Abstract
Over 35% of the adult US population is obese. In turn, excess adiposity increases the risk of multiple complications including type 2 diabetes (T2D), insulin resistance, and cardiovascular disease; yet, obesity also independently heightens risk of Alzheimer’s Disease (AD), even after adjusting for other important confounding risk factors including blood pressure, sociodemographics, cholesterol levels, smoking status, and Apolipoprotein E (ApoE) genotype. Among patients over the age of 65 with dementia, 37% have coexisting diabetes, and an estimated 7.3% of cases of AD are directly attributable to midlife obesity. Clusterin, also known as apolipoprotein J (ApoJ), is a multifunctional glycoprotein that acts as a molecular chaperone, assisting folding of secreted proteins. Clusterin has been implicated in several physiological and pathological states, including AD, metabolic disease, and cardiovascular disease. Despite long-standing interest in elucidating clusterin’s relationship with amyloid beta (Aβ) aggregation/clearance and toxicity, significant knowledge gaps still exist. Altered clusterin expression and protein levels have been linked with cognitive and memory function, disrupted central nervous system lipid flux, as well as pathogenic brain structure; and its role in cardiometabolic disease suggests that it may be a link between insulin resistance, dyslipidemia, and AD. Here, we briefly highlight clusterin’s relevance to AD by presenting existing evidence linking clusterin to AD and cardiometabolic disease, and discussing its potential utility as a biomarker for AD in the presence of obesity-related metabolic disease.
Keywords: Alzheimer’s Disease, amyloid beta, adipose tissue, clusterin
Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia, and is expected to rise precipitously with an increasingly aging population.1,2 Unfortunately, treatments that prevent or slow the progression of AD are lacking, likely secondary to an incomplete understanding of AD pathogenesis. The etiology of AD is multifactorial, with contributions including genetic risk polymorphisms,3 lifestyle and dietary factors,4 and advancing age, among others.5 Although the hallmark neuropathological changes in AD include intracellular accumulation of hyperphosphorylated tau (p-tau), all forms of AD also exhibit overproduction and/or reduced clearance of amyloid-beta (Aβ).6 Therefore, one of the prominent signs of AD is the excess formation of diffuse senile plaques, composed of both aggregating and non-aggregating Aβ derived from endoproteolytic cleavage of the Aβ precursor protein (APP) by beta- and gamma-secretase.
Interestingly, acquired risk factors for AD include numerous factors associated with the metabolic syndrome (hypertension, dyslipidemia, cerebro- and cardiovascular disease, and insulin resistance and Type 2 diabetes [T2D]).7,8 Patients with ⩾ 2 vascular risk factors in midlife have a threefold higher risk of brain Aβ deposition later in life9; and T2D, which is closely linked with obesity,10 has long been associated with a higher risk of cognitive decline and AD.11–21 Obesity-related comorbidities have important effects on Aβ production and deposition, underscoring the importance of better elucidating their role in AD pathogenesis. Yet, despite the large impact that AD has on individuals, families, society, and the health care system,1 the underlying mechanisms connecting obesity and AD remain largely unknown.
Potential mechanism(s) linking excess adiposity, insulin resistance, and AD risk
The role of insulin and insulin signaling in AD
One potential reason for the connection between dysregulated metabolism and AD is that insulin has direct effects on neurotransmission and neuropathology in the brain,22–25 including alterations in the production, degradation and clearance of Aβ that subsequently lead to plaque deposition.26 Raising peripheral insulin levels acutely elevates brain and cerebrospinal fluid (CSF) insulin levels,27 as illustrated by the finding that peripheral intravenous infusion of different concentrations of insulin in 8 normal, lean subjects over 4.5 hours not only increased mean plasma concentrations of insulin (12 ± 1.2 to 268 ± 35 μU/ml), but CSF insulin levels as well (0.9 ± 0.1 to 2.8 ± 0.4 μU/ml) (P < .006). In contrast, prolonged peripheral hyperinsulinemia (as seen in obesity and T2D) down-regulates blood-brain barrier (BBB) insulin receptors and reduces insulin transport into the brain.28 In obese Zucker rats, Stanley et al observed a 65% reduction in brain capillary insulin binding sites compared to controls, with the degree of insulin binding negatively correlating with circulating plasma insulin levels (P < .05).29 However, data supporting this contention in humans is limited and oftentimes conflicting.29 In the sole study reporting brain insulin protein levels in AD patients, there was an equivalent decrease in both AD patients and age-matched controls, suggesting that the lower insulin protein levels may be related to aging, and not AD pathology per se.30 Yet, 2 other studies reported significant reductions in insulin mRNA gene expression in age-matched AD patients.31,32
Patients with mild cognitive impairment (MCI) and AD also have documented dysregulation in central nervous system (CNS) insulin signaling. This is evident in postmortem brain samples, even in the absence of diabetes, including decreased phosphorylation of protein kinase B (Akt) and reduced activation of phosphoinositide 3-kinase (PI3K),32–34 key factors in the canonical insulin signaling pathway.35 Inhibiting PI3K activity increases Aβ production, and restoring proper signaling, even peripherally, leads to reduced amyloid deposition.36 As a result of these conflicting findings, a better understanding of insulin’s effects in the human brain is needed, particularly accounting for the severity of AD and relative to age-matched controls.
