Abstract
Background: The diagnostic role of Wilms’ tumor 1 (WT1) is well known in gynaeco-pathological setting, since it is considered a specific marker of serous histotype and adnexal origin. Moreover, its oncogenic role has been recently highlighted in many cancers and it has also been regarded as a promising target antigen for cancer immunotherapy. However, the relationship between its expression and prognostic role in uterine cancer remains unclear. We analyzed the diagnostic and prognostic role of WT1 expression in patients with uterine carcinoma by completing a search using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the PICOS (Participants, Intervention, Comparison, Outcomes, Study Design) model through PubMed, Scopus and Web of Science databases to identify studies that fit our search criteria. The objective of the current meta-analysis was to investigate the diagnostic and prognostic role of WT1 expression in patients with uterine carcinoma. Materials and Methods: A literature search was performed of the PubMed, Scopus, and Web of Science databases for English-language studies published from January 2000 to April 2020. Studies were considered eligible if they evaluated the WT1 expression in uterine carcinoma. Results: In total, 35 articles were identified that used uterine carcinoma criteria and provided data for 1616 patients. The overall rate of WT1 expression in uterine carcinoma was 25%. The subgroup analysis of uterine cancer types revealed that WT1 was expressed differently among different histotypes (endometrioid, clear cell, serous carcinoma and carcinosarcoma). Discussion and Conclusions: The WT1 immunohistochemical expression is not limited to serous histotype and/or ovarian origin. In fact, a significant proportion of endometrial adenocarcinomas can also show WT1 immunoreactivity. Moreover, our study suggests that WT1 may be a potential marker to predict the prognosis of patients with uterine cancer, but more studies are needed to confirm its role in clinical practice.
Keywords: endometrial carcinoma, WT1, diagnosis and prognosis, immunohistochemistry, serous carcinoma, carcinosarcoma, clear cell carcinoma, endometrioid carcinoma
1. Introduction
Endometrial carcinomas (EC) is the most common gynecological malignant neoplasm in industrialized countries and its incidence and mortality has been constantly increasing [1].
To date, it is largely recognized that EC represents a heterogeneous group of diseases with different morphological and molecular features. The first pathogenetic model proposed by Bokhman stratified EC patients in two subgroups: Type I, with high expression of hormonal receptors and a better prognosis; and Type II, which lacks hormone receptors expression and a worse prognosis [2].
A large-scale molecular analysis published in 2013 by the Cancer Genome Atlas (TCGA), defined four molecular categories of endometrial cancer: POLE mutated, hypermutated secondary to microsatellite instability (MSI), low copy number, and high copy number (serous-like) [3].
Despite all these novel pathogenetic and molecular discoveries, EC still carries a high mortality rate and an increase in incidence and mortality is expected over the next few years [4]. Therefore, novel diagnostic and prognostic bio-markers are needed to improve the clinical and therapeutic management of EC patients.
The Wilms’ tumor gene (WT1) was first identified in the urogenital system. It encodes a transcription-regulating protein of 52–54 kDa with homology to the prototypic transcription factor family of early growth response genes [5]. It has been shown that WT1 is expressed in various kinds of human cancer including leukemia and myelodysplastic syndrome, brain tumors, neuroblastoma, lung cancer, breast cancer, soft tissue sarcoma as well as in gynecological tumors such as ovarian carcinoma [6,7]. Data from the literature have also revealed that WT1 can promote invasion, migration and metastasis, facilitate angiogenesis and confer drug resistance to cancer cells [5,6].
In the gynecological tract, WT1 is expressed in the surface epithelial cells of the ovaries and fallopian tubes, as well as granulosa cells, myometrium and endometrial stromal cells [8]. Moreover, in gynecological pathology, the immunohistochemical expression of WT1 is useful in the diagnosis of ovarian serous carcinoma (both high grade and low grade histotypes) and is also helpful to distinguish carcinoma of ovarian origin from carcinoma with other primary sites [9]. However, recent papers showed that WT1 immunoexpression can be observed in different histotypes of endometrial carcinoma also suggesting that WT1 may represent a potential prognostic marker in endometrial carcinoma [10].
In the present paper, we conducted a systematic meta-analysis with the aim to elucidate the diagnostic and prognostic role of WT1 immunoexpression in patients with endometrial carcinoma.
2. Materials and Methods
2.1. Search Strategy
A systematic literature search was performed to identify articles regarding WT1 and prognosis of endometrial carcinoma. Pubmed, Web of Science, and Scopus were used simultaneously, with the combination of terms “WT1 or Wilms’ tumor 1 or Wilms’ tumor gene 1 or Wilms’ tumor protein 1 or Wilms’ tumor suppressor gene 1” and “gynaecological or uterine or endometrial” and “cancer or tumor or neoplasm or carcinoma” (from January 2000 up to April 2020). All articles were initially reviewed by abstract and title browsing to select the relevant reports, which were subjected to further screening.
2.2. Study Eligibility
Data retrieved from the studies included the following: author, country, year of publication, follow-up time, total number of patients, mean age, outcome model, overall survival (OS), progression free survival (PFS), relapse/recurrence-free survival (RFS), disease free survival (DFS), WT1 expression in uterine carcinoma, cut-off value of WT1, and stage/grade of tumor according to International Federation of Gynecology and Obstetrics (FIGO) grading and staging system. The language was limited to English only.
2.3. Data Extraction
Starting from 140 identified references, 60 duplicates were removed. The first step consisted in an accurate reading of titles and abstracts and the analysis of all the references denoted high intra-rate reliability (98.62% agreement; Cohen K: 0.97). A total of 45 references were then retained and a full-text assessment was performed. Finally, 35 references which met the eligibility criteria were retained and included in the current work [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
The present meta-analysis was conducted according to Guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and PICOS (Participants, Intervention, Comparison, Outcomes, Study Design) model. Data from each eligible study were extracted without modification of original data according to the PICOS (Population, Intervention or risk factor, Comparator, Outcomes, Study design) items. “Population” of our study was represented by patients diagnosed with EC. “Intervention” (or risk factor) was the EC group with WT1 expression, assessed by immunohistochemical analysis. “Comparator” was the EC group without WT1 immunohistochemical expression. “Outcomes” were overall survival (OS), progression free survival (PFS), relapse/recurrence-free survival (RFS) and disease free survival (DFS). “Study design” was the study design of the included studies. The PRISMA checklist is shown in Table S1.
