Abstract
Objective
To investigate the risk factors of early postoperative recurrence in patients with single and small (≤3 cm) hepatitis B virus-associated primary hepatocellular carcinoma (HBV-HCC).
Methods
This retrospective study analyzed patients with single and small HBV-HCC. All patients were followed up for 1 year after surgery.
Results
Among 182 patients, 54 patients had early recurrence within 1 year. The recurrence group had higher proportions of men, drinking history, Child–Turcotte–Pugh (CTP) class C, patients who underwent transarterial chemoembolization (TACE), and serum alpha-fetoprotein (AFP) >10 ng/mL as well as higher gamma-glutamyl transpeptidase (GGT) levels and lower total protein (TP) and CD8+ T lymphocyte levels than the no recurrence group. Cox multivariate regression analysis demonstrated that drinking history (HR, 1.312; 95% CI, 1.042–1.652), CTP class C (HR, 1.236; 95% CI, 1.037–1.473), TACE treatment (HR, 1.241; 95% CI, 1.026–1.501), GGT (HR, 1.138; 95% CI, 1.042–1.243), TP (HR, 0.729; 95% CI, 0.555–0.957), and AFP (HR, 2.519; 95% CI, 1.343–4.726) were independently associated with early postoperative recurrence.
Conclusion
Drinking history, CTP class C, TACE, serum AFP, GGT, and TP levels were independently associated with early postoperative recurrence in patients with single and small HBV-HCC.
Keywords: Hepatitis B virus, primary hepatocellular carcinoma, recurrence, prognosis, drinking history, Child–Turcotte–Pugh, transarterial chemoembolization, alpha-fetoprotein, gamma-glutamyl transpeptidase, total protein
Introduction
A survey that was conducted in 2015 showed that there were 782,500 new cases of hepatocellular carcinoma (HCC) and 745,500 HCC-related deaths that occurred worldwide in 2012. The proportion of new-onset HCC and death in China was high, accounting for more than 50% of the global total cases.1 About 350 million people worldwide are infected with chronic hepatitis B virus (HBV).2 In China, HBV infection is the main cause of HCC. Therefore, treatment, postoperative recurrence, and survival of hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC) are hotspots and remain a top priority for researchers.3 With the improvement of imaging technology, the detection rate of primary small HCC has been increasing.4,5 Studies have shown that the recurrence rate of small HCC is still high after treatment.6,7 Previous studies have shown that risk factors affecting postoperative recurrence of HCC included tumor size, tumor with or without capsule, microvascular invasion, cirrhosis, alpha-fetoprotein (AFP) >400 μg/L, and antiviral drugs.8–11
There have been few studies on the risk factors of survival or recurrence in patients with single and small (≤3 cm) hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC). Most of the patients with single and small HCC were in the early stage, and the clinical symptoms were mild. The survival rate of these patients after treatment was acceptable. However, the occurrence of HBV-HCC is a multistep process that involves multiple genetic alterations.12,13 Early or late recurrence after primary HCC resection directly influenced the long-term prognosis.14 Therefore, it is important to explore the factors that influence postoperative recurrence in patients with single and small (≤3 cm) HBV-HCC, to provide evidence for screening of patients who are at a high-risk of recurrence and to predict clinical prognosis.
The definition of early and late recurrence after HCC surgery remains controversial. Cheng et al.15 studied 918 patients with HCC resection in the Shanghai Eastern Hepatobiliary Surgery Hospital between 2000 and 2010 in China, and recurrence within 2 years after surgery was considered to be early recurrence. However, Park et al.16 studied 356 Korean patients who underwent hepatectomy in the Samsung Medical Center between 2000 and 2003, and the results showed that the overall survival rate of patients who had recurrence within 6 months was significantly lower than for those who had recurrence after 6 months. Therefore, recurrence within 6 months was considered to be early recurrence and recurrence beyond 6 months was considered to be the late recurrence. Imamura et al.17 suggested that 1 year after surgery was the dangerous period for postoperative recurrence in HCC patients. Clinical practice guidelines recommend 2 years as the cutoff point between early and late recurrence based on the viewpoint that early recurrence (<2 years) is caused by intrahepatic metastases, and late recurrence (>2 years) is caused by multicentric occurrence.18 Because we retrospectively analyzed Asian patients with single and small (≤3 cm) HBV-HCC, early recurrence within 1 year after surgery was considered to be early recurrence. The aim of this study was to explore the risk factors affecting the postoperative recurrence within 1 year after surgery in patients with single and small (≤3 cm) HBV-HCC.
