Abstract
The search for suitable fish meal replacements in aqua-diets is a salient agenda in the constant effort of making aquaculture practices more sustainable. In this study, we tested four customised diets composed by systematic inclusion of pre-selected fish meal substitutes, lupin kernel meal, BSF meal, TH and PBM on growth, metabolism, cytokine profile, gut morphology and microbiota of juvenile Lates calcarifer. Five isoproteic and isoenergetic diets were prepared viz. FM100 as a control (without fish meal substitute), while FM75, FM50, FM25 and FM0 indicates replacement of fish meal (FM) at 25%, 50%, 75%, and 100%, respectively by a mixture of four different pre-selected non-fish meal (NFM) ingredients. Fish fed FM100, FM75, FM50, FM25 exhibited consistent growth and haematological response, while the fish fed no fishmeal (FM0) showed significant decline in final body weight (FBW) and specific growth rate (SGR). The poor growth performance was correlated with a decrease in villous width, microvilli height and goblet cells density. A significant shift in abundance profile of Psychrobacter in the gut microbial profile of fish fed FM50 was noticed compared to fish fed FM100. The results of qRT-PCR showed up-regulated expression of innate immune responsive genes in the FM50 group. The adverse impacts on growth performance and gut health of fish fed FM0 suggest that the complete substitution of fishmeal is not advisable and the inclusion range of these alternatives should be decided for a species only after examining their effect on maximal physiological performance.
Subject terms: Biotechnology, Computational biology and bioinformatics, Immunology, Molecular biology, Physiology, Zoology
Introduction
Fishmeal (FM) is an exemplary source of dietary protein for aquaculture species due to its high protein content, omega-3 polyunsaturated fatty acid, balanced amino acid composition and easy digestibility1. However, the dependence of aquaculture on FM for protein source is a rising concern for this industry with regards to its economics and sustainability. Aquaculturists worldwide have been conducting trials to come up with alternative ingredients for FM that can be cost-effective, easily available and yet provides the same nutritional value for the target species2,3. Attempts have been made to exploit both plant and animal protein sources as total or partial FM replacements in aqua-diets to achieve results comparable to diets with FM4–9. FM replacement, solely, by plant-based alternatives has been a challenge mainly due to the presence of anti-nutritional factors (ANFs), including alkaloids, lectins, protease inhibitors, phytates, saponins and tannins, deficiency of lysine and methionine and concerns about digestibility and palatability10. On the other hand, animal-based sources like poultry by meal (PBM), blood/ bone/ meat meal etc. despite having high protein content and lack of ANFs demonstrated a deficit in lysine, methionine and isoleucine4. Researchers are trying to navigate these concerns by exogenous supplementation of essential amino acids or biochemical manipulation of the constituents10–14. Inclusion of both plant and animal meal has also exhibited promising results in various species15,16. Recycling of marine by-products is another tested alternative for FM. The marine fish processing industry utilises only raw flesh for packaging while about two-thirds of the fish is discarded17. As one of the most lucrative species on the international fish market, tuna (Thunnus spp.) also contributes to a huge load of by-product. Hydrolysis of skipjack tuna (Katsuwonus pelamis) waste provides tuna hydrolysate (TH) which is rich in low molecular weight peptides and free amino acids and holds great potential for economic viability of aquaculture industry18. Addition of yellowfish TH to the diet of Persian sturgeon (Acipenser persicus) larvae resulted in maximised growth and feed utilisation at 25% FM replacement while showing no impact on gut microflora count or immunity19. TH also proved to be effective in improving immunity and dietary protein digestibility in red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus)20. With high protein and cholesterol content, ubiquitous availability and ease of species palatability, PBM is another promising FM replacement for considerable cost-cutting in aqua-feed production21,22. Up to 65% incorporation of PBM has been feasible in channel catfish (Ictalurus punctatus) without hampering its growth23. A recent study by Sabbagh et al., demonstrated the possibility of total FM replacement by PBM in gilthead seabream (Sparus aurata) showing comparable growth performance, fillet quality and haematological parameters to that of fish fed 100% FM24. Galkanda-Arachchige et al. performed a meta-study on assessment of PBM as a FM substitute that showed insignificant weight variations between various dietary treatments while significant in-study variations were noted between fish species25. Another promising FM substitute is insect meal, especially black soldier fly (BSF) (Hermetia illucens) larvae meal that has led to improvement in growth performance and immune indices in yellow catfish (Pelteobagrus fulvidraco) at partial incorporation levels26. BSF meal has also demonstrated potent results in juvenile Nile tilapia, (Oreochromis niloticus) and marron (Cherax cainii) but its commercial application still requires consideration on issue of mass production and cost management27,28.
Lates calcarifer or barramundi, distributed across the Indo-specific and Australia, is a commercially viable species due to its high physiological tolerance, fast growth, easy maintenance and production29. As an obligate carnivore, it requires high quality protein for maintenance of its optimum growth. In the recent years, a variety of FM substitutes have been tested on barramundi as well, amongst other marine carnivore species. L. calcarifer showed an improvement in feed intake and growth performance with skipjack TH -supplemented diets30. Sixty percent inclusion of fermented lupin (Lupinus angustifolius) in the diet of juvenile barramundi had no adverse effect on its body weight or fish carcass composition31. Replacement of FM was also found to be feasible with H. illucens meal in the range of 28.4–50%32. Though the results from these studies indicate the potential of these raw ingredients for commercial aqua-diet preparation, yet the majority are focussed on testing the efficacy of a stand-alone plant and animal based protein source. The mixture of various protein sources may be able to meet the nutritional requirements comparable to FM. Hence, there is a need for a thorough assessment of impacts of dietary inclusion of a mixture of raw plant and animal based proteins to replace FM. Reports on implication of gut microbiota profile as a determinant of physiological aspects like digestion and immunity highlight the critical relationship between aquadiets and gut health33. With high throughput sequencing technologies becoming advanced and cheaper by the day, the analysis of the data via high processor speed and computational biology algorithms would prove insightful in understanding the regulation of fish physiological systems by outlining gut microbes34.
The current study was undertaken to assess the impact of including various concentrations of a combination of non-fish meal ingredients such as lupin kernel, tuna hydrolysate, BSF meal, and PBM on the growth performance, biochemical indices, cytokine profile of distal gut and its microbiota composition. Starting from FM100 which had 100% FM, step by step replacement of FM was done in each of the subsequent diet preparations to formulate FM75, FM50, FM25 and FM0 diets by increasing the variety of non-FM ingredients.
Results
Growth parameters
Growth performance, food conversion ratio (FCR), hepatosomatic index (HSI) and survival of barramundi fed with four different non-fish meal experimental diets and the control are shown in Table 1. Significantly lower final body weight (FBW) and specific growth rate (SGR) were observed in the barramundi fed without fish meal than the other dietary treatment groups. Similarly, biomass gain (BG) was significantly decreased in the group fed with completely replaced fish meal fed group (FM0), however, non-significant change was observed compared to control and 25% fish meal substituted group (FM75). FCR and HSI of juvenile barramundi were not affected by any dietary treatments. Although survival of juveniles was the highest in FM50 compared to other treatment groups, but it was non-significant.
Table 1.
