Abstract
The activity-dependent neuroprotective protein (ADNP), a double-edged sword, sex-dependently regulates multiple genes and was previously associated with the control of early muscle development and aging. Here we aimed to decipher the involvement of ADNP in versatile muscle gene expression patterns in correlation with motor function throughout life. Using quantitative RT-PCR we showed that Adnp+/− heterozygous deficiency in mice resulted in aberrant gastrocnemius (GC) muscle, tongue and bladder gene expression, which was corrected by the Adnp snippet, drug candidate, NAP (CP201). A significant sexual dichotomy was discovered, coupled to muscle and age-specific gene regulation. As such, Adnp was shown to regulate myosin light chain (Myl) in the gastrocnemius (GC) muscle, the language acquisition gene forkhead box protein P2 (Foxp2) in the tongue and the pituitary-adenylate cyclase activating polypeptide (PACAP) receptor PAC1 mRNA (Adcyap1r1) in the bladder, with PACAP linked to bladder function. A tight age regulation was observed, coupled to an extensive correlation to muscle function (gait analysis), placing ADNP as a muscle-regulating gene/protein.
Keywords: ADNP, NAP, muscle function, CatWalk, gene expression
1. Introduction
The activity-dependent neuroprotective protein (ADNP) [1,2], partly controlled by vasoactive intestinal peptide (VIP) and pituitary-adenylate cyclase activating polypeptide (PACAP) [3,4,5,6,7,8,9], is a known major regulator of gene function [10]. For example, our original studies showed that Adnp regulates more than 400 genes during embryonic development including genes controlling the development of the visceral endoderm, the heart and organogenesis in general [11]. In the adult mouse, Adnp regulates hundreds of genes important for brain and immune functions [12,13]. Importantly, there is a significant resemblance between mouse and human ADNP gene regulation [13,14,15]. The resemblance is further accentuated by the fact that mouse Adnp mRNA is 90% identical to human ADNP mRNA [2]. Indeed, the Adnp-deficient heterozygous (haploinsufficient) mouse model [16] predicted the autism-intellectual disability—associated ADNP syndrome [13].
Delayed motor development characterizes a large majority (96%) of ADNP syndrome children, suffering from de novo mutations in ADNP [14,17,18]. ADNP syndrome motor deficiencies manifest as hypotonia as well as opposing acute muscle tightness, signs of atrophy and abnormal gait [17,18]. The human pathological condition was mimicked by the mouse model of Adnp haploinsufficiency [13], exhibiting reduced muscle tone, as was demonstrated in the hanging wire and grip strength tests, along with gait deficits as established by the CatWalk gait analyses [13].
We have also shown that the expression levels of ADNP and its paralogue protein ADNP2 in the vastus lateralis and bicep brachii muscles are significantly upregulated in the human elderly population, compared to young subjects, in a sex dependent manner. Thus, in the vastus lateralis ADNP is increased with aging in males and females, while ADNP2 is increased with aging in females only. In the bicep brachii muscles, ADNP is increased with aging only in males and not in females, while ADNP2 is increased with aging exclusively in females [19]. ADNP expression was highly correlated with 24 genes, with nicotinamide nucleotide adenylyl (NAD) transferase 1 (NMNAT1) being the leading gene/protein [19]. As such, NMNAT1-associated regulation of NAD+ salvage capacity in human skeletal muscle declines with aging, suggesting a causative or a compensatory role for ADNP content [20].
Importantly, not only the skeletal limb muscles are affected in the ADNP syndrome. For example, the bladder is slow in development with 81% of children suffering from the syndrome, exhibiting bladder training delay, and many are still not toilet trained when approaching puberty [17]. Another key characteristic of ADNP syndrome is speech delay, which presented in 98.6% of individuals and 19% had no language development at all [17]. Apraxia and absence of tongue movement was also found [18].
Mechanistically, ADNP is found in the nucleus as well as in the cytoplasm, and cytoplasmic representation increases in mature neurons, where ADNP is essential for neurite maintenance [21]. As such, ADNP exerts its control by regulating microtubule dynamics, binding to microtubule end binding proteins EB1 and EB3 [22], also regulating Tau-microtubule interaction [23,24] and axonal transport [12]. Importantly, cytoskeletal reorganization is associated with stretch-induced gene expression, implicating a role for cytoplasmic ADNP in gene expression regulation as well [25], especially in stretch-associated muscle cells.
Adnp deficiency is also associated with reduced autophagy that is dependent on microtubule integrity [26], with ADNP binding the microtubule associated protein 1 light chain 3B (LC3B), forming the autophagosome [27]. Interestingly, several muscle diseases present microtubule/autophagy deficits. For example, Duchenne muscular dystrophy (DMD) [28,29,30,31], Becker muscular dystrophy (BMD) [30,32,33], exhibiting absence or mutations in dystrophin, and tibial muscular dystrophy (TMD), exhibiting mutations in titin [34]. Furthermore, the ADNP regulating neuropeptide PACAP [3] was also shown to protect muscle function in a model of spinobulbar muscular atrophy (SBMA) [35].
The cytoplasmic interactions of ADNP with LC3 and EB1/EB3 are enhanced in the presence of the ADNP snippet, NAP (NAPVSIPQ, also known as CP201) containing an EB1/EB3 and self-interacting SxIP motif [12,23,24,26,27]. Moreover, through EB1/EB3 interactions, NAP enhances Tau-microtubule binding, protecting against tauopathy [23,24], which has also been found in the SOD1-G93A mouse model of the neuromuscular disorder, amyotrophic lateral sclerosis (ALS) [36].
Given the significant effect of ADNP mutations and ADNP deficiency on motor functions [13], and the strong association of ADNP with gene regulation (e.g., [10,11,21]), we hypothesized that it is involved in direct regulation of versatile muscle genes, with expression levels correlating with motor function throughout development. Our results have proven our hypothesis and identified multiple biomarkers for Adnp muscle activity.
2. Results
2.1. Adnp+/– Mice Display Age-Dependent Aberrant Gene Expression in Skeletal Muscle, Tongue, and Bladder, Compared with Adnp+/+ Mice, Corrected by NAP Treatment
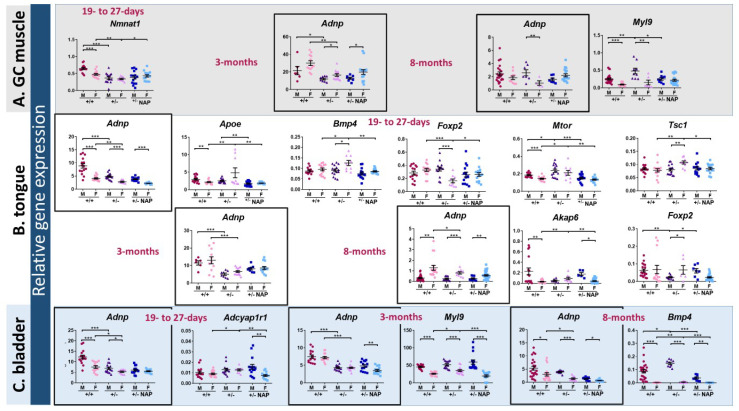
To evaluate a potential mechanistic basis for the effects of ADNP on muscle development/maintenance, we examined the water-based DD- formulated/NAP-treated Adnp+/– and Adnp+/+ mice, previously extensively studied for brain function and behavior (methods) [13]. We chose to assess gene expression patterns in skeletal muscle, tongue, and bladder as ADNP syndrome patients suffer from delays in motor development, language acquisition, and bladder training [17]. Given the developmental regulation attributed to Adnp as well as the sex-dependency [12], males and females at three age groups were chosen (methods): 19–27-days (youngest), 3-months (young adults), and 8-months (old group) (Figure 1). Based on previous gene arrays and RNA-seq experiments addressing Adnp gene regulation, 18 genes were chosen (Table S1). Using qRT-PCR, we measured transcript expression levels of these 18 genes including Adnp and the reference gene Hprt [37] in the youngest mouse group (19–27-day-old) in the gastrocnemius (GC) muscle (Figure 1A), tongue (Figure 1B), and bladder (Figure 1C). At 3- and 8-months of age, we selected the following genes, Adnp, Adnp2, Akap6, Bmp4, Foxp2, Myl2 and Myl9.