2.4. Risk of Bias across Studies
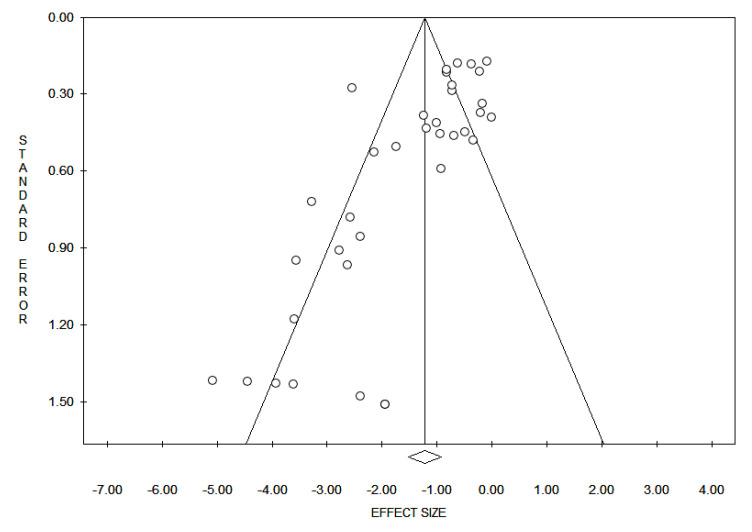
Reporting bias across studies was evaluated by a graphic diagnostic tool named funnel plot Figure 1. The x-axis in the present analysis is the WT1 expression and the y-axis is the standard error. In the absence of bias, a funnel plot should be a symmetrical inverted funnel. In the presence of bias, smaller studies with no expression would be missing, thus creating an asymmetrical funnel. Asymmetry in a funnel plot suggests that there is a systematic difference between larger and smaller studies and/or that there is publication bias.
Figure 1.
Funnel plot for evaluation of bias across studies: The x-axis in the present analysis is the Wilms’ tumor 1 (WT1) expression and the y-axis is the standard error. In the absence of bias, a funnel plot should be a symmetrical inverted funnel. In the presence of bias, smaller studies with no expression would be missing, thus creating an asymmetrical funnel. Asymmetry in a funnel plot suggests that there is a systematic difference between larger and smaller studies and/or that there is publication bias.
2.5. Data Analysis
The rate of WT1 expression in endometrial cancer was calculated for each study included in the meta-analysis, and the results were aggregated using the meta-analytic software ProMeta 2.0 (Internovi, Cesena, Italy). Statistical analysis was performed using MedCalc version 10.2.0.0 (StataCorp LP, College Station, TX, USA) and the GraphPad-Prism 5 software (Graph Pad Software, San Diego, CA, USA). The inverse-variance method was utilized to obtain an overall effect size of the pooled rates of malignancy across studies. Following this, a random-effects model was used as a conservative approach to discriminate the different sources of variation among studies (i.e., within-study variance and between-studies variance) [44].
Q and I2 statistics were then conducted to evaluate heterogeneity across studies [45]. In detail, a significant Q value denotes the lack of homogeneity among studies; on the other hand, the proportion of observed variance, which indicates real differences in effect sizes was calculated with I2 statistics: values of 25%, 50%, and 75% were considered as low, moderate, and high, respectively [46]. Moreover, heterogeneity across study findings was determined using a moderator analysis.
Sensitivity analyses were also performed to determine the stability of study results, computing how the overall rates would change by removing one study at a time. Finally, publication bias analyses were established with two tests: the regression method reported by Egger et al. and the Begg and Mazumdar rank correlation test [46,47,48]. The absence of publication bias is indicated in both tests by non-significant results.
3. Results
On the basis of our criteria, the articles that were published between 2000 and 2020 were analyzed and reported in Table 1.
Table 1.
Characteristics of Included Studies in the Meta-Analysis.
| Author | Year | Country | Cancer Type | No of Patients | Age (Mean) | Stage/Grade | Follow up Time (Months) | Outcome | WT1 Positive Expression (%); Cutoff Value |
|---|---|---|---|---|---|---|---|---|---|
| Coosemans, et al. [10] | 2008 | Belgium | EC SC CCC |
24 9 3 |
NA | I–IV | NA | NA | 17/24 (71) 7/9 (77.7) 2/3 (66.6); score ≥ 3 |
| Acs, et al. [11] | 2003 | USA | EC CCC SC |
35 18 16 |
63.1 | I–III | NA | NA | 0/35 0/18 10/16; ≥50% |
| Al-Hussaini, et al. [12] | 2003 | UK | EC SC |
7 25 |
NA | NA | NA | NA | 0/7 (0) 2/25 (8); ≥50% |
| Atik, et al. [14] | 2016 | Turkey | EC | 50 | 56 | I–III | NA | OS | 40/50 (80); score ≥ 3 |
| Baek, et al. [15] | 2016 | Korea | EC | 10 | 50 | I–IV | 0–40 | OS/DFS | 4/10 (40); score ≥ 3 |
| Chen, et al. [16] | 2016 | Canada | EC CCC |
113 17 |
66 | I–IV | NA | DFS | 23/113 (18.5); score ≥ 1 0/17 (0) |
| Chitale, et al. [17] | 2005 | USA | EC CCC CS |
35 12 13 |
NA | I–III | NA | OS | 11/35 (31.4) 2/12(16.6) 7/13(53.8); ≥50% score ≥ 3 |
| Coosemans, et al. [18] | 2011 | Belgium | CS | 71 | 65 | I–IV | ≥12 m | OS/PFS | 49/71 (69%); score ≥ 20 |
| Dohi, et al. [19] | 2009 | Japan | EC | 70 | 57.3 | I–IV | NA | OS | 64/70 (91); ≥50% |
| Dupont, et al. [20] | 2004 | USA | EC CCC SC CS |
99 4 9 10 |
65 | I–IV | 1–241 | OS | 20/99 (20) 2/4 (50) 3/9 (33.3) 7/10 (70); ≥50% |
| Egan, et al. [21] | 2003 | USA | EC SC |
39 31 |
NA | I–III | NA | NA | 0/39 (0) 2/31 (6.4); score ≥ 2 |
| Espinosa, et al. [22] | 2017 | Spain | EC | 3 | 58.6 | I–IV | 0–48 | OS | 0/3 (0) |
