Abstract
Introduction
Serum amino acid (AA) profiles represent a valuable tool in the metabolic assessment of cancer patients; still, information on the AA pattern in head and neck cancer (HNC) patients is insufficient. The aim of the study was to assess whether serum AA levels were associated with the stage of neoplastic disease and prognosis in primary HNC patients.
Methods
Two hundred and two primary HNC patients were included in the study. Thirty-one AAs and derivatives were measured in serum through an ultraperformance liquid chromatography-mass spectrometry (UPLC-MS). The association between AA concentrations and the stage (advanced versus early) of HNC was estimated using a multivariable logistic regression model. A multivariable Cox regression model was used to evaluate the prognostic significance of each AA.
Results
At the multivariable logistic regression analysis, increased levels of alpha-aminobutyric acid, aminoadipic acid, histidine, proline, and tryptophan were associated with a reduced risk of advanced stage HNC, while high levels of beta-alanine, beta-aminobutyric acid, ethanolamine, glycine, isoleucine, 4-hydroxyproline, and phenylalanine were associated with an increased risk of advanced stage HNC. Furthermore, at multivariate analysis, increased levels of alpha-aminobutyric acid were associated with increased overall survival (OS), while high levels of arginine, ethanolamine, glycine, histidine, isoleucine, 4-hydroxyproline, leucine, lysine, 3-methylhistidine, phenylalanine, and serine were associated with decreased OS.
Conclusions
Our study suggests that AA levels are associated with the stage of disease and prognosis in patients with HNC. More study is necessary to evaluate if serum AA levels may be considered a hallmark of HNC and prove to be clinically useful markers of disease status and prognosis in HNC patients.
1. Introduction
Head and neck cancers (HNCs) are highly aggressive, multifactorial tumors affecting more than 800,000 new patients worldwide each year [1]. Tobacco and alcohol are the main risk factors for HNCs, with human papillomavirus type 16 (HPV16) being a well-established risk factor for oropharyngeal cancer [2]. Furthermore, specific nutrient deficiencies (vitamin C, folates, and carotenoids), food groups, and dietary behaviors are related to the risk for HNC [3, 4].
The majority of HNC present with advanced stage (III-IV) at the time of diagnosis [5]. Treatment involves the standard therapy options of surgery, radiotherapy, and chemotherapy, but all modalities are largely ineffective. Following diagnosis of HNC, 5-year survival varies substantially across countries. In Europe, five-year age standardized relative survival is the highest for laryngeal cancer and the poorest for hypopharyngeal cancer (59% for larynx, 45% for oral cavity, 39% for oropharynx, and 25% for hypopharynx). The risk of death is approximately 2-3 times greater among patients with stage III-IV, respectively, than those with stage I at diagnosis. While HNC can be often cured when diagnosed at an early stage, late-stage disease may be untreatable or involves aggressive multimodality treatment that often leads to severe physical and psychological disabilities [6].
Recently, metabolomics has been used to identify changes in metabolite profiles in the different stages of cancer in order to introduce new noninvasive molecular tools for staging [7].
The past decade has seen rapid advances in our understanding of the metabolic reprogramming that occurs during tumorigenesis. It is well established that tumors display different metabolic phenotypes than normal tissues [8]. Cancer cells develop an altered nutrient utilization to obtain metabolic fuels. Glucose and then glutamine are the most rapidly consumed nutrients by many cultured cancer cell lines [9]. In addition to glucose, there has also been a long-standing interest in understanding the unique amino acid (AA) requirements of cancer cells [10]. Indeed, like glucose, there are major differences in the uptake and secretion of several AAs in tumors relative to normal tissues [11]. Furthermore, it is now appreciated that AAs, rather than glucose, account for the majority of the carbon-based biomass production in rapidly proliferating cancer cells [12]. AAs also contain nitrogen and have been demonstrated to be the dominant nitrogen source for hexosamines, nucleotides, and other nitrogenous compounds in rapidly proliferating cells [13]. Because of these important roles in tumor metabolism, there continues to be significant interest in studying AA metabolism for cancer marker detection. Several investigators have reported changes in peripheral blood AAs in various types of cancer patients, including lung, ovarian, and also HNC patients [14, 15]. The only study which specifically addressed the link between the basal serum AA levels and long-term prognosis in HNC patients concluded that increased serum levels of methionine had a favourable prognostic impact in terms of overall survival (OS) and relapse-free survival, while increased serine showed borderline significance as a negative prognostic factor for OS [15].
The aim of this study was to quantitate a wider panel of serum AAs in primary HNC patients using ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) in order to provide an expanded view of the associations between increased AA levels, stage of disease, and OS.
