Abstract
Background
Human intestinal protozoan parasitic infections (HIPPIs) are a series of public health problems in developing countries like Ethiopia. The overall prevalence of HIPPIs in Ethiopia is not known. Therefore, this systematic review and meta-analysis study is aimed at determining the overall prevalence of HIPPIs in Ethiopia.
Methods
Articles written in English were searched from online public databases. Searching terms used were “prevalence,” “intestinal protozoan parasite,” “associated factors,” and “Ethiopia.” We used Stata version 14 for meta-analysis and Cochran's Q test statistics and the I2 test for heterogeneity.
Result
A total of 286 articles were reviewed, but only 45 of them fulfilled the inclusion criteria. The pooled prevalence of HIPPIs in Ethiopia was 25.01% (95% CI: 20.08%-29.95%) where Entamoeba histolytica/dispar is the most prevalent (14.09%, 95% CI: 11.03%-17.14%) followed by Giardia lamblia (10.03%, 95% CI: 7.69%-12.38%) and Cryptosporidium spp. (5.93%, 95% CI: 2.95%-8.91%). This meta-analysis showed that family size (OR: 3.7, 95% CI: 1.45-5.85), source of drinking water (OR: 3.33, 95% CI: 1.30-5.36), open field defecation (OR: 2.91, 95% CI: 1.60-4.21), handwashing habit (OR: 2.82, 95% CI: 2.01-3.63), playing with soil (OR: 2.15, 95% CI: 1.01-3.29), the habit of eating raw vegetables (OR: 1.77, 95% CI: 1.03-2.51), and fingernail trimming (OR: 1.70, 95% CI: 0.89-2.25) were strongly associated with the HIPPIs in Ethiopia. High heterogeneity on the prevalence of HIPPIs was observed among studies within and among regions (I2 > 99% and P ≤ 0.01).
Conclusion
The prevalence of HIPPIs was significantly high among the Ethiopian population. Family size, source of drinking water, open field defecation, handwashing habit, the habit of eating raw vegetables, and fingernail trimming habits were significantly associated with HIPPIs.
1. Background
Parasitic infection is one of the major health problems where more than 3.5 billion people are infected globally. Parasitic infections result in 450 million and 200,000 annual morbidities and mortalities, respectively [1]. Protozoan infections are among such infections. They are highly prevalent in preschool children in developing countries [2, 3]. E. histolytica/dispar, G. lamblia/duodenalis, and Cryptosporidium spp. are the major common pathogenic intestinal protozoan species globally reported [4].
E. histolytica has an annual incidence rate of five million cases, affects approximately 500 million people worldwide, and results in 50 million annual symptomatic diseases and 100,000 deaths [5]. It too results in 2.2 million disability-adjusted life years [6, 7]. G. lamblia/duodenalis infects 280 million people annually. It results in two and half million cases of diarrhea every year in resource-poor countries alone. In these countries, the prevalence of giardia infection is acquired during early infancy and it reaches up to 30% in children younger than 10 years of age [8]. The global prevalence of cryptosporidiosis is 1 to 4.5% in developed countries and 3 to 20% in developing countries. Its infection rate in AIDS patients ranges from 3 to 20% in the United States and 50 to 60% in Africa and Haiti [9]. It results in an estimate of 8.37 million DALYs with cryptosporidiosis [10].
Transmission of E. histolytica, G. lamblia/duodenalis, and cryptosporidiosis is through the oral-fecal route following direct or indirect contact with the infectious stages, including human-to-human, zoonotic, waterborne, and foodborne transmission [11]. However, Cryptosporidium may also be transmitted airborne [12]. Eating unwashed fruits, nail-biting, sucking fingers, and contact with infected family members are also key factors contributing to the increased HIPPIs [13, 14].
Protozoan infections are serious public health concerns and are responsible for iron deficiency anemia, growth retardation, and physical and mental health problems among children. They also lead to nutritional depletion, poor immunity in infants, mucosal loss and lymphatic leakages, and local hemorrhages [2].
Despite remarkable development in medical science in recent years, protozoan parasitic infections remain serious health issues in developing countries like Ethiopia [15]. Low level of environmental sanitation, personal and food hygiene, contamination of water with human excreta, and lack of awareness about simple health promotion practices such as personal hygiene and food hygiene make HIPPIs the most common problems in Ethiopia [16–19]. The prevalence of HIPPIs in Ethiopia is different in different parts of the country. However, there is no summarized pooled overall prevalence of HIPPIs. Therefore, this systematic review and meta-analysis study is aimed at producing the pooled prevalence and factors associated with HIPPIs from available studies in Ethiopia.
2. Methods
2.1. Study Design and Setting
Ethiopia is located in the horn of Africa. It is bounded by Eritrea to the north, Djibouti and Somalia to the east, Sudan and South Sudan to the west, and Kenya to the south. Currently, the Ethiopian population is estimated to be 113,620,337 with 21.3% (24,463,423) people living in the urban area and a median age of 19.5 years. Ethiopia's population is equivalent to 1.47% of the total world population. The population density in Ethiopia is 115/km2 (298 people/mi2) [20]. The total land area is 1,104,300 km2 [21].
2.2. Search Strategies
Articles written in English were searched from online public databases, namely, PubMed/MEDLINE, ScienceDirect, Web of Science, Google Scholar, Hinari, WorldCat, and Cochrane Library [22], using core search terms and phrases: “prevalence,” “intestinal protozoan parasite,” “associated factors,” and “Ethiopia.” The search terms were used separately and in combination using Boolean operators like “OR” or “AND.” Besides, Gray literature was searched through the review of available references. Searching of pieces of literature included in this meta-analysis was conducted from December 2019 to January 2020.
2.3. Inclusion and Exclusion Criteria
Studies that were written in the English language, reporting about the prevalence of HIPPIs and their associated risk factors in Ethiopia, published from 2008 to January 2020, with sample sizes above 100 [23] were included in the study. Studies conducted on HIV/AIDS patients, meta-analysis, and review articles were excluded from this systematic review and meta-analysis study.
2.4. Data Extraction
The data extraction protocol was prepared and evaluated by all authors. The data extraction protocol consists of the name of the author and year of publication, region, nature of study subjects (school-age children, food handlers, preschool-age children, patients, rural dwellers, and street dwellers), total sample size, number of positive cases, estimated prevalence, species of intestinal parasites, and potential risk factors associated with individual species of HIPPIs.
2.5. Quality Assessment of Individual Studies Included in the Meta-Analysis
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the overall quality of evidence [24]. The quality of each study was declared using the three major assessment tools (methodological quality, comparability, and outcome and statistical analysis of the study). Two points were given to each criterion. Publications with a total score of 5–6 points were considered to be high, 4 points to be moderate, and 0–3 points to be low-quality publications [24]. The association between HIPPIs and associated risk factors was calculated in the form of the log odds ratio. The odds ratio was calculated for the common associated risk factors of the reported studies.
2.6. Risk of Publication Bias across Studies Included in This Meta-Analysis
The risks of publication bias across studies were assessed using funnel plot symmetry and Egger's test. Egger's test (P value < 0.05) was used to determine the presence of publication bias across studies.
2.7. Outcomes of the Study
Intestinal protozoan parasitic infection and associated risk factors of HIPPIs were the two major outcome variables.
2.8. Data Analysis
The pooled prevalence of HIPPIs was calculated by dividing the total positive cases to the total study subjects included in this meta-analysis. Cochran's Q test and I2 statistics were used to assess heterogeneity among the studies [25]. There was a clear heterogeneity on the prevalence of HIPPIs across studies included in this meta-analysis; we used a random-effects model to estimate the pooled effect size. To sort out the causes of heterogeneity, we conduct a subgroup analysis based on the region of the study, the nature of study participants, study year, and sample size included in individual studies. The presence of publication bias was assessed using Egger's test and symmetry of the funnel plot. The cause of publication bias was assessed using a sensitivity test and regression test. Forest plot format was used to present the pooled point prevalence with 95% CI. A log odds ratio was used to decide the association between associated risk factors and HIPPIs among respondents included in the studies. The meta-analysis was conducted using Stata software (version 14, StataCorp, College Station, TX), where P < 0.05 was considered statistically significant.
3. Results
A total of 286 articles on the prevalence and associated risk factors of intestinal parasitic infections in Ethiopia were retrieved. Forty-eight of these articles were excluded due to duplicates. From the remaining 238 articles, 65 were excluded after review of their titles (titles were not related to HIPPIs) and 80 were excluded after review of abstracts (lack of full information about protozoan parasites). The remaining 93 full-text articles were accessed and assessed for eligibility based on the inclusion criteria and information indicated in the data extraction protocol. As a result, 48 articles were further excluded in the data extraction process primarily due to the outcome of interest, and they did not have OR, 95% CI, and the number of positive cases (meaning the report was only based on estimated prevalence percent). Thus, only 45 (15.7%) of the studies met the eligibility criteria and were included in the final systematic review and meta-analysis study (Figure 1).
Figure 1.