| Fadare, et al. [23] | 2013 | USA | SC | 22 | NA | I–II | NA | NA | 8/22 (36); ≥50% |
| Franko, et al. [24] | 2010 | Canada | CS | 16 | NA | I–IV | NA | NA | 13/16 (81); score ≥ 3 |
| Goldstein, et al. [25] | 2002 | USA | SC | 18 | NA | NA | NA | NA | 0/18 (0) |
| Guntupalli, et al. [26] | 2013 | USA | CS | 87 | 68.8 | I–IV | 1–187 | OS | 47/87 (54%); score > 21 |
| Hashi, et al. [27] | 2003 | Japan | SC | 13 | NA | I–IV | 6–142 | OS | 13/13(100); ≥50%/score ≥ 3 |
| Hedley, et al. [28] | 2014 | UK | EC | 77 | 69 | I–IV | 0–56 | DFS | 34/77 (44); ≥50% |
| Hirschowit, et al. [29] | 2009 | UK | SC | 34 | 68.7 | NA | NA | NA | 4/34 (12); score ≥ 3 |
| Jones, et al. [30] | 2019 | USA | CS | 43 | 67 | I–IV | NA | OS | 21/43 (49); score ≥ 3 |
| Kitade, et al. [31] | 2019 | Japan | SC | 5 | 52.4 | I–IV | 26–210 | NA | 0/5 (0) |
| Lu, et al. [32] | 2016 | China | SC | 3 | 58 | I–III | Median 44 | NA | 0/3 (0) |
| Matalka, et al. [33] | 2012 | Jordan | EC | 53 | 57.8 | I–III | NA | NA | 2/53 (8.1); score ≥ 3 |
| Nofech-Mozes, et al. [34] | 2008 | Canada | SC | 37 | 71.1 | I–IV | NA | NA | 18/37 (48.6); ≥50% score ≥ 3 |
| Nafisi, et al. [35] | 2015 | Canada | EC SC |
23 17 |
NA | NA | NA | NA | 4/23 (17.3) 3/17 (17.6); ≥ 50% |
| Ohno, et al. [36] | 2009 | Japan | EC | 70 | 57.3 | I–IV | Median 61 m | OS/RFS | 31/70 (44%); score ≥ 5 |
| Ruba, et al. [37] | 2020 | Australia | EC | 14 | 64 | I–IV | NA | NA | 7/14 (50); >10% |
| Stanescu, et al. [38] | 2014 | Romania | EC | 79 | 62 | I–III | NA | NA | 0/79 (0) |
| Sumathi, et al. [39] | 2004 | UK | EC | 19 | NA | NA | NA | NA | 16/19 (84.2); score ≥ 3 |
| Tanvir, et al. [40] | 2014 | Pakistan | EC | 42 | 63 | NA | NA | NA | 0/42 (0) |
| Togami, et al. [41] | 2015 | Japan | EC SC |
29 12 |
NA | NA | NA | NA | 6/29 (21) 0/12 (0); score ≥ 2 |
| Trinh, et al. [42] | 2019 | Canada | EC SC |
37 25 |
66.8 | I–IV | NA | NA | 26/37 (70.2) 3/25 (12); ≥50% |
| Yan, et al. [43] | 2013 | USA | SC | 13 | 62.2 | NA | NA | NA | 8/13 (61.5); score ≥ 3 |
EC: endometrioid carcinoma; CCC: clear cells carcinoma; CS: carcinosarcoma; SC: serous carcinoma; WT1: Wilms’ tumor 1; NA: not available; OS: overall survival; PFS: progression free survival; RFS: relapse/recurrence-free survival; DFS: disease free survival.
In detail, a total of 35 studies with 1616 patients assessed the role of WT1 expression in patients with uterine carcinoma. The median age was 62.1 years (range 50–71.1). The main characteristics of the studies are reported in Table 1. It is worth noting that some studies reported rates of WT1 expression for endometrioid and serous carcinoma (n. 5 studies), for endometrioid and clear cell carcinoma (n. 1 study), for endometrioid, clear cell and serous carcinoma (n. 2 study), and for endometrioid, clear cell carcinoma and carcinosarcoma (n. 1 study), whereas other studies were selective only for one tumor type (n. 26 studies). The shapes of the funnel plots did not reveal evidence of obvious asymmetry (Figure 1).
The shapes of the funnel plots did not reveal evidence of obvious asymmetry.
The results indicated that, in a highly heterogeneous set of 35 studies that compared endometrioid, serous, clear cells carcinoma and carcinosarcoma, the overall rate of WT1 expression was 25% (95% CI = 0.20–0.30; Q = 120.4; I2 = 71.7), with p < 0.05. Following this, we selected each tumor type and computed the rate of expression.
3.1. Analyses of Endometrioid, Serous, Clear Cell Carcinoma and Carcinosarcoma
To provide a comprehensive understanding of the WT1 expression for the single cancer type, additional analyses that included both studies that reported data on the all carcinoma and studies that focused on only a single carcinoma were conducted (Table 1 and Table 2).
Table 2.
Summary of Meta-Analytic Results.
| K | N | Overall Rate of WT1 Expression (95% CI), % |
Q | I2 | |
|---|---|---|---|---|---|
| Endometroid Carcinoma | 23 | 985 | 21 (16−29) | 117.07 | 81.21 |
| Serous Carcinoma | 17 | 307 | 21 (14−29) | 42.3 | 62.2 |
| Clear Cell Carcinoma | 6 | 59 | 15 (6−33) | 6.99 | 28.4 |
| Carcinosarcoma | 6 | 240 | 38 (33−43) | 2.31 | 0.00 |
K: number of studies; N: total number of patients; CI: confidence interval; I2: index for quantifying the degree of heterogeneity; Q: test for heterogeneity; p < 0.001.
Details of the overall rates were tested through moderator analyses. Table 3 illustrates the cut-off values for WT1 in the selected studies.
Table 3.
