Abstract
Reactive oxygen species (ROS) are critical signaling molecules for neuronal physiology that stimulate growth and development and play vital roles in several pathways when in a balanced state, but they cause neurodegeneration when unbalanced. As ROS levels above a certain threshold cause the activation of the autophagy system, moderate levels of ROS can be used as treatment strategies. Currently, such treatments are used together with low-level laser or photodynamic therapies, photo-bio modulation, or infrared treatments, in different chronic diseases but not in the treatment of neurodegeneration. Recently, non-thermal plasma has been successfully used in biomedical applications and treatments, and beneficial effects such as differentiation, cell growth, and proliferation, stimulation of ROS based pathways have been observed. Besides the activation of a wide range of biological signaling pathways by generating ROS, plasma application can be an effective treatment in neuronal regeneration, as well as in neuronal diseases. In this review, we summarize the generation and role of ROS in neurons and provide critical insights into their potential benefits on neurons. We also discuss the underlying mechanisms of ROS on neuronal development. Regarding clinical applications, we focus on ROS-based neuronal growth and regeneration strategies and in the usage of non-thermal plasma in neuronal and CNS injury treatments.
Keywords: reactive species, neuronal growth, neuronal stem cells, neurodegeneration, gas plasma
1. Introduction
Reactive oxygen species (ROS) are group of molecules which are generated from oxygen and are very reactive in nature. The properties and activity of different reactive species as signaling molecules are most widely studied signaling molecules and evident to be responsible for both positive and negative impacts on both human physiology and disease pathology, as well as in treatment of a variety of health conditions [1,2]. Because of the double-edged sword property of ROS [3], maintaining its balance and regulation is crucial [4]. ROS is found to be an essential molecule for maintaining the normal physiology of the brain by stimulating many receptors and metabolic functions [5,6]. ROS can influence multiple aspects of neural differentiation and function, including the survival and plasticity of neurons [7], the proliferation of neural precursors, as well as their differentiation into specific neuronal cell types [8,9]. On the other hand, disruption of the ROS balance and quantity of excessive amount of ROS in a specific region of the brain may cause defects in hippocampal plasticity and learning paradigms [10].The brain is the most energy-demanding organ, requiring 20% of the body’s energy [11], and mitochondrial oxidative phosphorylation is a significant source of ROS [12]. ROS and the accumulation of ROS-related damage are also associated with aging [13], oxidized lipids [14], and DNA damage [15]. However, recent studies have provided ample evidence of ROS-regulating neuronal development and function, including the establishment of neuronal polarity, growth cone pathfinding [16], and the regulation of connectivity and synaptic transmission [17].
In recent times, non-thermal plasma (NTP) has gained popularity as a great source of reactive species. NTP is an ionized gas condition that consists of a considerable quantity of reactive oxygen (ROS), hydrogen (RHS), and nitrogen species (RNS) [18,19]. With the development of physical plasma technologies, NTP has been widely investigated in cancer treatment [20], decontamination [21], dental treatment [22], wound healing [23], and other health areas, founding a new research field called plasma medicine. Recent studies with NTP have shown that it enhances proliferation of cells, cell migration, tube forming in endothelial cells, and wound healing in vitro, which is related to plasma-generated ROS and the stimulation of several growth factors, e.g., the vascular endothelial growth factor (VEGF) [24] and fibroblast growth factor-2 (FGF-2) [25]. Since limited levels of ROS promote cell proliferation, survival, migration, invasion, and angiogenesis, but may also induce autophagy, NTP-associated ROS could activate autophagy as well. As a result, NTP may be beneficial in the treatment of neurodegenerative (NDs) diseases, but it may also be detrimental. A common pathology of diverging NDs, such as Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s (HD) diseases, is the presence of misfolded proteins and the accumulation of denatured proteins inside or outside of cells [26,27]. Accumulated proteins usually generate protein aggregates and eventually cause neurodegeneration. Emerging evidence suggests that NDs develop due to defects in autophagy regulation [28,29]. Therefore, activation of autophagy has been proposed as a potential mechanism to clear abnormal protein aggregations [30,31], and thus as an effective way to cope with NDs. Applying a limited amount of NTP to initiate autophagy, without causing any cell or tissue damage, could constitute a potential treatment for NDs.
Although the role of NTP in neuronal growth and development has not been deeply studied, a few works have reported that cold plasma could improve differentiation of neuronal stem cells and increase neuronal regeneration following trauma [32,33,34]. Another modulator of neuronal outgrowth and regeneration following injury can be found in glial cell microenvironment. Glial cells contain different non-neuronal cells, including astrocytes, that promote neuronal health and viability by sustaining homeostasis and by assuring support and protection for neurons. In a healthy central nervous system (CNS), astrocytes maintain neuronal health by secreting proteins and neurotrophic factors [35], and plasma application has been shown to have a significant effect on astrocyte growth. However, the studies performed on neuronal growth, development, and disease treatment using NTP have not been enough to determine the underlying mechanism of action. In addition, there are a considerable number of factors that should be considered for further research studies. The objective of this review is to highlight the role of reactive species in neuron growth and development and to focus on the underlying mechanism by which NTP acts on neuronal growth, differentiation, and regeneration to promote post-injury healing, together with functional regeneration.
2. Role of ROS Generated in the Neuronal Environment
The term ROS defines a group of reactive free radicals that originate from molecular oxygen (O2). ROS, such as the superoxide anion (O2•−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and the hydroxyl radical (HO•), consist of radical and non-radical oxygen species generated by a limited reduction of oxygen [36]. These are species of oxygen that can exist independently with one or more unpaired electrons. ROS are highly reactive molecules that undergo several reduction reactions in normal cells [37]. To maintain the normal balance of ROS in cells, several defense mechanisms have been developed that include both enzymatic and non-enzymatic machineries. The enzymatic defense system includes glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT), whereas non-enzymatic antioxidants include glutathione (GSH), uric acid, melatonin, vitamins C and E, polyphenols, and other molecules. In the glutathione redox cycle, GPX utilizes GSH to reduce organic peroxides and H2O2, while the glutathione reductase reduces the oxidized form of GSH with concomitant oxidation of nicotinamide adenine dinucleotide phosphate [38]. In addition, SODs are enzymes that require metal cofactors for the conversion of O2•− into O2 and H2O2 [39], and CAT is a heme-containing peroxisomal enzyme important in the decomposition of intracellular H2O2 [40]. CAT catalytically decomposes H2O2 into water and oxygen (α phase), or peroxidatively, by oxidizing alcohols, formate, or nitrate (β phase) [41]. ROS generation is in subtle balance with these antioxidative defense mechanisms. If this balance is disrupted, the ROS accumulation could become deleterious, causing different disease states. ROS directly activate oxidative stress responsive mechanisms.
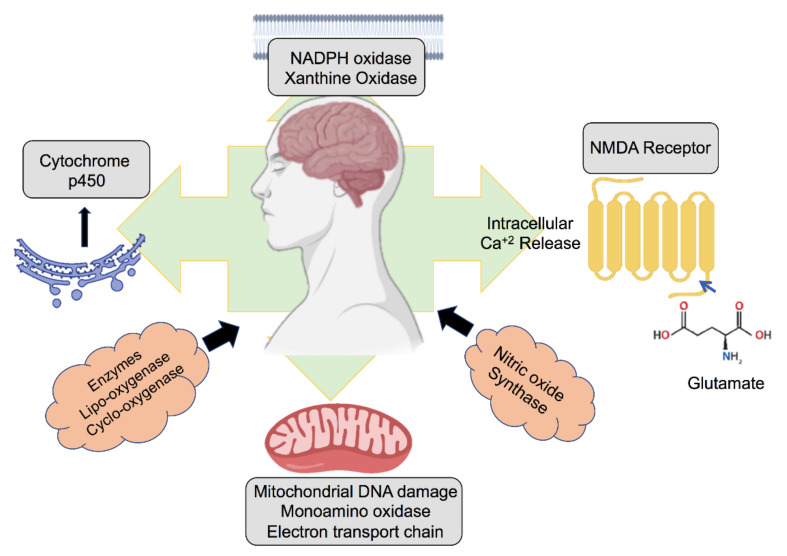
In the brain tissue, microglia and astrocytes produce ROS and regulate synaptic and non-synaptic communication between neurons and glia. Although ROS are widely related to a number of ND pathologies, reported studies also suggest that ROS play an essential role in several physiological processes [42]. Endogenously, ROS can be produced in cellular organelles. Mitochondria and the NOX system are major contributors to cellular ROS production. Mitochondrial ROS have two sources: the mitochondrial respiratory or electron transport chain (ETC) and the mitochondrial outer membrane flavoprotein, also known as monoamine oxidase (MAO). Mitochondrial ETC is a powerful supplier of ROS during increased Ca2+ signaling. It has been reported that elevating Ca2+ and Na+ is sufficient to produce free radicals from isolated rat mitochondria [43]. Again, ROS are inevitable by-products of cellular respiration, during which an electron that escapes from the ETC binds oxygen to form O2•− [44]. Superoxide anion generation occurs mainly at two points of the ETC, at Complex I (NADH dehydrogenase) and Complex III (ubiquinone-cytochrome c reductase). Thus, ROS formation is excessive due to metabolic demand and excitotoxicity.
