Abstract
Epigenetics has provided a new dimension to our understanding of nuclear factor erythroid 2–related factor 2/Kelch-like ECH-associated protein 1 (human NRF2/KEAP1 and murine Nrf2/Keap1) signaling. Unlike the genetic changes affecting DNA sequence, the reversible nature of epigenetic alterations provides an attractive avenue for cancer interception. Thus, targeting epigenetic mechanisms in the corresponding signaling networks represents an enticing strategy for therapeutic intervention with dietary phytochemicals acting at transcriptional, post-transcriptional, and post-translational levels. This regulation involves the interplay of histone modifications and DNA methylation states in the human NFE2L2/KEAP1 and murine Nfe2l2/Keap1 genes, acetylation of lysine residues in NRF2 and Nrf2, interaction with bromodomain and extraterminal domain (BET) acetyl “reader” proteins, and non-coding RNAs such as microRNA (miRNA) and long non-coding RNA (lncRNA). Phytochemicals documented to modulate NRF2 signaling act by reversing hypermethylated states in the CpG islands of NFE2L2 or Nfe2l2, via the inhibition of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), through the induction of ten-eleven translocation (TET) enzymes, or by inducing miRNA to target the 3′-UTR of the corresponding mRNA transcripts. To date, fewer than twenty phytochemicals have been reported as NRF2 epigenetic modifiers, including curcumin, sulforaphane, resveratrol, reserpine, and ursolic acid. This opens avenues for exploring additional dietary phytochemicals that regulate the human epigenome, and the potential for novel strategies to target NRF2 signaling with a view to beneficial interception of cancer and other chronic diseases.
Keywords: antioxidant response element, cancer interception, DNA methylation, histone acetylation, histone methylation, lncRNA, miRNA
1. Introduction
The NRF2 signaling axis has received widespread attention from the research community due to its critical role in responding to xenobiotic and electrophilic stress [1]. Binding of NRF2 to antioxidant response element (ARE) sequences in gene promoters activates antioxidant and xenobiotic detoxifying enzymes [2]. Activation of NRF2 by various dietary phytochemicals provides a promising strategy to prevent cancer, and the protective role of Nrf2/NRF2 activators has been verified in preclinical models and in human clinical trials [3,4].
However, inactivating mutations in KEAP1 that lead to constitutive NRF2 activation [5,6] can provide a growth advantage in some cancer cells. The yin/yang aspect of NRF2 signaling was first elaborated by Wang et al., reporting that knockdown of NRF2 using siRNA, or stable overexpression of KEAP1, sensitized human cancer cells to chemotherapy [7]. There is growing evidence linking elevated NRF2 expression with chemoresistance and poor prognosis in various cancer types [8,9].
In view of this functional duality, NRF2 has been discussed both as a “friend or foe” or a “double-edged sword” in cancer etiology [10,11]. However, the context and timing of NRF2 activation plays an important role in determining beneficial outcomes and highlights the critical need for a thorough mechanistic understanding of NRF2 signaling [12].
Regulation of NRF2 signaling occurs at the level of transcription, post-transcription, and protein stability. Although genetic alterations initially were reported in NFE2L2/KEAP1, more recently, epigenetic mechanisms have added a new dimension and an element of fine-tuning to the NRF2 signaling axis. Thus, the present review aims to provide an update on the various phytochemicals that regulate NRF2 via the “epigenetic trinity” of DNA methylation, histone modifications, and non-coding RNAs.
2. Multilayer Regulation of NRF2 Signaling
2.1. Transcriptional Regulation
A wide array of stimuli can activate NRF2 signaling, including oxidative, inflammatory, and metabolic stressors [13]. The NFE2L2 and Nfe2l2 genes are regulated at the transcriptional level by multiple transcription factors [14]. For example, the Aryl hydrocarbon receptor/Aryl hydrocarbon receptor nuclear translocator (AhR/Arnt) heterodimer can interact with a xenobiotic response element in the NFE2L2 promoter, leading to transactivation [15]. Also, in response to inflammatory stimuli, Nfe2l2 can be activated by nuclear factor kappa B (NF-κB) [16], whereas a c-Jun binding site in Nfe2l2 was implicated in oncogenic Nrf2 activation via K-ras, B-Raf, and c-Myc [17]. Notch signaling directly activated Nrf2 by recruiting Notch Intracellular Domain-recombination signal binding protein for the immunoglobulin kappa J region complex to a conserved site in the promoter of Nfe2l2, which promoted cytoprotective outcomes during liver development and hepatic stress responses [18]. Interestingly, Nrf2 also can regulate its own transcription by binding to ARE-like sequences in the Nfe2l2 promoter [19].
2.2. Post-Transcriptional Regulation
Modifications of the NFE2L2 mRNA transcript also play essential roles in the activation and correct functioning of NRF2. Post-transcriptional processing includes regulation by microRNAs (miRNAs), long non-coding RNAs (lncRNAs), adenosine methylation, and alternative splicing of the NFE2L2 transcript. Several miRNAs downregulate or upregulate NRF2 protein expression and activity by directly targeting 3′-UTR sequences of NFE2L2 or KEAP1 mRNA, respectively. Evidence also has accrued linking lncRNAs to NRF2 regulation, as discussed in Section 3.4.
Alternative splicing of NFE2L2 is another key mechanism regulating NRF2 activity. Some tumors express transcript variants of NFE2L2 that lack exons coding for the KEAP1 interacting domain, resulting in hyperactivation of the NRF2 pathway [20]. A recent study reported regulation of Nfe2l2 mRNA nuclear export and stabilization by two mRNA binding proteins, HuR and AUF1, targeting the 3′-UTR of the nascent transcript [21].
2.3. Regulation of NRF2 Protein Stability
Protein levels of NRF2 often are tightly regulated by proteasomal degradation complexes, in particular via the Cullin 3/RING-box protein 1 (Cul3/Rbx1)/Keap1 complex. KEAP1 acts as a linker protein between NRF2 and the Cul3/Rbx1-based ubiquitin ligase and causes continuous degradation under basal conditions, resulting in low constitutive NRF2 levels under physiological conditions. Regulation of NRF2 protein stability also is mediated by KEAP1-independent proteasomal degradation mechanisms, such as through the S-phase kinase-associated protein 1/Cullin/F-box (SCF)-β-transducin repeats-containing proteins (β-TrCP) complex, or HMG-CoA reductase degradation protein 1 (HRD1) [14]. Degradation of NRF2 via the SCF-β-TrCP complex is facilitated by phosphorylation of the Neh6 domain involving glycogen synthase kinase-3β (GSK-3β), which is recognized by β-TrCP ubiquitin ligase [22].
On the other hand, inhibitory phosphorylation of GSK-3β by extracellular signal-regulated kinase (ERK), p38 MAP kinase (MAPK), phosphoinositide 3-kinase (PI3K), protein kinase C (PKC), and protein kinase B/Akt kinase stabilizes and activates NRF2 [14]. 3-Hydroxy-3-methylglutaryl reductase degradation 1 (Hrd-1) is another ubiquitin ligase that facilitates proteasomal degradation of NRF2 [23]. The degradation complexes also can be influenced by post-translational modifications on NRF2. For example, PKC has been reported to phosphorylate NRF2 at Ser40, promoting its dissociation from KEAP1, thereby increasing NRF2 stability [24].
3. Epigenetic Mechanisms Regulating NRF2 Signaling
Mechanisms of NRF2 regulation discussed above can be influenced both by genetic and epigenetic alterations. Biallelic inactivation of KEAP1, resulting in NRF2 hyperactivation, was identified as a relatively common event in lung cancer [25]. Nuclear accumulation of NRF2 and low expression of KEAP1 correlated with tumor aggressiveness, although the expected phenotypic outcomes were not necessarily consistent in cases of somatic mutation in KEAP1 vs. NFE2L2 [26].
The discovery of KEAP1 promoter hypermethylation, leading to gene silencing in lung cancer [27], prompted widespread research on epigenetic regulation of the NRF2 signaling pathway. This topic has been covered in detail in several excellent review articles [28,29,30]. Prior to discussion of the various phytochemicals reported to act via epigenetic mechanisms, a brief description of the epigenetic regulation of NRF2 signaling is presented first.
3.1. DNA Methylation
Several CpG islands were identified in the promoters of NFE2L2 and Nfe2l2 [31]. Hypermethylation of these CpG sites markedly lowered NRF2 expression in prostate tumorigenesis [32]. As discussed above, KEAP1 promoter methylation was first reported in lung cancer, but similar observations have been linked to poor prognosis in glioma, breast cancer, non-small cell lung carcinoma, colorectal cancer, clear renal cell carcinoma, and pancreatic cancer [33,34,35,36,37,38].
3.2. Histone Modifications
In addition to DNA methylation, histone methylation and acetylation, among other changes, also plays a vital role in regulating gene expression. Methylation of histones occurs primarily on the basic amino acids lysine and arginine. Gene activation or repression depends on the amino acid that is methylated and the degree of methylation, i.e., monomethylation, dimethylation, or trimethylation. Enhancer of zeste homolog 2 (EZH2), which is a member of the Polycomb group of proteins that catalyze trimethylation of histone H3 lysine 27 (H3K27me3), was downregulated in lung cancer and associated with NFE2L2 gene silencing [39].
Another study showed that increased binding of transcription factor Specificity protein 1 (Sp1) on the KEAP1 promoter increased methylation of histone H3K4 by the histone methyltransferase (HMT) SET Domain Containing 7 (SETD7, Set7/9) [40]; thus, KEAP1 also is regulated by changes in histone methylation.
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) also are viewed as critical epigenetic “writers” and “erasers” that catalyze, respectively, the addition and removal of acetyl groups from histone and non-histone proteins. Acetylation of NRF2 at the Neh1 DNA binding domain by the HAT p300/CREB binding protein (CBP) is required for Nrf2-dependent gene transcription [41]. Similarly, p300/CBP was implicated in the regulation of subunit p65 in NF-κB-mediated NRF2 activation, and in recruiting HDAC3 to inhibit ARE-dependent gene transcription [42]. On the other hand, HDAC2 increased the stability of NRF2 protein by deacetylating lysine residues, thereby preventing NRF2 protein degradation [43].
3.3. Epigenetic “Readers”
In addition to the “writers” and “erasers”, epigenetic “reader” proteins are gaining attention in the context of Nrf2 signaling. Bromodomain and extraterminal domain (BET) proteins interact with acetylated lysine residues in histones, regulating genes such as MYC, but also with acetylated non-histone proteins such as Nrf2 to inhibit Nrf2-dependent signaling, in species as diverse as mammals and Drosophila (reviewed in [44]).
