Key Points
Transplant recipients with low-risk TERS score have better OS and lower NRM than patients with high-risk TERS score.
Psychosocial TERS score impact on survival is independent of disease risk and Hematopoietic Cell Transplantation–Specific Comorbidity Index.
Abstract
To evaluate the impact of psychosocial risks on post–hematopoietic stem cell transplantation (HSCT) outcomes, we prospectively conducted psychosocial assessment of 556 consecutive allogeneic HSCT patients who received their first allogeneic transplant at our center between 2003 and 2017. The Transplant Evaluation Rating Scale (TERS) score was prospectively assessed by a psychologist before transplantation, and patients were categorized as low, intermediate, or high risk based on their TERS score. Patients in the high-risk TERS group had significantly longer hospital stays during the first 180 days and 1 year post–allogeneic HSCT compared with the low-risk group (16 vs 13 and 21 vs 16 days; P = .05 and .02, respectively). The survival estimates for low-, intermediate-, and high-risk TERS groups at 3 year were as follows: overall survival (OS), 73%, 60%, and 65%; disease-free survival (DFS), 63%, 55%, and 60%; nonrelapse mortality (NRM), 11%, 20%, and 17%; and relapse, 26%, 25%, and 23%, respectively. In a multivariable analysis, intermediate- and high-risk TERS scores predicted for inferior OS, similar DFS, and higher NRM compared with low-risk TERS score. In a subset analysis of patients with low/intermediate risk per Disease Risk Index, multivariable analysis showed that high- and intermediate-risk TERS scores predicted for significantly worse OS, worse DFS, higher NRM, and similar relapse rates compared with low-risk TERS score. Our findings show that psychosocial factors as measured by TERS score are strong predictors of morbidity and mortality after HSCT among patients with low/intermediate disease risk.
Visual Abstract
Introduction
Although psychological and social factors are recognized as being important in the evaluation of patients for hematopoietic stem cell transplantation (HSCT), the significance of these psychosocial factors on objective outcome variables has yet to be determined. In HSCT programs, psychosocial evaluations have been modeled after those completed in solid organ transplantation. In solid organ transplantation, psychosocial assessments have been integrated into the selection of appropriate candidates. The focus of psychosocial evaluations in solid organ transplantation are to provide better understanding of the transplantation process, identify potential risk factors, develop a plan for high-risk patients, and obtain a baseline of the patient’s neuropsychiatric and cognitive functioning.
Several studies have been performed to analyze the challenges and variations in psychosocial assessment before solid organ transplantation.1 The goal of psychosocial assessment is to identify risk factors that place patients at higher risk for negative outcomes and help focus psychosocial intervention pre- and posttransplantation to minimize such risk. For this purpose, screening tools have been developed, such as the Psychological Assessment of Candidates for Transplantation (PACT) and Transplant Evaluation Rating Scale (TERS).2 These tools assess areas such as family support and availability, history of psychiatric problems, chemical dependency, knowledge about transplantation, and compliance. TERS has become a common tool to identify psychosocial distress. Studies have examined the potential impact of psychosocial functioning as identified by TERS on quality of life and overall survival (OS). The TERS score has been found to have significant interrater reliability, and significant correlations have been found between TERS score and rating of outcome.2-5
Psychosocial issues found pre-HSCT are only important if they correlate with outcome. As with solid organ transplantation programs, psychosocial evaluations are routinely performed before transplantation in almost all HSCT programs. Variations in assessment procedures and goals also depend on which instruments are used and who conducts the assessment (social worker, RN, psychologist). At most centers, these evaluations are used to identify potential risk factors, develop a plan for high-risk patients, and educate the patient and caregivers on what to expect during the transplantation process.
A survey of bone marrow (BM) transplantation (BMT) professionals found that despite psychosocial concerns, many BMT professionals would still recommend proceeding with HSCT.6 The main reasons for not proceeding with BMT in this survey were suicidal ideation (86.8%), use of addictive illicit drugs (81.7%), history of noncompliance (80.5%), no caregiver (69.3%), alcoholism (64.8%), and mild dementia/Alzheimer’s (64.4%). Given the results of this survey, certain programs will move forward with HSCT in patients regardless of the presence of psychosocial issues. This is due in part to the unknown significance of psychosocial factors on outcome. Among HSCT patients, several variables across some but not all studies have been found to be associated with better OS, including nonsmoking status and being better adjusted, less depressed, and more educated before initiating BMT. Other studies have documented that patient coping style, affective functioning, compliance, and psychosocial support have an impact on survival in BMT patients.4,7-10
Both PACT and TERS are being used in the assessment of HSCT recipients.11,12 PACT score was recently analyzed among adult HSCT patients and found to be associated with nonrelapse mortality (NRM) in multivariate analysis models that included patient and disease factors but not in models that also included transplantation-related factors and performance status.13 TERS score, meanwhile, was found to be correlated with readmission rate among HSCT patients11
The purpose of this study was to prospectively evaluate consecutive allogeneic HSCT patients who received their first allogeneic transplant at our center using TERS and assessing the impact of TERS score on transplantation outcome.
Patients and methods
Objective and definition
The objective of this single-institution study was to assess the impact of psychosocial factors as measured by TERS score on outcome after allogeneic HSCT. The TERS score is a compilation of scores on 10 weighted factors14,15: psychiatric history of Axis I disorder, psychiatric history of Axis II disorder, substance abuse, health behaviors, compliance, quality of family/social support, history of coping, coping with disease and treatment, quality of affect, and mental status.
TERS is completed by a mental health provider, in this case, the health psychologist. Each factor receives a score of 1, 2, or 3 based on the level of presence of symptoms within each factor. For example, a patient who has never abused substances would receive a score of 1 in factor 3. A patient who stopped using substances when he or she became ill would receive a score of 2 in factor 3, and a patient who was still using substances would receive a score of 3 in factor 3. After each factor is scored following the clinical interview, the weight for each factor is calculated, and a total score is compiled. The lowest possible score a patient can receive on TERS is 26.5. A lower number equals fewer psychosocial risk factors. The score was prospectively assessed for all patients before transplantation. On the basis of a patient’s total score, patients were divided into 3 tertile groups: low-, intermediate-, and high-risk TERS scores.