2. Methods
Subjects with histologically confirmed primary squamous cell carcinoma of the head and neck were included. The study was approved by the Ethical Committee of the Policlinico Universitario Agostino Gemelli (protocol ID 2435). HNC tumors were classified into anatomic site according to the following ICD-0-2 categories: oral cavity, oropharynx, hypopharynx, larynx, and not specified site. The tumors were staged according to the tumor, node, metastasis (TNM) classification [16]. The recruitment was conducted from 2002 to 2012 in Rome (Institute of Otorhinolaryngology, Università Cattolica Sacro Cuore, Fondazione Policlinico Universitario Agostino Gemelli). A total of 202 patients gave their consent to have blood withdrawn and processed for research studies and also had a 5-year follow-up. The characteristics of these patients (rates of sex, tumor sites, etc.) did not significantly differ from those of the total number of cases treated in the same period.
2.1. Blood Samples
Blood samples were obtained by venipuncture and collected in 8 mL serum collection tubes Vacuette (VACUETTE® TUBE Greiner Bio-One, Kremsmünster, Austria). Every sample was taken once in each patient prior to any treatment. The blood samples were centrifuged at 2000 g at 4°C for 10 min within 60 min after collection. Serum was aliquoted and stored at −80°C until analysis.
2.2. Data Collection
Patients were interviewed face-to-face by medical doctors, on demographics and alcohol and tobacco consumption. Participants were followed from the date of diagnosis to the date of death or end of follow-up at June 2017, whichever occurred first. Death certificate data were also used for mortality, and the cause of death was coded according the International Classification of Diseases, Ninth Revision. Data on tumor pathology and treatment were obtained from pathology records.
2.3. Ultraperformance Liquid Chromatography-Mass Spectrometry (UPLC-MS)
Thirty-one AAs and derivatives (alanine, alpha-aminobutyric acid, aminoadipic acid, anserine, arginine, asparagine, beta-alanine, beta-aminobutyric acid, carnosine, citrulline, cystathionine, ethanolamine, gamma-aminobutyric acid, glycine, histidine, isoleucine, 4-hydroxyproline, leucine, lysine, methionine, 1-methylhistidine, 3-methylhistidine, phenylalanine, phosphoethanolamine, proline, sarcosine, serine, threonine, tryptophan, tyrosine, and valine) were measured in serum through an ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) validated methodology. Briefly, 50 μL of sample was mixed with 100 μL 10% (w/v) sulfosalicylic acid containing an internal standard mix (50 μM) and centrifuged at 1000 × g for 15 min. 10 μL of the supernatant was transferred into a vial containing 70 μL of borate buffer to which 20 μL of AccQ Tag reagents (Waters Corporation, Milford, MA) was subsequently added. Samples were then vortexed for 10 s and heated at 55°C for 10 min. The chromatographic separation was performed by ACQUITY H-Class (Waters Corporation) using an ACQUITY CORTECS C18 column (Waters Corporation) eluted at a flow rate of 500 μL/min with a linear gradient (9 min) from 99 to 1 water 0.1% formic acid in acetonitrile 0.1% formic acid. MS was an ACQUITY QDa single quadrupole equipped with electrospray source operating in positive mode (Waters Corporation). Analytical process was monitored using Kairos™ Amino Acid Quality Control (level 1 and level 2) manufactured by Waters.
2.4. Statistical Analysis
Descriptive statistics were conducted to describe the study participants. Categorical variables were reported as absolute frequencies and percentages, and continuous variables were reported as mean and standard deviation. Tumor stage was categorized as early stage (I and II) and advanced stage (III and IV). Smoking (and drinking) habits were categorized as never, former, and current smoker (or drinker). OS was defined as the time from the date of diagnosis to the date of death. AA concentrations were dichotomized according to cut-off points calculated by maximally selected rank statistics [17]. A multivariable logistic regression model was used to assess the association with the risk of advanced versus early stage HNC. A multivariable Cox regression model was used to evaluate the prognostic significance of each AA, including the following terms: age, sex, tumor stage, treatment type, smoking status, and alcohol-drinking status. The Kaplan-Meier method was used to plot the survival curves. Statistical analyses were conducted using Stata software (Stata Statistical Software Release 16; StataCorp LP, College Station, Texas, USA) and the R packages Survival [18] and Maxstat [19]. All tests were two sided, and a p value < 0.05 was considered as statistically significant.
3. Results
3.1. Participants' Characteristics
Participants' demographic, clinical, and behavioral characteristics are reported in Table 1. Two hundred and two participants were included in the study, with a higher prevalence of males (75.2%) and an average age of 63 years old. A higher prevalence of participants had a laryngeal tumor subsite (52.0%) and an advanced stage of tumor (64.4%). A higher prevalence of participants underwent surgery and radio- or radio/chemotherapy treatment (38.6%), followed by surgery only (30.7%) and by radio/chemotherapy or radiotherapy (30.2%). A higher prevalence of participants was categorized as former smokers (61.4%) and as current drinkers (67.3%).