Evidence linked upregulation of KEAP1 expression with increased levels of the BET protein BRD4, and with concomitant downregulation of NRF2 in prostate cancer cell lines, where integration of RNA-seq data with chromatin immunoprecipitation (ChIP) assays correlated NRF2-dependent gene expression with KEAP1 among the top genes interacting with BRD4 [45].
3.4. Regulation by Non-Coding RNAs
Non-coding RNAs linked to NRF2 signaling include miRNAs, which contain ~22 nucleotides, and lncRNAs, which are greater than 200 nucleotides in length. Other aspects of non-coding RNA biology will not be discussed here in detail, although they represent interesting avenues for future research. For example, overexpression of small nucleolar RNA ACA11 was linked to a hyper-proliferative phenotype, reactive oxygen species generation, and NRF2 nuclear import in multiple myeloma [46]. Post-transcriptional changes to RNAs, such as 6-methyladenosine (m6A) modification of NFE2L2 mRNA, also warrant further investigation [47].
The contribution of miRNAs to the regulation of NRF2 signaling is widely recognized. For example, downregulation by targeting the 3′untranslated region (3′UTR) “seed” sequences in NFE2L2 mRNA has been reported for miR-153, miR142-5p, mi-R27a, miR-144, miR34a, and miR-93 (reviewed in [48,49]). On the other hand, an increase in NRF2 expression and activity was observed by miRNAs targeting the 3′UTR of KEAP1 mRNA. The first miRNA reported to regulate KEAP1 in this manner was miR-200a [50], followed by other examples, such as miR-455-3p, miR-141, miR-7, and miR-432-3p [51,52,53,54].
Several lncRNAs have been identified with regulatory roles in NRF2 activation. These lncRNAs include: (i) UCA1, MEG3, and NRAL acting as competing endogenous RNAs (ceRNA) for mRNA by “sponging” the respective miRNAs, (ii) HOTAIR increasing histone H4 acetylation at the NFE2L2 promoter, (iii) MALAT1 negatively regulating KEAP1, (iv) TUG1 interacting directly with the NRF2 protein, and (v) NMRAL2P serving as a novel functional pseudogene both upstream and downstream of NRF2 (reviewed in [12]).
4. Phytochemicals and the Epigenetic Regulation of NRF2 Signaling
The role of dietary phytochemicals in cancer prevention and interception is well documented, and NRF2 signaling is considered a major target for many bioactive natural compounds [55,56,57]. Such agents affect the expression and activities of downstream target genes of NRF2, and the crosstalk with other signaling pathways, not only in cancer but also in other chronic diseases. However, the impact of phytochemicals on epigenetic mechanisms regulating NRF2 is an emerging area. In this section, we provide an update on the current state of knowledge regarding epigenetic regulation of NRF2 signaling by specific phytochemicals.
4.1. 3,3′-Diindolylmethane (DIM)
Indole-3-carbinol (I3C) is derived from the breakdown of glucobrassicin in cruciferous vegetables such as cabbage, cauliflower and broccoli [57]. Under low pH conditions in the stomach, I3C forms oligomers [58,59], including the dimer 3,3′-diindolylmethane (DIM), which have been investigated for cancer preventive and therapeutic activities [60,61,62,63,64,65,66,67,68,69,70,71,72], including in human [73,74,75,76,77,78].
In a study designed to assess epigenetic regulation of Nrf2, DIM increased Nfe2l2 mRNA in transgenic adenocarcinoma mouse prostate (TRAMP)-C1 prostate cancer cells by reversing the methylation status of the first five CpGs in the Nfe2l2 promoter. DIM inhibited the mRNA and protein expression of Dnmt1, Dnmt3a, and Dnmt3b, and well as Hdac2 and Hdac3 in vitro. In vivo, DIM reduced 5-methylcytosine immunostaining in prostate cancer tissues of the TRAMP mouse, which mirrors the pathogenesis of human prostate cancer, and DIM-supplemented diet lowered the incidence of palpable tumors and lymph node metastasis compared to controls [79] (Table 1).
Table 1.
List of phytochemicals reported to regulate NRF2 signaling epigenetically.
| Sl No. | Phytochemical | Chemical Name | Epigenetic Mechanism of Nrf2 Regulation | Molecular Targets | Cell Type | Reference |
|---|---|---|---|---|---|---|
| 1. | 3,3′-diindolylmethane | 3,3′-Methylenebis(1H-indole) | Decreased methylation of CpG sites in the promoter region of mouse Nfe2l2 | Suppressed mRNA and protein expression of Dnmt1, Dnmt3a, and Dnmt3b; inhibited protein expression of Hdac2 and Hdac3 | TRAMP-C1 prostate cells | [79] |
| 2. | Apigenin | 5,7-Dihydroxy-2-(4hydroxy-phenyl)-4H-chromen-4-one | Decreased Nfe2l2 hyper-methylation; induced expression of miR-101, targeting Nfe2l2 mRNA | Inhibited Dnmt1, Dnmt3a and Dnmt3b; inhibited Hdacs; induced miR101 | Mouse epidermal JB6 P+ cells BEL-7402/ADM cells | [80,81] |
| 3. | Corosolic acid | 2α,3β-2,3-dihydroxyurs-12-en-28-oic acid | Decreased Nfe2l2 hypermethylation; increased histone H3 lysine 27 acetylation; decreased H3 lysine 27 trimethylation | Decreased levels of Dnmt1, Dnmt3a and Dnmt3b; reduced levels of Hdac1, Hdac2, Hdac3, Hdac4, Hdac7 and Hdac8 | TRAMP-C1 prostate cells | [82] |
| 4. | Curcumin | 1, 7-bis (4-hydroxy-3-methoxy-phenyl)-1, 6 heptadiene-3, 5-dione | Decreased Nfe2l2 hypermethylation | Inhibited enzymatic activity of Dnmt enzymes | TRAMP-C1 prostate cells | [83] |
| 5. | Delphinidin | 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl) chromenium | Demethylation of 15 CpG sites in the mouse Nfe2l2 promoter region | Decreased protein expression of Dnmt1, Dnmt3a, and class I/II Hdacs | Mouse epidermal JB6 P+ cells | [84] |
| 6. | Fucoxanthin | 3,5′-Dihydroxy-8-oxo-6′,7′-didehydro-5,5′,6,6′,7,8-hexahydro-5,6-epoxy-β,β-caroten-3′-yl acetate | Decreased Nfe2l2 hypermethylation | Reduced Dnmt activity | Mouse epidermal JB6 P+ cells | [85] |
| 7. | Luteolin | 2-(3,4dihydroxyphenyl)-5,7-dihydroxy-chromen-4-one | Decreased NFE2L2 hypermethylation | Decreased expression of DNMT1, DNMT3A and DNMT3B; decreased HDAC1, HDAC2, HDAC3, HDAC6, HDAC7; reduced activities of DNMTs and HDACs; increased ten-eleven translocation 1, 2 and 3 (TET1, TET2, and TET3) | Human colon cancer cells and SNU-407 cells | [86,87] |
| 8. | Pelargonidin | 3,5,7-Trihydroxy-2-(4hydroxyphenyl) chromenium | Decreased methylated CpGs in Nfe2l2 promoter | Decreased Dnmt1 and Dnmt3b expression; reduced levels of Hdacs 1–4 and Hdac7 | JB6 P+ cells | [88] |
| 9. | Polydatin | 3-Hydroxy-5-[(E)-2-(4-hydroxyphenyl)vinyl] phenyl β-d-glucopyranoside | Enhanced miR-200a targeting KEAP1 to activate NRF2 signaling | Increased miR-200a expression under high fructose induction; downregulated KEAP1 mRNA and protein | Buffalo rat liver (BRL-3A) and human HepG2 cells | [89] |
| 10. | Reserpine | Methyl 18β-hydroxy-11,17α-dimethoxy-3β,20α-yohimban-16βcarboxylate 3,4,5-trimethoxybenzoate | Decreased proportion of methylated CpG sites in the Nfe2l2 promoter | Concentration-dependent decreased mRNA and protein expression of Dnmt1, Dnmt3a, and Dnmt3b | JB6 P+ Cell | [90] |
| 11. | Resveratrol | 3,4′,5-trihydroxystilbene | Decreased methylation of the NFE2L2 promoter | Inhibited expression and activity of DNMT1, DNMT3a, and DNMT3b; miR93 implicated | HepG2 cells and estradiol-induced breast cancer | [91,92] |
| 12. | Sulforaphane | 1-Isothiocyanato-4-(methanesulfinyl)butane | CpG demethylation and histone acetylation at the Nfe2l2 promoter; lncRNA upregulation | Inhibition of Dnmt1, Dnmt3a, Dnmt3b, Hdacs 1–5, and Hdac7; upregulated functional pseudogene NMRAL2P | JB6 P+ cells; TRAMP C1 cells; human colon cancer cells | [93,94] |
| 13. | Tanshinone IIA | 1,6,6-trimethyl-8,9- dihydro-7H-naphtho [1,2-g] benzofuran-10,11-dione | Decreased methylated CpGs in Nfe2l2 promoter; increased recruitment of RNA polymerase complex II at the NFE2L2 transcription start site | Decreased mRNA and protein levels of HDAC1, HDAC3, and HDAC8, as well as DNMT1, DNMT3a, and DNMT3b; induced expression of TET2 | JB6 P+ cells, human normal hepatocyte and Hepa RG cells; rifampicin-induced liver injury in mice | [95,96] |
| 14. | Taxifolin | (2S,3S)-2-(3,4dihydroxy-phenyl)-3,5,7-trihydroxy-2,3dihydro-4H-chromen-4-one | Decreased proportion of methylated CpGs in the Nfe2l2 promoter | Reduced protein levels of Dnmt1, Dnmt3a and Dnmt3b as well as Hdacs 1, 3, 4, and 8 | JB6 P+ cells | [97] |
| 15. | Ursolic acid | (3β)-3-Hydroxyurs-12-en-28-oic acid | Nfe2l2 mouse promoter demethylation; increased acetylation and K4 monomethylation of histone H3 in human cells | Reduced DNMT1 and DNMT3a protein levels; inhibited expression of HDACs 1-3 and 8 (Class I) and HDAC 6 and 7 (Class II); induced Setd7 | JB6 P+ cells PC3 and LnCaP cells | [98,99] |
| 16. | γ Tocopherol–rich mixture of tocopherols (γ-TmT) | (2R)-2,5,7,8-tetramethyl-2-[ (4R,8R)-4,8,12-trimethyl-tridecyl]-6-chromanol | Reversed hyper-methylation in the Nfe2l2 promoter | Inhibited protein levels of Dnmt1, Dnmt3a, and Dnmt3b | Prostate tissues of C57BL/TGN TRAMP mice | [100] |
| 17. | Z-Ligustilide | (3E)-3-butylidene-4,5-dihydro-2-benzofuran-1-one | Decreased methylation of the first five CpGs of the Nfe2l2 promoter | Inhibited Dnmt activity | TRAMP C1 cells | [101] |
4.2. Apigenin
Apigenin is a natural flavonoid derived from chamomile flowers, oranges, parsley, celery, and other natural sources, with potential antioxidant, anti-inflammatory, and anticancer properties [102]. Anticancer properties of apigenin were exhibited in many types of malignancy, and some were linked to epigenetic mechanisms [80,81,103,104]. One study showed restoration of Nrf2 expression and activity in the murine preneoplastic epidermal JB6 P+ cell line by decreasing the methylation status of the Nfe2l2 promoter, and by inhibiting the expression of Dnmts and Hdacs [105].