Study population
Included in this analysis were 556 consecutive allogeneic HSCT patients who received their first allogeneic transplant using an HLA-matched related donor (MRD; n = 238), 7/8 of 8 HLA-A, -B, -C, and -DRB1 allele matched unrelated donor (MUD; n = 95), or T cell–replete haploidentical graft using posttransplantation cyclophosphamide (haplo; n = 223) at our center. The transplantations were performed consecutively between January 2003 and December 2017. Cord blood recipients were excluded, because they constituted a small number of transplantations in the identified time. Patients were analyzed in 3 separate groups based on TERS score: low, ≤29.5 (n = 199); intermediate, 30 to 37.5 (n = 181); and high, ≥38 (n = 176). All patients underwent transplantation in the outpatient setting, with admission reserved only for those experiencing complications that could not be handled in the clinic. Patients were required to have a fulltime caregiver for 3 months and stay within a 50-mile radius of the center. The local institutional review board at Northside Hospital approved this study.
Treatment regimens
Conditioning regimen intensity was assigned based on standard published criteria. Patients were defined as myeloablative conditioning (MAC) recipients if they underwent total-body irradiation at ≥8 Gy fractionated or received an oral busulfan dose >8 mg/kg or IV equivalent.16,17 Reduced-intensity conditioning (RIC) haplo transplantation regimens used included the Johns Hopkins University protocol18 or a locally developed regimen at our institution that consisted of 30mg/m2 of fludarabine on days −6 to −2, 140mg/m2 of melphalan on day −1, and 50 mg/kg of cyclophosphamide on days +3 and +4. MAC haplo transplantation was performed using 2 different regimens that were consecutively developed at our institution19,20: regimen 1: 25 mg/m2 of fludarabine IV once per day on days −6 to −2, 110 to 130 mg/m2 of busulfan IV once per day on days −7 to −4, and 14.5 mg/kg of cyclophosphamide IV once per day on days −3 and −2 and 50 mg/kg once per day on days +3 and +4; regimen 2: 30 mg/m2 of fludarabine once per day on days −7 to −5 and total-body irradiation at 1.5 Gy twice daily on days −4 to −1 (total dose, 12 Gy), with the same posttransplantation therapy as in regimen 1. Supportive care and infectious disease prophylaxis were similar among all donor types, except for extended quinolone therapy in haplo recipients for BK cystitis prophylaxis.
Covariates
TERS score was prospectively assessed and calculated for all patients before commencing a high-dose conditioning regimen by a single psychologist. Patient-, disease-, and transplantation-related variables were prospectively documented and obtained for this analysis from our comprehensive institutional database. Clinical factors examined included age (<55 or ≥55 years), sex, diagnosis (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], non-Hodgkin lymphoma [NHL]/Hodgkin disease/chronic lymphocytic leukemia [CLL], or other), donor type (MRD, MUD, or haplo), source (BM or peripheral blood stem cells), intensity (myeloablative or nonmyeloablative/RIC), Karnofsky performance score (60-80 or 90-100), year of transplantation (2003-2009, 2010-2014, or 2015-2017), Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI),21 and Disease Risk Index (DRI).22 HCT-CI was retrospectively calculated for patients undergoing transplantation before 2006.
End points
Outcomes analyzed were OS (time from transplantation to death), disease-free survival (DFS; survival without evidence of relapse of the underlying malignancy after transplantation), NRM, relapse/progression of malignancy, acute graft-versus-host disease (GVHD), and chronic GVHD. Because of the possibility of delayed onset of clinical acute GVHD after transplantation performed using RIC/nonablative regimens, cumulative incidence of acute GVHD was assessed at 6 months after transplantation. Chronic GVHD was classified as mild, moderate, or severe by 2005 National Institutes of Health consensus criteria.23 Acute and chronic GVHD were prospectively evaluated, graded, and documented by a single practitioner within the program.
Statistical analysis
The Kaplan-Meier method was used to estimate probabilities of OS and DFS. The cumulative incidences of treatment-related mortality (TRM), relapse, and acute and chronic GVHD were computed to accommodate competing risks. TRM and relapse were considered competing risks. Death was considered the competing risk for GVHD end points. Log-rank and Gray’s tests were used to compare survival probabilities and cumulative incidence probabilities, respectively. The Cox model was used to model the hazard functions of OS and DFS and cause-specific hazard functions of TRM, relapse, and GVHD end points. The following variables were considered in multivariate analysis: TERS score category (≤29.5, 30-37.5, and ≥38), conditioning intensity, age, patient sex, diagnosis (AML, ALL, NHL/Hodgkin disease/CLL, or other), stem cell source, donor type, HCT-CI (0-2 or ≥3), disease risk (low, intermediate, or high), Karnofsky performance score (60-80 or 90-100), and year of transplantation (2003-2009, 2010-2014, or 2015-2017). TERS scores were categorized by tertiles. The tertile method does not cause inflation on P values compared with outcome-dependent cut points. The tertile method was also suitable for our study so that TERS groups had sufficient sizes. TERS score category was retained in the models. Other variables were evaluated by the forward stepwise algorithm. A variable was selected if its significance was below the 5% threshold. Proportionality of selected variables was tested by including time-dependent covariate Z × log(t) in Cox models. Violation of proportionality was detected only for stem cell source in the Cox model for grade 2 to 4 acute GVHD based on the whole cohort. We used a Cox model stratified on stem cell source to model this end point. Two-sided P values were reported, and P < .05 was significant. All statistical analyses were performed by using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 556 patients with low- (n = 199), intermediate- (n = 181), and high-risk TERS scores (n = 176) who underwent allogeneic HSCT at our center between 2003 and 2017 were included in this analysis. Donor type was MRD (n = 238), 7/8 or 8/8 MUD (n = 95), or haplo (n = 223). The most common indication for transplantation was AML, followed by ALL, myelodysplastic syndrome/myeloproliferative disease, and NHL (Table 1). Patients in the high-risk TERS group were less likely to have high-risk DRI (13% vs 23% for low-risk TERS; P = .01) and less likely to have undergone transplantation in recent years (23% vs 48%; P < .001) compared with those in the low-risk TERS group. The median follow-up times for survivors were 51, 79, and 83 months for low-, intermediate-, and high-risk TERS groups, respectively.
