Abstract
Diagnosis of Parkinson’s disease (PD) relies on clinical history and physical examination, but misdiagnosis is common in early stages. Identification of biomarkers for PD may allow for early and more precise diagnosis and provide information about prognosis. Developments in analytical chemistry allow for the detection of a large number of molecules in cerebrospinal fluid (CSF), which are known to be associated with the pathogenesis of PD. Given the pathophysiology of PD, CSF α-synuclein species have the strongest rationale for use, also providing encouraging preliminary results in terms of early diagnosis. In the field of classical Alzheimer’s disease (AD) biomarkers, low CSF Aβ42 levels have shown a robust prognostic value in terms of development of cognitive impairment. Other CSF biomarkers including lysosomal enzymes, neurofilament light chain, markers of neuroinflammation and oxidative stress, although promising, have not proved to be reliable for diagnostic and prognostic purposes yet. Overall, the implementation of CSF biomarkers may give a substantial contribution to the optimal use of disease-modifying drugs.
Keywords: Cerebrospinal fluid biomarkers, Parkinson’s disease, alpha-synuclein, Alzheimer’s disease biomarkers, lysosomal enzymes, neurofilament light chain, inflammation, oxidative stress
Introduction
Current diagnostic criteria for Parkinson’s disease (PD) allow for identification of only clinically manifested disease, which occurs years after the neurodegenerative process has started.1 However, in view of having effective disease modifying treatments, early diagnosis represents a priority. In this perspective, diagnostic and prognostic biomarkers are needed in the field of neurodegeneration. Alzheimer’s disease (AD), the most common neurodegenerative disorder, provides an example of the usefulness and application of cerebrospinal fluid (CSF) biomarkers for diagnosis, independent of clinical stage.2 Indeed, the proximity of CSF to the central nervous system (CNS) makes this biofluid the ideal source for markers of the ongoing pathological processes. Over the past 5 years, research on biofluid biomarkers in PD has markedly expanded. In this review, we will first provide a general overview on PD pathophysiology. Then, updated evidences about CSF biomarkers for PD diagnosis and prognosis are reported, with major attention on those closely related to the main pathological processes. According to the relevance of pathophysiological mechanisms, we will first discuss markers of synucleinopathy, followed by markers of amyloid pathology, tauopathy, lysosomal dysfunction, and finally of axonal damage and neuroinflammation.
Parkinson’s Disease Pathophysiology
Even if sporadic forms represent the majority of PD cases, so far, knowledge about mechanisms underlying the pathophysiology of PD derive from genetic forms, which account for 5% to 10% of all cases.3 Genetic PD include forms with autosomal dominant (ie, SNCA, LRRK2), and autosomal recessive (ie, PINK1, DJ1) inheritance. Furthermore, several other genetic alterations (the most prevalent and important of which are heterozygous mutations in GBA) have been associated to specific forms of disease (as young onset PD and faster impairment of cognitive performances).4,5 Susceptibility gene variants are of utmost importance in understanding the complex link between genetics and environmental factors.6,7 Functional characterization of the products of these genes has revealed that α-synuclein (α-syn) production (SNCA) and degradation (GBA), mitochondrial function (PINK1), oxidative stress (DJ-1), and neuroinflammation (LRRK2)3 can be considered as the key molecular mechanisms in spreading pathology of the disease that may be shared between familial and sporadic forms of PD.6
The widespread accumulation of the intracellular protein α-syn is a core feature in the neuropathology of PD.8 Lewy bodies (LBs), which mainly consist of aggregated α-syn, were firstly described over a century ago. More recently, the neurotoxic effects of other α-syn aggregates, as oligomers and protofibrils, have also been described.9 The physiological functions of α-syn are still not completely known. It is present in the cytosol, possibly also in mitochondria and in the nucleus, and it is thought to play a role in synaptic vesicle dynamics, mitochondrial function, intracellular trafficking and as a potential chaperone.10-12 α-syn neurotoxic properties are acquired during a process in which soluble monomers of the protein start forming oligomers, that combine into small protofibrils and eventually large, insoluble α-syn fibrils (the aggregates responsible for Lewy pathology).13,14 Accumulation and aggregation of α-syn can be due to relative overproduction (SNCA duplication or triplication),15 mutations increasing the likelihood for its misfolding and oligomerization (A53T, A53V, E46K, and H50Q mutations in SNCA),16 or impairment in the clearance of the protein (as, for example, mutations in LRRK2, GBA and VPS35).