Figure 1.
Activity-dependent neuroprotective protein (Adnp) haploinsufficiency alters muscle (GC muscle (A), tongue (B) and bladder (C)) gene expression in a sex- and age-dependent manner: NAP amelioration. Total RNA was extracted from 3 groups of age youngest group: 19–27-day-old mice (males: Adnp+/+ n = 5, Adnp+/– n = 5, Adnp+/+ NAP n = 4, Adnp+/– NAP n = 5; females: Adnp+/+ n = 5, Adnp+/– n = 3, Adnp+/+ NAP n = 4, Adnp+/– NAP n = 5). Young adults: 3-month-old mice (for muscle and tongue tissues males: Adnp+/+ n = 3, Adnp+/– n = 4, Adnp+/+ NAP n = 4, Adnp+/– NAP n = 4; females: Adnp+/+ n = 6, Adnp+/– n = 7, Adnp+/+ NAP n = 6, Adnp+/– NAP n = 8/9, respectively. For bladder of both sexes: Adnp+/+ n = 5, Adnp+/– n = 5, Adnp+/+ NAP n = 5, Adnp+/– NAP n = 5). Old group: 8-month-old mice (males: Adnp+/+ n = 8, Adnp+/– n = 3, Adnp+/+ NAP n = 4, Adnp+/– NAP n = 3; females: Adnp+/+ n = 6, Adnp+/– n = 3, Adnp+/+ NAP n = 5, Adnp+/– NAP n = 10/11 (in bladder and tongue n = 11)). Results were normalized to Hprt. The comparative Ct method was implemented here (2−ΔCT) for quantification of transcripts (indicated as relative gene expression and further explained in the Methods). A two-way ANOVA with Tukey’s post hoc test revealed significant differences between vehicle-treated Adnp+/+ and Adnp+/– mice and between NAP and vehicle-treated Adnp+/– mice (* p < 0.05, ** p < 0.01 and *** p < 0.001). Sex differences were determined by an unpaired Student’s t-test.
We chose to concentrate on gene transcripts changed as a consequence of Adnp heterozygosity (haploinsufficiency), which were corrected by the ADNP snippet, drug candidate NAP, further attesting for specificity.
Adnp reduction as a consequence of Adnp gene copy deficiency (Adnp+/−) was significant in all tested tissues at the youngest tested age in both males and females, with significantly higher expression in Adnp+/+ males compared with females (Figure 1A–C, boxes, the data for the GC muscle are not shown). This gene copy deficiency was maintained in the young adults (Figure 1A–C, boxes). However, it was abolished in the GC muscle of 8-month-old mice, while being maintained only in the female tongue and bladder (Figure 1A–C, boxes). Sex differences coupled to developmental and organ differences were observed in Adnp transcript content. For example, higher tongue Adnp expression was displayed in Adnp+/+ males compared to other groups, in the youngest age tested, whereas, no sex differences were observed in the young adults and higher female expression was exhibited in the older group (Figure 1B, boxes). In contrast, increased male Adnp expression was observed in the bladder in selected genotype and treatment groups, in all tested ages, with all tested groups exhibiting this finding in the oldest tested age (Figure 1C, boxes). Of the 16 mRNAs assessed at the youngest age (Table S1 excluding Adnp and Hprt), Myl2 (not shown, in preparation), Myl9, and Nmnat1 showed Adnp/NAP as well as age/sex-dependent regulation in the GC muscle (Figure 1A).
Tongue sex/age/Adnp genotype/NAP-dependent regulation was shown in 5 out of the 16 tested genes at the youngest age tested (Apoe, Bmp4, Foxp2, Tsc1 all in females and Mtor, in females and males) and two (Akap6, females and Foxp2, males) at the oldest age tested (Figure 1B), while only one transcript change per age group was shown in the bladder (Figure 1C, Adcyap1r1, young group females, Myl9, young adult females and Bmp4, old females and males).
As indicated above, we chose to concentrate on gene transcripts that have changed as a consequence of Adnp heterozygosity (haploinsufficiency), which were corrected by the ADNP snippet, drug candidate NAP (Figure 1). Additionally, Table S2 summarizes relative gene expression changes affected either by the Adnp genotype, or by NAP treatment and/or sex in muscle, tongue, and bladder in the three tested age groups. A most extensive sex difference was observed in the Adnp+/+ mice indicating that the chosen studied gene transcripts present an inherent sexual difference, which was maintained, in part, in the Adnp+/− mice and also upon treatment with NAP. While most of the changes were subtle, there were a few exceptions as follows. Akap6 increased by 3.71 folds in the 8-month-old Adnp+/− female GC muscle compared to Adnp+/+ and Myl2 decreased by 0.24 folds in the 3-month-old Adnp+/− vs. Adnp+/+ male tongue, suggesting partial, but not complete NAP amelioration.
In conclusion, Adnp+/– mice displayed age-and sex-dependent aberrant gene expression in skeletal muscle, tongue, and bladder, compared with Adnp+/+ mice and correction by NAP treatment. Of the selected tissues, the tongue showed the most robust changes, potentially connected with the ADNP syndrome phenotype of deficient language acquisition.
2.2. Muscle Aberrant Gene Expression Is Correlated with Adnp Deficiency in a Sex and Age-Dependent Manner
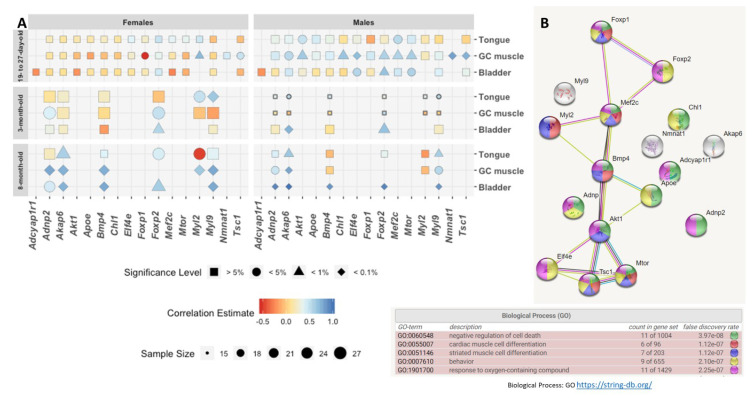
Further comparisons of the expression levels of the 16 tested transcripts with Adnp expression revealed extensive correlations. Akap6, Foxp2, and Myl9 showed the most abundant correlations across tissues, age, and sex (Figure 2A). Interestingly Nmnat1, which was correlated before with ADNP expression in human muscle [19], showed a highly significant correlation with Adnp in the GC muscle in the youngest group of males (Figure 2A). Comparing males to females suggested a more significant Adnp transcript correlation effect in males.