Patients and methods
Study design and patient population
Data from patients who were initially diagnosed with single and small (≤3 cm) HBV-HCC at the Department of Hepatology, Qingdao No. 6 People’s Hospital between January 2016 and February 2018 were retrospectively analyzed. The inclusion criteria were as follows: (i) patients with single and small (≤3 cm) HBV-HCC; (ii) 18 to 75 years of age; and (iii) patients received surgical treatment and were followed up for at least 1 year. The exclusion criteria were as follows: (i) patients with hepatitis C virus (HCV) or human immunodeficiency virus (HIV) infection; (ii) patients with severe heart, lung, brain, kidney insufficiency or other serious organ diseases; (iii) patients with severe mental illness; (iv) pregnant or lactating women; or (v) patients with incomplete clinical data. Diagnostic criteria for HCC in most patients was confirmed by histopathology, and some patients had a clinical diagnosis (using at least two kinds of imaging [hepatic angiography, magnetic resonance imaging, computed tomography, or liver ultrasound] that showed a definite lesion or one kind of imaging that indicated HCC with a serum AFP level ≥400 ng/mL).19
The study was approved by the ethics committee of Qingdao No. 6 People’s Hospital. The need for individual consent was waived by the committee due to the retrospective nature of the study.
Data extraction
Baseline data were collected from patients’ medical records, including age, gender, surgical method, smoking history (defined as current smokers and patients who quit smoking within the last 5 years), drinking history (defined as patients who had consumed 50 mL or more of alcohol per day for more than 1 year), family medical history, tumor size, with or without cirrhosis, Child–Turcotte–Pugh (CTP) score, and Model for End-Stage Liver Disease (MELD) score.20 Relevant laboratory indicators were also collected, including white blood cells (WBC), hemoglobin (HGB), CD8+ lymphocytes, neutrophil-to-lymphocyte ratio (NLR), alanine transaminase (ALT), aspartate amino, transferase (AST), gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), total protein (TP), serum albumin (ALB), creatinine (Cr), prothrombin time (PT), HBV-DNA, and AFP.
Treatment
In accordance with international guidelines,21 patients were recommended to receive radical treatment to remove the tumor, including resection and radiofrequency ablation (RFA). However, there were still some patients who underwent transarterial chemoembolization (TACE) as the locoregional treatment for various reasons including generally poor hepatic function and surgical contraindications, and this treatment strategy was consistent with the Chinese guidelines for HCC treatment.22,23
Follow-up and outcomes
The follow-up period was 1 year after surgery in patients with single and small HBV-HCC, and the outcome was recurrence within the first year after surgery. Serum AFP was measured and liver ultrasound was conducted every 3 to 6 months, and CT/MRI examinations were conducted if necessary. Recurrence was defined as follows: (i) new lesions that were found in the original tumor bed and in its surroundings or in other parts of the liver; or (ii) imaging findings of new lesions that were consistent with primary HCC.24,25
Statistical analyses
The Kolmogorov–Smirnov method was performed for the normality test. Quantitative data with a normal distribution were presented as the mean ± standard deviation (mean ± SD), and two groups were compared using the Student’s t-test. Data that were not normally distributed were expressed as the median (range), and the Mann–Whitney U test was used for comparisons between the two groups. Count data were expressed as the frequency and compared using the chi-squared or Fisher’s exact test. The Kaplan–Meier method was used to draw the recurrence rate curve, and the log-rank method was used to compare the difference of the recurrence rate between the two groups. Univariate and multivariate analyses (Entered method) were performed using the Cox proportional hazard model to explore the independent risk factors affecting postoperative early recurrence within 1 year in single and small HBV-HCC. SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. A two-sided P value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
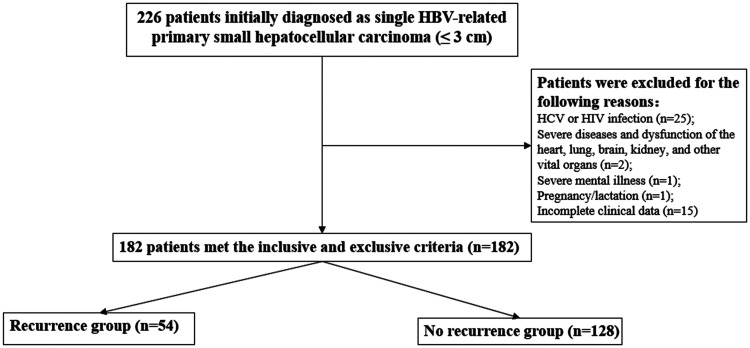
As illustrated in Figure 1, 44 patients were excluded during the screening process (25 patients had concomitant HCV or HIV infection; two patients had concomitant severe systemic diseases or other important dysfunction in organs such as heart, lung, brain, or kidney; one patient had concomitant severe mental illness; one patient was pregnant or in the lactation period; and 15 patients had incomplete clinical data). Finally, 182 patients who met the inclusion and exclusion criteria were analyzed.