Homeostasis of α-syn is maintained by the ubiquitin–proteasome system and by the lysosomal autophagy system. In the substantia nigra of patients and experimental models of PD, lysosomal enzyme levels are reduced, particularly in neurons containing α-syn inclusions.17 Alterations of the lysosomal hydrolase β-glucocerebrosidase (GCase), transcriptional product of GBA (the most common genetic risk factor for PD),18 lead to accumulation of its substrate, glucosylceramide. As previously demonstrated,19 this has been associated to α-syn accumulation, which in turn may induce a reduction of GCase activity, suggesting the existence of a vicious, neurotoxic cycle between α-syn and GCase. Lysosomal enzymes other than GCase, such as cathepsin D (CTSD), are known to be directly involved in α-syn degradation. As a consequence, degradation processes are impaired when the activity of these enzymes is decreased.20 Finally, the co-occurrence of AD-associated pathology as Aβ plaques and neurofibrillary tangles (NFTs) could facilitate α-syn aggregation.21-23 Mitochondrial dysfunction, which may be triggered by genetically driven loss of function of PINK1, DJ-1, or LRRK2, can exacerbate α-syn aggregation cascade leading to neuronal degeneration and LBs deposition. It has been proposed that α-syn accumulation inside mitochondria leads to mitochondrial complex I deficits and oxidative stress, and energy failure.24
In parallel with these processes, α-syn aggregation boosts a microglial reaction that, through inflammatory mediators, attracts peripheral immune cells within the CNS. After astrocytes have taken up neuronally released α-syn via endocytosis, cellular gene expression changes, indicating an inflammatory response.25 Microglial cells are particularly inclined to the expression of the major histocompatibility complex (MHC) II, a key regulator of the immune response, which appears to be critical for α-syn-induced neurodegeneration.26 As a result, microglia cause persistent and dysfunctional microglial activation, facilitating both mitochondrial dysfunction and neuronal degeneration. Oxidative stress, a consequence of both microglial activation and mitochondrial dysfunction, is increased in the brain tissue of patients with PD.27 Mutations in DJ1 (also known as PARK7) encoding for a putative antioxidant cause early-onset autosomal recessive PD, and are associated with increased cellular oxidative stress.28
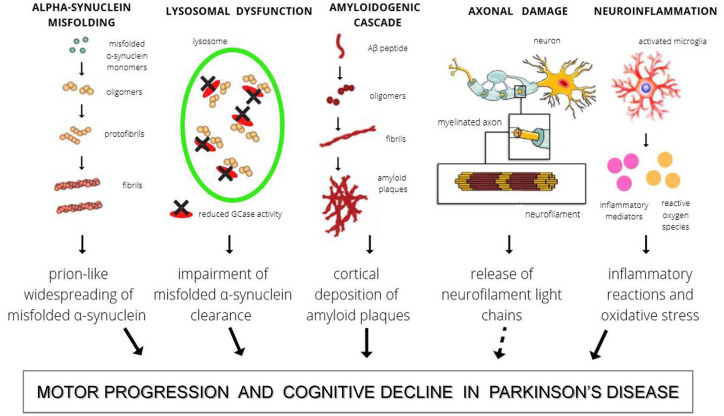
Despite the fact that each of the aforementioned molecular processes is playing a unique role in the pathogenesis of PD, their pathways all converge to the final event of axonal damage. This consequence, common to many other neurodegenerative diseases, can be reliably reflected by the increased peripheral levels of neurofilament light chains (NfL).29 In Figure 1 these pathophysiological pathways are schematically illustrated.
Figure 1.
The figure shows the main pathophysiological mechanisms involved in PD and reflected in CSF.
Possible Role of CSF Biomarkers in Diagnosis and Prognosis of PD
At present, diagnosis of PD mostly relies on clinical criteria.1 However, biological changes leading to neurodegeneration start several years before the appearance of clinical manifestations. Ideally, this asymptomatic phase represents the most suitable phase for treatment with disease-modifying drugs.30 Thus, reliable biomarkers reflecting biological changes occurring early in the disease are strongly desirable. CSF may reflect the molecular changes taking place in PD brain, thus representing an optimal source of diagnostic and prognostic biomarkers31 (Table 1). In this context, among neurodegenerative diseases, AD represents a valuable example.2
Table 1.
The main neurobiological mechanisms involved in PD physiopathology are summarized. For each of them, related CSF biomarkers are presented, with a focus on their diagnostic and prognostic values.
| Pathophysiological mechanisms | CSF biomarkers | Diagnostic value | Prognostic value |
|
|---|---|---|---|---|
| Motor progression | Cognitive decline | |||
| α-Syn misfolding | t-α-syn | ↓ in PD versus HC | ↑/↓ possible association with worsening of motor symptoms | ___ |
| o-α-syn, p-α-syn | ↑ in PD versus HC | ___ | ___ | |
| α-syn aggregates | ↑ in PD and other synucleinopathies versus HC and non-synucleinopathies | ___ | ___ | |
| Amyloidosis | Aβ42 | ↓ in PDD and ↓↓ in DLB versus PD | ___ | ↓ predicts earlier cognitive decline |
| Tauopathy | p-tau | ___ | ___ | ___ |
| Neurodegeneration | t-tau | ___ | ↑ correlation with faster motor progression | ___ |
| Axonal damage | NfL | ↑ AP in versus PD | ___ | ↑ predicts cognitive decline |
| Autophagic-lysosomal pathway dysfunctions | GCase | ↓ in PD and other synucleinopathies versus HC | ↓↓ in the most advanced stages | ___ |
| CTSD, β-hexoxaminidase | ↓ in PD versus HC | ___ | ↓ correlates with worse cognitive performances | |
| Neuroinflammation | MCP-1 | ↑ in PD versus HC | ___ | ___ |
| YKL-40 | ↑ in AP versus PD | ___ | ↑ associates to cognitive decline | |
Abbreviations: Aβ42, amyloid-β peptide 42; APS, atypical parkinsonisms; CTSD, cathepsin D activity; CSF, cerebrospinal fluid; DLB, dementia with Lewy bodies; GCase, glucocerebrosidase activity; HC, healthy control subjects; YKL-40, chitinase-3-like protein 1; MCP-1, monocyte chemoattractant protein-1; o-α-syn, oligomeric α-synuclein; PD, Parkinson’s disease; PDD, Parkinson’s-dementia complex; p-α-syn, phosphorylated α-synuclein; p-tau, phosphorylated tau protein; t-α-syn, total α-synuclein; t-tau, total tau protein.
___, insufficient evidences; ↑, increased levels; ↓, decreased levels.
CSF biomarkers for PD are actively investigated. Increasing evidences support their potential role in both diagnostic and prognostic terms. Safety of lumbar puncture (LP) as a “routine” procedure in neurodegenerative diseases besides AD may represent an issue. Indeed, there is a large body of literature demonstrating feasibility of LP.32,33 PD patients have higher prevalence of spinal deformities like kyphoscoliosis that could influence the feasibility and safety of LP. To this purpose, an interesting contribution has been provided by the Parkinson’s Progression Markers Initiative (PPMI), which is a longitudinal observation study designed to identify PD progression biomarkers.34 The study enrolled a large cohort of participants with de novo PD (at baseline) compared to healthy controls (HC). All PPMI participants underwent LP at baseline, 6, 12 months and yearly thereafter. Analyzing safety data from baseline LPs, 23% participants reported any related adverse events (AEs), 68% of all AEs were mild while 5.6% were severe. There were no serious LP related AEs, and complications like iatrogenic meningitis or arachnoiditis were not observed. As expected, the most common AEs were headaches (13%) and low back pain (6.5%), with the lower rate in PD subgroup. Factors associated with higher incidence of AEs included female gender, younger age and use of traumatic needles with larger diameter. Lying down LP position reduced the risk of AEs with respect to sitting LP position. Additionally, CSF volume had no significant effect on the incidence of AEs. In summary, consistent with the data reported in the AD literature, PPMI study showed that LP is overall safe and feasible in PD patients.