Figure 2.
Heatmap correlations between Adnp expression levels and the expression levels of genes crucial for the proper function of GC muscle, tongue, and bladder. (A) Correlative analyses were performed using either the Pearson correlation coefficient method or the Spearman’s rank correlation coefficient, if at least one of the data sets was not normally distributed. Significant correlations were observed in a sex-dependent manner. The correlations were performed in the three tested age groups: 19–27-day-old, 3-month-old, and 8-month-old mice in three different tissues: GC muscle, tongue, and bladder. (B) Functional enrichment and network analysis of the gene protein products presented in panel A + Adnp. The genes/proteins presented a crucial network biological process role for proper muscle function as also previously revealed by mouse and human RNA-seq [12,14], as well as Affymetrix array [16] (Table S1).
Developmentally, focusing on females, at the youngest age tested, almost no significant correlations were discovered, except for Foxp1, Myl2, and Tsc1 in the GC muscle. This finding was in contrast to males, where 9 of 15 tested GC muscle gene transcripts showed significant correlation to Adnp, excluding Foxp1 and Myl2. Again contrasting females, showing no Adnp gene transcript correlations at the youngest tested age in the bladder and the tongue, male results indicated Akt1 and Mef2c correlations to Adnp in the tongue and of Elf4e, Mtor, and Foxp2 in the bladder (Figure 2A). Additionally, Nmnat1 correlated with Adnp in the GC muscle only in males and not in females. Finally, Adcyap1r1, encoding the PACAP-specific, PAC1 receptor, did not correlate with Adnp in the bladder (Figure 2A), despite the significant increase Adcyap1r1 seen in the Adnp+/− versus Adnp+/+ females and the correction of the female Adnp+/− levels to the Adnp+/+ levels by NAP treatment (Figure 1C).
Surprisingly, at 3 months of age, no correlations were seen in the male GC muscle, while female Adnp correlated with Adnp2 and Foxp2. Foxp2 also correlated with Adnp in the bladder in 3-month-old males and females, whereas Akap6 also correlated with Adnp only in males. Additionally, Akap6 correlated with Adnp in the 3-month-old male tongue (but not female tongue), while Myl9 correlated with Adnp in both sexes, and Myl2 correlated with Adnp only in females (Figure 2A).
At 8 months of age, all gene transcripts tested showed significant correlations with Adnp in the bladder of males and females, with the highest correlations (almost 1) detected in males. Similarly, all transcripts tested showed significant correlations with Adnp in the female GC muscle, while only Adnp2, Akap6, and Myl9 were significant in the males. Akap6, Foxp2 and Myl2 correlated with Adnp in the old female tongue, with Akap6 also correlating with Adnp in males, and Myl9 correlating with Adnp only in the male and not in the female tongue.
Interestingly, the most extensive (and only) negative correlation was observed in the females for Foxp1 in the youngest GC muscle and for Myl2 in the oldest tested tongue tissue (red, Figure 2A). Molecular interaction/STRING analysis showed the expected result indicating that a great majority of the tested transcripts protein products were linked to muscle development and differentiation (Figure 2B).
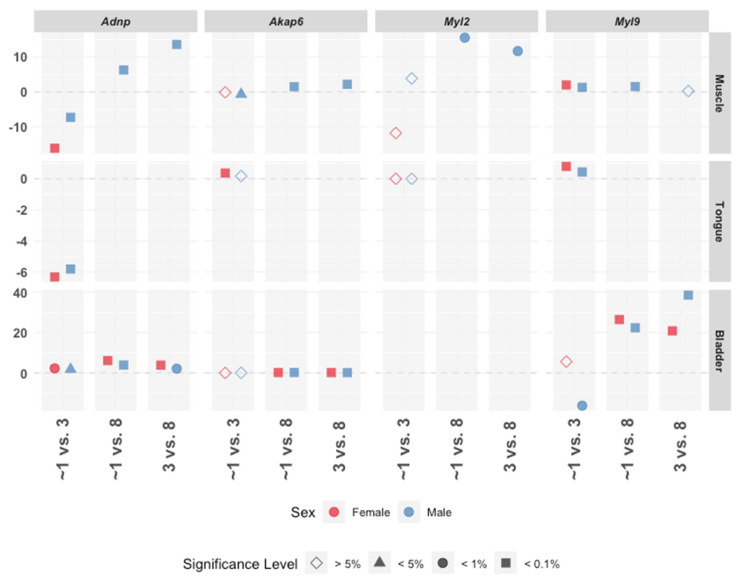
Further analysis of GC muscle, tongue, and bladder gene expression across the three tested ages showed increased expression of Adnp and Myl2 with age in the male muscle (blue), contrasting a decrease of Adnp in the female (pink), while a decrease in Adnp between one (i.e., 19–27 days) and three months of age was apparent in both sexes in the tongue (Figure 3). Age-dependent effects only on Myl9 in the bladder were shown decreasing only in males at 3 months of age, compared to 1 month, and increasing thereafter in both males and females (Figure 3).
Figure 3.
Age-dependent correlative gene expression patterns. Pairwise comparisons between age in month groups (x-axis), where the estimated difference (y-axis) is tested for statistical significance (shape) using post-hoc t-test with BH adjustment, for males and females separately (color). The most considerable differences were detected in Adnp, Akap6, Myl2, and Myl9. The majority of the comparisons were found statistically significant at 5% except for in 19–27-day-old mice = ~1 month vs. 3 months for Akap6, Myl2, and Myl9 in muscle, tongue and bladder. In addition, 3- and 8-month-old males were found to be similar in the respective Myl9 muscle expression levels.
Together, the results here showed that muscle aberrant gene expression was correlated with Adnp deficiency in a sex, tissue, and age, development-dependent manner and associated key muscle regulating genes with Adnp function. The finding of the age, tissue, and female specific negative regulation of Foxp1 and Myl2, emphasized age and sex-separation of the Adnp genotype.
