TO THE EDITOR:
Aggressive lymphoma arising in the setting of underlying chronic lymphocytic leukemia (CLL), known as Richter syndrome (RS), is associated with poor outcomes with current standard-of-care therapies,1 particularly among patients with clonally related disease, prior ibrutinib treatment, and complex karyotype (CK).1,2 In our institutional experience, the median overall survival following RS diagnosis was only 5.9 months, with <40% of patients achieving an objective response to anthracycline-based chemoimmunotherapy.2 Two anti-CD19 chimeric antigen receptor (CAR) T-cell (CAR-T) therapies, axicabtagene ciloleucel (axi-cel) and tisagenlecleucel, are approved by the US Food and Drug Administration (FDA) for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma. Both provide durable disease control in roughly 50% of patients, including cases of chemorefractory disease.3,4 Although anti-CD19 CAR-T therapy was first studied in CLL and achieves an antitumor response in some cases, response rates in CLL are lower compared with DLBCL, which may be explained by CLL-induced impairment in T-cell fitness and persistence.5,6 Owing in part to concerns related to CLL-induced immune dysfunction, patients with RS were excluded from the pivotal trials of axi-cel and tisagenlecleucel in DLBCL,3,4 and literature regarding the efficacy of approved CAR-T products in patients with RS is lacking. Herein, we describe the experience of our institution of treating patients with RS with commercial axi-cel.
After obtaining institutional review board approval, we identified 9 patients treated at our center for RS with axi-cel between 1 January 2019 and 15 May 2020. Baseline patient and disease characteristics along with subsequent clinical course were obtained from the electronic medical record by review of progress notes, pathology reports, and imaging studies. Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded and reported using American Society for Transplantation and Cellular Therapy (ASTCT) Consensus Grading as standard practice at our center7 and responses were assessed according to the 2014 Lugano Classification.8 The timing of response evaluation was in accordance with institutional practice: computed tomography (CT) imaging was performed ∼30 days and positron emission tomography imaging ∼90 days after axi-cel infusion. Commercial axi-cel was administered off-label.
Baseline patient characteristics (Table 1) included a median age of 64 years (range, 40-77 years) and a median of 4 (2-6) prior lines of therapy for CLL and/or RS. Eight patients had prior acalabrutinib or ibrutinib treatment: of these 8 patients who had received Bruton tyrosine kinase inhibitor (BTKi), 5 also received prior venetoclax, 1 patient had prior programmed cell death protein 1 inhibitor therapy, and 1 patient had previously received anti-CD19 CAR-T therapy in an investigational trial for CLL. Five patients were previously treated with anthracycline-based chemoimmunotherapy for RS. Five cases of RS were clonally related as assessed by IGH gene polymerase chain reaction testing; in the remaining cases, clonality was unable to be assessed. High-risk features included deletion 17p in 3 patients, CK in 6 patients, and MYC gene rearrangement in 4 patients. Molecular testing was available for 5 patients and revealed pathogenic TP53 mutations in 2 patients and a pathogenic NOTCH1 mutation in 1 patient. Four patients had progressive disease prior to leukapheresis; 5 patients had stable disease or partial response (PR) to the prior line of therapy.
Table 1.
Patient characteristics
| Pt no. | Age, y | Time: CLL Dx to RS, mo | Histology | Pertinent genetic aberrations by FISH | CK | IGHV mutational status | Clonally related | No. of prior Tx | Most recent prior line of Tx* | Time on BTKi prior to leuka-pheresis, d | Time from RT diagnosis to leuka-pheresis, d | Concurrent BTKi | Disease status at leuka-pheresis | Max CRS grade | Max ICANS grade | Grade 3/4 events | Best response (Lugano) | Time to best response, d | Follow-up, d | Clinical status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 24 | Non-GCB | Del(13q) | Yes | Unable to assess | Unable to assess | 3 | RDHAP/ RDHAX | N/A | 1111 | No | PR | 1 | 4 | No | CR | 72 | 345 | Ongoing CR |