CSF Alpha Synuclein (α-syn) Species
As previously described, the accumulation, misfolding and aggregation of α-syn with progressive deposition in large intracellular aggregates represent the main pathological hallmark of most genetic and sporadic forms of PD.8 Although the presence of LBs has been associated to the death of dopaminergic neurons, smaller aggregates rather than LBs, like protofibrils and soluble oligomers of α-syn, are currently considered to be the most toxic species.9,35 Nigro-striatal damage due to α-syn misfolding processes is always accompanied by extensive extranigral pathology, from the caudal brainstem (dorsal motor nucleus of glossopharyngeal and vagal nerves, reticular formation, raphe system, coeruleus–subcoeruleus complex) in patients with premotor PD to the cortex (temporal mesocortex, allocortex and associative areas of neocortex) in the most advanced clinical stages. Thus, the neuronal damage does not develop randomly, rather it follows a predetermined sequence marked by characteristic changes in topographical extent.36 Furthermore, both in-vitro and in-vivo studies evidenced that amplification of misfolded α-syn happens in a prion-like fashion, which accounts for the cell-to-cell transmission of α-synuclein fibrils and the spread of selective neurodegeneration. Synthetic α-syn fibrils and abnormal α-synaggregates extracted from patients’ brain were able to induce template-dependent aggregation of α-syn both in cultured cells and rodent brains, with the final fibrils showing the same biochemical and morphological characteristics as the added aggregates.37 Seed-dependent accumulation of misfolded α-syn with subsequent distinct spreading patterns of α-syn pathology occurred in brains of transgenic mice expressing human α-syn and in wild-type mice. Otherwise, no α-syn pathology was observed in α-syn knock-out mice after the inoculation of α-syn fibrils.38 These data indicate that synthetic α-syn fibrils act as a template to convert endogenous α-syn into an abnormal form, and then abnormal α-syn is transmitted cell to cell throughout neuronal networks.39
Several investigations into biomarker identification and validation have therefore focused on the measurement of total α-syn species in CSF. Furthermore, specific α-syn species (ie, oligomeric α-syn, phosphorylated α-syn at residue Ser129, and proaggregating forms of α-syn) have been considered as potential CSF biomarkers for PD.
CSF total α-syn (t-α-syn): research on CSF α-syn has reached consistent results with several studies, including meta-analyses. As a global result, it can be claimed that t-α-syn levels are significantly decreased in PD patients compared to healthy controls.40-45 However, the diagnostic accuracy of CSF t-α-syn in distinguishing patients with PD from controls remains unsatisfactory, with a pooled sensitivity between 88% and 78%, and a specificity between 40% and 57%.41,43
The performance of CSF t-α-syn in other neurodegenerative disorders is quite controversial. Similarly to PD, changes toward low CSF t-α-syn have been found both in other synucleinopathy (multiple system atrophy, MSA and dementia with Lewy bodies, DLB), and, more interestingly, in tauopathies as well as (progressive supranuclear palsy, PSP, corticobasal syndrome CBS, and frontotemporal dementia).46-48 Noteworthy, in AD and Creutzfeldt-Jakob disease, a significant increase in CSF t-α-syn has been reported compared with controls.41-44
Longitudinal changes in CSF α-syn and other biomarkers in PD have been examined in different cohorts, with conflicting results. One study employed the cohort from the deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP) study, which is a placebo-controlled clinical trial on individuals with early PD.49 It was observed that t-α-syn levels significantly decreased over approximately 2 years of follow-up in PD patients, but they did not predict the progression of motor symptoms, with some evidences of prediction of cognitive decline.49 On the other hand, according to another study based on DATATOP cohort, t-α-syn levels significantly increased during the 2-year follow-up period, also showing an association with the worsening of motor signs, especially in the group of postural instability and gait disorder (PIGD) patients.50 In this context, discrepant dynamics of CSF α-syn could be due to different assays and inclusion criteria. Similarly to what observed by Majbour et al, in the Swedish BioFINDER study, levels of a-syn resulted stable early in the symptomatic disease process, increasing over time later in the disease course and in more severe disease.51 In the PPMI study, CSF α-syn decreased longitudinally in PD at 24 and 36 months. However, the decrease in PD was not shown when CSF samples with high hemoglobin concentration were removed from analysis. CSF a-syn changes did not correlate with longitudinal MDS-UPDRS motor scores or dopamine transporter scan.52 Similarly, CSF total α-syn levels displayed no substantial changes during a period of up to 4 years according to a recent study, with no correlation to subsequent motor or cognitive decline.53
Overall, all these results suggest that t-α-syn could serve as a marker of synucleinopathy, when its CSF concentrations decrease, as well as a non-specific marker of synaptic damage, when its CSF concentrations increase.54 However, several limitations hamper the routine clinical use of CSF α-syn as potential diagnostic biomarker. Values of α-syn concentration in the CSF strongly vary in distinct studies and among different laboratories. First of all, it could be attributable to differences in clinical and demographical features of heterogeneous cohorts of patients as well as differences related to control populations which can be represented by either completely healthy subjects or patients with other neurological conditions.55 Then, pre-analytical confounding factors such as blood contamination and analytical variability related to methodological processing and use of different immunoassays should be inevitably considered.56,57 In conclusion, CSF t-α-syn alone does not seem to be a reliable diagnostic or prognostic marker for PD, while the study of other species of α-syn, and the combination with other CSF biomarkers could provide promising results.