Table 1.
Description of the 202 patients included in the analysis.
| Parameter | N | % |
|---|---|---|
| Age at diagnosis (mean, sd) | 63.0 | 11.4 |
| Gender | ||
| Male | 152 | 75.2 |
| Female | 50 | 24.8 |
| Missing | 0 | — |
| Tumor stage | ||
| Early | 72 | 35.6 |
| Advanced | 130 | 64.4 |
| Missing | 0 | — |
| Tumor subsite | ||
| Oral cavity | 54 | 26.7 |
| Oropharynx | 31 | 15.3 |
| Hypopharynx | 10 | 5.0 |
| Larynx | 105 | 52.0 |
| Other | 2 | 1.0 |
| Missing | 0 | — |
| Treatment type | ||
| Surgery | 62 | 30.7 |
| Chemo or radio | 61 | 30.2 |
| Surgery and radio or chemo | 78 | 38.6 |
| Missing | 1 | 0.5 |
| Smoking status | ||
| Never smoker | 32 | 15.8 |
| Former smoker | 124 | 61.4 |
| Current smoker | 38 | 18.8 |
| Missing | 8 | 4.0 |
| Alcohol drinking status | ||
| Never drinker | 47 | 23.3 |
| Former drinker | 12 | 5.9 |
| Current drinker | 136 | 67.3 |
| Missing | 7 | 3.5 |
3.2. Association between AA Serum Levels and Risk for Advanced Stage HNC
In Table 2, we reported the odds ratios (ORs) and 95% CI for the associations between the AA concentrations and the risk of advanced stage of HNC. High levels of alpha-aminobutyric acid (OR: 0.40; 95% CI: 0.16-0.98), aminoadipic acid (OR: 0.36; 95% CI: 0.17-0.78), histidine (OR: 0.40; 95% CI: 0.19-0.85), proline (OR: 0.24; 95% CI: 0.09-0.62), and tryptophan (OR: 0.41; 95% CI: 0.22-0.76) were associated with a reduced risk of advanced stage HNC, while high levels of beta-alanine (OR: 2.55; 95% CI: 1.17-5.60), beta-aminobutyric acid (OR: 1.91; 95% CI: 1.00-3.65), ethanolamine (OR: 2.25; 95% CI: 1.20-4.24), glycine (OR: 2.35; 95% CI: 1.17-4.73), isoleucine (OR: 2.39; 95% CI: 1.20-4.76), 4-hydroxyproline (OR: 3.16; 95% CI: 1.02-9.78), and phenylalanine (OR: 2.72; 95% CI: 1.42-5.21) were associated with an increased risk of advanced stage HNC.
Table 2.
Odds ratios (ORs) and 95% confidence interval (95% CI) for advanced vs. early stage of HNC.
| Amino acid | ORs (95% CI) | p value |
|---|---|---|
| Alanine | 0.47 (0.19-1.12) | 0.089 |
| Alpha-aminobutyric acid | 0.40 (0.16-0.98) | 0.045 |
| Aminoadipic acid | 0.36 (0.17-0.78) | 0.010 |
| Arginine | 0.59 (0.32-1.11) | 0.101 |
| Asparagine | 0.67 (0.37-1.23) | 0.195 |
| Beta-alanine | 2.55 (1.17-5.60) | 0.019 |
| Beta-aminobutyric acid | 1.91 (1.00-3.65) | 0.048 |
| Citrulline | 0.68 (0.36-1.27) | 0.228 |
| Ethanolamine | 2.25 (1.20-4.24) | 0.012 |
| Gamma-aminobutyric acid | nc | nc |
| Glycine | 2.35 (1.17-4.73) | 0.016 |
| Histidine | 0.40 (0.19-0.85) | 0.018 |
| Isoleucine | 2.39 (1.20-4.76) | 0.014 |
| 4-Hydroxyproline | 3.16 (1.02-9.78) | 0.047 |
| Leucine | 1.65 (0.85-3.19) | 0.141 |
| Lysine | 2.13 (0.95-4.77) | 0.066 |
| Methionine | 2.15 (0.73-6.26) | 0.163 |
| 1-Methylhistidine | 0.59 (0.32-1.11) | 0.102 |
| 3-Methylhistidine | 0.77 (0.38-1.58) | 0.473 |
| Phenylalanine | 2.72 (1.42-5.21) | 0.003 |
| Phosphoethanolamine | nc | nc |
| Proline | 0.24 (0.09-0.62) | 0.003 |
| Sarcosine | 0.43 (0.15-1.21) | 0.109 |
| Serine | 1.76 (0.95-3.26) | 0.075 |
| Threonine | 0.42 (0.17-1.05) | 0.064 |
| Tryptophan | 0.41 (0.22-0.76) | 0.005 |
| Tyrosine | 0.44 (0.13-1.46) | 0.181 |
| Valine | 1.30 (0.71-2.39) | 0.399 |
aOdds ratio adjusted for age, sex, smoking status, and alcohol drinking status. nc: not computable.