There have been numerous studies suggesting that peripheral hyperinsulinemia also alters the risk of AD via its effects on degradation and/or clearance of Aβ. In the CNS, insulin and Aβ are degraded by insulin degrading enzymes (IDEs); and with elevated insulin levels, IDEs preferentially degrade insulin in favor of Aβ, which could lead to Aβ deposition.37 Aβ clearance is also significantly reduced in the setting of elevated insulin levels.38,39 In fact, small interventional trials with intravenous insulin infusion,26,40,41 inhaled insulin,42,43 the insulin-sensitizing agent pioglitazone,44,45 metformin,46,47 and incretin-based therapies48 have shown beneficial effects on memory.
Brain activity may be affected by obesity and peripheral insulin resistance. In various murine models of obesity and diabetes (including after high-fat diet feeding),49–52 there exists a strong relationship between peripheral and “brain” insulin resistance; and in humans, altered metabolic brain activity occurs in peripherally insulin-resistant subjects.53–55 In a study of lean vs. obese humans (scanned by functional magnetic resonance imaging (fMRI) while simultaneously completing memory testing),56 regions of the brain known to be important for recollecting episodic memories (ie, the hippocampus, angular gyrus, and dorsolateral prefrontal cortex) had significantly impaired functional activity in the obese subjects.
The role of CNS lipid flux in AD
Distinct from insulin resistance and insulin signaling defects, lipid flux and cholesterol metabolism in the CNS are also involved in the pathogenesis of AD and Aβ pathology. Apolipoprotein E (ApoE) is the principal carrier of cholesterol in the CNS, the ApoE epsilon 4 (ε4) genetic polymorphism is the most prominent genetic determinant of AD risk,57 and ApoE from human stem-cell derived neurons is directly related to increased Aβ production and secretion.58,59 In both mice and human stem-cell derived astrocytes and microglia, ApoE isoforms alter Aβ clearance from the brain.59–61 Although early, more limited studies suggested that polymorphisms in apolipoprotein A1 (ApoA1), a major component of protective high density lipoprotein (HDL) cholesterol in the CNS, were related to early onset and late onset AD,62–64 this finding has not been replicated in larger genome wide association (GWAS) studies. Nevertheless, low plasma and CSF levels of ApoA1 have been observed in AD patients65 and are connected to earlier AD onset in non-demented elderly patients.65,66 To further illustrate clinically that Aβ may be affected by the amount and type of cholesterol in the CNS, its production in hippocampal neurons can be inhibited by the lipid lowering medication lovastatin.67
Clinical implications of obesity on AD risk
Despite the above mechanistic findings, the relationship between obesity, insulin resistance, T2D and pathological markers of AD, such as Aβ and p-tau, remains controversial. Studies assessing the relationship between T2D and accumulation of Aβ, either by PET imaging or by histology, have been mixed. While one study showed a close association between insulin resistance and Aβ by imaging in middle-age subjects,68 others have found no relationship between glucose tolerance and Aβ in diabetic vs. nondiabetic elderly patients with normal cognition, MCI, or AD,42,43,69 and no association with systemic insulin resistance and CSF levels of Aβ69 or histological Aβ burden.70,71 However, these cross-sectional studies included only elderly patients as controls, and after the onset of clinical dementia symptoms. As Aβ deposition begins up to 20 years before clinical symptomatology,72 the negative findings may not accurately reflect the typical time course of AD pathophysiologic progression. In fact, brain insulin levels and insulin receptor density are reduced in older patients with AD compared to middle-aged controls even without clinical symptomatology,30 suggesting the need for further human studies that compare insulin resistance-associated Aβ pathology in younger high risk, potentially “preclinical” patients to older patients who have already developed clinically-evident disease.