Table 1.
Patient characteristics by TERS group (N = 556)
| TERS score, n (%) | P | |||
|---|---|---|---|---|
| ≤29.5 (n = 199) | 30-37.5 (n = 181) | ≥38 (n = 176) | ||
| Age, y | .06 | |||
| Median | 52 | 52 | 50.5 | |
| Range | 18-75 | 19-77 | 19-73 | |
| Male sex | 116 (58) | 92 (51) | 98 (56) | .34 |
| Race | .30 | |||
| White | 142 (71) | 138 (76) | 128 (73) | |
| Black/African American | 53 (27) | 36 (20) | 39 (22) | |
| Asian | 4 (2) | 7 (4) | 9 (5) | |
| Education level | .85 | |||
| High school | 42 (21) | 42 (23) | 43 (24) | |
| Associate/bachelor’s degree | 87 (44) | 81 (45) | 80 (45) | |
| Master’s/doctorate degree | 21 (11) | 18 (10) | 21 (12) | |
| Unknown | 49 (25) | 40 (22) | 32 (18) | |
| Marital status | .35 | |||
| Single | 33 (17) | 32 (18) | 41 (23) | |
| Married | 143 (72) | 130 (72) | 108 (61) | |
| Divorced/separated | 19 (9) | 14 (7) | 21 (12) | |
| Widowed | 4 (2) | 5 (3) | 6 (4) | |
| Diagnosis | .63 | |||
| AML | 78 (39) | 71 (39) | 72 (41) | |
| ALL | 32 (16) | 27 (15) | 26 (15) | |
| MDS/MPD/CML | 44 (22) | 36 (20) | 25 (14) | |
| NHL/HD/CLL | 33 (17) | 36 (20) | 37 (21) | |
| Other | 12 (6) | 11 (6) | 16 (9) | |
| Donor type | .011 | |||
| MRD | 80 (40) | 78 (43) | 80 (45) | |
| MUD | 22 (11) | 37 (20) | 36 (21) | |
| Haplo | 97 (49) | 66 (37) | 60 (34) | |
| Source | .86 | |||
| BM | 31 (16) | 29 (16) | 30 (17) | |
| PBSCs | 168 (84) | 152 (83) | 146 (82) | |
| Intensity | .52 | |||
| Myeloablative | 104 (52) | 84 (46) | 86 (49) | |
| Nonmyeloablative/RIC | 95 (52) | 97 (54) | 90 (51) | |
| Disease risk | .010 | |||
| Low | 98 (49) | 96 (53) | 83 (47) | |
| Intermediate | 52 (26) | 63 (35) | 65 (37) | |
| High | 46 (23) | 21 (12) | 23 (13) | |
| N/A | 3 (2) | 1 (1) | 5 (3) | |
| HCT-CI | .22 | |||
| 0-2 | 114 (57) | 111 (61) | 92 (52) | |
| ≥3 | 85 (43) | 70 (39) | 84 (48) | |
| KPS | .009 | |||
| 60-80 | 146 (73) | 115 (64) | 103 (59) | |
| 90-100 | 53 (27) | 66 (36) | 73 (41) | |
| Year of transplantation | <.001 | |||
| 2003-2009 | 12 (6) | 52 (29) | 63 (36) | |
| 2010-2014 | 91 (46) | 79 (44) | 72 (41) | |
| 2015-2017 | 96 (48) | 50 (28) | 41 (23) | |
| No. of survivors | 142 | 105 | 105 | |
| Survivor follow-up, mo | <.001 | |||
| Median | 51 | 79 | 83 | |
| Range | 15-181 | 14-184 | 16-182 | |
CML, chronic myeloid leukemia; HD, Hodgkin disease; KPS, Karnofsky performance score; MDS, myelodysplastic syndrome; MPS, myeloproliferative disease; PBSC, peripheral blood stem cell.
Length of stay
All patients underwent transplantation in the outpatient setting, with admission reserved for those with complications that could not be handled in the clinic. Patients in the high-risk TERS group had significantly longer hospital stays compared with those in low- and intermediate-risk TERS groups. The median lengths of hospital stay for those with low-risk TERS score were 12 (range, 0-100), 13 (range, 0-132), and 16 days (range, 0-246 days) in the first 100 and 180 days and 1 year post-HSCT, respectively. Compared with the low-risk TERS group, the length of hospital stay for the high-risk TERS group was significantly longer during the first 180 days and 1 year post-HSCT, at 16 (range, 1-32; P = .05) and 21 days (range, 1-253 days; P = .02), respectively.
Survival and incidence estimates
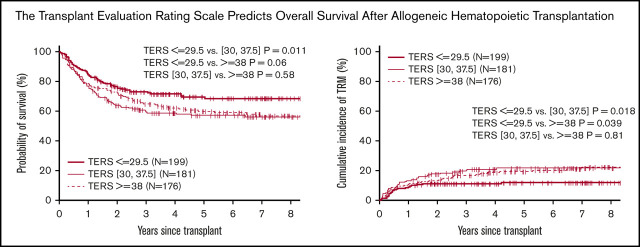
The 3-year probabilities of OS and DFS were 73% and 63% for low-, 60% and 55% for intermediate-, and 65% and 60% for high-risk TERS groups. The Kaplan-Meier estimates showed that patients in the low-risk TERS group had better OS (Figure 1) and similar DFS compared with intermediate- and high-risk TERS groups. The cumulative incidence of NRM at 3 years posttransplantation was significantly lower for the low-risk TERS group, at 11%, compared with intermediate- (20%; P = .018) and high-risk TERS groups (17%; P = .04). The relapse rate at 3 years was similar between the different TERS groups, at 26% for those with low-, 25% for those with intermediate-, and 23% for those with high-risk TERS scores (P nonsignificant for all). There was no difference in the cumulative incidence for grade 2 to 4 acute GVHD (36% vs 33% and 31%; P = .30) and moderate to severe chronic GVHD (30% vs 29% and 27%; P = .46) for low-, intermediate-, and high-risk TERS groups, respectively.