CSF oligomeric and phosphorylated α-synuclein (o-α-syn and p-α-syn): recently, specific α-syn species, such as o-α-syn and p-α-syn, have been considered as potential diagnostic biomarkers. Indeed, the oligomerization of α-syn precedes its aggregation into mature amyloid fibrils in LBs, and studies provide evidences that soluble oligomers of α-syn are neurotoxic in vitro and in vivo.10 Also, since approximately 90% of accumulated α-syn in LBs consists of p-α-syn, it has been considered an interesting biomarker candidate of synucleinopathies.58
CSF o-α-syn has been consistently found to be higher in PD patients compared to other neurological disorders59,60 and healthy controls.50 In the comparison between Parkinson’s disease-dementia complex (PDD) and AD, CSF levels of o-α-syn resulted significantly higher in patients with PDD, and also the o-α-syn/t-α-syn ratio was elevated in PDD patients compared with AD patients.61 Also, an association between a change of the o-α-syn/t-α-syn ratio and a worsening of motor signs, in particular in the postural-instability and gait-difficulty PD patients has been noted.50
Only a few studies have focused on CSF p-α-syn as a diagnostic marker, finding increased levels in PD compared to controls and to progressive supranuclear palsy (PSP).41 Similar to o-α-syn, its diagnostic accuracy increases when considered together with other α-syn species and neurodegenerative biomarkers (o-α-syn/t-α-syn ratio together with p-α-syn and p-tau).62 All these results need confirmation in larger cohorts.
CSF α-synuclein aggregates: in the last years, 2 novel ultrasensitive protein amplification assays, named seeding aggregation assays (SAA) and represented by Protein-Misfolding Cyclic Amplification (PMCA) and Real-Time Quaking-Induced Conversion (RT-QuIC), were applied for the detection of “pro-aggregating” α-syn in CSF.63 In 2016, Fairfoul et al reported the first application of RT-QuIC on CSF samples of patients affected by synucleinopathies and controls obtaining a sensitivity of 95% for PD and 92% for DLB, with both specificities of 100% with respect to HC and AD patients.64 Similarly, in 2018, Groveman and colleagues performed RT-QuIC on CSF samples from patients with PD, DLB, and non-synucleinopathy controls (including patients with AD and HC). In patients with PD and DLB, 93% of individuals had positive RT-QuIC responses, while none of the non-synucleinopathy controls had positive RT-QuIC responses, with a specificity of 100%.65 These results suggest that measurement of CSF α-syn aggregates is a promising diagnostic marker for synucleinopathies. Interestingly, RT-QuIC showed also the potential to diagnose synucleinopathies in a pre-symptomatic phase, since it was able to detect aggregates in CSF samples of patients affected by rapid eye movement sleep behavior disorder (RBD) who developed synucleinopathies a few years later.64 However, available data suggest that CSF levels of misfolded α-synuclein species capable of seeding aggregation do not correlate with clinical parameters such as disease stage and duration.66,67 Likewise, further studies including a wider spectrum of PD stages and longitudinal cohorts will be relevant to address whether SAA can be useful in tracking disease progression and severity.
Classical CSF AD Biomarkers
Investigations into the neuropathological basis of PD and other Lewy body disorders (ie, DLB) have demonstrated that, while synuclein pathology is the defining feature of these diseases, it is often accompanied by other age-related neurodegenerative pathologies. Many studies have focused on co-occurrence of AD pathology, characterized by amyloid plaques and NFTs, as these aspects may contribute to clinical manifestations, in particular to the development of cognitive impairment and dementia. Indeed, less than 10% of PD without dementia has significant co-morbid AD at autopsy, with increasing rates in PDD (35%-50%) and DLB (up to 70%).68-73 These findings are of utmost interest as clinical distinction of PDD and DLB is currently a matter of debate, given that they share many prodromal and clinical features, with the distinction that the cognitive impairment in DLB starts within a year of the onset of motor symptoms. These findings support the assessment of the role of CSF AD biomarkers in the diagnosis and prognosis of PD.
With respect to CSF Aβ42, no significant differences were found when PD patients were compared with controls subjects.40,74 With respect to other parkinsonisms, CSF Aβ42 showed reduced values in MSA but not in PD and PSP, if compared to controls.75 Also, both patients with PD and PDD showed higher CSF Aβ42 compared with patients with DLB, even if with poor diagnostic accuracy.76 These findings are in line with a faster progressing dementia in DLB with respect to PD and PDD.
Studies on CSF t-tau and p-tau have not shown a distinctive PD profile. In distinguishing PD patients from controls, several authors found no changes in t-tau or p-tau in PD.77,78 However, only one study74 found an increase in t-tau, and another one in patients with PDD.79 Three studies80-82 found a decrease in p-tau and 1 found a decrease in t-tau.83 When comparing PD to atypical parkinsonisms, Abdo et al described significantly increased t-tau in MSA-P compared with PD.84 Conversely, Herbert et al and Sussmuth et al found a decrease in t-tau in MSA patients77,85 and Sussmuth et al also in PSP with predominant parkinsonism (PSP-P).77 Larger autopsy confirmed studies are needed in order to confirm the validity of these data.