Monoamine oxidases (MAO) are also potential sources of ROS in the brain. MAO, in the mitochondrial outer membrane, induce oxidative stress by producing hydrogen peroxide by oxidation of monoamine substrates [45,46].
ROS generation also occurs by the action of several enzymatic systems, such as lipoxygenases, xanthine oxidases, cyclooxygenases, monooxygenases, nitric oxide synthases (NOS), and NADPH oxidases (NOX). Among them, NOX has been described as a critical enzyme that utilizes molecular oxygen as substrate and regulates the production of ROS. Different research groups have reported seven NOX paralogs comprising NOX1–5 and dual oxidase 1/2 (Duox1/2) [47]. Noteworthy, NOX1 and NOX2 are overexpressed in microglia [48].
Glutamate is a well-known excitatory neurotransmitter engaged in neural function. Excessive buildup of intercellular glutamate leads to increased concentrations of ROS and RNS in neuronal cells [49]. Activation of the NMDA receptor by glutamate application to cultured forebrain neurons stimulates a localized ROS formation. It is known that glutamate reduces intracellular pH in a Ca2+-dependent manner [50], while NOS also plays a role in superoxide generation. In addition, stimulating NMDA receptor results in the production of superoxide [51].
Other cytosolic sources of ROS are cytochrome p-450 enzymes in the endoplasmic reticulum, which generate these compounds during fatty acid oxidation. With the degradation of cellular material, ROS are moved to lysosomes via autophagic or endocytic pathways. Besides, hydrogen peroxide can freely diffuse into the lysosome from the cytoplasm. In the lysosome, low pH and high iron concentrations build a supreme environment for the formation of ROS from Fenton reactions; thus, if there is an accumulation of oxidants, lysosomes must face oxidative stress [52]. The physiological sources of ROS in the brain are shown in Figure 1.
Figure 1.
Different sources of reactive oxygen species (ROS) in the brain and neurons.
The generation of oxidative stress resulting from an excessive production of ROS is closely related to age. As age increases, in the brain, the usual antioxidant defense machinery is reduced, leading to an increase in the susceptibility of the brain to the destructive effects of oxidative molecules [53]. It is assumed that mitochondrial DNA (mtDNA) damage is caused primarily by free radicals of mitochondrial origin. Several studies have reported elevated levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) in mtDNA in the aged brain, which is a biomarker of oxidative DNA damage [54]. Besides, there is an increased accumulation of mtDNA mutations in the aging brain; thus, mitochondrial energy production-related genes become less active, and dysfunctional mitochondria are observed [55]. In addition, age per se is a risk factor that makes mitochondria more vulnerable to oxidative stress and gives rise to dysfunctional mitochondria. Eventually, these changes create a vicious cycle between mitochondrial dysfunction and oxidative damage [55]. Moreover, the “mitochondrial theory of aging” by Harman suggests that mitochondria play a vital role in aging; it indicates that aging results from accumulated damage caused by mitochondrial ROS in both cells and tissues [56].
3. ROS and Neurogenerative Disease Pathology
ROS have long been studied and have been established as damaging agents from the perspective of the nervous system, aging, and degeneration [57]. Oxidative stress is caused by an imbalanced production of ROS and RNS and antioxidants in cells and tissues. ROS and RNS are overproduced for several reasons, such as aging and disease, and they eventually cause cell damage by inducing chemical modifications on lipids, proteins, and nucleic acids. This type of oxidative modification may be a triggering event, ultimately leading to neuronal injury. Moreover, oxidative stress is a significant cause of neuronal disorders. Thus, excessive production of ROS/RNS has been considered a mechanism for the neurodegeneration associated with other problems of neurons, such as hypoxia and hypoglycemia [58], as well as with the neurodegeneration seen in AD [59], PD [60], and amyotrophic lateral sclerosis (ALS) [61]. In depressive disorders, oxidative stress also plays a crucial role in disease pathology. All the neurodegenerative disorders (NDs) possess similar alterations, such as abnormally aggregated protein deposition and oxidative damage caused by mitochondrial dysfunction [62]. ROS-induced oxidative stress plays a key role in the pathogenesis of AD, as it is a critical factor in Aβ peptide accumulation [63]. Moreover, oxidative damage, resulting in lipid peroxidation and nitration, reactive carbonyl species formation, and nucleic acid oxidation, is observed at increased ratios in neurons of patients with AD [64]. Cytochrome oxidase, the pyruvate dehydrogenase complex, and the α-ketoglutarate dehydrogenase complex show decreased activity as a result of oxidative damage in AD [65].
In the development of PD, ROS play a preeminent role. Excessive ROS accumulation is crucial not only in the pathology of the PD-related gene PINK1, but also in the physiology of PINK1/Parkin-related mitophagy [66]. PD also shows a general increase in end-product markers of oxidative and nitrosative stress, reflecting excessive damage to biomolecules. ALS shows similar hallmarks of protein accumulation and oxidative damage. Mitochondrial oxidative damage has also been demonstrated in patients affected by ALS [67]. Mutations in the ALS-related genes TDP-43, FUS/TLS, and p62 also increase mitochondrial ROS and oxidative stress [68,69]. Apart from these neurodegenerative disorders, ROS also plays a major role in depression [70].
4. ROS in Neuronal Growth, Differentiation, and Synaptic Plasticity
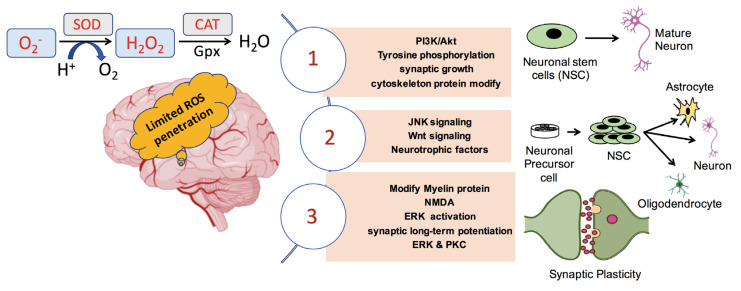
From the discussion above, it is clear that ROS and subsequent oxidative stress are responsible for neurodegeneration, which has also been established in previous studies [71,72]. However, ROS have also been considered as regulators and modulators of signaling pathways and gene expression, many of which are known to cause neuronal growth, differentiation, and synaptic plasticity. There are redox-dependent mechanisms that promote neuronal differentiation. While the major ROS in cells are O2•−, •OH, peroxynitrite (ONOO−), and H2O2, recent studies have suggested that high, but sublethal, levels of O2•− and H2O2 can control intracellular signaling pathways in neuronal cells by acting on gene expression, cellular growth, and differentiation [73,74]. As described in Section 2, NOX enzymes are a regulated source of ROS in neurons [75]. ROS production during normal neuronal development does not alter the probability of a cell of becoming a neuron, but it affects neuronal maturation in terms of morphology, physiology, and biochemistry. The possible mechanism behind this role is that ROS reversibly oxidize enzymes such as protein tyrosine phosphatases. ROS can influence tyrosine phosphorylation and subsequent signaling, controlling protein stabilization [76] during early neurogenesis and the outgrowth stage [8]. Studies also suggest that ROS, in particular H2O2, are essential for activity-induced synaptic terminal growth and sufficient to drive this process [77]. It has been found that nerve cells activate a process of self-renewal when ROS are present in the brain via the PI3K/Akt pathway [78], and it is suspected that this process may induce synapse growth. ROS can also maintain cytoskeletal changes by direct redox modification of structural cytoskeletal proteins and by indirect modification of the proteins or signaling pathways controlling cytoskeletal dynamics. All major cytoskeletal elements and cytoskeleton-associated proteins are subject to direct redox alterations [79], mainly actin, tubulin, and neurofilament fractions. Redox modification of cytoskeletal proteins likely affects other signaling pathways [80], directly or indirectly; for example, the cellular redox state guides neuronal growth cone responses to extracellular cues [81].
Similarly, stem cell differentiation is significantly controlled by the action of the redox state in various signaling pathways. Recent evidence suggests that mesenchymal stem cells (MSCs) can be used to replace injured neurons and support endogenous neuronal cell repair or survival by releasing neurotrophic factors [82,83]. Other studies also suggest that ROS-mediated neurogenesis is based on JNK signaling activation [84]. Wnt5a promotes neurogenic differentiation in human adipose derived stem cells (ADSCs), binding to Fz3 and Fz5, and signaling through the Wnt5a-JNK pathway [85]. Different members of the Wnt signaling pathway also play important roles in ROS-mediated neuronal cell differentiation, including Wnt-3a and Wnt-7a. Additionally, the Wnt/β-catenin pathway is activated in response to ROS [86]. Finally, Wnt can stimulate the expression of neuroD, Brn3a, and neurogenin 1 (Ngn1),which are sensory neuron markers, via activation of Tlx3 [87].