Clusterin as a potential biomarker for Alzheimer’s disease
Clusterin (encoded by the gene CLU) was originally isolated from ram testis fluid in 1983,73 and has since been identified as a molecular chaperone expressed by a wide-ranging number of tissues.74,75 Its traditionally identified function has been to assist folding of secreted proteins; as such, clusterin overexpression protects cells from apoptosis induced by chemotherapy, radiotherapy, and androgen/estrogen depletion.65,66 Its relationship with Aβ has been well studied (summarized in Foster et al76), but the precise contribution(s) of clusterin to AD pathology remains confounded by complexities involving its biogenesis, the role of extra- vs. intracellular clusterin, and its vast number of attributable functions. In addition to its direct role in Aβ pathology, clusterin appears to play a role in neurodegeneration. The ApoE-ε4 allele exacerbates synapse degeneration and leads to accumulation of toxic oligomeric Aβ. Interestingly, synapses containing higher amounts of clusterin are seen in APOE-ε4 carriers compared to ApoE-ε3 carriers, with correspondingly higher oligomeric Aβ burden.77 This finding potentially explains the synergistic effect of 2 prominent genetic risk factors on synapse degeneration in AD.
The role of clusterin genetic variants on AD
Clusterin is highly expressed in the brain by both astrocytes and neurons,78 and has been linked with an increased risk of AD.79 In large-scale GWAS studies, polymorphisms in the CLU gene strongly associate with late-onset AD (LOAD),3,80 although this finding has since been questioned when analyzed specifically in different ethnic/racial groups.81–83 In turn, polymorphisms in CLU may have critical implications on brain structure. An MRI-based study of nearly 400 young healthy carriers of the CLU (rs11136000) allele showed a distinct deterioration in white matter integrity, suggesting increased vulnerability to developing AD later in life.84
Few studies have evaluated the relationship between CLU genetic changes and coexisting metabolic disease. In 550 women with a history of gestational diabetes mellitus (GDM), T2D, or impaired glucose tolerance (IGT) compared to controls, no significant association with CLU rs11136000 was observed in any of the groups.85 Another study in 418 individuals (236 with MCI and 192 control subjects), however, did report a relationship between CLU and metabolic disease, with T2D prevalence higher in individuals carrying the CLU variant, and rs11136000 specifically associated with elevated MCI risk (OR 1.79, P = .019).86 In patients with clinical AD, the relationship between dysregulated metabolism and CLU genetic variations is largely unknown and requires further investigation.
Clusterin levels in the pathogenesis of AD
In patients with both MCI and AD, a majority of studies have documented elevated brain, CSF and circulating clusterin levels.87–90 In the study by Nilselid et. al., involving CSF analyses from Alzheimer patients (n = 99) and controls (n = 39), clusterin was significantly higher in AD patients, quantified both before and after deglycosylation using sandwich enzyme-linked immunosorbent assay (ELISA) (Before deglycosylation: 7.17 ± 2.43 versus 5.73 ± 2.09 AU; p=0.002; After deglycosylation: 12.19 ± 5.00 vs 9.68 ± 4.38 AU; P = .004).87 In a separate cohort of 44 subjects representing a continuum of disease (27 with mild to moderate AD and 17 with MCI) plasma clusterin by ELISA ( coefficient of variation 3.5%) was associated with entorhinal cortex atrophy, baseline disease severity, and quicker clinical progression.88 In addition, plasma clusterin levels predicted higher Aβ burden in the medial temporal lobe. These findings were confirmed in a meta-analysis of 28 studies that demonstrated higher clusterin concentration both in plasma (SDM = 0.73, P = .002) and brain tissue (SDM = 0.71, P = .022) compared to normal controls.89 Yet interestingly, CSF clusterin was not different by patient group. In a separate study of 231 T2D patients, including 126 with MCI and 105 cognitively healthy controls, plasma clusterin was significantly higher in MCI patients vs. controls (P = .007), and negatively correlated with the Montreal cognitive assessment and auditory verbal learning test, and delayed recall scores (P = .027 and P = .020, respectively).91 Multivariable regression modeling showed that educational attainment, duration of diabetes, high-density lipoprotein cholesterol (HDL-c), and plasma clusterin levels were all associated with MCI in T2D patients.