Figure 1.
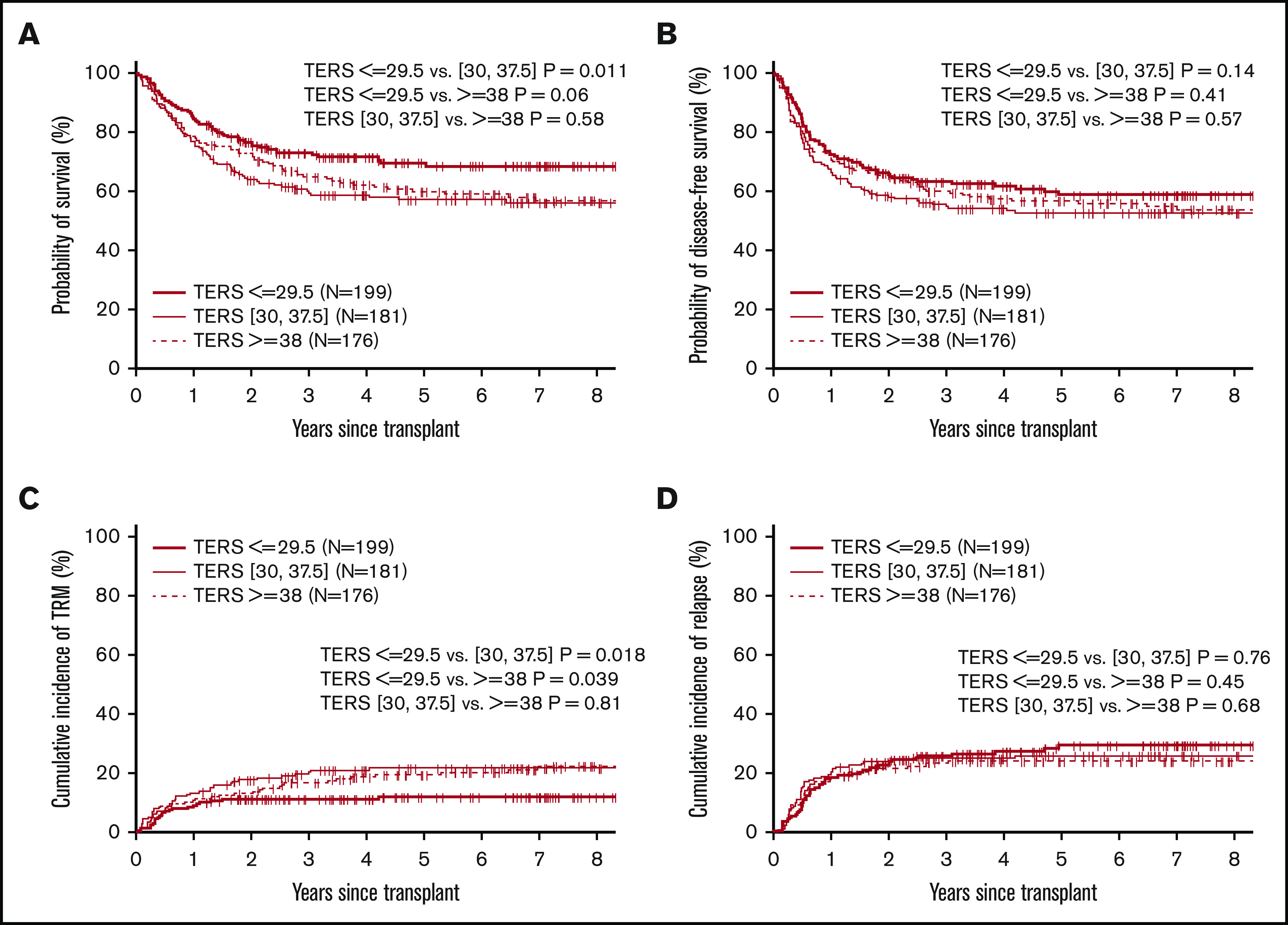
Survival endpoints by TERS score. Probability of OS (A) and DFS (B) and cumulative incidence of TRM (C) and relapse (D) by TERS score.
Among patients with low/intermediate disease risk by DRI, high-risk TERS score was associated with inferior OS and DFS rates at 1 year: 63% and 55%, compared with 83% and 75% for those with low-risk TERS score (OS, P < .01; PFS, P = .04). For patients with low HCT-CI (0-2), those in the high-risk TERS group had worse OS and TRM at 1 year: 67% and 25%, compared with 87% and 7% for those in the low-risk TERS group.
Multivariate analysis
Entire cohort.
The factors that affected OS in multivariate analysis were TERS score, age, DRI, and year of transplantation. Patients in the high-risk TERS group had higher mortality, with a hazard ratio (HR) of 1.42 (95% confidence interval [CI], 1.02-2.06; P = .05), compared with those in the low-risk TERS group (Table 2). TERS score was not associated with DFS. Factors associated with worse DFS included age (≥55 vs <55 years) and disease risk (high vs low). High-risk TERS score was significant for worse NRM (HR, 1.80; 95% CI, 1.07-3.04; P = .028) compared with low-risk TERS score. Other factors predictive of worse NRM were age (≥55 vs <55 years). Cox model for grade 2 to 4 acute GVHD and moderate to severe chronic GVHD showed that for acute GVHD, transplantation type (MUD vs MRD; HR, 2.30; 95% CI, 1.54-3.43; P < .001) and year of transplantation from 2015 to 2017 vs 2003 to 2009 (HR, 1.82; 95% CI, 1.18-3.03; P = .01) were associated with higher risk of developing acute GVHD. For moderate to severe chronic GVHD, transplantation type (haplo vs other; HR, 0.58; 95% CI, 0.40-0.83; P = .003) and later year of transplantation, from 2015 to 2017 vs 2003 to 2009 (HR, 0.55; 95% CI, 0.35-0.85; P = .008) had lower risk of chronic GVHD.