3.3. Association between AA Serum Levels and OS
In Table 3, we reported the HRs and 95% CI for the association between the AA concentrations and the OS. High levels of alpha-aminobutyric acid (HR: 0.41; 95% CI: 0.24-0.69) were associated with increased OS, while high levels of arginine (HR: 2.13; 95% CI: 1.07-4.24), ethanolamine (HR: 2.28; 95% CI: 1.07-4.84), glycine (HR: 2.49; 95% CI: 1.17-5.32), histidine (HR: 2.98; 95% CI: 1.44-6.19), isoleucine (HR: 3.08; 95% CI: 1.49-6.40), 4-hydroxyproline (HR: 1.66; 95% CI: 1.00-2.74), leucine (HR: 2.29; 95% CI: 1.17-4.48), lysine (HR: 2.34; 95% CI: 1.19-4.60), 3-methylhistidine (HR: 1.83; 95% CI: 1.07-3.13), phenylalanine (HR: 2.06; 95% CI: 1.19-3.57), and serine (HR: 2.71; 95% CI: 1.39-5.31) (Figure 1) were associated with decreased OS.
Table 3.
Predictors of OS among 202 HNC cases by multivariate analysis.
| Amino acid | HRs (95% CI)a | p value |
|---|---|---|
| Alanine | 0.64 (0.35-1.15) | 0.135 |
| Alpha-aminobutyric acid | 0.41 (0.24-0.69) | 0.001 |
| Aminoadipic acid | 1.13 (0.65-1.94) | 0.671 |
| Arginine | 2.13 (1.07-4.24) | 0.030 |
| Asparagine | 2.76 (0.99-7.74) | 0.053 |
| Beta-alanine | 0.70 (0.35-1.40) | 0.309 |
| Beta-aminobutyric acid | 1.04 (0.63-1.72) | 0.882 |
| Citrulline | 0.65 (0.39-1.08) | 0.099 |
| Ethanolamine | 2.28 (1.07-4.84) | 0.032 |
| Gamma-aminobutyric acid | nc | nc |
| Glycine | 2.49 (1.17-5.32) | 0.018 |
| Histidine | 2.98 (1.44-6.19) | 0.003 |
| Isoleucine | 3.08 (1.49-6.40) | 0.002 |
| 4-Hydroxyproline | 1.66 (1.00-2.74) | 0.048 |
| Leucine | 2.29 (1.17-4.48) | 0.015 |
| Lysine | 2.34 (1.19-4.60) | 0.014 |
| Methionine | 1.40 (0.85-2.31) | 0.189 |
| 1-Methylhistidine | 0.59 (0.35-1.00) | 0.050 |
| 3-Methylhistidine | 1.83 (1.07-3.13) | 0.027 |
| Ornithine | 1.56 (0.93-2.63) | 0.091 |
| Phenylalanine | 2.06 (1.19-3.57) | 0.010 |
| Phosphoethanolamine | nc | nc |
| Proline | 1.63 (0.92-2.88) | 0.092 |
| Sarcosine | 1.54 (0.91-2.62) | 0.107 |
| Serine | 2.71 (1.39-5.31) | 0.004 |
| Threonine | 0.67 (0.41-1.11) | 0.121 |
| Tryptophan | 0.68 (0.39-1.19) | 0.175 |
| Tyrosine | 1.52 (0.82-2.82) | 0.187 |
| Valine | 1.30 (0.74-2.29) | 0.360 |
aHazard ratio adjusted for age, sex, stage, treatment type, smoking status, and alcohol drinking status; nc: not computable.
Figure 1.