Figure 1.
Flowchart of this study.
There were 182 patients with single and small HBV-HCC who met the inclusion and exclusion criteria, and they were included in the study. Among these patients, 129 patients (70.9%) were men and 53 patients (29.1%) were women. Fifty-four patients had early recurrence within 1 year, and the recurrence rate was 29.7%. The clinical characteristics between the two groups were compared, and baseline data are shown in Table 1. The average age of disease onset was 56.6 ± 9.3 years. Compared with the no recurrence group, the recurrence group had higher proportions of men (44 [81.5%] vs. 85 [66.4%], P = 0.041) or drinking history (21 [38.9%] vs. 28 [21.9%], P = 0.027). Compared with patients in the no recurrence group, the recurrence group had higher proportions of patients with CTP class C (18.5% vs. 6.3%; P = 0.038) or TACE (44.4% vs. 24.9%; P = 0.011). However, the proportion of patients with radical therapy (including RFA [15.6% vs. 7.4%] and radical resection [18.8% vs. 9.3%]; P = 0.011) was higher in the no recurrence compared with the recurrence group. TACE as the initial therapy was performed in 52 patients because they had poor hepatic function (n = 25) and surgical contraindications (n = 27).
Table 1.
Comparison of baseline data between the recurrence and no recurrence groups.
| Variables | Recurrence group (n = 54) | No recurrence group (n = 128) | P value |
|---|---|---|---|
| Age (year), mean ± SD | 55.6 ± 10.2 | 57.3 ± 8.4 | N.S. |
| Gender (male), n (%) | 44 (81.5%) | 85 (66.4%) | 0.041 |
| Drinking history, n (%) | 21 (38.9%) | 28 (21.9%) | 0.027 |
| Smoking history, n (%) | 18 (33.3%) | 36 (28.1%) | N.S. |
| Family history, n (%) | 19 (35.2%) | 38 (29.7%) | N.S. |
| CTP class, n (%) | 0.038 | ||
| A | 30 (55.6%) | 78 (60.9%) | |
| B | 14 (25.9%) | 42 (32.8%) | |
| C | 10 (18.5%) | 8 (6.3%) | |
| Combined with liver cirrhosis, n (%) | 50 (92.6%) | 116 (90.6) | N.S. |
| Portal thrombosis and/or vascular filtration, n (%) | 2 (3.7%) | 3 (2.3%) | N.S. |
| Combined with portal hypertension, n (%) | 13 (24.1%) | 34 (26.6%) | N.S. |
| Tumor size, n (%) | N.S. | ||
| ≤2 cm | 30 (55.6%) | 62 (48.4%) | |
| 2–3 cm | 24 (44.4%) | 66 (51.6%) | |
| Treatment, n (%) | 0.011 | ||
| RFA | 4 (7.4%) | 20 (15.6%) | |
| TACE | 24 (44.4%) | 28 (21.9%) | |
| RFA+TACE | 21 (38.9%) | 56 (43.7%) | |
| Resection | 5 (9.3%) | 24 (18.8%) | |
| HBV-DNA (positive), n (%) | 23 (42.6%) | 42 (32.8%) | N.S. |
| Antiviral drugs, n (%) | 26 (48.1%) | 62 (48.4%) | N.S. |
| MELD score, mean ± SD | 5.6 ± 3.7 | 5.2 ± 4.0 | N.S. |
SD, standard deviation; CTP, Child–Turcotte–Pugh; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; HBV, hepatitis B virus; MELD, model for end-stage liver disease; N.S., not significant.