Growth performance of juvenile barramundi fed with different non-fish meal diets for 7 weeks.
| Growth parameters | Experimental diets | ||||
|---|---|---|---|---|---|
| FM100 | FM75 | FM50 | FM25 | FM0 | |
| IBW (g) | 2.51 ± 0.01 | 2.53 ± 0.03 | 2.54 ± 0.02 | 2.53 ± 0.02 | 2.60 ± 0.06 |
| FBW (g) | 63.70a ± 3.14 | 63.19a ± 1.96 | 62.68a ± 2.71 | 60.72a ± 3.01 | 44.27b ± 1.55 |
| SGR (%/d) | 6.60a ± 0.10 | 6.56a ± 0.06 | 6.53a ± 0.09 | 6.48a ± 0.10 | 5.79b ± 0.03 |
| BG (%) | 1041.91ab ± 68.21 | 1070.20ab ± 28.06 | 1231.36b ± 40.35 | 1136.36b ± 98.87 | 854.10a ± 19.02 |
| FCR | 1.26 ± 0.03 | 1.28 ± 0.05 | 1.17 ± 0.02 | 1.28 ± 0.09 | 1.53 ± 0.17 |
| HSI | 1.69 ± 0.11 | 1.91 ± 0.16 | 1.77 ± 0.21 | 2.19 ± 0.31 | 2.10 ± 0.17 |
| Survival (%) | 81.67a ± 1.67 | 85.00ab ± 5.00 | 98.33b ± 1.67 | 93.33ab ± 4.41 | 96.67ab ± 3.33 |
Different superscript letters (a, b) in the same row denote significant differences (p < 0.05). Data were represented as mean ± SE.
Serum and blood biochemical indices
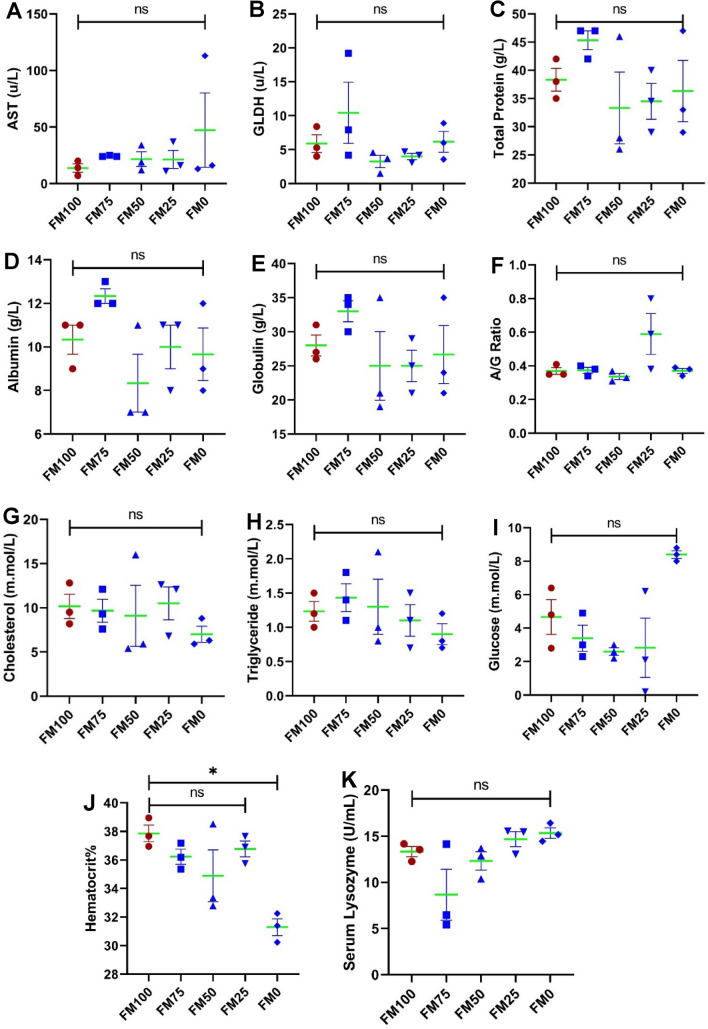
Apart from haematocrit % (Fig. 1J) none of the measured blood and serum biochemical indices including, aspartate aminotransferase (AST), glutamate dehydrogenase (GLDH), total protein, albumin, globulin, albumin and globulin ratio (A/G ratio) cholesterol, triglyceride, glucose and serum lysozyme were significantly affected by inclusion of various non-fish meal ingredients in the diets of juvenile barramundi (Fig. 1A–K). Significant decline in the haematocrit level was observed between fish meal (FM100) and non-fish meal (FM0) fed groups, however, non-significant change was noticed among other treatments (Fig. 1J).
Figure 1.
Serum and blood biochemical indices of juvenile barramundi fed with different protein sources for 7 weeks. X-axis represents the different non protein source 0 (control, FM100), 25, 50, 75 and 100 are considered as experimental treatments. (A) AST, aspartate transaminase, (B) GLDH, glutamate dehydrogenase, (C) total protein, (D) albumin, (E) globulin, (F) A/G ratio (albumin/globulin ratio), (G) Cholesterol, (H) Triglyceride, (I) Glucose, (J) Heamatocrit % and (K) Serum lysozyme. Data were represented as mean ± S.E., n = 3. Post ANOVA Turkey multiple comparison test was applied to compare the mean value of each treatment with the mean value of the control. Mean values significantly different from the control are noted with P < 0.05.
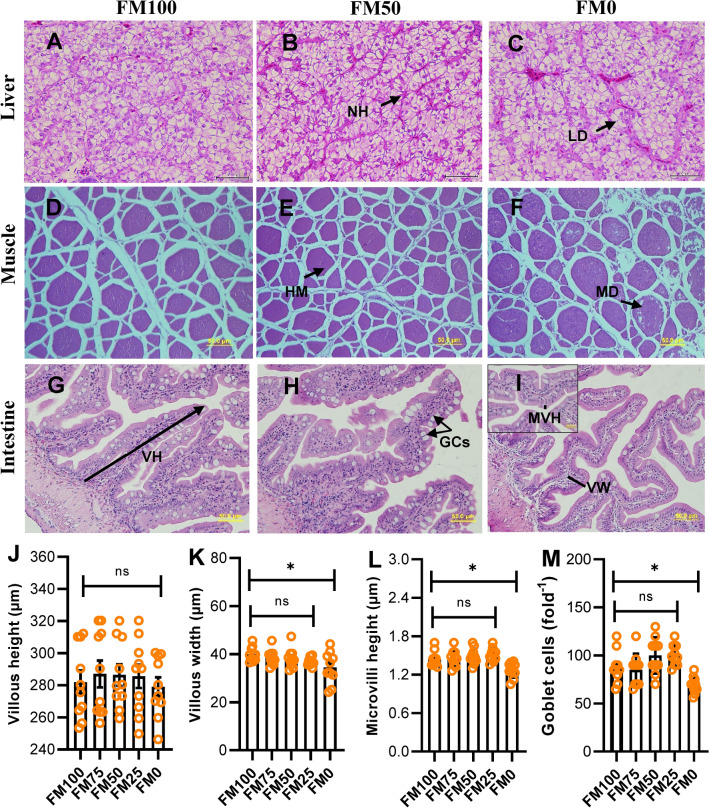
Histomorphology
Histomorphological changes in the liver, muscle and intestinal mucosal morphological examination of juvenile barramundi fed with FM100, FM50 and FM0 diets were demonstrated in Fig. 2. Liver histology of barramundi fed FM100 exhibited increased lipid deposition in hepatocytes while normal cells as indicated by clear hexagonal hepatocytes with prominent nuclei and rare cytoplasmic vacuolization were observed in FM50 fed fish. However, cellular degeneration and necrotic foci were observed in the liver of fish fed FM0. Muscle tissues of juvenile barramundi fed different non FM protein sources showed healthy myotomes characterised by rounded, packed and uniformly identical muscle fibres in fish fed FM50 while necrosis and myodegeneration was observed in fish fed FM0 and FM100. The distal intestine of fish fed FM0 showed notable alteration including reduced villous width, microvilli height and goblet cells density whereas intestinal mucosal morphology were unchanged in fish fed with other treatment diets.
Figure 2.
Representative micrographs of liver (A–C), muscle (D–F) and intestine (G–I) of juvenile barramundi after 7 weeks of experimental trials. All sections are stained with H&E with 40 magnification. Variation in villous height (J), villous width (K), microvilli height (L) and goblet cells density (M) in fish fed test diets, compared by Turkey multiple comparison test at P < 0.05. NH, normal hepatocyte; LD, lipid droplet; HM, healthy myotome; DM, myodigeneration; VH, villous height; GCs, goblet cells; VW, villous width and MVH, microvilli height.