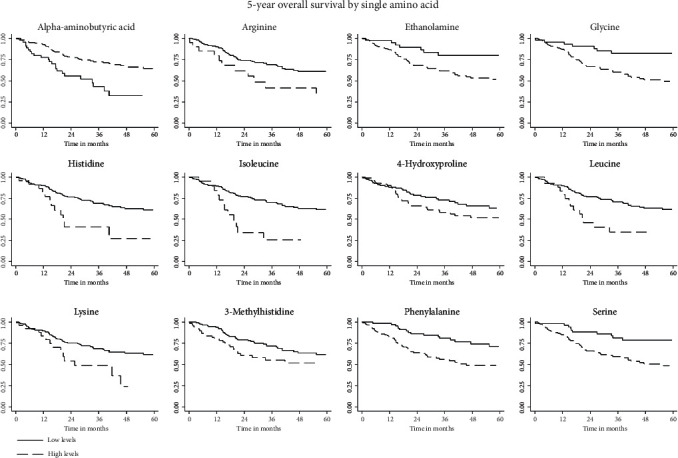
Kaplan-Meier curves for amino acids whose levels were significantly associated with OS.
3.4. Comparison of AA Levels among Tumor Subsites
In Table 4, we reported the p value obtained from the comparison of the AA levels among HNC tumor subsites. A significant difference was observed for ethanolamine levels (p = 0.026) and phenylalanine levels (p = 0.003). Post hoc comparison showed higher levels of ethanolamine in patients with oral cavity cancer compared to those with larynx (p = 0.013) cancer and higher levels of phenylalanine in patients with oral cavity cancer compared to those with hypopharynx (p = 0.029) and larynx (p = 0.002) cancers (data not shown).
Table 4.
Comparison of amino acid levels among HNC tumor subsites.
| Amino acid | p value∗ |
|---|---|
| Alanine | 0.452 |
| Alpha-aminobutyric acid | 0.116 |
| Aminoadipic acid | 0.071 |
| Arginine | 0.447 |
| Asparagine | 0.699 |
| Beta-alanine | 0.663 |
| Beta-aminobutyric acid | 0.764 |
| Citrulline | 0.474 |
| Ethanolamine | 0.026 |
| Gamma-aminobutyric acid | nc |
| Glycine | 0.075 |
| Histidine | 0.505 |
| Isoleucine | 0.483 |
| 4-Hydroxyproline | 0.585 |
| Leucine | 0.346 |
| Lysine | 0.380 |
| Methionine | 0.302 |
| 1-Methylhistidine | 0.300 |
| 3-Methylhistidine | 0.953 |
| Phenylalanine | 0.003 |
| Phosphoethanolamine | nc |
| Proline | 0.698 |
| Sarcosine | 0.712 |
| Serine | 0.087 |
| Threonine | 0.306 |
| Tryptophan | 0.066 |
| Tyrosine | 0.740 |
| Valine | 0.369 |
a p value obtained from the Kruskal-Wallis test; nc: not computable.
4. Discussion
Various factors can influence serum AA pattern in cancer, depending on the type and the stage of the disease: muscle wasting, malnutrition, systemic inflammation, and insulin resistance [20, 21]. To our knowledge, only one study has specifically addressed the link between the basal serum AA levels and long-term prognosis in HNC patients [15]. Our study was done on a larger number of patients, with a less unbalanced female/male patient ratio, measuring a wider panel of serum AAs and evaluating the impact of AA profiles on both the OS and the risk of advanced stage. In our group of patients with HNC, we found that higher serum levels of alpha-aminobutyric acid, aminoadipic acid, histidine, proline, and tryptophan were associated with a reduced risk of advanced stage HNC, while high levels of beta-alanine, beta-aminobutyric acid, ethanolamine, glycine, isoleucine, 4-hydroxyproline, and phenylalanine were associated with an increased risk of advanced stage HNC. Moreover, at multivariate analysis, increased levels of alpha-aminobutyric acid were associated with increased OS, while high levels of arginine, ethanolamine, glycine, histidine, isoleucine, 4-hydroxyproline, leucine, lysine, 3-methylhistidine, phenylalanine, and serine were associated with decreased OS.
It has been suggested that increased muscle breakdown in cancer provides substrates for enhanced gluconeogenesis in the liver and enhanced branched-chain amino acid (BCAA) oxidation in muscle, and plasma levels of these AAs might fluctuate with the stage of tumor [22]. Elevated levels of BCAA in plasma have been reported in different types of cancer [23] and have been associated with insulin resistance [20, 24], which is a recognized feature in cancer patients, particularly in late stages with cachexia. In our patients, higher levels of BCAA reflected both the risk of a more severe stage of HNC (isoleucine) and a lower OS (leucine and isoleucine).
It is interesting to note that in our patients we found high levels of aminoadipic acid in the serum of patients with reduced risk of advanced stage of HNC. Aminoadipic acid is a poorly characterized product of lysine degradation, and it may appear in the circulation from degradation of whole tissue or plasma proteins. It has been described experimentally that plasma aminoadipic acid has a role in modulating glucose levels, being augmented as a compensatory response to hyperglycemia, probably by upregulating insulin secretion in early insulin resistance [25].