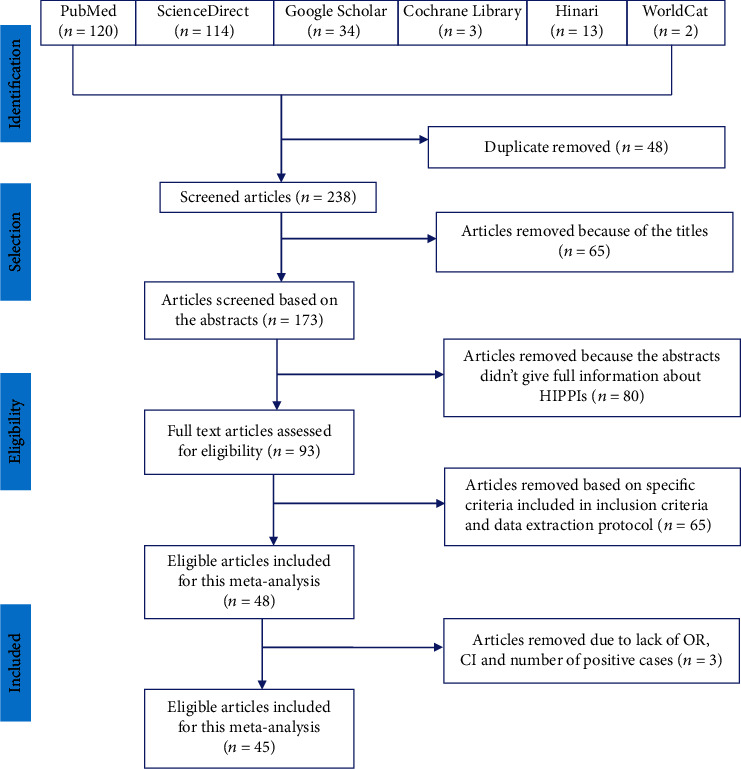
PRISMA flow diagram of articles considered for the review on HIPPIs among the Ethiopian population.
3.1. Characteristics of Original Studies Included in the Meta-Analysis
Out of the 45 screened studies, 44 (97.5%) of them were cross-sectional and one (2.5%) was a case-control study. A total of 131,916 study participants were involved, and the sample size of the studies ranged from 127 to 89,423. Twenty (44.44%) of the studies were from Amhara, 8 (17.77%) from SNNPR, 6 (13.33%) from Oromia, 2 (4.44%) from Addis Ababa, 1 (2.22%) from Dire Dawa, 2 (4.44%) from Benishangul-Gumuz, and 6 (13.33%) from Tigray regions (Table 1). Unfortunately, there were no studies reported from Afar, Harari, Gambela, and Somali regional states of Ethiopia.
Table 1.
Characteristics of the study subjects included in the eligible articles used for this review.
| Source | Year | Region | Study area | Sample size | Cases | Prevalence (95% CI) | Quality score |
|---|---|---|---|---|---|---|---|
| Andargie et al. | 2008 | Amhara | Gondar food handler | 127 | 3 | 2.4% (0.48-6.90) | 4 |
| Ayalew et al. | 2008 | Dire Dawa | Lege Dini watershed | 655 | 311 | 47.50% (42.30-53.10) | 3 |
| Abera et al. | 2010 | Amhara | Bahir Dar food handler | 384 | 76 | 19.79% (15.60-24.80) | 6 |
| Tigabu et al. | 2010 | Benishangul | Pawi | 384 | 133 | 34.6% (28.90-41.04) | 6 |
| Ayalew et al. | 2011 | Amhara | Delgi primary school children | 704 | 487 | 69.20% (63.10-75.60) | 6 |
| Yihenew | 2011 | Amhara | Fogera Awuramba | 392 | 58 | 14.8% (11.20-19.10) | 3 |
| Gelaw et al. | 2013 | Amhara | Gondar health center | 304 | 40 | 13.20% (9.40-17.90) | 6 |
| Wegayehu et al. | 2013 | Oromia | North Shewa Zone children | 384 | 95 | 24.70% (20.00-30.00) | 6 |
| Wegayehu et al. | 2013 | SNNPR | Gamo rural residence | 858 | 189 | 22.0% (18.90-25.40) | 6 |
| Abossie and Seid | 2014 | SNNPR | Chencha town primary school | 400 | 112 | 28% (23.05-33.60) | 6 |
| Andualem | 2014 | Amhara | Motta Town primary school | 364 | 70 | 19.50% (15.20-24.60) | 6 |
| Beyene and Tasew | 2014 | Oromia | Jimma health center | 260 | 43 | 16.50% (11.90-22.20) | 5 |
| Firdu et al. | 2014 | SNNPR | Yirgalem health center | 230 | 39 | 16.95 (12.05-23.20) | 6 |
| Kidane et al. | 2014 | Tigray | Wukro Town health center | 384 | 163 | 42% (36.20-49.50) | 6 |
| Mekonnen et al. | 2014 | Addis Ababa | Addis Ababa | 355 | 63 | 17.7% (13.60-22.70) | 6 |
| Tulu et al. | 2014 | Oromia | Delo-Mena, Bale Zone | 340 | 26 | 3.60% (4.90-11.20) | 6 |
| Workneh et al. | 2014 | Amhara | Debre Elias primary school | 541 | 44 | 8.13% (5.90-10.90) | 6 |
| Aklilu et al. | 2015 | Addis Ababa | Addis Ababa University | 172 | 86 | 50% (39.90-61.70) | 3 |
| Aleka et al. | 2015 | Amhara | Gondar University Hospital | 277 | 10 | 3.6% (1.70-6.60) | 6 |
| G/Silassie et al. | 2015 | Tigray | Aksum Town primary school | 404 | 127 | 31.4% (26.20-37.40) | 4 |
| Deneke | 2016 | Amhara | Ankober | 403 | 240 | 60.0% (52.20-67.60) | 3 |
| Tulu et al. | 2016 | Oromia | Dolomena (Balie Zone) | 492 | 48 | 9.8% (7.10-12.90) | 6 |
| Birmeka et al. | 2017 | SNNPR | Gurage zone primary school | 450 | 97 | 21.60% (17.40-26.20) | 6 |
| Gebretsadik | 2017 | Benishangul | Homsha district | 395 | 106 | 26.8% (21.90-32.40) | 6 |
| Hailegebriel | 2017 | Amhara | Bahir Dar | 359 | 129 | 35.9% (30.00-42.60) | 6 |
| Senbeta | 2017 | Tigray | Adigrat primary school | 309 | 21 | 6.80% (4.20-10.38) | 5 |
| Berhe et al. | 2018 | Tigray | Mekele | 226 | 101 | 45.3% (36.40-54.30) | 5 |
| Dobo | 2018 | SNNPR | Hawasa | 89423 | 39895 | 44.6% (44.20-45.05) | 3 |
| Gebreyohanns et al. | 2018 | Tigray | Addiremets town | 411 | 13 | 3.2% (1.68-5.40) | 5 |
| Tegegne et al. | 2018 | Amhara | Gondar University Hospital | 256 | 14 | 5.5% (2.90-9.17) | 5 |
| Alemu et al. | 2019 | SNNPR | Gamogofa Zone primary school | 351 | 26 | 7.4% (4.80-10.80) | 6 |
| Asires et al. | 2019 | Amhara | East and West Gojjam | 344 | 96 | 27.9% (22.60-34.07) | 5 |
| Ayalew et al. | 2019 | Amhara | Bahir Dar primary school | 418 | 71 | 16.98% (13.05-21.15) | 5 |
| Ayelgn et al. | 2019 | Amhara | Gondar poly health center | 13329 | 3760 | 28.2% (27.30-29.10) | 5 |
| Eshetu et al. | 2019 | Oromia | Nekemit | 240 | 73 | 30.4% (23.80-38.20) | 6 |
| Gebrecherkos et al. | 2019 | Amhara | University of Gondar | 150 | 45 | 30% (21.80-40.10) | 6 |
| Kumma et al. | 2019 | SNNPR | Wolayta Sodo University | 233 | 47 | 20.2% (14.80-26.80) | 6 |
| Lewetegn et al. | 2019 | Amhara | Senbete and Bete Towns | 214 | 60 | 28.1% (21.30-36.08) | 6 |
| Menjetta et al. | 2019 | SNNPR | Hawasa University Clinic | 13679 | 3782 | 27.6% (26.70-28.50) | 4 |
| Sewunet and Tekelia | 2019 | Amhara | Woreta | 310 | 52 | 16.8% (12.50-21.90) | 5 |
| Shimeles et al. | 2019 | Amhara | Chagni food handler | 400 | 40 | 10% (7.10-13.60) | 6 |
| Sitotaw et al. | 2019 | Amhara | Jawi primary school | 406 | 105 | 25.9% (21.10-31.30) | 6 |
| Tadesse et al. | 2019 | Oromia | Bamo no. 2 primary school | 417 | 74 | 17.7% (13.90-22.20) | 5 |
| Tigabu et al. | 2019 | Amhara | Shahura health center | 364 | 145 | 39.84% (33.60-46.90) | 6 |
| Berhe et al. | 2020 | Tigray | Adigrat | 418 | 248 | 59.30 (52.20-67.20) | 6 |
SNNPR: Southern Nations, Nationalities, and People's Region.
3.2. Quality of Studies Included in the Meta-Analysis
The quality score of each original study ranged between three and the highest six (Table 1). The overall quality of the articles included in this meta-analysis is very good.
3.3. Prevalence of Intestinal Protozoan Parasitic Infections in Ethiopia
The lowest prevalence of IPPIs was reported from Gondar (2.4%) [26] while the highest prevalence (69.2%) was reported from Delgi primary school, Amhara region [27]. The overall pooled prevalence of HIPPIs was 25.01% (95% CI: 20.08%-29.95%) (Figure 2). High heterogeneity (I2 = 99.4%, P ≤ 0.001) was observed across studies included in the studies.