Comparison of baseline serum biomarkers between the recurrence and no recurrence groups
Baseline serum biomarkers indicated that patients with early recurrence had higher GGT levels (42.5 [26.2, 80.1] vs. 33.7 [21.5, 58.2] U/L, P = 0.016), lower TP levels (67.3 [63.1, 70.2] vs. 69.3 [63.9, 75.2] g/L, P = 0.024), and a higher proportion of patients with AFP (>10 ng/mL) (32 [59.3%] vs. 46 [35.9%], P = 0.004) compared with those with no recurrence. Compared with patients in the no recurrence group, the levels of CD8+ T lymphocytes in the recurrence group were lower than that in the no recurrence group, and the difference was statistically significant (250.2±140.7 vs. 312.4±213.1, P = 0.022). The results are shown in Table 2.
Table 2.
Comparison of baseline serum biomarkers between the recurrence and no recurrence groups.
| Variables | Recurrence group (n = 54) | No recurrence group (n = 128) | P value |
|---|---|---|---|
| AFP (>10 ng/mL), n (%) | 32 (59.3%) | 46 (35.9%) | 0.004 |
| WBC (×109/L), mean ± SD | 4.2 ± 2.2 | 4.3 ± 2.1 | N.S. |
| HGB (g/L), mean ± SD | 132 ± 24.7 | 125.8 ± 23.8 | N.S. |
| ALT (U/L), median (range) | 30.2 (20.7, 46.3) | 28.3 (18.5, 42.1) | N.S. |
| TBil (μmol/L), median (range) | 17.0 (12.3, 25.8) | 17.1 (12.4, 25.7) | N.S. |
| GGT (U/L), median (range) | 42.5 (26.2, 80.1) | 33.7 (21.5, 58.2) | 0.016 |
| TP (g/L), median (range) | 67.3 (63.1, 70.2) | 69.3 (63.9, 75.2) | 0.024 |
| ALB (g/L), median (range) | 36.5 (31.5, 41.6) | 38.6 (32.2, 42.3) | N.S. |
| CR (μmol/L), mean ± SD | 70.8 ± 15.9 | 68.4 ± 16.2 | N.S. |
| PT (s), mean ± SD | 13.7 ± 3.0 | 13.4 ± 2.5 | N.S. |
| NLR, median (range) | 2.0 (0.7, 6.0) | 1.9 (0.6, 5.8) | N.S. |
| CD8+ T lymphocytes, mean ± SD | 250.2 ± 140.7 | 312.4 ± 213.1 | 0.022 |
AFP, α-fetoprotein; WBC, white blood cells; HGB, hemoglobin; NLR, neutrophil-lymphocyte ratio; ALT, alanine transaminase; TBil, total bilirubin; GGT, γ-glutamyl transpeptidase; TP, total protein; ALB, albumin; CR, creatinine; PT, prothrombin time; NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation; N.S., not significant.
