Abstract
Off-label tocilizumab use in COVID-19 patients reflects concern for cytokine release syndrome. Comparison of matched COVID-19 pneumonia patients found elevated IL-6 levels correlated with mortality that did not change with tocilizumab administration. Correlating mortality with increased IL-6 doesn’t imply causality however lack of improvement by tocilizumab requires further clinical trial alterations.
Subject terms: Diseases, Outcomes research
Introduction
The lack of proven medications for COVID-19 beget the widespread use of off label therapeutics based on theoretical efficacy. Early data correlated elevated levels of IL-6 with increased mortality1. This finding led clinicians to use the humanized anti-IL6 receptor antagonist, tocilizumab, to attenuate IL-6 levels in infected patients based on its proven benefit in patients suffering from cytokine release syndrome after chimeric antigen receptors (CAR)-T cell infusion2,3. In COVID-19, the question of whether elevated IL-6 levels reflect an overwhelming viral infection or directly cause immunopathology responsible for a patient’s poor outcome remains unanswered. We present our experience with tocilizumab in conjunction with the published literature to argue for the urgent need to understand a disease before carte blanche application of unproven therapies in single arm trials. We aimed to find the effect of tocilizumab on mortality of COVID19 in a well-matched population cohort study.
We performed a retrospective cohort study of matched patients admitted to the University of Miami Hospital with a confirmed diagnosis of COVID-19 approved by University of Miami.
Institutional Review Board (IRB #20,200,441). All procedures in this study were performed in accordance with relevant guidelines and regulations. The IRB waived a requirement for informed consent in the context of minimal risk research.
Tocilizumab was administered to patients after consultation with an Infectious Disease driven committee for COVID19 who required oxygen ≥ 4 L per minute via nasal cannula and had specific levels of at least 4 biomarkers; IL-6 > 40 pg/mL, CRP > 10 mg/dL, D- dimer > 1 mcg/mL FEU, ferritin > 1,000 ng/mL, or LDH > 350 units. Out of 250 confirmed COVID-19 patients, 32 (12.5%) received Tocilizumab during hospitalization and enrolled in the study. Patients received 400 mg tocilizumab as a single intravenous infusion. 24 patients received tocilizumab when started mechanically ventilation and rest on supplemental oxygen. Thirty patients who did not receive tocilizumab were matched for gender, age, ICU admission, and qSOFA score and included in the control group. Demographic, clinical, laboratory data, and in-hospital mortality data were collected from the medical records. For descriptive analysis of ordinal variables, we used Mantel–Haenszel methods. Continuous variables were reported as median and interquartile range (IQR). Medians of two groups were tested using Wilcoxon-Mann–Whitney test. We used Cox's proportional hazards model to analyze survival time and multivariate analysis to test the effect of each independent variable on mortality. A p value less than 0.05 was considered statistically significant.
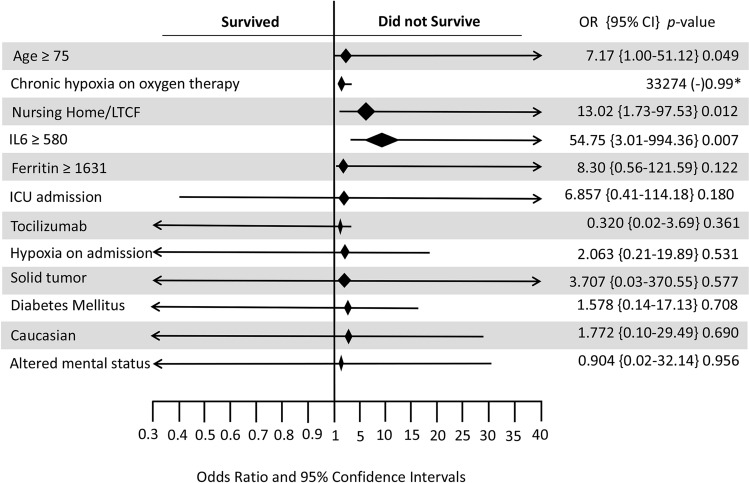
Nineteen patients (30.6%) died during this study with 14 (22.6%) dying in the ICU. The majority of deaths occurred in subjects older than 75 years (OR 7.1, p = 0.049). Subjects with IL-6 levels over 580 pg/mL had an increased mortality (OR 54.7, p = 0.007) (Table 1). In multivariate analysis, tocilizumab administration had no discernible effect on mortality (OR 0.3, p value 0.36). Additional variables that were conclusive using the univariate analysis but did not hold up in multivariate analysis include; residents of nursing homes/long term care facility (OR 13.0, p = 0.12), diabetes mellitus (OR1.5, p = 0.70) solid tumors (OR 3.70, p = 0.57), ferritin ≥ 1631 (OR 8.3, p = 0.12), and altered mental status (OR 0.9, p = 0.95) (Fig. 1). Figure 2 shows survival analysis result and showing no significant difference in mortality between subjects treated and untreated with tocilizumab.