Evaluation the cut-off value for Wilms’ tumor 1 (WT1) in the selected studies.
| Author | Cancer Type | WT1 Positive Expression (%); Cutoff Value |
Cut-Off Value for WT1 |
|---|---|---|---|
| Coosemans, et al. [10] | CS | 49/71 (69) | A score for each slide was calculated by multiplying the percentage and intensity of positive cells and then categorized as negative (0–20), weak (21–80), moderate (81–180), and strong (181–300). |
| Acs, et al. [11] | EC CCC SC |
0/35 (0) 0/18 (0) 10/16 (62.5) |
Score (out of maximum of 300) = sum 1 × percentage of weak, 2 × percentage of moderate, 3 × percentage of strong staining. |
| Al-Hussaini, et al. [12] | EC SC |
0/7 (0) 2/25 (8) |
Cases were scored as 0 (totally negative or only occasional scattered positive cells), 1+ (<10% cells positive), 2+ (10–50% of cells positive) or 3+ (>50% of cells positive). |
| Atik, et al. [14] | EC | 40/50 (80) | The total score was calculated by multiplying the intensity and percentage of staining: negative (0), 0–20; weak (1), 21–80; moderate (2), 81–180; and strong (3), 181–300. |
| Baek, et al. [15] | EC | 4/10 (40) | Cases were divided by the intensity of cell staining, given as values of 0, 1, 2, and 3. The percentage stained area was multiplied by this number to calculate the overall score (negative 0–20, weakly positive 21–80, moderately positive 81–180, and strongly positive 181–300). |
| Chen, et al. [16] | EC CCC |
23/113 (18.5) 0/17 (0) |
Any staining ≥1% of tumor cells were categorized as positive. |
| Chitale, et al. [17] | EC CCC CS |
11/35 (31.4) 2/12 (16.6) 7/13 (53.8) |
The extent of tumor staining was estimated on the basis of numbers of tumor cells stained and graded as follows: Focal, approximately ˂5%; +, 5–25%; ++, 26–50%; +++, 51–75%; and ++++, >75%. Staining in ˂50% of the tumor (+ to ++) was considered heterogeneous staining. |
| Coosemans, et al. [18] | EC SC CCC |
17/24 (71) 7/9 (77.7) 2/3 (66.6) |
A scoring system was based on the multiplication of percentage and intensity of positive cells, being negative (0–20), weak (21–80), moderately (81–180) and strong (181–300). |
| Dohi, et al. [19] | EC | 64/70 (91) | Staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). The extent of staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%) according to the percentage of positive staining area in relation to the whole carcinoma area. The sum of the intensity and extent score was used as the final staining score (0–7) for WT1. Tumors having a final staining score of ≥5 were considered to exhibit strong expression. |
| Dupont, et al. [20] | EC CCC SC CS |
20/99 (20) 2/4 (50) 3/9 (33.3) 7/10 (70) |
An adaptation of the German immunoreactive score (IRS), negative or weak immunoreactivity (scores 0–3) was considered negative, while moderate or strong immunoreactivity (scores 4–12) was considered positive. |
| Egan, et al. [21] | EC SC |
0/39 (0) 2/31 (6.4) |
WT1 was scored on the intensity and localization of the staining of tumor cell nuclei and was graded 0, 1+, 2+, and 3+, representing absent, focal/weak, moderate, and intense expression. Average scores of 0 to 1 were considered negative. Scores of 2 to 3 were interpreted as positive. |
| Espinosa, et al. [22] | EC | 0/3 (0) | Strong expression in tumor cell nuclei. |
| Fadare, et al. [23] | SC | 8/22 (36) | The extent of staining was semi-quantitatively assessed as follows: 0 (0–9%), 1 (10–25%), 2 (26–50%), 3 (51–100%). Any composite score above 0 was considered to be positive. |
| Franko, et al. [24] | CS | 13/16 (81) | Staining intensity was scored as 0 = none, 1 = weak, 2 = intermediate, and 3 = strong and amount as 0 = none, 1 = less than 1%, 2 = 1% to 10%, 3 = 11% to 33%, 4 = 34% to 67% and 5 = more than 67%. Intensity and amount were multiplied to yield a score. |
| Goldstein, et al. [25] | SC | 0/18 (0) | Tumor staining was estimated on the basis of numbers of tumor cells stained and graded as follows: Focal, approximately ˂5%; +, 5–25%; ++, 26–50%; +++, 51–75%; and ++++, >75%. |
| Guntupalli, et al. [26] | CS | 47/87 (54) | WT1 was stratified by absent/low expression (score 0–2), moderate expression (score 3–4), and strong expression (5–6). |
| Hashi, et al. [27] | SC | 13/13 (100) | Staining intensity was scored as 0 = none, 1 = weak, 2 = intermediate, and 3 = strong. |
| Hedley, et al. [28] | EC | 34/77 (44) | Expression of WT1 was considered positive when nuclear staining was identified. |
| Hirschowit, et al. [29] | SC | 4/34 (12) | Immunoreactivity was scored as follows: no reactivity = 0; <10% nuclei positive = 1+; 10–49% positive = 2+; 50–74% positive = 3+; 75–100% positive = 4+. |
| Jones, et al. [30] | CS | 21/43 (49) | Staining intensity was scored as 0 = none, 1 = weak, 2 = intermediate, and 3 = strong. The score ranged to 0 (no immunoreactivity) to 300 (highest immunoreactivity). |
| Kitade, et al. [31] | SC | 0/5 (0) | Staining intensity was scored as 0 = none, 1 = weak, 2 = intermediate, and 3 = strong. |
| Lu, et al. [32] | SC | 0/3 (0) | The percentage of positive cells was scored as follows: − for no immunoreactivity; focally + for 1% to 5%; + for 6% to 25%; ++ for 26% to 50%; +++ for 51% to 75%; ++++ for 76% to 100%. |
| Matalka, et al. [33] | EC | 2/53 (8.1) | WT1 scoring system was based on the multiplication of percentage and intensity of positive cells: negative (0–20), weak (21–80), moderate (81–180), and strong (181–300). Negative or weak immunoreactivity was considered negative, while moderate or strong immunoreactivity was considered positive. |
| Nofech-Mozes, et al. [34] | SC | 18/37 (48.6) | The proportion of positive cells and classified as: negative: 0%; 1+ = 1–25%; 2+ = 25–50% and 3 += strong (>50%). |
| Nafisi, et al. [35] | EC SC |
4/23 (17.3) 3/17 (17.6) |
Staining intensity was scored as 0 = none, 1 = weak, 2 = intermediate, and 3 = strong. |
| Ruba, et al. [37] | EC | 7/14 (50) | Positive WT1 expression was defined as moderate to strong nuclear immunoreactivity in >10% of tumor cells. |
| Stanescu, et al. [38] | EC | 0/79 (0) | Immunohistochemical results were either evaluated in a semi-quantitative manner and scored according to the percentages of positively staining cells or in a qualitative manner and appreciated as being positive or negative, paying attention to scoring only tumor cells stained in the appropriate nuclear/membrane position. |
| Sumathi, et al. [39] | EC | 16/19 (84.2) | Cases scored as 0 (negative or only an occasional cell staining), 1+ (˂5% cells positive), 2+ (5% to 25% cells positive), 3+ (26% to 50% cells positive), and 4% (>50% cells positive). |
| Tanvir, et al. [40] | EC | 0/42 (0) | The positive cells were classified as: negative: 0%; 1+ = 1–25%; 2+ = 25–50% and 3+ = strong (>50%). |
| Togami, et al. [41] | EC SC |
6/29 (21) 0/12 (0) |
The level of expression was graded according to the percentage of immunoreactive neoplastic cells component as follows: 0, <10%; 1+, 10–25%; 2+, 26–50%; 3+, >50%. Tumors with >10% stained cells were considered positive for expression of that antigen. |
| Trinh, et al. [42] | EC SC |
26/37 (70.2) 3/25 (12) |
The positive cells were classified as: negative: 0%; 1+ = 1–25%; 2+ = 25–50% and 3+ = strong (>50%). |
| Yan, et al. [43] | SC | 8/13 (61.5) | The level of expression was graded according to the percentage of immunoreactive neoplastic cells component as follows: 0, <10%; 1+, 10–25%; 2+, 26–50%; 3+, >50%. |
EC: endometrioid carcinoma, SC: serous carcinoma, CCC: clear cells carcinoma, CS: carcinosarcoma.