2.3. Adnp+/– Mice Display of Age-Dependent Aberrant Gene Expression in Skeletal Muscle is Correlated with Behavior
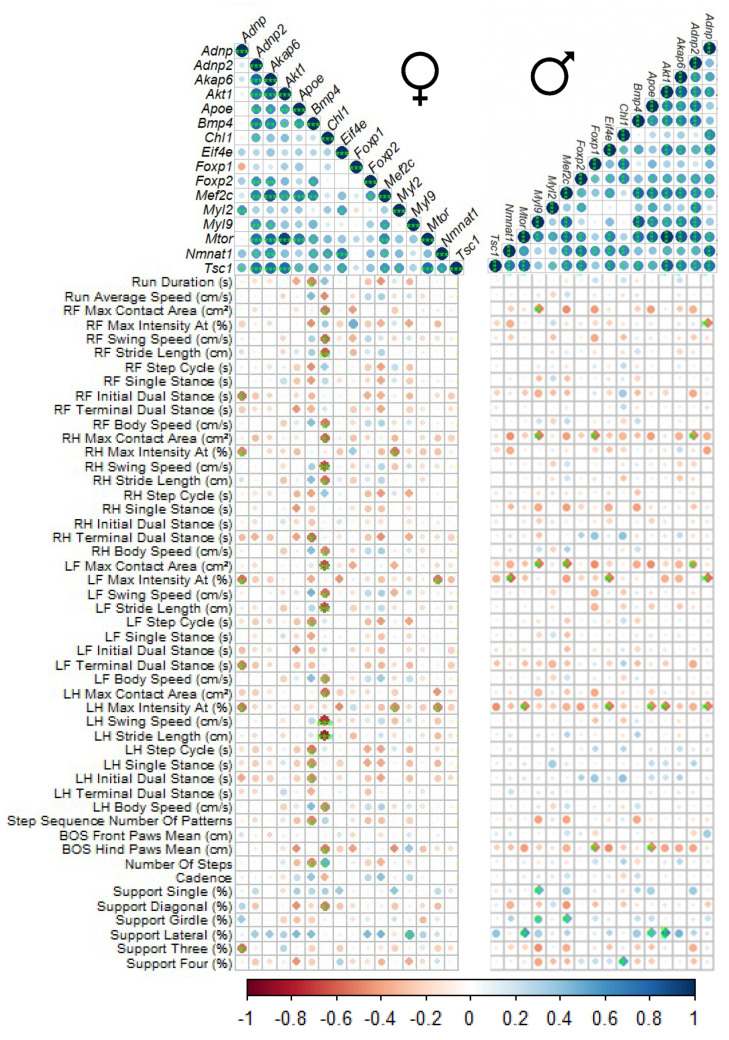
The youngest age examined (19–27-day-old) exhibited significant positive correlations with Adnp in 9 of the 15 tested genes (Figure 2) in the GC muscle of the male mouse. Hence, we further correlated specific RNA expression levels with previously published behavioral outcomes in the CatWalk gait measurements [13], (Table S3) addressing GC muscle function.
As a word of introduction, the automated CatWalk gait analysis is an exceedingly sensitive tool allowing the identification of extensive number of gait and locomotion parameters with a minimal human interference [38]. This tool was previously implemented in the assessment of static and dynamic gait parameters in a variety of nerve injury models [39,40,41] including muscular dystrophies [39]. We show here numerous correlations between the different behavioral outcomes comparing males to females, with both sexes showing a similar, but not identical distribution patterns (Figure 4). Most tested gene transcripts showed correlations amongst each other and also with assorted CatWalk behaviors, displaying different patterns in males and females and implicating the involvement of these genes in muscle function. Interestingly, Bmp4 and Chl1 correlated with a number of different CatWalk behaviors in females (Figure 4, vertical view), while in the males, several genes correlated with selected CatWalk behaviors, e.g., maximal contact area (Figure 4, horizontal view).
Figure 4.
Correlative analysis between gene expression in GC muscle of 19–27-day old Adnp mice and the respective performance in the CatWalk apparatus. Correlative analyses were performed using Pearson correlation coefficient method. Positive correlations are displayed in a blue scale and negative correlations are displayed in a red-brown scale. The asterisks represent the significance level: * p < 0.05, ** p < 0.01 and *** p < 0.001. Please see Supplementary Table S3 for further explanation of the CatWalk measurements. R = right, L = left, H = hind paw, F = front paw.
Taken together, the Adnp genotype affected age-dependent gene expression in skeletal muscle in a sex-dependent correlation with gait parameters, with motor development/function being a major impediment in the ADNP syndrome patients.
3. Discussion
In the current paper we have discovered extensive correlation between Adnp expression and multi-muscle gene expression throughout life, with sex-specific patterns. Adnp-deficient gene regulation was corrected by its active site protein fragment, drug candidate NAP. Mechanistically, Adnp gene regulation was correlated with CatWalk motor behavioral outcomes.
Further referring to the mechanism, our original studies identified a high degree of correlation between ADNP and NMNAT1 in the human muscle [19]. Here, a high correlation was discovered between Adnp and Nmnat1 expression in the young male mouse muscle. NMNAT1 regulates NAD+ salvage capacity in human skeletal muscle, which is declining with aging [20].
Additionally, here, in the GC muscle, Adnp correlated with Adnp2 only in the older mouse groups in a sex-dependent manner (appearing earlier and showing stronger correlations in the females). Previous findings linked dysregulation of Adnp/Adnp2 correlations with aberrant synaptic function in neuropsychiatric diseases [42]. Interestingly, sexual differences were found in ADNP, ADNP2 expression in Alzheimer’s disease (lymphocytes) [43] and in schizophrenia (postmortem brains and lymphocytes) [27,42]. While ADNP2 is a less studied gene compared to ADNP [2,44,45], recent studies have also linked ADNP2 deletion (together with other genes) to autism [46]. Further studies tied ADNP2 to osteoblast regulation [47], suggesting pleiotropic activities, similar, and potentially complementary to ADNP.
Adcyap1r1 encoding the PACAP-specific, PAC1 receptor, did not correlate with Adnp in the bladder, despite the significant increase seen in the Adnp+/− females compared to Adnp+/+ females and the amelioration with NAP treatment. These findings are of significant interest, as intra-bladder administration of the PAC1 receptor antagonist, PACAP(6–38), reduces urinary bladder frequency and pelvic sensitivity in mice exposed to repeated variate stress [48], with PACAP ameliorating Adnp-deficiency exacerbated stress response [3]. While ADNP was directly linked to cognitive impairment/language acquisition in humans [14,17,49] and in mice (vocalization) [13], indirect evidence also ties PACAP to the vocalization response [50]. We also participated in a study showing that PACAP regulates muscle function in protection against outcome measures in a mouse model of spinobulbar muscular atrophy (SBMA) [35].
Interestingly, here, we also found sexually/developmentally differential expression of Myl2 and Myl9 in correlation with Adnp expression. In this respect, MYL2 is linked to cardiac development and function [51], while MYL9 is involved in smooth muscle and non-muscle cell contractile activity e.g., in the gut and urinary tract [52], with ADNP syndrome children suffering cardiac as well as gastrointestinal problems [17]. Furthermore, a recent study identified Myl9 as highly important for skeletal muscle development [53] as well as to bladder and gastrointestinal smooth muscle contraction, with homozygous deletion leading to megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS), a severe disease characterized by functional obstruction in the urinary and gastrointestinal tract [52,54]. Anatomically, the bladder divides into two parts: the dome and the base. The dome of the bladder is made up of smooth muscle, and the base consists of a trigone and neck that are closely connected to the pelvic floor. There are two urethral sphincters at the bladder outlet that are necessary for normal voluntary micturition [55]. Myl9 may be associated with the sphincters as well as the smooth muscle function. Together with the current findings, MYL9 may play a significant role in skeletal as well as smooth muscle regulation in the ADNP syndrome.