Table 1.
Demographic and clinical characteristics, and outcomes of subjects with COVID19.
| Demographics (N = 62) | Survived N (%) | Did not survive N (%) | p value |
|---|---|---|---|
| Age, Median (IQR) year | 62 (42–82) | 75 (58–92) | 0.006 |
| Age ≥ 75 (N = 15) | 5 (11.6) | 10 (52.6) | 0.001 |
| Male (N = 44) | 30 (69.8) | 14 (73.7) | 0.754 |
| Hispanics (N = 34) | 23 (56.1) | 11 (64.7) | 0.545 |
| White (N = 33) | 20 (51.3) | 13 (76.5) | 0.085 |
| Black (N = 15) | 13 (33.3) | 2 (11.8) | 0.109 |
| Asian (N = 2) | 1 (2.6) | 1 (5.9) | 0.55 |
| More than one race (N = 6) | 5 (12.8) | 1 (5.9) | 0.452 |
| Tobacco cigarette use (N = 39) | 27 (62.8 | 12 (63.2) | 0.978 |
| Vaping (N = 51) | 37 (86) | 14 (73.7) | 0.247 |
| Alcohol (N = 50) | 35 (81.4) | 15 (78.9) | 0.822 |
| Marijuana (N = 49) | 32 (74.4) | 17 (89.5) | 0.194 |
| Symptoms at or after 48 h of hospital admission | |||
| Temperature > = 100°F (N = 26) | 18 (41.9) | 8 (42.1) | 0.986 |
| Cough (N = 44) | 32 (76.2) | 12 (66.7) | 0.447 |
| Sore throat (N = 2) | 1 (2.4) | 1 (5.6) | 0.553 |
| Rhinorrhea (N = 4) | 3 (7.3) | 1 (6.3) | 0.887 |
| Dyspnea (N = 43) | 30 (69.8) | 13 (68.4) | 0.916 |
| Fever (N = 42) | 29 (67.4) | 13 (72.2) | 0.713 |
| Chills (N = 21) | 13 (31) | 8 (44.4) | 0.318 |
| Myalgias (N = 16) | 10 (24.4) | 6 (33.3) | 0.478 |
| Abdominal pain (N = 5) | 4 (9.8) | 1 (5.9) | 0.636 |
| Diarrhea (N = 5) | 3 (7.3) | 2 (11.1) | 0.632 |
| Nausea/vomiting (N = 7) | 5 (12.2) | 2 (11.8) | 0.963 |
| Altered mental status (N = 8) | 3 (7.1 | 5 (29.4) | 0.035 |
| Pre-admission oxygen use (N = 5) | 1 (2.4) | 4 (21.1) | 0.039 |
| Inhaled steroid use (N = 6) | 4 (9.5) | 2 (11.1) | 0.851 |
| qSOFA, Median (IQR) | 0.001 (0–0.5) | 1 (0–2) | 0.582 |
| Medications prior to hospital admission | |||
| Prednisone | 6 (14.3) | 2 (11.1) | 0.741 |
| ACE inhibitors | 4 (9.5) | 3 (17.6) | 0.389 |
| Angiotensin Receptor Blockers | 7 (16.7) | 3 (17.6) | 0.928 |
| Statins | 10 (23.8) | 7 (41.2) | 0.187 |
| Emergency Room visit within 12 months | 10 (24.4) | 4 (22.2) | 0.857 |
| Hospital admission within last 12 months | 11 (26.8) | 5 (27.8) | 0.94 |
| Nursing home/long term care facility residents | 5 (11.9) | 9 (47.4) | 0.004 |
| Findings at Chest X-Ray or CT images obtained while in hospital | |||
| Ground glass opacities | 9 (22.0) | 5 (26.3) | 0.71 |
| Consolidations | 10 (24.4) | 8 (42.1) | 0.168 |
| Pleural effusions | 5 (12.2) | 5 (26.3) | 0.181 |
| Bilateral infiltrates | 27 (65.9) | 19 (31.7) | 0.552 |