Risk factors of early postoperative recurrence in patients with single and small HBV-HCC
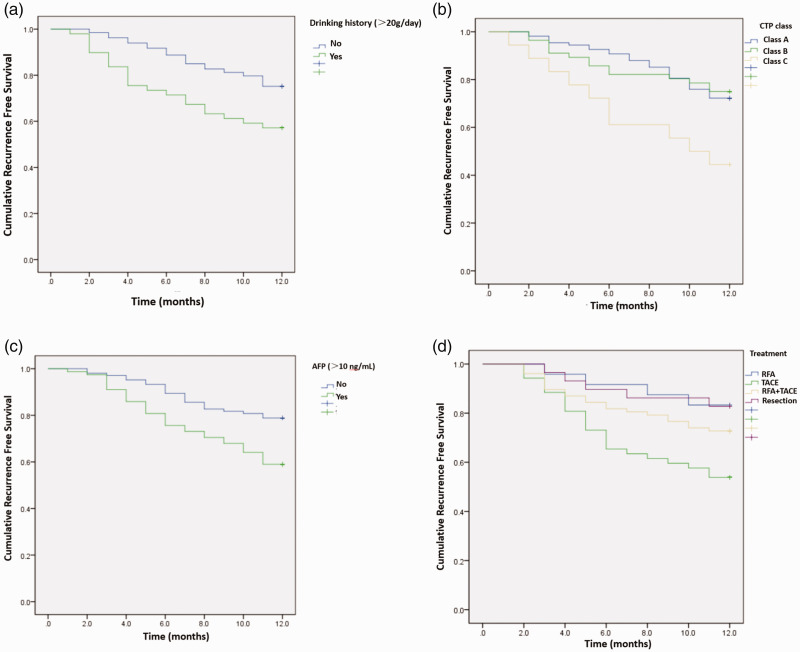
The recurrence rate in patients with and without a drinking history were 42.9% and 24.8%, respectively. Patients with a drinking history had a higher recurrence rate than those without a drinking history (P = 0.007, log-rank test, Figure 2a). The early postoperative recurrence rate of patients with CTP class A or B was 26.8%, and the recurrence rate of patients with CTP class C was 55.6%. The recurrence rate of patients with grade C was significantly higher than that of patients with grade A or B (P = 0.011, log-rank test, Figure 2b). The recurrence rate was 41.0% in patients with AFP (>10 ng/mL) and 21.2% in patients with negative AFP. Patients with AFP >10 ng/mL had a higher early postoperative recurrence rate than those with AFP <10 ng/mL (P = 0.003, log-rank test, Figure 2c). The distributions of the therapeutic methods for patients with single and small HBV-HCC were different between the recurrence and no recurrence groups (P = 0.011). Patients with RFA had the lowest recurrence rate (16.7%) within 1 year, followed by patients with hepatectomy (17.2%), and the recurrence rate was highest in patients with TACE treatment (46.2%). The early recurrence rate of patients with TACE treatment was significantly higher than those receiving RFA, RFA plus TACE, or hepatectomy (P = 0.009, log-rank test, Figure 2d).
Figure 2.
Drinking history, CTP class, AFP levels, and treatment methods were associated with early recurrence of single and small HBV-HCC. The Kaplan–Meier method was used to draw the cumulative recurrence free survival and the differences were compared between groups using the log-rank test. (a) Drinking history, (b) CTP class, (c) AFP level, and (d) Treatment method.
CTP, Child–Turcotte–Pugh grade; AFP, α-fetoprotein; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
As shown in Table 3, Cox multivariate regression analysis demonstrated that drinking history (HR, 1.312; 95% CI, 1.042–1.652; P = 0.021), CTP class C (HR, 1.236; 95% CI, 1.037–1.473; P = 0.018), TACE treatment (HR, 1.241; 95% CI, 1.026–1.501; P = 0.026), GGT (HR, 1.138; 95% CI, 1.042–1.243; P = 0.004), TP levels (HR, 0.729; 95% CI, 0.555–0.957; P = 0.023), and AFP (HR, 2.519; 95% CI, 1.343–4.726; P = 0.004) were independently associated with early postoperative recurrence in patients with single and small HBV-HCC.
Table 3.
Risk factors for early postoperative recurrence in patients with single and small HBV-HCC.