Table 2.
Cox models for OS, DFS, NRM, and relapse
| Variable | HR | 95% CI | P |
|---|---|---|---|
| OS | |||
| TERS score | |||
| 30-37.5 vs ≤29.5 | 1.50 | 1.05-2.14 | .028 |
| ≥38 vs ≤29.5 | 1.42 | 1.02-2.06 | .049 |
| Age, y | |||
| ≥55 vs <55 | 1.94 | 1.45-2.59 | <.001 |
| Disease risk | |||
| Intermediate vs low | 1.39 | 0.99-1.95 | .056 |
| High vs low | 1.68 | 1.10-2.58 | .018 |
| Year of transplantation | |||
| 2010-2014 vs 2003-2009 | 0.67 | 0.46-0.96 | .029 |
| 2015-2017 vs 2003-2009 | 0.56 | 0.35-0.88 | .011 |
| DFS | |||
| TERS score | |||
| 30-37.5 vs ≤29.5 | 1.36 | 0.99-1.87 | .055 |
| ≥38 vs ≤29.5 | 1.31 | 0.95-1.80 | .102 |
| Age, y | |||
| ≥55 vs <55 | 1.67 | 1.28-2.17 | <.001 |
| Disease risk | |||
| High vs low | 1.74 | 1.21-2.49 | .003 |
| TRM | |||
| TERS score | |||
| 30-37.5 vs ≤29.5 | 1.91 | 1.14-3.21 | .014 |
| ≥38 vs ≤29.5 | 1.80 | 1.07-3.04 | .028 |
| Age, y | |||
| ≥55 vs <55 | 1.72 | 1.15-2.56 | .008 |
| Relapse | |||
| TERS score | |||
| 30-37.5 vs ≤29.5 | 1.05 | 0.70-1.57 | .820 |
| ≥38 vs ≤29.5 | 0.99 | 0.66-1.49 | .950 |
| Diagnosis | |||
| AML vs other | 1.90 | 1.21-2.98 | .005 |
| ALL vs other | 2.33 | 1.31-4.13 | .004 |
| Disease risk | |||
| High vs low | 2.13 | 1.38-3.27 | <.001 |
| Intensity | |||
| Myeloablative vs nonmyeloablative/RIC | 0.58 | 0.40-0.84 | .004 |
Impact of TERS based on disease risk.
Among the cohort of 457 patients with low/intermediate disease risk by DRI, multivariate analysis showed that high- and intermediate-risk TERS scores were associated with lower OS, lower DFS, and higher NRM but similar relapse and GVHD rates compared with low-risk TERS score (Table 3). Patients with high-risk TERS score had worse OS (HR, 1.99; 95% CI, 1.31-3.02; P = .001), worse DFS (HR, 1.52; 95% CI, 1.05-2.20; P = .029), and higher NRM (HR, 2.40; 95% CI, 1.31-4.39; P = .005) compared with those with low-risk TERS score. Among 90 patients with high DRI status, multivariate analysis had no impact on OS, DFS, NRM, or relapse.
Table 3.
Cox models of OS, DFS, TRM, and relapse for low/intermediate disease risk and low HCT-CI 0-2 subgroups
| TERS score | HR | 95% CI | P |
|---|---|---|---|
| Low/intermediate disease risk cohort | |||
| OS | |||
| 30-37.5 vs ≤29.5 | 1.99 | 1.32-2.99 | .001 |
| ≥38 vs ≤29.5 | 1.99 | 1.31-3.02 | .001 |
| DFS | |||
| 30-37.5 vs ≤29.5 | 1.54 | 1.07-2.22 | .020 |
| ≥38 vs ≤29.5 | 1.52 | 1.05-2.20 | .029 |
| TRM | |||
| 30-37.5 vs ≤29.5 | 2.26 | 1.24-4.10 | .008 |
| ≥38 vs ≤29.5 | 2.40 | 1.31-4.39 | .005 |
| Relapse | |||
| 30-37.5 vs ≤29.5 | 1.20 | 0.76-1.91 | .44 |
| ≥38 vs ≤29.5 | 1.13 | 0.70-1.82 | .61 |
| Low HCT-CI (0-2) cohort | |||
| OS | |||
| 30-37.5 vs ≤29.5 | 1.51 | 0.92-2.48 | .107 |
| ≥38 vs ≤29.5 | 1.29 | 0.74-2.26 | .364 |
| DFS | |||
| 30-37.5 vs ≤ 29.5 | 1.51 | 0.99-2.31 | .056 |
| ≥38 vs ≤29.5 | 1.28 | 0.81-2.04 | .294 |
| TRM | |||
| 30-37.5 vs ≤29.5 | 2.93 | 1.30-6.59 | .010 |
| ≥38 vs ≤29.5 | 2.67 | 1.14-6.26 | .024 |
| Relapse | |||
| 30-37.5 vs ≤29.5 | 0.98 | 0.58-1.67 | .952 |
| ≥38 vs ≤29.5 | 0.75 | 0.41-1.36 | .338 |
Effect: high- and intermediate-risk TERS score vs low-risk TERS score. TERS score was retained in all models. In low/intermediate risk cohort, age and donor type were included in Cox models for OS, DFS, and TRM; Cox model for OS also included year of transplantation; diagnosis was included in Cox model for relapse. In HCT-CI 0-2 cohort, Cox model for OS included age, diagnosis, donor type, and year of transplantation; Cox model for DFS included age and diagnosis; Cox model for TRM included age and donor type; Cox model for relapse included diagnosis and intensity.
TERS risk score and HCT-CI.
Multivariate analysis of 317 patients with HCT-CI of 0 to 2 showed that high-risk TERS score predicted for higher TRM (HR, 2.67; 95% CI, 1.14-6.26; P = .024) but had no impact on OS, DFS, relapse, or GVHD compared with low-risk TERS score (Table 3). Among patients with high HCT-CI (n = 239), multivariate analysis showed that TERS score had no association with OS, DFS, NRM, or relapse.