Contrary to this induction of Nrf2 activity, another investigation with apigenin reported increased miR-101 levels targeting the 3′UTR of NFE2L2 mRNA, with enhanced chemosensitivity of doxorubicin-resistant human hepatocellular carcinoma BEL-7402/ADM cells [106]. These findings point to possible species-specific differential methylation signatures for the corresponding target gene(s) in mouse epidermal vs. human hepatoma cells that have become drug-resistant.
4.3. Corosolic Acid
Corosolic acid is found in medical herbs, such as Lagerstroemia speciose, Eriobotrta japonica, and Tiarella polyphylla [107], and has gained attention for its beneficial effects in the prevention or treatment of metabolic disease, including diabetes. This triterpenoid also is reported to possess anticancer activity, and one study demonstrated effects on Nrf2 via epigenetic modifications. Specifically, corosolic acid induced Nfe2l2 at the transcriptional level by decreasing CpG methylation in the corresponding promoter region of TRAMP-C1 cells. Increased histone H3 lysine 27 acetylation (H3K27ac) was also observed, as well as decreased trimethylation of H3K27 (H3K27Me3). Protein levels of Dnmts (Dnmt1, Dnmt3a and Dnmt3b) and Hdacs (Hdac1, Hdac2, Hdac3, Hdac4, Hdac7 and Hdac8) were inhibited in corosolic acid-treated TRAMP-C1 cells [82]. The study suggested that upregulation of Nrf2 was responsible for the inhibitory effects of corosolic acid on anchorage-independent growth of TRAMP-C1 cells, which was abrogated following Nrf2 knockdown [82].
4.4. Curcumin
Curcumin is the principal curcuminoid found in the rhizomes of turmeric (Curcuma longa), linked to anti-inflammatory, antioxidant, antitumor, and anti-diabetic effects. Curcumin is a known inducer of NRF2 and its downstream transcriptional targets, with emerging evidence for epigenetic regulation. Using bisulfite sequencing, Khor et al. [83] demonstrated that curcumin reversed the methylation status of the first five hypermethylated CpG islands in the Nfe2l2 promoter, thereby restoring epigenetically-silenced Nrf2 in TRAMP-C1 cells. Curcumin had negligible effects on DNA methyltransferases (Dnmts) at the RNA or protein level, but rather inhibited their enzymatic activity [83]. It was concluded that the epigenetic restoration of Nrf2 activity by curcumin might play a role in the prevention of prostate cancer in TRAMP-C1 mice [83].
4.5. Delphinidin
Delphinidin is an anthocyanidin flavonoid that contributes to the intense blue coloration in many fruits and vegetables, such as blackcurrant, eggplant, black grapes, red cabbage, and blackberries, and is abundant in pomegranate fruit extract [108,109]. Delphinidin is an anthocyanidin exhibiting potent antioxidant, anti-inflammatory, and antitumor properties. A study investigated the effects of delphinidin against skin cell neoplastic transformation by modulating the Nrf2 pathway. Delphinidin inhibited the neoplastic transformation of mouse epidermal JB6 P+ cells by 12-O-tetradecanoylphorbol-13-acetate (TPA). The anthocyanidin decreased the CpG methylation ratio at the Nfe2l2 promoter resulting in upregulated mRNA and protein expression levels of Nrf2 and its target genes. The study further demonstrated downregulation of Dnmts (Dnmt1a and Dnmt3a), and class I and II Hdacs linked to reactivation of the Nrf2 pathway in JB6 P+ cells [84].
4.6. Fucoxanthin
Fucoxanthin is a xanthophyll carotenoid abundantly available in seaweeds. Its unique chemical structure provides a variety of biological activities, and it has been ascribed health benefits against chronic diseases such as cancer, obesity, and diabetes [110]. This carotenoid was found to activate Nrf2 signaling by causing demethylation of CpG islands in the Nfe2l2 promoter, resulting in inhibition of TPA-induced transformation of JB6 P+ cells. Mechanistically, fucoxanthin decreased Dnmt1 mRNA and proteins levels, but was not found to alter Hdac expression levels [85].
4.7. Luteolin
Luteolin is a natural flavonoid present in the leaves, roots, stems, and fruits of several species of plants, such as chrysanthemum flowers, onion leaves, and celery [111]. Health benefits of luteolin have been linked to interfering with “hallmark features” of carcinogenesis such as angiogenesis, cell invasion, and metastasis. Regulation of the Nrf2 signaling pathway by luteolin is well studied and is a key mechanism through which the flavonoid is thought to exert health benefits. A few recent studies have pointed towards epigenetic modifications as a key underlying mechanism of action for luteolin. One investigation showed that luteolin inhibited proliferation of human colorectal HCT116 cells by upregulating NFE2L2 mRNA and NRF2 protein expression. Bisulfite sequencing revealed a marked reduction in CpG methylation at the NFE2L2 promoter, which was associated with significant reduction in expression and activities of DNMTs (DNMT1, DNMT3a, and DNMT3b) and HDACs (HDAC1, HDAC2, HDAC3, HDAC6, and HDAC7) [86]. Another study corroborated the epigenetic regulation of NRF2 by luteolin and showed reduced DNA methylation of the NFE2L2 promoter in luteolin-treated human colon adenocarcinoma HT29 cells. In luteolin-treated cells, ChIP assays showed reduced DNMT1 binding and increased TET1 interaction at the NFE2L2 promoter [87].
4.8. Pelargonidin
Pelargonidin is a plant anthocyanindin pigment producing a characteristic red-orange color in various fruits and vegetables, such as pomegranate, red radish, and strawberry. Pelargonidin is reported to possess antioxidant and anti-inflammatory properties and shows strong cytotoxicity towards various cancer cell lines. A molecular modeling approach in silico revealed that pelargonidin might inhibit the catalytic binding sites of human DNMT1 and DNMT3a [112]. In accordance with this work, pelargonidin reduced DNA methylation of the Nfe2l2 promoter to activate Nrf2-driven gene expression in JB6 P+ cells, leading to suppression of TPA-induced neoplastic transformation. Treatment with pelargonidin decreased the expression of Dnmt1 and Dnmt3b, and Hdac1, Hdac2, Hdac3, Hdac4, and Hdac7) [88].
4.9. Polydatin
Polydatin is isolated from the Chinese herb Polygonum cuspidatum, and is a natural precursor of resveratrol, with potent anti-inflammatory properties that are beneficial against many pathologies, including atherosclerosis, neurological disorders and cancer. Polydatin was reported to induce Nrf2 by inhibiting Keap1 [113]. To elucidate the mechanisms behind the activation of Nrf2 by polydatin, an in vivo and in vitro model of non-alcoholic fatty liver disease (NAFLD) induced by high fructose was employed. Polydatin reduced fructose-induced oxidative stress and inflammation by inhibiting Keap1 and activating Nrf2. Polydatin also caused a marked increase in the levels of miR-200a, which targeted KEAP1 to activate NRF2 signaling in response to high fructose-induced oxidative stress and inflammation in BRL-3A and HepG2 cells, and in the liver of high fructose-fed rats [89]. The study demonstrated that polydatin provided protection against fructose-induced liver inflammation and lipid deposition by activating Nrf2 and reducing oxidative stress [89].
4.10. Reserpine
Reserpine is an indole alkaloid, which is the principal active component found in the plant Rauwolfia serpentina and in other species of Rauwolfia sp. Reserpine is an anti-hypertensive drug and widely used to treat neurological disorders, and also possesses anticancer activity. A study showed that reserpine induced Nrf2-driven target genes to inhibit TPA-stimulated neoplastic transformation in mouse epidermal JB6 P+ cells. The relative methylation status of the CpG island at the Nfe2l2 promoter was found to decrease with increasing concentrations of reserpine. Expression levels of Dnmt1, Dnmt3a, and Dnmt3b were decreased by reserpine treatment in JB6 P+ cells [90].
4.11. Resveratrol
Resveratrol is a widely investigated plant polyphenol due to its antioxidant, anti-inflammatory, and antimicrobial properties. Several studies have pointed towards a role as an epigenetic modifier, most notably in anti-aging research, with resveratrol classified mechanistically as a class III HDAC/ sirtuin-activating compound [114,115,116]. Other work in HepG2 cells treated with high glucose and in high-fat models of NAFLD found that the methylation status of the NFE2L2 gene was increased, while that of KEAP1 was decreased, leading to decreased NRF2 expression and activity [91]. Effects of resveratrol on the methylation status of the Nfe2l2 promoter were shown in an earlier study involving a rat model of estrogen-induced mammary cancer [92]. Singh et al. noted “inhibition of 17β-estradiol-mediated alterations in NRF2 promoter methylation and expression of NRF2 targeting miR-93 after resveratrol treatment” as evidence for “resveratrol-mediated epigenetic regulation of NRF2 during E2-induced breast carcinogenesis” [92].
4.12. Sulforaphane
Sulforaphane is an isothiocyanate, abundant in the form of its precursor glucoraphanin in cruciferous vegetables such as broccoli [57,117,118] and acting via several anticancer mechanisms [93,119,120,121,122,123,124,125,126]. One major mechanism of action is through the induction of NRF2 and its downstream target antioxidant and detoxifying enzymes. In addition to modification of sulfhydryl groups in Keap1 [127], sulforaphane has been reported to act via epigenetic mechanisms [57,123,128,129,130,131,132,133], including inhibition of Dnmts and Hdacs, that also affect the methylation and acetylation status at the Nfe2l2 gene-level. This mechanism has been investigated in TRAMP C1 prostate cancer cells and in TPA-induced mouse skin JB6 P+ cells (Reviewed in [110]).