| Comorbidities | |||
| Chronic Obstructive Pulmonary Disease | 4 (9.5) | 1 (5.3) | 0.58 |
| Supportive oxygen before admission | 16 (42.1 | 13 (68.4) | 0.066 |
| Congestive Heart Failure | 1 (2.4) | 2 (10.5) | 0.211 |
| Atrial fibrillation | 4 (9.5) | 2 (10.5) | 0.903 |
| Hypertension | 23 (54.8) | 14 (73.7) | 0.167 |
| Stroke | 4 (9.5) | 2 (10.5) | 0.903 |
| Dementia | 2 (4.8) | 1 (5.3) | 0.933 |
| Chronic Renal Failure | 2 (4.8) | 1 (5.3) | 0.933 |
| Diabetes Mellitus | 10 (23.8) | 13 (68.4) | 0.02 |
| Lymphoma | 2 (4.8) | 1 (5.3) | 0.933 |
| Solid tumor | 1 (2.4) | 4 (21.1) | 0.039 |
| Laboratory findings at or within 48 h of admission to hospital | |||
| Procalcitonin ≥ 1.15 ng/mL | 7 (16.3 | 5 (26.3) | 0.36 |
| Fibrinogen ≥ 649 mg/dL | 2 (4.7) | 1 (5.3) | 0.918 |
| IL-6 > 580 pg/mL | 1 (2.3) | 4 (21.1) | 0.037 |
| IL-6 Median (IQR) pg/mL | 81.8 (0–264.9) | 1197.3 (0–3738.9) | 0.097 |
| Ferritin > 1631 ng/mL | 6 (14.0) | 7 (36.8) | 0.048 |
| C-Reactive Protein ≥ 21 | 7 (16.3) | 6 (31.6) | 0.179 |
| Mechanical ventilation use | 21 (65.6) | 15 (83.3) | 0.19 |
| Positive blood culture obtained after tocilizumab | 5 (16.1) | 2 (11.8) | 0.683 |
| Medications for COVID19 | |||
| Tocilizumab | 22 (51.2) | 10 (52.6) | 0.915 |
| Tocilizumab administration post-admission, Median (IQR) day | 2 (0–5) | 2 (0–6) | 0.703 |
| Tocilizumab for more than 4 days | 6 (14.0) | 2 (10.5) | 0.711 |
| Chloroquine/Hydroxychloroquine | 31 (72.1 | 15 (32.6) | 0.571 |
| Macrolides | 33 (76.7) | 16 (84.2) | 0.508 |
| Steroids | 26 (60.5) | 10 (52.6) | 0.565 |
| Outcome | |||
| Hospital stay duration, Median (IQR) day | 22 (0–47) | 11 (0–30) | 0.102 |
| ICU stay duration, Median (IQR) day | 5 (0–24) | 7 (0–21) | 0.582 |
| Patient delay*, Median (IQR) day | 4 (0–10) | 4 (0–10) | 0.703 |
| Physician delay**, mean (SD) day | 3 (0–10) | 4.0 (0–11) | 0.609 |
| Readmission | 3 (7.0) | 1 (5.3) | 0.801 |
| ICU admission | 29 (67.4) | 17 (89.5) | 0.083 |
Figure 1.
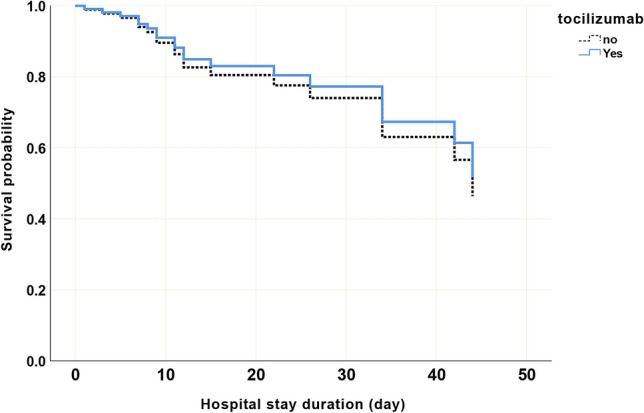
Cox regression showing no statistical difference in mortality between COVID 19 subjects treated and untreated tocilizumab therapy. Blue: shows treated with Tocilizuman, Black: shows untreated with Tocilizumab. Variables in the model: Age > = 75, IL6 > = 580, and Tocilizumab. p value, HR (95%CI) for Tocilizumab: 0.75, 0.9 (0.3–2.2).
Figure 2.
Forest plot showing the variables used in the multivariate model among COVID19 subjects. Hosmer lameshow score > 0.05, IL6 and Ferritin units: pg/ml.