Serum phenylalanine was highly predictive in our group for both risks of advanced stage and decreased OS. It is generally accepted that phenylalanine plasma concentrations are elevated in cancer, as a reflection of increased muscle proteolysis [26]. Together with BCAA, increased levels of phenylalanine have been observed in head and neck squamous carcinoma cells from different patients [27].
Higher levels of glycine were associated in our patients with decreased OS and with increased risk of advanced stage, while higher serine was significantly associated with decreased OS and, with near-borderline significance, with increased risk of advanced stage. Glycine and serine are closely linked; biosynthesis and uptake of both AAs are usually increased in cancer cells [28], and their relation to one-carbon metabolism is a highly relevant aspect of tumor metabolism for a variety of reasons [29]. Glycine uptake and catabolism promote tumorigenesis and malignancy [30], and its biosynthesis is deemed as a central process in sustaining rapid proliferation [11, 28]; serine serves as a central hub in the metabolic network for many aspects of cancer cell survival and proliferation [28]. Higher concentrations of glycine and serine have been detected in the malignant head and neck squamous cell carcinoma tissue samples compared to surrounding normal tissues [15, 31].
Also, increased levels of 4-hydroxyproline and ethanolamine reflected the risk of a more severe stage of HNC and of a lower OS. 4-Hydroxyproline production from proline is quite critical for tumor survival by stabilizing HIF-1alpha (hypoxia-inducible factor-1 alpha) under hypoxia. Clinically, elevated HIF-1alpha levels in a number of cancers, including HNC, have been associated with aggressive tumor progression and thus implicated as a predictive and prognostic marker [32, 33]. Ethanolamine is not an AA but a primary amine and a primary alcohol [34], and its clinical significance in human blood has not been clarified [35]. Anyhow, ethanolamine has been shown to stimulate the rapid growth of mammalian cells in culture, and therefore, it has been called a growth factor [36]; it also has an important role in cell proliferation as phosphatidylethanolamine participates in the promotion of synthetic DNA [35, 36].
In our patients, higher levels of beta-alanine and beta-aminobutyric acid, both nonproteogenic beta AAs, were associated with increased risk of advanced stage of cancer. Beta-aminobutyric acid, an isomer of aminobutyric acid, is mainly known for its function in plant disease resistance; its role in human physiology is presently unclear, and its significance in the context of human cancer has yet to be clarified. Increased levels of beta-alanine have been described in oral wash samples of patients with head and neck squamous cell carcinoma compared to healthy controls [37]. Sources for beta-alanine include pyrimidine catabolism of cytosine and uracil [38].
Increased levels of arginine were associated with reduced OS. Arginine is among the AAs for which elevated tumor utilization has been established in various other processes, in addition to protein synthesis. In particular, the rapid proliferation rate of tumor cells also requires enhanced production of bioactive products of arginine metabolism, including polyamines and nitric oxide [26]; hence, modulation of the enzymes arginase and nitric oxide synthase can modulate tumor proliferation. Various malignant tumor tissues contain a considerable amount of arginase which converts arginine to ornithine and urea; ornithine is the precursor of polyamines, which are essential components of cell proliferation [39]. Nitric oxide can play variable roles in tumor growth, being potentially toxic for malignant cells, depending on the stage and biology of tumors [39–41]. Arginine metabolism is therefore highly dysregulated in cancer [42], and the prevailing route determines the fate of arginine in tumor promotion or regression; in patients with oral cancer, significantly increased serum arginase activity and nitric oxide levels have been reported [39]. Since arginine is known to be essentially required for the growth of HNC cells, arginine deprivation has been considered as a potential anticancer approach [43, 44].
Associations between high levels of histidine and lysine and reduced OS were found in our patients. Mukherji and colleagues reported that elevated levels of these AAs were more likely found in head and neck tumor tissue compared to normal tissue [45]. Increased serum levels of lysine have been reported in patients with HNC compared to controls [46]. In our study, high lysine also approached borderline significance for increased risk of advanced stage HNC. With regard to the stage of HNC, the result for histidine appeared at variance with that on OS, and this aspect may deserve further investigation.
Serum 3-methylhistidine levels were higher in our patients with reduced OS. High circulating 3-methylhistidine is considered generally as a marker of muscle proteolysis, and increased levels have been proposed as biomarkers of frailty [47]. Significantly higher levels of serum 3-methylhistidine have been described in patients with HNC compared to healthy subjects [46].
A limitation of our study is that there was no control group of healthy individuals to compare the AA levels against. However, this should not alter the reliability of the described results, as these address, within the whole pool of patient measurements, differences in AA values associated with differences in the stage of disease and in overall survival.