In a recent study, Loc344887 was the most highly upregulated transcript in sulforaphane-treated human HCT116 colon cancer cells [94]. This non-coding RNA was identified as a novel functional pseudogene and renamed NMRAL2P, with 62% homology to the protein-coding gene NmrA-like redox sensor 1 (NMRAL1). In addition to being a direct transcriptional target of NRF2, NMRAL2P was a downstream coactivator of NRF2-dependent NQO1 expression in human colon cancer cells. It was further shown that NMRAL2P knockout HCT116 cells were less responsive to sulforaphane-induced growth inhibitory effects. This report added a further layer of epigenetic regulation to the NRF2 network [94], which also involves other non-coding RNAs altered by sulforaphane [134,135,136,137].
4.13. Tanshinone IIA
Tanshinone IIA is a lipid-soluble natural compound isolated from the medicinal herb Salvia miltiorrhiza Burge and associated with cardiovascular and cerebrovascular protective effects. The involvement of epigenetic mechanisms in the induction of Nfe2l2 by Tanshinone IIA has been demonstrated in TPA-induced neoplastic transformation of JB6 P+ cells [95] and in in vitro and in vivo models of rifampicin-induced liver injury [96]. Hypomethylation of the Nfe2l2 promoter was mechanistically linked to potent induction of Nfe2l2 mRNA and Nrf2 protein levels by Tanshinone IIA. While one study reported decreased expression of Dnmts and Hdac1, Hdac3, and Hdac8 by Tanshinone IIA in JB6 P+ cells [95], another found no significant changes in DNMTs, but elevated expression of DNA demethylases, especially ten-eleven translocation 2 (TET2), in human hepatocyte L02 and Hepa RG cells [96]. The latter investigation showed that Tanshinone IIA could prevent rifampicin-induced liver injury by inducing the expression of bile salt efflux pump (BSEP) and Na+/taurocholate cotransporter (NTCP), which were directly regulated by NRF2 [96].
4.14. Taxifolin
Taxifolin is a flavonoid found in onion, milk thistle, and in Pinaceae plants, with diverse pharmacological activities, including antioxidant, anti-inflammatory, and antimicrobial properties. Induction of Nrf2 and its downstream target genes is considered crucial for the beneficial effects of taxifolin [138]. Taxifolin was found to inhibit TPA-induced neoplastic transformation of JB6 P+ cells by epigenetically inducing Nrf2. Bisulfite sequencing showed that the flavonoid reduced the proportion of methylated CpG sites in the Nfe2l2 promoter. At the molecular level, the protein expression levels of Dnmt1, Dnmt3a Dnmt3b, Hdac1, Hdac3, and Hdac8 were significantly decreased by taxifolin [97].
4.15. Ursolic Acid
Ursolic acid is a pentacyclic triterpenoid found ubiquitously in fruits, vegetables and herbs, such as cranberry, apple peel, basil, and rosemary [139]. Two studies have investigated the epigenetic regulation of Nrf2 by ursolic acid. In one investigation, the triterpenoid activated the Nrf2 pathway by demethylating the Nfe2l2 promoter, accompanied by a reduction in the expression levels of Dnmts and Hdacs, which was linked to inhibition of TPA-induced neoplastic transformation in mouse epidermal cells [98]. In the second study, ursolic acid induced the expression of the protein methyltransferase SETD7, and knockdown of SETD7 decreased NRF2 protein and its downstream target genes in LNCaP and PC-3 cells. Also, H3K4me1 monomethylation at the NFE2L2 promoter was reduced by SETD7 knockdown. On the other hand, treatment with ursolic acid enriched H3K4me1 at the NFE2L2 promoter, leading to increased NRF2 signaling [99]. It was hypothesized that direct methylation of the NRF2 protein by SETD7 might be mechanistically relevant.
4.16. γ. Tocopherol–Rich Mixture of Tocopherols (γ-TmT)
The major forms of vitamin E comprise α-, β-, γ- and δ-tocopherols and related tocotrienols [140, 141, 142]. These lipophilic compounds are abundant in vegetable oils and nuts and are widely studied as agents with an impact on human health and disease pathogenesis [143,144,145,146,147]. Numerous reviews have covered the topic of vitamin E and cancer [148,149,150,151]. Recently, γ-TmT, a commercially available by-product of vegetable oil refinery containing 57% γ-tocopherol, was linked to induction of the Nrf2 pathway via hypomethylation of the Nfe2l2 promoter in the TRAMP mouse and in TRAMP-C1 cells in vitro. This study also noted decreased protein levels of Dnmt1, Dnmt3a, and Dnmt3b in vivo compared to controls [100].
4.17. Z-Ligustilide
Z-Ligustilide is a natural benzoquinone derivative found in Chinese medicinal herbs, including Radix Angelicae Sinensis, and is reported to possess diverse pharmacological activities. Several studies [101, 152, 153, 154] noted upregulation of Nrf2 and its downstream antioxidant enzymes by Z-Ligustilide. Su et al. [101] demonstrated induction of Nrf2 by Radix Angelicae Sinensis and purified Z-Ligustilide. Bisulfite sequencing revealed decreased methylation at the Nfe2l2 promoter, accompanied by inhibition of Dnmts by both the plant extract and its isolated bioactive components [101].
5. Discussion
In addition to genetic alterations, i.e., mutations or chromosomal rearrangements in germline and somatic cells, epigenetic mechanisms also play a crucial role in cancer development. For instance, promoter hypermethylation is associated with the silencing of many tumor suppressor genes. The preponderance of epigenetic deregulation in cancer and the reversible nature of these alterations [155,156] has focused attention on epigenetic changes as viable targets for prevention or therapeutic strategies. Epigenetic alterations represent promising targets for cancer interception across multiple stages, from early to late disease pathogenesis [156,157,158,159]. Relatively few “epigenetic” drugs have been approved as anticancer agents, including the DNMT inhibitor 5-azacytidine (VidazaTM, AzadineTM) and the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA, VorinostatTM, ZolinzaTM), with recent attention also shifting to the BET inhibitor JQ1 (clinicaltrials.gov). In general, these therapeutics are not without side-effects as monotherapies, and combination approaches with standard-of-care practices provide the most viable way forward to minimize toxicity and related concerns [157].
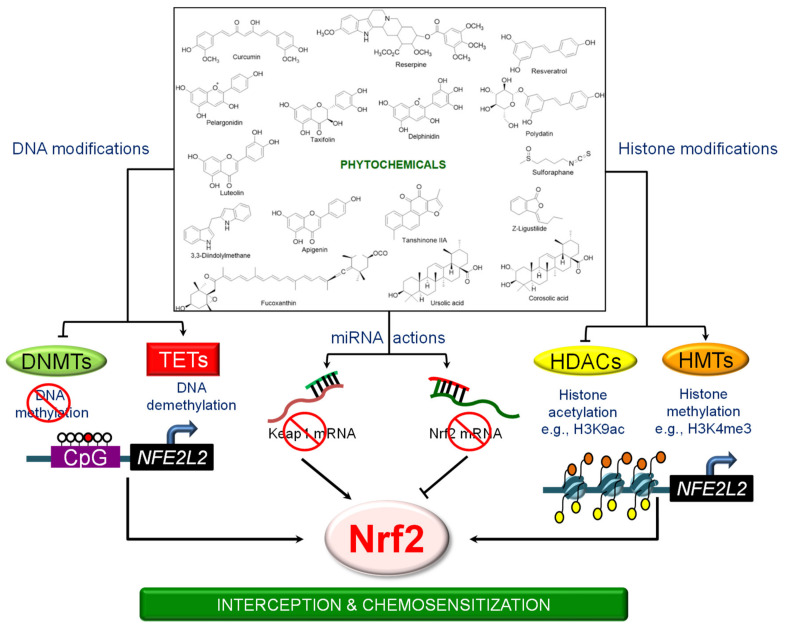
Many of the studies reviewed here involved analyses of CpG methylation sites in the NFE2L2 promoter and inhibition of DNMTs and/or HDACs, without probing more deeply into other mechanistic aspects, such as the involvement of chromatin coactivator/corepressor complexes, long-range chromatin interactions, and non-coding RNAs. This leaves plenty of scope for future research. KEAP1-NRF2 signaling is a key molecular target of cancer preventive agents, including an array of phytochemicals (Table 1). These phytochemicals can induce NRF2 either by acting as Michael acceptors that interact with KEAP1 sensor thiols, or by activating phosphorylation cascades that stabilize NRF2 [55,160]. However, research also has demonstrated that dietary phytochemicals can regulate NRF2 signaling by diverse epigenetic mechanisms (Figure 1).
Figure 1.
Phytochemicals and the epigenetic mechanisms linked to Nrf2-dependent signaling.
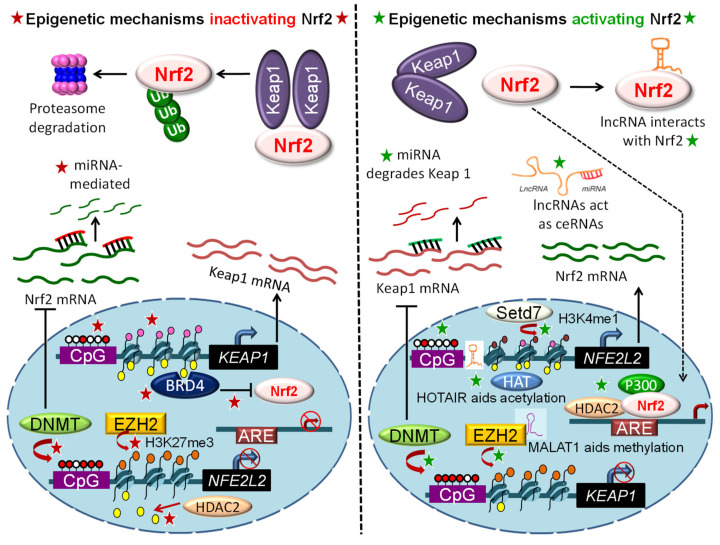
The best known NRF2 activator that has obtained clinical approval is dimethyl fumarate (Tecfidera), for the treatment of multiple sclerosis [161]. However, Tecfidera has several side-effects, including allergic reactions and gastrointestinal disturbance (www.PDR.net). There are a few related agents in clinical trials, such as Bardoxolone and SFX-01, a synthetic derivative of sulforaphane [161], which also exhibit less than desirable outcomes. Despite the promise from preclinical models and early clinical trials, safety, specificity, and potency issues must be resolved. All of these agents act by preventing the proteasomal degradation pathway. Given the multifactorial epigenetic regulation of NRF2, exploring other modulators that target NRF2 signaling at the transcription or post-transcription level warrants further attention (Figure 1 and Figure 2).