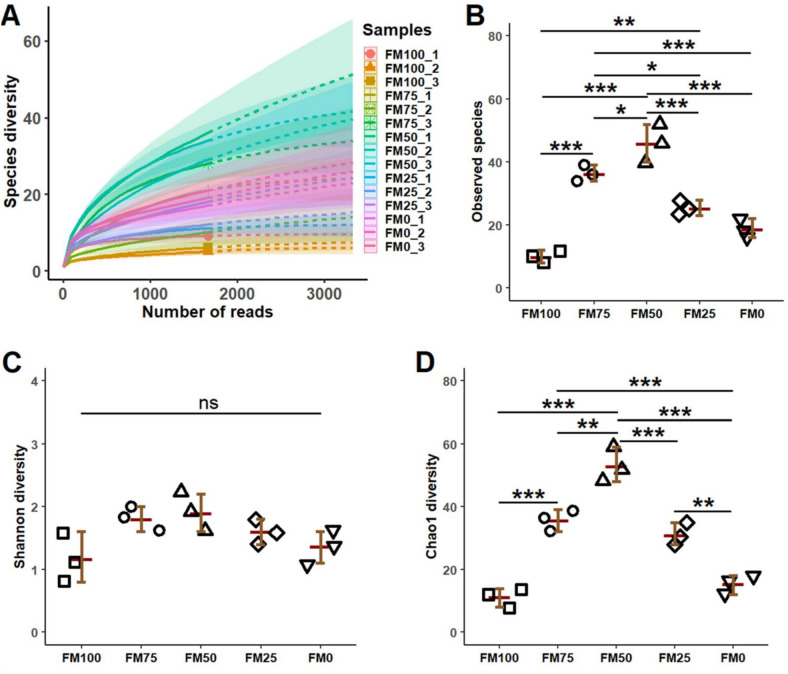
Alpha diversity of microbial communities
After quality trimming, 15 samples generated 878,788 high quality Illumina pair-end reads that were classified into 125 OTUs, 6 phyla and 56 genera. The rarefaction curve indicated that the 16S rRNA sequence captured enough depth and diversity for 15 samples from five different treatment groups (Fig. 3A). The curve revealed significant influences of bacterial communities in the hindgut of barramundi fed FM50. The alpha diversity measurements revealed that FM50 diet had significant positive influence on observed species and Chao1 indices, followed by FM75, FM25, FM0 and FM100, respectively. The Shannon index on the other hand remained unchanged with all five test diets. The diversity data also showed that FM100 and FM0 diets generated inadequate results in terms of microbial diversity (Fig. 3A–D).
Figure 3.
Rare faction curve and diversity indices (mean ± S.E., n = 3) of bacterial genera in juvenile barramundi fed with different non-fish meal diets for 7 weeks of feeding trials. (A) Species diversity, (B) Observed species, (C) Shanon diversity and (D) Chaol 1 diversity (ns = Non-significant).
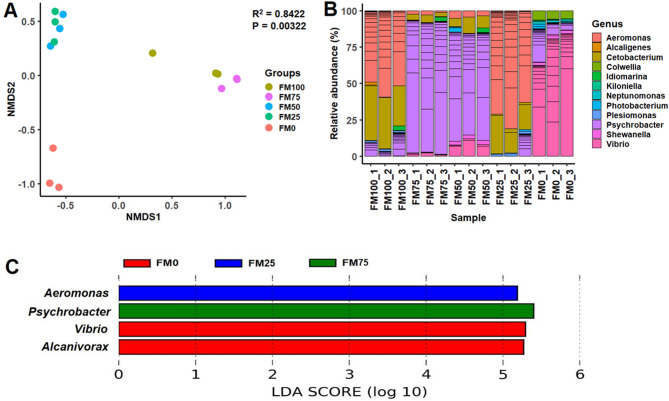
Beta diversity and microbial communities
Beta dispersion of samples based on non-metric multidimensional scaling (nMDS) is shown in Fig. 4A. An R2 value of 0.8422 and P value of 0.00322 showed significant effects of different dietary protein ingredients on barramundi gut. Compare to FM0, FM75 and FM50 had almost same effect while FM25 and FM100 had similar kind of effects on the gut microbiota of juvenile barramundi (Fig. 4A).
Figure 4.
Non-metric multidimensional scaling (nMDS) representing the clustering of samples for five different dietary protein fed groups based on relative abundance of bacterial OTUs (A). Relative abundance of bacterial OTUs at Genus level of five different dietary protein fed groups (B). Wisconsin non-parametric t-test at 0.05 level of significance and stringent LDA cut-off value of 3.0. (C).
At phylum level, Proteobacteria was the most abundant (88.2–98.6%) bacteria in all five diet groups wherein Fusobacteria observed only for FM75 (6.8%) and FM50 (4.8%) (Fig. 4B). At genus level, FM25 (75.9%) and FM100 (48.2%) were dominated by Aeromonas, FM75 (88.6%) and FM50 (72.4%) had Psychrobacter profusion and FM0 dominated by Vibrio (74.6%) (Fig. 3B). The differential abundance at 0.05 level of significance revealed that Vibrio and Alcanivorax were the indicator microbes in FM0, Psychrobacter in FM75 and Aeromonas in FM25, respectively (Fig. 4C).
Impacts on immune gene expression
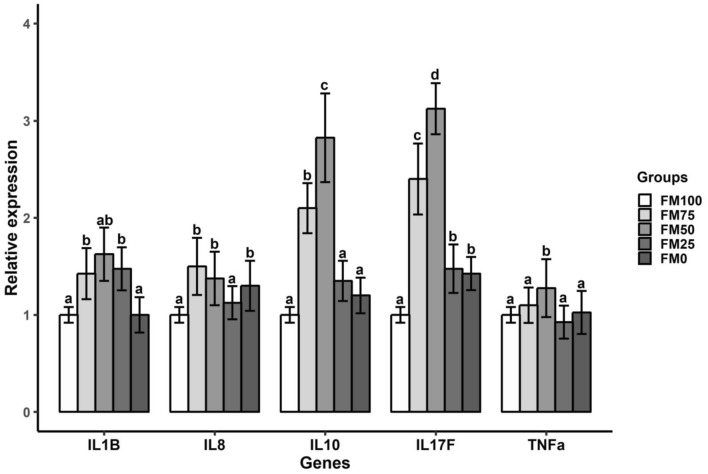
Relative to control, the results of qRT-PCR showed up-regulation of innate immune responsive genes in the FM50 group. The expression level of immune genes in the juvenile barramundi fed FM75 had similar patterns to the FM50 group. There was 3.1 and 2.4-fold increase in the expression of pro-inflammatory cytokine, interleukin-17 (IL-17) with FM50 and FM75 diets, respectively (Fig. 5). On the other hand, 2.8 and 2.1-fold increase in the expression of anti-inflammatory cytokine, interleukin 10 (IL10) was observed in the group fed with FM50 and FM75 diets, respectively. Compared to control, 1.6-fold, 1.4-fold and 1.3-fold upregulated expression level of interleukin 1β (IL-1β), interleukin 8 (IL-8) and tumour necrosis factor (TNF-α) genes were observed in FM50 group. The expression of IL-1β, IL10 and TNF-α in the group fed without fish meal inclusion (FM0) was relatively static in compare to control group fed solely with fish meal protein ingredients (FM100).
Figure 5.
Relative expression level of some cytokine genes in distal intestine of barramundi at the end of 7 weeks feeding trial. Super script letter (a, b and c) on the top of error bar represents significant difference with P < 0.05.