| 2† | 46 | 38 | Non-GCB | Del(11q), MYC | Yes | Unmutated | Unable to assess | 6 | BTKi | 51 | 182 | Yes‡ | PD | 2 | 4 | No | CR | 92 | 296 | Received consolidation with MUD PBSCT, with ongoing CR |
| 3 | 64 | 197 | Non-GCB | del(13q), del(17p), MYC | Yes | Unmutated | Yes | 5 | BTKi | 24 | 50 | Yes | PR | 2 | 0 | No | CR | 93 | 210 | Ongoing CR, continues on BTKi |
| 4 | 76 | 89 | HGBCL | del(11q), del(13q), del(17p) | Yes | Unable to assess | Unable to assess | 3 | REPOCH | 3 | 119 | Yes | PD | 1 | 0 | Yes | PR | 107 | 210 | Relapsed disease at day 201 |
| 5 | 70 | 109 | Non-GCB | MYC | Yes | Unmutated | Yes | 3 | BTKi | 98 | 124 | Yes§ | PD | 4 | 3 | Yes | N/A | N/A | 49 | Died of infection |
| 6 | 72 | 36 | Non-GCB | del(11q) | Not done | Mutated | Yes | 2 | BTKi | N/A | 30 | Yes|| | SD | 2 | 1 | Yes | PR | 64 | 183 | Ongoing PR, continues on BTKi |
| 7 | 40 | 131 | Non-GCB | MYC | Not done | Mutated | Unable to assess | 5 | REPOCH | N/A | 120 | Yes¶ | SD | 2 | 0 | No | PR | 37 | 155 | Ongoing PR |
| 8 | 63 | 157 | GCB | None | Not done | Unmutated | Yes | 4 | BTKi | 120 | 143 | Yes | PD | 1 | 0 | No | CR | 81 | 86 | Ongoing CR, continues BTKi |
| 9 | 69 | 117 | HGBCL | Del (17p) | Yes | Unmutated | Yes | 5 | REPOCH | N/A | 215 | No | PR | 1 | 0 | No | CR | 92 | 93 | Ongoing CR |
BTKi, Bruton tyrosine kinase inhibitor; CR, complete response; del/Del, deletion; Dx, diagnosis; FISH, fluorescent in situ hybridization; GCB, germinal center B; HGBCL, high-grade B-cell lymphoma; IGHV, immunoglobulin heavy chain variable region; Max, maximum; MUD PBSCT, matched unrelated donor peripheral blood stem cell transplant; MYC, MYC gene rearrangement; N/A, not applicable; PD, progressive disease; PR, partial response; Pt, patient; RDHAP, rituximab, dexamethasone, cytarabine, cisplatin; RDHAX, rituximab, dexamethasone, cytarabine, oxaliplatin; REPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; SD, stable disease; Tx, treatment.
Denotes prior line of therapy immediately preceding leukapheresis.
Patient received prior CAR-T therapy for CLL before developing RS.
Stopped BTKi when patient received MUD PBSCT.
Stopped BTKi 5 days after axi-cel due to toxicity.
Started on BTKi after leukapheresis and continued.
Started on BTKi after leukapheresis stopped prior to axi-cel infusion.
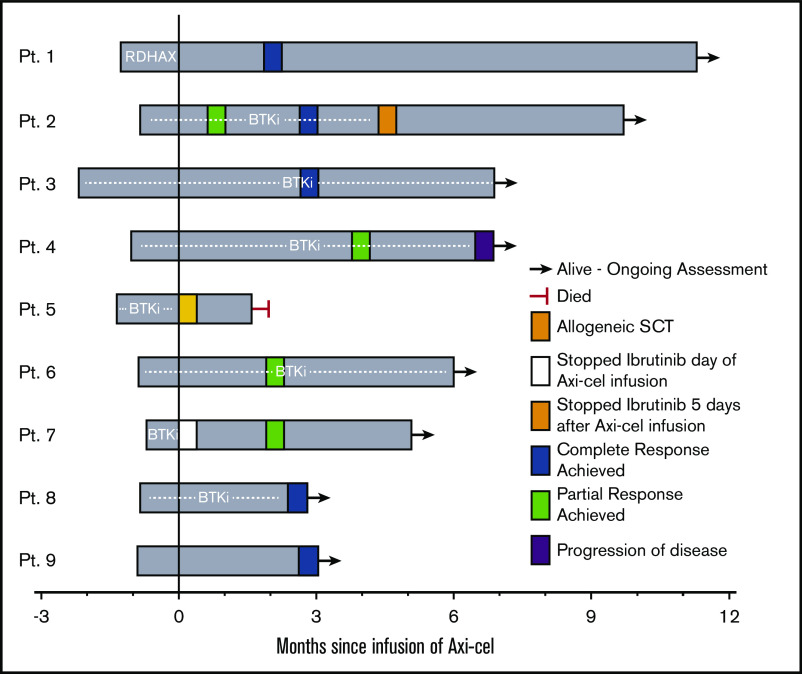
Five patients received a BTKi leading up to leukapheresis (Table 1), and 7 patients received a BTKi as bridging therapy (Figure 1). Of these 7 patients, 2 stopped BTKi shortly after axi-cel infusion (1 due to development of grade 3 ICANS [patient 5], and 1 for unknown reasons [patient 7]), the remaining 5 patients continued BTKi for at least 30 days after axi-cel infusion. One patient received rituximab, dexamethasone, cytarabine, oxaliplatin (R-DHAX) as bridging therapy; 1 patient received no bridging therapy. All patients received fludarabine and cyclophosphamide lymphodepletion at standard dosing as previously described.3 CRS occurred in all patients; CRS was grade ≤2 in 8 patients and grade 4 in 1 (patient 5) whose course is subsequently described in detail. Grade ≥3 ICANS occurred in 3 patients. ICANS improved rapidly and resolved with high-dose corticosteroids in 2 patients. One patient (patient 1) with a history of secondary central nervous system (CNS) involvement by RS experienced grade 4 ICANS beginning day +6, which initially resolved with corticosteroids, but developed grade 3 ICANS on day +16 and underwent a lumbar puncture with intrathecal administration of cytarabine, hydrocortisone, and methotrexate as empiric treatment of possible CNS recurrence on day +17. Cerebrospinal fluid studies were negative for CNS involvement by lymphoma and the patient experienced resolution of ICANS symptoms following intrathecal therapy.9 Patient 5 experienced grade 3 CRS beginning on day +3 requiring repeated tocilizumab administration and developed grade 3 ICANS