Figure 2.
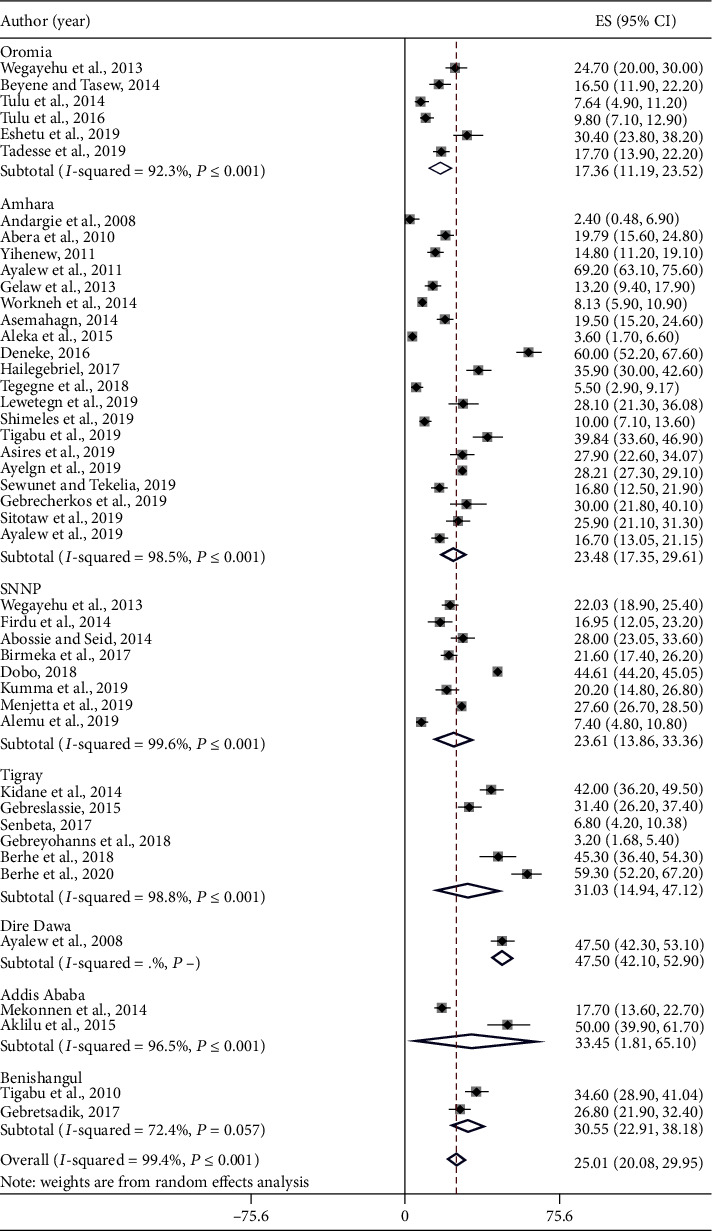
Pooled prevalence of HIPPIs in Ethiopia including the regional level. Each square represents the effect size (ES) of individual studies, and the horizontal line represents the 95% CI. The diamond indicates the pooled effect, and the vertical dash lines indicate the overall estimate.
3.4. Subgroup Analysis
High pooled prevalence of HIPPIs was reported from Dire Dawa (47.5%, 95% CI: 42.10-52.9), followed by Addis Ababa (33.45%, 95% CI: 1.81-65.10), Tigray (31.03%, 95% CI: 14.94-47.12), and Benishangul-Gumuz (30.55%, 95% CI: 22.91-38.18), whereas the low prevalence of HIPPIs was observed in Oromia (17.35%, 95% CI: 11.17-23.53) followed by Amhara (23.48%, 95% CI: 17.35-29.61) and SNNPR (23.61%, 95% CI: 13.86-33.36). The pooled prevalence of HIPPIs of studies with sample sizes > 200 (24.89%, 95% CI: 19.86-29.93) was lower than that of studies having sample sizes ≤ 200 (27.12%, 95% CI:-2.2-56.45) (Table 2).
Table 2.
Prevalence of HIPPIs in Ethiopia by subgroups.
| Variables | Characteristics | Number of studies | Sample size | Prevalence (95% CI) | I 2, P value |
|---|---|---|---|---|---|
| Sample size | ≤200 | 3 | 449 | 27.12% (95% CI: -2.2-56.45) | 99.4%, P < 0.01 |
| >200 | 42 | 131,467 | 24.89% (95% CI: 19.86-29.93) | 97.8%, P < 0.01 | |
|
| |||||
| Pooled prevalence of HIPPIs by year | 2008-2012 | 6 | 2646 | 31.28% (95% CI: 12.78-49.77) | 99.0%, P < 0.01 |
| 2013-2017 | 20 | 7681 | 22.58% (95% CI: 17.52-27.64) | 96.7%, P = 0.01 | |
| 2018-2020 | 19 | 121,589 | 25.30% (95% CI: 18.33-32.27) | 99.6%, P < 0.01 | |
|
| |||||
| Nature of study participants | Food handlers | 7 | 1900 | 22.24% (95% CI: 12.94-31.54) | 96%, P < 0.01 |
| Patients | 13 | 118,738 | 32.65% (95% CI: 25.64-39.67) | 99.%, P < 0.01 | |
| Preschool children | 3 | 858 | 15.81% (95% CI: 2.23-29.40) | 96%, P < 0.01 | |
| School children | 18 | 8404 | 24.21% (95% CI: 17.89-30.52) | 97.7%, P < 0.01 | |
| Urban dwellers | 1 | 355 | 17.7% (95% CI: 13.15-22.25) | — | |
| Rural dwellers | 3 | 1661 | 13.29% (95% CI: 0.85-25.72) | 98.1%, P < 0.01 | |
|
| |||||
| Overall | 45 | 131,916 | 25.01% (95% CI: 20.08-29.95) | 99.4%, P < 0.01 | |
The highest pooled prevalence of HIPPIs was observed among patients (32.65%, 95% CI: 25.64-39.67) followed by school children (24.21%, 95% CI: 17.89-30.52), food handlers (22.24%, 95% CI: 12.94-31.54), urban dwellers (17.7%, 95% CI: 13.15-22.25), preschool children (15.81%, 95% CI: 2.23-29.40), and rural dwellers (13.29%, 95% CI: 0.85-25.72) (Table 2).
The pooled prevalence of HIPPIs was 31.28% (95% CI: 12.78-49.77), 25.30% (95% CI: 18.33-32.27), and 22.58% (95% CI: 17.52-27.64) observed among studies conducted from 2008 to 2012, 2018 to 2020, and 2013 to 2017, respectively (Table 2).
3.5. Common HIPPIs in Ethiopia
The pooled prevalence of E. histolytica/dispar was 14.09% (95% CI: 11.03-17.14) (Figure 3) followed by G. lamblia 10.03% (95% CI: 7.69-12.38) (Figure 4) and Cryptosporidium spp. 5.93% (95% CI: 2.95-8.91) (Figure 5) among study subjects in Ethiopia. There is no significant difference in the pooled prevalence of E. histolytica/dispar, G. lamblia, and Cryptosporidium spp. among regions included in this meta-analysis (P = 0.322, P = 0.168, and P = 0.088, respectively).
Figure 3.
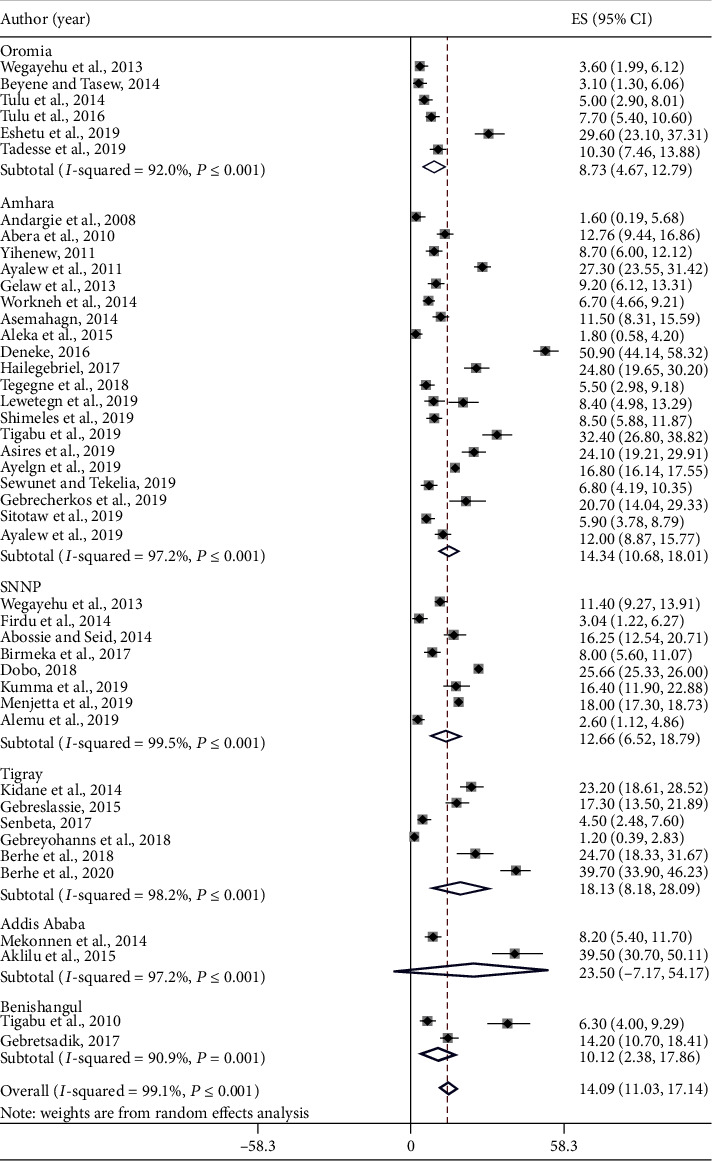
The pooled prevalence of E. histolytica/dispar among study participants in Ethiopia.