Synaptic plasticity is the ability of synapses to regulate their strength, connectivity, and structure based on previously experienced activity [88]. Current evidence suggests that synaptic plasticity is regulated by both direct and indirect modes of ROS action [89]. ROS interfere with increased neuronal activity by altering the myelin basic protein. They can also induce synaptic long-term potentiation (LTP), which guides activity-dependent synaptic plasticity and memory consolidation [90]. One of the most widely studied varieties of synaptic plasticity in hippocampal LTP [91], which is a persistent increase in synaptic efficacy elicited by brief, high-frequency stimulation. This requires nitric oxide synthase activity, providing compelling evidence that nitric oxide (NO), which is an ROS, can act as an intercellular messenger during LTP. Synaptic plasticity can also be regulated by the N-methyl-D-aspartate (NMDA)-mediated pathway, where ROS play an important role [92]. In this process, NMDA receptor (NMDAR) activation causes insertion of AMPA receptors into the postsynaptic membrane. As a result, the ERK mitogen-activated protein kinase signaling cascade is activated, and it phosphorylates the cAMP-responsive element binding protein, a transcription factor that can intervene in the transcription of multiple “synapse-associated genes” necessary for memory consolidation. NMDAR activation promotes O2•− production by NOX, which is vital for the activation of the NMDAR-mediated ERK pathway, for the full expression of NMDAR-mediated LTP, and to trigger hippocampal-dependent memory tasks [93]. Furthermore, ROS control canonical synaptic plasticity mechanisms by direct oxidative modification, by inhibiting phosphatases PP1, PP2, PTEN, and calcineurin, causing elevated kinase signaling, including those involving ERK and PKC [94].
A common pathology between diverging NDs such as AD, PD, and HD, and type II diabetes is the accumulation of denatured proteins inside or outside the cell. As previously mentioned, accumulated proteins usually form aggregates. Emerging evidence suggests that neurodegenerative diseases develop due to defects in autophagy regulation [95]. Therefore, autophagy activation has been proposed as a possible means of clearing abnormal protein aggregates [30]; consequently, as an effective way to cope with NDs. Many medicines have been designed to trigger autophagy for treating neurological disorders [96]. Physiological ROS induce autophagy [97] to maintain cellular homeostasis in different types of cells, whereas dysregulation of redox signaling can disrupt autophagic activity [98,99]. Many mechanisms and pathways are involved in ROS-induced autophagy, e.g., JNK/AP-1 pathway initiation by oxidative stress is thought to reinforce autophagy, and many genes encoding autophagy proteins are transcriptional targets of AP-1 [65]. Additionally, activation of autophagy via the oxidative stress-induced JNK/AP-1 mechanism can control synaptic terminal size and strength. Figure 2 summarizes the pathways related to neuronal growth, differentiation, and synaptic plasticity mediated by ROS. It would be of great importance if future studies were focused on the mechanisms underlying controlled autophagy initiated by ROS, aiming to assist in neuronal disease treatments.
Figure 2.
ROS mediated pathways that influence neuronal maturation and differentiation as well as synaptic plasticity in the brain.
5. ROS-Mediated Therapies for Neuronal Injuries
Recently, physical therapeutics whose mechanisms of action are related to ROS generation, such as different types of radiation and laser, photodynamic, non-thermal plasma, and infrared light treatments, have being used against several diseases, mainly for cancer treatment. However, a strict control of the therapeutic regimen should be followed when treating patients, given that any of these treatments can reach extreme levels of toxicity and can cause permanent damage if not carefully controlled. Such negative effects have been observed in a number of studies regarding neuronal treatment and growth induction light therapy or photo biomodulation (PBM) [100,101].
Low-level laser therapy (LLLT), also known as laser light therapy, is a method that generates shallow levels of ROS in cells, which are beneficial [102]. It is a non-thermal method of low-intensity light application. In recent studies, LLLT has been applied to neurons to treat neurotoxic [103], peripheral and central nerve, and spinal cord injuries [104], and to increase axonal growth and nerve regeneration [105]. Studies have also reported that LLLT stimulates the release of ROS, which eventually activates the NF-kB factor and releases NO in normal murine cortical neurons, resulting in a stimulation of beneficial effects, such as neuroprotection and growth [106].
Multi-watt near-infrared light therapy (NILT) is another therapeutic approach whose effects on neuronal injuries and growth have been studied [107]. Similarly, studies have focused in the mechanism of action for generating ROS and NO at beneficial levels only, those that activate the NF-kB signaling pathway and that lead to increased synaptogenesis, neurogenesis, and generation of inflammatory mediators and growth factors [108]. For NILT, the wavelength that is generally used oscillates between 800 and 1100 nm. It is evident that infrared light can penetrate the brain and has a beneficial effect on traumatic brain injury [109]. In addition, this therapy has been proven to be effective in patients suffering from anxiety or depression [110].
It has recently been shown that the human gut microbiota plays a vital role in the physiology and neurochemistry of the CNS [111]. However, the mechanism underlying the effects of microbiota on CNS disorders is yet to be discovered. Nevertheless, a recent hypothesis states that, by the influence of gut microbiota, the gut–brain axis generates physiological levels of ROS. Such controlled levels of ROS stimulate the activation of the antioxidant defense system, reducing the possibility of cell injury [112].
ROS-mediated therapies for neuronal regeneration or disease treatment may situate patients in susceptible situations because there is a thin line between having beneficial and harmful effects. Therefore, the dose of treatment and the level of ROS generation must be carefully controlled and maintained to an optimum level. Ideally, future research should be focused on the application of these therapies to patients to determine specific doses to be applied to keep them safe from possible side effects.
6. Application of NTP in Biomedicine
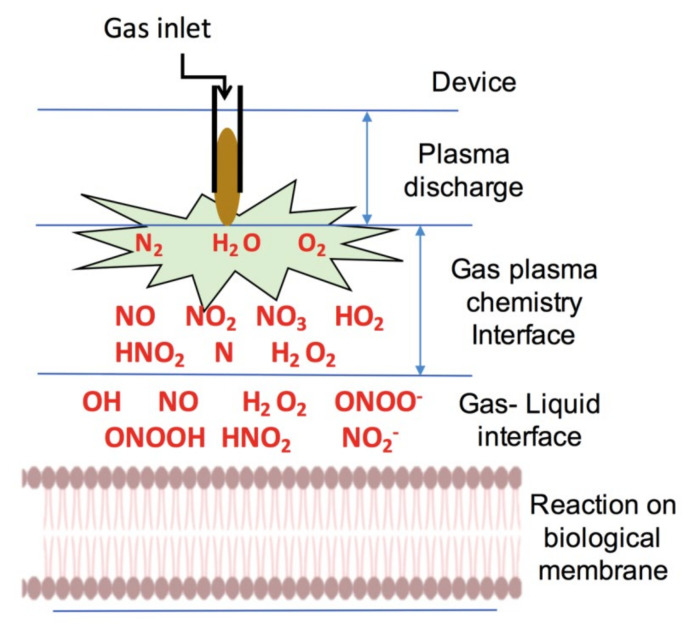
Non-thermal plasma, the fourth state of matter, is becoming a useful tool with an increasing number of biomedical applications; currently, it has been applied to many disease treatments such as hair loss and cancers [113]. One of the underlying mechanisms of action of NTP over biological systems relies on the fact that it is an excellent source of reactive species [114]. In liquid–air surfaces, such as those around the cell, NTP interacts with the liquid phase of the biological membrane and produces a large variety of reactive species, as demonstrated in Figure 3.
Figure 3.
Different reactive species generated by gas plasma in air and in gas–liquid interfaces.
Generally, the efficiency achieved by indirect NTP application via treatment media or water depends on the generation of reactive species in liquid. When a liquid and plasma react, hydrogen peroxide, hydroxyl radicals, hydrogen radicals, singlet oxygen, nitrogen oxide, and some other species are formed [115]. The amount, nature, and types of reactive species generated by plasma mainly depend on the type of gas device used to generate the plasma [18]. In addition, the generation of reactive species can be controlled by modifying gas flow rate, plasma treatment time, plasma discharge distance, and other parameters. Beside reactive species, plasma also generates free electrons, UV radiation, and excited ions in the treated surface [116].
For biomedical applications, plasma jet devices and dielectric-barrier discharge (DBD) sources are the most commonly used devices [117,118,119]. Some of the different types of plasma devices and gases used for biomedical applications are listed in Table 1.
Table 1.
