Abstract
Background
Rituximab (RTX) and ocrelizumab (OCR) are two anti-CD20 biologics used in MS; however, comparisons on safety and efficacy are rare.
Objective
To compare treatment outcomes over the first year with RTX and OCR.
Methods
Retrospective cohort study comprising MS patients initiating RTX at the Karolinska University Hospital (Sweden; n = 311) and OCR at Rocky Mountain MS Clinic (Utah, USA; n = 161), respectively.
Results
Levels of immunoglobulin G measured in blood dropped 0.16 g/L (95% confidence interval 0.01 to 0.31) with each OCR infusion, but remained stable with RTX. In contrast, levels of immunoglobulin M decreased to a similar extent with both drugs. Ten and 15% of patients discontinued treatment with RTX and OCR, respectively (n.s), however, adverse events leading to treatment discontinuation were more common with OCR (6.8% vs 2.6%; p = 0.026). Only 3.1 and 1.6% discontinued OCR and RTX, respectively, due to lack of effect (n.s). The degree of B cell depletion was superior with OCR.
Conclusion
Overall, differences between the two treatments were small. Although the study design precludes robust conclusions regarding the risk-benefit with the studied therapies, our findings indicate that the tolerability and safety with RTX is not inferior to OCR.
Keywords: Multiple sclerosis, biomarkers, ocrelizumab, rituximab, relapsing/remitting, immunoglobulins
Introduction
The number of approved disease modulatory treatments (DMTs) for relapsing-remitting MS (RMMS) has grown considerably over the last decade, while treatment options in progressive MS remains much more limited.1 A growing body of evidence supports the notion of a strong suppressive effect on inflammatory disease activity in MS with B cell depleting therapies.2–4 Unlike other MS DMTs, anti-CD20 biologics have been shown to be effective both in relapsing-remitting (RRMS) and primary progressive (PPMS).1,5,6 Rituximab (RTX, Roche) is a mouse chimeric monoclonal antibody approved for the treatment of RA, lymphoma and systemic vasculitis.2 While it is not formally approved for MS, it has undergone early clinical trials both in RRMS and PPMS and is increasingly used off-label in certain parts of the world, e.g. in Sweden. Ocrelizumab (OCR, Roche) is a fully humanized anti-CD20 antibody that recently became the first MS DMT to be approved both for RRMS and PPMS after successfully completing phase III clinical trial programs in both indications.5,6 Both RTX and OCR are of the immunoglobulin G (IgG) subtype 1 and bind overlapping epitopes on the CD20 antigen.7 RTX has more complement-dependent cytotoxicity and less antibody dependent cellular cytotoxicity than OCR.7 An advantage of this DMT class is that anti-CD20 therapies have been used for decades in other disease areas, where especially the rheumatoid arthritis (RA) indication for RTX provides valuable information on the safety profile with long-term use. Safety concerns include infusion-reactions and increased susceptibility to infections, due to interference with the normal physiological functions of the immune defense.8 In contrast, there is no indication that RTX is associated with increased risk of malignancies.9 Although both RTX and OCR have been tested in similar clinical contexts in MS, no direct comparisons of the tolerability, safety and immunosuppressive effects between the two DMTs exist.
In this study we compared a real-world cohort of patients initiating treatment with RTX or OCR in order to determine effects on Ig-levels, B cell depletion measured in blood and treatment outcomes over the first year.
Materials and methods
Study population
The study was performed at two specialized MS clinics; the Karolinska University Hospital Huddinge, Sweden, and Rocky Mountain Multiple Sclerosis Clinic (RMMSC), Utah, USA. In Sweden RTX is extensively used off-label since several years, while the use of OCR has been limited to clinical trials. The Karolinska cohort comprised all patients with RRMS or secondary progressive MS (SPMS) initiating RTX between 2010 and 28 May 2018. Patients were identified through the nationwide Swedish MS register, which was started in 2000 and has a high coverage and validity of registered data, especially regarding therapy episodes.10 The RMMSC cohort comprised all patients with RRMS or SPMS initiating OCR between 1 May 2017 and 30 November 2018. They were identified through a database search of electronic medical records. In both groups medical charts were reviewed to validate and collect data according to a pre-specified data collection protocol. Inclusion criteria were: a diagnosis of RRMS or SPMS, that treatment was initiated due to MS and that infusions had been given in intervals of 5–7 months. All patients fulfilled the revised 2017 McDonald criteria for MS, in addition the definition of SPMS relied on the 2013 Lublin criteria.11,12
The study was approved by the Regional Ethical Board of Stockholm (Dnr: 2009/2107-31-2) and IRB number Pro00038748.