TERS component distribution and outcome.
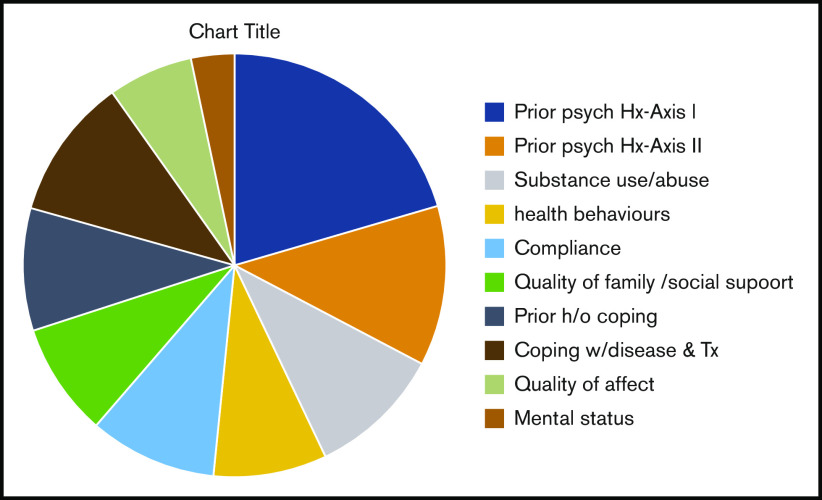
The distribution of the mean weighted score for each component of the total TERS score is shown in Figure 2. Axis I psychiatric history had the highest mean score, at 6.8 (range, 4-12), followed by Axis II psychiatric history (4.035; range, 4-8) and coping skills (3.6; range, 2.5-7.5). Individual factor analysis of the impact of each component on OS showed that poor coping skills and impairment in mental status, such as impaired cognitive function, attention, sleep-wake cycle, and/or responsiveness, were associated with worse survival (Figure 3).
Figure 2.
Pie chart of the distribution of mean weighted scores of TERS components. h/o, history of; Hx, history; Tx, treatment.
Figure 3.
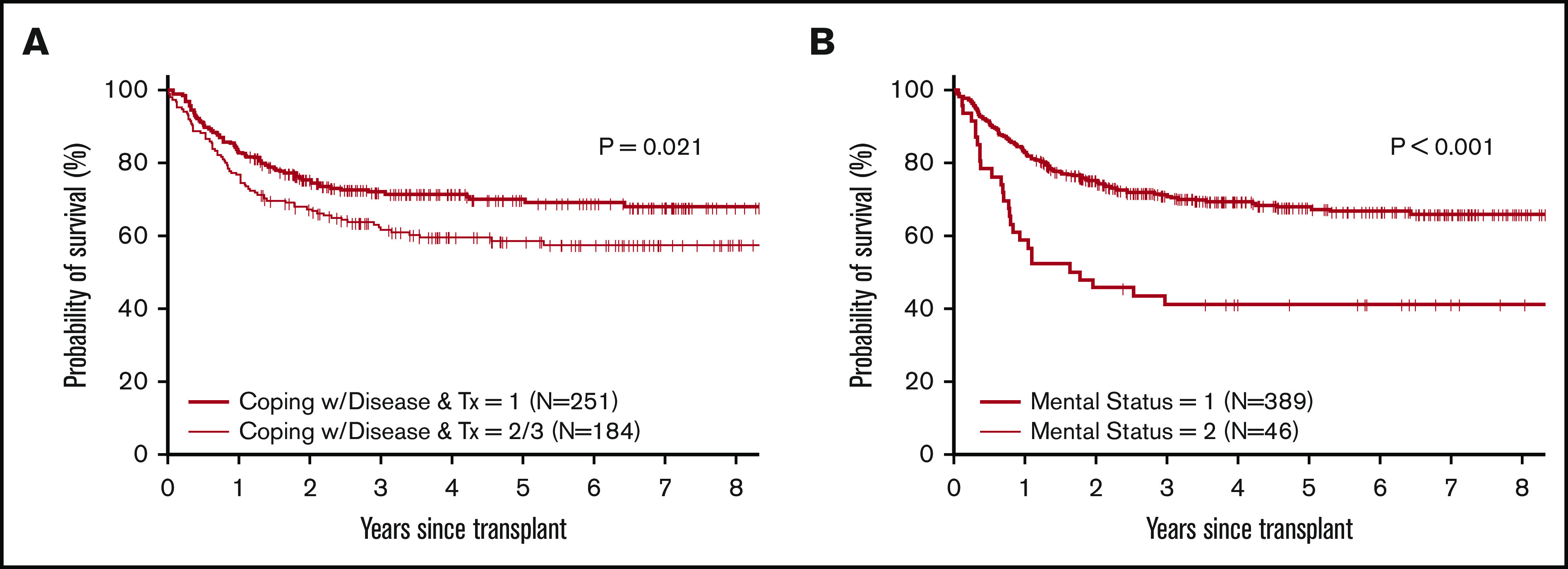
Survival by TERS components. Probability of survival based on coping with disease (A) and mental status (B).
Discussion
This large single-center analysis of 556 allogeneic HSCT patients shows that psychosocial factors as measured by TERS score are predictive of transplantation outcome. More specifically, patients with low psychosocial risk have better OS and lower NRM compared with patients with intermediate- or high-risk psychosocial TERS score. We also show that for patients without high-risk disease, as measured by DRI, higher psychosocial risks are associated with worse OS, DFS, and NRM. The impact of TERS on NRM is also seen among patients with low HCT-CI score. Patients with high-risk TERS score have longer hospital stays during the first 6 months and 1 year after HSCT. The impact of TERS seems to be limited to the low-risk groups (low/intermediate DRI and low HCT-CI), and this may limit the wide use of such scores among all patient groups. The lack of effect of TERS score in the high-risk group may have been influenced by the high mortality and relapse seen in such patients, where the increase in mortality attributed to TERS is not high enough to reach significant levels.