In PD patients the prognostic role of CSF AD biomarkers has been extensively investigated in terms of development of cognitive impairment and dementia. Several studies have provided support that lower baseline CSF concentrations of Aβ42 are associated with worse cognition and might predict cognitive decline.59,78,86 By contrast, CSF t-tau and p-tau have shown conflicting findings.59,79 Total tau and p-tau have been reported to be either equivalent or lower than healthy controls in nondemented PD patients, but higher in PDD.87,88 Across the Lewy Body diseases spectrum, the CSF signature of AD was demonstrated to be more common in PDD, and even more in DLB, as compared to PD (reviewed in Irwin et al), which resembles frequencies of AD co-pathology seen in large autopsy studies.73 Of interest, low CSF Aβ42 has been found to predict early psychosis in patients with PD, being associated with the appearance of illusions or hallucinations within 3 to 4 years of follow-up.89
With respect to motor progression, reduced CSF Aβ42 levels at baseline seemed to be an independent predictor of development of early L-dopa-resistant gait impairment.90 Zhang et al83 interestingly found that baseline Aβ42 negatively correlates with baseline UPDRS score in patients from the DATATOP study. Furthermore, baseline p-tau/t-tau and p-tau/Aβ resulted to be negatively correlated with the rates of the UPDRS change.91
In conclusion, although CSF AD classic biomarkers alone do not have a specific role for PD diagnosis, they can add precious information in terms of prognosis and cognition, in particular CSF Aβ42. Furthermore, patients with mixed pathology, identified using AD biomarkers, may benefit from AD-directed therapies as they are developed.68 Further data are needed to clarify all these associations, longitudinal progression of these markers over time and biomarkers cut-offs, similarly to what is needed for CSF syn species.
CSF Lysosomal Enzymes
More than 10 years ago lysosomal enzymes activities were assessed in the CSF of PD patients for the first time.92 As main explanation, it was proposed that decreased lysosomal enzymes activities in the CSF reflect the derangement of autophagic-lysosomal system occurring in the brain, leading to reduced degradation of α-syn. Different studies with independent cohorts found that CSF GCase activity is lower in PD patients, irrespectively of their GBA mutation carrier status, as compared to healthy controls.93,94 Even if GCase activity in the CSF mirrors one of the most important pathophysiological mechanisms involved in PD, its diagnostic accuracy is poor when considered alone. Diagnostic accuracy can be improved with the association of either other lysosomal enzymes activities (CTSD and β-hexoxaminidase) or other biomarkers (α-syn and Aβ42).93,94 In terms of clinical correlations, when PD patients were stratified according to disease stage (H&Y<2 and H&Y⩾2), GCase and CTSD activities were found to be significantly reduced in the more advanced stages of disease. Furthermore, lower CTSD and β-hexoxaminidase activities were significantly associated with worse cognitive performances, as evaluated by means of Montreal Cognitive Assessment (MoCA).94
CSF Neurofilament Light Chain
Neurofilaments are exclusively expressed in neurons and are released in the interstitial space within the CNS following axonal damage or degeneration. Indeed, they reach abnormal levels in neurodegenerative, inflammatory, vascular and traumatic diseases both in the CSF and in blood.95 The precise functions of neurofilaments remain unknown, but they are thought to have a direct role in radial growth and stability of axons, which is critical for effective nerve conduction, organelle distribution along axons and synaptic plasticity.95 Among neurofilaments, CSF concentration of the light subunit (NfL) has been extensively studied as a biomarker in various neurological diseases.
In patients with PD, no strong evidence for a difference in CSF NfL concentrations has been found, compared with controls. Nevertheless, higher baseline levels of NfL seem to predict the subsequent development of dementia in patients with PD.96 In the context of atypical parkinsonisms, CSF NfL concentrations seem to be significantly increased in MSA and PSP with respect to PD, helping to separate PD from atypical parkinsonian syndromes.84,97 These results have been more recently confirmed by a meta-analysis.98 Similarly, high CSF NfL levels have been described in CBS.99 All these findings may suggest a more rapid neurodegeneration in these diseases if compared to PD.
CSF Markers of Inflammation and Oxidative Stress
The ascertained role of glial activation and inflammation contributing to the development and progression of PD led to the investigation of inflammatory markers in the CSF of patients with PD. In this field, MCP-1 (monocyte chemoattractant protein-1, also called CCL2) and YKL-40 (also called Chitinase-3-like protein 1) are 2 promising biomarkers. MCP-1 is one of the most important chemokines regulating microglial activation. α-syn is able to stimulate pericytes to produce MCP-1 and other inflammatory molecules including interleukine (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and matrix metalloproteinase-9 (MMP-9).100 Accordingly, higher CSF MCP-1 levels were described in PD, in association with increased concentrations of both pro-inflammatory cytokines (IL-2, IL-6, and TNFα) and activated T-lymphocytes.101 YKL-40 is a non-CNS specific glycoprotein released by peripheral cell-types (including cancer cells), microglia, and astrocytes. It is involved in tissue remodeling during inflammation, angiogenic processes, macrophages differentiation and maturation.102 YKL-40 is overexpressed in a variety of neurological diseases including stroke, viral encephalitis, traumatic brain injury, amyotrophic lateral sclerosis, multiple sclerosis, prion diseases, and AD.103 However, different studies found comparable levels of CSF YKL-40 between PD patients and healthy controls. Instead, higher concentrations were demonstrated in patients with atypical parkinsonism compared to PD patients.104,105 Furthermore, increased levels were found in PD patients with lower MMSE scores, suggesting the potential role of YKL-40 as biomarker of cognitive impairment in PD.106 Other studies focusing on CSF biomarkers associated inflammation with cognitive decline in PD. CSF levels of IL-6, IL-8, and C Reactive Protein (CRP) were found to be increased in PDD patients compared to non-demented PD and healthy controls.107 CSF biomarkers reflecting oxidative stress can be expression of immune system activation, as well as representative of mitochondrial derangement. Reduced levels of superoxide dismutase associated with increased levels of catalase activity were found in the CSF of PD patients.108 Studies focusing on DJ-1 provided no conclusive results. Hong and coll. showed decreased concentration of CSF DJ-1 in a cohort of PD patients with respect to controls.109 Before then, its levels were demonstrated increased in PD, with higher value in the early stage of disease,110 suggesting that DJ-1 up-regulation occurs early in the disease course to counteract oxidative stress. For this reason, CSF biomarkers reflecting oxidative stress might be useful to select early-stage PD patients for potential disease modifying strategies. The reliability of CSF biomarkers of inflammation and oxidative stress, when considered alone, is scarce for PD in terms of both diagnostic and prognostic properties. However, they could add valuable information if included in a panel of multiple biomarkers. For instance, Magdalinou et al assessed CSF levels of MCP-1 and YKL-40, together with other biomarkers (α-syn, Aβ42, p-tau, t-tau, amyloid precursor protein soluble metabolites α and β, NfL), in a cohort of patients with neurodegenerative disorders including PD, atypical parkinsonisms, AD and fronto-temporal dementia. This panel of biomarkers was able to differentiate PD from atypical parkinsonian syndromes with high accuracy.48 Further interesting findings will derive from proteomics-based assessments, which show the possibility of testing not a single biomarker but simultaneously a set of putative proteins characterized even by very low concentrations in the CSF and involved in specific biological pathways like neuroinflammation and oxidative stress.111
Conclusion
PD pathophysiology is characterized by a variety of biological pathways which differently contribute to its clinical development and progression. Molecular changes underlying these pathways anticipate the appearance of clinical manifestations by several years. In front of this, diagnostic criteria still mostly rely on clinical features, thus making it difficult to recognize the disease in its earliest phases. CSF biomarkers, by providing a realistic picture of what happens in brain, might be suitable to identify the disease at pre-motor stages, also showing the high potential for molecular and clinical characterization of PD subtypes. Thus, their implementation may give a substantial contribution to the optimal use of disease-modifying drugs, in terms of patients selection and treatment outcomes. To do this, further efforts to standardize CSF biomarkers assessment in PD, thus validating their use in routine clinical practice both for diagnostic and prognostic purposes, are urgently needed.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contributions: LP conceived and designed the content and structure of the review; LF, FPP and SS drafted, revised, and edited the review; LF, FPP, SS and LP revised and edited the manuscript.