Discussion
Multiple studies have been carried out for testing the efficiency of alternatives for FM in L. calcarifer32,35–37. The choice of raw ingredient is often guided by the ease and cost of availing it in bulk locally, however, with respect to impact assessment, growth parameters should not be the sole criterion for its selection, physiological factors like metabolism, immunity and gut microbial composition should also be taken into account while optimizing the inclusion range of a certain FM substitute. The present study assessed the impact of four experimental diets customised by systematic inclusion of four FM substitutes—lupin kernel meal, BSF meal, TH and PBM on growth, metabolism, immunity and gut microflora of L. calcarifer juveniles.
The raw ingredients chosen for this study have been studied earlier individually or in dual combination and have shown significant potential for inclusion in juvenile barramundi diet36–38. Upto 45% replacement of fish meal protein could be replaced by lupin meal without compromising growth and protein metabolism parameters39. At 10% TH inclusion in the diet of juvenile barramundi, there was a significant increase in the fish’s body weight and SGR38. The fish fed on this diet also exhibited improved intestinal health and enhanced resistance to Streptococcus iniae infection. BSF-supplemented PBM could replace up to 45% FM in juvenile barramundi while improving growth, gut health and immune parameters36. In corroboration with the afore mentioned studies on juvenile barramundi, our study also vividly indicated that the complete replacement of fish meal from the diet of barramundi without adversely impacting its health and physiology is not advisable. The fish fed FM0 containing LKM, PBM, BSF and TH exhibited significant decline in growth parameters and hematocrit %. This dietary group also showed distinct fatty liver with intense vacuoles in the hepatocytes, necrosis and myodegeneration in muscle and a decrease in villous width, microvilli height and goblet cells density. The intestinal micomorphology, in particular, villous width, microvilli height and goblet cells density is related to absorptive surface area of intestine that have been reported to have correlation with digestion and absorption38, 40. Such changes in the intestinal micromorphology might have induced the depressed growth of fish fed FM0. In addition,the adverse impact on health parameters in FM0 may be endowed upon the presence of anti-nutritional components in lupin in this diet composition. The lupin meal used in our study was not subjected to any pre-treatment (heat, fermentation), which may have allowed the inherent quantities of phytates and tannins to mediate these ill-effects31. For instance, Ilham et al., reported depressed growth performance, lower level of leucocrit, multifocal necrosis in muscle and hepatic steatosis in liver in juvenile barramundi when fed with 25 and 75% of lupin meal over a period of 60 days41. Meanwhile, survival rate was significantly higher in FM50 compare to control and there was no adverse effect on weight gain of the other groups except FM0. It is interesting to note that the diet compositions with TH exhibited normal myotome in muscle and improved intestinal histomorphology with no implications on weight gain, except for the last group (FM0) that was composed of LKM along with PBM, BSF and TH. Elevated survival rate in FM50 group and improved histomorphology may be attributed to TH courtesy of its wide array of functional properties particularly presence of greater than 90% of low molecular weight peptides (10 kDa)42 that stimulate immune response and disease resistance17,20. Despite the health promoting capacity of TH, its inclusion percentage was not enhanced in any of the diets. A previous study by Siddik et al., highlights the negative impacts of excess inclusion of TH in juvenile barramundi diet that include reduced growth performance, feed utilisation and digestibility, cytoplasm vacuolization, necrosis and lipid accumulation in hepatic tissue43. Proximate composition analysis showed a considerable increase in ash% with the replacement of FM in each subsequent diet formulation. Previous study on barramundi has highlighted the negative correlation between ash content v/s protein digestibility and mineral utilization44.
Amongst the biochemical indices analysed in this study, only haematocrit% seemed to vary significantly between the dietary groups FM100 and FM0. The results conformed with those obtained by Khosravi et al., and Siddik et al., where most of the biochemical parameters were unaffected by inclusion of fish hydrolysates in diets of olive flounder and barramundi, respectively20,38. For the quantitative assay of immune genes, FM25 (containing BSF, TH, and PBM) and FM50 (containing TH and PBM) groups showed a similar cytokine profile. The inclusion of BSF in the FM25 diet has led to an enhanced inflammatory response which is in congruence with the findings on BSF + PBM/ BSF + FM supplementation in marron28. The inflammatory stimulation (upregulated IL-1β and TNF-α, downregulated IL-10) of fermented PBM + TH (from industrial residues) diet has been recently reported in juvenile barramundi, however IL-8 and IL-17F did not vary significantly in comparison to the control groups45. The spike in immunomodulatory effect in FM50 and FM25 groups in this study may be due to the presence of antibacterial peptides in fish protein hydrolysates and BSF46,47.
Studies on gut microbiota of Sparus aurata, Dicentrarchus labrax and Onchorhynchus mykiss have highlighted that gut microflora varies significantly in diversity or composition, depending on the diet48,49. Recently, Zheng et al., published a study on the effect of rearing salinity on diversity of gut microbiota of barramundi, reporting Proteobacteria and Firmicutes as the most abundant phyla50. These two phyla along with Actinobacteria dominate the gut microbial population of fish, with variations in relative compositions37. The impact of variation in the diet on the composition of gut microbiota is evident from the results of our study. The phyla composition of our control and experimental groups are in congruence with the results of Siddik et al.’s study on effect of fish protein hydrolysate on juvenile barramundi37. Our observations show a 70:30 abundance ratio of Vibrio: Psychrobacter in the FM100 fed fish, while the FM50 and FM25 diets are seen to transform this ratio to 10:90, increasing the abundance of Psychrobacter. This genus has been associated with secretion of omega fatty acids and metabolites for enhancing immunity and antioxidant status51. Therefore, in correlation with Smith et al.’s (2017) study on killfish, this may be an indicator of a healthy gut microflora community in FM50 and FM25 group51. In grouper, Epinephelus coioides, Psychrobacter sp. was shown to improve the immunity as well as gut microbial diversity52,53. This may be the possible cause for significant enrichment in microbial diversity index and elevated cytokine levels in FM25 fed group. The gut microbiota profile of FM75 and FM0 showed abundance of Aeromonas and Cetobacterium. While Aeromonas is a commonly found genus in gut of freshwater fish33, the abundance of vitamin B12 producing Cetobacterium in the two groups is noteworthy which may be attributed to variation in nutritional components of diets54,55.
The results of the current study indicate that FM replacement of 50 to 75% could be incorporated in the diet of juvenile barramundi using the appropriate composition of FM substitutes – PBM, BSF and TH. The fish in FM50 and FM25 groups showed a boost in cytokine profile as well as healthy hepatic and intestinal histomorphology. The diets also influenced the gut microbiota composition considerably with enrichment of Proteobacteria at the phylum level and Psychrobacter at genus level. FM25 also showed significant enrichment of Shannon and Simpson indices in comparison to control. Overall, findings from this study would be cornerstone to ingredient selection for FM replacement in other related carnivore fish56, though one has to be mindful of the impact of species-specific diet manipulation on gut microbiota and select the diet composition judiciously49. Further extension of this study would be the assessment of bioprocessed non-FM ingredients mixture on physiological parameters of barramundi to see if their inclusion level in the fish diet can be increased.
Material and methods
Ethic statements
This experiment was conducted in a recirculating aquaculture system (RAS) facility at Curtin Aquatic Research Laboratory (CARL), Curtin University as per the recommendations of the Guide for the Care and Use of Laboratory Animals of Australia. The study plan and protocols were reviewed and approved by the Ethics Committee in Animal Experimentation of the Curtin University (Approval number ARE2018-33). AQUI-S (8 mg/L) was used as an anaesthetic for proper handling of fish in accordance with the statement of purpose (SOP) of the Curtin Research Laboratories on anaesthetizing fish. Maximum efforts were devoted to alleviate stress and pain for fish for performing all the experimental protocols.