|
Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95%CI | P |
| Gender (Female vs. male) | 0.512 | 0.272–0.964 | 0.038 | 0.319 | 0.097–1.054 | N.S. |
| Drinking history | 1.283 | 1.018–1.617 | 0.035 | 1.312 | 1.042–1.652 | 0.021 |
| CTP class | ||||||
| A or B | 1 | Reference | 1 | Reference | ||
| C | 1.167 | 1.022–1.333 | 0.023 | 1.236 | 1.037–1.473 | 0.018 |
| Combined with liver cirrhosis (yes vs no) | 1.124 | 0.981–1.288 | N.S. | 1.077 | 0.974–1.191 | N.S. |
| Treatment | ||||||
| Other methods | 1 | Reference | 1 | Reference | ||
| TACE | 1.364 | 1.052–1.768 | 0.019 | 1.241 | 1.026–1.501 | 0.026 |
| GGT | 1.113 | 1.012–1.224 | 0.027 | 1.138 | 1.042–1.243 | 0.004 |
| TP | 0.833 | 0.716–0.969 | 0.018 | 0.729 | 0.555–0.957 | 0.023 |
| NLR | 1.031 | 0.958–1.110 | N.S. | 1.121 | 0.900–1.396 | N.S. |
| AFP | 1.875 | 1.091–3.224 | 0.023 | 2.519 | 1.343–4.726 | 0.004 |
| CD8+ T lymphocytes | 0.825 | 0.703–0.968 | 0.018 | 0.726 | 0.513–1.028 | N.S. |
HR, hazard ratio; 95% CI, 95% confidence interval; CTP, Child–Turcotte–Pugh; TACE, transarterial chemoembolization; AFP, α-fetoprotein; GGT, γ-glutamyl transpeptidase; TP, total protein; NLR, neutrophil-to-lymphocyte ratio; N.S., not significant.
Discussion
The results of this study indicated that AFP, GGT, and drinking history were independent risk factors, and TP was a protective factor for early postoperative recurrence in single and small HBV-HCC patients. Our results may provide the theoretical basis for close surveillance among high-risk patients in clinical practice.
Recurrence after treatment was a key factor influencing the long-term survival of HCC patients. The influencing factors of postoperative recurrence in HBV-HCC patients included tumor factors, vascular factors around the tumor, and liver function. Hirokawa et al.26 believed that patients with early recurrence (within 6 months after hepatectomy) had a lower survival rate, and vascular invasion, indocyanine green retention rate at 15 minutes (ICGR15) ≥16% were independent risk factors for early tumor recurrence. Cheng et al.15 proposed that the potential independent risk factors for early recurrence after hepatectomy were tumor diameter >5 cm, tumor without capsule, and microvascular invasion, while cirrhosis, AFP >400 μg/L were independent associated with late recurrence. Truant et al.27 considered that portal vein invasion and tumors size >8 cm were independent risk factors for survival in HCC patients. Our study included patients with single and small HBV-HCC as the study population.
Long-term heavy drinking could directly or indirectly damage the liver, resulting in liver fibrosis or HCC. Marrero et al.28 demonstrated that both smoking and drinking could promote tumorigenesis. Chavez et al.29 revealed that long-term chronic excessive intake of ethanol could reduce the level of insulin-like growth factor 1 (IGF-1) in a mouse model, which influenced proliferation of normal hepatocytes and promoted tumorigenesis. Sakamoto et al.30 also showed that relatively high doses of alcohol were related with HCC. Our results also showed that single and small HBV-HCC patients with drinking history were more likely to have early postoperative recurrence.
Our results indicated that GGT levels were positively correlated with the early postoperative recurrence of single and small HBV-HCC, which was consistent with previously published studies.31,32 Studies have also shown that GGT levels were associated with hepatocarcinogenesis and prognosis of patients with HCC.32–34 Moreover, previous studies also proposed that alcohol intake could increase ALT and GGT levels in HCC patients independent of smoking,35 and alcohol consumption had a deleterious effect on HCC prognosis, especially in patients with hepatitis virus infection.36
TP consists of globulin and albumin. Globulin and albumin levels are both important indicators to reflect liver function. Our results indicated that TP levels were independently associated with early postoperative recurrence in single and small HBV-HCC patients, which was consistent with previous results that were reported by Deng et al.37 This suggests that the reduction of either immunoglobulin or albumin could influence the prognosis. It has been demonstrated that a low pretreatment albumin-to-globulin ratio (AGR) is a useful biomarker for identifying HCC patients who have a poor prognosis.38
AFP was a vital biomarker for detecting HCC occurrence. Many studies have demonstrated that AFP was also a biomarker for predicting the prognosis of HCC. Marubashi et al.39 believed that AFP mRNA-expressing cells were independent risk factors for postoperative recurrence in patients receiving liver transplantation. Uchino et al.40 indicated that recurrence and metastasis were more likely to occur after surgery in patients with serum AFP levels >100 μg/L. Lee et al.41 considered that AFP and Des-gamma-carboxy prothrombin (DCP) levels were influencing factors in predicting survival after TACE treatment. Lu et al.42 suggested that AFP exerted an important role in promoting HCC metastasis. This study confirmed that AFP was significantly elevated in patients with single and small HBV-HCC, and was the independent risk factor for early postoperative recurrence.