Figure 2.
Regulation of Nrf2 signaling via epigenetically-mediated transcriptional, post-transcriptional, and post-translational mechanisms (star symbols). Red and white circles, DNA methylation and unmethylation; yellow ovals, histone acetylation; pink, brown and orange ovals, histone unmethylation, H3K4me1 and H3K27me3, respectively.
Among the phytochemicals reviewed here, most were shown to reverse NFE2L2 promoter methylation by inhibiting DNMTs and attenuating certain HDACs. It is noteworthy that inhibitors directed against DNMTs and HDACs are used for the treatment of several malignancies, and the combination has been reported to produce synergistic growth inhibitory effects in cancer cells [162]. Therefore, it would be worthwhile to compare the combination of DNMT and HDAC inhibitors vis-à-vis Nrf2 induction by dietary phytochemicals (e.g., tea polyphenols plus sulforaphane). Moreover, a recent study showed that the combined inhibition of DNMT and HDAC activity caused de novo transcription of long-terminal repeats (LTRs) of the LTR12 family, linked to LTR-derived immunogens presented on major histocompatibility complex class I molecules [163]. Thus, phytochemical-mediated inhibition of DNMT and HDAC activities might dovetail NRF2 signaling with immunoprevention and immunotherapy.
Sulforaphane is an NRF2 inducer, as well as an inhibitor of HDAC activity and protein expression. One interesting observation is that Hdac3 expression was reduced by dietary sulforaphane in 1,2-dimethylhydrazine-treated wild-type (WT) mice, but less so in Nrf2−/+ mice, and that WT mice were more susceptible to carcinogen-induced colon tumorigenesis [164]. Thus, Nrf2 exerted an apparent oncogenic role in the gut, and Nrf2 status dictated Hdac inhibitory responses to sulforaphane and the extent of tumor growth suppression [164]. Another interesting avenue is the synergistic combination of sulforaphane with the BET inhibitor JQ1 in colon cancer models, targeting the non-histone protein Cell cycle and apoptosis regulator 2 (CCAR2) for acetylation and altered Wnt coactivator functionality [165]. As BET proteins are reported to interact with and inhibit NRF2 [44], the prospect of combined deacetylase and bromodomain inhibition affecting NRF2 regulation is worthy of further mechanistic investigation.
The phytochemicals reviewed here typically activate murine Nfe2l2 epigenetically, coincident with Nrf2 induction, except in the case of apigenin. Whereas one report noted Nfe2l2 activation via promoter hypomethylation in mouse skin epidermal cells [80], another showed miRNA-mediated inhibition of human NFE2L2 by apigenin, leading to chemosensitization of adriamycin-resistant hepatocellular carcinoma cells [106]. It would be interesting to explore the underlying circumstances for this epigenetic regulation of NRF2 by apigenin and, for example, whether DNA methylation predominates over non-coding RNA-mediated mechanisms. Activation of NRF2 is highly regulated, both temporally and spatially, and it will be important to decipher the epigenetic mechanisms in various cell types and the respective context-specific regulation of NRF2. From the current update, the majority of studies involved a single cell type and one of two preclinical models: JB6+ mouse skin epidermal cells and TRAMP prostate tumor cells. This calls for further investigation in different cancer scenarios to better characterize the epigenetic regulation of Nrf2/Keap1 by phytochemicals.
Finally, NRF2 is a complex regulator in cancer etiology because of its yin/yang roles in prevention and promotion, dictated in part by early vs. late stages of disease pathogenesis [10,11]. Epigenetic mechanisms add an additional layer of regulation, with numerous readers, writers and erasers that interact to affect histone states and chromatin access. Epigenetic aspects of NRF2 signaling by natural phytochemicals are worthy of further investigation to better understand context-dependent mechanisms that might provide new avenues for cancer prevention and interception.
6. Conclusions
The use of dietary components as cancer preventive agents continues to be of great interest, and experimental evidence supports the role of nutritional factors in modulating deregulated signaling pathways during cancer initiation and progression. Our understanding of the complex mechanisms of action of dietary factors is constantly evolving. One major take-home message from the accrued literature is that no agent, dietary or otherwise, is likely to act by one mechanism alone, or to affect a single molecular target without influencing other components of a signaling network. It is increasingly documented that diet or dietary components can influence gene expression through epigenetic mechanisms, but further work is needed to truly appreciate how these mechanisms can be manipulated in a beneficial manner for disease interception in the context of NRF2 signaling.
Abbreviations
| Nrf2 | murine nuclear factor erythroid 2-related factor 2 |
| Keap1 | murine Kelch-like ECH-associated protein 1 |
| NRF2 and KEAP1 | the corresponding human proteins |
| NFE2L2 | human gene encoding NRF2 protein |
| Nfe2l2 | murine gene encoding Nrf2 protein |
| KEAP1 | human gene encoding KEAP1 protein |
| Keap1 | murine gene encoding Keap1 protein |
| BET | bromodomain and extraterminal domain |
| miRNA | microRNA |
| lncRNA | long non-coding RNA |
| ARE | antioxidant response element |
| DNMT | DNA methyltransferase |
| HDAC | histone deacetylase |
| TET | ten-eleven translocation |
| AhR | aryl hydrocarbon receptor |
| Arnt | Aryl hydrocarbon receptor nuclear translocator |
| NF-κB | nuclear factor kappa B |
| Cul3 | Cullin 3 |
| Rbx1 | RING-box protein 1 |
| SCF | S-phase kinase-associated protein 1/Cullin/F-box |
| β-TrCP | β-transducin repeats-containing proteins |
| HRD1 | HMG-CoA reductase degradation protein 1 |
| GSK-3β | glycogen synthase kinase-3β |
| ERK | extracellular signal-regulated kinase |
| MAPK | p38 MAP kinase |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| Hrd-1 | 3-hydroxy-3-methylglutaryl reductase degradation 1 |
| EZH2 | Enhancer of zeste homolog 2 |
| Sp1 | Specificity protein 1 |
| SETD7/Set7/9 | SET Domain Containing 7 |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| CBP | CREB binding protein |
| ChIP | chromatin immunoprecipitation |
| 3′UTR | 3′untranslated region |
| I3C | indole-3-carbinol |
| DIM | 3,3′-diindolylmethane |
| NAFLD | non-alcoholic fatty liver disease |
| TRAMP | transgenic adenocarcinoma mouse prostate |
| SAHA | suberoylanilide hydroxamic acid |
Author Contributions
S.B. and R.H.D. contributed equally to the conceptualization, writing and review of this paper. All authors have read and agreed to the published version of the manuscript.
Funding
Original research was supported by grants CA090890 and CA122959 from the National Cancer Institute, by the John S. Dunn Foundation, and by a Chancellor’s Research Initiative from Texas A&M University. An Indo-US Overseas Fellowship, supported by Department of Science and Technology (DST) Govt. of India and implemented by Indo-US Science and Technology Forum (IUSSTF), was provided to S.B to undertake research in the Center for Epigenetics & Disease Prevention, Texas A&M Health Science Center, Houston, Texas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 3.Yates M.S., Kensler T.W. Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 2007;20:109–117. doi: 10.1358/dnp.2007.20.2.1083437. [DOI] [PubMed] [Google Scholar]
- 4.Kensler T.W., Chen J.G., Egner P.A., Fahey J.W., Jacobson L.P., Stephenson K.K., Ye L., Coady J.L., Wang J.B., Wu Y. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrenetetraols in a randomized clinical trial in HeZuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 5.Gong M., Li Y., Ye X., Zhang L., Wang Z., Xu X., Shen Y., Zheng C. Loss-of-function mutations in KEAP1 drive lung cancer progression via KEAP1/NRF2 pathway activation. Cell Commun. Signal. 2020;18:98. doi: 10.1186/s12964-020-00568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padmanabhan B., Tong K.I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M.I., Kobayashi A., Yokoyama S., Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G.T. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harder B., Jiang T., Wu T., Tao S., De La Vega M.R., Tian W., Chapman E., Zhang D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015;43:680–686. doi: 10.1042/BST20150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J.S., Nam L.B., Yoo O.K., Keum Y.S. Molecular mechanisms and systemic targeting of NRF2 dysregulation in cancer. Biochem. Pharmacol. 2020:114002. doi: 10.1016/j.bcp.2020.114002. [DOI] [PubMed] [Google Scholar]
- 10.Kensler T.W., Wakabayashi N. Nrf2: Friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S., Lu H., Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharjee S., Li J., Dashwood R.H. Emerging crosstalk between long non-coding RNAs and Nrf2 signaling. Cancer Lett. 2020;490:154–164. doi: 10.1016/j.canlet.2020.07.011. [published online ahead of print, 24 July 2020] [DOI] [PubMed] [Google Scholar]