Test diets
Five isoproteic (crude protein approx. 45%) and isolipidic diets (13% crude lipid) were prepared by encompassing fishmeal, lupin meal, black soldier fly meal, poultry by-product meal and tuna hydrolysate (TH) as the main protein sources. All these ingredients except liquid TH used for preparing experimental diets were supplied by Glen Forest Specialty Feeds, Pvt. Ltd, Perth, Western Australia. Liquid TH was procured from SAMPI, Port Lincoln, Australia. Diet formulations were performed using the Feed LIVE—version 1.52 from Live Informatics Company Limited, Thailand (Table 2).
Table 2.
Feed ingredients and proximate composition (% dry weight).
| Ingredients (% dry weight)a | Experimental diets | ||||
|---|---|---|---|---|---|
| FM100 | FM75 | FM50 | FM25 | FM0 | |
| Wheat flour | 10.13 | 8.92 | 6.99 | 5.92 | 2.38 |
| Soybean meal | 6.99 | 7.00 | 7.00 | 7.00 | 7.00 |
| Fish meal (FM) | 62.98 | 46.00 | 30.00 | 16.00 | 0.00 |
| Poultry by-product meal (PBM) | 0.00 | 16.00 | 16.00 | 16.00 | 19.00 |
| Tuna hydrolysate (TH) | 0.00 | 0.00 | 18.00 | 18.00 | 18.00 |
| Black soldier fly meal (BSF) | 0.00 | 0.00 | 0.00 | 19.00 | 18.00 |
| Lupin kernel meal (LKM) | 0.00 | 0.00 | 0.00 | 0.00 | 21.24 |
| Wheat Starch | 6.99 | 7.00 | 7.00 | 5.00 | 1.20 |
| Trout Px | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Canola Oil | 3.99 | 4.00 | 4.00 | 4.00 | 4.00 |
| Fish oil | 8.49 | 8.70 | 8.70 | 6.70 | 6.8 |
| Sargassum Linearfolium meal | 0.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Dicalcium phosphate(DCP) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Oxicap E2b | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Stay C (35% Vit C) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Proximate composition (g Kg−1 dry weight basis) | |||||
| CP% | 44.77 | 44.63 | 44.71 | 44.76 | 44.59 |
| Lipid%* | 12.91 | 13.08 | 13.05 | 13.12 | 13.05 |
| Fiber%* | 0.38 | 0.77 | 0.75 | 0.72 | 0.65 |
| Ash%* | 0.57 | 1.10 | 3.12 | 4.49 | 5.02 |
aSpecialty Feeds, Glen Forrest Stockfeeders, 3150 Great Eastern Highway, Glen Forrest, Western Australia 6071.; CP (Crude protein).
bOxicap E2 contains ethoxyquin, butylated hydroxytoluene, and synergistic chelating agents. *Data were obtained from MixFeed software database program (https://softimbra.com/home).
The raw materials of feed were weighed, crushed and sieved through a 100 μm mesh to obtain fine, uniform sized particles. These ingredients were mixed thoroughly followed by addition of distilled water to the mix to enhance the moisture level for easy pelletizing. The dough was subsequently processed through a pellet extruder, and the pellets were dried in the oven, overnight at 60 °C. The protein content of each of the dietary feeds was confirmed by modifying the formulation until a minima of ± 44.50% was obtained. These diets were labelled as FM100 as a control, FM75, FM50, FM25 and FM0 to replace fishmeal by non-fishmeal protein ingredients at 25% with (PBM), 50% with (25% PBM + 25% TH), 75% with (25% PBM + 25% TH + 25% BSF), and 100% with (25% PBM + 25% TH + 25% BSF + 25% LKM), respectively. Except control (FM100), all the diets were supplied with 2% Sargassum Linearfolium meal as mineral supplement source. The pelleted formulations were stored in air tight plastic containers in the feed storage unit until further use.
Fish husbandry and management
In total, 320 barramundi juveniles were procured from the Mainstream Aquaculture Pty Ltd., Werribee VIC 3030 after size grading. The fish were acclimated for a period of 14 days at Curtin aquaculture research laborarory (CARL) during which they were fed commercially formulated diet (470 g protein kg−1 diet and 20.0 MJ kg−1 dietary gross energy), twice a day. On the 15th day, 300 juveniles (pool weight of 2.52 ± 0.11 g/ fish) were randomly distributed into 15 independent tanks (300-L water capacity) at a stocking density of 20 fish/ tank. Each tank was equipped with an aerator, electric heater and an external bio-filter (Astro 2212, China). The water quality parameters like temperature (27.60–29.40 °C), salinity (5–6 ppt), dissolved oxygen (5.90–7.51 mgL−1), ammonia nitrogen (< 0.50 mg L−1) were monitored on daily basis and observed to be within the suitable range for culture of the barramundi fry in recirculating aquaculture systems57. The light regime of 14-h light/10-h dark cycle was set using an automatic indoor light switch (Clipsal, Australia).
According to the experimental feed regime, the juveniles in each group were fed respective diets, twice a day at 09:00 and 17:00 h, for a duration of 7 weeks. Feed intake was calculated by subtracting the dry weight of siphoned, uneaten food in the tank from the weight of food provided. The mortality, if any, in each group was recorded during the course of 7 weeks and the weight of dead fish were recorded. The fish were starved for 24 h, and bulk weight and individual weight was recorded for each group of fries for evaluation of growth parameters.
Biochemical indices of blood and serum
At the end of 7 week of feeding, the fish were anesthetized with AQUI-S (8 mg L−1) and blood was drawn from caudal vein of 2 fish per tank (n = 6) using a 1 mL non-heparinized syringe. The blood from one animal was then transferred to a heparinised tube to determine hematocrit level as per the protocol of McLeay and Gordon and expressed as a Hematocrit% (Ht%)58. The blood from second animal was transferred to non-heparinised tubes, kept at room temperature until coagulation and finally centrifuged (3000 rpm, 15 min) at 4 °C to obtain serum. The collected serum samples were stored at − 80 °C until further use for determination of hematological parameters. Biochemical indices like triglyceride (TG), cholesterol, glutamate dehydrogenase (GLDH), aspartate aminotransferase (AST), total protein and albumin were determined using an automated blood analyzer (SLIM; SEAC Inc, Florence, Italy) in accordance with the protocols from Blanc et al.59. The albumin and globulin ratio (A/G ratio) was calculated by dividing the total albumin content by the difference of total serum and albumin protein values. Cholesterol and TG content was determined according to the method of Siddik, et al.38. For recording serum lysozyme, the previously described protocol of Le and Fotedar60 was followed.
Histomorphology
Liver, muscle and distal intestine were excised from ten sacrificed fish in each treatment group (two fish/replicate) and processed for histological analysis. The excised tissues were fixed in 10% neutral buffered formalin before dehydrating them in graded alcohol dilutions. Tissues were treated with xylene before embedding in paraffin wax. Wax-embedded tissue blocks were sectioned at 5 µm on a rotary microtome. The sections were processed for hematoxylin and eosin (H&E) staining following the standard histological protocols. The sections were scanned under a light imaging microscope (BX40F4, Olympus, Tokyo, Japan) for obtaining digital photographs. Intestinal mucosal morphology in terms of villous height, villous width, microvilli height and goblet cells density were measured and quantified randomly from ten intact villi following our earlier studies38,61.