As a result of these and other reports, clusterin has been proposed as a potential biomarker of AD.79 Yet from a physiologic perspective, the existing evidence supports both neurotoxic and neuroprotective effects, and may be dependent on the balance of clusterin and Aβ in the CNS,92 and, importantly, its cell distribution.76 Clusterin is capable of binding to Aβ, preventing aggregation,93 and enabling LRP2 (megalin)-mediated Aβ removal.94 In the physiologic state, it therefore exhibits neuroprotective properties.95–97 In contrast, a decreased clusterin/Aβ ratio, observed in AD patients, appears to be neurotoxic92 by raising soluble oligomeric Aβ peptides.98
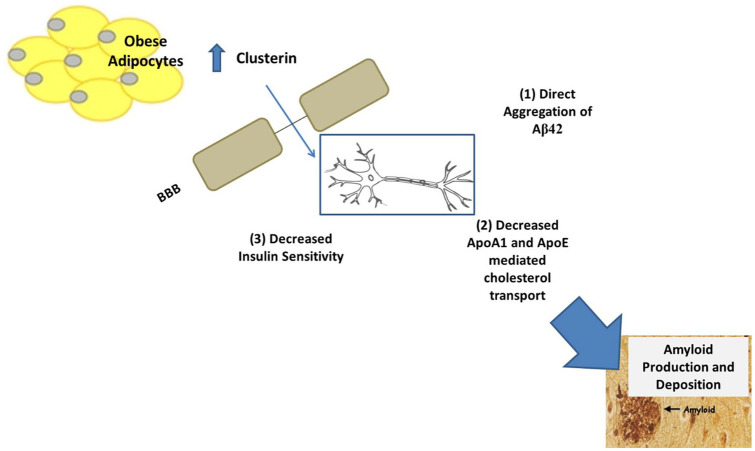
Adipocyte clusterin as a potential novel player in human AD (Figure 1)
Figure 1.
Summary of hypothesized mechanisms (labeled 1-3) responsible for clusterin-mediated amyloid beta (Aβ) deposition.
Abbreviations: ApoA1, apolipoprotein A1; ApoE, apolipoprotein E; BBB, blood brain barrier; CSF, cerebrospinal fluid; HDL, high density lipoprotein.
The adipose tissue (AT) microenvironment is increasingly recognized as a determinant of systemic insulin action and inflammation, and is to some extent determined by the adipocyte.99 Recent studies indicate that adaptive and innate immune functions of the adipocyte regulate the overall immune cell composition of AT, which is enhanced during the transition from a lean to obese state.100 As the major storage depot for excess calories, and comprised of a variety of metabolically-active immune cells, AT is uniquely poised to influence systemic inflammation as well as key systemic metabolic pathways such as insulin action. Several adipokines, comprised of a number of cell signaling proteins secreted by AT,101 have been proposed as biomarkers of AD.102,103
Clusterin is not only expressed by astrocytes and neurons, but by a large number of peripheral tissues, including AT104 and it readily crosses the BBB.105 Indeed, the cognitive decline in MCI and AD has been related to circulating, and not CNS clusterin,106 and the meta-analysis of 12 studies comparing patients with AD to controls showed that plasma clusterin was increased, but not CSF clusterin.89 A recent study expanded on these findings by classifying 59 total participants by cognitive status (normal cognition, MCI, or AD) and by degree of metabolic impairment (healthy, prediabetes, or T2D) in order to determine associations with circulating clusterin levels.107 They found that plasma clusterin levels were not only higher in participants with both AD and prediabetes/T2D compared to those without metabolic impairment, but that clusterin was negatively associated with cognitive scores, and positively with worsening metabolic parameters (hemoglobin A1c, insulin resistance by HOMA-IR, and fasting C-peptide levels) and brain pathology by MRI (medial temporal atrophy and white matter lesions). Although the study involved a cross-sectional, observational design, in mediation analysis plasma clusterin was determined to be a direct mediator of these important associations. Mechanistically, substantial weight loss induced by gastric bypass surgery reduces clusterin expression in peripheral blood mononuclear cells (PBMCs) in association with attenuated expression of amyloid precursor protein (APP) and presenilin-2.108 These studies suggest that peripherally-derived clusterin may have a unique role in CNS AD pathology, particularly with coexistent metabolic impairment.