on day +6 requiring high-dose corticosteroids. This patient’s hospital course was complicated by Enterococcus faecalis pneumonia and bacteremia, and subsequent acute respiratory distress syndrome. The patient ultimately died due to complications of acute respiratory distress syndrome on day +49 with no formal assessment of response to therapy. Chest CT scans performed on day +31 demonstrated resolution of prior lymphadenopathy including a large (previously 6 × 4 cm) retrocrural lymph node conglomerate. On autopsy, the largest visible lymph node measured 3 cm in diameter; on histopathologic examination of the lymph node, necrotic tissue without viable lymphoma cells was seen.
Figure 1.
Clinical course and response following axi-cel infusion. Swimmer plot of patients with RS treated with axi-cel. Plot begins at time of leukapheresis. “0” time point represents day of axi-cel infusion. SCT, stem cell transplantation.
Eight patients underwent formal response assessment. All evaluable patients achieved an objective response including 5 patients with complete response (CR) and 3 patients with PR as best response (Figure 1). Response assessment was by positron emission tomography in 7 patients; 1 evaluable patient was assessed by CT scan only (patient 6, classified as PR). The median follow-up was 6 months (range, 2.8-11.3 months). One case of disease progression was observed (patient 4), with the remaining 7 patients in remission at last follow-up. One patient (patient 2) whose CLL was refractory to ibrutinib and venetoclax, and whose recent relapse with RS occurred following CAR-T on an investigational study, received a matched unrelated donor allogeneic stem cell transplantation as consolidation. Flow cytometry of the peripheral blood and bone marrow assessment was not performed routinely after CAR-T infusion, and when performed was not done in a uniform manner. Hence, CLL response to therapy could not be determined.
Data regarding the efficacy of anti-CD19 CAR-T therapy in RS are limited and with commercially available agents are very limited. A study reported by Cruz et al included 2 patients with RS treated with allogeneic (donor-derived) CAR-Ts; 1 PR was observed with progression within 2 months of treatment.10 In an early study of axi-cel at the National Cancer Institute, 1 patient with RS was included and achieved a PR but progressed after 1 month.11 Finally, Turtle et al conducted a study of the anti-CD19 CAR-T product JCAR017 in 24 patients with relapsed/refractory CLL, including 5 patients with RS. Of the 5 patients with RS, best response was CR in 2 patients, PR in 1 patient, and progressive disease in 2 patients.12
To our knowledge, this is the largest published cohort of patients with RS treated with a commercial anti-CD19 CAR-T therapy. Prior reports on the efficacy of anti-CD19 CAR-T therapy in RS, although limited by small numbers, suggest either lack of response or brief duration of response. We observed encouraging response rates with axi-cel in patients with RS, including 1 case of deepening response with ongoing follow-up; however, the short duration of follow-up limits evaluation of long-term durability of response. In our series, 7 patients received a BTKi concurrent with axi-cel therapy. Preclinical evidence supports enhanced ex vivo CAR-T expansion in CLL patients receiving BTKi treatment, and improved CAR-T engraftment and tumor clearance with BTKi continuation after CAR-T infusion in murine models; prospective clinical data support the safety of this combination.13-16 Whether concurrent BTKi treatment contributed to successful CAR-T manufacturing and/or efficacy in our study cannot be determined due to heterogeneity in this series regarding the administration of BTKi. Prospective study is warranted to determine the optimal timing of BTKi treatment in the context of CAR-T therapy for RS and to develop strategies to mitigate potential toxicity. In conclusion, further study is warranted to evaluate the efficacy of anti-CD19 CAR-T treatment in RS given these promising results and the lack of effective standard therapies.