Ramzan et al.43 revealed that high densities of liver-infiltrating CD8+ T lymphocytes could contribute to tumorigenesis and tumor recurrence in HCC patients with the hepatitis C virus. A meta-analysis suggested that an increased NLR indicated a poor outcome for HCC patients.44 However, in our study, NLR and CD8+ T lymphocytes were not identified as independent risk factors in patients with single and small HBV-HCC.
This study had several limitations. First, tumor location, distance from the portal vein, and tumor pathological features were not included for further analysis. However, our study mainly focused on the clinically available serum biomarkers. Second, although many patients with HCC were treated at our hospital, our study only specifically analyzed the patients with single and small HBV-HCC, which limited the sample size. Third, the follow-up time was only 1 year, and data on late postoperative recurrence at 2 or 3 years were not evaluated. Therefore, the recurrence and survival during long-term follow-up warrants further exploration. Fourth, the radical treatment for HCC patients include RFA and surgical resection, and TACE is still regarded as a palliative option. However, some patients underwent TACE as a first-line treatment for reasons such as poor liver function or surgery contraindications, and this treatment strategy was consistent with the Chinese guidelines for HCC treatment.22 Finally, analysis of the risk factors for early postoperative recurrence (within 1 year) in patients with single and small HBV-HCC was mainly based on Qingdao and its surrounding population, which includes patients who were of Asian ethnicity, and studies on other regions and ethnic groups are required.
In conclusion, the independent risk factors for the early postoperative recurrence of single and small HBV-HCC were drinking history, AFP, GGT, and TP, providing the basis for early clinical diagnosis and prognosis evaluation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Fuhui Liu https://orcid.org/0000-0001-7524-6476
References
- 1.Harris PS, Hansen RM, Gray ME, et al. Hepatocellular carcinoma surveillance: an evidence-based approach. World J Gastroenterol 2019; 25: 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda T, Goto T, Hirotsu Y, et al. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci 2019; 20: 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou HY, Luo Y, Chen WD, et al. Hepatitis B virus mutation may play a role in hepatocellular carcinoma recurrence: a systematic review and meta-regression analysis. J Gastroenterol Hepatol 2015; 30: 977–983. [DOI] [PubMed] [Google Scholar]
- 4.Miller ZA, Lee KS. Screening for hepatocellular carcinoma in high-risk populations. Clin Imaging 2016; 40: 311–314. [DOI] [PubMed] [Google Scholar]
- 5.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med 2017; 130: 1099–1106.e1. [DOI] [PubMed] [Google Scholar]
- 6.Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016; 65: 719–726. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Han X, Ding YB, et al. Prediction and prophylaxis of hepatocellular carcinoma occurrence and postoperative recurrence in chronic hepatitis B virus-infected subjects. World J Gastroenterol 2016; 22: 6565–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang Q, Bi JB, Wang ZX, et al. Simple models based on gamma-glutamyl transpeptidase and platelets for predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther 2016; 9: 2099–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Q, Tian H, Luo HX, et al. Better prognosis of hepatic resection combined with antiviral therapy for HBV-related hepatocellular carcinoma with BCLC Stage B/C. Asian J Surg 2017; 40: 453–462. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Zhao X, Jiang P, et al. HBV is a risk factor for poor patient prognosis after curative resection of hepatocellular carcinoma: a retrospective case-control study. Medicine (Baltimore) 2016; 95: e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debes JD, Chan AJ, Balderramo D, et al. Hepatocellular carcinoma in South America: evaluation of risk factors, demographics and therapy. Liver Int 2018; 38: 136–143. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Ai J, Zhang W. Current clinical evidence for nucleos(t)ide analogues in patients with HBV-related hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2017; 11: 925–937. [DOI] [PubMed] [Google Scholar]
- 13.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016; 64: S84–S101. [DOI] [PubMed] [Google Scholar]