5. Conclusion
The significance of our study stems from the scarceness of knowledge regarding changes in serum AAs associated with the stage of disease and prognosis in HNC. We showed some findings which may be generically related to the information provided by previous clinical and experimental studies and totally new findings which deserve deeper assessment. Deeper assessment is also needed to specifically characterize serum AA profiles in HNC patients and their relationship with underlying metabolic changes and already available predictive biomarkers. Characterizations should even address specific landmarks for tumor type, disease stage, prognosis, and the implications for nutritional intervention and other treatments.
Data Availability
The data could be obtained by contacting the corresponding author.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A., Karamouzis M. V., Raben D., Ferris R. L. Head and neck cancer. The Lancet. 2008;371(9625):1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leoncini E., Edefonti V., Hashibe M., et al. Carotenoid intake and head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. European Journal of Epidemiology. 2016;31(4):369–383. doi: 10.1007/s10654-015-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almadori G., Bussu F., Galli J., et al. Serum levels of folate, homocysteine, and vitamin B12 in head and neck squamous cell carcinoma and in laryngeal leukoplakia. Cancer. 2005;103(2):284–292. doi: 10.1002/cncr.20772. [DOI] [PubMed] [Google Scholar]
- 5.Leoncini E., Vukovic V., Cadoni G., et al. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. European Journal of Epidemiology. 2018;33(12):1205–1218. doi: 10.1007/s10654-018-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahão R., Anantharaman D., Gaborieau V., et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: the ARCAGE study. International Journal of Cancer. 2018;143(1):32–44. doi: 10.1002/ijc.31294. [DOI] [PubMed] [Google Scholar]
- 7.Yonezawa K., Nishiumi S., Kitamoto-Matsuda J., et al. Serum and tissue metabolomics of head and neck cancer. CANCER GENOMICS and PROTEOMICS. 2013;10(5):233–238. [PubMed] [Google Scholar]
- 8.Pavlova N. N., Thompson C. B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukey M. J., Greene K. S., Erickson J. W., Wilson K. F., Cerione R. A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nature Communications. 2016;7(1):1–14. doi: 10.1038/ncomms11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122(3168):501–504. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 11.Jain M., Nilsson R., Sharma S., et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosios A. M., Hecht V. C., Danai L. V., et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Developmental Cell. 2016;36(5):540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Z., Vosseller K. O-GlcNAc in cancer biology. Amino Acids. 2013;45(4):719–733. doi: 10.1007/s00726-013-1543-8. [DOI] [PubMed] [Google Scholar]
- 14.Shingyoji M., Iizasa T., Higashiyama M., et al. The significance and robustness of a plasma free amino acid (PFAA) profile-based multiplex function for detecting lung cancer. BMC Cancer. 2013;13(1) doi: 10.1186/1471-2407-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vsiansky V., Svobodova M., Gumulec J., et al. Prognostic significance of serum free amino acids in head and neck cancers. Cells. 2019;8(5):p. 428. doi: 10.3390/cells8050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brierley J. D., Gospodarowicz M. K., Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2017. [Google Scholar]
- 17.Lausen B., Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48(1):73–85. doi: 10.2307/2532740. [DOI] [Google Scholar]
- 18.Therneau T. M., Grambsch P. M. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [DOI] [Google Scholar]
- 19.Hothorn T., Lausen B. On the exact distribution of maximally selected rank statistics. Computational Statistics and Data Analysis. 2003;43(2):121–137. doi: 10.1016/S0167-9473(02)00225-6. [DOI] [Google Scholar]
- 20.Bi X., Henry C. J. Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutrition & Diabetes. 2017;7(3):p. e249. doi: 10.1038/nutd.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scioscia K. A., Snyderman C. H., Wagner R. Altered serum amino acid profiles in head and neck cancer. Nutrition and Cancer. 1998;30(2):144–147. doi: 10.1080/01635589809514654. [DOI] [PubMed] [Google Scholar]
- 22.van der Meij B. S., Teleni L., Engelen M. P. K. J., Deutz N. E. P. Amino acid kinetics and the response to nutrition in patients with cancer. International Journal of Radiation Biology. 2019;95(4):480–492. doi: 10.1080/09553002.2018.1466209. [DOI] [PubMed] [Google Scholar]
- 23.Siddik M. A. B., Shin A. C. Recent progress on branched-chain amino acids in obesity, diabetes, and beyond. Endocrinology and Metabolism. 2019;34(3):234–246. doi: 10.3803/EnM.2019.34.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8(7):p. 405. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T. J., Ngo D., Psychogios N., et al. 2-Aminoadipic acid is a biomarker for diabetes risk. Journal of Clinical Investigation. 2013;123(10):4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cynober L. A. Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition. CRC Press; 2004. [Google Scholar]