In fact, metabolic effects of clusterin have recently emerged. In obese patients, plasma clusterin levels are elevated and associate with BMI, waist circumference, markers of inflammation (hsCRP and retinol-binding protein-4), and insulin resistance.109 In mice, skeletal muscle and hepatic gene expression of CLU is increased after high-fat diet feeding, and whole-body clusterin knockout (KO) mice are insulin sensitive compared to wild-type (WT) mice.110 On array analysis, we have previously identified subcutaneous adipocyte CLU as one of the top 15 extracellular matrix-related genes overexpressed in human obesity.104 In addition, we found in 54 obese patients compared to 18 lean patients that human adipocyte expression, protein levels and serum concentrations of clusterin were higher in obesity and directly associated with multiple sequelae of obesity-related cardiometabolic disease: systemic insulin resistance, dyslipidemia, elevated blood pressure, hepatic steatosis/steatohepatitis, key biomarkers of cardiovascular disease and risk, and atherosclerotic lesions.104 We also demonstrated that clusterin in vivo has a progressive abrogating effect on hepatic ApoA1 expression (HepG2 cells cultured with increasing levels of recombinant clusterin). ApoA1 a major component of HDL cholesterol and a biomarker of reduced myocardial infarction risk,111 through binding to low density lipoprotein-related protein 2 (LRP2/Megalin).104 Similar to the liver, LRP2 is the main clusterin receptor found in the brain and high concentrations of CNS clusterin are internalized and degraded via LRP2.94 Although far from conclusive, these results open the possibility that clusterin derived from obese adipocytes may play a role in the heightened risk of AD in the setting of obesity.
Conclusion
The development of AD is complex and multifactorial. However, one of the prominent risk factors includes obesity-related cardiometabolic disease, particularly when these comorbidities develop in midlife. Therefore, factors such as adipokines from adipose tissue, linking obesity and AD, may prove useful as biomarkers of AD risk and development. One of these potential biomarkers is clusterin, which is elevated in the blood and CNS of patients with both MCI and AD. However, studies have attributed both a neuroprotective and neurotoxic role to clusterin. This discrepancy may be due to the myriad of functions that have been ascribed to clusterin and its cellular distribution.
Despite its known association with AD, and findings that clusterin is increased in AD, T2D, and obesity, the role of clusterin as a biomarker in AD pathophysiology remains an enigma, especially its relationship to metabolic disease. In particular, a better understanding of insulin’s effects in the human brain is needed, accounting for both the severity of AD and relative to age-matched controls. In addition, well-designed studies that compare insulin resistance-associated Aβ pathology in younger patients at risk (potentially in “preclinical” stages) to older patients with clinically-evident disease will shed more light on the contribution of clusterin to AD pathogenesis. Although adipocyte-derived clusterin may play a role in Aβ pathology, future studies are needed to determine if is a viable biomarker for AD, and if it offers a key link between obesity, metabolic disease, and AD.
Footnotes
Funding:The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the American Diabetes Association 1-16-ICTS-049), The National Institutes of Health KL2 Scholar Award KL2TR001068, and the Ohio State University College of Medicine Office of Research Bridge Funding Program.
Declaration of conflicting interests:The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Alzheimer’s Association. Alzheimer’s disease facts and figures [Ebook]. https://www.alz.org/documents_custom/2018-facts-and-figures.pdf. Accessed June 20, 2020.
