtzman D.M., Herzog E.D., Imai S. SIRT1 Promotes the Central Adaptive Response to Diet Restriction through Activation of the Dorsomedial and Lateral Nuclei of the Hypothalamus. J. Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moraes D.S., Moreira D.C., Andrade J.M.O., Santos S.H.S. Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep. 2020;9:46–51. doi: 10.1016/j.ibror.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Radak Z., Suzuki K., Posa A., Petrovszky Z., Koltai E., Boldogh I. The systemic role of SIRT1 in exercise mediated adaptation. Redox Biol. 2020;35:101467. doi: 10.1016/j.redox.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jang B.K. Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012;18:357–359. doi: 10.3350/cmh.2012.18.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar R., Rastogi A., Maras J.S., Sarin S.K. Unconjugated hyperbilirubinemia in patients with non-alcoholic fatty liver disease: A favorable endogenous response. Clin. Biochem. 2012;45:272–274. doi: 10.1016/j.clinbiochem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 183.Lin L.-Y., Kuo H.-K., Hwang J.-J., Lai L.-P., Chiang F.-T., Tseng C.-D., Lin J.-L. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis. 2009;203:563–568. doi: 10.1016/j.atherosclerosis.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 184.Puri K., Nobili V., Melville K., Corte C.D., Sartorelli M.R., Lopez R., Feldstein A.E., Alkhouri N. Serum bilirubin level is inversely associated with nonalcoholic steatohepatitis in children. J. Pediatr. Gastroenterol. Nutr. 2013;57:114–118. doi: 10.1097/MPG.0b013e318291fefe. [DOI] [PubMed] [Google Scholar]
- 185.Herskovits A.Z., Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chandrasekaran K., Salimian M., Konduru S.R., Choi J., Kumar P., Long A., Klimova N., Ho C.-Y., Kristian T., Russell J.W. Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain. 2019;142:3737–3752. doi: 10.1093/brain/awz324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nassir F., Ibdah J.A. Sirtuins and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016;22:10084–10092. doi: 10.3748/wjg.v22.i46.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vítek L., Majer F., Muchová L., Zelenka J., Jirásková A., Branný P., Malina J., Ubik K. Identification of bilirubin reduction products formed by Clostridium perfringens isolated from human neonatal fecal flora. J. Chromatogr. B. 2006;833:149–157. doi: 10.1016/j.jchromb.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 189.Sedlak T., Snyder S. Bilirubin Benefits: Cellular Protection by a Biliverdin Reductase Antioxidant Cycle. Pediatrics. 2004;113:1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 190.Jasprova J., Dal Ben M., Vianello E., Goncharova I., Urbanova M., Vyroubalova K., Gazzin S., Tiribelli C., Sticha M., Cerna M., et al. The Biological Effects of Bilirubin Photoisomers. PLoS ONE. 2016;11:e0148126. doi: 10.1371/journal.pone.0148126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garcia E., Aguilar-Cevallos J., Silva-Garcia R., Ibarra A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. [(accessed on 27 July 2020)]; doi: 10.1155/2016/9476020. Available online: https://www.hindawi.com/journals/mi/2016/9476020/ [DOI] [PMC free article] [PubMed]
- 192.Kempuraj D., Thangavel R., Natteru P., Selvakumar G., Saeed D., Zahoor H., Zaheer S., Iyer S., Zaheer A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine. 2016;1:1003. [PMC free article] [PubMed] [Google Scholar]
- 193.Dietzschold B., Richt J.A., editors. Protective and Pathological Immune Responses in the CNS. Springer; Berlin/Heidelberg, Germany: 2002. Current Topics in Microbiology and Immunology. [Google Scholar]
- 194.Hirota H. Accelerated Nerve Regeneration in Mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J. Exp. Med. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hagman S., Mäkinen A., Ylä-Outinen L., Huhtala H., Elovaara I., Narkilahti S. Effects of inflammatory cytokines IFN-γ, TNF-α and IL-6 on the viability and functionality of human pluripotent stem cell-derived neural cells. J. Neuroimmunol. 2019;331:36–45. doi: 10.1016/j.jneuroim.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 196.Vodret S., Bortolussi G., Jašprová J., Vitek L., Muro A.F. Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1 (-/-) mouse model. J. Neuroinflamm. 2017;14:64. doi: 10.1186/s12974-017-0838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vodret S., Bortolussi G., Iaconcig A., Martinelli E., Tiribelli C., Muro A.F. Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia. Brain Behav. Immun. 2018;70:166–178. doi: 10.1016/j.bbi.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 198.Shimoharada K., Inoue S., Nakahara M., Kanzaki N., Shimizu S., Kang D., Hamasaki N., Kinoshita S. Urine Concentration of Biopyrrins: A New Marker for Oxidative Stress in Vivo. Clin. Chem. 1998;44:2554–2555. doi: 10.1093/clinchem/44.12.2554a. [DOI] [PubMed] [Google Scholar]
- 199.Vítek L., Kráslová I., Muchová L., Novotný L., Yamaguchi T. Urinary excretion of oxidative metabolites of bilirubin in subjects with Gilbert syndrome. J. Gastroenterol. Hepatol. 2007;22:841–845. doi: 10.1111/j.1440-1746.2006.04564.x. [DOI] [PubMed] [Google Scholar]
- 200.Jašprová J., Dvořák A., Vecka M., Leníček M., Lacina O., Valášková P., Zapadlo M., Plavka R., Klán P., Vítek L. A novel accurate LC-MS/MS method for quantitative determination of Z-lumirubin. Sci. Rep. 2020;10:4411. doi: 10.1038/s41598-020-61280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ndayisaba A., Kaindlstorfer C., Wenning G.K. Iron in Neurodegeneration—Cause or Consequence? Front. Neurosci. 2019;13:180. doi: 10.3389/fnins.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li J.-J., Zou Z.-Y., Liu J., Xiong L.-L., Jiang H.-Y., Wang T.-H., Shao J.-L. Biliverdin administration ameliorates cerebral ischemia reperfusion injury in rats and is associated with proinflammatory factor downregulation. Exp. Ther. Med. 2017;14:671–679. doi: 10.3892/etm.2017.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lu X., Gu R., Hu W., Sun Z., Wang G., Wang L., Xu Y. Upregulation of heme oxygenase-1 protected against brain damage induced by transient cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2018;15:4629–4636. doi: 10.3892/etm.2018.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pehar M., Vargas M.R., Cassina P., Barbeito A.G., Beckman J.S., Barbeito L. Complexity of Astrocyte-Motor Neuron Interactions in Amyotrophic Lateral Sclerosis. NDD. 2005;2:139–146. doi: 10.1159/000089619. [DOI] [PubMed] [Google Scholar]
- 205.van Horssen J., Schreibelt G., Drexhage J., Hazes T., Dijkstra C.D., van der Valk P., de Vries H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008;45:1729–1737. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 206.Parfenova H., Leffler C.W. Cerebroprotective functions of HO-2. Curr. Pharm. Des. 2008;14:443–453. doi: 10.2174/138161208783597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gandini N.A., Fermento M.E., Salomón D.G., Obiol D.J., Andrés N.C., Zenklusen J.C., Arevalo J., Blasco J., López Romero A., Facchinetti M.M., et al. Heme oxygenase-1 expression in human gliomas and its correlation with poor prognosis in patients with astrocytoma. Tumor Biol. 2014;35:2803–2815. doi: 10.1007/s13277-013-1373-z. [DOI] [PubMed] [Google Scholar]