Abstract
Background
Cardiac contractility modulation (CCM) is non-excitatory electrical stimulation for improving cardiac function. This study aimed to evaluate the effects of CCM on structural and electrical remodeling in a rabbit model of chronic heart failure (CHF).
Methods
Thirty rabbits were randomly divided into the sham, CHF, and CCM groups. The CHF model was induced 12 weeks after trans-aortic constriction by pressure unloading and CCM was delivered to the myocardium for 4 weeks. Corrected QT intervals, the ventricular effective refractory period, and inducibility of ventricular tachycardia were measured by an electrophysiological examination. Connective tissue growth factor, galectin-3, Kv4.3, KCNQ1, KCNH2, and connexin 43 protein levels were measured by western blotting.
Results
The CHF group had a significantly prolonged corrected QT interval and ventricular effective refractory period, and increased inducibility of ventricular tachycardia. Prominent myocardial fibrosis and increased hydroxyproline content were observed in the CHF group, but these were suppressed in the CCM group. Kv4.3, KCNQ1, KCNH2, and connexin 43 protein levels were significantly lower in the CHF group, but treatment with CCM partially restored their levels.
Conclusions
CCM attenuates myocardial structural and electrical remodeling during CHF. These findings provide evidence for clinical use of CCM in treating CHF.
Keywords: Cardiac contractility modulation, chronic heart failure, structural remodeling, electrical remodeling, QT interval, connective tissue growth factor, ventricular tachycardia
Introduction
Chronic heart failure (CHF) is a common cardiovascular disease with a continuously increasing prevalence. Despite recent progress in treating CHF, the morbidity and mortality rates of patients with CHF are still high.1 A poor prognosis of CHF is thought to be related to increased susceptibility to lethal ventricular arrhythmia. Development of heart failure (HF) is accompanied by complex changes in cardiac electrophysiology and functional properties of cardiomyocytes, which cause structural and electrical remodeling of the ventricles.2
Cardiac contractility modulation (CCM) is a device-based therapy for HF. CCM involves application of high-voltage, long-duration, biphasic electric signals to the myocardium during the absolute myocardial refractory period.3–5 In particular, the majority of patients with HF have a narrow QRS complex and are not candidates for cardiac resynchronization therapy. For these patients, alternative electrical therapy, such as CCM, could significantly improve contractility, functional capacity, and symptoms.6 Evidence from animal models and patients with CHF has also shown that CCM therapy has beneficial effects by regulating calcium handling, the cytoskeleton, the extracellular matrix, and potentially the autonomous nervous system.7,8 However, the association between CCM and arrhythmic complications, and the effects of CCM on electrical remodeling and structural remodeling have rarely been investigated.9–12
Connective tissue growth factor (CTGF) and galectin-3 (Gal-3) are novel pro-fibrotic markers involved in myocardial structural remodeling.13,14 In addition to alterations in connexin 43 (Cx43) expression, changes in potassium currents are important contributors to myocardial electrical remodeling.15,16 These pathologies are associated with an increasing propensity for arrhythmia.
In our previous study, we established a rabbit model of HF and found beneficial effects of CCM on cardiac contractile function.17 Therefore, in the current study, we used the same rabbit HF model to further investigate the effects of CCM on structural and electrical remodeling by using histological and electrophysiological analysis. Furthermore, we detected protein levels of CTGF, Gal-3, Kv4.3 (subunit of the Ito channel), KCNH2 (subunit of the Ikr channel), KCNQ1 (subunit of the Iks channel), and Cx43 to investigate the underlying mechanism.
Methods
Rabbits
The study was approved by the Animal Care and Use Committee of Hebei Medical University and all experimental protocols were conducted in accordance with the Guidelines for Animal Experiments of Hebei Medical University (Approval No. H2015307037). Thirty healthy New Zealand white rabbits (6 months old, weight: 2.5–3.5 kg, both male and female rabbits) were provided by the Experimental Animal Center of Hebei Medical University. The rabbits were randomly divided into three groups (n = 10) as follows: the sham operation group (sham group), the HF group (CHF group), and the CCM group. Rabbits in the sham group only received thoracotomy. Thoracotomy and ascending aortic cerclage were performed in the CHF group. In the CCM group, rabbits underwent thoracotomy and ascending aortic cerclage, and received 4-week CCM after formation of CHF.