- 13.Dodson M., De La Vega M.R., Cholanians A.B., Schmidlin C.J., Chapman E., Zhang D.D. Modulating NRF2 in disease: Timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related Factor (NRF2) expression by the Aryl Hydrocarbon Receptor-Xenobiotic Response Element signaling pathway direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 16.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 17.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Kenneth H.Y., Yeo C.J., Calhoun E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi N., Skoko J.J., Chartoumpekis D.V., Kimura S., Slocum S.L., Noda K., Palliyaguru D.L., Fujimuro M., Boley P.A., Tanaka Y. Notch-Nrf2 axis: Regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 2014;34:653–663. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak M.K., Itoh K., Yamamoto M., Kensler T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the Nrf2 promoter. Mol. Cell. Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein L.D., Lee J., Gnad F., Klijn C., Schaub A., Reeder J., Daemen A., Bakalarski C.E., Holcomb T., Shames D.S. Recurrent loss of NFE2L2 exon 2 is a mechanism for Nrf2 pathway activation in human cancers. Cell Rep. 2016;16:2605–2617. doi: 10.1016/j.celrep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Poganik J.R., Long M.J., Disare M.T., Liu X., Chang S.H., Hla T., Aye Y. Post-transcriptional regulation of Nrf2-mRNA by the mRNA-binding proteins HuR and AUF1. FASEB J. 2019;33:14636–14652. doi: 10.1096/fj.201901930R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/β-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T., Zhao F., Gao B., Tan C., Yagishita N., Nakajima T., Wong P.K., Chapman E., Fang D., Zhang D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 25.Singh A., Misra V., Thimmulappa R.K., Lee H., Ames S., Hoque M.O., Herman J.G., Baylin S.B., Sidransky D., Gabrielson E. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS. Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solis L.M., Behrens C., Dong W., Suraokar M., Ozburn N.C., Moran C.A., Corvalan A.H., Biswal S., Swisher S.G., Bekele B.N. Nrf2 and Keap1 abnormalities in non–small cell lung carcinoma and association with clinicopathologic features. Clin. Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo D., Wu B., Yan J., Li X., Sun H., Zhou D. A possible gene silencing mechanism: Hypermethylation of the Keap1 promoter abrogates binding of the transcription factor Sp1 in lung cancer cells. Biochem. Biophys. Res. Commun. 2012;428:80–85. doi: 10.1016/j.bbrc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y., Yu S., Zhang C., Kong A.N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free. Radic. Biol. Med. 2015;88:337–349. doi: 10.1016/j.freeradbiomed.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng D., Wu R., Guo Y., Kong A.N.T. Regulation of Keap1–Nrf2 signaling: The role of epigenetics. Curr. Opin. Toxicol. 2016;1:134–138. doi: 10.1016/j.cotox.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabrizio F.P., Sparaneo A., Trombetta D., Muscarella L.A. Epigenetic versus genetic deregulation of the KEAP1/NRF2 axis in solid tumors: Focus on methylation and noncoding RNAs. Oxid. Med. Cell. Longev. 2018;2018:2492063. doi: 10.1155/2018/2492063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S., Khor T.O., Cheung K.L., Li W., Wu T.Y., Huang Y., Foster B.A., Kan Y.W., Kong A.N. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS ONE. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khor T.O., Fuentes F., Shu L., Paredes-Gonzalez X., Yang A.Y., Liu Y., Smiraglia D.J., Yegnasubramanian S., Nelson W.G., Kong A.N.T. Epigenetic DNA methylation of antioxidative stress regulator NRF2 in human prostate cancer. Cancer Prev. Res. 2014;7:1186–1197. doi: 10.1158/1940-6207.CAPR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muscarella L.A., Barbano R., D’Angelo V., Copetti M., Coco M., Balsamo T., la Torre A., Notarangelo A., Troiano M., Parisi S. Regulation of KEAP1 expression by promoter methylation in malignant gliomas and association with patient’s outcome. Epigenetics. 2011;6:317–325. doi: 10.4161/epi.6.3.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbano R., Muscarella L.A., Pasculli B., Valori V.M., Fontana A., Coco M., la Torre A., Balsamo T., Poeta M.L., Marangi G.F. Aberrant Keap1 methylation in breast cancer and association with clinicopathological features. Epigenetics. 2013;8:105–112. doi: 10.4161/epi.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscarella L.A., Parrella P., D’Alessandro V., la Torre A., Barbano R., Fontana A., Tancredi A., Guarnieri V., Balsamo T., Coco M. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6:710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 36.Gao L., Yuan F., Che G., Xiao X., Nie X., Wang Y., Jia J., Kong A.-N., Zhang L. Epigenetic modifications but not genetic polymorphisms regulate KEAP1 expression in colorectal cancer. J. Cell. Biochem. 2019;120:12311–12320. doi: 10.1002/jcb.28495. [DOI] [PubMed] [Google Scholar]
- 37.Fabrizio F.P., Costantini M., Copetti M., la Torre A., Sparaneo A., Fontana A., Poeta L., Gallucci M., Sentinelli S., Graziano P. Keap1/Nrf2 pathway in kidney cancer: Frequent methylation of KEAP1 gene promoter in clear renal cell carcinoma. Oncotarget. 2017;8:11187. doi: 10.18632/oncotarget.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Xu J., Li C., Shi S., Ji S., Xu W., Liu J., Jin K., Liang D., Liang C. MBD1 is an epigenetic regulator of KEAP1 in pancreatic cancer. Curr. Mol. Med. 2016;16:404–411. doi: 10.2174/1566524016666160316154150. [DOI] [PubMed] [Google Scholar]
- 39.Li Z., Xu L., Tang N., Xu Y., Ye X., Shen S., Niu X., Lu S., Chen Z. The polycomb group protein EZH2 inhibits lung cancer cell growth by repressing the transcription factor Nrf2. FEBS Lett. 2014;588:3000–3007. doi: 10.1016/j.febslet.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 40.Mishra M., Zhong Q., Kowluru R.A. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2014;55:7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell. Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G.H., Qu J., Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Mercado N., Thimmulappa R., Thomas C.M., Fenwick P.S., Chana K.K., Donnelly L.E., Biswal S., Ito K., Barnes P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011;406:292–298. doi: 10.1016/j.bbrc.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee N., Bohmann D. BET-ting on Nrf2: How Nrf2 Signaling can Influence the Therapeutic Activities of BET Protein Inhibitors. Bioessays. 2018;40:1800007. doi: 10.1002/bies.201800007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussong M., Börno S.T., Kerick M., Wunderlich A., Franz A., Sültmann H., Timmermann B., Lehrach H., Hirsch-Kauffmann M., Schweiger M.R. The bromodomain protein BRD4 regulates the KEAP1/NRF2-dependent oxidative stress response. Cell. Death. Dis. 2014;5:e1195. doi: 10.1038/cddis.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahajan N., Wu H.J., Bennett R.L., Troche C., Licht J.D., Weber J.D., Maggi L.B., Jr., Tomasson M.H. Sabotaging of the oxidative stress response by an oncogenic noncoding RNA. FASEB J. 2017;31:482–490. doi: 10.1096/fj.201600654R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao T.X., Wang J.K., Shen L.J., Long C.L., Liu B., Wei Y., Han L.D., Wei Y.X., Wu S.D., Wei G.H. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 2020;259:113911. doi: 10.1016/j.envpol.2020.113911. [DOI] [PubMed] [Google Scholar]
- 48.Kurinna S., Werner S. NRF2 and microRNAs: New but awaited relations. Biochem. Soc. Trans. 2015;43:595–601. doi: 10.1042/BST20140317. [DOI] [PubMed] [Google Scholar]
- 49.Ashrafizadeh M., Ahmadi Z., Samarghandian S., Mohammadinejad R., Yaribeygi H., Sathyapalan T., Sahebkar A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020;244:117329. doi: 10.1016/j.lfs.2020.117329. [DOI] [PubMed] [Google Scholar]
- 50.Eades G., Yang M., Yao Y., Zhang Y., Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J. Biol. Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S., Wu W., Jiao G., Li C., Liu H. MiR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects osteoblasts from oxidative stress. Int. J. Biol. Macromol. 2018;107:2094–2101. doi: 10.1016/j.ijbiomac.2017.10.080. [DOI] [PubMed] [Google Scholar]
- 52.Van Jaarsveld M.T.M., Helleman J., Boersma A.W.M., Van Kuijk P.F., Van Ijcken W.F., Despierre E., Vergote I., Mathijssen R.H.J., Berns E., Verweij J. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 53.Kabaria S., Choi D.C., Chaudhuri A.D., Jain M.R., Li H., Junn E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free. Radic. Biol. Med. 2015;89:548–556. doi: 10.1016/j.freeradbiomed.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akdemir B., Nakajima Y., Inazawa J., Inoue J. miR-432 induces NRF2 stabilization by directly targeting KEAP1. Mol. Cancer Res. 2017;15:1570–1578. doi: 10.1158/1541-7786.MCR-17-0232. [DOI] [PubMed] [Google Scholar]
- 55.Kwak M.K., Kensler T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad A., Li Y., Sarkar F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food. Res. 2016;60:1251–1263. doi: 10.1002/mnfr.201500867. [DOI] [PubMed] [Google Scholar]
- 57.Higdon J.V., Delage B., Williams D.E., Dashwood R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grose K.R., Bjeldanes L.F. Oligomerization of indole-3-carbinol in aqueous acid. Chem. Res. Toxicol. 1992;5:188–193. doi: 10.1021/tx00026a007. [DOI] [PubMed] [Google Scholar]
- 59.Dashwood R.H., Fong A.T., Arbogast D.N., Bjeldanes L.F., Hendricks J.D., Bailey G.S. Anticarcinogenic activity of indole-3-carbinol acid products: Ultrasensitive bioassay by trout embryo microinjection. Cancer Res. 1994;54:3617–3619. [PubMed] [Google Scholar]
- 60.Wattenberg L.W. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. doi: 10.1006/pmed.1996.0015. [DOI] [PubMed] [Google Scholar]
- 61.Dashwood R.H., Arbogast D.N., Fong A.T., Pereira C., Hendricks J.D., Bailey G.S. Quantitative inter-relationships between aflatoxin B1 carcinogen dose, indole-3-carbinol anti-carcinogen dose, target organ DNA adduction and final tumor response. Carcinogenesis. 1989;10:175–181. doi: 10.1093/carcin/10.1.175. [DOI] [PubMed] [Google Scholar]
- 62.Dashwood R.H., Fong A.T., Williams D.E., Hendricks J.D., Bailey G.S. Promotion of aflatoxin B1 carcinogenesis by the natural tumor modulator indole-3-carbinol: Influence of dose, duration, and intermittent exposure on indole-3-carbinol promotional potency. Cancer Res. 1991;51:2362–2365. [PubMed] [Google Scholar]
- 63.Guo D., Schut H.A., Davis C.D., Snyderwine E.G., Bailey G.S., Dashwood R.H. Protection by chlorophyllin and indole-3-carbinol against 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1995;16:2931–2937. doi: 10.1093/carcin/16.12.2931. [DOI] [PubMed] [Google Scholar]
- 64.Xu M., Bailey A.C., Hernaez J.F., Taoka C.R., Schut H.A., Dashwood R.H. Protection by green tea, black tea, and indole-3-carbinol against 2-amino-3-methylimidazo[4,5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- 65.Dashwood R.H. Indole-3-carbinol: Anticarcinogen or tumor promoter in brassica vegetables? Chem. Biol. Interact. 1998;110:1–5. doi: 10.1016/S0009-2797(97)00115-4. [DOI] [PubMed] [Google Scholar]
- 66.Xu M., Orner G.A., Bailey G.S., Stoner G.D., Horio D.T., Dashwood R.H. Post-initiation effects of chlorophyllin and indole-3-carbinol in rats given 1,2-dimethylhydrazine or 2-amino-3-methylimidazo[4,5-f]quinoline. Carcinogenesis. 2001;22:309–314. doi: 10.1093/carcin/22.2.309. [DOI] [PubMed] [Google Scholar]
- 67.Blum C.A., Xu M., Orner G.A., Fong A.T., Bailey G.S., Stoner G.D., Horio D.T., Dashwood R.H. beta-Catenin mutation in rat colon tumors initiated by 1,2-dimethylhydrazine and 2-amino-3-methylimidazo[4,5-f]quinoline, and the effect of post-initiation treatment with chlorophyllin and indole-3-carbinol. Carcinogenesis. 2001;22:315–320. doi: 10.1093/carcin/22.2.315. [DOI] [PubMed] [Google Scholar]