Different types of NTP devices used in biomedical applications.
| Year | Name of Device | Gas Used | Biomedical Application | Reference |
|---|---|---|---|---|
| 2019 | KINpen Jet | Argon | Bone Cancer | [120] |
| 2019 | MiniJet-R | Argon | Bone Cancer | [120] |
| 2019 | Plasma Jet | Argon | Skin Cancer | [121] |
| 2017 | DBD | Nitrogen | Cervical cancer | [122] |
| 2018 | Micro Plasma | Helium | Breast Cancer | [123] |
| 2018 | Plasma Jet | Helium | Breast Cancer | [124] |
| 2018 | Micro Plasma | Helium | Brain Cancer | [123] |
| 2020 | Plasma jet | Helium | Prostate | [125] |
| 2019 | DBD | Helium and air | Wound healing | [126] |
| 2018 | DBD | Helium | Wound healing | [127] |
| 2009 | Plasma Jet | Helium, Nitrogen, Oxygen | Dentistry | [128] |
| 2020 | DBD | Helium | Dentistry | [129] |
| 2012 | Microsecond pulse plasma jet | Helium and Oxygen | Disinfection | [130] |
| 2019 | Surface micro-discharge plasma | Air | Sanitation | [131] |
There is evidence that NTP enhances cell proliferation [132] and controls cell migration [133], tube formation in endothelial cells [134], and wound healing in vitro [135,136]; all these effects have been associated with plasma-generated ROS and the stimulation of growth-related factors, such as the vascular endothelial growth factor (VEGF) and the fibroblast growth factor-2 (FGF-2) [137,138]. NTP has also been used as a reliable tool for surface decontamination and sterilization of medical devices because it can inactivate microorganisms [21,116,139]. Recently, NTP has also been used in dentistry, and its beneficial effects have also been related to the generation of ROS. NTP can be used to reduce the difficulties of various dental complications, such as the elimination of caries, root canal sterilization, and bleaching. The application of this type of plasma can be done by both direct and indirect methods [140]. Several studies on the emerging field of plasma medicine have established that NTP is an emerging therapeutic agent for cancer treatment [97,141,142]. Moreover, because of the safe and effective action of plasma, research related to plasma medicine is gaining more attention. However, future research should focus on the controlled generation of different reactive species to minimize any possibility of harmful effects when treating human pathologies.
7. Present Scenario of Plasma Medicine Applied to Neuronal Growth
Previous studies have shown that low levels of ROS promote cell proliferation, survival, migration, invasion, and angiogenesis; therefore, NTP-associated ROS/RNS could promote neuronal growth and migration. It is also known that NDs and traumatic CNS damage are currently difficult to treat. However, neural stem cells (NSCs) can improve their treatment, and several studies have been investigating NSC proliferation induced by NTP [143].
Recent evidence suggests that NTP regulates diverse cellular processes; it can also regulate neural differentiation. However, the exact mechanisms behind the physicochemical signaling process elicited by ROS/RNS on biological systems remains elusive. Among all the plasma produced reactive species, NO plays a significant role in the CNS. NO is an essential signaling molecule required for many biological processes and plays a dual role in the physiological system, especially in the case of neurotoxicity and neuroprotection [144,145,146]. NO, at a physiologically minimal amount, can provide neuroprotection by regulating diverse signaling pathways, such as the PI3K/Akt [147] and the NO/cGMP/PKG pathways [148]. Additionally, NO is also a strong cerebral vasodilator agent [149] that can enhance cerebral blood flow (CBF) supply during ischemic brain injury or hypoxia [150].
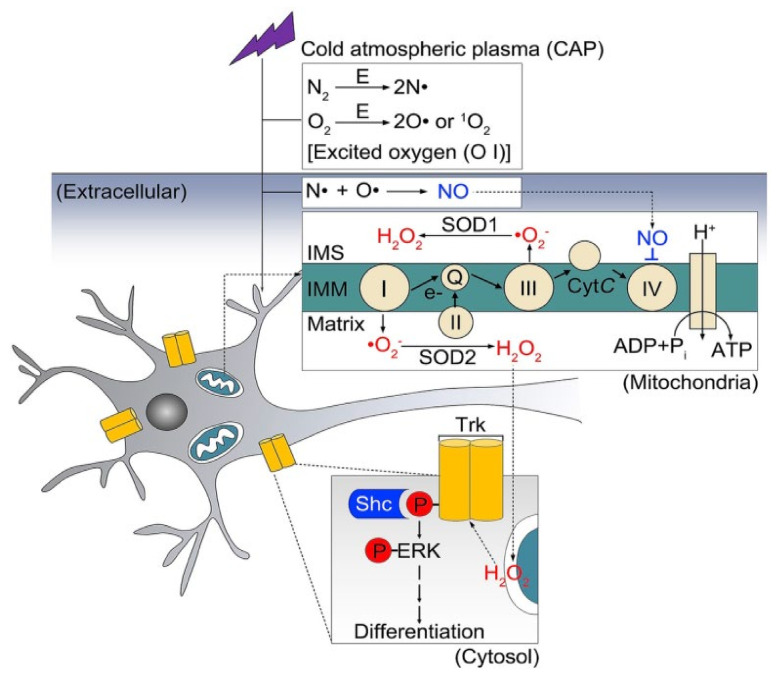
Neural differentiation by plasma can have some advantages. First, the differentiation process with NBP treatment is faster than without any treatment. Second, plasma application increases differentiation efficiency noticeably by upregulating specific genes. Finally, NTP treatment can differentiate a large percentage of cells with or without other chemical inducers [151]. Interestingly, in a recent study, it was found that there are physicochemical and biological connections between the non-thermal plasma cascade and the Trk/Ras/ERK signaling pathway; what is known about the underlying mechanism is summarized in Figure 4 [152]. Besides, it was seen that the nerve growth factor exerted its effects mainly by interacting with the specific receptor TrkA [153]. Therefore, the stimulation by NTP resulted in neural differentiation. The authors considered that mitochondrial O2 and cytosolic H2O2 must have acted cooperatively because the experimental cytosolic increase in H2O2 by itself was not sufficient to initiate differentiation. Moreover, the mechanisms of phosphorylation of the TrkA receptor at specific sites remain unknown. Excited atomic oxygen generated in plasma eventually form reactive oxygen nitrogen species (RONS) and interact in the extracellular liquid phase with reactive atoms, generating NO. Large amounts of (O2•−) in the cell’s mitochondria exposed to plasma treatment showed that reversible inhibition of mitochondrial Complex IV is increased by extracellular NO [152].
Figure 4.
CAP-based mechanism for neuron stimulation differentiation by Trk/Ras/ERK signaling pathway [154]. IMS, intermembrane space; IMM, inner mitochondrial membrane.
A recent study also found that treatment by nanosecond-pulsed dielectric barrier discharge (nspDBD) plasma showed significant outcomes on astrocyte regrowth or neurite regeneration. The observed enhancement in neurite outgrowth as a result of low-intensity plasma stimulation in non-contact cocultures was probably because of soluble factors produced between neurons and astrocytes [155]. One possible mechanism of NTP enhancing neural outgrowth would be similar to that noticed in the stress preconditioning mechanism. It is evident that in the hypoxia/ischemia field that exposure to mild “mini-insults” causes injury tolerance makes neurons more resilient to damage in the future [156]. It is believed that ROS present in a low dose of NTP induce transient oxidative stress conditions, protecting cells against stronger stresses that may present later. This cytoprotective effect of non-thermal plasma has already been reported for other types of cells [157].
Au-Xiong et al., showed that NTP treatment can significantly elevate the proliferation and differentiation rates in C17.2 murine NSC lines [158]. Moreover, almost 75% of NSCs differentiated into neuronal cell lines after exposure to NTP; this percentage is higher than that achieved by specific growth factors. Differentiated neurons showed high β-tubulin III protein expression levels; this protein is considered a neuron marker [159]. Studies made on the usage of NTP in neuronal treatments are summarized in Table 2.
Table 2.
Non-thermal plasma application for neuronal treatments.
| Year | Plasma Device | Cell Line | Mechanism | Activity | Reference |
|---|---|---|---|---|---|
| 2017 | Plasma Jet | SH-SY5Y | Reducing cell apoptosis | Neuroprotection | [33] |
| 2013 | Micro-plasma jet | Neural stem cells | NO species induce gene expression | Cell Differentiation | [32] |
| 2019 | Nanosecond-pulsed dielectric barrier discharge | Cortical neurons | Stress preconditioning mechanism | Neurite re-growth | [155] |
| 2018 | DBD (dielectric barrier discharge) plasma | Mouse neuroblastoma Neuro 2A (N2a) cells | activate the Trk/Ras/ERK signaling pathway | Cell Differentiation | [152] |
| 2017 | Plasma Jet | SH-SY5Y | Cytoprotection by supplying RONS | Treating diseases in the CNS related to glucose deprivation | [160] |
| 2018 | Plasma jet | SH-SY5Y | Neuroprotective effect by NO accumulation | Neuroprotection from hypoxic cell injury | [161] |
| 2019 | Plasma Bubbling system | PC12 cells | Neurite growth | Erk and CREB activation | [162] |
8. Future Perspectives
Low levels of ROS can be helpful for neuronal growth and disease management, and a number of therapies can generate a controlled amount of ROS. Photo biomodulation or PBM therapy has already been applied to several disease conditions as a successful treatment strategy [163] and has also been studied for neuronal treatments. This phenomenon needs to be further studied for optimization and for introducing it as a new strategy for neuronal regeneration. PBM and various light therapies such as infrared and laser therapies stimulate stem cell proliferation and differentiation. The underlying mechanism can be related to ROS production because the stem cell niche is hypoxic, and, when stem cells encounter ROS, their differentiation program is activated [164]. This knowledge could be applied in neuronal stem cell treatments to increase differentiation.