Similarly, Foxp2 is implicated as critical for central control for normal bladder voiding behavior [56] and may be important to tongue movement function [57], both affected in the ADNP syndrome. Indeed, Foxp2 showed distinct sexual dichotomy in the tongue. Further sexual differences were observed in Foxp2 also in correlation with Catwalk performance. Previous studies have indicated a close correlation of the Foxp1/Foxp2 transcript expression and regulation of ultrasonic vocalization in mice. Foxp1 and the androgen receptor are co-expressed in striatal medium spiny neurons and brain-specific androgen receptor KO (ArNesCre) mice exhibit reduced Foxp1 expression in the striatum at E17.5 and P7.5 and an increased Foxp2 level in the cortex at P7.5 [58]. Our previous bioinformatics results showed ADNP regulation of steroid pathways [15] and our current results suggest that this may be extended to the specific muscle cells. Furthermore, this steroid regulation may be linked with the higher prevalence of autism in boys compared to girls [58], with ADNP being a major autism driving gene [59,60]. Future research should further aim to decipher ADNP precise involvement in muscle function including cardiac and gastrointestinal muscles and use age and sex-dependency as major impacting study design and research outcome covariates.
While NAP treatment corrected many of the Adnp+/− associated deficiencies, it did not correct all (Table S2), as we have previously observed, also in other organs [13]. This could be due to additional differential temporal and sex-dependent controls exerted on these genes by Adnp and other regulatory proteins. One example is Akap6 suggested as an important regulator of myoblast differentiation, myotube formation, and muscle regeneration [61]. Thus, further studies are required to elucidate the potential interactions between Akap6 and Adnp.
4. Materials and Methods
4.1. Animals
The Adnp+/− mice, on a mixed C57BL and 129/Sv background, were previously described [13,16,59,62,63]. For continuous breeding, an ICR outbred mouse line was used [12,59]. Genotyping was performed by Transnetyx (Memphis, TN, USA). All animal groups were housed in a 12-h light/12-h dark cycle animal facility, with free access to rodent chow and water. All procedures involving animals were conducted under the supervision and approval of the IACUC of Tel Aviv University and the Israeli Ministry of Health (ethics certificate number: 01-18-018, valid dates: 15 March 2018–15 March 2021; ethics certificate number: 01-17-029: valid dates: 18 May 2018–6 April 2021). Adnp+/+ littermates served as controls.
4.2. Peptide Synthesis and Formulations
NAP peptide was custom synthesized using conventional methods [64], as before [13]. The peptide was dissolved in a vehicle solution termed DD, in which each milliliter included 7.5 mg of NaCl, 1.7 mg of citric acid monohydrate, 3 mg of disodium phosphate dihydrate, and 0.2 mg of benzalkonium chloride solution (50%) [13,65]. In days of scheduled behavioral tests, NAP was applied 2 h before the behavioral tests. For intranasal administration, each mouse was grasped in a vertical position and the solution was applied using a pipette and 5 μL tip. One droplet was released at the exterior naris and then inhaled by the mouse, and following a short break, the drug was administered into the other naris. This method has been extensively validated as producing reliable brain levels of NAP (CP201 also called davunetide) [66,67,68].
4.3. NAP Age Treatment Groups
Mice were divided to three age and treatment groups and sacrificed for RNA extraction by the end of the treatment period:
(1) A DD-group consisting of mouse pups that were subcutaneously administrated NAP (25 μg NAP/1 mL saline) for 21 consecutive days at the following doses: 40, 80, 120, and 160 μL on P9–P10, P11–P14, P15–P18, and P19–P21, respectively. At 21 days of age, these mice were treated daily with intranasal NAP for another 6 days [13] (tested at 19–27 days of age).
(2) 1-month-old mice were treated daily with intranasal NAP for 2 months (0.5 μg NAP/5 μL DD per mouse) (tested at 3 months of age) [13].
(3) 1-month-old mice (from group 1), were continuously treated daily with intranasal NAP for additional 7 months (0.5 μg NAP/5 μL DD per mouse) (tested at 8 months of age) [13].
4.4. Gait Analysis
CatWalk XT (Noldus Information Technology, Wageningen, the Netherlands) was used [13,69,70]. Importantly, parameters used are further described in Table S3.
4.5. RNA Extraction and cDNA Synthesis
Mice described above were sacrificed and the following tissues were immediately frozen with liquid nitrogen after excision: GC muscle, tongue, and bladder. Total RNA was isolated using TRI Reagent (T9424, Sigma-Aldrich, St. Louis, MO, USA) kit according to the manufacturer’s instructions, with slight modifications as described before [71]. Briefly, the tissues were added to tubes containing 1 mL of TRI Reagent and 5 × 2.8 mm stainless steel beads (D1033-28, Benchmark Scientific, Sayreville, NJ, USA). Subsequently, samples were homogenized for 10 cycles of 5 min using BeadBug microtube homogenizer (D1030-E, Daniel Biotech, Rehovot, Israel), subjected to centrifugation (12,000× g, 10 min, 4 °C) to pellet cell debris, and the supernatants were transferred to new tubes. All samples were processed as per the manufacturer’s protocol supplied with the TRI Reagent. The quantity and quality of RNA were analyzed by Nanodrop (ND-1000UV-Vis spectrophotometer, NanoDrop Technologies, Thermo Fisher Scientific, Waltham, MA, USA) [71]. Total RNA (1μg/sample) was subjected to reverse transcription with the qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD, USA).
4.6. Quantitative Real-Time PCR
Real-time PCR was performed using PerfeCTa SYBR Green FastMix, Low ROX (Quanta Biosciences, Gaithersburg, MD, USA) and QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA), employing the default thermocycler program for all genes. RNA expression levels of each gene in each tissue were normalized to Hprt [37]. Primer pairs were designed using the IDT SciTools [72] and synthesized by Sigma (Sigma-Aldrich, St. Louis, MO, USA) (Table S1). Real-time PCR reactions were carried out in a total volume of 10μL in a 384-well plate (Applied Biosystems, Foster, CA, USA), containing Quanta PerfeCTa SYBR X2 and 300/350nM of each sense and antisense primers. All real-time PCR reactions were carried out in duplicates/triplicates. The relative expression of each gene in each sample was calculated with the 2−ΔCT method [43,71], with ΔCT = CT (a target gene)−CT (the reference gene, i.e., Hprt) [73].
Biological and technical replicates were used for gene expression analysis as detailed in the Figure legends.
5. Conclusions
Despite the obvious study limitation of reliance upon subtle, albeit significant, gene expression changes, our results suggest a connection between Adnp and muscle regulation, during development and aging, including a potential involvement with the acto-myosin muscle system (Myl2 and Myl9), energy metabolism (Nmnat1), and language acquisition Foxp1/Foxp2 tongue expression. Our discovery of the extensive sexual dichotomy paves the path to better experimental design, looking separately at males and females both in basic research and most importantly in clinical studies. Our studies further suggest biomarkers for Adnp’s crucial activities regarding muscle function such as gait regulation and suggest a feedback mechanism with the ADNP regulator PACAP, with Adnp regulating bladder PAC1.
Acknowledgments
We thank Yoav Benjamini, mentor of IJ for excellent collaborations. We thank Gal Hacohen-Kleiman for the help with Adnp+/− mouse colony and Lior Bikovski, head of the Myers Neuro-Behavioral Core Facility. Gratitude also goes to Noy Amram, Irena Voinsky, Shahar Laks, Adi Linevitz Ovsiovich and Adi Zaslavsky for technical and editorial valuable help.