Figure 4.
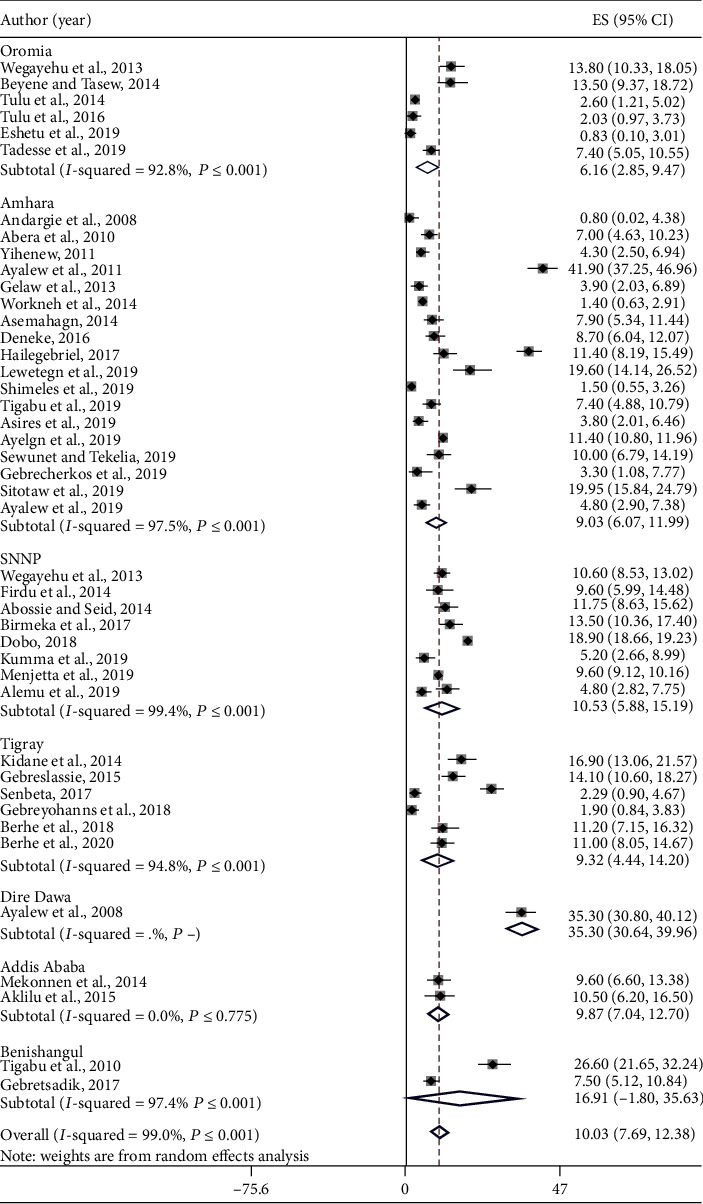
The pooled prevalence of G. lamblia among study participants in Ethiopia.
Figure 5.
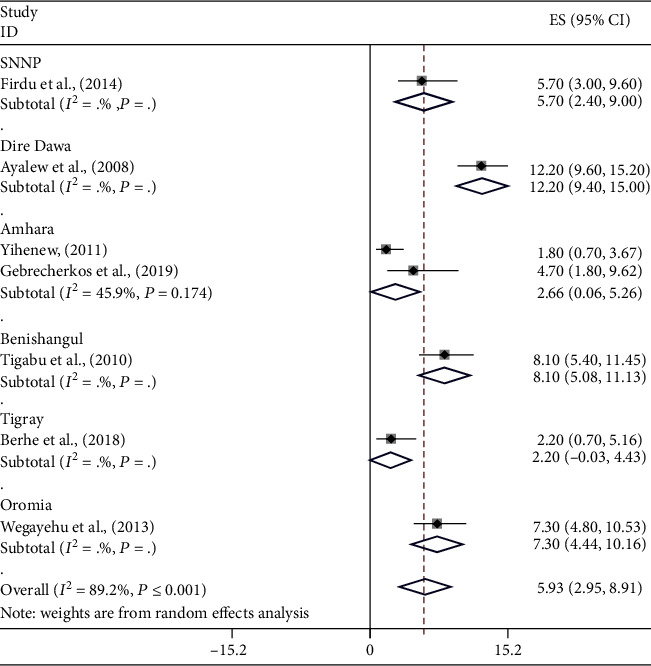
The pooled prevalence of Cryptosporidium spp. among study subjects in Ethiopia.
We perform a metaregression analysis to identify the sources of heterogeneity across studies. The analysis showed that the region of study (regression coefficient: 0.104, 95% CI: -0.032-0.239, and P = 0.131), nature of study subjects (regression coefficient: -0.158, 95% CI: -0.322-0.006, and P = 0.058), year of publication (regression coefficient: -0.029, 95% CI: -0.365-0.306, and P = 0.861), and sample size (regression coefficient: 0.041, 95% CI: -1.024-0.941, and P = 0.933) did not contribute for the heterogeneity.
3.6. Risk of Publication Bias across Studies Included in the Meta-Analysis
The funnel plot symmetry demonstrates the presence of publication bias among studies included in this meta-analysis (Figure 6). Similarly, Egger's test results (P ≤ 0.001) indicate publication bias among studies. Sensitivity analysis was performed by recalculating the pooled prevalence of HIPPIs by sequentially removing one by one to identify the cause of publication bias. The pooled prevalence remained stable, and the result was not driven by individual studies included in the meta-analysis.
Figure 6.
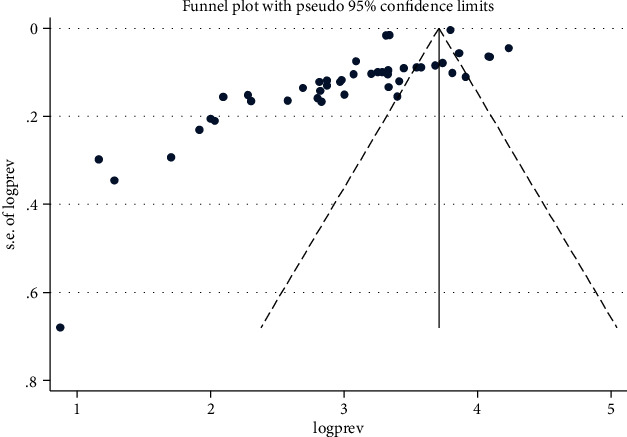
Funnel plot indicates publication bias among studies.
3.7. Factors Associated with HIPPIs in Ethiopia
In this meta-analysis, we have reviewed several potential risk factors associated with HIPPIs in Ethiopia. Family size, source of drinking water, open field defecation, handwashing habit, the habit of eating raw vegetables, and fingernail trimming and cleanness habits were associated with HIPPIs.
The association between fingernail trimming and cleanness habits and intestinal protozoan parasitic infection in people of Ethiopia was computed from 12 studies [18, 28–38]. The pooled results showed that individuals with poor fingernail trimming and cleanness habits were 1.7 times more likely to be infected with HIPPIs than their counterparts (OR: 1.7, 95% CI: 0.89-2.25, and P ≤ 0.001) (Supplementary 1 = S1).
The pooled results of 20 studies [17, 18, 27, 28, 31–34, 36–47] showed that handwashing habits were strongly associated with infection with intestinal protozoan parasitic infections in Ethiopia.
The odds of having an intestinal parasitic infection was 2.82 times higher among people who did not wash their hands after defecation than people who wash their hands (OR: 2.82, 95% CI: 2.01-3.63) (S2).
The association between age and intestinal protozoan parasitic infections was analyzed from nine studies [18, 29, 31, 34, 41, 42, 47–49]. Age (children up to 14 years) was significantly associated with the prevalence of intestinal protozoan parasitic infections. The odds of having HIPPIs in children (up to 14 years) were 1.54 times higher than those in adults (OR: 1.54, 95% CI: 0.91-2.18) (S3).
The association between open field defecation and intestinal protozoan parasitic infection among people in Ethiopia was computed from six studies [3, 34, 35, 38, 42, 50]. People who practiced open field defecation were 2.91 times more likely to have intestinal protozoan parasitic infections than those who did not practice open field defecation (OR: 2.91, 95% CI: 1.60-4.21) (S4).
The association between the habits of eating raw, unwashed, contaminated, leftover fruits and vegetables with intestinal protozoan parasitic infections was evaluated from nine studies [18, 27–29, 34, 35, 38, 44, 45]. The odds of having intestinal protozoan parasitic infections was 1.77 times higher among people who had habits of eating raw, unwashed, contaminated, leftover fruits and vegetables as compared with the counterparts (OR: 1.77, 95%; CI: 1.03-2.51) (S5).
The association between family education and intestinal protozoan parasitic infection among people in Ethiopia was computed from ten studies [3, 17, 27–29, 31, 42, 46, 48, 50]. The pooled results showed that uneducated mothers, fathers, and children were 1.69 times more likely to have intestinal protozoan parasitic infections than those who were educated (OR: 1.69, 95% CI: 0.84-2.54) (S6).