We also divided all outcomes into two groups including OS, and DFS/RFS/PFS (Table 4). Following this, we presented the main results according to different groups.
Table 4.
Combined Hazard Ratio (HR) for OS and PFS in different histotypes of Endometrial Carcinoma.
| Histotypes | Combined HR OS | Combined HR PFS |
|---|---|---|
| Endometrioid | 27% | 24% |
| Serous | 40% | 3% |
| Clear Cell | 21% | 5% |
| Carcinosarcoma | 35% | 41% |
3.2. Endometrioid Carcinoma
The analyses indicated that the expression of WT1 was 21% (95% CI = 0.16–0.29), in a highly heterogeneous set of 23 studies involving a total of 928 patients (Table 2). The result of publication bias analyses was: Egger test, −3.42; p = 0.005; Begg and Mazumdar test, −1.79; p = 0.074. For stage assessments, data extracted from studies revealed that tumors classified the FIGO Stage IV had a greater expression of WT1 (28%) than FIGO Stage III (7%) (p < 0.05). The combined HR estimate of OS was 27% (95% CI = 0.15–0.44). The combined HR estimate of DFS/RFS/PFS was 24% (95% CI = 0.15–0.35).
3.3. Serous Carcinoma
The analyses indicated that the expression of WT1 was 21% (95% CI = 0.14–0.29) in a heterogeneous set of 17 studies involving a total of 289 patients (Table 2). The result of publication bias analyses was: Egger test, −4.01; p = 0.001; Begg and Mazumdar test, −1.69; p = 0.091. For stage assessments, data extracted from studies revealed that tumors classified the FIGO Stage IV had a greater expression of WT1 (27%) than FIGO Stage III (17%) (p < 0.05). The combined HR estimate of OS was 40% (95% CI = 0.19–0.65). The combined HR estimate of DFS/RFS/PFS was 3% (95% CI = 0.00–0.31).
3.4. Clear Cell Carcinoma
The analyses indicated that the expression of WT1 was 15% (95% CI = 0.06–0.33); in a set of 6 studies involving a total of 54 patients (Table 2). The result of publication bias analyses was: Egger test, −2.05; p = 0.11; Begg and Mazumdar test −0.56; p = 0.57. For stage assessments, data extracted from studies revealed that tumors classified the FIGO Stage IV had a greater expression of WT1 (20%) than FIGO Stage III (9%) (p < 0.05). The combined HR estimate of OS was 21% (95% CI = 0.08–0.45). The combined HR estimate of DFS/RFS/PFS was 5% (95% CI = 0.01–0.27). Datasets analysis showed that WT1 expression was associated with OS.
3.5. Carcinosarcoma
The analyses indicated that the expression of WT1 was 38% (95% CI = 0.33–0.43) in a set of 6 studies involving a total of 240 patients (Table 2). The result of publication bias analyses was: Egger test, 0.34; p = 0.75 Begg and Mazumdar test, 0.19; p = 0.85. For stage assessments, data extracted from studies revealed that tumors classified the FIGO Stage IV and III had similar levels of WT1 expression (38% and 35%, respectively) (p < 0.05). The combined HR estimate of OS was 35% (95% CI = 0.29–0.41). The combined HR estimate of DFS/RFS/PFS was 41% (95% CI = 0.32–0.50). Datasets analysis showed that WT1 expression was associated with DFS/RFS/PFS.
4. Discussion
Increasing literature evidence suggests WT1 gene implications in the pathogenesis and prognosis of several solid tumors [5,6,7,8]. Regarding therapeutical strategies, some pilot clinical studies, performed on different types of malignancy, expressing WT1, showed also encouraging results by immunotherapeutic targeting of WT1 [49,50,51,52,53,54]. However, for EC, data concerning safety and tolerability of immunotherapeutic protocols or peptide vaccine with WT1 are limited [55,56,57].
Nevertheless, the clinical-prognostic implications of WT1 expression in endometrial cancer are still controversial. Therefore, to better clarify this issue, we conducted a systematic review and meta-analysis, including all published papers on the WT1 immunohistochemical expression across all histotypes of endometrial carcinoma. The present paper included a total of 35 eligible studies with 52 datasets and 1616 patients for qualitative analysis [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Carcinosarcoma and serous histotypes showed the higher rates of WT1 IHC expression (38% and 21% respectively), followed by endometrioid and clear cell histotypes (19% and 15% respectively).