References
- 1. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591-1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1-21. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 4. Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989-993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD The CORE-PD study. Neurology. 2012;78:1434-1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karimi-Moghadam A, Charsouei S, Bell B, Jabalameli MR. Parkinson disease from mendelian forms to genetic susceptibility: new molecular insights into the neurodegeneration process. Cell Mol Neurobiol. 2018;38:1153-1178. doi: 10.1007/s10571-018-0587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pang SYY, Ho PWL, Liu HF, et al. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl Neurodegener. 2019;8:23. doi: 10.1186/s40035-019-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150-1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 9. Ingelsson M. Alpha-synuclein oligomers—neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front Neurosci. 2016;10:408. doi: 10.3389/fnins.2016.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015-1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- 11. Wales P, Pinho R, Lázaro DF, Outeiro TF. Limelight on alpha-synuclein: pathological and mechanistic implications in neurodegeneration. J Parkinsons Dis. 2013;3:415-459. doi: 10.3233/JPD-130216. [DOI] [PubMed] [Google Scholar]
- 12. Burré J. The synaptic function of α-synuclein. J Parkinsons Dis. 2015;5:699-713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim C, Lee S-J. Controlling the mass action of α-synuclein in Parkinson’s disease. J Neurochem. 2008;107:303-316. doi: 10.1111/j.1471-4159.2008.05612.x. [DOI] [PubMed] [Google Scholar]
- 14. Melki R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J Parkinsons Dis. 2015;5:217-227. doi: 10.3233/JPD-150543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross OA, Braithwaite AT, Skipper LM, et al. Genomic investigation of α-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743-750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehra S, Sahay S, Maji SK. α-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta - Proteins Proteomics. 2019;1867:890-908. doi: 10.1016/j.bbapap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 17. Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385-398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 18. Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651-1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzulli JR, Xu Y-H, Sun Y, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37-52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sevlever D, Jiang P, Yen S-HC. Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species†. Biochemistry. 2008;47:9678-9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between Aβ, tau, and -synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281-7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pletnikova O, West N, Lee MK, et al. Aβ deposition is associated with enhanced cortical α-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26:1183-1192. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23. Masliah E. Recent advances in the understanding of the role of synaptic proteins in Alzheimer’s Disease and other neurodegenerative disorders. J Alzheimer’s Dis. 2001;3:121-129. doi: 10.3233/JAD-2001-3117. [DOI] [PubMed] [Google Scholar]
- 24. Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089-9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee H-JJ, Suk J-EE, Patrick C, et al. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262-9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harms AS, Cao S, Rowse AL, et al. MHCII is required for -synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33:9592-9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3:461-491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonifati V. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256-259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 29. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90:870-881. doi: 10.1136/jnnp-2018-320106. [DOI] [PubMed] [Google Scholar]
- 30. Mahlknecht P, Seppi K, Poewe W. The concept of prodromal Parkinson’s disease. J Parkinsons Dis. 2015;5:681-697. doi: 10.3233/JPD-150685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parnetti L, Castrioto A, Chiasserini D, et al. Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol. 2013;9:131-140. doi: 10.1038/nrneurol.2013.10. [DOI] [PubMed] [Google Scholar]
- 32. Alcolea D, Martínez-Lage P, Izagirre A, et al. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer’s disease: a multicenter study in Spain. J Alzheimer’s Dis. 2014;39:719-726. doi: 10.3233/JAD-131334. [DOI] [PubMed] [Google Scholar]
- 33. Duits FH, Martinez-Lage P, Paquet C, et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimer’s Dement. 2016;12:154-163. doi: 10.1016/j.jalz.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 34. Prakash N, Caspell-Garcia C, Coffey C, et al. Feasibility and safety of lumbar puncture in the Parkinson’s disease research participants: Parkinson’s Progression Marker Initiative (PPMI). Parkinsonism Relat Disord. 2019;62:201-209. doi: 10.1016/j.parkreldis.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Volles MJ, Lee S-J, Rochet J-C, et al. Vesicle permeabilization by protofibrillar α-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease†. Biochemistry. 2001;40:7812-7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 36. Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197-211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 37. Yonetani M, Nonaka T, Masuda M, et al. Conversion of wild-type α-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284:7940-7950. doi: 10.1074/jbc.M807482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975-988. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128-1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parnetti L, Chiasserini D, Bellomo G, et al. Cerebrospinal fluid Tau/α-synuclein ratio in Parkinson’s disease and degenerative dementias. Mov Disord. 2011;26:1428-1435. doi: 10.1002/mds.23670. [DOI] [PubMed] [Google Scholar]
- 41. Eusebi P, Giannandrea D, Biscetti L, et al. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2017;32:1389-1400. doi: 10.1002/mds.27110. [DOI] [PubMed] [Google Scholar]
- 42. Sako W, Murakami N, Izumi Y, Kaji R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: evidence from a meta-analysis. Mov Disord. 2014;29:1599-1605. doi: 10.1002/mds.26036. [DOI] [PubMed] [Google Scholar]