- 68.Beaver L.M., Yu T.W., Sokolowski E.I., Williams D.E., Dashwood R.H., Ho E. 3,3′-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012;263:345–351. doi: 10.1016/j.taap.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shorey L.E., Madeen E.P., Atwell L.L., Ho E., Löhr C.V., Pereira C.B., Dashwood R.H., Williams D.E. Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: Maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol. Appl. Pharmacol. 2013;270:60–69. doi: 10.1016/j.taap.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong C.P., Hsu A., Buchanan A., Palomera-Sanchez Z., Beaver L.M., Houseman E.A., Williams D.E., Dashwood R.H., Ho E. Effects of sulforaphane and 3,3′-diindolylmethane on genome-wide promoter methylation in normal prostate epithelial cells and prostate cancer cells. PLoS ONE. 2014;9:e86787. doi: 10.1371/journal.pone.0086787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolluri S.K., Jin U.H., Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017;91:2497–2513. doi: 10.1007/s00204-017-1981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujioka N., Fritz V., Upadhyaya P., Kassie F., Hecht S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food. Res. 2016;60:1228–1238. doi: 10.1002/mnfr.201500889. [DOI] [PubMed] [Google Scholar]
- 73.Yerushalmi R., Bargil S., Bar Y., Ozlavo R., Tuval S., Rapson Y., Pomerantz A., Zoref D., Sharon E., Caspi O., et al. 3,3-Diindolylmethane (DIM): A nutritional intervention and its impact on breast density in healthy BRCA carriers. A prospective clinical trial. Carcinogenesis. 2020 doi: 10.1093/carcin/bgaa050. [published online ahead of print 27 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradlow H.L., Michnovicz J.J., Halper M., Miller D.G., Wong G.Y., Osborne M.P. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer. Epidemiol. Biomarkers. Prev. 1994;3:591–595. [PubMed] [Google Scholar]
- 75.Bradlow H.L., Sepkovic D.W., Telang N.T., Osborne M.P. Indole-3-carbinol. A novel approach to breast cancer prevention. Ann. N.Y. Acad. Sci. 1995;768:180–200. doi: 10.1111/j.1749-6632.1995.tb12121.x. [DOI] [PubMed] [Google Scholar]
- 76.Michnovicz J.J. Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int. J. Obes. Relat. Metab. Disord. 1998;22:227–229. doi: 10.1038/sj.ijo.0800573. [DOI] [PubMed] [Google Scholar]
- 77.Bell M.C., Crowley-Nowick P., Bradlow H.L., Sepkovic D.W., Schmidt-Grimminger D., Howell P., Mayeaux E.J., Tucker A., Turbat-Herrera E.A., Mathis J.M. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol. Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 78.Sepkovic D.W., Bradlow H.L., Bell M. Quantitative determination of 3,3′-diindolylmethane in urine of individuals receiving indole-3-carbinol. Nutr. Cancer. 2001;41:57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 79.Wu T.Y., Khor T.O., Su Z.Y., Saw C.L.L., Shu L., Cheung K.L., Huang Y., Yu S., Kong A.N.T. Epigenetic modifications of Nrf2 by 3, 3′-diindolylmethane in vitro in TRAMP C1 cell line and in vivo TRAMP prostate tumors. AAPS J. 2013;15:864–874. doi: 10.1208/s12248-013-9493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pandey M., Kaur P., Shukla S., Abbas A., Fu P., Gupta S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012;51:952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng T.H., Chien M.H., Lin W.L., Wen Y.C., Chow J.M., Chen C.K., Kuo T.C., Lee W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21WAF1/CIP1 expression. Environ. Toxicol. 2017;32:434–444. doi: 10.1002/tox.22247. [DOI] [PubMed] [Google Scholar]
- 82.Yang J., Wu R., Li W., Gao L., Yang Y., Li P., Kong A.-N. The triterpenoid corosolic acid blocks transformation and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells. Mol. Carcinog. 2018;57:512–521. doi: 10.1002/mc.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 84.Kuo H., Wu R., Li S., Yang A.Y., Kong A.N. Anthocyanin delphinidin prevents neoplastic transformation of mouse skin JB6 P+ cells: Epigenetic re-activation of Nrf2-ARE pathway. AAPS J. 2019;21:83. doi: 10.1208/s12248-019-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y., Yang I., Cao M., Su Z., Wu R., Guo Y., Fang M., Kong A.N. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018;20:32. doi: 10.1208/s12248-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuo Q., Wu R., Xiao X., Yang C., Yang Y., Wang C., Lin L., Kong A.N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell. Biochem. 2018;119:9573–9582. doi: 10.1002/jcb.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang K.A., Piao M.J., Hyun Y.J., Zhen A.X., Cho S.J., Ahn M.J., Yi J.M., Hyun J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019;51:1–14. doi: 10.1038/s12276-019-0238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S., Li W., Wang C., Wu R., Yin R., Kuo H.C., Wang L., Kong A.N. Pelargonidin reduces the TPA induced transformation of mouse epidermal cells–potential involvement of Nrf2 promoter demethylation. Chem. Biol. Interact. 2019;309:108701. doi: 10.1016/j.cbi.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao X.J., Yu H.W., Yang Y.Z., Wu W.Y., Chen T.Y., Jia K.K., Kang L.L., Jiao R.Q., Kong L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong B., Su Z., Zhang C., Yang Y., Guo Y., Li W., Kong A.N.T. Reserpine inhibit the JB6 P+ cell transformation through epigenetic reactivation of Nrf2-mediated anti-oxidative stress pathway. AAPS J. 2016;18:659–669. doi: 10.1208/s12248-016-9901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hosseini H., Teimouri M., Shabani M., Koushki M., Khorzoughi R.B., Namvarjah F., Izadi P., Meshkani R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell. Biol. 2020;119:105667. doi: 10.1016/j.biocel.2019.105667. [DOI] [PubMed] [Google Scholar]
- 92.Singh B., Shoulson R., Chatterjee A., Ronghe A., Bhat N.K., Dim D.C., Bhat H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis. 2014;35:1872–1880. doi: 10.1093/carcin/bgu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su X., Jiang X., Meng L., Dong X., Shen Y., Xin Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2018;2018:5438179. doi: 10.1155/2018/5438179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson G.S., Li J., Beaver L.M., Dashwood W.M., Sun D., Rajendran P., Williams D.E., Ho E., Dashwood R.H. A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells. Mol. Nutr. Food. Res. 2017;61:1600769. doi: 10.1002/mnfr.201600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L., Zhang C., Guo Y., Su Z.Y., Yang Y., Shu L., Kong A.N.T. Blocking of JB6 cell transformation by tanshinone IIA: Epigenetic reactivation of Nrf2 antioxidative stress pathway. AAPS J. 2014;16:1214–1225. doi: 10.1208/s12248-014-9666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y., Liu L., Zhang X., Jiang X., Wang L. Tanshinone IIA prevents rifampicin-induced liver injury by regulating BSEP/NTCP expression via epigenetic activation of NRF2. Liver Int. 2020;40:141–154. doi: 10.1111/liv.14262. [DOI] [PubMed] [Google Scholar]
- 97.Kuang H., Tang Z., Zhang C., Wang Z., Li W., Yang C., Wang Q., Yang B., Kong A.N. Taxifolin Activates the Nrf2 Anti-Oxidative Stress Pathway in Mouse Skin Epidermal JB6 P+ Cells through Epigenetic Modifications. Int. J. Mol. Sci. 2017;18:1546. doi: 10.3390/ijms18071546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim H., Ramirez C.N., Su Z.Y., Kong A.N.T. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016;33:54–62. doi: 10.1016/j.jnutbio.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C., Shu L., Zhang C., Li W., Wu R., Guo Y., Yang Y., Kong A.N. Histone methyltransferase Setd7 regulates Nrf2 signaling pathway by phenethyl isothiocyanate and ursolic acid in human prostate cancer cells. Mol. Nutr. Food Res. 2018;62:e1700840. doi: 10.1002/mnfr.201700840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y., Khor T.O., Shu L., Saw C.L.L., Wu T.Y., Suh N., Yang C.S., Kong A.N.T. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012;142:818–823. doi: 10.3945/jn.111.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su Z.Y., Khor T.O., Shu L., Lee J.H., Saw C.L.L., Wu T.Y., Huang Y., Suh N., Yang C.S., Conney A.H., et al. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem. Res. Toxicol. 2013;26:477–485. doi: 10.1021/tx300524p. [DOI] [PubMed] [Google Scholar]
- 102.Madunić J., Madunić I.V., Gajski G., Popić J., Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 103.Kanwal R., Datt M., Liu X., Gupta S. Dietary Flavones as Dual Inhibitors of DNA Methyltransferases and Histone Methyltransferases. PLoS ONE. 2016;11:e0162956. doi: 10.1371/journal.pone.0162956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L., Xie W., Xie W., Zhuang W., Jiang C., Liu N. Apigenin attenuates isoflurane-induced cognitive dysfunction via epigenetic regulation and neuroinflammation in aged rats. Arch. Gerontol. Geriatr. 2017;73:29–36. doi: 10.1016/j.archger.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Paredes-Gonzalez X., Fuentes F., Su Z.Y., Kong A.N.T. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P+ cells through epigenetics modifications. AAPS J. 2014;16:727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao A.M., Zhang X.Y., Ke Z.P. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8:82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu S., Wang G., Peng W., Xu Y., Zhang Y., Ge Y., Jing Y., Gong Z. Corosolic acid isolated from Eriobotrya japonica leaves reduces glucose level in human hepatocellular carcinoma cells, zebrafish and rats. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-40934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y., Ge Z., Huang S., Zhou L., Zhai C., Chen Y., Hu Q., Cao W., Weng Y., Li Y. Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway. Aging. 2020;12:5362. doi: 10.18632/aging.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Afaq F., Syed D.N., Malik A., Hadi N., Sarfaraz S., Kweon M.-H., Khan N., Zaid M.A., Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 110.Bae M., Kim M.B., Park Y.K., Lee J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 2020;1865:158618. doi: 10.1016/j.bbalip.2020.158618. [DOI] [PubMed] [Google Scholar]