Treatment and follow-up monitoring
The dosing regimen for RTX consisted of a single infusion 500 or 1000 mg of RTX, followed by a single infusion of 500 mg every 5–7 month thereafter. The OCR dosing regimen consisted of two 300 mg infusions two weeks apart and a single infusion of 600 mg every 5–7 months thereafter. The clinical follow up routine consisted of clinical examinations and brain 1.5 or 3 T magnetic resonance imaging (MRI) before treatment and at every 6–12 months, or as clinically indicated. In general, blood analyses were done before start of therapy and before each additional DMT infusion.
Data collection and outcomes
The data extraction protocol included data on IgG, IgM and Ig A levels, total lymphocyte numbers and flow cytometric determination of the number of T (CD3+CD4+ or CD3+CD8+) and B (CD19+CD3-) cells, all measured in blood. The intervals differed somewhat between Sweden and RMMSC due to follow up routines. In Sweden, blood tests were carried out before every RTX dose with an interval of 0–45 days (mean = 10.7; SD = 6.4). At RMMSC, the interval between blood samples and treatment was 0–192 days (mean = 63,9; SD = 40). We also collected data on age, sex, MS-duration at start of treatment with RTX or OCR, all prior treatments, EDSS at baseline, relapses within 3 months of start of therapy. Blood test data for patients treated with intravenous Ig (IVIG) or plasma exchange (PE) within 100 days of start of therapy or re-infusion were excluded as this could affect the values of the parameters mentioned above. Reasons for discontinuation were stratified into the following groups; lack of effect, adverse events (AE), stable disease, confirmed or planned pregnancy, and other reasons. In patients with two or more reasons for discontinuation only one was considered according to the priority order listed above. Lack of effect was defined as a verified clinical relapse or contrast-enhancing lesions on T1-weighed MRI at least three months after first anti-CD20 DMT infusion or a new lesion on T2-weighed MRI compared with a reference scan performed at least three months after first anti-CD20 DMT administration. AEs as reason for discontinuation were specified according to the medical records. The definition of an AE was up to the treating physician and the decision to discontinue the treatment was take after discussion with the patient.
Statistical analyses
Data was analyzed using a linear mixed effect model (MEM) (IBM SPSS Statistics for Windows, Version 25.0), which included number of doses of administered DMT (i.e. OCR or RTX) as random effect, while age, sex, previous DMT, administered DMT and the interaction of doses and administered DMT as fixed effects. Covariance type was unstructured. We also performed Generalized Estimating Equations (GEE) to get more robust estimations and to confirm or reject our initial findings. The correlation matrix was independent. In order to compensate for variability in sample times between RTX and OCR the data was also analyzed with days between first dose and sample collection (baseline was set to 0) instead of samples coupled to doses. The immunological biomarkers at baseline were compared with outcomes after one and two doses of RTX and OCR, respectively, and subsequently compared using chi-square test. Mann-Whitney test was used to compare CD19+ B cell counts. P-values <0.05 were considered statistically significant. Non-parametric variables were presented as median (range), and normally distributed variables as mean (SD).
Results
Study population
We identified a total of 311 MS patients starting RTX who fulfilled the inclusion criteria, of which 225 had RRMS and the remaining 86 SPMS who had received RTX every 5–7 months (mean interval 188 days between doses; SD = 11). The OCR cohort consisted of 161 patients, all of which were classified as RRMS, who had received DMT infusions with a mean interval of 190 (SD = 11) days. Detailed data on the two cohorts are given in Table 1. Blood test values for two RTX patients were excluded due to treatment with IVIG and PE, respectively, within 100 days prior to baseline. In the OCR group, six values were excluded due to IVIG treatment prior to re-infusion.
Table 1.