Abbreviations
| ADNP | Activity-dependent neuroprotective protein |
| ALS | Amyotrophic lateral sclerosis |
| BMD | Becker muscular dystrophy |
| DMD | Duchenne muscular dystrophy |
| EB’s | Microtubule end-binding proteins |
| GC | Gastrocnemius |
| LC3B | Microtubule associated protein 1 light chain 3B |
| mRNA | Messenger ribonucleic acid |
| NAP | NAPVSIPQ |
| NMNAT1 | Nicotinamide nucleotide adenylyl (NAD) transferase 1 |
| PACAP | Pituitary adenylate cyclase activating polypeptide |
| PAC1 | Pituitary adenylate cyclase-activating polypeptide type I receptor |
| qPCR | Quantitative real time PCR |
| SBMA | Spinobulbar muscular atrophy |
| VIP | Vasoactive intestinal peptide |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/18/6715/s1.
Author Contributions
O.K. provided input in establishment of research hypothesis and project design, she performed experiments, and provided impact to data analysis and article writing. S.S. supplied important input into project design, provided tissues from Adnp+/− mice and contributed to CatWalk gait analysis performance. I.J. performed extensive statistical analyses. A.H. contributed to statistical and graphical preparations. E.G. supported animal daily treatment, mouse sacrifice and tissue excision. I.G. led and orchestrated the entire project, provided funding, designed experiments, and wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the following grants (IG): European Research Area Network (ERA-NET) Neuron ADNPinMED, the US–Israel Binational Science Foundation—US National Science Foundation (BSF-NSF 2016746), the Alberto Moscona Nisim (AMN) Foundation for the Advancement of Science, Art and Culture in Israel, as well as by Ronith and Armand Stemmer and Arthur Gerbi (French Friends of Tel Aviv University). I.G. is the former first incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors. O.K. is and S.S. was supported by the Israeli BioInnovators Fellowship and Mentors by Teva. SS is a former Levi Eshkol fellow, supported by the Israel Ministry of Science and Technology, the Tel Aviv University GRTF and The Naomi Foundation, as well as The Eldee Foundation/Bloomfield Family of Montreal awards for student exchange (Tel Aviv University/McGill University). This work is in partial fulfilment of O.K. Ph.D. thesis requirements at the Miriam and Sheldon G. Adelson Graduate School of Medicine, Sackler Faculty of Medicine, Tel Aviv University.
Conflicts of Interest
The authors declare no conflict of interest. Professor Illana Gozes is the Chief Scientific Officer of Coronis Neurosciences. NAP (CP201) use is under patent protection (US patent nos. US7960334, US8618043, and USWO2017130190A1). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Bassan M., Zamostiano R., Davidson A., Pinhasov A., Giladi E., Perl O., Bassan H., Blat C., Gibney G., Glazner G., et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- 2.Zamostiano R., Pinhasov A., Gelber E., Steingart R.A., Seroussi E., Giladi E., Bassan M., Wollman Y., Eyre H.J., Mulley J.C., et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- 3.Sragovich S., Ziv Y., Vaisvaser S., Shomron N., Hendler T., Gozes I. The autism-mutated ADNP plays a key role in stress response. Transl. Psychiatry. 2019;9:235. doi: 10.1038/s41398-019-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamachi T., Tsuchida M., Kagami N., Yofu S., Wada Y., Hori M., Tsuchikawa D., Yoshikawa A., Imai N., Nakamura K., et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J. Mol. Neurosci. 2012;48:518–525. doi: 10.1007/s12031-012-9819-0. [DOI] [PubMed] [Google Scholar]
- 5.Castorina A., Giunta S., Scuderi S., D’Agata V. Involvement of PACAP/ADNP signaling in the resistance to cell death in malignant peripheral nerve sheath tumor (MPNST) cells. J. Mol. Neurosci. 2012;48:674–683. doi: 10.1007/s12031-012-9755-z. [DOI] [PubMed] [Google Scholar]
- 6.Nakamachi T., Ohtaki H., Yofu S., Dohi K., Watanabe J., Hayashi D., Matsuno R., Nonaka N., Itabashi K., Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul. Pept. 2008;145:88–95. doi: 10.1016/j.regpep.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Nakamachi T., Li M., Shioda S., Arimura A. Signaling involved in pituitary adenylate cyclase-activating polypeptide-stimulated ADNP expression. Peptides. 2006;27:1859–1894. doi: 10.1016/j.peptides.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Li M., David C., Kikuta T., Somogyvari-Vigh A., Arimura A. Signaling cascades involved in neuroprotection by subpicomolar pituitary adenylate cyclase-activating polypeptide 38. J. Mol. Neurosci. 2005;27:91–105. doi: 10.1385/JMN:27:1:091. [DOI] [PubMed] [Google Scholar]
- 9.Zusev M., Gozes I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul. Pept. 2004;123:33–41. doi: 10.1016/j.regpep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Kaaij L.J.T., Mohn F., Van der Weide R.H., De Wit E., Buhler M. The ChAHP Complex Counteracts Chromatin Looping at CTCF Sites that Emerged from SINE Expansions in Mouse. Cell. 2019;178:1437–1451. doi: 10.1016/j.cell.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Mandel S., Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J. Biol. Chem. 2007;282:34448–34456.e14. doi: 10.1074/jbc.M704756200. [DOI] [PubMed] [Google Scholar]
- 12.Amram N., Hacohen-Kleiman G., Sragovich S., Malishkevich A., Katz J., Touloumi O., Lagoudaki R., Grigoriadis N.C., Giladi E., Yeheskel A., et al. Sexual divergence in microtubule function: The novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry. 2016;21:1467–1476. doi: 10.1038/mp.2015.208. [DOI] [PubMed] [Google Scholar]
- 13.Hacohen-Kleiman G., Sragovich S., Karmon G., Gao A.Y.L., Grigg I., Pasmanik-Chor M., Le A., Korenkova V., McKinney R.A., Gozes I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Investig. 2018;128:4956–4969. doi: 10.1172/JCI98199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gozes I., Van Dijck A., Hacohen-Kleiman G., Grigg I., Karmon G., Giladi E., Eger M., Gabet Y., Pasmanik-Chor M., Cappuyns E., et al. Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Transl. Psychiatry. 2017;7:e1043. doi: 10.1038/tp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grigg I., Ivashko-Pachima Y., Hait T.A., Korenkova V., Touloumi O., Lagoudaki R., Van Dijck A., Marusic Z., Anicic M., Vukovic J., et al. Tauopathy in the young autistic brain: Novel biomarker and therapeutic target. Transl. Psychiatry. 2020;10:228. doi: 10.1038/s41398-020-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulih-Shultzman I., Pinhasov A., Mandel S., Grigoriadis N., Touloumi O., Pittel Z., Gozes I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- 17.Van Dijck A., Vulto-van Silfhout A.T., Cappuyns E., Van der Werf I.M., Mancini G.M., Tzschach A., Bernier R., Gozes I., Eichler E.E., Romano C., et al. Clinical Presentation of a Complex Neurodevelopmental Disorder Caused by Mutations in ADNP. Biol. Psychiatry. 2019;85:287–297. doi: 10.1016/j.biopsych.2018.02.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozes I., Patterson M.C., Van Dijck A., Kooy R.F., Peeden J.N., Eichenberger J.A., Zawacki-Downing A., Bedrosian-Sermone S. The Eight and a Half Year Journey of Undiagnosed AD: Gene Sequencing and Funding of Advanced Genetic Testing Has Led to Hope and New Beginnings. Front. Endocrinol. 2017;8:107. doi: 10.3389/fendo.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapitansky O., Gozes I. ADNP differentially interact with genes/proteins in correlation with aging: A novel marker for muscle aging. Geroscience. 2019;41:321–340. doi: 10.1007/s11357-019-00079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Guia R.M., Agerholm M., Nielsen T.S., Consitt L.A., Sogaard D., Helge J.W., Larsen S., Brandauer J., Houmard J.A., Treebak J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol. Rep. 2019;7:e14139. doi: 10.14814/phy2.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandel S., Spivak-Pohis I., Gozes I. ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J. Mol. Neurosci. 2008;35:127–141. doi: 10.1007/s12031-007-9013-y. [DOI] [PubMed] [Google Scholar]