Our data show survivors had mean IL-6 levels of 177.9 + /- 227.9 whereas non-survivors had mean levels of 1384.2 + /- 1234.7 congruent with reports that elevated IL-6 levels are associated with poor outcomes in patients with COVID19 viral pneumonia4,5. The literature showing this correlation predominantly only measures admission IL-6 levels (most < 50 pg/mL) but does not follow them throughout the clinical course5. A recent study in Chest followed IL-6 levels after tocilizumab administration and found an immediate increase followed by a steady decrease but did not differentiate IL-6 levels between survivors and non-survivors4. Data from China found patients with rising IL-6 levels greater than 4000 pg/mL perished despite receiving tocilizumab6. These IL-6 levels begin to reach those seen in cytokine release syndrome (CRS) following CAR T cell infusion where levels reach a median value of 8,309 pg/mL for grade 4 or 5 CRS7.
The finding of an elevated cytokine level in disease does not prove causality regardless of the degree of correlation. However, rapid reversal of a clinical syndrome through use of cytokine specific blockade provides good data linking that cytokine to disease pathogenesis. Use of tocilizumab for CRS in patients treated with CAR T cells causes a dramatic improvement in disease usually within 48 h8,9. To date, tocilizumab has not had the same dramatic effect on reversing COVID-19 pneumonia like it does in CRS from CAR-T cell infusion despite papers suggesting possible benefit when compared to historical controls10–14.
Comparison of IL-6′s role in viral infections versus CRS after CAR-T cell infusion is informative. IL-6 is an important cytokine responsible for promoting anti-viral T cell responses, inhibiting viral replication and resolving inflammation to promote tissue repair15. However, mouse models of viral infections that artificially create supraphysiologic IL-6 levels (10,000 pg/mL) show viral persistence and increased immunopathology16. Immune stimulating agents in cancer, e.g. CAR T cells, are efficacious partly because they are impervious to negative immune regulation and allow unfettered immune reactions against tumor cells. Not surprisingly, the supraphysiologic IL-6 levels in CAR-T infusion both correlate with high tumor burdens17 and appear to be directly responsible for disease as administration of tocilizumab dramatically improves clinical status usually within 48 h3. Our data and another retrospective cohort trial of matched patients showed tocilizumab had no effect on mortality18.
There are several limitations to this retrospective cohort study including small sample size and retrospective cohort design. Tocilizumab was given based on clinical parameters and biomarkers assumed to indicate IL-6 mediated immunopathology. Although IL-6 is correlated with poorer outcomes, in COVID19 we do not know if, at what level or at what time point IL-6 leads to immunopathology. Tocilizumab may have failed to influence mortality because IL-6 may not be either responsible for, or the only cytokine involved in immunopathology. If IL-6 is responsible for CRS in COVID-19, it is unknown at what level or time point it changes from having anti-viral properties to causing immunopathology. Use of tocilizumab in patients regardless of IL-6 levels may have diluted out patients for whom tocilizumab may have benefited obscuring its effect on mortality. Tocilizumab would not help patients who did not produce pathologic IL-6 levels but could be detrimental if lower IL-6 levels were necessary to fight the viral infection15.
We believe the need to “do something” has superseded the need to evaluate disease to apply clinical trials based on data. We argue that evaluation of immune parameters in COVID19 patients need to first be studied to ensure that IL-6 is involved in immunopathology and second to determine at what level or time point in the clinical course of infection, IL-6 produces immunopathology. Serial measurements of key cytokines in COVID19 may characterize IL-6 and additional cytokine levels to correlate with clinical outcomes, before administration of tocilizumab. After measuring the levels of cytokines in the clinical course of a COVID19 infection, trials should be attempted to apply immune modulating therapy to patients for whom the immune system is causing disease via dysregulation.
Acknowledgments
The authors would like to thank the physicians and nurses of the COVID-19 units of University of Miami and all of the patients for their participation.
Author contributions
G.H. conducted literature review and helped in manuscript preparation. M.B., M.M., S.K., K.S., M.V.P.B., H.A., S.H., S.A. conducted literature review and collected data from medical records and helped to develop first draft of manuscript. M.M. conducted literature review, designed the study, conducted exploratory analysis, performed data analysis, and manuscript preparation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens. Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kewan T, et al. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grifoni E, et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020;81:452–482. doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo P, et al. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teachey DT, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia; Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia; Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 374, 998, doi:10.1056/NEJMx160005 (2016). [DOI] [PubMed]
- 9.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colaneri M, et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020 doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rossi N, et al. Early use of low dose tocilizumab in patients with COVID-19: A retrospective cohort study with a complete follow-up. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer-Gould A, et al. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortegiani A, et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canziani LM, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu W, et al. TLR ligand induced IL-6 counter-regulates the anti-viral CD8(+) T cell response during an acute retrovirus infection. Sci. Rep. 2015;5:10501. doi: 10.1038/srep10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay KA, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campochiaro C, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]