RNA extraction and qRT-PCR analysis
After collection of blood from the anesthetized fish, they were euthanized (AQUI-S, 175 mg l−1) (two/replicate) and distal gut was excised and stored in RNA Later (Sigma-Aldrich, Germany) at − 80 °C until RNA extraction. The tissue (~ 5 mg) was thawed and homogenised to a fine powder before processing for RNA extraction as per the manufacturer’s instructions RNeasy Mini Kit (Qiagen, Hilden, Germany). DNase treatment was carried out using RNase free DNase-I (Qiagen, Hilden, Germany) before RNA QC via agarose gel electrophoresis and NanoDrop spectrophotometer 2000c (Thermo Fisher Scientific, USA). cDNA was prepared from 1 µg of RNA as per the manufacturer’s protocol of Omnicript RT kit (Qiagen, Hilden, Germany).
qPCR for IL-1β, IL-8, IL-10, IL-17F, and β-actin was carried out using cDNA samples of the distal intestine in 7500 Real-Time PCR System (Applied Biosystems, USA) employing PowerUp SYBR Green Master Mix (Thermo Scientific, USA) as per the manufacturer’s protocols after standardization. The total volume of PCR reaction was 20 µl consisting of 10 μl TransStart Top Green qPCR SuperMix (2 ×), 0.6 μl of each primer, 1 μl cDNA, and 7.8 μl RNase-free H2O, 4 μM of each forward and reverse gene-specific primer (Table 3). geNorm (v3.5) was used to normalize the quantitative real-time PCR data and the expression levels of IL-1β, IL-8, IL-10, IL-17F were calculated using 2 −△△CT method. One-way ANOVA with Tukey’s HSD was used to compare the mean ± SE of relative expression among the treatment groups.
Table 3.
List of gene specific primers used for qRT-PCR analysis.
| Primers | Sequence (Forward, 5ʹ–3ʹ) | Sequence (Reverse, 5ʹ–3ʹ) | References |
|---|---|---|---|
| IL-1β | ACAACGTCATGGAGCTCTGG | TCTTTGTCCTTCACCGCCTC | Zoccola et al.71 |
| IL-8 | GTCTGAGAAGCCTGGGAGTG | GCAATGGGAGTTAGCAGGAA | Miao et al.72 |
| IL-10 | CGACCAGCTCAAGAGTGATG | AGAGGCTGCATGGTTTCTGT | Miao et al.72 |
| IL-17F | GTCTCTGTCACCGTGGAC | TGGGCCTCACACAGGTACA | Miao et al.72 |
| TNF-α | GCCATCTATCTGGGTGCAGT | AAAGTGCAAACACCCCAAAG | Zoccola et al.71 |
| β-actin | CTTCACCACCACAGCCGAGA | TGCCGATGGTGATGACCTGT | Alhazzaa et al.73 |
DNA extraction, 16S amplification and high-throughput sequencing
For DNA extraction, two randomly picked fish from each tank (n = 6) were selected, followed by careful excision of gut inside biological safety hood, and separation of distal gut. The samples were then homogenized in Qiagen Tissue LyserII (Qiagen, Hilden, Germany) and two distal gut samples from each respective tank were pooled together, and transferred to 1.5-mL Eppendorf tube for DNA extraction62. Total bacterial DNA was extracted from the pooled distal intestine sample using commercial DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Nano-Drop spectrophotometer 2000c (Thermo Fisher Scientific, Waltham, CA, USA) was used to measure the concentration of DNA. To make the final concentration to 30 ng/µL, the extracted DNA was subsequently diluted with nuclease-free water. V3-V4 hypervariable region of bacterial 16S was amplified using PCR in a 50 µL reaction mix consisting of 2µL of template DNA, 1µL each of forward and reverse primers, 25 µL of Hot Start 2X Master Mix (New England BioLabs Inc., Ipswich, MA, USA) and 21 µL of nuclease-free water. BioRad S1000 Gradient Thermal Cycler (Bio-Rad Laboratories, Inc., Foster City, California, USA) was used for 30 cycles of amplification reactions. The products were resolved on 1% agarose gel and purified using AMPure beads methods, followed by library preparation and secondary PCR amplicon barcoding according to the Illumina standard protocol (Part # 15044223 Rev. B)63. Sequencing (~ 50,000 reads) of each sample was carried out on Illumina MiSeq platform (Illumina Inc., San Diego, California, USA), using Illumina MiSeq v3 kits (600 cycles, Part # MS-102-3003).
Bioinformatics
The quality of Illumina Miseq generated data was checked using FastQC pipelines64. The reads were trimmed to meet quality parameters (–q20 –l 200) using Sickle65. Overlapping pair-end sequences were merged using MeFiT pipeline with default parameters66. MICCA(version1.7.0)67 was used for filtering the chimeric sequences and de novo assembly of 16S rRNA sequences into Operational Taxonomic Units (OTUs) at 97% similarity threshold. The taxonomic classification of detected OTUs was carried out at 97% similarity check against the SILVA database (SILVA 132 release) (www.arb-silva.de)68. QIIME (version 1.9.1) and R packages (Verson V3.5.1) (https://www.r-project.org/) were used for calculating the microbial diversity in each sample at a rarefaction depth value of 3696 bp69. The calculation of alpha diversity was performed in terms of observed species, Shannon and chao1 indices. ANOSIM was used for the non-parametric statistical analysis of the distance metric at 1000 permutations. Clustering of sample in terms of non-metric multi-dimensional scaling (NMDS) was performed using Bray–Curtis dissimilarity of weighted UniFrac distance metric. Linear discriminant analysis (LDA) with default parameters was used to identify the differential abundance of bacterial genera in different treatment groups70.
Statistical analysis
Statistical analysis of the data was performed using SPSSfor Windows version 25, IBMCurtin University, Australia. The results of growth performance, serum and blood biometry and immune indices were expressed as mean ± standard error, and checked for normality and homogeneity of variances with Shapiro–Wilk’s and Levene’s tests. Post confirming these two tests, an ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to test the significant difference at 0.05 < P < 0.001 where diet was used as an illustrative variable.
Acknowledgements
This work is supported through the Australian Government Endeavour Scholarships and Fellowships program with recipient ID (ERF PDR 6379 2018) to the first author. We sincerely thank Australian Centre for Applied Aquaculture Research (ACAAR), Fremantle, Australia for providing fish and Rowan Kleindienst for technical assistance during the wet laboratory work. First author sincere acknowledges the support received from Indian Council of Agricultural Research (ICAR) for availing the Endeavour post doctoral research program.
Author contributions
S.K.G. and R.F. conceptualized the experimental designed, conducting experiments and reviewed the manuscript. M.J.F assisted in the microbiome and immune gene expression data analysis and edited the manuscript. M.P. assisted in data compilation and draft manuscript preparation. M.A.B.S. and M.R.C. helped in haematological and histological analysis of collected samples. T.T.T.D. assisted in stocking of fish, collecting samples, conducting the experiment and analysis of growth performance data. JH assisted in feed formulation and designing of experiment and reviewed the manuscript. All authors reviewed and approved the final manuscript.
Data availability
Altogether datasets produced through the study have been presented in the form of figures and tables but are accessible from the corresponding author on considerable demand. The gut microbiome data is available on NCBI database under BioProject No.- PRJNA608700.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miles R.D. & Chapman F. The Benefits of Fish Meal in Aquaculture Diets. Univ. Florida IFAS Ext. 1–6 (2006).