Induction of HF
The CHF model was established using the procedure of trans-aortic constriction. Briefly, all rabbits were anesthetized with 3% sodium pentobarbital (30 mg/kg) via an ear vein. The thoracic cavity was opened, and penicillin was injected to prevent infection. After the ascending aorta was dissected, it was occluded to allow constriction to 60% of its original circumference. A pediatric temporary pacing lead (Medtronic 6491; Medtronic Inc., Minneapolis, MN, USA) for delivering CCM was sutured to the left ventricular anterior wall and the other end of the electrode was fixed to the neck for later use. The left ventricular ejection fraction was assessed at 12 weeks. A left ventricular ejection fraction ≤40% indicated that the CHF model was successfully established.
CCM protocols
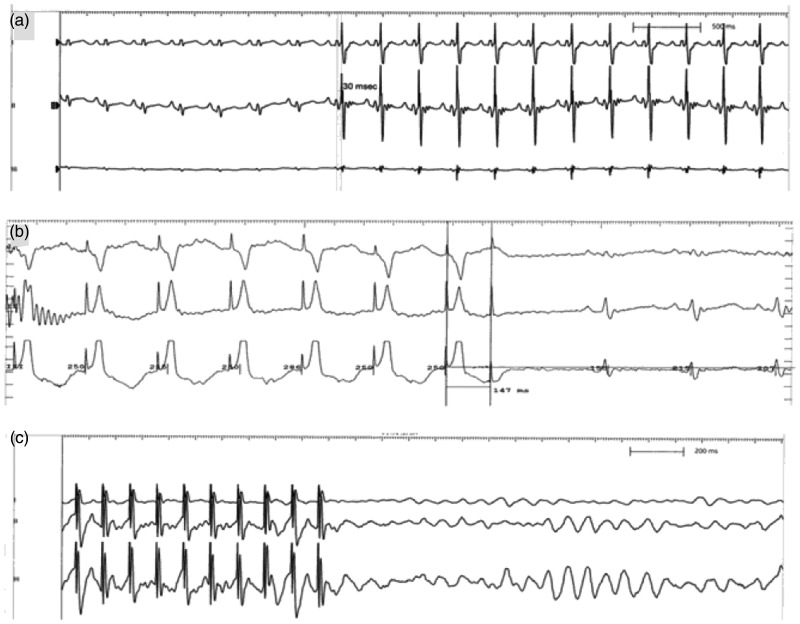
The EPS320 Cardiac Stimulator (Micro Pace; Bard Inc., Lowell, MA, USA) was used to deliver CCM stimulation to the absolute refractory period of the heart by the sensed R wave. The signals consisted of biphasic square wave pulses with a phase duration of 2 ms, stimulus amplitude of 7 V, and a 30-ms delay after R-wave sensing.18,19 CCM signals were conducted 6 hours per day for 4 consecutive weeks (Figure 1a).
Figure 1.
Effect of cardiac contractility modulation on myocardial electrophysiological characteristics. (a) Representative electrocardiographic recordings of cardiac contractility modulation signals. (b) Representative measurement of the ventricular effective refractory period. (c) Representative inducibility of ventricular tachycardia.
Electrocardiography
Needle electrodes were placed subcutaneously in all four limbs for surface electrocardiographic (ECG) recordings as described previously.20 Lead II of the ECG was selected for further analysis. The QT interval mainly focuses on repolarization of the action potential of the heart. Currently, beat-to-beat instability of the QT interval is viewed as an ECG biomarker that detects cardiac arrhythmia, mainly focusing on repolarization of the action potential of the heart. The QT interval was defined as the duration from the beginning of the Q wave to the end of the T wave. Additionally, heart rate-corrected QT (QTc) calculation was obtained by using Carlson’s formula as follows: QTc = (QT−0.175) × (RR interval−300).21
Electrophysiological analysis
Programmed electrical stimulation protocols were performed using the EPS320 Cardiac Stimulator (Bard Inc.) to assess electrophysiological characteristics. The effective refractory prior (ERP) was measured (2 times the diastolic threshold, 2-ms pulse width) at basic cycle lengths of 250 ms with a train of eight basic (S1) stimuli followed by a premature (S2) stimulus with 2-ms decrements (Figure 1b). The longest S1–S2 interval that failed to produce a propagated ventricular response was taken as the ERP.22 The inducibility of ventricular tachycardia (VT) was assessed with eight beat drive trains (S1) at 200 ms, followed by one to three ventricular extra-stimuli. Single (S2) or double (S2–S3) premature stimuli were applied with a coupling interval of 160 ms, and were progressively shortened in 2-ms steps until induction of VT or until the ventricular ERP was reached (Figure 1c). VT was defined as eight consecutive ventricular beats at a cycle length of ≤150 ms.23
Histological analysis
At the end of the study, the rabbits were sacrificed by exsanguination under anesthesia. The hearts from anesthetized rabbits were rapidly excised and fixed in 4% paraformaldehyde solution. The heart was sectioned transversely across myocardial papillary muscle and then embedded with paraffin. Myocardial tissue sections (5 μm) were cut and stained with picrosirius red liquid dye for 60 minutes, and then counterstained with hematoxylin. The stained sections were observed in a blinded manner under polarized light, which could distinguish type I from type III collagen by different colors. Yellow and red indicated type I collagen, and green indicated collagen III. Digital photographs were taken at ×400 magnification for 10 random fields from each section. The percentage of the collagen volume fraction (collagen area/total area ×100%) was detected by Image-ProPlus 5.1 (Media Cybernetics Inc., Bethesda, MD, USA).