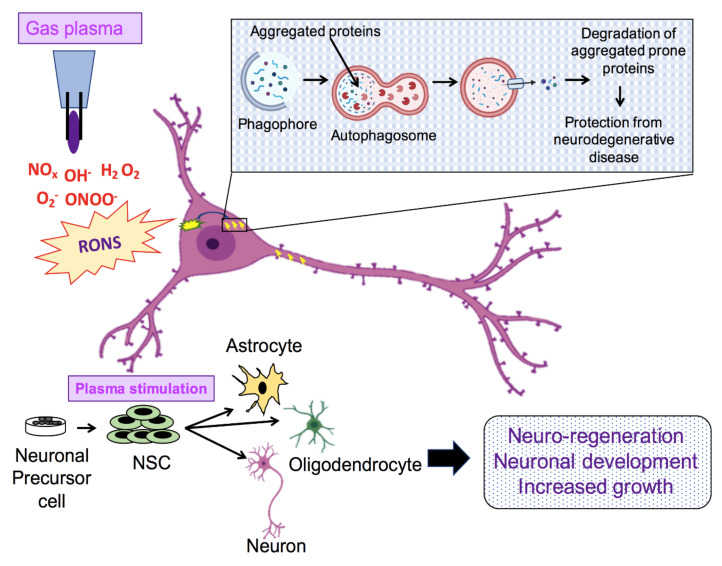
In biomedical applications and treatments, NTP has an immense potential. In various fields, NTP has already proved to be a great treatment strategy. Although in the field of neuronal growth and in the treatment of neurology-related diseases NTP has not been explored enough to find a specific mechanism of action and to describe its related effects, it can be a promising new strategy. It is already well established that NTP can generate a wide range of ROS and RNS, which is the main reason for influencing biological systems and samples. We discuss above the significance of ROS in different aspects of neural development. Thus, it can be assumed that NTP can act in the same way to initiate signaling pathways to promote neural development and neuroprotection. In addition, some studies have already reported that NTP can significantly stimulate NSC differentiation. Ideally, future studies should focus on finding the possible mechanisms by which NTP acts and how plasma can be potentially applied in growth and neural regeneration and to heal the neuronal injury or trauma post-injury (Figure 5). NTP has a very significant role in activating autophagy, which has been reported in a number of studies on different disease treatment strategies [97]. In the case of NDs, autophagy activation could play a significant role in the clearance of accumulated proteins. Therefore, NTP could be used to temporarily trigger autophagy to accelerate the clearance system. Activation of autophagy by NTP efficiently removes intracellular denatured proteins and promotes neuronal growth and development (Figure 5).
Figure 5.
Possible mechanism of action of non-thermal plasma over neuronal differentiation and neurodegenerative diseases.
From the discussion, it is clear that plasma treatments could become successful therapeutic strategies to protect neurons from degenerative cascades. However, plasma treatments still face several challenges in the field of neuroregeneration. Recent studies have shown that plasma at low doses can enhance antioxidant activity, stimulate immune cells such as macrophages to clear plaque, and stimulate stem cells. Therefore, future plasma medicine research could focus in the study of neurodegenerative disease-targeted approaches by using non-thermal plasmas for stimulating immune and stem cells, enhancing the antioxidant capacity of cells, and improving cell–cell communication. Considering the potential of these ROS-based therapies in the neuron, future studies should be performed using in vivo models and should involve clinical studies on new therapeutics, balancing the positive and negative effects of NTP.
Acknowledgments
The authors thank Raju Dash at Dongguk University College of Medicine (Korea) for the helpful discussions.
Author Contributions
Conceptualization, S.M. and N.K.K.; methodology, S.M.; software, N.K.; investigation, S.M. and N.K.; writing—original draft preparation, S.M., N.K., and N.K.K.; writing—review and editing, N.K.K., I.S.M. and E.H.C.; and supervision, N.K.K., I.S.M. and E.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation (NRF) of Korea, funded by the Korea government (NRF-2016K1A4A3914113), and also the present research has been conducted using a Research Grant from Kwangwoon University in 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bardaweel S.K., Gul M., Alzweiri M., Ishaqat A., HA A.L., Bashatwah R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018;50:193–201. doi: 10.5152/eurasianjmed.2018.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin K.R., Barrett J.C. Reactive oxygen species as double-edged swords in cellular processes: Low-dose cell signaling versus high-dose toxicity. Hum. Exp. Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Kim J., Bae J.-S. ROS homeostasis and metabolism: A critical liaison for cancer therapy. Exp. Mol. Med. 2016;48:e269. doi: 10.1038/emm.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massaad C.A., Klann E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2010;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsatmali M., Walcott E.C., Makarenkova H., Crossin K.L. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol. Cell. Neurosci. 2006;33:345–357. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olguín-Albuerne M., Morán J. ROS produced by NOX2 control in vitro development of cerebellar granule neurons development. ASN Neuro. 2015;7:1759091415578712. doi: 10.1177/1759091415578712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oswald M.C.W., Brooks P.S., Zwart M.F., Mukherjee A., West R.J.H., Giachello C.N.G., Morarach K., Baines R.A., Sweeney S.T., Landgraf M. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. eLife. 2018;7:e39393. doi: 10.7554/eLife.39393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magistretti P.J., Allaman I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz A. OP-20—Mitochondrial ROS and ageing. Free Radic. Biol. Med. 2017;108:S9. doi: 10.1016/j.freeradbiomed.2017.04.059. [DOI] [Google Scholar]
- 14.Chmielowska-Bąk J., Izbiańska K., Deckert J. Products of lipid, protein and RNA oxidation as signals and regulators of gene expression in plants. Front. Plant Sci. 2015;6:405. doi: 10.3389/fpls.2015.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davalli P., Marverti G., Lauriola A., D’Arca D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxidative Med. Cell. Longev. 2018;2018:2389523. doi: 10.1155/2018/2389523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson C., Muñoz-Palma E., Henríquez D.R., Palmisano I., Núñez M.T., Di Giovanni S., González-Billault C. A Feed-Forward Mechanism Involving the NOX Complex and RyR-Mediated Ca2+ Release During Axonal Specification. J. Neurosci. 2016;36:11107. doi: 10.1523/JNEUROSCI.1455-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauvin J., Judée F., Yousfi M., Vicendo P., Merbahi N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017;7:4562. doi: 10.1038/s41598-017-04650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorbanev Y., Privat-Maldonado A., Bogaerts A. Analysis of Short-Lived Reactive Species in Plasma–Air–Water Systems: The Dos and the Do Nots. Anal. Chem. 2018;90:13151–13158. doi: 10.1021/acs.analchem.8b03336. [DOI] [PubMed] [Google Scholar]
- 20.Dubuc A., Monsarrat P., Virard F., Merbahi N., Sarrette J.-P., Laurencin-Dalicieux S., Cousty S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Adv. Med. Oncol. 2018;10:1758835918786475. doi: 10.1177/1758835918786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau M., Orange N., Feuilloley M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Cha S., Park Y.-S. Plasma in dentistry. Clin. Plasma Med. 2014;2:4–10. doi: 10.1016/j.cpme.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubinova S., Zaviskova K., Uherkova L., Zablotskii V., Churpita O., Lunov O., Dejneka A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017;7:45183. doi: 10.1038/srep45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H.-Y., Lee H.-J., Kim G.-C., Choi J.-H., Hong J.-W. Plasma cupping induces VEGF expression in skin cells through nitric oxide-mediated activation of hypoxia inducible factor 1. Sci. Rep. 2019;9:3821. doi: 10.1038/s41598-019-40086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalghatgi S.U., Fridman A., Friedman G., Clyne A.M. Non-thermal plasma enhances endothelial cell proliferation through fibroblast growth factor-2 release; Proceedings of the 2009 IEEE International Conference on Plasma Science—Abstracts; San Diego, CA, USA. 1–5 June 2009; p. 1. [Google Scholar]
- 26.Sweeney P., Park H., Baumann M., Dunlop J., Frydman J., Kopito R., McCampbell A., Leblanc G., Venkateswaran A., Nurmi A., et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017;6:6. doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo E.H., Lansbury P.T., Kelly J.W. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA. 1999;96:9989. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nah J., Yuan J., Jung Y.-K. Autophagy in neurodegenerative diseases: From mechanism to therapeutic approach. Mol. Cells. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frake R.A., Ricketts T., Menzies F.M., Rubinsztein D.C. Autophagy and neurodegeneration. J. Clin. Investig. 2015;125:65–74. doi: 10.1172/JCI73944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mputhia Z., Hone E., Tripathi T., Sargeant T., Martins R., Bharadwaj P. Autophagy Modulation as a Treatment of Amyloid Diseases. Molecules. 2019;24:3372. doi: 10.3390/molecules24183372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komatsu M., Ueno T., Waguri S., Uchiyama Y., Kominami E., Tanaka K. Constitutive autophagy: Vital role in clearance of unfavorable proteins in neurons. Cell Death Differ. 2007;14:887–894. doi: 10.1038/sj.cdd.4402120. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Z., Zhao S., Mao X., Lu X., He G., Yang G., Chen M., Ishaq M., Ostrikov K. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Res. 2014;12:387–399. doi: 10.1016/j.scr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Yan X., Qiao Y., Ouyang J., Jia M., Li J., Yuan F. Protective effect of atmospheric pressure plasma on oxidative stress-induced neuronal injuries: Anin vitrostudy. J. Phys. D Appl. Phys. 2017;50:095401. doi: 10.1088/1361-6463/aa5603. [DOI] [Google Scholar]