These reported data have important differential diagnostic implications since WT1 IHC is generally believed as the most reliable tool in the distinction between ovarian and endometrial origin of gynecological tumors [8]. In particular, we are aware of the diagnostic challenge encountered in small peritoneal biopsies in cases of peritoneal carcinosis. In this setting immunoreactivity of WT1, particularly if diffuse, could favor tubo-ovarian origin but it is not exclusive of adnexal origin and/or serous histotype. In fact, considering the possibility of WT1 expression also in uterine cancers, difficulty in assigning tumor origin can persist in a minority of cases. In addition, in these contexts, we retain that clinical history, instrumental findings, laboratory markers and a wider immunohistochemistry panel are fundamental to define the correct diagnosis [58,59].
Regarding the impact of WT1 on the cancer patient prognosis most of the scientific studies have shown that positive expression of WT1 was linked with an unfavorable biological behavior.
In an article by Miyoshi et al., a significantly lower disease-free survival rate was observed in breast cancer patients with high levels of WT1 mRNA compared to those with low levels [60]. Similar results were reported in leukemia patients by Inoue et al. In fact, strong WT1 mRNA expression was related to a lower rate of complete remission and worse overall survival [61]. Moreover, the prognostic role of WT1 was also documented in hepatocellular carcinoma patients by Sera et al. In this paper, WT1 protein overexpression, confirmed by Western blotting and immunohistochemistry, represented an independent prognostic factor for disease-free survival [62]. By contrast, Høgdall et al. demonstrated a significantly shorter disease-specific survival in patients affected by ovarian cancer with positive WT1 protein expression [63]. Similarly, Netinatsunthorn et al. reported the prognostic role of WT1 immunohistochemical expression in patients with advanced serous ovarian carcinoma [64].
To date, on the other hand, only few reports are available on the prognostic impact of WT1 expression in endometrial cancer patients. In the present meta-analysis, we observed a worse prognosis in term of OS and DFS/RFS/PFS in EC cases showing strong WT1 expression. In detail, we found that uterine carcinosarcoma with high WT1 expression showed the worst outcome, as also highlighted by Coosemans et al. [18], especially regarding DFS/RFS/PFS. Overall, WT1 expression showed association with OS and DFS/RFS/PFS in endometrioid carcinoma and with OS, especially for serous carcinoma and clear cell carcinoma patients. Moreover, we noted that WT1 showed higher rates of expression in advance FIGO staged cancers (33%) in all histotypes.
It should be noted that there are some limitations to the analysis presented here. First, publication bias should be considered because more positive results tended to be published, thus potentially exaggerating the association between WT1 expression and poor prognosis. Second, there is limited number of studies reporting outcome results, therefore further larger cohorts of EC patients are needed to validate results of the present meta-analysis. Third, we combined DFS/RFS/PFS as a group. Although definitions among DFS/RFS/PFS are not standardized in the majority of our analysis, we consider them equivalent, and the combination can lead a bias.
Finally, we were unable to carry out stratified analysis according to cut-off values of WT1 expression due to numerous methodological variations among selected studies.
5. Conclusions
In summary, our study suggests the potential diagnostic and prognostic utility of WT1 in EC patients. Moreover, strong expression of WT1 is associated with poor outcome in this category of affected women. Therefore, we retain that it is important to validate pathological assessment of WT1 expression and its clinical utility by large multicenter prospective studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/9/637/s1.
Author Contributions
Conceptualization, G.F.Z., A.S. and G.A.; methodology, F.I., A.M. and P.S.; software, M.V. and S.S.; validation, N.D., D.A. and G.F.Z.; formal analysis, F.C. and P.S.; investigation, G.F.Z.; data curation, A.S.; writing—original draft preparation, A.S., P.S. and G.A.; writing—review and editing, A.S. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Goebel E.A., Vidal A., Matias-Guiu X. The evolution of endometrial carcinoma classification through application of immunohistochemistry and molecular diagnostics: Past, present and future. Virchows Arch. 2018;472:885–896. doi: 10.1007/s00428-017-2279-8. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Kandoth C., Schultz N. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felix A.S., Yang H.P., Bell D.W., Sherman M.E. Epidemiology of Endometrial Carcinoma: Etiologic Importance of Hormonal and Metabolic Influences. Adv. Exp. Med. Biol. 2017;943:3–46. doi: 10.1007/978-3-319-43139-0_1. [DOI] [PubMed] [Google Scholar]
- 5.Hohenstein P., Hastie N.D. The many facets of the Wilms’ tumour gene, WT1. Hum. Mol. Genet. 2006;15:R196–R201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- 6.Nakatsuka S., Oji Y., Horiuchi T. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 7.Magro G., Longo F.R., Angelico G., Spadola S., Amore F.F., Salvatorelli L. Immunohistochemistry as potential diagnostic pitfall in the most common solid tumors of children and adolescents. Acta Histochem. 2015;117:397–414. doi: 10.1016/j.acthis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Bárcena C., Oliva E. WT1 expression in the female genital tract. Adv. Anat. Pathol. 2011;18:454–465. doi: 10.1097/PAP.0b013e318234aaed. [DOI] [PubMed] [Google Scholar]