Hydroxyproline assay
Myocardial hydroxyproline content was analyzed using a commercial kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Briefly, myocardial tissue (30–100 mg) was digested at 95°C for 20 minutes in acidic buffer, and then centrifuged at 1370 ×g for 10 minutes. Absorbance of the final solution was determined using a microplate spectrophotometer at 550 nm, and hydroxyproline content was calculated as μg per mg of tissue.
Western blot analysis
Total protein was extracted from myocardial tissue and quantified using a bicinchoninic acid kit (Beyotime, Shanghai, China). Samples were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After being blocked with 5% fat-free milk for 2 hours, the membrane was incubated overnight at 4°C with the following primary antibodies: Gal-3 (1:1000 dilution; Abcam, Cambridge, MA, USA), CTGF (1:1000 dilution; Santa Cruz, Dallas, TX, USA), Kv4.3 (1:400 dilution; Santa Cruz), KCNH2 (1:500 dilution; Santa Cruz), KCNQ1 (1:500 dilution; Santa Cruz), and Cx43 (1:500 dilution; Abcam). An antibody against β-actin (1:1000 dilution; Santa Cruz) was used as an internal control. After washing with phosphate-buffered saline, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:1000 dilution; Santa Cruz). The bands were visualized using an enhanced chemiluminescence kit (ECL Millipore Corp., Bedford, MA, USA). Developed films were scanned and Image-ProPlus 5.1 was used for quantitative analysis.
Statistical analysis
Continuous data are reported as mean ± standard deviation. Categorical data are presented as absolute values and percentages. Differences among multiple groups were compared with single factor analysis of variance, while comparison between two groups was tested with the least significant difference method. Fisher’s exact test was used for significance of frequency data. Statistical analyses were performed using the Software Package for Statistical Science (IBM SPSS for Windows, version 22; IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Establishment of the HF model
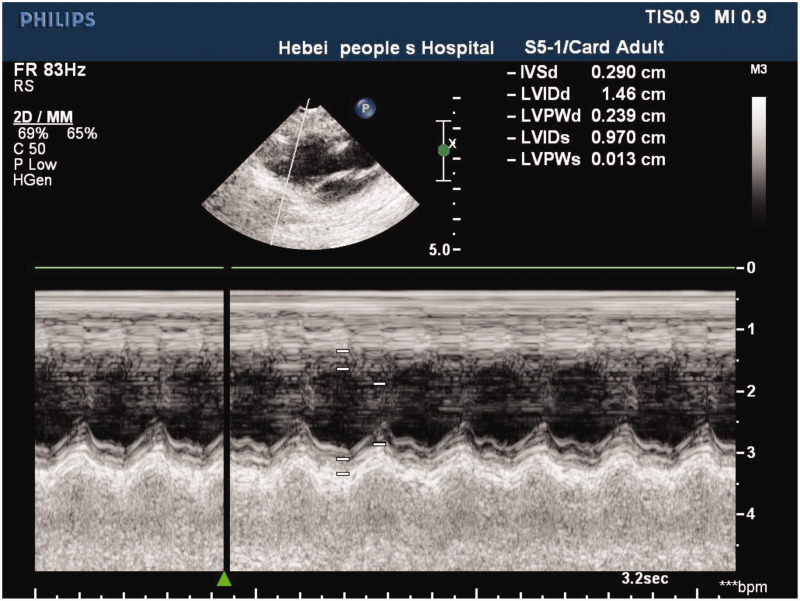
There were no deaths in the sham group during surgery. One rabbit died during surgery owing to pneumothorax in the CHF group and one rabbit died as a result of artery rupture in the CCM group. Echocardiography confirmed that the HF model was successfully established (Figure 2).