Acknowledgment:
The authors thank the patients and their families as well as the nurses, pharmacists, advance practitioners, and other support staff from The Ohio State University James Comprehensive Cancer Center who provided patient care.
Footnotes
Data-sharing requests may be e-mailed to the corresponding author, David A. Bond, at david.bond@osumc.edu.
Contribution: A.S.K., D.A.B., and S.M.J. collected and interpreted data; A.S.K., D.A.B., B.W., A.S., S.P., Y.E., K.L., S.A.W., H.K.C., B.B., S.V., J.B., P.S., M.L., A.M., L.O., S.A.B., K.A.R., J.A.W., J.C.B., and S.M.J. provided patient care; A.S.K., D.A.B., J.A.W., and S.M.J. were involved in the conception and design of this study; A.S.K., D.A.B, and S.M.J. wrote the manuscript; and all authors analyzed the data, edited and revised the manuscript, provided critical intellectual content, and approved the revised manuscript.
Conflict-of-interest disclosure: D.A.B. received travel funding from Novartis and receives research funding from Bristol-Myers Squibb. B.W. receives research support from Incyte, Dova, and Merck; consulted for Guidepoint Global and Techspert; and is on the advisory board for Kyowa Kirin. A.S. receives research support from Amgen, Kadmon, and OrcaBio, and consulted for Magenta Therapeutics. B.B. receives research support from Karyopharm Therapeutics and Cell Therapeutics, and is on the advisory board for Novartis, Astellas, Kite, and Cell Therapeutics. S.V. participated in the advisory board of Omeros Therapeutics, and receives research support from Kiadis Inc. P.S. receives research support from Seattle Genetics via contractor company Leidos Biomedical Research Inc. A.M. consulted for AbbVie, Agios, Astellas, Jazz, and PTC Therapeutics. L.O. is the vice president of the International Society of Cell and Gene Therapy, and is a member of their board of directors. S.A.B. participated in advisory boards for Pharmacyclics and Jansen. K.A.R. receives research funding from Genentech, AbbVie, and Janssen; participated in advisory boards for Acerta Pharma, AstraZeneca, and Pharmacyclics; and received travel funding from AstraZeneca. J.A.W. receives research laboratory support from Loxo and AbbVie, and consulted for Pharmacyclics, Janssen, Acerta, AstraZeneca, and Arqule. J.C.B. receives research support from Janssen, Genentech, Pharmacyclics, and Acerta. S.M.J. receives research support from Novartis, Kite, Unum Therapeutics, and consulted for Novartis, Kite, Juno, and CRISPR Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: David A. Bond, The Ohio State University, 320 W 10th Ave, Columbus, OH 43210; e-mail: david.bond@osumc.edu.
References
- 1.Tsimberidou AM, O’Brien S, Khouri I, et al. . Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343-2351. [DOI] [PubMed] [Google Scholar]
- 2.Rogers KA, Huang Y, Ruppert AS, et al. . A single-institution retrospective cohort study of first-line R-EPOCH chemoimmunotherapy for Richter syndrome demonstrating complex chronic lymphocytic leukaemia karyotype as an adverse prognostic factor. Br J Haematol. 2018;180(2):259-266. [DOI] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 5.Fraietta JA, Lacey SF, Orlando EJ, et al. . Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Bruggen JAC, Martens AWJ, Fraietta JA, et al. . Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8+ T cells and impede CAR T-cell efficacy. Blood. 2019;134(1):44-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Santomasso BD, Locke FL, et al. . ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yucebay F, Maakaron J, Grana A, Jaglowski S, Roddy J. Intrathecal chemotherapy: an alternative treatment strategy to prolonged corticosteroids for severe CAR T associated neurotoxicity [abstract]. Biol Blood Marrow Transplant. 2020;26(suppl 3):S312. [Google Scholar]
- 10.Cruz CR, Micklethwaite KP, Savoldo B, et al. . Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study [published correction appears in Blood. 2014;123(21):3364]. Blood. 2013;122(17):2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Dudley ME, Kassim SH, et al. . Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turtle CJ, Hay KA, Hanafi LA, et al. . Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraietta JA, Beckwith KA, Patel PR, et al. . Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin JS, Johnstone TG, Baturevych A, et al. . Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother. 2020;43(4):107-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill SVV, Frey NV. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate [abstract] Blood. 2018;132(suppl 1). Abstract 298. [Google Scholar]
- 16.Gauthier J, Hirayama AV, Purushe J, et al. . Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]