- 9.McCluggage W.G. WT1 is of value in ascertaining the site of origin of serous carcinomas within the female genital tract. Int. J. Gynecol. Pathol. 2004;23:97–99. doi: 10.1097/00004347-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Coosemans A., Moerman P., Verbist G. Wilms’ tumor gene 1 (WT1) in endometrial carcinoma. Gynecol. Oncol. 2008;111:502–508. doi: 10.1016/j.ygyno.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Acs G., Pasha T., Zhang P. WT1 Is Differentially Expressed in Serous, Endometrioid, Clear Cell, and Mucinous Carcinomas of the Peritoneum, Fallopian Tube, Ovary, and Endometrium. Int. J. Gynecol. Pathol. 2004;23:110–118. doi: 10.1097/00004347-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hussaini M., Stockman A.A., Foster H., McCluggage W.G. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004;44:109–115. doi: 10.1111/j.1365-2559.2004.01787.x. [DOI] [PubMed] [Google Scholar]
- 13.Assem H., Rambau P.F., Lee S. High-grade Endometrioid Carcinoma of the Ovary: A Clinicopathologic Study of 30 Cases. Am. J. Surg. Pathol. 2018;42:534–544. doi: 10.1097/PAS.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 14.Atik Y., Demir B.C., Ozan H., Baykara S., Usubutun A., Erturk F.Y. Wilms’ tumor 1 protein expression in endometrial adenocarcinoma and endometrial intra-epithelial neoplasia. J. Obstet. Gynaecol. Res. 2016;42:870–875. doi: 10.1111/jog.12981. [DOI] [PubMed] [Google Scholar]
- 15.Baek M.H., Park J.Y., Rhim C.C. Investigation of New Therapeutic Targets in Undifferentiated Endometrial Sarcoma. Gynecol. Obstet. Investig. 2017;82:329–339. doi: 10.1159/000454769. [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Husain A., Nelson G.S. Immunohistochemical Profiling of Endometrial Serous Carcinoma. Int. J. Gynecol. Pathol. 2017;36:128–139. doi: 10.1097/PGP.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 17.Chitale D.A., Jungbluth A.A., Marshall D.S. Expression of cancer-testis antigens in endometrial carcinomas using a tissue microarray. Mod. Pathol. 2005;18:119–126. doi: 10.1038/modpathol.3800232. [DOI] [PubMed] [Google Scholar]
- 18.Coosemans A., Van Calster B., Verbist G. Wilms Tumor Gene 1 (WT1) Is a Prognostic Marker in High-Grade Uterine Sarcoma. Int. J. Gynecol. Cancer. 2011;21:302–308. doi: 10.1097/IGC.0b013e318207cab5. [DOI] [PubMed] [Google Scholar]
- 19.Dohi S., Ohno S., Ohno Y., Soma G., Kyo S., Inoue M. Correlation between WT1 Expression and Cell Proliferation in Endometrial Cancer. Anticancer Res. 2009;29:4887–4892. [PubMed] [Google Scholar]
- 20.Dupont J., Wang X., Marshall D.S. Wilms Tumor Gene (WT1) and p53 Expression in Endometrial Carcinomas: A Study of 130 Cases Using a Tissue Microarray. Gynecol. Oncol. 2004;94:449–455. doi: 10.1016/j.ygyno.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Egan J.A., Ionescu M.C., Eapen E., Jones J.G., Marshall D.S. Differential Expression of WT1 and p53 in Serous and Endometrioid Carcinomas of the Endometrium. Int. J. Gynecol. Pathol. 2004;23:119–122. doi: 10.1097/00004347-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Espinosa I., D’Angelo E., Corominas M., Gonzalez A., Prat J. Mixed endometrial carcinomas with a “low-grade serous”—Like component: A clinicopathologic, immunohistochemical, and molecular genetic study. Hum. Pathol. 2018;71:65–73. doi: 10.1016/j.humpath.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Fadare O., James S., Desouki M.M., Khabele D. Coordinate Patterns of, ER, PR and WT1 Expression in the Histopathologic Distinction of Ovarian from Endometrial Serous Adenocarcinomas. Ann. Diagn. Pathol. 2013;17:430–433. doi: 10.1016/j.anndiagpath.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franko A., Magliocco A.M., Duan Q., Duggan M.A. WT1 Immunoprofiling and Comparison of Malignant Mullerian Mixed Tumors of the Female Genital Tract. Int. J. Gynecol. Pathol. 2010;29:452–458. doi: 10.1097/PGP.0b013e3181d55597. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein N.S., Uzieblo A. WT1 Immunoreactivity in Uterine Papillary Serous Carcinomas Is Different From Ovarian Serous Carcinomas. Am. J. Clin. Pathol. 2002;117:541–545. doi: 10.1309/K84K-005F-TCB8-FV4B. [DOI] [PubMed] [Google Scholar]
- 26.Guntupalli S.R., Cao D., Shroff R. Wilms’ Tumor 1 Protein and Estrogen Receptor Beta Expression Are Associated With Poor Outcomes in Uterine Carcinosarcoma. Ann. Surg. Oncol. 2013;20:2373–2379. doi: 10.1245/s10434-012-2838-9. [DOI] [PubMed] [Google Scholar]
- 27.Hashi A., Yuminamochi T., Murata S.I., Iwamoto H., Honda T., Hoshi K. Wilms Tumor Gene Immunoreactivity in Primary Serous Carcinomas of the Fallopian Tube, Ovary, Endometrium, and Peritoneum. Int. J. Gynecol. Pathol. 2003;22:374–377. doi: 10.1097/01.pgp.0000092130.10100.88. [DOI] [PubMed] [Google Scholar]
- 28.Hedley C., Sriraksa R., Showeil R., Van Noorden S., El-Bahrawy M. The frequency and significance of WT-1 expression in serous endometrial carcinoma. Hum. Pathol. 2014;45:1879–1884. doi: 10.1016/j.humpath.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Hirschowitz L., Ganesan R., McCluggage W.G. WT1, p53 and Hormone Receptor Expression in Uterine Serous Carcinoma. Histopathology. 2009;55:478–482. doi: 10.1111/j.1365-2559.2009.03390.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones T.E., Pradhan D., Dabbs D.J., Bhargava R., Onisko A., Jones M.W. Immunohistochemical Markers With Potential Diagnostic, Prognostic, and Therapeutic Significance in Uterine Carcinosarcoma: A Clinicopathologic Study of 43 Cases. Int. J. Gynecol. Pathol. 2019 doi: 10.1097/PGP.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 31.Kitade S., Ariyoshi K., Taguchi K. Serous Carcinoma of the Uterine Cervix: Clinicopathological Features Differing From Serous Carcinomas of Other Female Organs. J. Obstet. Gynaecol. Res. 2020;46:153–160. doi: 10.1111/jog.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B., Chen Q., Zhang X., Cheng L. Serous Carcinoma Arising From Uterine Adenomyosis/Adenomyotic Cyst of the Cervical Stump: A Report of 3 Cases. Diagn. Pathol. 2016;11:46. doi: 10.1186/s13000-016-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matalkaa I., Obeidatb B., Mohtaseba A., Awamlehc A. The significance of Wilms Tumor Gene (WT1) and p53 expression in curettage specimens of patients with endometrial carcinomas. Pathol. Res. Pract. 2013;209:19–23. doi: 10.1016/j.prp.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Nofech-Mozes S., Khalifa M.A., Ismiil N. Immunophenotyping of Serous Carcinoma of the Female Genital Tract. Mod. Pathol. 2008;21:1147–1155. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- 35.Nafisi H., Ghorab Z., Ismill N. Immunophenotypic Analysis in Early Müllerian Serous Carcinogenesis. Int. J. Gynecol. Pathol. 2015;34:424–436. doi: 10.1097/PGP.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 36.Ohno S., Dohi S., Ohno Y. Immunohistochemical Detection of WT1 Protein in Endometrial Cancer. Anticancer Res. 2009;29:1691–1696. [PubMed] [Google Scholar]