Demographics of OCR (n = 161) and RTX (n = 311).
| OCR n (%) | RTX n (%) | |
|---|---|---|
| Aubagio | 9 (5.59) | 3 (0.96) |
| Gilenya | 1 (0.62) | 13 (4.18) |
| Injectable | 14 (8.69) | 124 (39.8) |
| Naive | 7 (4.34) | 80 (25.7) |
| Rituxan | 23 (14.2) | – |
| Tecfidera | 40 (24.8) | 19 (6.10) |
| Tysabri | 66 (40.9) | 72 (23.1) |
| Unknown | 1 (0.62) | – |
| Total | 161 (100) | 311 (100) |
| Sex female | 95 (59.0) | 222 (71.3) |
| RRMS | 161 (100) | 225 (72) |
| Age mean (SD) | 49.8 (11.9) | 44.0 (11.7) |
| EDSS Median (IQR; min; max) | – | 2.5 (2.125; 0; 8.5) |
| MS duration mean (SD) | 12.5 (8.32) | 11.3 (8.87) |
Adverse events
In the RTX group the reasons where: suicidal ideation (n = 1), hair loss and painful sensations (n = 1), anemia (n = 1), increased fatigue (n = 1), swollen hands (n = 1), infusion-reactions (n = 2) and unknown (termed adverse event, but not specified; n = 1).
In the OCR group the reasons where: infections (n = 10; comprising sinusitis, appendicitis, urinary tract infection, pneumonia, diverticulitis, bronchitis and sepsis), infusion-reactions (n = 2), lymphopenia (n = 1), Stevens Johnson syndrome (n = 1) and anemia (n = 1).
Immunoglobulin concentrations and B cells
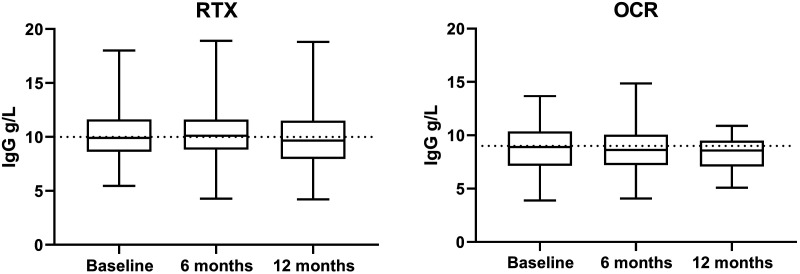
Calculating data with MEM showed that total levels of IgG in blood decreased with a mean of 0.16 g/L (CI 0.01 to 0.31; p = 0.039) with each OCR infusion, but remained stable with RTX (Figure 1). Using the alternative GEE approach and instead of doses using days from treatment dose to sample time resulted in a trend for lowered IgG with OCR (0.001 g/L per day; CI 0.000 to -0.003), corresponding to 0.18 g/L by each infusion; p = 0.102). Finally, using MEM with days between first dose and blood sample collection showed a significant decrease in the OCR group (p = 0.036), other IgG decreased to similar extent as those before. Factoring in other characteristics showed that higher age was associated both with lower starting IgG levels and a stronger decreasing effect. This was not linear, as the effect became evident only from the age span of 40 to 50 years of age, see Table 2 for age stratified IgG levels. In addition, patients who had received RTX before OCR had a lower starting value of IgG (p = 0.030) compared to natalizumab. These patients in general had received doses of 500 mg every two weeks for two months and repeated doses with six months’ intervals after this initial cycle. Other DMTs were not different from natalizumab. No difference between genders. At the group level, IgG was lower in the OCR group before treatment start and this difference remained even when patients who previously had been treated with RTX and switched to OCR were excluded.
Figure 1.
Median IgG, 25–75% in the box, whiskers show max and min.
Table 2.
IgG levels (g/L) stratified by age and treatment.
| Age | Baseline mean (SD) | 12 Months mean (SD) | |
|---|---|---|---|
| <40 | OCR | 8.82 (2.0) | 8.55 (1.4) |
| RTX | 10.7 (2.4) | 10.9 (2.2) | |
| 50–40 | OCR | 8.79 (2.2) | 8.75 (2.8) |
| RTX | 10.0 (2.1) | 10.1 (2.1) | |
| >50 | OCR | 8.76 (2.2) | 7.99 (2.0) |
| RTX | 9.85 (2.3) | 9.48 (2.6) |
OCR: ocrelizumab; RTX: rituximab.