- 27.Tripathi P., Kamarajan P., Somashekar B. S., et al. Delineating metabolic signatures of head and neck squamous cell carcinoma: phospholipase A2, a potential therapeutic target. The International Journal of Biochemestry and Cell Biology. 2012;44(11):1852–1861. doi: 10.1016/j.biocel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuvalov O., Petukhov A., Daks A., Fedorova O., Vasileva E., Barlev N. A. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017;8(14):23955–23977. doi: 10.18632/oncotarget.15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi B.-H., Coloff J. L. The diverse functions of non-essential amino acids in cancer. Cancers (Basel) 2019;11(5):p. 675. doi: 10.3390/cancers11050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locasale J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature Reviews Cancer. 2013;13(8):572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leme I., Portari G., Padovan G., Rosa F. T., Mello-Filho F. V., Marchini J. S. Amino acids in squamous cell carcinomas and adjacent normal tissues from patients with larynx and oral cavity lesions. Clinics. 2012;67(10):1225–1227. doi: 10.6061/clinics/2012(10)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L., Zeng J., Geng P., et al. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clinical Cancer Research. 2018;24(2):474–485. doi: 10.1158/1078-0432.CCR-17-1707. [DOI] [PubMed] [Google Scholar]
- 33.Aebersold D. M., Burri P., Beer K. T., et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Research. 2001;61(7):2911–2916. [PubMed] [Google Scholar]
- 34.Dereziński P., Klupczynska A., Sawicki W., Pałka J. A., Kokot Z. J. Amino acid profiles of serum and urine in search for prostate cancer biomarkers: a pilot study. International Journal of Medical Sciences. 2017;14(1):1–12. doi: 10.7150/ijms.15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta E., Hokazono E., Tateishi T., Kawano M., Kayamori Y. Development of an enzymatic assay for ethanolamine in plasma. International Journal of Analytical Bio-Science. 2016;4(4):110–116. [Google Scholar]
- 36.Patel D., Witt S. N. Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxidative Medicine and Cellular Longevity. 2017;2017:18. doi: 10.1155/2017/4829180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee P. K., Funchain P., Retuerto M., et al. Metabolomic analysis identifies differentially produced oral metabolites, including the oncometabolite 2-hydroxyglutarate, in patients with head and neck squamous cell carcinoma. BBA Clinical. 2017;7:8–15. doi: 10.1016/j.bbacli.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehninger A. L., Nelson D. L., Cox M. M. In: Lehninger principles of biochemistry. Freeman W. H., editor. Macmillan; 2005. [Google Scholar]
- 39.Prachi M. S., Iyer C. M. Evaluation of nitric oxide levels and arginase activity in oral cancer patients. International Journal of Current Research and Review. 2016;8(11):34–37. [Google Scholar]
- 40.Buijs N., van Bokhorst-de van der Schueren M. A. E., Langius J. A. E., et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. American Journal of Clinical Nutrition. 2010;92(5):1151–1156. doi: 10.3945/ajcn.2010.29532. [DOI] [PubMed] [Google Scholar]
- 41.Wu G., Bazer F. W., Burghardt R. C., et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40(4):1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S.-H., Roszik J., Grimm E. A., Ekmekcioglu S. Impact of L-arginine metabolism on immune response and anticancer immunotherapy. Frontiers in Oncology. 2018;8 doi: 10.3389/fonc.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C.-C., Tsai S.-T., Kuo C.-C., et al. Arginine deprivation as a new treatment strategy for head and neck cancer. Oral Oncology. 2012;48(12):1227–1235. doi: 10.1016/j.oraloncology.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Patil M. D., Bhaumik J., Babykutty S., Banerjee U. C., Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35(38):4957–4972. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherji S. K., Schiro S., Castillo M., Kwock L., Muller K. E., Blackstock W. Proton MR spectroscopy of squamous cell carcinoma of the extracranial head and neck: in vitro and in vivo studies. AJNR American Journal of Neuroradiology. 1997;18(6):1057–1072. [PMC free article] [PubMed] [Google Scholar]
- 46.Cobo Dols M., Domínguez López M., Ramírez Plaza C., et al. Specific alterations in the serum amino acid profile of patients with lung cancer and head and neck cancer. Oncología (Barcelona) 2006;29(7):283–290. doi: 10.4321/s0378-48352006000700002. [DOI] [Google Scholar]
- 47.Kochlik B., Stuetz W., Pérès K., et al. Associations of plasma 3-methylhistidine with frailty status in French cohorts of the FRAILOMIC initiative. Journal of Clinical Medicine. 2019;8(7):p. 1010. doi: 10.3390/jcm8071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data could be obtained by contacting the corresponding author.