- 2.Henry M, Gasco L, Piccolo G, Fountoulaki E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015;203:1–22. doi: 10.1016/j.anifeedsci.2015.03.001. [DOI] [Google Scholar]
- 3.Camacho-Rodríguez J, Macías-Sánchez MD, Cerón-García MC, Alarcón FJ, Molina-Grima E. Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: nutrient stability under different storage conditions. J. Appl. Phycol. 2018;30:1049–1059. doi: 10.1007/s10811-017-1281-5. [DOI] [Google Scholar]
- 4.Ogello EO, Munguti JM, Sakakura Y, Hagiwara A. Complete replacement of fish meal in the diet of Nile Tilapia (Oreochromis niloticus L.) grow-out with alternative protein sources. A review. Int. J. Adv. Res. 2014;2:962–978. [Google Scholar]
- 5.Xie SW, Liu YJ, Zeng S, Niu J, Tian LX. Partial replacement of fish-meal by soy protein concentrate and soybean meal based protein blend for juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 2016;464:296–302. doi: 10.1016/j.aquaculture.2016.07.002. [DOI] [Google Scholar]
- 6.Moutinho S, et al. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture. 2017;468:271–277. doi: 10.1016/j.aquaculture.2016.10.024. [DOI] [Google Scholar]
- 7.Tschirner M, Kloas W. Increasing the sustainability of aquaculture systems: Insects as alternative protein source for fish diets. Gaia. 2017;26:332–340. doi: 10.14512/gaia.26.4.10. [DOI] [Google Scholar]
- 8.Daniel D. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018;6:164–179. [Google Scholar]
- 9.Lazzarotto V, Médale F, Larroquet L, Corraze G. Long-term dietary replacement of fishmeal and fish oil in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth, whole body fatty acids and intestinal and hepatic gene expression. PLoS ONE. 2018;13:1–25. doi: 10.1371/journal.pone.0190730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis G, Makkar HPS, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199:197–227. doi: 10.1016/S0044-8486(01)00526-9. [DOI] [Google Scholar]
- 11.Khattab RY, Arntfield SD. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT Food Sci. Technol. 2009;42:1113–1118. doi: 10.1016/j.lwt.2009.02.004. [DOI] [Google Scholar]
- 12.Salze GP, Davis DA. Taurine: A critical nutrient for future fish feeds. Aquaculture. 2015;437:215–229. doi: 10.1016/j.aquaculture.2014.12.006. [DOI] [Google Scholar]
- 13.Renna M, et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017;8:1–13. doi: 10.1186/s40104-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis MJ, et al. A comparison of in-vivo and in-vitro methods for assessing the digestibility of poultry by-product meals using barramundi (lates calcarifer); impacts of cooking temperature and raw material freshness. Aquaculture. 2019;498:187–200. doi: 10.1016/j.aquaculture.2018.08.032. [DOI] [Google Scholar]
- 15.Havasi M, et al. Effect of total fish meal replacement with vegetal protein alone or combined with rendered animal protein on growth performance and tissue composition of European catfish (Silurus glanis L.) Isr. J. Aquacult-Bamid. 2015;67:1–8. [Google Scholar]
- 16.Gao Y, Li X, Dong Y, Zhu X, Li W, Wu X. Replacement of fishmeal by mixed animal and plant protein sources in diets for juvenile mangrove red snapper (Lutjanus argentimaculatus)(Forsskål, 1775) Isr. J. Aquacult-Bamid. 2018;70:1–8. [Google Scholar]
- 17.Herpandi NH, Rosma A, Nadiah WA. The tuna fishing industry: A new outlook on fish protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2011;10:195–207. doi: 10.1111/j.1541-4337.2011.00155.x. [DOI] [Google Scholar]
- 18.Klomklao S, Benjakul S. Utilization of Tuna processing byproducts: protein hydrolysate from Skipjack Tuna (Katsuwonus pelamis) Viscera. J. Food Process. Preserv. 2017;41:e12970. doi: 10.1111/jfpp.12970. [DOI] [Google Scholar]
- 19.Ovissipour M, Abedian Kenari A, Nazari R, Motamedzadegan A, Rasco B. Tuna viscera protein hydrolysate: Nutritive and disease resistance properties for Persian sturgeon (Acipenser persicus L.) larvae. Aquac. Res. 2014;45:591–601. doi: 10.1111/j.1365-2109.2012.03257.x. [DOI] [Google Scholar]
- 20.Khosravi S, et al. Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus) Aquaculture. 2015;435:371–376. doi: 10.1016/j.aquaculture.2014.10.019. [DOI] [Google Scholar]
- 21.Gunben EM, Senoo S, Yong A, Shapawi R. High potential of poultry by-product meal as a main protein source in the formulated feeds for a commonly cultured grouper in Malaysia (Epinephelus fuscoguttatus) Sains Malaysiana. 2014;43:399–405. [Google Scholar]
- 22.Hernández C, et al. Replacement of fish meal by poultry by-product meal, food grade, in diets for juvenile spotted rose snapper (Lutjanus guttatus) Lat. Am. J. Aquat. Res. 2014;42:111–120. doi: 10.3856/vol42-issue1-fulltext-8. [DOI] [Google Scholar]
- 23.García-Pérez OD, Cruz-Valdez JC, Ramírez-Martínez C, Villarreal-Cavazos D, Gamboa-Delgado J. Exploring the contribution of dietary protein from poultry by-product meal and fish meal to the growth of catfish Ictalurus punctatus by means of nitrogen stable isotopes. Lat. Am. J. Aquat. Res. 2018;46:37–44. doi: 10.3856/vol46-issue1-fulltext-5. [DOI] [Google Scholar]
- 24.Sabbagh M, et al. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture. 2019;511:734220. doi: 10.1016/j.aquaculture.2019.734220. [DOI] [Google Scholar]
- 25.Galkanda-Arachchige HSC, Wilson AE, Davis DA. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: a meta-analysis. Rev. Aquac. 2019 doi: 10.1111/raq.12401. [DOI] [Google Scholar]
- 26.Xiao X, et al. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco) Aquac. Res. 2018;49:1569–1577. doi: 10.1111/are.13611. [DOI] [Google Scholar]
- 27.Devic E, Leschen W, Murray F, Little DC. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing Black Soldier Fly (Hermetia illucens) larvae meal. Aquac. Nutr. 2018;24:416–423. doi: 10.1111/anu.12573. [DOI] [Google Scholar]
- 28.Foysal MJ, Fotedar R, Tay CY, Gupta SK, Kumarnsit E. Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ. 2019;2019:e6891. doi: 10.7717/peerj.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FAO. FAO Fisheries & Aquaculture—Cultured Aquatic Species Information Programme - Lates calcarifer (Block, 1790).