Figure 2.
Echocardiography shows that the heart failure model was successfully established in the rabbit.
Effect of CCM on electrophysiological characteristics
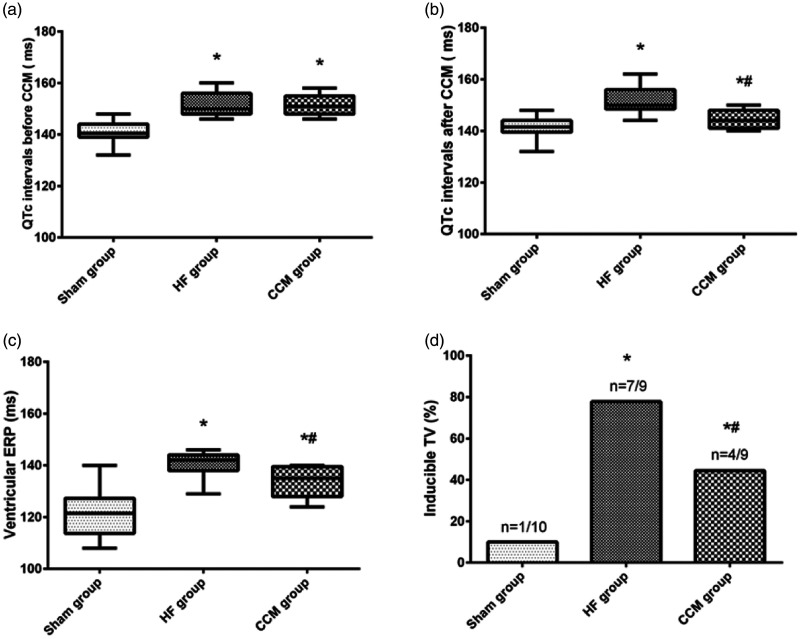
Rabbits in the HF and CCM groups with 12 weeks of trans-aortic constriction showed a significant decrease in the ejection fraction and met the criteria of HF. All rabbits survived after the CCM protocol. Rabbits in the HF (152.00 ± 4.80 ms) and CCM groups (151.44 ± 3.97 ms) developed a significantly prolonged QTc compared with the sham group (140.70 ± 4.42 ms, both P < 0.05) before the CCM protocol (Figure 3a). After 4 weeks of CCM stimulation, the mean QTc interval in the CCM group (144.67 ± 3.84 ms) showed significant shortening compared with that in the CHF group (152.11 ± 5.49 ms, P < 0.05) (Figure 3b).
Figure 3.
Results of CCM on myocardial electrophysiological characteristics.
(a) Comparison of the QTc interval before CCM among the three groups. (b) Comparison of the QTc interval after CCM among the three groups. (c) Comparison of ventricular ERP among the three groups. (d) VT inducibility in the sham group, HF group, and CCM group. *P < 0.05 vs. the sham group (n = 5); #P < 0.05 vs. the HF group (n = 5).
CCM, cardiac contractility modulation; QTc, corrected QT interval; HF, heart failure; ERP, effective refractory period; VT, ventricular tachycardia.
Consistent with the change in QTc, the ERP was significantly longer in CHF group (140.56 ± 5.10 ms) compared with the sham group (121.50 ± 9.54 ms, P < 0.05). CCM significantly reduced the ERP in rabbits with CHF (133.33 ± 6.12 ms, P < 0.05) (Figure 3c).
Inducibility of VT was significantly higher in rabbits in the CHF group (8/9, 89%) compared with those in the sham group (1/10, 10%, P < 0.05), while VT inducibility was lower in the CCM group than in the CHF group (4/9, 44%, P < 0.05) (Figure 3d).