- 34.Zhao S., Han R., Li Y., Lu C., Chen X., Xiong Z., Mao X. Investigation of the mechanism of enhanced and directed differentiation of neural stem cells by an atmospheric plasma jet: A gene-level study. J. Appl. Phys. 2019;125:163301. doi: 10.1063/1.5060650. [DOI] [Google Scholar]
- 35.Dowell J.A., Johnson J.A., Li L. Identification of Astrocyte Secreted Proteins with a Combination of Shotgun Proteomics and Bioinformatics. J. Proteome Res. 2009;8:4135–4143. doi: 10.1021/pr900248y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray P.D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa T., Sato E.F., Choudhury T., Nagata K., Kasahara E., Matsui H., Watanabe K., Inoue M. Effect of nitric oxide on the oxygen metabolism and growth of E. faecalis. J. Clin. Biochem. Nutr. 2009;44:178–184. doi: 10.3164/jcbn.08-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patlevič P., Vašková J., Švorc P., Jr., Vaško L., Švorc P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016;5:250–258. doi: 10.1016/j.imr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasauer A., Chandel N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014;92:90–101. doi: 10.1016/j.bcp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Aksoy Y., Balk M., ÖĞÜŞ H., Özer N. The mechanism of inhibition of human erythrocyte catalase by azide. Turk. J. Biol. 2005;28:65–70. [Google Scholar]
- 41.Lardinois O.M., Rouxhet P.G. Peroxidatic degradation of azide by catalase and irreversible enzyme inactivation. Biochim. Biophys. Acta. 1996;1298:180–190. doi: 10.1016/S0167-4838(96)00130-6. [DOI] [PubMed] [Google Scholar]
- 42.Atkins C.M., Sweatt J.D. Reactive Oxygen Species Mediate Activity-Dependent Neuron–Glia Signaling in Output Fibers of the Hippocampus. J. Neurosci. 1999;19:7241–7248. doi: 10.1523/JNEUROSCI.19-17-07241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dykens J.A. Isolated Cerebral and Cerebellar Mitochondria Produce Free Radicals when Exposed to Elevated Ca2+ and Na+: Implications for Neurodegeneration. J. Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen R., Lai U.H., Zhu L., Singh A., Ahmed M., Forsyth N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018;6:132. doi: 10.3389/fcell.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naoi M., Maruyama W., Akao Y., Yi H., Yamaoka Y. Involvement of type A monoamine oxidase in neurodegeneration: Regulation of mitochondrial signaling leading to cell death or neuroprotection. J. Neural Transm. 2006:67–77. doi: 10.1007/978-3-211-33328-0_8. [DOI] [PubMed] [Google Scholar]
- 46.Cai Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review) Mol. Med. Rep. 2014;9:1533–1541. doi: 10.3892/mmr.2014.2040. [DOI] [PubMed] [Google Scholar]
- 47.Eun H.S., Cho S.Y., Joo J.S., Kang S.H., Moon H.S., Lee E.S., Kim S.H., Lee B.S. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci. Rep. 2017;7:11060. doi: 10.1038/s41598-017-11280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Infanger D.W., Sharma R.V., Davisson R.L. NADPH oxidases of the brain: Distribution, regulation, and function. Antioxid. Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 49.Xin H., Cui Y., An Z., Yang Q., Zou X., Yu N. Attenuated glutamate induced ROS production by antioxidative compounds in neural cell lines. RSC Adv. 2019;9:34735–34743. doi: 10.1039/C9RA03848E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds I.J., Hastings T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. Off. J. Soc. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinzel B., John M., Klatt P., Böhme E., Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Pt 3Biochem. J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terman A., Kurz T. Lysosomal iron, iron chelation, and cell death. Antioxid. Redox Signal. 2013;18:888–898. doi: 10.1089/ars.2012.4885. [DOI] [PubMed] [Google Scholar]
- 53.Ren X., Zou L., Zhang X., Branco V., Wang J., Carvalho C., Holmgren A., Lu J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017;27:989–1010. doi: 10.1089/ars.2016.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Sun N., Youle R.J., Finkel T. The Mitochondrial Basis of Aging. Mol. Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harman D. The free radical theory of aging: Effect of age on serum copper levels. J. Gerontol. 1965;20:151–153. doi: 10.1093/geronj/20.2.151. [DOI] [PubMed] [Google Scholar]
- 57.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 58.Halliwell B., Gutteridge J.M., Cross C.E. Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 59.Su B., Wang X., Nunomura A., Moreira P.I., Lee H.g., Perry G., Smith M.A., Zhu X. Oxidative stress signaling in Alzheimer’s disease. Curr. Alzheimer Res. 2008;5:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenner P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl. 3):S26–S36, discussion S36–S28. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 61.Carrí M.T., Ferri A., Cozzolino M., Calabrese L., Rotilio G. Neurodegeneration in amyotrophic lateral sclerosis: The role of oxidative stress and altered homeostasis of metals. Brain Res. Bull. 2003;61:365–374. doi: 10.1016/S0361-9230(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 62.Cenini G., Lloret A., Cascella R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019;2019:2105607. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonda D.J., Wang X., Perry G., Nunomura A., Tabaton M., Zhu X., Smith M.A. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Castellani R.J., Harris P.L.R., Sayre L.M., Fujii J., Taniguchi N., Vitek M.P., Founds H., Atwood C.S., Perry G., Smith M.A. Active glycation in neurofibrillary pathology of Alzheimer disease: Nε-(Carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001;31:175–180. doi: 10.1016/S0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 65.Atzori C., Ghetti B., Piva R., Srinivasan A.N., Zolo P., Delisle M.B., Mirra S.S., Migheli A. Activation of the JNK/p38 Pathway Occurs in Diseases Characterized by Tau Protein Pathology and Is Related to Tau Phosphorylation But Not to Apoptosis. J. Neuropathol. Exp. Neurol. 2001;60:1190–1197. doi: 10.1093/jnen/60.12.1190. [DOI] [PubMed] [Google Scholar]
- 66.Xiao B., Goh J.-Y., Xiao L., Xian H., Lim K.-L., Liou Y.-C. Reactive oxygen species trigger Parkin/PINK1 pathway–dependent mitophagy by inducing mitochondrial recruitment of Parkin. J. Biol. Chem. 2017;292:16697–16708. doi: 10.1074/jbc.M117.787739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaw I.C., Fitzmaurice P.S., Mitchell J.D., Lynch P.G. Studies on Cellular Free Radical Protection Mechanisms in the Anterior Horn from Patients with Amyotrophic Lateral Sclerosis. Neurodegeneration. 1995;4:391–396. doi: 10.1006/neur.1995.0047. [DOI] [PubMed] [Google Scholar]
- 68.Bartolome F., Esteras N., Martin-Requero A., Boutoleau-Bretonniere C., Vercelletto M., Gabelle A., Le Ber I., Honda T., Dinkova-Kostova A.T., Hardy J., et al. Pathogenic p62/SQSTM1 mutations impair energy metabolism through limitation of mitochondrial substrates. Sci. Rep. 2017;7:1666. doi: 10.1038/s41598-017-01678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onesto E., Colombrita C., Gumina V., Borghi M.O., Dusi S., Doretti A., Fagiolari G., Invernizzi F., Moggio M., Tiranti V., et al. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol. Commun. 2016;4:47. doi: 10.1186/s40478-016-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bajpai A., Verma A.K., Srivastava M., Srivastava R. Oxidative stress and major depression. J. Clin. Diagn. Res. 2014;8:CC04–CC07. doi: 10.7860/JCDR/2014/10258.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hancock J.T., Desikan R., Neill S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001;29:345–350. doi: 10.1042/bst0290345. [DOI] [PubMed] [Google Scholar]
- 74.Klann E., Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: Implications for hippocampal synaptic plasticity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1999;23:359–376. doi: 10.1016/S0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 75.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller S., Hoege C., Pyrowolakis G., Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat. Reviews. Mol. Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 77.Lee S.H., Na S.I., Heo J.S., Kim M.H., Kim Y.H., Lee M.Y., Kim S.H., Lee Y.J., Han H.J. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: Involvement of Ca2+/PKC and MAPKs-induced EGFR transactivation. J. Cell. Biochem. 2009;106:787–797. doi: 10.1002/jcb.22013. [DOI] [PubMed] [Google Scholar]