Results from six studies revealed that the level of household income was strongly associated with HIPPIs [31, 33, 41, 42, 44]. People who had low household income were 1.64 times more likely to have intestinal protozoan parasitic infections than those who had higher family income (OR: 1.64, 95% CI: 0.96-2.32) (S7).
The pooled results of eleven studies [17, 27, 28, 30, 31, 38, 42, 46, 47, 50, 51] revealed that people who drink unprotected water were 3.33 times more likely to have intestinal protozoan parasitic infections than those who drink protected water (OR: 3.33, 95% CI: 1.30-5.36) (S8).
The pooled analysis of two studies conducted in Ethiopia [3, 35] showed that people who had the habit of playing with soil were 2.15 times more likely to have intestinal protozoan parasitic infections than those who did not (OR: 2.15, 95% CI: 1.01-3.29) (S9).
The pooled results of five studies [31, 45–47, 50] showed that family sizes were strongly associated with intestinal protozoan parasitic infection among people in Ethiopia. The likelihood of intestinal parasitic infection was 3.7 times higher among people with a family size of above 5 that those with low family sizes (OR: 3.7, 95% CI: 1.45-5.85) (S10).
4. Discussion
Human intestinal protozoan infections are the major IPIs and are the common causes of morbidity and mortality in Ethiopia [23]. Knowing the exact national pooled prevalence of HIPPIs is useful for policymakers. The pooled prevalence of HIPPIs in this systematic review and meta-analysis study was 25.01% (95% CI: 20.08-29.95). It was higher than studies conducted in Côte d'Ivoire (18.7%) [52], Tanzania (17.4%) [53], Saudi Arabia (18.7%) [54], and Qatar (5.93%) [55]. However, it was almost similar to the studies from Bulgaria (25.53%) [56], Spain (28%) [57], the Democratic Republic of São Tomé and Príncipe (28.6%) [58], and Iran (21%) [59].
The prevalence of HIPPIs in the present study was lower than that of Libya (85%) [14]; Shahura Health Center, Amhara region, Ethiopia (39.84%) [49]; Cambodia (53.9%) [58]; Senegal (32.6%) [60]; Thailand (37.8%) [61]; Palestine (39.21%) [62]; Ghana (42.9%) [63]; Sudan (54.2%) [64]; Tripoli, Kenya (56%) [65]; Kut city, Iraq (57.5%) [66]; Iraq (98.8%) [67]; Cameroon (74.3%) [68]; Malaysia (72.3%) [69]; Mexico (65%) [70]; Mexico (60%) [71]; and Burkina Faso (84.7%) [72]. The differences may be attributed to methodological, social, economic, demographic, hygienic, weather and climatic, environmental, and political factors.
The highest HIPPI prevalence was observed among patients (32.65%, 95% CI: 25.64-39.67) and schoolchildren (24.21%, 95% CI: 17.89-30.52) while the lowest prevalence was observed among rural dwellers (13.29%, 95% CI: 0.85-25.72) and preschool children (15.81%, 95% CI: 2.23-29.40). Parental care among preschool children may be the reason for their lower prevalence. But school-age children have higher prevalence due to their habit of playing with soil [3, 35]. The high prevalence of HIPPIs among patients might be due to their high susceptibility to protozoan parasites that might be associated with low immunity. Intestinal parasitic infections were more prevalent among the poor divisions of the population with poor handling of personal and environmental sanitation [46].
The trend of HIPPIs in Ethiopia from 2008 to 2012, 2013 to 2017, and 2018 to 2020 were 31.28% (95% CI: 12.78-49.77), 22.58% (95% CI: 17.52-27.64), and 25.30% (95% CI: 18.33-32.27), respectively. Accordingly, the prevalence of HIPPIs in the first ten years was reduced from 31.28% to 22.58%. However, it rose from 2018 to 2020 by close to 2.72%. This finding disagreed with the study done in Qatar in which HIPPI prevalence reduced from 2005 to 2008 (7.98%, 95% CI: 7.429-8.536), 2009 to 2011 (5.13%, 95% CI: 4.673-5.593), and 2012 to 2014 (4.89%, 95% CI: 4.488-5.286) [55]. The rise of HIPPIs from 2018 to 2020 in this study may be due to a lack of mass treatment, especially school deworming [73].
The pooled prevalence of G. lamblia was 10.03% in this meta-analysis, which is in line with the studies conducted in Côte d'Ivoire (13.1%) [52], Tanzania (10.6%) [53], Ghana (12.2%) [63], Iraq (10.8%) [67], and Nepal (12.5%) [74]. However, it was lower than studies in Tripoli, Libya (28.5%) [14]; Bulgaria (62.05%) [56]; Spain (18%) [57]; Cambodia (31.5%) [58]; Senegal (20.4%) [60]; Sudan (22.9%) [64]; Mexico (24%) [70]; Burkina Faso (28.1%) [72]; Dhaka (17.6%) [75]; the Philippines (19.2%) [76]; and Turkey (47.97%) [77]. But, G. lamblia prevalence (10.03%) in this study was much higher than that of Saudi Arabia (3%) [54], Thailand (4.2%) [61], Kenya (6.5%) [65], Iraq (4%) [66], Cameroon (3.3%) [68], Iran (1.7%) [59], Libya (4.9%) [78], Turkey (6.1%) [79], and Thailand (0.6%) [80]. The variations might be due to variations in the quality of drinking water sources and environmental conditions [81].
The pooled prevalence of E. histolytica/dispar was 14.09% in the present meta-analysis. It agrees with the reports from Pahang, Malaysia (18.5%) [69]; the Philippines (12.1%) [76]; and Karnataka, India (9%) [82]. However, the result of this study was higher than that of studies in Saudi Arabia (2%) [54], Iran (0.6%) [59], Thailand (0.73%) [61], Ghana (0.21%) [63], Cameroon (7.3%) [68], and Mexico (5%) [70]. In contrast, the results of the present study were lower than those of studies from Côte d'Ivoire (56%) [52]; Tanzania (28.5%) [53]; Sudan (31.2%) [64]; Kenya (23.9%) [65]; Kut city, Iraq (41%) [66]; Iraq (88%) [67]; Burkina Faso (66.5%) [72]; and Libya (21.14%) [78]. The variation might be due to the quality of food and water and the environmental condition of the different study localities. E. histolytica/dispar is an environmental contaminant of drinking water supply and food. It can be transmitted by drinking infected water and by consuming contaminated vegetables and food [81].
The pooled prevalence of Cryptosporidium species was 5.93%, which is higher than other studies from Saudi Arabia (3%) [54], Bulgaria (1.69%) [56], Spain (1%) [57], Iran (0.4%) [59], Senegal (0.3%) [60], Dhaka (0.5%) [75], Libya (0.8%) [78], and China (2.4%) [83]. The result of this meta-analysis was lower than that of studies conducted in Iraq (10.4%) [14], Ghana (8.5%) [63], Kenya (13%) [65], Iraq (12.5%) [66], Cameroon (44%) [68], and the Philippines (22%) [76]. The reason for the low prevalence of Cryptosporidium infection in Ethiopia might be associated with the types of laboratory diagnostic procedures. About 84% studies included in this meta-analysis did not use appropriate methods for detecting opportunistic parasites such as Cryptosporidium.
This meta-analysis and systematic review study showed that there was a clear variation in the prevalence of HIPPIs in Ethiopia. The highest pooled prevalence was observed from Dire Dawa while the lowest prevalence was obtained from the Oromia region. The potential justification for this difference might be due to the peculiarity in sociodemographic, environmental, geographical, and behavioral characteristics. Similar observations were reported from Nepal [84].
The odds of HIPPI occurrence was 1.7 times higher among groups who did not have regular fingernail trimming and cleanness habits compared to their counterparts. This finding is supported by the studies conducted in Sri Lanka [13] and Nepal [74]. It is known that unclean fingernails may contain cysts of protozoan parasites that lead to higher infection.
The habit of handwashing was significantly associated with the prevalence of HIPPIs in Ethiopia. The odds of having HIPPIs among people who did not wash their hands after defecation was about 2.8-fold higher than that among people who used to wash their hands regularly. This finding is opposed to the studies conducted in Sudan [64] and Nepal [74]. This might be due to fecal-oral contamination through unwashed hands.
Age (children up to 14 years) was significantly associated with the prevalence of intestinal protozoan parasitic infections. The result was supported by the studies conducted in Iran [59], Palestine [62], Ghana [63], Sudan [64], Cameroon [68], Libya [78], and Karachi [85]. This might be because children have weak immunity than adults. Their complex nutritional requirements and less developed immune systems make children the principal sufferers of the intestinal parasitic infections [1].
The habit of eating raw, unwashed, contaminated, and leftover fruits and vegetables was significantly associated with intestinal protozoan parasitic infections. This was supported by the studies done in Sri Lanka [13] and Tripoli, Libya [14]. This is because raw, unwashed, contaminated, and leftover fruits and vegetables carry intestinal protozoan parasites [81].
Families with low-level education were 1.3 times more likely to have HIPPIs than educated families. This result was supported by studies done in Iran [59], Cameroon [68], Mexico [70], and Nepal [74]. The reason may be uneducated people lack the necessary knowledge and practices towards the transmission and prevention of HIPPs [70].