- 43. Gao L, Tang H, Nie K, et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int J Neurosci. 2015;125:645-654. doi: 10.3109/00207454.2014.961454. [DOI] [PubMed] [Google Scholar]
- 44. Zhou B, Wen M, Yu WF, Zhang CL, Jiao L. The diagnostic and differential diagnosis utility of cerebrospinal fluid α -synuclein levels in Parkinson’s disease: a meta-analysis. Parkinsons Dis. 2015;2015. doi: 10.1155/2015/567386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mollenhauer B, Zimmermann J, Sixel-Döring F, et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov Disord. 2019;34:67-77. doi: 10.1002/mds.27492. [DOI] [PubMed] [Google Scholar]
- 46. Wennström M, Surova Y, Hall S, et al. Low CSF levels of both α-synuclein and the α-synuclein cleaving enzyme neurosin in patients with synucleinopathy. Ginsberg SD, ed. PLoS One. 2013;8:e53250. doi: 10.1371/journal.pone.0053250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bäckström DC, Eriksson Domellöf M, Linder J, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident parkinson disease. JAMA Neurol. 2015;72:1175-1182. doi: 10.1001/jamaneurol.2015.1449. [DOI] [PubMed] [Google Scholar]
- 48. Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86:1240-1247. doi: 10.1136/jnnp-2014-309562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart T, Liu C, Ginghina C, et al. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol. 2014;184:966-975. doi: 10.1016/j.ajpath.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Majbour NK, Vaikath NN, Eusebi P, et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord. 2016;31:1535-1542. doi: 10.1002/mds.26754. [DOI] [PubMed] [Google Scholar]
- 51. Hall S, Surova Y, Öhrfelt A, Blennow K, Zetterberg H, Hansson O. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson’s disease. Mov Disord. 2016;31:898-905. doi: 10.1002/mds.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mollenhauer B, Caspell-Garcia CJ, Coffey CS, et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov Disord. 2019;34:1354-1364. doi: 10.1002/mds.27806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Førland MG, Öhrfelt A, Dalen I, et al. Evolution of cerebrospinal fluid total α-synuclein in Parkinson’s disease. Parkinsonism Relat Disord. 2018;49:4-8. doi: 10.1016/j.parkreldis.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 54. Parnetti L, Gaetani L, Eusebi P, et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019;18:573-586. doi: 10.1016/S1474-4422(19)30024-9. [DOI] [PubMed] [Google Scholar]
- 55. Mollenhauer B, Parnetti L, Rektorova I, et al. Biological confounders for the values of cerebrospinal fluid proteins in Parkinson’s disease and related disorders. J Neurochem. 2016;139:290-317. doi: 10.1111/jnc.13390. [DOI] [PubMed] [Google Scholar]
- 56. Barbour R, Kling K, Anderson JP, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55-59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 57. Mollenhauer B, Bowman FD, Drake D, et al. Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid—method comparison and round robin study. J Neurochem. 2019;149:126-138. doi: 10.1111/jnc.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739-29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 59. Parnetti L, Farotti L, Eusebi P, et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s disease. Front Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tokuda T, Qureshi MM, Ardah MT, et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766-1772. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 61. Hansson O, Hall S, Öhrfelt A, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther. 2014;6:25. doi: 10.1186/alzrt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Majbour NK, Vaikath NN, van Dijk KD, et al. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol Neurodegener. 2016;11:7. doi: 10.1186/s13024-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paciotti S, Bellomo G, Gatticchi L, Parnetti L. Are we ready for detecting α-synuclein prone to aggregation in patients? The case of “protein-misfolding cyclic amplification” and “real-time quaking-induced conversion” as diagnostic tools. Front Neurol. 2018;9:415. doi: 10.3389/fneur.2018.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3:812-818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Groveman BR, Orrù CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018;6:7. doi: 10.1186/s40478-018-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kang UJ, Boehme AK, Fairfoul G, et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord. 2019;34:536-544. doi: 10.1002/mds.27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol. 2019;85:777-781. doi: 10.1002/ana.25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coughlin DG, Hurtig HI, Irwin DJ. Pathological influences on clinical heterogeneity in Lewy body diseases. Mov Disord. 2020;35:5-19. doi: 10.1002/mds.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16:55. doi: 10.1016/S1474-4422(16)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409-415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 71. Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm. 2002;109:329-339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 72. Jellinger KA. Neuropathological aspects of Alzheimer disease, Parkinson disease and frontotemporal dementia. Neurodegener Dis. 2008;5:118-121. doi: 10.1159/000113679. [DOI] [PubMed] [Google Scholar]
- 73. Irwin DJ, Lee VM-Y, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626-636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Přikrylová Vranová H, Mareš J, Nevrlý M, et al. CSF markers of neurodegeneration in Parkinson’s disease. J Neural Transm. 2010;117:1177-1181. doi: 10.1007/s00702-010-0462-z. [DOI] [PubMed] [Google Scholar]
- 75. Holmberg B, Johnels B, Blennow K, Rosengren L. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson’s disease and progressive supranuclear palsy. Mov Disord. 2003;18:186-190. doi: 10.1002/mds.10321. [DOI] [PubMed] [Google Scholar]
- 76. Kaerst L, Kuhlmann A, Wedekind D, Stoeck K, Lange P, Zerr I. Using cerebrospinal fluid marker profiles in clinical diagnosis of dementia with Lewy bodies, Parkinson’s disease, and Alzheimer’s disease. J Alzheimer’s Dis. 2013;38:63-73. doi: 10.3233/JAD-130995. [DOI] [PubMed] [Google Scholar]
- 77. Süssmuth SD, Uttner I, Landwehrmeyer B, et al. Differential pattern of brain-specific CSF proteins tau and amyloid-beta in Parkinsonian syndromes. Mov Disord. 2010;25:1284-1288. doi: 10.1002/mds.22895. [DOI] [PubMed] [Google Scholar]