Effect of CCM on myocardial fibrosis
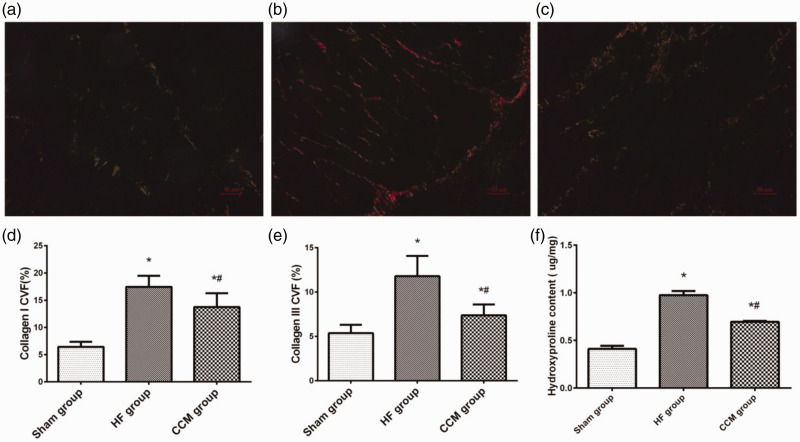
Hydroxyproline is unique to collagen and is a well-recognized marker for fibrosis. Picrosirius red staining was performed to differentiate depositions of collagen I and collagen III (Figure 4a, b, c). We observed that types I and III collagen in the CHF group were significantly higher compared with those in the sham group (both P < 0.05), but collagen was suppressed by CCM treatment (both P < 0.05, CHF group vs CCM group; Figure 4d, e). Mean hydroxyproline content in the CHF group was significantly higher than that in the sham group (P < 0.05). After CCM treatment, hydroxyproline content was remarkably reduced compared with that in the CHF group (P < 0.05; Figure 4f).
Figure 4.
Effect of CCM on myocardial fibrosis. (a–c) Representative picrosirius red staining of the myocardium in the sham, HF, and CCM groups. Magnification, ×400, scale bar: 50 µm. (d) Percentage of areas of collagen I among the three groups. (e) Percentage of areas of collagen III among the three groups. (f) Comparison of hydroxyproline content among the three groups. Each bar represents the mean ± standard deviation. *P < 0.05 vs. the sham group (n = 5); #P < 0.05 vs. the HF group (n = 5).
CVF, collagen volume fraction; HF, heart failure; CCM, cardiac contractility modulation.
Effect of CCM on cardiac protein expression of CTGF and Gal-3
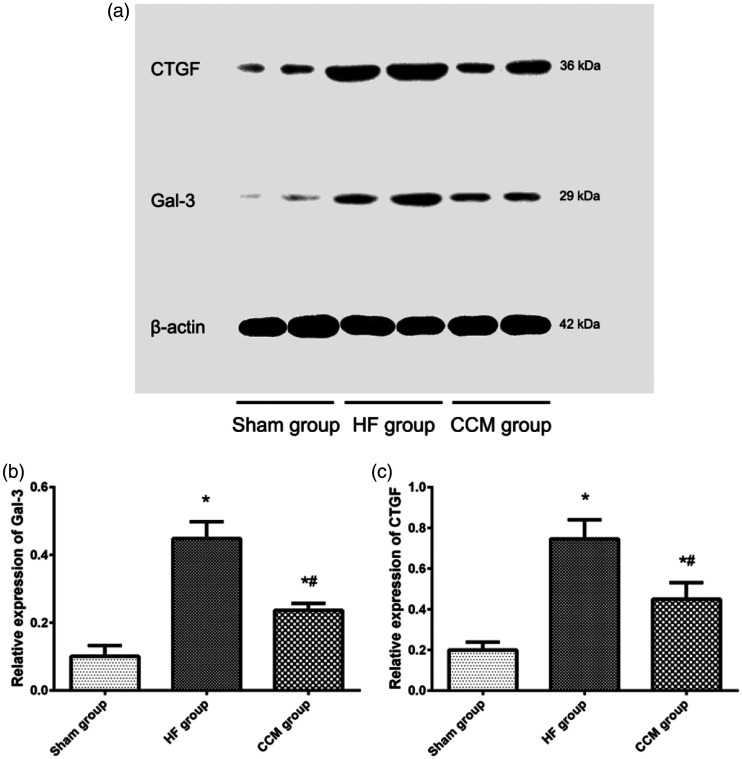
CTGF and Gal-3 increase the production of extracellular matrix components and contribute to the pathogenesis of myocardial fibrosis. CTGF and Gal-3 protein levels were significantly higher in heart tissue in the CHF group compared with the sham group (both P < 0.05), but they were reduced in the CCM group compared with the CHF group (both P < 0.05; Figure 5).