In this meta-analysis, drinking of unprotected water was significantly associated with the occurrence of HIPPIs in Ethiopia. The people who drink unprotected water were 3.3 times more likely to have intestinal protozoan parasitic infections than those who use protected water. This finding was in line with the studies done in Ethiopia [46], Sudan [64], Cameroon [68], Mexico [71], Turkey [77], and China [83]. This is because unprotected water would have a pool of intestinal protozoan parasites and can be a source of infection. Water is one of the important vehicles for pathogen dissemination [86]. E. histolytica/dispar, Cryptosporidium, and G. lamblia are environmental contaminants of drinking water supplies [82].
This meta-analysis showed that open field defecation was significantly associated with the presence of human intestinal protozoan parasitic infections. People who practiced open field defecation were 2.74 times more likely to have intestinal protozoan parasitic infections than their counterparts. The result of this study was in accordance with the study done in Ethiopia [46], Mexico [70], and South India [87]. In developing countries, poor hygiene and the use of untreated human feces are important factors that contribute to the contamination of food and water. Due to this, E. histolytica, G. lamblia, and Cryptosporidium spp. could be transmitted to humans [88]. Intestinal parasitic infections also occur via contaminated material such as earth, water, uncooked, or cross-contaminated food that has been in contact with the feces of an infected individual or animal [89].
People who had poor levels of income were 1.64 times more likely to have intestinal protozoan parasitic infections than their counterparts. This might be due to lack of treatment, medication, and quality food and water and poor living conditions among the people who had poor economic levels. This result is in agreement with the study from Mexico [70]. On the contrary, this finding was opposed to the study done in Gondar, Ethiopia; the level of income is not associated with the prevalence of intestinal parasitic infections [41].
The people who had the habit of playing with soil were about 2-fold more likely to have HIPPIs than their counterparts. The study was in accordance with the study conducted in North Shewa, Ethiopia [3]. This is because the soil contains eggs and cysts of protozoan intestinal parasites which could contaminate food and water [5].
The people who had a large family size (>5) were nearly 4-fold more likely to have intestinal protozoan parasitic infections than those who have a small family size. This might be because a large family size would increase the chance of more contact with each other and could be a source of transmission of protozoan infections [2]. The outcome was in line with studies done in Ethiopia [46] and Turkey [77].
5. Limitations of the Study
This meta-analysis and systematic review study produced a lot of valuable data about intestinal parasites in Ethiopia, but it has also several limitations. The articles included in this meta-analysis were not derived from all regions (information about HIPPIs was lacking from Afar, Gambela, Somali, and Harari regions). Besides, the information used in this meta-analysis was not uniformly distributed in the regions included in this meta-analysis. Therefore, the result may not fully represent the national prevalence of intestinal parasitic infection.
6. Conclusion
The pooled prevalence of intestinal protozoan parasitic infections according to this review was found to be 25.01%. There was a clear difference in the prevalence of HIPPIs across regions in the country. Fingernail trimming, handwashing habits, age, open field defecation, the habit of eating raw fruits and vegetables, level of family education, levels of income, source of drinking water, playing with soil, and family size were significantly associated with the prevalence of intestinal protozoan parasitic infections. This study highlights the importance of proper health education on personal hygiene, handwashing practice, open field defecation, handling of food, selections of living rooms of animals to prevent animal contact, and food safety. Therefore, all stakeholders should give proper attention to increasing awareness of the community and proper treatments of infected patients.
Abbreviations
- AOR:
Adjusted odds ratio
- CDC:
Center for Disease Controlling program
- CI:
Confidence interval
- DALYs:
Disability-adjusted life years
- GRADE:
Grading of Recommendations Assessment, Development and Evaluation
- HIPPIs:
Human intestinal protozoan parasitic infections
- HIPPs:
Human intestinal protozoan parasites
- IPIs:
Intestinal parasitic infections
- OR:
Odds ratio
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- spp.:
Species
- WHO:
World Health Organization.
Data Availability
All related data has been presented within the manuscript and on supplementary data. The dataset supporting the conclusions of this article is available from the authors on request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
DT developed the draft proposal under the supervision of DD. TH guided the statistical analysis. All authors (DT, DD, and TH) critically reviewed, provided substantive feedback, contributed to the intellectual content of this paper, and made substantial contributions to the conception, conceptualization, and manuscript preparation of this systematic review. All authors read and approved the final manuscript.
Supplementary Materials
S1: the pooled odds ratio of the association between fingernail trimming and cleanness and HIPPIs in Ethiopia. S2: the pooled odds ratio of the association between handwashing habits and HIPPIs in Ethiopia. S3: the pooled odds ratio of the association between age and HIPPIs in Ethiopia. S4: the pooled odds ratio of the association between open field defecation habit and HIPPIs in Ethiopia. S5: the pooled odds ratio of the association between eating raw, undercooked, contaminated, and leftover food and HIPPIs in Ethiopia. S6: the pooled odds ratio of the association between the level of education and HIPPIs in Ethiopia. S7: the pooled odds ratio of the association between the level of family income and HIPPIs in Ethiopia. S8: the pooled odds ratio of the association between the unprotected drinking water sources and HIPPIs in Ethiopia. S9: the pooled odds ratio of the association between playing with soil and HIPPIs in Ethiopia. S10: the pooled odds ratio of the association between the number of family size and HIPPIs in Ethiopia.
References
- 1.Abdullah I., Tak H., Ahmad F. Predominance of gastrointestinal protozoan parasites in children: a brief review. Journal of Health Education Research and Development. 2016;4(4) doi: 10.4172/2380-5439.1000194. [DOI] [Google Scholar]
- 2.Norhayati M., Fatmah M. S., Yusof S., Edariah A. B. Intestinal parasitic infections in man: a review. Medical Journal of Malaysia. 2003;58(2):296–305. [PubMed] [Google Scholar]
- 3.Lewetegn M. Prevalence of intestinal parasites among preschool children and maternal KAP on prevention and control in Senbete and Bete Towns, North Shoa, Ethiopia. International Journal of Biomedical Materials Research. 2019;7(1, article 1511220):1–7. doi: 10.11648/j.ijbmr.20190701.11. [DOI] [Google Scholar]
- 4.WHO. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. 2010 [PubMed]
- 5.Ayed L. B., Sabbahi S. Entamoeba histolytica. In: Rose J. B., Jimenez-Cisneros B., editors. Global water pathogen project. E. Lansing, MI, UNESCO.: Michigan: Michigan University; 2017. [Google Scholar]
- 6.Murray C. J. L., Vos T., Lozano R., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laishram S., Kang G., Ajjampur S. S. R. Giardiasis: a review on assemblage distribution and epidemiology in India. Indian Journal of Gastroenterology. 2012;31(1):3–12. doi: 10.1007/s12664-012-0161-9. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed A., Degefu H., Jilo K. Cryptosporidium and its public health importance: review. International Journal of Research Studies in Microbiology and Biotechnology. 2017;3(4):12–31. doi: 10.20431/2454-9428.0304004. [DOI] [Google Scholar]
- 10.Pisarski K. The global burden of disease of zoonotic parasitic diseases: top 5 contenders for priority consideration. Tropical Medicine of Infectious Diseases. 2019;4(1):p. 44. doi: 10.3390/tropicalmed4010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacciò S. M., Thompson R. C., McLauchlin J., Smith H. V. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitology. 2005;21(9):430–437. doi: 10.1016/j.pt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Sponseller J. K., Griffiths J. K., Tzipori S. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clinical Microbiology Review. 2014;27(3):575–586. doi: 10.1128/cmr.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galgamuwa L., Iddawela D., Dharmaratne S. Intestinal protozoa infections, associated risk factors and clinical features among children in a low-income tea plantation community in Sri Lanka. International Journal of Community Medicine and Public Health. 2016;3(9):2452–2458. doi: 10.18203/2394-6040.ijcmph20163053. [DOI] [Google Scholar]
- 14.Osman M., el Safadi D., Cian A., et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among Schoolchildren in Tripoli, Lebanon. Public Library of Science Neglected Tropical Diseases. 2016;10(3, article e0004496) doi: 10.1371/journal.pntd.0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basualdo J. A., Córdoba M. A., de Luca M. M., et al. Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002-2003. Revista do Instituto de Medicina Tropical de Sao Paulo. 2007;49(4):251–255. doi: 10.1590/s0036-46652007000400011. [DOI] [PubMed] [Google Scholar]
- 16.Mengistu A., Gebre-Selassie S., Kassa T. Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiopian Journal of Health Development. 2007;21(1):12–17. doi: 10.4314/ejhd.v21i1.10026. [DOI] [Google Scholar]
- 17.Abossie A., Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BioMed Central Public Health. 2014;14(1):p. 166. doi: 10.1186/1471-2458-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebretsadik G. Current status of intestinal parasites and associated risk factors among schoolchildren of Homesha District in Northwest Ethiopia. International Journal of Current Research. 2019;9(7):54369–54374. [Google Scholar]
- 19.Alemu M., Anley A., Tedla K. Magnitude of intestinal parasitosis and associated factors in rural school children, Northwest Ethiopia. Ethiopian Journal of Health Sciences. 2018;29(1):p. 923. doi: 10.4314/ejhs.v29i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethiopia population (2020)-Worldometer. 2020.
- 21.2020. https://www.cia.gov/library/publications/the-world-factbook/attachments/summaries/ET-s ummary.pdf.