- 78. Alves G, Bronnick K, Aarsland D, et al. CSF amyloid- and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080-1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 79. Compta Y, Martà MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24:2203-2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 80. Hall S, Öhrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 81. Montine TJ, Shi M, Quinn JF, et al. CSF Aβ 42 and tau in Parkinson’s disease with cognitive impairment. Mov Disord. 2010;25:2682-2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kang J-H, Mollenhauer B, Coffey CS, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s progression markers initiative study. Acta Neuropathol. 2016;131:935-949. doi: 10.1007/s00401-016-1552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang J, Sokal I, Peskind ER, et al. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J Clin Pathol. 2008;129:526-529. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Abdo WF, Bloem BR, Van Geel WJ, Esselink RAJJ, Verbeek MM. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol Aging. 2007;28:742-747. doi: 10.1016/j.neurobiolaging.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 85. Herbert MK, Eeftens JM, Aerts MB, et al. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat Disord. 2014;20:112-115. doi: 10.1016/j.parkreldis.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 86. Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055-1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mollenhauer B, Trenkwalder C, Von Ahsen N, et al. Beta-amlyoid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson’s disease dementia. Dement Geriatr Cogn Disord. 2006;22:200-208. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- 88. Liu C, Cholerton B, Shi M, et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Park Relat Disord. 2015;21:271-276. doi: 10.1016/j.parkreldis.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson’s progression markers initiative. J Neurol Neurosurg Psychiatry. 2017;88:325-331. doi: 10.1136/jnnp-2016-314832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rochester L, Galna B, Lord S, et al. Decrease in Aβ42 predicts dopa-resistant gait progression in early Parkinson disease. Neurology. 2017;88:1501-1511. doi: 10.1212/WNL.0000000000003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang J, Mattison HA, Liu C, et al. Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol. 2013;126:671-682. doi: 10.1007/s00401-013-1121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Balducci C, Pierguidi L, Persichetti E, et al. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov Disord. 2007;22:1481-1484. doi: 10.1002/mds.21399. [DOI] [PubMed] [Google Scholar]
- 93. Parnetti L, Chiasserini D, Persichetti E, et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov Disord. 2014;29:1019-1027. doi: 10.1002/mds.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Parnetti L, Paciotti S, Eusebi P, et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in parkinson’s disease patients. Mov Disord. 2017;32:1423-1431. doi: 10.1002/mds.27136. [DOI] [PubMed] [Google Scholar]
- 95. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577-589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 96. Olsson B, Portelius E, Cullen NC, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76: 318-325. doi: 10.1001/jamaneurol.2018.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Holmberg B, Rosengren L, Karlsson J-EE, Johnels B. Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov Disord. 1998;13:70-77. doi: 10.1002/mds.870130116. [DOI] [PubMed] [Google Scholar]
- 98. Sako W, Murakami N, Izumi Y, Kaji R. Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. J Neurol Sci. 2015;352:84-87. doi: 10.1016/j.jns.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 99. Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson’s disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord. 2010;16:142-145. doi: 10.1016/j.parkreldis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 100. Dohgu S, Takata F, Matsumoto J, Kimura I, Yamauchi A, Kataoka Y. Monomeric α-synuclein induces blood–brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc Res. 2019;124:61-66. doi: 10.1016/j.mvr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 101. Schröder JB, Pawlowski M, Hörste GMZ, et al. Immune cell activation in the cerebrospinal fluid of patients with Parkinson’s disease. Front Neurol. 2018;9:1081. doi: 10.3389/fneur.2018.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shao R, Hamel K, Petersen L, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456-4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Llorens F, Thüne K, Tahir W, et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener. 2017;12:83. doi: 10.1186/s13024-017-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Olsson B, Constantinescu R, Holmberg B, Andreasen N, Blennow K, Zetterberg H. The glial marker YKL-40 is decreased in synucleinopathies. Mov Disord. 2013;28:1882-1885. doi: 10.1002/mds.25589. [DOI] [PubMed] [Google Scholar]
- 105. Magdalinou NK, Noyce AJ, Pinto R, et al. Identification of candidate cerebrospinal fluid biomarkers in parkinsonism using quantitative proteomics. Park Relat Disord. 2017;37:65-71. doi: 10.1016/j.parkreldis.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 106. Wennström M, Surova Y, Hall S, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PLoS One. 2015;10:e0135458. doi: 10.1371/journal.pone.0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci Reports 2018 81. 2018;8:1-9. doi: 10.1038/s41598-018-31517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pandey S, Singh B, Yadav SK, Mahdi AA. Novel biomarker for neurodegenerative diseases- motor neuron disease (MND), cerebellar ataxia (CA) and Parkinson’s disease (PD). Clin Chim Acta. 2018;485:258-261. doi: 10.1016/j.cca.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 109. Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010;133(Pt 3):713-726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Waragai M, Wei J, Fujita M, et al. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson’s disease. 2006;345:967-972. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 111. Bader JM, Geyer PE, Müller JB, et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol Syst Biol. 2020;16:e9356. doi: 10.15252/msb.20199356. [DOI] [PMC free article] [PubMed] [Google Scholar]