- 37.Ruba S., Doherty D., Stewart C.J.R. A detailed morphological and immunohistochemical comparison of primary endometrial and tubo-ovarian highgrade serous carcinomas and their corresponding omental metastases. Pathology. 2020;52:197–205. doi: 10.1016/j.pathol.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Stănescu A.D., Nistor I., Potecă A.G., Diţescu D., Comănescu M. Prognostic Biomarkers in Endometrial Adenocarcinoma. Rom. J. Morphol. Embryol. 2014;55:1339–1344. [PubMed] [Google Scholar]
- 39.Sumathi V.P., Al-Hussaini M., Connolly L.E., Fullerton L., McCluggage W.G. Endometrial Stromal Neoplasms Are Immunoreactive With WT-1 Antibody. Int. J. Gynecol. Pathol. 2004;23:241–247. doi: 10.1097/01.pgp.0000130051.04396.13. [DOI] [PubMed] [Google Scholar]
- 40.Tanvir I., Riaz S., Hussain A., Mehboob R., Shams M.U., Khan H.A. Hospital-based Study of Epithelial Malignancies of Endometrial Cancer Frequency in Lahore, Pakistan, and Common Diagnostic Pitfalls. Pathol. Res. Int. 2014;2014:179384. doi: 10.1155/2014/179384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Togami S., Sasajima Y., Kasamatsu T. Immunophenotype and Human Papillomavirus Status of Serous Adenocarcinoma of the Uterine Cervix. Pathol. Oncol. Res. 2016;21:487–494. doi: 10.1007/s12253-014-9854-y. [DOI] [PubMed] [Google Scholar]
- 42.Trinh V.Q.-H., Pelletier M.P., Echelard P. Distinct Histologic, Immunohistochemical and Clinical Features Associated With Serous Endometrial Intraepithelial Carcinoma Involving Polyps. Int. J. Gynecol. Pathol. 2020;39:128–135. doi: 10.1097/PGP.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 43.Yan Z., Hui P. Minimal Uterine Serous Carcinoma with Extrauterine Tumor of Identical Morphology: An Immunohistochemical Study of 13 Cases. Appl. Immunohistochem. Mol. Morphol. 2010;18:75–79. doi: 10.1097/PAI.0b013e3181b1d10e. [DOI] [PubMed] [Google Scholar]
- 44.Huedo-Medina T.B., Sanchez-Meca J., Marìn-Martìnez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 45.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed S., Ahmad M., Khan M.A. The interobserver reproducibility of thyroid cytopathology using Bethesda reporting system: Analysis of 200 cases. J. Pak. Med. Assoc. 2013;63:1252–1255. [PubMed] [Google Scholar]
- 49.Iiyama T., Udaka K., Takeda S. WT1 (Wilms’ tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol. Immunol. 2007;51:519–530. doi: 10.1111/j.1348-0421.2007.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 50.Mailander V., Scheibenbogen C., Thiel E., Letsch A., Blau I.W., Keilholz U. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of haematological or renal toxicity. Leukemia. 2004;18:165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 51.Oka Y., Tsuboi A., Taguchi T. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oka Y., Tsuboi A., Elisseeva O.A. WT1 peptide cancer vaccine for patients with hematopoietic malignancies and solid cancers. Sci. World J. 2007;7:649–665. doi: 10.1100/tsw.2007.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Driessche A., Gao L., Stauss H.J. Antigen-specific cellular immunotherapy of leukemia. Leukemia. 2005;19:1863–1871. doi: 10.1038/sj.leu.2403930. [DOI] [PubMed] [Google Scholar]
- 54.Izumoto S., Tsuboi A., Oka Y. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 55.Ohno S., Kyo S., Myojo S. Wilms’ tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29:4779–4784. [PubMed] [Google Scholar]
- 56.Coosemans A., Vanderstraeten A., Tuyaerts S. Wilms’ Tumor Gene 1 (WT1)-loaded dendritic cell immunotherapy in patients with uterine tumors: A phase I/II clinical trial. Anticancer Res. 2013;33:5495–5500. [PubMed] [Google Scholar]
- 57.Di Tucci C., Capone C., Galati G. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol. Oncol. 2019;30:e46. doi: 10.3802/jgo.2019.30.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zannoni G.F., Santoro A., Angelico G. Clear cell carcinoma of the endometrium: An immunohistochemical and molecular analysis of 45 cases. Hum. Pathol. 2019;92:10–17. doi: 10.1016/j.humpath.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Santoro A., Angelico G., Inzani F., Spadola S., Arciuolo D., Valente M., Musarra T., Capelli G., Fanfani F., Gallotta V., et al. Pathological features, immunoprofile and mismatch repair protein expression status in uterine endometrioid carcinoma: Focus on MELF pattern of myoinvasion. Eur. J. Surg. Oncol. 2020 doi: 10.1016/j.ejso.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 60.Miyoshi Y., Ando A., Egawa C. High expression of Wilms’ tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin. Cancer Res. 2002;8:1167–1171. [PubMed] [Google Scholar]
- 61.Inoue K., Sugiyama H., Ogawa H. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84:3071–3079. doi: 10.1182/blood.V84.9.3071.3071. [DOI] [PubMed] [Google Scholar]
- 62.Sera T., Hiasa Y., Mashiba T. Wilms’ tumour 1 gene expression is increased in hepatocellular carcinoma and associated with poor prognosis. Eur. J. Cancer. 2008;44:600–608. doi: 10.1016/j.ejca.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Høgdall E.V., Christensen L., Kjaer S.K. Expression level of Wilms tumor 1 (WT1) protein has limited prognostic value in epithelial ovarian cancer: From the Danish “MALOVA” ovarian cancer study. Gynecol. Oncol. 2007;106:318–324. doi: 10.1016/j.ygyno.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 64.Netinatsunthorn W., Hanprasertpong J., Dechsukhum C., Leetanaporn R., Geater A. WT1 gene expression as a prognostic marker in advanced serous epithelial ovarian carcinoma: An immunohistochemical study. BMC Cancer. 2006;6:90. doi: 10.1186/1471-2407-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.