- 22.Moher D., Pham B., Lawson M., Klassen T. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technology Assessment. 2003;7(41):1–90. doi: 10.3310/hta7410. [DOI] [PubMed] [Google Scholar]
- 23.Alemnew B., Gedefaw G., Diress G., Bizuneh A. D. Prevalence and factors associated with intestinal parasitic infections among food handlers working at higher public University student’s cafeterias and public food establishments in Ethiopia: a systematic review and meta-analysis. BMC Infectious Diseases. 2020;20(1) doi: 10.1186/s12879-020-4884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The GRADE Working Group, Atkins D., Eccles M., et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BioMedCentral health services research. 2004;4(1) doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rücker G., Schwarzer G., Carpenter J. R., Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology. 2008;8(1) doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andargie G., Kassu A., Moges F., Tiruneh M., Huruy K. Prevalence of bacteria and intestinal parasites among food-handlers in Gondar Town, Northwest Ethiopia. Journal of Health Population and nutrition. 2008;26(4):451–455. doi: 10.3329/jhpn.v26i4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayalew A., Debebe T., Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. Journal of Parasitology and Vector Biology. 2011;3(5):75–81. [Google Scholar]
- 28.Tulu B., Taye S., Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Research Notes. 2014;7(1) doi: 10.1186/1756-0500-7-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mekonnen B., Erko B., Legesse M. Prevalence of Intestinal Parasitic Infections and Related Risk Factors among Street Dwellers in Addis Ababa, Ethiopia. Journal of Tropical Diseases. 2014;2(2) doi: 10.4172/2329-891x.1000132. [DOI] [Google Scholar]
- 30.Department of Biology, College of Natural and Computational Science, Adigrat University PO Box 50, Adigrat, Ethiopia, Maru D. S. Prevalence of intestinal parasitic infections and associated risk factors among school children in Adigrat town, Northern Ethiopia. International Journal of Emerging Trends in Science and Technology. 2015;4(1):4943–4948. doi: 10.18535/ijetst/v4i1.03. [DOI] [Google Scholar]
- 31.Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. Ethiopia. BMC Infectious Diseases. 2017;17(1) doi: 10.1186/s12879-017-2466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alemu A. S., Baraki A. G., Alemayehu M., Yenit M. K. The prevalence of intestinal parasite infection and associated factors among food handlers in eating and drinking establishments in Chagni Town, Northwest Ethiopia. BNC Research Notes. 2019;12(1) doi: 10.1186/s13104-019-4338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asires A., Wubie M., Reta A. Prevalence and associated factors of intestinal parasitic infections among food handlers at prison, East and West Gojjam, Ethiopia. Advances in Medicine. 2019;2019:8. doi: 10.1155/2019/2101089.2101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitotaw B., Mekuriaw H., Damtie D. Prevalence of intestinal parasitic infections and associated risk factors among Jawi primary school children, Jawi town, northwest Ethiopia. BMC Infectious Diseases. 2019;19(1) doi: 10.1186/s12879-019-3971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekonnen H. S., Ekubagewargies D. T. Prevalence and factors associated with intestinal parasites among under-five children attending Woreta Health Center, Northwest Ethiopia. BMC Infectious Diseases. 2019;19(1) doi: 10.1186/s12879-019-3884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshetu L., Dabsu R., Tadele G. Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte town, west Oromia, Ethiopia. Research and Reports in Tropical Medicine. 2019;10:25–30. doi: 10.2147/RRTM.S186723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumma W., Meskele W., Admasie A. Prevalence of intestinal parasitic infections and associated factors among food handlers in Wolaita Sodo University students caterings, Wolaita Sodo, Southern Ethiopia: a cross-sectional study. Frontiers in Public Health. 2019;7 doi: 10.3389/fpubh.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berhe B., Mardu F., Tesfay K., et al. More than half prevalence of protozoan parasitic infections among diarrheic outpatients in Eastern Tigrai, Ethiopia, 2019; a cross-sectional study. Infection and Drug Resistance. 2020;13:27–34. doi: 10.2147/IDR.S238493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleka Y., G/egziabher S., Tamir W., Birhane M., Alemu A. prevalence and associated risk factors of intestinal parasitic infection among under five children in University of Gondar Hospital, Gondar, Northwest Ethiopia. Biomedical Research and Therapy. 2015;2(8):347–353. doi: 10.7603/s40730-015-0020-2. [DOI] [Google Scholar]
- 40.Abera B., Biadegelgen F., Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiopian Journal of Health Development. 2010;24(1) doi: 10.4314/ejhd.v24i1.62944. [DOI] [Google Scholar]
- 41.Gelaw A., Anagaw B., Nigussie B., et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13(1) doi: 10.1186/1471-2458-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andualem Asemahagn M. Parasitic infection and associated factors among the primary school children in Motta Town, Western Amhara, Ethiopia. American Journal of Public Health Research. 2014;2(6):248–254. doi: 10.12691/ajphr-2-6-6. [DOI] [Google Scholar]
- 43.Firdu T., Abunna F., Girma M. Intestinal protozoal parasites in diarrheal children and associated risk factors at Yirgalem Hospital, Ethiopia: a case-control study. International Scholarly Research Notices. 2014;2014:8. doi: 10.1155/2014/357126.357126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebreslassie M., Dejenie T., Tomass Z. Prevalence of intestinal parasites and associated risk factors in schoolchildren of Aksum Town Northern Ethiopia. Acta Parasitologica Globalis. 2015;6(1):42–48. [Google Scholar]
- 45.Deneke A. A thesis submitted to Addis Ababa University to fullfi MSSs.in biology department in Biology. Addis Ababa University.; 2016. Intestinal parasitic infection among patients visiting Gorebella Health Center, North Centrel Ethiopia. [Google Scholar]
- 46.Birmeka M., Urga K., Petros B. Prevalence and determinants of intestinal parasitic infections among primary schoolchildren in Gurage zone, South central Ethiopia. ECronicon Microbiology. 2017;8(2):59–70. [Google Scholar]
- 47.Alemu G., Abossie A., Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC Infectious Diseases. 2019;19(1) doi: 10.1186/s12879-019-3879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebreyohanns A., Legese M. H., Wolde M., Leta G., Tasew G. Prevalence of intestinal parasites versus knowledge, attitude and practices (KAPs) with special emphasis to Schistosoma mansoni among individuals who have river water contact in Addiremets town, Western Tigray, Ethiopia. PLOS ONE. 2018;13(9, article e0204259) doi: 10.1371/journal.pone.0204259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tigabu A., Taye S., Aynalem M., Adane K. Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest Ethiopia. BMC Research Notes. 2019;12(1) doi: 10.1186/s13104-019-4377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kidane E., Menkir S., Kebede A., Desta M. Prevalence of intestinal parasitic infections and their associations with anthropometric measurements of school children in selected primary schools, Wukro Town, Eastern Tigray, Ethiopia. International Journal of Current Microbiology and Applied Science. 2014;3(3):11–29. [Google Scholar]
- 51.Workneh T., Esmael A., Ayichiluhm M. Prevalence of intestinal parasitic infections and associated factors among Debre Elias primary schools children, East Gojjam Zone, Amhara Region, North West Ethiopia. Journal of Bacteriology and Parasitology. 2014;5(1) doi: 10.4172/2155-9597.1000181. [DOI] [Google Scholar]
- 52.Coulibaly G., Ouattara M., Dongo K., et al. Epidemiology of intestinal parasite infections in three departments of south-central Côte d’Ivoire before the implementation of a cluster-randomised trial. Parasite Epidemiology and Control. 2018;3(2):63–76. doi: 10.1016/j.parepi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Former, Senior Lecturer, Department of Microbiology, Faculty of Medicine AIMST University, Kedah Darul Aman, Malaysia, Venkatajothi D. R. Incidence of intestinal protozoa infections among school going children. International Journal of Current Research in Medical Sciences. 2017;3(4):54–58. doi: 10.22192/ijcrms.2017.03.04.008. [DOI] [Google Scholar]
- 54.KA I. Prevalence of intestinal parasitic infection among school children in Taif. Insights in Biomedicine. 2018;3(2) doi: 10.21767/2572-5610.10045. [DOI] [Google Scholar]
- 55.Abu-Madi M. A., Behnke J. M., Boughattas S., Al-Thani A., Doiphode S. H. A decade of intestinal protozoan epidemiology among settled immigrants in Qatar. BMC Infectious Diseases. 2016;16(1) doi: 10.1186/s12879-016-1728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harizanov R., Rainova I., Tsvetkova N., et al. Prevalence of intestinal parasitic infections among the Bulgarian population over a three year period (2015 – 2017) Helminthologia. 2020;57(1):12–18. doi: 10.2478/helm-2020-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reh L., Muadica A. S., Köster P. C., et al. Substantial prevalence of enteroparasites Cryptosporidium spp., Giardia duodenalis and Blastocystis sp. in asymptomatic schoolchildren in Madrid, Spain, November 2017 to June 2018. Eurosurveillance. 2019;24(43, article 1900241) doi: 10.2807/1560-7917.es.2019.24.43.1900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao C. W., Fu C. J., Kao C. Y., et al. Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of São Tomé and Príncipe, West Africa. African Health Sciences. 2016;16(3, article 690):690–697. doi: 10.4314/ahs.v16i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bahrami F., Haghighi A., Zamini G., Khadem-Erfan M. B., Azargashb E. Prevalence and associated risk factors of intestinal parasitic infections in Kurdistan province, northwest Iran. Cogent Medicine. 2018;5(1, article 1503777) doi: 10.1080/2331205x.2018.1503777. [DOI] [Google Scholar]