- 2. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnard ND, Bush AI, Ceccarelli A, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol Aging. 2014;35 Suppl 2:S74-S78. [DOI] [PubMed] [Google Scholar]
- 5. Bendlin BB, Carlsson CM, Gleason CE, et al. Midlife predictors of Alzheimer’s disease. Maturitas. 2010;65:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masliah E, Mallory M, Deerinck T, et al. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J Neuropathol Exp Neurol. 1993;52:619-632. [DOI] [PubMed] [Google Scholar]
- 7. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788-794. [DOI] [PubMed] [Google Scholar]
- 8. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 2017;74:1246-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bray GA. Complications of obesity. Ann Intern Med. 1985;103:1052-1062. [DOI] [PubMed] [Google Scholar]
- 11. Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panza F, Frisardi V, Capurso C, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691-724. [DOI] [PubMed] [Google Scholar]
- 13. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology. 1999;53:1937-1942. [DOI] [PubMed] [Google Scholar]
- 14. Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301-308. [DOI] [PubMed] [Google Scholar]
- 15. Arvanitakis Z, Wilson RS, Bennett DA. Diabetes mellitus, dementia, and cognitive function in older persons. J Nutr Health Aging. 2006;10:287-291. [PubMed] [Google Scholar]
- 16. Wu JH, Haan MN, Liang J, et al. Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: a population-based cohort study. Ann Epidemiol. 2003;13:369-376. [DOI] [PubMed] [Google Scholar]
- 17. Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of diabetes on cognitive function among older Latinos: a population-based cohort study. J Clin Epidemiol. 2003;56:686-693. [DOI] [PubMed] [Google Scholar]
- 18. Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195-1202. [DOI] [PubMed] [Google Scholar]
- 19. Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658-663. [DOI] [PubMed] [Google Scholar]
- 20. Stoeckel LE, Arvanitakis Z, Gandy S, et al. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res. 2016;5:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ. 2004;328:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke DW, Boyd FT, Jr., Kappy MS, Raizada MK. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J Biol Chem. 1984;259:11672-11675. [PubMed] [Google Scholar]
- 23. Raizada MK, Shemer J, Judkins JH, Clarke DW, Masters BA, LeRoith D. Insulin receptors in the brain: structural and physiological characterization. Neurochem Res. 1988;13:297-303. [DOI] [PubMed] [Google Scholar]
- 24. Smythe GA, Bradshaw JE, Nicholson MV, Grunstein HS, Storlien LH. Rapid bidirectional effects of insulin on hypothalamic noradrenergic and serotoninergic neuronal activity in the rat: role in glucose homeostasis. Endocrinology. 1985;117:1590-1597. [DOI] [PubMed] [Google Scholar]
- 25. Uemura E, Greenlee HW. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp Neurol. 2006;198:48-53. [DOI] [PubMed] [Google Scholar]
- 26. Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809-822. [DOI] [PubMed] [Google Scholar]
- 27. Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190-194. [DOI] [PubMed] [Google Scholar]
- 28. Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467-472. [DOI] [PubMed] [Google Scholar]
- 29. Stanley M, Macauley SL, Holtzman DM. Changes in insulin and insulin signaling in Alzheimer’s disease: cause or consequence? J Exp Med. 2016;213:1375-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423-438. [DOI] [PubMed] [Google Scholar]
- 31. Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247-268. [DOI] [PubMed] [Google Scholar]
- 32. Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63-80. [DOI] [PubMed] [Google Scholar]
- 33. Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stohr O, Schilbach K, Moll L, et al. Insulin receptor signaling mediates APP processing and beta-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer’s disease. Age. 2013;35:83-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730-32738. [DOI] [PubMed] [Google Scholar]
- 38. Shiiki T, Ohtsuki S, Kurihara A, et al. Brain insulin impairs amyloid-beta(1-40) clearance from the brain. J Neurosci. 2004;24:9632-9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270-280. [DOI] [PubMed] [Google Scholar]
- 41. Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123-130. [DOI] [PubMed] [Google Scholar]
- 42. Thambisetty M, Metter EJ, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore longitudinal study of aging. JAMA Neurol. 2013;70:1167-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moran C, Beare R, Phan TG, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85:1123-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626-1633. [DOI] [PubMed] [Google Scholar]
- 45. Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177-179. [DOI] [PubMed] [Google Scholar]
- 46. Luchsinger JA, Perez T, Chang H, et al. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis. 2016;51:501-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koenig AM, Mechanic-Hamilton D, Xie SX, et al. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord. 2017;31:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y, Li L, Holscher C. Incretin-based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev Neurosci. 2016;27:689-711. [DOI] [PubMed] [Google Scholar]