- 30.Chotikachinda R, Tantikitti C, Benjakul S, Rustad T, Kumarnsit E. Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer) Aquac. Nutr. 2013;19:773–784. doi: 10.1111/anu.12024. [DOI] [Google Scholar]
- 31.Van Vo B, Bui DP, Nguyen HQ, Fotedar R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture. 2015;444:62–69. doi: 10.1016/j.aquaculture.2015.03.019. [DOI] [Google Scholar]
- 32.Katya K, et al. Efficacy of insect larval meal to replace fish meal in juvenile barramundi, Lates calcarifer reared in freshwater. Int. Aquat. Res. 2017;9:303–312. doi: 10.1007/s40071-017-0178-x. [DOI] [Google Scholar]
- 33.Butt RL, Volkoff H. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. (Lausanne) 2019;10:6–8. doi: 10.3389/fendo.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraoka S, Yang CC, Iwasaki W. Metagenomics and bioinformatics in microbial ecology: Current status and beyond. Microbes Environ. 2016;31:204–212. doi: 10.1264/jsme2.ME16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Z, et al. Comparison of partial replacement of fishmeal with soybean meal and EnzoMeal on growth performance of Asian seabass Lates calcarifer. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019;216:29–37. doi: 10.1016/j.cbpc.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Chaklader MR, Siddik MAB, Fotedar R, Howieson J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-53018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddik MAB, Chungu P, Fotedar R, Howieson J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch) PLoS ONE. 2019;14:1–18. doi: 10.1371/journal.pone.0215025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddik MAB, Howieson J, Partridge GJ, Fotedar R, Gholipourkanani H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katersky RS, Carter CG. Growth and protein synthesis of barramundi, Lates calcarifer, fed lupin as a partial protein replacement. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009;152:513–517. doi: 10.1016/j.cbpa.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Van Vo B, et al. Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture. 2020 doi: 10.1016/j.aquaculture.2020.735741. [DOI] [Google Scholar]
- 41.Ilham I, Hapsari F, Fotedar R. Growth, enzymatic glutathione peroxidase activity and biochemical status of juvenile barramundi (Lates calcarifer) fed dietary fermented lupin meal supplemented with organic selenium. Aquac. Res. 2018;49:151–164. doi: 10.1111/are.13444. [DOI] [Google Scholar]
- 42.Chaklader MR, Fotedar R, Howieson J, Siddik MAB, Foysal MJ. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 2020;104:567–578. doi: 10.1016/j.fsi.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Siddik MAB, Howieson J, Ilham I, Fotedar R. Growth, biochemical response and liver health of juvenile barramundi (Lates calcarifer) fed fermented and nonfermented tuna hydrolysate as fishmeal protein replacement ingredients. PeerJ. 2018;2018:e4870. doi: 10.7717/peerj.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaimongkol A, Boonyaratpalin M. Effects of ash and inorganic phosphorus in diets on growth and mineral composition of seabass Lates calcarifer (Bloch) Aquac. Res. 2001;32:53–59. doi: 10.1046/j.1355-557x.2001.00035_32_s1.x. [DOI] [Google Scholar]
- 45.Siddik MAB, et al. Influence of fish protein hydrolysate produced from industrial residues on antioxidant activity, cytokine expression and gut microbial communities in juvenile barramundi Lates calcarifer. Fish Shellfish Immunol. 2020;97:465–473. doi: 10.1016/j.fsi.2019.12.057. [DOI] [PubMed] [Google Scholar]
- 46.Zamora-Sillero J, Gharsallaoui A, Prentice C. Peptides from Fish by-product protein hydrolysates and its functional properties: an overview. Mar. Biotechnol. 2018;20:118–130. doi: 10.1007/s10126-018-9799-3. [DOI] [PubMed] [Google Scholar]
- 47.Vogel H, Müller A, Heckel DG, Gutzeit H, Vilcinskas A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018;78:141–148. doi: 10.1016/j.dci.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Estruch G, et al. Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA gene. PLoS ONE. 2015;10:1–22. doi: 10.1371/journal.pone.0136389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonopoulou E, et al. Reshaping gut bacterial communities after dietary Tenebrio molitor larvae meal supplementation in three fish species. Aquaculture. 2019;503:628–635. doi: 10.1016/j.aquaculture.2018.12.013. [DOI] [Google Scholar]
- 50.Zheng X, et al. The gut microbiota community and antioxidant enzymes activity of barramundi reared at seawater and freshwater. Fish Shellfish Immunol. 2019;89:127–131. doi: 10.1016/j.fsi.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 51.Smith P, et al. Regulation of life span by the gut microbiota in the short-lived african turquoise killifish. Elife. 2017;6:1–26. doi: 10.7554/eLife.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang HL, Sun YZ, Ma RL, Li JS, Huang KP. Probiotic psychrobacter sp. improved the autochthonous microbial diversity along the gastrointestinal tract of grouper Epinephelus coioides. J. Aquac. Res. Dev. 2011 doi: 10.4172/2155-9546.S1-001. [DOI] [Google Scholar]
- 53.Sun YZ, Yang HL, Ma RL, Zhang CX, Lin WY. Effect of dietary administration of Psychrobacter sp. On the growth, feed utilization, digestive enzymes and immune responses of grouper Epinephelus coioides. Aquac. Nutr. 2011;17:e733–e740. doi: 10.1111/j.1365-2095.2010.00837.x. [DOI] [Google Scholar]
- 54.Pedrotti FS, et al. The autochthonous microbiota of the freshwater omnivores jundiá (Rhamdia quelen) and tilapia (Oreochromis niloticus) and the effect of dietary carbohydrates. Aquac. Res. 2015;46:472–481. doi: 10.1111/are.12195. [DOI] [Google Scholar]
- 55.Gatesoupe FJ, et al. Intestinal microbiota in rainbow trout, Oncorhynchus mykiss, fed diets with different levels of fish-based and plant ingredients: A correlative approach with some plasma metabolites. Aquac. Nutr. 2018;24:1563–1576. doi: 10.1111/anu.12793. [DOI] [Google Scholar]
- 56.Glencross B. A comparison of the digestibility of diets and ingredients fed to rainbow trout (Oncorhynchus mykiss) or barramundi (Lates calcarifer)—the potential for inference of digestibility values among species. Aquac. Nutr. 2011;17:28. [Google Scholar]
- 57.Ardiansyah, Fotedar R. Water quality, growth and stress responses of juvenile barramundi (Lates calcarifer Bloch), reared at four different densities in integrated recirculating aquaculture systems. Aquaculture. 2016;458:113–120. doi: 10.1016/j.aquaculture.2016.03.001. [DOI] [Google Scholar]
- 58.McLeay DJ, Gordon MR. Leucocrit: A simple hematological technique for measuring acute stress in salmonid fish, including stressful concentrations of pulpmill efflent. J. Fish. Res. Board Canada. 1977;34:2164–2175. doi: 10.1139/f77-285. [DOI] [Google Scholar]
- 59.Blanc MC, Neveux N, Laromiguiere M, Berard MP, Cynober L. Evaluation of a newly available biochemical analyzer: The Olympus AU 600. Clin. Chem. Lab. Med. 2000;38:465–475. doi: 10.1515/CCLM.2000.067. [DOI] [PubMed] [Google Scholar]
- 60.Le KT, Fotedar R. Dietary selenium requirement of yellowtail kingfish (Seriola lalandi) Agric. Sci. 2013;04:68–75. [Google Scholar]
- 61.Van Vo B, et al. Growth and health of juvenile barramundi (Lates calcarifer) challenged with DO hypoxia after feeding various inclusions of germinated, fermented and untreated peanut meals. PLoS ONE. 2020;15:1–19. doi: 10.1371/journal.pone.0232278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahlhausen KE, Doroud L, Firl AJ, Polkinghorne A, Eisen JA. Characterization of shifts of koala (Phascolarctos cinereus) intestinal microbial communities associated with antibiotic treatment. PeerJ. 2018;2018:1–20. doi: 10.7717/peerj.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gajardo K, et al. A high-resolution map of the gut microbiota in Atlantic salmon (Salmo salar): A basis for comparative gut microbial research. Scientific Reports. 2016;6:1–10. doi: 10.1038/srep30893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrews, S. FastQC: a quality control tool for high throughput sequence data, 5. https://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 65.Joshi NA, F. J. A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.33)[Software]. (2011).
- 66.Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: Merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinform. 2016;17:1–6. doi: 10.1186/s12859-016-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albanese D, Fontana P, De Filippo C, Cavalieri D, Donati C. MICCA: A complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 2015;5:1–7. doi: 10.1038/srep09743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quast C, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caporaso JG, et al. correspondence QIIME allows analysis of high- throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing. Nat. Publ. Gr. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. etagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoccola E, Kellie S, Barnes AC. Leucocyte integrins, but neither caspases nor NLR inflammasome are associated with lipopolysaccharide recognition and response in barramundi (Lates calcarifer) Fish & shellfish immunology. 2019;91:172–179. doi: 10.1016/j.fsi.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Miao S, Zhao C, Zhu J, Hu J, Dong X, Sun L. Dietary soybean meal affects intestinal homeostasis by altering the microbiota, morphology, and inflammatory cytokine gene expression in northern snakehead. Sci Rep. 2018;8:113. doi: 10.1038/s41598-017-18430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alhazzaa R, Bridle AR, Nichols PD, Carter CG. Up-regulated desaturase and elongase gene expression promoted accumulation of polyunsaturated fatty acid (PUFA) but not long-chain PUFA in Lates calcarifer, a tropical euryhaline fish, fed a stearidonic acid- and γ-linoleic acidenriched diet. J Agric. food chem. 2011;59(15):8423–8434. doi: 10.1021/jf201871w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Altogether datasets produced through the study have been presented in the form of figures and tables but are accessible from the corresponding author on considerable demand. The gut microbiome data is available on NCBI database under BioProject No.- PRJNA608700.