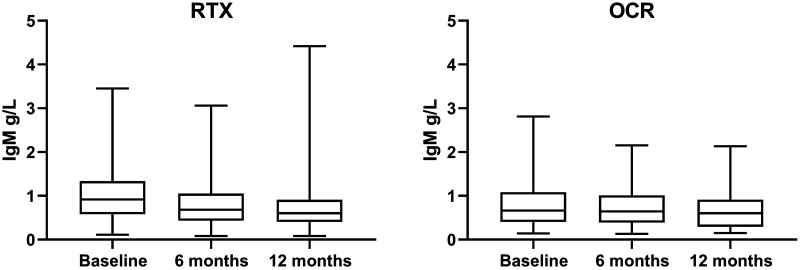
In contrast to IgG, IgM dropped to a similar extent in both groups with each additional infusion calculated with MEM; 0.12 g/L (CI 0.10 to 0.15) and 0.11 g/L (CI 0.09 to 0.14) for RTX and OCR, respectively (Figure 2). Using GEE and days between first dose and sample collection both treatments displayed a decreased of-0.18g/L (p < 0.001). Baseline levels of IgM was affected by sex, since females displayed higher starting values (+0.18 g/L; CI 0.06 to 0.29 compared to males; p = 0.003). Treatment naïve patients and those previously exposed to dimethyl fumarate had higher starting values than those switching from natalizumab (0.21 g/L, CI 0.04 to 0.37, p = 0.014; 0.28 g/L, CI 0.10 to 0.46, p = 0.002, respectively). The effect of previous treatment with RTX was even greater than with natalizumab (–0.24 g/L, CI –0.06 to –0.42, p = 0,009). In contrast to IgG, there was only a trend for lower values with increasing age (p = 0.053). These findings were consistent to those calculated with MEM.
Figure 2.
Median IgM, 25–75% in the box, whiskers show max and min.
Levels of IgA in blood were not affected by treatment with either OCR or RTX, however females displayed lower baseline concentrations compared to males (–0.35 g/L, CI –0.08 to –0.63, p = 0,011).
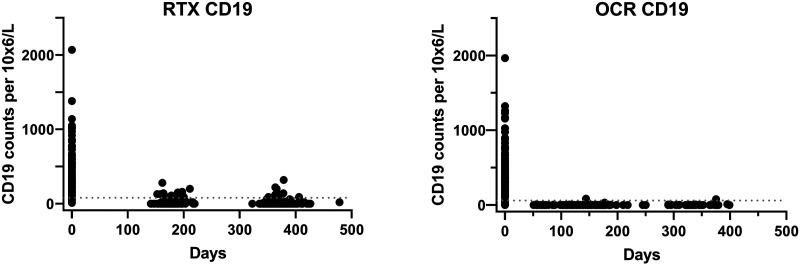
CD19+ B cell counts were greatly depleted with both RTX and OCR. In most patients CD19+ B cell were below the detection limit before next infusion. A subgroup analysis was performed using Mann-Whitney test for CD19+ B cell counts in the time span of 150–180 days after the first infusion of OCR (median = 0 × 106/L) and RTX (median = 10 × 106/L), which showed a significant decrease in CD19+ B cell counts compared to RTX (U = 1170, p < 0.001) (Figure 3).
Figure 3.
CD19 cell counts in blood. Lower levels of normal (LLN) are indicated in the graphs. Note that LLN differed slightly between centra (80 and 61 cells 106/L at Karolinska and Rocky Mountain, respectively).
Treatment discontinuations
There was no statistical difference in the proportion of patients terminating treatment within the first year (10 and 15% with RTX and OCR, respectively, p = 0.11). Reasons to discontinue treatment are depicted in Table 3. AEs as stated reason to discontinue DMT were more common with OCR than RTX (9.3% vs 2.6%, p < 0.01). The difference retained statistical significance when patients switching to OCR from RTX were excluded (5.6% vs 2.6%, p = 0.043). In contrast, only 2.5% and 1.6% discontinued OCR and RTX, respectively, due to lack of effect, a difference that was not significant between the two treatments.
Table 3.
Side effects for OCR and RTX divided into groups.
| OCR n | RTX n | |
|---|---|---|
| Lack of effect | 5 | 5 |
| Adverse event(infusion reactions) | 15 (2) | 8 (2) |
| Lost to follow up | 3 | 2 |
| Other reasons | 2 | 5 |
| Stable disease | 0 | 10 |
| Pregnancy confirmed | 0 | 1 |
| Pregnancy planned | 0 | 0 |
n: number.