- 111.Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 112.Karthi N., Karthiga A., Kalaiyarasu T., Stalin A., Manju V., Singh S.K., Cyril R., Lee S.M. Exploration of cell cycle regulation and modulation of the DNA methylation mechanism of pelargonidin: Insights from the molecular modeling approach. Comput. Biol. Chem. 2017;70:175–185. doi: 10.1016/j.compbiolchem.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 113.Huang K., Chen C., Hao J., Huang J., Wang S., Liu P., Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronectin and transforming growth factor-β1 in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2015;399:178–189. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 115.Bonkowski M.S., Sinclair D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell. Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 117.Yagishita Y., Fahey J.W., Dinkova-Kostova A.T., Kensler T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules. 2019;24:3593. doi: 10.3390/molecules24193593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., Talalay P., Cho C.G., Posner G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Houghton C.A. Sulforaphane: Its “Coming of Age” as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxid. Med. Cell. Longev. 2019;2019:2716870. doi: 10.1155/2019/2716870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ho E., Beaver L.M., Williams D.E., Dashwood R.H. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv. Nutr. 2011;2:497–510. doi: 10.3945/an.111.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nian H., Delage B., Ho E., Dashwood R.H. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009;50:213–221. doi: 10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dashwood R.H., Ho E. Dietary agents as histone deacetylase inhibitors: Sulforaphane and structurally related isothiocyanates. Nutr. Rev. 2008;66:S36–S38. doi: 10.1111/j.1753-4887.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Myzak M.C., Dashwood R.H. Chemoprotection by sulforaphane: Keep one eye beyond Keap1. Cancer Lett. 2006;233:208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dashwood R.H., Myzak M.C., Ho E. Dietary HDAC inhibitors: Time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27:344–349. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Myzak M.C., Karplus P.A., Chung F.L., Dashwood R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 127.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Myzak M.C., Dashwood R.H. Histone deacetylases as targets for dietary cancer preventive agents: Lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr. Drug Targets. 2006;7:443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 129.Dashwood R.H., Ho E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin. Cancer Biol. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:405–410. doi: 10.1097/MCO.0b013e328362014e. [DOI] [PubMed] [Google Scholar]
- 131.Tollefsbol T.O. Dietary epigenetics in cancer and aging. Cancer Treat. Res. 2014;159:257–267. doi: 10.1007/978-3-642-38007-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Myzak M.C., Ho E., Dashwood R.H. Dietary agents as histone deacetylase inhibitors. Mol. Carcinog. 2006;45:443–446. doi: 10.1002/mc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsu A., Wong C.P., Yu Z., Williams D.E., Dashwood R.H., Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics. 2011;3:3. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beaver L.M., Kuintzle R., Buchanan A., Wiley M.W., Glasser S.T., Wong C.P., Johnson G.S., Chang J.H., Löhr C.V., Williams D.E., et al. Long noncoding RNAs and sulforaphane: A target for chemoprevention and suppression of prostate cancer. J. Nutr. Biochem. 2017;42:72–83. doi: 10.1016/j.jnutbio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rafiei H., Ashrafizadeh M., Ahmadi Z. MicroRNAs as novel targets of sulforaphane in cancer therapy: The beginning of a new tale? Phytother. Res. 2020;34:721–728. doi: 10.1002/ptr.6572. [DOI] [PubMed] [Google Scholar]
- 136.Gao L., Cheng D., Yang J., Wu R., Li W., Kong A.N. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J. Nutr. Biochem. 2018;56:109–115. doi: 10.1016/j.jnutbio.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin S.L., Kala R., Tollefsbol T.O. Mechanisms for the inhibition of colon cancer cells by sulforaphane through epigenetic modulation of microRNA-21 and human Telomerase Reverse Transcriptase (hTERT) down-regulation. Curr. Cancer Drug Targets. 2018;18:97–106. doi: 10.2174/1568009617666170206104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manigandan K., Manimaran D., Jayaraj R.L., Elangovan N., Dhivya V., Kaphle A. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie. 2015;119:103–112. doi: 10.1016/j.biochi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 139.Zhang C., Wang C., Li W., Wu R., Guo Y., Cheng D., Yang Y., Androulakis I.P., Kong A.N. Pharmacokinetics and pharmacodynamics of the triterpenoid ursolic acid in regulating the antioxidant, anti-inflammatory, and epigenetic gene responses in rat leukocytes. Mol. Pharm. 2017;14:3709–3717. doi: 10.1021/acs.molpharmaceut.7b00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brigelius-Flohé R., Traber M.G. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–1155. doi: 10.1096/fasebj.13.10.1145. [DOI] [PubMed] [Google Scholar]
- 141.Burton G.W., Traber M.G. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 142.Podda M., Weber C., Traber M.G., Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid. Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 143.Ju J., Picinich S.C., Yang Z., Zhao Y., Suh N., Kong A.N., Yang C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kayden H.J., Traber M.G. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J. Lipid. Res. 1993;34:343–358. [PubMed] [Google Scholar]
- 145.Traber M.G., Sies H. Vitamin E in humans: Demand and delivery. Annu. Rev. Nutr. 1996;16:321–347. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 146.Traber M.G., Ramakrishnan R., Kayden H.J. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 1994;91:10005–10008. doi: 10.1073/pnas.91.21.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Burton G.W., Traber M.G., Acuff R.V., Walters D.N., Kayden H., Hughes L., Ingold K.U. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am. J. Clin. Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 148.Yang C.S., Luo P., Zeng Z., Wang H., Malafa M., Suh N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020;59:365–389. doi: 10.1002/mc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Montagnani M.M., Marzagalli M., Fontana F., Raimondi M., Moretti R.M., Limonta P. Anticancer properties of tocotrienols: A review of cellular mechanisms and molecular targets. J. Cell. Physiol. 2019;234:1147–1164. doi: 10.1002/jcp.27075. [DOI] [PubMed] [Google Scholar]
- 150.Abraham A., Kattoor A.J., Saldeen T., Mehta J.L. Vitamin E and its anticancer effects. Crit. Rev. Food. Sci. Nutr. 2019;59:2831–2838. doi: 10.1080/10408398.2018.1474169. [DOI] [PubMed] [Google Scholar]
- 151.Jiang Q. Natural forms of vitamin E as effective agents for cancer prevention and therapy. Adv. Nutr. 2017;8:850–867. doi: 10.3945/an.117.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu Y., Zhang Y., Huang X., Xie Y., Qu Y., Long H., Gu N., Jiang W. Z-Ligustilide protects vascular endothelial cells from oxidative stress and rescues high fat diet-induced atherosclerosis by activating multiple NRF2 downstream genes. Atherosclerosis. 2019;284:110–120. doi: 10.1016/j.atherosclerosis.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 153.Wu Z., Uchi H., Morino-Koga S., Shi W., Furue M. Z-ligustilide ameliorated ultraviolet B-induced oxidative stress and inflammatory cytokine production in human keratinocytes through upregulation of Nrf2/HO-1 and suppression of NF-κB pathway. Exp. Dermatol. 2015;24:703–708. doi: 10.1111/exd.12758. [DOI] [PubMed] [Google Scholar]
- 154.Peng B., Zhao P., Lu Y.P., Chen M.M., Sun H., Wu X.M., Zhu L. Z-ligustilide activates the Nrf2/HO-1 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Brain. Res. 2013;1520:168–177. doi: 10.1016/j.brainres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 155.Mohammad H.P., Barbash O., Creasy C.L. Targeting epigenetic modifications in cancer therapy: Erasing the roadmap to cancer. Nat. Med. 2019;25:403–418. doi: 10.1038/s41591-019-0376-8. [DOI] [PubMed] [Google Scholar]
- 156.Gerhauser C. Cancer chemoprevention and nutriepigenetics: State of the art and future challenges. Top. Curr. Chem. 2013;329:73–132. doi: 10.1007/128_2012_360. [DOI] [PubMed] [Google Scholar]
- 157.Ganesan A., Arimondo P.B., Rots M.G., Jeronimo C., Berdasco M. The timeline of epigenetic drug discovery: From reality to dreams. Clin. Epigenetics. 2019;11:174. doi: 10.1186/s13148-019-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DiMarco-Crook C., Xiao H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food. Sci. Technol. 2015;6:505–526. doi: 10.1146/annurev-food-081114-110833. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y., Kutateladze T.G. Diet and the epigenome. Nat. Commun. 2018;9:3375. doi: 10.1038/s41467-018-05778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stefanson A.L., Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients. 2014;6:3777–3801. doi: 10.3390/nu6093777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernández Ó., Giovannoni G., Fox R.J., Gold R., Phillips J.T., Potts J., Okwuokenye M., Marantz J.L. Efficacy and safety of delayed-release dimethyl fumarate for relapsing-remitting multiple sclerosis in prior interferon users: An integrated analysis of DEFINE and CONFIRM. Clin. Ther. 2017;39:1671–1679. doi: 10.1016/j.clinthera.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 162.Zhu W.G., Otterson G.A. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr. Med. Chem. Anti-cancer. Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 163.Brocks D., Schmidt C.R., Daskalakis M., Jang H.S., Shah N.M., Li D., Li J., Zhang B., Hou Y., Laudato S., et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat. Genet. 2017;49:1052–1060. doi: 10.1038/ng.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rajendran P., Dashwood W.M., Li L., Kang Y., Kim E., Johnson G., Fischer K.A., Löhr C.V., Williams D.E., Ho E., et al. Nrf2 status affects tumor growth, HDAC3 gene promoter associations, and the response to sulforaphane in the colon. Clin. Epigenetics. 2015;7:102. doi: 10.1186/s13148-015-0132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rajendran P., Johnson G., Li L., Chen Y.S., Dashwood M., Nguyen N., Ulusan A., Ertem F., Zhang M., Li J. Acetylation of CCAR2 establishes a BET/BRD9 acetyl switch in response to combined deacetylase and bromodomain inhibition. Cancer Res. 2019;79:918–927. doi: 10.1158/0008-5472.CAN-18-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]