The importance of TERS score assessment is that it measures factors beyond the patient’s emotional wellbeing. Such factors of family and social support are usually not assessed by most comorbidity scores such as HCT-CI that are measured before transplantation. Additionally, this score investigates other factors such as compliance and adaptation skills that can help predict future behavior as patients go through the stressful months of allogeneic HSCT.15 Richardson et al11 evaluated 395 adult HSCT patients (allogeneic, n = 177; autologous, n = 218) and found that TERS score predicted hospital readmission independent of disease, comorbidities, and type of transplantation. Our data also show that, in an outpatient transplantation program, TERS score predicts length of inpatient hospital stay.
A review by Hoodin et al24 summarizing the evidence of the relation between pretransplantation emotional status and TRM revealed that most studies (10 of 15) identified emotional status to be highly linked to transplantation survival. Depression was the most frequently identified risk factor and had the most effect on posttransplantation mortality. Lower level of optimism and increased anxiety, depression, and posttraumatic symptoms in the peritransplantation period are associated with impaired white count cell recovery, and anxiety has been associated with acute GVHD.25-27 Although not all studies have established a strong relation between depression and posttransplantation impact, this correlation is believed to be well established in most recent large studies and reviews,28 as opposed to older studies, which were limited by small samples and lack of taking into account other factors that affect posttransplantation survival.29
Social effects, such as social support, socioeconomic status, optimism, coping skills, and spirituality, were investigated as predictors of mortality post-HSCT.30-33 The exact factors that lead to worse outcomes among patients with low socioeconomic status are not well defined. It is hypothesized that stress associated with low socioeconomic status can lead to altered neural, endocrine, and immune activation. Among recipients of unrelated transplants, low socioeconomic status is associated with a gene expression pattern representative of chronic adversity. This profile is predictive of increased relapse and worse leukemia-free survival.34
BMTCTN 0902 was a randomized 2 × 2 factorial study of whether an exercise and/or stress intervention vs usual care improved quality of life after HSCT.35,36 In this study, participants also reported survey data before HSCT, including Cancer and Treatment Distress, the Pittsburgh Sleep Quality Index, and the 36-Item Short Form Survey (a quality of life measure). Socioeconomic status was measured based on marital status, employment status, household income, and educational level. In a secondary analysis of BMTCTN 0902, lower socioeconomic status was associated with worse pretransplantation physical functioning and increased distress and poor sleep quality. However, neither the socioeconomic status nor the pretransplantation patient-reported outcomes were associated with the primary end point of engraftment or the secondary end points of GVHD and life outside the hospital by day 100.37 This unexpected finding could be attributed to the small sample of individuals representative of lower socioeconomic status.
A recent publication from the Cleveland clinic group assessed PACT score and its impact on transplantation outcome.13 PACT measures similar factors as TERS; however, TERS gives more consideration to coping skills and history of coping with disease and illness. In the mentioned study, PACT score was associated with NRM only in models that excluded transplantation-related factors and performance status. Additionally, TERS score encompasses more psychosocial elements than have been individually assessed in prior studies, such as socioeconomic status and race,38 psychiatric comorbidities,39,40 and caregiver availability.41 In our study, of the individual components, coping skills and mental status were both strong predictors of survival outcome.
Our study has several strong aspects, including the prospective collection of all TERS data by a single provider (this can help avoid interrater variability), similar supportive care measures, and similar algorithms for treating patients for posttransplantation complications. Moreover, intervention for distress and psychosocial challenges was addressed by the program psychologist throughout the study period. One limitation is that some patients with high-risk TERS score who were considered unfit for transplantation may have not been offered transplantation. The data for such patients are not captured in our database.
The goal of TERS in our program is to recognize risk factors to decrease risk and enable us to offer transplantation to all potential candidates. Once a patient is shown to be at high risk by TERS score, program physicians and psychologist discuss the interventions required to be able to safely offer transplantation. Interventions can include increased number of visits and counseling with program behavioral health staff, intensive outpatient programs for substance abuse, increased caregiver involvement or change of caregiver, and/or smoking cessation treatment. TERS allows us to focus on high psychosocial risk factors that can be treated or minimized before transplantation so that patients can remain candidates if their disease requires them to undergo transplantation. It is not clear that such interventions affect the outcomes of high-risk patients, given that we did not reassess TERS score after interventions took place and patients achieved acceptable goals to proceed with transplantation.
In conclusion, this study shows that psychological and social factors as measured by TERS score are predictive of survival after allogeneic HSCT. The impact is seen among patients with low/intermediate DRI and low HCT-CI. We believe such assessment should be incorporated into the survival predictive models of patients undergoing allogeneic HSCT.
Footnotes
Interim results of this study were presented orally at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
Send data sharing requests via e-mail to the corresponding author, Melhem M. Solh (msolh@bmtga.com).
Authorship
Contribution: M.M.S. performed the analysis, designed the study, and wrote the manuscript; D.S. performed the TERS assessment and approved the manuscript; S.R.S., A.B., and L.E.M. analyzed data and helped finalize the manuscript; X.Z. performed the statistical analysis and approved the manuscript; and H.K.H. designed the study, was part of the analysis, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Melhem M. Solh, Blood and Marrow Transplant Program, Northside Hospital, 5670 Peachtree Dunwoody Rd NE, Suite 1000, Atlanta, GA 30342; e-mail: msolh@bmtga.com.