Discussion
We here report the first direct comparison between RTX and OCR in context of MS. Although both treatments are similar in their mode of action, this does not exclude the existence of clinically relevant differences in effect or safety/tolerability. Both RTX and OCR have been studied in clinical trials in RRMS and PPMS.5,6,13,14 However, due to differences in design and methodology, comparisons across studies should be done with caution. In addition, randomized controlled trials have known limitations regarding generalizability to real-world patient populations due to restrictive inclusion criteria. A strength with this study is that we included all patients starting RTX or OCR at either site and, thus, were able to study a heterogeneous patient population similar to that seen in clinical practice. The fact that no patient started OCR outside of clinical trials in the Swedish cohort and the insurance companies decided whether the patient should receive RTX or OCR in the RMMSC cohort ensured that there was no concealed confounding factor affecting channeling to the two DMTs. However, inevitably this also introduced a possible bias regarding baseline patient characteristics. For example, the US cohort only included patients with RRMS, since OCR is currently not approved for SPMS. Since baseline characteristics regarding age, disease duration and EDSS did not reveal major differences between the two groups, it is possible that criteria for classifying a disease course as secondary progressive were more liberal in Sweden. Furthermore, a higher proportion of patients in the Swedish cohort were treatment naïve, while the proportion switching from natalizumab was higher in the RMMSC cohort. Together with differences in follow up routines regarding relapse documentation and the way disability ratings and imaging were carried out, all assumptions on possible differences in effectiveness outcomes between RTX and OCR should be interpreted with caution. Therefore, we here limited this to the proportion of patients terminating either DMT due to lack of effect that likely is less sensitive to such differences. This showed that few patients terminated either treatment due to lack of effect, which is in line with previous observations regarding B cell depleting therapies in context both of clinical trials and real world evidence.5,15–18
In contrast to clinical outcomes, our analysis of immunoglobulin and B cell counts represent objective measures of impact of therapy. Here we found that OCR, but not RTX, was associated with a decrease in IgG measured in blood with increasing number of infusions, even if differences reached significance only with one of the statistical approaches (MEM). In contrast, both DMTs reduced IgM levels to a similar extent. Hypogammaglobulinemia is a well-known consequence of B cell depleting therapies and is associated with increased susceptibility to infections, in turn believed to be relating to total exposure to RTX.19 The frequency of hypogammaglobulinemiawith RTX used in context of RA has been estimated at 2.7 events per 100 patient years.20 An important difference is that we here studied a low dose regimen for RTX, since a large majority started with a single 500 mg infusion while the label for RA states a cycle with two 1000 mg infusions over two weeks. In an earlier real world study of MS patients the 500 mg RTX dose was associated with a trend of fewer side effects compared to 1000 mg, although differences in impact on levels of IgG between the two doses were not significant.21 However, we here used a more precise statistical model, which included exact information on dates of drug administration and blood sampling and it is therefore unclear if the higher dosage of RTX affect levels of IgG to a greater extent. However, further studies are needed to describe more in detail how different doses and dosing intervals affect immunolglobulin levels. Further studies are also needed to address if the difference we see here regarding IgG between OCR and RTX mostly depends on the relative efficacy of B cell depletion, since a higher proportion of patients treated with RTX had detectable levels of B cells before next infusion. Longer observations will be required to determine if the early effects on IgG we see here also translate into differences in risks of hypogammaglobulinemia, in turn relating to risks of infections.19,20 A potentially important observation here was that older age was associated with both lower baseline levels of IgG and a greater drop with successive infusions. This may suggest that age should be considered when deciding on doses and dosing intervals with B cell depleting drugs in order to reduce the risks of developing IgG deficiency, since it tends to be long lasting even after drug administration has been terminated. This also underscores the importance of monitoring of immunoglobulin levels with anti-CD20 therapies, since this offers an opportunity for earlier detection of patients at risk, who may benefit from immunoglobulin replacement.
A theoretical advantage with OCR compared to RTX is that the former likely is less immunogenic, with fewer patients developing anti-drug antibodies (ADA). In other contexts, ADAs have been associated with reduced efficacy and possibly also increased risks of infusion reactions. The fact that we here found that a higher proportion of patients that terminated OCR due to AEs suggests that this is of limited clinical importance for anti-CD20 biologicals. In a previous study we also found that while a significant proportion of patients treated with RTX developed anti-RTX antibodies, we did not find an association between ADAs and infusion reactions, adverse events or lack of effect.22
Limitations with the study apart from those mentioned above, include that there were certain differences in follow up procedures between the two centers, e.g. time to blood sample, EDSS missing in the OCR group and different laboratories performing blood analyses. Furthermore, since information was collected from clinical routine, not all patients had complete data sets. We also lacked data on certain parameters such as body mass index and concomitant diseases, which may have affected the studied outcomes. Although we adjusted for age in the statistical analysis we cannot completely rule out a degree of residual confounding that could explain some of the difference in IgG levels between the RTX and OCR groups. The difference in baseline IgG levels between the sites suggests that the laboratories measurement is not completely the same. Still, the statistical methods used here, Mixed effect model (MEM) and General estimating equations (GEE), are less sensitive to such differences (provided that laboratory measurements are stable over time) since modelling is based on an individual´s starting value and look at difference over time rather than exact starting values. CD19+ B cell counts were also measured by different labs, which might impact on results. Finally, the non-randomized design makes the study susceptible to bias by additional confounders not accounted for.