- 22.Oz S., Kapitansky O., Ivashco-Pachima Y., Malishkevich A., Giladi E., Skalka N., Rosin-Arbesfeld R., Mittelman L., Segev O., Hirsch J.A. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry. 2014;19:1115–1124. doi: 10.1038/mp.2014.97. [DOI] [PubMed] [Google Scholar]
- 23.Ivashko-Pachima Y., Sayas C.L., Malishkevich A., Gozes I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: A novel avenue for protection against tauopathy. Mol. Psychiatry. 2017;22:1335–1344. doi: 10.1038/mp.2016.255. [DOI] [PubMed] [Google Scholar]
- 24.Ivashko-Pachima Y., Maor-Nof M., Gozes I. NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies. PLoS ONE. 2019;14:e0213666. doi: 10.1371/journal.pone.0213666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger R.C., Taylor W., Glucksberg M.R., Dean D.A. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006;13:725–731. doi: 10.1038/sj.gt.3302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esteves A.R., Gozes I., Cardoso S.M. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson’s disease. Biochim. Biophys. Acta. 2014;1842:7–21. doi: 10.1016/j.bbadis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Merenlender-Wagner A., Malishkevich A., Shemer Z., Udawela M., Gibbons A., Scarr E., Dean B., Levine J., Agam G., Gozes I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry. 2015;20:126–132. doi: 10.1038/mp.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yiu E.M., Kornberg A.J. Duchenne muscular dystrophy. J. Paediatr. Child Health. 2015;51:759–764. doi: 10.1111/jpc.12868. [DOI] [PubMed] [Google Scholar]
- 29.Belanto J.J., Mader T.L., Eckhoff M.D., Strandjord D.M., Banks G.B., Gardner M.K., Lowe D.A., Ervasti J.M. Microtubule binding distinguishes dystrophin from utrophin. Proc. Natl. Acad. Sci. USA. 2014;111:5723–5728. doi: 10.1073/pnas.1323842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capitanio D., Moriggi M., Torretta E., Barbacini P., De Palma S., Vigano A., Lochmuller H., Muntoni F., Ferlini A., Mora M., et al. Comparative proteomic analyses of Duchenne muscular dystrophy and Becker muscular dystrophy muscles: Changes contributing to preserve muscle function in Becker muscular dystrophy patients. J. Cachexia Sarcopenia Muscle. 2020;11:547–563. doi: 10.1002/jcsm.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Call J.A., Nichenko A.S. Autophagy: An essential but limited cellular process for timely skeletal muscle recovery from injury. Autophagy. 2020;16:1344–1347. doi: 10.1080/15548627.2020.1753000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews J.G., Wahl R.A. Duchenne and Becker muscular dystrophy in adolescents: Current perspectives. Adolesc. Health Med. Ther. 2018;9:53–63. doi: 10.2147/AHMT.S125739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catalani E., Bongiorni S., Taddei A.R., Mezzetti M., Silvestri F., Coazzoli M., Zecchini S., Giovarelli M., Perrotta C., De Palma C., et al. Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy. Cell Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savarese M., Sarparanta J., Vihola A., Udd B., Hackman P. Increasing Role of Titin Mutations in Neuromuscular Disorders. J. Neuromuscul. Dis. 2016;3:293–308. doi: 10.3233/JND-160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polanco M.J., Parodi S., Piol D., Stack C., Chivet M., Contestabile A., Miranda H.C., Lievens P.M., Espinoza S., Jochum T., et al. Adenylyl cyclase activating polypeptide reduces phosphorylation and toxicity of the polyglutamine-expanded androgen receptor in spinobulbar muscular atrophy. Sci. Transl. Med. 2016;8:370ra181. doi: 10.1126/scitranslmed.aaf9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouroukhin Y., Ostritsky R., Assaf Y., Pelled G., Giladi E., Gozes I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: Protection against impairments in axonal transport. Neurobiol. Dis. 2013;56:79–94. doi: 10.1016/j.nbd.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Zhao H., Ni J., Pan J., Hua H., Wang Y. Identification of suitable reference genes for gene expression studies in rat skeletal muscle following sciatic nerve crush injury. Mol. Med. Rep. 2019;19:4377–4387. doi: 10.3892/mmr.2019.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballero-Garrido E., Pena-Philippides J.C., Galochkina Z., Erhardt E., Roitbak T. Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system. Behav. Brain Res. 2017;331:282–296. doi: 10.1016/j.bbr.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoes G.F., Benitez S.U., Oliveira A.L. Granulocyte colony-stimulating factor (G-CSF) positive effects on muscle fiber degeneration and gait recovery after nerve lesion in MDX mice. Brain Behav. 2014;4:738–753. doi: 10.1002/brb3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamers F.P., Koopmans G.C., Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 41.Deumens R., Jaken R.J., Marcus M.A., Joosten E.A. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J. Neurosci. Methods. 2007;164:120–130. doi: 10.1016/j.jneumeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Dresner E., Agam G., Gozes I. Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: Deregulation in schizophrenia. Eur. Neuropsychopharmacol. 2011;21:355–361. doi: 10.1016/j.euroneuro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Malishkevich A., Marshall G.A., Schultz A.P., Sperling R.A., Aharon-Peretz J., Gozes I. Blood-Borne Activity-Dependent Neuroprotective Protein (ADNP) is Correlated with Premorbid Intelligence, Clinical Stage, and Alzheimer’s Disease Biomarkers. J. Alzheimers Dis. 2016;50:249–260. doi: 10.3233/JAD-150799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kushnir M., Dresner E., Mandel S., Gozes I. Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J. Neurochem. 2008;105:537–545. doi: 10.1111/j.1471-4159.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 45.Dresner E., Malishkevich A., Arviv C., Leibman B.S., Alon S., Ofir R., Gothilf Y., Gozes I. Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J. Biol. Chem. 2012;287:40173–40185. doi: 10.1074/jbc.M112.387027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He X., Zhao P., Huang Y., Cai X., Bi B., Lin J. [Genome-wide copy number microarray analysis for a boy with autism] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2019;36:157–160. doi: 10.3760/cma.j.issn.1003-9406.