- 14.Cauchy F, Soubrane O, Belghiti J. Liver resection for HCC: patient’s selection and controversial scenarios. Best Pract Res Clin Gastroenterol 2014; 28: 881–896. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015; 17: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JH, Koh KC, Choi MS, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg 2006; 192: 29–33. [DOI] [PubMed] [Google Scholar]
- 17.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003; 38: 200–207. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw 2017; 15: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhir M, Melin AA, Douaiher J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg 2016; 263: 1112–1125. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Kim SH, Kim KS, et al. Predictors of mortality in cirrhotic patients undergoing extrahepatic surgery: comparison of Child–Turcotte–Pugh and model for end-stage liver disease-based indices. ANZ J Surg 2014; 84: 832–836. [DOI] [PubMed] [Google Scholar]
- 21.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017; 11: 317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer 2014; 120: 2824–2838. [DOI] [PubMed] [Google Scholar]
- 23. Clinical practice guidelines for hepatocellular carcinoma differ between Japan, United States, and Europe. Liver Cancer 2015; 4: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arslanoglu A, Seyal AR, Sodagari F, et al. Current guidelines for the diagnosis and management of hepatocellular carcinoma: a comparative review. AJR Am J Roentgenol 2016; 207: W88–W98. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016; 25: 24–29. [DOI] [PubMed] [Google Scholar]
- 27.Truant S, Boleslawski E, Duhamel A, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol 2012; 38: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 28.Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005; 42: 218–224. [DOI] [PubMed] [Google Scholar]
- 29.Chavez PR, Lian F, Chung J, et al. Long-term ethanol consumption promotes hepatic tumorigenesis but impairs normal hepatocyte proliferation in rats. J Nutr 2011; 141: 1049–1055. DOI: 10.3945/jn.110.136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto T, Hara M, Higaki Y, et al. Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int J Cancer 2006; 118: 1501–1507. [DOI] [PubMed] [Google Scholar]
- 31.Zhang LX, Lv Y, Xu AM, et al. The prognostic significance of serum gamma-glutamyltransferase levels and AST/ALT in primary hepatic carcinoma. BMC Cancer 2019; 19: 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu SJ, Zhao Q, Ji F, et al. Elevated preoperative serum gamma-glutamyltranspeptidase predicts poor prognosis for hepatocellular carcinoma after liver transplantation. Sci Rep 2016; 6: 28835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu XS, Wan Y, Song SD, et al. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol 2014; 20: 10944–10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D, Wang R, Meng X, et al. Prognostic value of serum γ-glutamyl transferase in unresectable hepatocellular carcinoma patients treated with transcatheter arterial chemoembolization combined with conformal radiotherapy. Oncol Lett 2014; 8: 2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Pang Q, Liu HC, et al. Alcohol consumption on liver function and its prognostic value in male patients with hepatocellular carcinoma: a retrospective cohort study. Int J Clin Exp Med 2017; 10: 11835–11845. [Google Scholar]
- 36.Shih WL, Chang HC, Liaw YF, et al. Influences of tobacco and alcohol use on hepatocellular carcinoma survival. Int J Cancer 2012; 131: 2612–2621. [DOI] [PubMed] [Google Scholar]
- 37.Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther 2016; 9: 5317–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Liu X, Yang Z, et al. The pretreatment albumin to globulin ratio, a validated biomarker, predicts prognosis in hepatocellular carcinoma. J BUON 2016; 21: 925–934. [PubMed] [Google Scholar]
- 39.Marubashi S, Dono K, Nagano H, et al. Detection of AFP mRNA-expressing cells in the peripheral blood for prediction of HCC recurrence after living donor liver transplantation. Transpl Int 2007; 20: 576–582. [DOI] [PubMed] [Google Scholar]
- 40.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 2011; 117: 4475–4483. [DOI] [PubMed] [Google Scholar]
- 41.Lee MH, Kim SU, Kim DY, et al. Early on-treatment predictions of clinical outcomes using alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol 2012; 27: 313–322. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Zhu M, Li W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med 2016; 20: 549–558. DOI: 10.1111/jcmm.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramzan M, Sturm N, Decaens T, et al. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int 2016; 36: 434–444. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem 2017; 44: 967–981. [DOI] [PubMed] [Google Scholar]