- 78.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D., Wu H., Kornblum H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson C., González-Billault C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015;9:381. doi: 10.3389/fncel.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Q., Huff L.P., Fujii M., Griendling K.K. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic. Biol. Med. 2017;109:84–107. doi: 10.1016/j.freeradbiomed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dent E.W., Gupton S.L., Gertler F.B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohtaki H., Ylostalo J.H., Foraker J.E., Robinson A.P., Reger R.L., Shioda S., Prockop D.J. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA. 2008;105:14638. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu K., Ge J., Summers J.B., Li F., Liu X., Ma P., Kaminski J., Zhuang J. TSP-1 Secreted by Bone Marrow Stromal Cells Contributes to Retinal Ganglion Cell Neurite Outgrowth and Survival. PLoS ONE. 2008;3:e2470. doi: 10.1371/journal.pone.0002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sart S., Song L., Li Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxidative Med. Cell. Longev. 2015;2015:105135. doi: 10.1155/2015/105135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang S., Park J.-S., Jeong H.-S. Neural Differentiation of Human Adipose Tissue-Derived Stem Cells Involves Activation of the Wnt5a/JNK Signalling. Stem Cells Int. 2015;2015:178618. doi: 10.1155/2015/178618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visweswaran M., Pohl S., Arfuso F., Newsholme P., Dilley R., Pervaiz S., Dharmarajan A. Multi-lineage differentiation of mesenchymal stem cells—To Wnt, or not Wnt. Int. J. Biochem. Cell Biol. 2015;68:139–147. doi: 10.1016/j.biocel.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Kondo T., Matsuoka A.J., Shimomura A., Koehler K.R., Chan R.J., Miller J.M., Srour E.F., Hashino E. Wnt Signaling Promotes Neuronal Differentiation from Mesenchymal Stem Cells Through Activation of Tlx3. Stem Cells. 2011;29:836–846. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oswald M.C.W., Garnham N., Sweeney S.T., Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–691. doi: 10.1002/1873-3468.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beckhauser T.F., Francis-Oliveira J., De Pasquale R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity:Supplementary Issue: Brain Plasticity and Repair. J. Exp. Neurosci. 2016;10:JEN.S39887. doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hidalgo C., Arias-Cavieres A. Calcium, Reactive Oxygen Species, and Synaptic Plasticity. Physiology (Bethesda, MD) 2016;31:201–215. doi: 10.1152/physiol.00038.2015. [DOI] [PubMed] [Google Scholar]
- 91.Larkman A.U., Jack J.J. Synaptic plasticity: Hippocampal LTP. Curr. Opin. Neurobiol. 1995;5:324–334. doi: 10.1016/0959-4388(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 92.Bórquez D.A., Urrutia P.J., Wilson C., van Zundert B., Núñez M.T., González-Billault C. Dissecting the role of redox signaling in neuronal development. J. Neurochem. 2016;137:506–517. doi: 10.1111/jnc.13581. [DOI] [PubMed] [Google Scholar]
- 93.Thiels E., Urban N.N., Gonzalez-Burgos G.R., Kanterewicz B.I., Barrionuevo G., Chu C.T., Oury T.D., Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. Off. J. Soc. Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferri A., Gabbianelli R., Casciati A., Paolucci E., Rotilio G., Carrì M.T. Calcineurin activity is regulated both by redox compounds and by mutant familial amyotrophic lateral sclerosis-superoxide dismutase. J. Neurochem. 2000;75:606–613. doi: 10.1046/j.1471-4159.2000.0750606.x. [DOI] [PubMed] [Google Scholar]
- 95.Fujikake N., Shin M., Shimizu S. Association Between Autophagy and Neurodegenerative Diseases. Front. Neurosci. 2018;12:255. doi: 10.3389/fnins.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem. Soc. Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- 97.Mitra S., Nguyen L.N., Akter M., Park G., Choi E.H., Kaushik N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers. 2019;11:1030. doi: 10.3390/cancers11071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petibone D.M., Majeed W., Casciano D.A. Autophagy function and its relationship to pathology, clinical applications, drug metabolism and toxicity. J. Appl. Toxicol. JAT. 2017;37:23–37. doi: 10.1002/jat.3393. [DOI] [PubMed] [Google Scholar]
- 99.Szumiel I. Autophagy, reactive oxygen species and the fate of mammalian cells. Free Radic. Res. 2011;45:253–265. doi: 10.3109/10715762.2010.525233. [DOI] [PubMed] [Google Scholar]
- 100.Hamblin M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anders J.J., Geuna S., Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol. Res. 2004;26:233–239. doi: 10.1179/016164104225013914. [DOI] [PubMed] [Google Scholar]
- 102.Huang Y.Y., Nagata K., Tedford C.E., McCarthy T., Hamblin M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics. 2013;6:829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong-Riley M.T., Liang H.L., Eells J.T., Chance B., Henry M.M., Buchmann E., Kane M., Whelan H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 104.Byrnes K.R., Waynant R.W., Ilev I.K., Wu X., Barna L., Smith K., Heckert R., Gerst H., Anders J.J. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 105.Rochkind S. Phototherapy in peripheral nerve regeneration: From basic science to clinical study. Neurosurg. Focus. 2009;26:E8. doi: 10.3171/FOC.2009.26.2.E8. [DOI] [PubMed] [Google Scholar]
- 106.Sharma S.K., Kharkwal G.B., Sajo M., Huang Y.Y., De Taboada L., McCarthy T., Hamblin M.R. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg. Med. 2011;43:851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morries L.D., Cassano P., Henderson T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015;11:2159. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henderson T.A. Multi-watt near-infrared light therapy as a neuroregenerative treatment for traumatic brain injury. Neural Regen. Res. 2016;11:563–565. doi: 10.4103/1673-5374.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quirk B.J., Torbey M., Buchmann E., Verma S., Whelan H.T. Near-Infrared Photobiomodulation in an Animal Model of Traumatic Brain Injury: Improvements at the Behavioral and Biochemical Levels. Photomed. Laser Surg. 2012;30:523–529. doi: 10.1089/pho.2012.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henderson T., Morries L.D. 192 Multi-Watt Near Infrared Phototherapy is an Effective Treatment for Depression. CNS Spectr. 2018;23:109–110. doi: 10.1017/S109285291800072X. [DOI] [Google Scholar]
- 111.Ma Q., Xing C., Long W., Wang H.Y., Liu Q., Wang R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dumitrescu L., Popescu-Olaru I., Cozma L., Tulbă D., Hinescu M.E., Ceafalan L.C., Gherghiceanu M., Popescu B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxidative Med. Cell. Longev. 2018;2018:2406594. doi: 10.1155/2018/2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanaka H., Hori M. Medical applications of non-thermal atmospheric pressure plasma. J. Clin. Biochem. Nutr. 2017;60:29–32. doi: 10.3164/jcbn.16-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ji W.-O., Lee M.-H., Kim G.-H., Kim E.-H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019;9:19837. doi: 10.1038/s41598-019-56160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gorbanev Y., O’Connell D., Chechik V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chem. Eur. J. 2016;22:3496–3505. doi: 10.1002/chem.201503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scholtz V., Pazlarova J., Souskova H., Khun J., Julak J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015;33:1108–1119. doi: 10.1016/j.biotechadv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Šimončicová J., Kryštofová S., Medvecká V., Ďurišová K., Kaliňáková B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019;103:5117–5129. doi: 10.1007/s00253-019-09877-x. [DOI] [PubMed] [Google Scholar]
- 118.Yan D., Sherman J., Keidar M. Cold Atmospheric Plasma, A Novel Promising Anti-cancer treatment modality. Oncotarget. 2016;8 doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaushik N.K., Kaushik N., Linh N.N., Ghimire B., Pengkit A., Sornsakdanuphap J., Lee S.-J., Choi E.H. Plasma and nanomaterials: Fabrication and biomedical applications. Nanomaterials. 2019;9:98. doi: 10.3390/nano9010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haralambiev L., Wien L., Gelbrich N., Lange J., Bakir S., Kramer A., Burchardt M., Ekkernkamp A., Gümbel D., Stope M.B. Cold atmospheric plasma inhibits the growth of osteosarcoma cells by inducing apoptosis, independent of the device used. Oncol. Lett. 2020;19:283–290. doi: 10.3892/ol.2019.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pereira S., Pinto E., Ribeiro P.A., Sério S. Study of a Cold Atmospheric Pressure Plasma jet device for indirect treatment of Squamous Cell Carcinoma. Clin. Plasma Med. 2019;13:9–14. doi: 10.1016/j.cpme.2018.09.001. [DOI] [Google Scholar]
- 122.Li Y., Ho Kang M., Sup Uhm H., Joon Lee G., Ha Choi E., Han I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017;7:45781. doi: 10.1038/srep45781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z., Lin L., Zheng Q., Sherman J.H., Canady J., Trink B., Keidar M. Micro-sized cold atmospheric plasma source for brain and breast cancer treatment. Plasma Med. 2018;8:203–215. doi: 10.1615/PlasmaMed.2018026588. [DOI] [Google Scholar]
- 124.Cheng X., Rowe W., Ly L., Shashurin A., Zhuang T., Wigh S., Basadonna G., Trink B., Keidar M., Canady J. Treatment of triple-negative breast cancer cells with the canady cold plasma conversion system: Preliminary results. Plasma. 2018;1:218–228. doi: 10.3390/plasma1010019. [DOI] [Google Scholar]