In conclusion, we find that OCR and older age are associated with greater effects on levels of IgG levels in blood, while RTX and OCR affected IgM to a similar extent. The proportion of patients terminating therapy due to lack of effect was small and did not differ between the two DMTs. In contrast, the risk of discontinuing therapy due to AEs was greater with OCR than RTX. These observations suggest that the tolerability and safety with RTX is not inferior to that of OCR. Further studies are warranted to address long term efficacy and safety outcomes with RTX and OCR.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Björn Evertsson: Received travel support for conference attendance from Roche.
John Foley sits on scientific advisory boards for Biogen, Genentech-Roche, Genzyme, Celgene, and EMD Serono. He is also on the Speakers Bureau for Biogen and Genentech-Roche. Dr Foley receives research support from Biogen, Genentech-Roche, Novartis, Genzyme, Mallinckrodt, Adamas and has equity interest in Abreos Bioscience.
Fredrik Piehl has received research grants from Biogen, Genzyme, Merck KGaA and Novartis, and fees for serving as Chair of DMC in clinical trials with Parexel
Contributor Information
Björn Evertsson, Neuroimmunology Unit, Karolinska Institutet, Clinical Neuroscience, Karolinska University Hospital, Stockholm, Sweden.
Angel Christensen, Rocky Mountain Multiple Sclerosis Clinic, Neurology, Salt Lake City, UT, USA.
Faiez AL Nimer, Neuroimmunology Unit, Karolinska Institutet, Clinical Neuroscience, Karolinska University Hospital, Stockholm, Sweden.
John Foley, Rocky Mountain Multiple Sclerosis Clinic, Neurology, Salt Lake City, UT, USA.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Björn Evertsson https://orcid.org/0000-0001-8799-9619 Faiez AL Nimer https://orcid.org/0000-0003-0937-5995
References
- 1.Piehl F. A changing treatment landscape for multiple sclerosis: challenges and opportunities. J Intern Med 2014; 275: 364–381. [DOI] [PubMed] [Google Scholar]
- 2.Ineichen BV, Moridi T, Granberg T, et al. Rituximab treatment for multiple sclerosis. Mult Scler 2020; 26: 137–152. [DOI] [PubMed] [Google Scholar]
- 3.Myhr K-M, Torkildsen Ø, Lossius A, et al. B cell depletion in the treatment of multiple sclerosis. Expert Opin Biol Ther 2019; 19: 261–271. [DOI] [PubMed] [Google Scholar]
- 4.Ancau M, Berthele A, Hemmer B. CD20 monoclonal antibodies for the treatment of multiple sclerosis: up-to-date. Expert Opin Biol Ther 2019; 19: 829–843. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 6.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 7.Klein C, Lammens A, Schafer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs 2013; 5: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vollenhoven RF, Fleischmann RM, Furst DE, et al. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol 2015; 42: 1761–1766. [DOI] [PubMed] [Google Scholar]
- 9.Wadstrom H, Frisell T, Askling J; Anti-Rheumatic Therapy in Sweden Study G. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med 2017; 177: 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alping P, Piehl F, Langer-Gould A, et al. ; COMBAT-MS Study Group. Validation of the Swedish multiple sclerosis register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology 2019; 30: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 12.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008; 63: 395–400. [DOI] [PubMed] [Google Scholar]
- 14.Hawker K, O'Connor P, Freedman MS, et al. ; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66: 460–471. [DOI] [PubMed] [Google Scholar]
- 15.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 2016; 79: 950–958. [DOI] [PubMed] [Google Scholar]
- 16.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 2018; 75: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boremalm M, Juto A, Axelsson M, et al. Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol 2019; 26: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011; 378: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 19.Barmettler S, Ong MS, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018; 1: e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boleto G, Avouac J, Wipff J, et al. Predictors of hypogammaglobulinemia during rituximab maintenance therapy in rheumatoid arthritis: a 12-year longitudinal multi-center study. Semin Arthritis Rheum 2018; 48: 149–154. [DOI] [PubMed] [Google Scholar]
- 21.Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016; 87: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn N, Juto A, Ryner M, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler 2018; 24: 1224–1233. [DOI] [PubMed] [Google Scholar]