References
- 1.Jowsey SG, Taylor ML, Schneekloth TD, Clark MM. Psychosocial challenges in transplantation. J Psychiatr Pract. 2001;7(6):404-414. [DOI] [PubMed] [Google Scholar]
- 2.Twillman RK, Manetto C, Wellisch DK, Wolcott DL. The Transplant Evaluation Rating Scale. A revision of the psychosocial levels system for evaluating organ transplant candidates. Psychosomatics. 1993;34(2):144-153. [PubMed] [Google Scholar]
- 3.Dieplinger G, Mokhaberi N, Wahba R, et al. Correlation between the Transplant Evaluation Rating Scale (TERS) and medical outcomes in living-donor kidney transplant recipients: a retrospective analysis [published correction appears in Transplant Proc. 2018;50(6):1922]. Transplant Proc. 2018;50(5):1276-1280. [DOI] [PubMed] [Google Scholar]
- 4.Hoodin F, Kalbfleisch KR, Thornton J, Ratanatharathorn V. Psychosocial influences on 305 adults’ survival after bone marrow transplantation; depression, smoking, and behavioral self-regulation. J Psychosom Res. 2004;57(2):145-154. [DOI] [PubMed] [Google Scholar]
- 5.Baranyi A, Krauseneck T, Rothenhäusler HB. Overall mental distress and health-related quality of life after solid-organ transplantation: results from a retrospective follow-up study. Health Qual Life Outcomes. 2013;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster LW, McLellan LJ, Rybicki LA, Dabney J, Welsh E, Bolwell BJ. Allogeneic BMT and patient eligibility based on psychosocial criteria: a survey of BMT professionals. Bone Marrow Transplant. 2006;37(2):223-228. [DOI] [PubMed] [Google Scholar]
- 7.Tschuschke V, Hertenstein B, Arnold R, Bunjes D, Denzinger R, Kaechele H. Associations between coping and survival time of adult leukemia patients receiving allogeneic bone marrow transplantation: results of a prospective study. J Psychosom Res. 2001;50(5):277-285. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigue JR, Pearman TP, Moreb J. Morbidity and mortality following bone marrow transplantation: predictive utility of pre-BMT affective functioning, compliance, and social support stability. Int J Behav Med. 1999;6(3):241-254. [DOI] [PubMed] [Google Scholar]
- 9.Broers S, Hengeveld MW, Kaptein AA, Le Cessie S, van de Loo F, de Vries T. Are pretransplant psychological variables related to survival after bone marrow transplantation? A prospective study of 123 consecutive patients. J Psychosom Res. 1998;45(4):341-351. [DOI] [PubMed] [Google Scholar]
- 10.Murphy KC, Jenkins PL, Whittaker JA. Psychosocial morbidity and survival in adult bone marrow transplant recipients—a follow-up study. Bone Marrow Transplant. 1996;18(1):199-201. [PubMed] [Google Scholar]
- 11.Richardson DR, Huang Y, McGinty HL, et al. Psychosocial risk predicts high readmission rates for hematopoietic cell transplant recipients. Bone Marrow Transplant. 2018;53(11):1418-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Presberg BA, Levenson JL, Olbrisch ME, Best AM. Rating scales for the psychosocial evaluation of organ transplant candidates. Comparison of the PACT and TERS with bone marrow transplant patients. Psychosomatics. 1995;36(5):458-461. [DOI] [PubMed] [Google Scholar]
- 13.Hong S, Rybicki L, Corrigan D, et al. Psychosocial Assessment of Candidates for Transplant (PACT) as a tool for psychological and social evaluation of allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant. 2019;54(9):1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoodin F, Kalbfleisch KR. How psychometrically sound is the transplant evaluation rating scale for bone marrow transplant recipients? Psychosomatics. 2001;42(6):490-496. [DOI] [PubMed] [Google Scholar]
- 15.Hoodin F, Kalbfleisch KR. Factor analysis and validity of the Transplant Evaluation Rating Scale in a large bone marrow transplant sample. J Psychosom Res. 2003;54(5):465-473. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859-1866. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SR, Sizemore CA, Sanacore M, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21(7):1299-1307. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 24.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38(4):255-264. [DOI] [PubMed] [Google Scholar]
- 25.McGregor BA, Syrjala KL, Dolan ED, Langer SL, Redman M. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav Immun. 2013;30(suppl):S142-S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight JM, Szabo A, Zhao S, et al. Circulating endocannabinoids during hematopoietic stem cell transplantation: a pilot study. Neurobiol Stress. 2015;2:44-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregurek R, Labar B, Mrsić M, et al. Anxiety as a possible predictor of acute GVHD. Bone Marrow Transplant. 1996;18(3):585-589. [PubMed] [Google Scholar]
- 28.Amonoo HL, Massey CN, Freedman ME, et al. Psychological considerations in hematopoietic stem cell transplantation. Psychosomatics. 2019;60(4):331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30(suppl):S68-S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(12):1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frick E, Motzke C, Fischer N, Busch R, Bumeder I. Is perceived social support a predictor of survival for patients undergoing autologous peripheral blood stem cell transplantation? Psychooncology. 2005;14(9):759-770. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Loberiza FR, Rizzo JD, Soiffer RJ, Antin JH, Weeks JC. Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389-396. [DOI] [PubMed] [Google Scholar]
- 33.Pereira DB, Christian LM, Patidar S, et al. Spiritual absence and 1-year mortality after hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2010;16(8):1171-1179. [DOI] [PubMed] [Google Scholar]
- 34.Knight JM, Rizzo JD, Logan BR, et al. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin Cancer Res. 2016;22(1):69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsen PB, Le-Rademacher J, Jim H, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer. 2016;122(1):91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight JM, Syrjala KL, Majhail NS, et al. Patient-reported outcomes and socioeconomic status as predictors of clinical outcomes after hematopoietic stem cell transplantation: a study from the Blood and Marrow Transplant Clinical Trials Network 0902 trial. Biol Blood Marrow Transplant. 2016;22(12):2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong S, Rybicki LA, Corrigan D, Schold JD, Majhail NS. Community risk score for evaluating health care disparities in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(4):877-879. [DOI] [PubMed] [Google Scholar]
- 39.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors associated with quality of life in allogeneic stem cell transplant patients prior to transplant. Psychooncology. 2014;23(6):642-649. [DOI] [PubMed] [Google Scholar]
- 40.Amonoo HL, Barclay ME, El-Jawahri A, Traeger LN, Lee SJ, Huffman JC. Positive psychological constructs and health outcomes in hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant. 2019;25(1):e5-e16. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich KB, Miller GE, Scheide T, et al. Pre-transplant emotional support is associated with longer survival after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(12):1594-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]