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Cai T., Wu B., Tang X., Zhou Z., Yang J., Ke R., Mu X. iTRAQ-Based Proteomic Analysis reveals possible target-related proteins and signal networks in human osteoblasts overexpressing FGFR2. Proteome Sci. 2018;16:12. doi: 10.1186/s12953-018-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girard B.M., Campbell S.E., Beca K.I., Perkins M., Hsiang H., May V., Vizzard M.A. Intrabladder PAC1 Receptor Antagonist, PACAP(6–38), Reduces Urinary Bladder Frequency and Pelvic Sensitivity in Mice Exposed to Repeated Variate Stress (RVS) J. Mol. Neurosci. 2020 doi: 10.1007/s12031-020-01649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnett A.B., Rhoads C.L., Hoekzema K., Turner T.N., Gerdts J., Wallace A.S., Bedrosian-Sermone S., Eichler E.E., Bernier R.A. The autism spectrum phenotype in ADNP syndrome. Autism Res. 2018;11:1300–1310. doi: 10.1002/aur.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delwig A., Majumdar S., Ahern K., LaVail M.M., Edwards R., Hnasko T.S., Copenhagen D.R. Glutamatergic neurotransmission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS ONE. 2013;8:e83974. doi: 10.1371/journal.pone.0083974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manivannan S.N., Darouich S., Masmoudi A., Gordon D., Zender G., Han Z., Fitzgerald-Butt S., White P., McBride K.L., Kharrat M. Novel frameshift variant in MYL2 reveals molecular differences between dominant and recessive forms of hypertrophic cardiomyopathy. PLoS Genet. 2020;16:e1008639. doi: 10.1371/journal.pgen.1008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno C.A., Sobreira N., Pugh E., Zhang P., Steel G., Torres F.R., Cavalcanti D.P. Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur. J. Hum. Genet. 2018;26:669–675. doi: 10.1038/s41431-017-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Xie S., Qian L., Cai C., Bi H., Cui W. Identification of genes related to skeletal muscle growth and development by integrated analysis of transcriptome and proteome in myostatin-edited Meishan pigs. J. Proteom. 2020;213:103628. doi: 10.1016/j.jprot.2019.103628. [DOI] [PubMed] [Google Scholar]
- 54.Gamboa H.E., Sood M. Pediatric Intestinal Pseudo-obstruction in the Era of Genetic Sequencing. Curr. Gastroenterol. Rep. 2019;21:70. doi: 10.1007/s11894-019-0737-y. [DOI] [PubMed] [Google Scholar]
- 55.Tayyeb M., Tadi P. Neurogenic Bladder. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 1 September 2020)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560617/ [Google Scholar]
- 56.Verstegen A.M.J., Vanderhorst V., Gray P.A., Zeidel M.L., Geerling J.C. Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J. Comp. Neurol. 2017;525:2287–2309. doi: 10.1002/cne.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsson M., Abbott B.W. Is the Capacity for Vocal Learning in Vertebrates Rooted in Fish Schooling Behavior? Evol. Biol. 2018;45:359–373. doi: 10.1007/s11692-018-9457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frohlich H., Rafiullah R., Schmitt N., Abele S., Rappold G.A. Foxp1 expression is essential for sex-specific murine neonatal ultrasonic vocalization. Hum. Mol. Genet. 2017;26:1511–1521. doi: 10.1093/hmg/ddx055. [DOI] [PubMed] [Google Scholar]
- 59.Malishkevich A., Amram N., Hacohen-Kleiman G., Magen I., Giladi E., Gozes I. Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Transl. Psychiatry. 2015;5:e501. doi: 10.1038/tp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satterstrom F.K., Kosmicki J.A., Wang J., Breen M.S., De Rubeis S., An J.Y., Peng M., Collins R., Grove J., Klei L., et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell. 2020;180:568–584 e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S.W., Won J.Y., Yang J., Lee J., Kim S.Y., Lee E.J., Kim H.S. AKAP6 inhibition impairs myoblast differentiation and muscle regeneration: Positive loop between AKAP6 and myogenin. Sci. Rep. 2015;5:16523. doi: 10.1038/srep16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinhasov A., Mandel S., Torchinsky A., Giladi E., Pittel Z., Goldsweig A.M., Servoss S.J., Brenneman D.E., Gozes I. Activity-dependent neuroprotective protein: A novel gene essential for brain formation. Brain Res. Dev. Brain Res. 2003;144:83–90. doi: 10.1016/S0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 63.Mollinedo P., Kapitansky O., Gonzalez-Lamuno D., Zaslavsky A., Real P., Gozes I., Gandarillas A., Fernandez-Luna J.L. Cellular and animal models of skin alterations in the autism-related ADNP syndrome. Sci. Rep. 2019;9:736. doi: 10.1038/s41598-018-36859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dangoor D., Biondi B., Gobbo M., Vachutinski Y., Fridkin M., Gozes I., Rocchi R. Novel glycosylated VIP analogs: Synthesis, biological activity, and metabolic stability. J. Pept. Sci. 2008;14:321–328. doi: 10.1002/psc.932. [DOI] [PubMed] [Google Scholar]
- 65.Alcalay R.N., Giladi E., Pick C.G., Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 2004;361:128–131. doi: 10.1016/j.neulet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Morimoto B.H., Fox A.W., Stewart A.J., Gold M. Davunetide: A review of safety and efficacy data with a focus on neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2013;6:483–502. doi: 10.1586/17512433.2013.827403. [DOI] [PubMed] [Google Scholar]
- 67.Magen I., Ostritsky R., Richter F., Zhu C., Fleming S.M., Lemesre V., Stewart A.J., Morimoto B.H., Gozes I., Chesselet M.F. Intranasal NAP (davunetide) decreases tau hyperphosphorylation and moderately improves behavioral deficits in mice overexpressing alpha-synuclein. Pharmacol. Res. Perspect. 2014;2:e00065. doi: 10.1002/prp2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sragovich S., Malishkevich A., Piontkewitz Y., Giladi E., Touloumi O., Lagoudaki R., Grigoriadis N., Gozes I. The autism/neuroprotection-linked ADNP/NAP regulate the excitatory glutamatergic synapse. Transl. Psychiatry. 2019;9:2. doi: 10.1038/s41398-018-0357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kyriakou E.I., Van der Kieft J.G., De Heer R.C., Spink A., Nguyen H.P., Homberg J.R., Van der Harst J.E. Automated quantitative analysis to assess motor function in different rat models of impaired coordination and ataxia. J. Neurosci. Methods. 2016;268:171–181. doi: 10.1016/j.jneumeth.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Liang Y., Zhang J., Walczak P., Bulte J.W.M. Quantification of motor neuron loss and muscular atrophy in ricin-induced focal nerve injury. J. Neurosci Methods. 2018;308:142–150. doi: 10.1016/j.jneumeth.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hacohen K.G., Barnea A., Gozes I. ADNP: A major autism mutated gene is differentially distributed (age and gender) in the songbird brain. Peptides. 2015;72:75–79. doi: 10.1016/j.peptides.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Idtdna. [(accessed on 16 September 2016)]; Available online: http://www.idtdna.com/SciTools/SciTools.aspx.
- 73.Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.