- 125.Fofana M., Buñay J., Judée F., Baron S., Menecier S., Nivoix M., Perisse F., Vacavant A., Balandraud X. Selective treatments of prostate tumor cells with a cold atmospheric plasma jet. Clin. Plasma Med. 2020;17–18:100098. doi: 10.1016/j.cpme.2020.100098. [DOI] [Google Scholar]
- 126.Cui H.S., Cho Y.S., Joo S.Y., Mun C.H., Seo C.H., Kim J.-B. Wound Healing Potential of Low Temperature Plasma in Human Primary Epidermal Keratinocytes. Tissue Eng. Regen. Med. 2019;16:585–593. doi: 10.1007/s13770-019-00215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J.-P., Guo L., Chen Q.-L., Zhang K.-Y., Wang T., An G.-Z., Zhang X.-F., Li H.-P., Ding G.-R. Effects and mechanisms of cold atmospheric plasma on skin wound healing of rats. Contrib. Plasma Phys. 2019;59:92–101. doi: 10.1002/ctpp.201800025. [DOI] [Google Scholar]
- 128.Rupf S., Lehmann A., Hannig M., Schäfer B., Schubert A., Feldmann U., Schindler A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010;59:206–212. doi: 10.1099/jmm.0.013714-0. [DOI] [PubMed] [Google Scholar]
- 129.Bisag A., Manzini M., Simoncelli E., Stancampiano A., Tonini R., Gherardi M., Colombo V. Cold atmospheric pressure plasma treatment to assist the restoration of the apical region of a root canal in endodontic procedures. Clin. Plasma Med. 2020;19–20:100100. doi: 10.1016/j.cpme.2020.100100. [DOI] [Google Scholar]
- 130.Jiang C., Schaudinn C., Jaramillo D.E., Gundersen M.A., Costerton J.W. Plasma for Bio-Decontamination, Medicine and Food Security. Springer; Dordrecht, The Netherlands: 2012. A sub-microsecond pulsed plasma jet for endodontic biofilm disinfection; pp. 179–190. [Google Scholar]
- 131.Theinkom F., Singer L., Cieplik F., Cantzler S., Weilemann H., Cantzler M., Hiller K.-A., Maisch T., Zimmermann J.L. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS ONE. 2019;14:e0223925. doi: 10.1371/journal.pone.0223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hasse S., Duong Tran T., Hahn O., Kindler S., Metelmann H.R., von Woedtke T., Masur K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2016;41:202–209. doi: 10.1111/ced.12735. [DOI] [PubMed] [Google Scholar]
- 133.Schmidt A., Bekeschus S., von Woedtke T., Hasse S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin. Plasma Med. 2015;3:24–31. doi: 10.1016/j.cpme.2015.05.003. [DOI] [Google Scholar]
- 134.Arjunan K.P., Clyne A.M. A nitric oxide producing pin-to-hole spark discharge plasma enhances endothelial cell proliferation and migration. Plasma Med. 2011;1:279–293. doi: 10.1615/PlasmaMed.2012006389. [DOI] [Google Scholar]
- 135.Kleineidam B., Nokhbehsaim M., Deschner J., Wahl G. Effect of cold plasma on periodontal wound healing—An in vitro study. Clin. Oral Investig. 2019;23:1941–1950. doi: 10.1007/s00784-018-2643-3. [DOI] [PubMed] [Google Scholar]
- 136.Nasruddin, Nakajima Y., Mukai K., Rahayu H.S.E., Nur M., Ishijima T., Enomoto H., Uesugi Y., Sugama J., Nakatani T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2014;2:28–35. doi: 10.1016/j.cpme.2014.01.001. [DOI] [Google Scholar]
- 137.Miller V., Lin A., Kako F., Gabunia K., Kelemen S., Brettschneider J., Fridman G., Fridman A., Autieri M. Microsecond-pulsed dielectric barrier discharge plasma stimulation of tissue macrophages for treatment of peripheral vascular disease. Phys. Plasmas. 2015;22:122005. doi: 10.1063/1.4933403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arjunan K.P., Friedman G., Fridman A., Clyne A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface. 2012;9:147–157. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.De Geyter N., Morent R. Nonthermal Plasma Sterilization of Living and Nonliving Surfaces. Annu. Rev. Biomed. Eng. 2012;14:255–274. doi: 10.1146/annurev-bioeng-071811-150110. [DOI] [PubMed] [Google Scholar]
- 140.Jha N., Ryu J.J., Choi E.H., Kaushik N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxidative Med. Cell. Longev. 2017;2017:7542540. doi: 10.1155/2017/7542540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nguyen N.H., Park H.J., Yang S.S., Choi K.S., Lee J.-S. Anti-cancer efficacy of nonthermal plasma dissolved in a liquid, liquid plasma in heterogeneous cancer cells. Sci. Rep. 2016;6:29020. doi: 10.1038/srep29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi J.-S., Kim J., Hong Y.-J., Bae W.-Y., Choi E.H., Jeong J.-W., Park H.-K. Evaluation of non-thermal plasma-induced anticancer effects on human colon cancer cells. Biomed. Opt. Express. 2017;8:2649–2659. doi: 10.1364/BOE.8.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Braný D., Dvorská D., Halašová E., Škovierová H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020;21:2932. doi: 10.3390/ijms21082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wink D.A., Hines H.B., Cheng R.Y.S., Switzer C.H., Flores-Santana W., Vitek M.P., Ridnour L.A., Colton C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiueh C.C. Neuroprotective properties of nitric oxide. Ann. N. Y. Acad. Sci. 1999;890:301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- 146.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 147.Molina-Holgado F., Pinteaux E., Heenan L., Moore J.D., Rothwell N.J., Gibson R.M. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol. Cell. Neurosci. 2005;28:189–194. doi: 10.1016/j.mcn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 148.Andoh T., Chock P.B., Chiueh C.C. Preconditioning-Mediated Neuroprotection. Ann. N. Y. Acad. Sci. 2002;962:1–7. doi: 10.1111/j.1749-6632.2002.tb04051.x. [DOI] [PubMed] [Google Scholar]
- 149.Faraci F.M., Brian J.E. Nitric oxide and the cerebral circulation. Stroke. 1994;25:692–703. doi: 10.1161/01.STR.25.3.692. [DOI] [PubMed] [Google Scholar]
- 150.Dormanns K., Brown R.G., David T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016;394:1–17. doi: 10.1016/j.jtbi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 151.Li Y., Choi E.H., Han I. Regulation of Redox Homeostasis by Nonthermal Biocompatible Plasma Discharge in Stem Cell Differentiation. Oxidative Med. Cell. Longev. 2019;2019:2318680. doi: 10.1155/2019/2318680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jang J.-Y., Hong Y.J., Lim J., Choi J.S., Choi E.H., Kang S., Rhim H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials. 2018;156:258–273. doi: 10.1016/j.biomaterials.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 153.Egea J., Espinet C., Soler R.M., Peiró S., Rocamora N., Comella J.X. Nerve growth factor activation of the extracellular signal-regulated kinase pathway is modulated by Ca(2+) and calmodulin. Mol. Cell Biol. 2000;20:1931–1946. doi: 10.1128/MCB.20.6.1931-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan X., Ouyang J., Zhang C., Shi Z., Wang B., Ostrikov K. Plasma medicine for neuroscience—An introduction. Chin. Neurosurg. J. 2019;5:25. doi: 10.1186/s41016-019-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Katiyar K.S., Lin A., Fridman A., Keating C.E., Cullen D.K., Miller V. Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Appl. Sci. 2019;9:3747. doi: 10.3390/app9183747. [DOI] [Google Scholar]
- 156.Rybnikova E., Samoilov M. Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Front. Neurosci. 2015;9:388. doi: 10.3389/fnins.2015.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Horiba M., Kamiya T., Hara H., Adachi T. Cytoprotective effects of mild plasma-activated medium against oxidative stress in human skin fibroblasts. Sci. Rep. 2017;7:42208. doi: 10.1038/srep42208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiong Z., Zhao S., Yan X. Nerve Stem Cell Differentiation by a One-step Cold Atmospheric Plasma Treatment In Vitro. JoVE. 2019:e58663. doi: 10.3791/58663. [DOI] [PubMed] [Google Scholar]
- 159.Jouhilahti E.M., Peltonen S., Peltonen J. Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2008;56:1113–1119. doi: 10.1369/jhc.2008.952002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yan X., Meng Z., Ouyang J., Qiao Y., Yuan F. New Application of an Atmospheric Pressure Plasma Jet as a Neuro-protective Agent Against Glucose Deprivation-induced Injury of SH-SY5Y Cells. J. Vis. Exp. 2017:56323. doi: 10.3791/56323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yan X., Meng Z., Ouyang J., Qiao Y., Li J., Jia M., Yuan F., Ostrikov K. Cytoprotective effects of atmospheric-pressure plasmas against hypoxia-induced neuronal injuries. J. Phys. D Appl. Phys. 2018;51:085401. doi: 10.1088/1361-6463/aaa867. [DOI] [Google Scholar]
- 162.Haccho T., Kanno A., Ichikawa H., Yamamoto K., Morita Y., Nakamachi E. Enhancement of PC12 Neurite Extension via Plasma-Activated Medium by Nonthermal Atmospheric-Pressure Plasma-Bubbling System. Plasma Med. 2019;9:129–146. doi: 10.1615/PlasmaMed.2020033059. [DOI] [Google Scholar]
- 163.Dompe C., Moncrieff L., Matys J., Grzech-Leśniak K., Kocherova I., Bryja A., Bruska M., Dominiak M., Mozdziak P., Skiba T.H.I. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020;9:1724. doi: 10.3390/jcm9061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shi Y., Hu Y., Lv C., Tu G. Effects of Reactive Oxygen Species on Differentiation of Bone Marrow Mesenchymal Stem Cells. Ann. Transplant. 2016;21:695–700. doi: 10.12659/AOT.900463. [DOI] [PubMed] [Google Scholar]