- 60.Sylla K., Kouly Tine R. C., Sow D., et al. Epidemiological profile of intestinal parasitic infection among preschool and school children living in a rural community in Senegal: a cross sectional survey. Journal of Bacteriology and Parasitology. 2018;9(4) doi: 10.4172/2155-9597.1000343. [DOI] [Google Scholar]
- 61.Sanprasert V., Srichaipon N., Bunkasem U., Srirungruang S., Nuchprayoon S. Prevalence of intestinal protozoan infections among children in Thailand: a large-scale screening and comparative study of three standard detection methods. Southeast Asian Journal of Tropical Medicine and Public Health. 2016;47(6):1123–1133. [PubMed] [Google Scholar]
- 62.Al-Jawabreh A., Ereqat S., Dumaidi K., Al-Jawabreh H., Abdeen Z., Nasereddin A. Prevalence of selected intestinal protozoan infections in marginalized rural communities in Palestine. BMC Public Health. 2019;19(1) doi: 10.1186/s12889-019-8024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walana W., Tay S. C. K., Tetteh P., Ziem J. B. Prevalence of intestinal protozoan infestation among primary school children in urban and peri-urban communities in Kumasi, Ghana. Science Journal of Public Health. 2014;2(2):52–57. [Google Scholar]
- 64.Suliman M. A., Magboul A. M., Mohammed H. Y., et al. Prevalence of intestinal parasitic infections and associated risk factors among school children in White Nile State, Sudan. Journal of Infectious Disease and Diagnosis. 2019;4(1) doi: 10.4172/2576-389X.1000125. [DOI] [Google Scholar]
- 65.Kimosop R. J., Mulambalah C. S., Ngeiywa M. M. Prevalence of enteric parasitic diseases among patients referred at a teaching hospital in Kenya. Journal of Health Research and Reviews. 2018;5(2):78–85. doi: 10.4103/jhrr.jhrr_7_18. [DOI] [Google Scholar]
- 66.Rahi A. A., Majeed L. Epidemiological study of intestinal protozoa at Wasit province Kut city, Iraq. EAS Journal of Orthopaedic and Physiotherapy. 2019;1:p. 3. [Google Scholar]
- 67.al-Taei A. . . The prevalence of intestinal parasite among the attending peoples to Al-Hashimyah hospitals for seven years, Babylon province, Iraq. Journal of Physics: Conference Series. 2019;1294, article 062022 doi: 10.1088/1742-6596/1294/6/062022. [DOI] [Google Scholar]
- 68.Nsagha D. Prevalence and predisposing factors to intestinal parasitic infections in HIV/AIDS patients in Fako division of Cameroon. BioMedical Journal of Global Health. 2017;2(Supplement 2):A41.2–A4A41. doi: 10.1136/bmjgh-2016-000260.109. [DOI] [Google Scholar]
- 69.Yusuf N. A., Yusri Y. M., Ismail N., Vythilingam I. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Tropical Biomedicine. 2007;24(1):55–62. [PubMed] [Google Scholar]
- 70.Quihui L., Valencia M. E., Crompton D. W. T., et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6(1):p. 225. doi: 10.1186/1471-2458-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quihui-Cota L., Morales-Figueroa G. G., Javalera-Duarte A., Ponce-Martínez J. A., Valbuena-Gregorio E., López-Mata M. A. Prevalence and associated risk factors for Giardia and Cryptosporidium infections among children of northwest Mexico: a cross-sectional study. BMC Public Health. 2017;17(1):p. 852. doi: 10.1186/s12889-017-4822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erismann S., Diagbouga S., Odermatt P., et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasites and Vectors. 2016;9(1) doi: 10.1186/s13071-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espinosa Aranzales A. F., Radon K., Froeschl G., Pinzón Rondón Á. M., Delius M. Prevalence and risk factors for intestinal parasitic infections in pregnant women residing in three districts of Bogotá, Colombia, Colombia. BMC Public Health. 2018;18(1) doi: 10.1186/s12889-018-5978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Associate Professor, School of Public Health and Community Medicine, BPKIHS, Dharan N., RB S., et al. A study of prevalence of intestinal protozoan infections and associated risk factors among the school children of Biratnagar Submetropolitan, eastern region of Nepal. Asian Pacific Journal of Health Science. 2016;3(1):181–197. doi: 10.21276/apjhs.2016.3.1.30. [DOI] [Google Scholar]
- 75.Shahid S. B., Wazib A., Chowdhury A., Shamsuzzam S. M., Mamuna K. Z. A study on different laboratory methods for diagnosis of intestinal protozoal infections. Bangladesh Medical Journal. 2014;40(2):47–49. doi: 10.3329/bmj.v40i2.18510. [DOI] [Google Scholar]
- 76.Weerakoon K. G., Gordon C. A., Williams G. M., et al. Co-parasitism of intestinal protozoa and Schistosoma japonicum in a rural community in the Philippines. Infectious Diseases of Poverty. 2018;7(1) doi: 10.1186/s40249-018-0504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doni N. Y., Gürses G., Şimşek Z., Zeyrek F. Y. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern Anatolian region of Turkey. Annal Agricultural and Environmental Medicien. 2015;22(3):438–442. doi: 10.5604/12321966.1167709. [DOI] [PubMed] [Google Scholar]
- 78.Ghenghesh K. S., Ghanghish K., BenDarif E. T., Shembesh K., Franka E. Libya: 2000–2015. 1, article 32088. Vol. 11. Libyan Journal of Medicine; 2016. Prevalence of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium spp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okyay P., Ertug S., Gultekin B., Onen O., Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health. 2004;4(1) doi: 10.1186/1471-2458-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Punsawad C., Phasuk N., Bunratsami S., Thongtup K., Siripakonuaong N., Nongnaul S. Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of southern Thailand. BMC Public Health. 2017;17(1) doi: 10.1186/s12889-017-4486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nasiri V., Esmailnia K., Karim G., Nasir M., Akhavan O. Intestinal parasitic infections among inhabitants of Karaj City, Tehran Province, Iran in 2006-2008. Korean Journal of Parasitology. 2009;47(3):265–268. doi: 10.3347/kjp.2009.47.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolhe V. S., More S. R. Study of opportunistic intestinal parasitic infections in HIV seropositive patients at a tertiary care teaching hospital in Karnataka, India. International Journal of Contemporary Medical Research. 2016;3(8):2219–2222. [Google Scholar]
- 83.Yang D., Yang Y., Wang Y., et al. Prevalence and risk factors of Ascaris lumbricoides, Trichuris trichiura and Cryptosporidium infections in elementary school children in Southwestern China: a school-based cross-sectional study. International Journal of Environmental Research and Public Health. 2018;15(9) doi: 10.3390/ijerph15091809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandrashekhar T. S., Joshi H. S., Gurung M., Subba S. H., Rana M. S., Shivananda P. G. Prevalence and distribution of intestinal parasitic infestations among school children in Kaski District, Western Nepal. Journal of Biomedical Science. 2005;4:78–82. [Google Scholar]
- 85.Mehraj V., Hatcher J., Akhtar S., Rafique G., Beg M. A. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11, article e3680) doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim Y. A. L., Sulaiman W. Y. W., Sawangjaroen N., et al. Comparative study on waterborne parasites between Malaysia and Thailand: a new insight. American Journal of Tropical Medicine and Hygiene. 2014;90(4):682–689. doi: 10.4269/ajtmh.13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vilwanathan S. A., Jayachandran A. L., Kandasamy B., Vijayalakshmi T. S. Prevalence of intestinal parasitic infections and predisposing factors among children in field practice area of tertiary care centre in South India. International Journal of Medical Microbiology and Tropical Diseases. 2017;3(2):45–49. doi: 10.18231/2455-6807.2017.0011. [DOI] [Google Scholar]
- 88.Dhawan V. K. Current diagnosis and treatment of amoebiasis. United States Infectious Disease. 2008;4(1):59–61. [Google Scholar]
- 89.Ortega M. T., Vergara A., Guimbao J., Clavel A., Gavín P., Ruiz A. Cryptosporidium hominis diarrhea outbreak and transmission linked to diaper infant use. Medicina Clínica. 2006;127(17):653–656. doi: 10.1016/s0025-7753(06)72352-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: the pooled odds ratio of the association between fingernail trimming and cleanness and HIPPIs in Ethiopia. S2: the pooled odds ratio of the association between handwashing habits and HIPPIs in Ethiopia. S3: the pooled odds ratio of the association between age and HIPPIs in Ethiopia. S4: the pooled odds ratio of the association between open field defecation habit and HIPPIs in Ethiopia. S5: the pooled odds ratio of the association between eating raw, undercooked, contaminated, and leftover food and HIPPIs in Ethiopia. S6: the pooled odds ratio of the association between the level of education and HIPPIs in Ethiopia. S7: the pooled odds ratio of the association between the level of family income and HIPPIs in Ethiopia. S8: the pooled odds ratio of the association between the unprotected drinking water sources and HIPPIs in Ethiopia. S9: the pooled odds ratio of the association between playing with soil and HIPPIs in Ethiopia. S10: the pooled odds ratio of the association between the number of family size and HIPPIs in Ethiopia.
Data Availability Statement
All related data has been presented within the manuscript and on supplementary data. The dataset supporting the conclusions of this article is available from the authors on request.