- 49. Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology. 2013;38:2462-2475. [DOI] [PubMed] [Google Scholar]
- 50. Arnold SE, Lucki I, Brookshire B, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Patil IY, Jiang T, et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One. 2015;10:e0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martins IV, Rivers-Auty J, Allan SM, Lawrence CB. Mitochondrial abnormalities and synaptic loss underlie memory deficits seen in mouse models of obesity and Alzheimer’s disease. J Alzheimers Dis. 2017;55:915-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tschritter O, Preissl H, Hennige AM, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103:12103-12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tschritter O, Preissl H, Yokoyama Y, Machicao F, Haring H-U, Fritsche A. Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia. 2007;50:2602-2603. [DOI] [PubMed] [Google Scholar]
- 55. Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986-2992. [DOI] [PubMed] [Google Scholar]
- 56. Cheke LG, Bonnici HM, Clayton NS, Simons JS. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia. 2017; 96:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hauser PS, Ryan RO. Impact of apolipoprotein E on Alzheimer’s disease. Curr Alzheimer Res. 2013;10:809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang YA, Zhou B, Wernig M, Sudhof TC. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and abeta secretion. Cell. 2017;168:427-441,e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin YT, Seo J, Gao F, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verghese PB, Castellano JM, Garai K, et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci USA. 2013;110:E1807-E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vollbach H, Heun R, Morris CM, et al. APOA1 polymorphism influences risk for early-onset nonfamiliar AD. Ann Neurol. 2005;58:436-441. [DOI] [PubMed] [Google Scholar]
- 63. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921-923. [DOI] [PubMed] [Google Scholar]
- 64. Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol Aging. 2000;21:27-30. [DOI] [PubMed] [Google Scholar]
- 66. Slot RE, Van Harten AC, Kester MI, et al. Apolipoprotein A1 in cerebrospinal fluid and plasma and progression to Alzheimer’s disease in non-demented elderly. J Alzheimers Dis. 2017;56:687-697. [DOI] [PubMed] [Google Scholar]
- 67. Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998;95:6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimer’s Dement J Alzheimer’s Assoc. 2015;11:504-510, e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Starks EJ, O’Grady JP, Hoscheidt SM, et al. Insulin resistance is associated with higher cerebrospinal fluid Tau levels in asymptomatic APOEvarepsilon4 carriers. J Alzheimers Dis. 2015;46:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nelson PT, Smith CD, Abner EA, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306-1311. [DOI] [PubMed] [Google Scholar]
- 72. Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173-1188. [DOI] [PubMed] [Google Scholar]
- 74. Rizzi F, Coletta M, Bettuzzi S. Chapter 2: Clusterin (CLU): from one gene and two transcripts to many proteins. Adv Cancer Res. 2009;104:9-23. [DOI] [PubMed] [Google Scholar]
- 75. de Silva HV, Stuart WD, Duvic CR, et al. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990;265:13240-13247. [PubMed] [Google Scholar]
- 76. Foster EM, Dangla-Valls A, Lovestone S, Ribe EM, Buckley NJ. Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front Neurosci. 2019;13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jackson RJ, Rose J, Tulloch J, Henstridge C, Smith C, Spires-Jones TL. Clusterin accumulates in synapses in Alzheimer’s disease and is increased in apolipoprotein E4 carriers. Brain Commun. 2019;1:fcz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Herring SK, Moon HJ, Rawal P, Chhibber A, Zhao L. Brain clusterin protein isoforms and mitochondrial localization. eLife. 2019;8:e48255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li X, Ma Y, Wei X, et al. Clusterin in Alzheimer’s disease: a player in the biological behavior of amyloid-beta. Neurosci Bull. 2014;30:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094-1099. [DOI] [PubMed] [Google Scholar]
- 81. Han Z, Qu J, Zhao J, Zou X. Analyzing 74,248 samples confirms the association between CLU rs11136000 polymorphism and Alzheimer’s disease in Caucasian but not Chinese population. Sci Rep. 2018;8:11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Du W, Tan J, Xu W, Chen J, Wang L. Association between clusterin gene polymorphism rs11136000 and late-onset Alzheimer’s disease susceptibility: a review and meta-analysis of case-control studies. Exp Ther Med. 2016;12:2915-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang S, Li X, Ma G, et al. CLU rs9331888 polymorphism contributes to Alzheimer’s disease susceptibility in Caucasian but not East Asian populations. Mol Neurobiol. 2016;53:1446-1451. [DOI] [PubMed] [Google Scholar]
- 84. Braskie MN, Jahanshad N, Stein JL, et al. Common Alzheimer’s disease risk variant within the CLU gene affects white matter microstructure in young adults. J Neurosci. 2011;31:6764-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vacinova G, Vejražková D, Lukášová P, et al. Associations of polymorphisms in the candidate genes for Alzheimer’s disease BIN1, CLU, CR1 and PICALM with gestational diabetes and impaired glucose tolerance. Mol Biol Rep. 2017;44:227-231. [DOI] [PubMed] [Google Scholar]
- 86. Aghajanpour-Mir M, Amjadi-Moheb F, Dadkhah T, et al. Informative combination of CLU rs11136000, serum HDL levels, diabetes, and age as a new piece of puzzle-picture of predictive medicine for cognitive disorders. Mol Biol Rep. 2019;46:1033-1041. [DOI] [PubMed] [Google Scholar]
- 87. Nilselid AM, Davidsson P, Nägga K, Andreasen N, Fredman P, Blennow K. Clusterin in cerebrospinal fluid: analysis of carbohydrates and quantification of native and glycosylated forms. Neurochem Int. 2006;48:718-728. [DOI] [PubMed] [Google Scholar]
- 88. Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang C, Wang H, Li C, Niu H, Luo S, Guo X. Association between clusterin concentration and dementia: a systematic review and meta-analysis. Metab Brain Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 90. Morgan AR, Touchard S, O’Hagan C, et al. The correlation between inflammatory biomarkers and polygenic risk score in Alzheimer’s disease. J Alzheimers Dis. 2017;56:25-36. [DOI] [PubMed] [Google Scholar]
- 91. Cai R, Han J, Sun J, et al. Plasma clusterin and the CLU gene rs11136000 variant are associated with mild cognitive impairment in type 2 diabetic patients. Front Aging Neurosci. 2016;8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yerbury JJ, Poon S, Meehan S, et al. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312-2322. [DOI] [PubMed] [Google Scholar]
- 93. Beeg M, Stravalaci M, Romeo M, et al. Clusterin binds to abeta1-42 oligomers with high affinity and interferes with peptide aggregation by inhibiting primary and secondary nucleation. J Biol Chem. 2016;291:6958-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nelson AR, Sagare AP, Zlokovic BV. Role of clusterin in the brain vascular clearance of amyloid-beta. Proc Natl Acad Sci USA. 2017;114:8681-8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bell RD, Sagare AP, Friedman AE, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cerebr Blood Flow Metab. 2007;27:909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J Biol Chem. 1997;272:18644-18649. [DOI] [PubMed] [Google Scholar]
- 97. Giannakopoulos P, Kövari E, French LE, Viard I, Hof PR, Bouras C. Possible neuroprotective role of clusterin in Alzheimer’s disease: a quantitative immunocytochemical study. Acta Neuropathol. 1998;95:387-394. [DOI] [PubMed] [Google Scholar]
- 98. Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deng T, Liu J, Deng Y, et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nat Commun. 2017;8:15725. [Google Scholar]
- 100. Ip BC, Hogan AE, Nikolajczyk BS. Lymphocyte roles in metabolic dysfunction: of men and mice. Trends Endocrinol Metab TEM. 2015;26:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteom Clin Appl. 2012;6:91-101. [DOI] [PubMed] [Google Scholar]
- 102. Lee TH, Cheng KK, Hoo RL, Siu PM, Yau SY. The novel perspectives of adipokines on brain health. Int J Mol Sci., 2019;20:5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Letra L, Matafome P, Rodrigues T, et al. Association between adipokines and biomarkers of Alzheimer’s disease: a cross-sectional study. J Alzheimers Dis. 2019;67:725-735. [DOI] [PubMed] [Google Scholar]
- 104. Bradley D, Blaszczak A, Yin Z, et al. Clusterin impairs hepatic insulin sensitivity and adipocyte clusterin associates with cardiometabolic risk. Diabetes Care. 2019;42:466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shayo M, McLay RN, Kastin AJ, Banks WA. The putative blood-brain barrier transporter for the beta-amyloid binding protein apolipoprotein j is saturated at physiological concentrations. Life Sci. 1997;60:PL115-PL118. [DOI] [PubMed] [Google Scholar]
- 106. Jongbloed W, van Dijk KD, Mulder SD, et al. Clusterin levels in plasma predict cognitive decline and progression to Alzheimer’s disease. J Alzheimers Dis. 2015;46:1103-1110. [DOI] [PubMed] [Google Scholar]
- 107. Ha J, Moon MK, Kim H, et al. Plasma clusterin as a potential link between diabetes and Alzheimer’s disease. J Clin Endocrinol Metab. 2020;105:dgaa378. [DOI] [PubMed] [Google Scholar]
- 108. Ghanim H, Monte SV, Sia CL, et al. Reduction in inflammation and the expression of amyloid precursor protein and other proteins related to Alzheimer’s disease following gastric bypass surgery. J Clin Endocrinol Metab. 2012;97:E1197-E1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014;9:e103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kwon MJ, Ju TJ, Heo JY, et al. Deficiency of clusterin exacerbates high-fat diet-induced insulin resistance in male mice. Endocrinology. 2014;155:2089-2101. [DOI] [PubMed] [Google Scholar]
- 111. McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224-233. [DOI] [PubMed] [Google Scholar]