Figure 5.
CTGF and Gal-3 protein levels in the myocardium. (a) Representative blots showing CTGF and Gal-3 protein levels. (b) Comparison of Gal-3 protein levels among the three groups. (c) Comparison of CTGF protein levels among the three groups. Each bar represents the mean ± standard deviation. *P < 0.05 vs. the sham group (n = 5); #P < 0.05 vs. the HF group (n = 5).
Gal-3, galectin-3; HF, heart failure; CCM, cardiac contractility modulation; CTGF, connective tissue growth factor.
Effect of CCM on cardiac protein expression of Kv4.3, KCNQ1, KCNH2, and Cx43
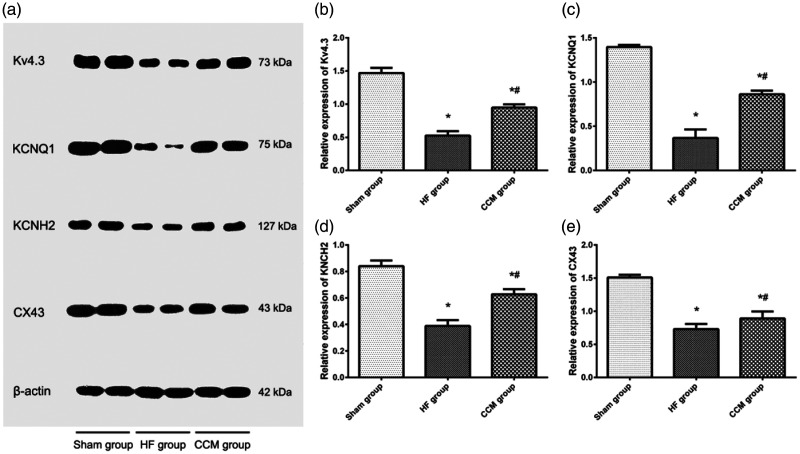
To investigate possible mechanisms by which CCM changed electrophysiological characteristics of the heart, we examined Kv4.3, KCNQ1, KCNH2, and Cx43 protein expression in heart tissue. Kv4.3, KCNQ1, KCNH2, and Cx43 protein levels were significantly lower in the CHF group compared with the sham group (all P < 0.05). Treatment with CCM partially restored protein levels of Kv4.3, KCNQ1, KCNH2, and Cx43 (all P < 0.05, CCM group vs CHF group; Figure 6).
Figure 6.
Kv4.3, KCNQ1, KCNH2, and Cx43 protein levels in the myocardium. (a) Representative blots showing Kv4.3, KCNQ1, KCNH2, and Cx43 protein levels. (b), (c), (d), (e) Comparison of Kv4.3, KCNQ1, KCNH2, and Cx43 protein levels among the three groups. Each bar represents the mean ± standard deviation. *P < 0.05 vs. the sham group (n = 5); #P < 0.05 vs. the HF group (n = 5).
Cx43, connexin 43; HF, heart failure; CCM, cardiac contractility modulation.
Discussion
CHF is a serious disease that may cause high mortality and disability in clinics. The remodeling process of myocardial structure and electricity deteriorates heart function and increases the susceptibility for arrhythmia. CCM is a novel alternative therapeutic approach for CHF in the clinic. The European Society of Cardiology guidelines suggest that patients with symptomatic HF should consider CCM therapy.1 We chose a rabbit CHF model induced by trans-aortic constriction to investigate the effects of CCM on structural and electrical remodeling of the heart because the rabbit heart shares some physiological similarity to the human heart.24 To the best of our knowledge, this is the first study to report that CCM affected protein expression of subunits of ion channels. The main findings of this study were as follows: (1) CCM therapy attenuated deposition of collagen, shortened the QTc interval and ERP, and decreased the inducibility of VT in rabbits with CHF; and (2) CTGF and Gal-3 protein expression was downregulated, and Cx43, KCNH2, and KCNQ1 protein expression was upregulated by CCM, which might contribute to the therapeutic effects of CCM on CHF.
Myocardial fibrosis is a hallmark of HF and plays a vital role in myocardial structural remodeling. Myocardial structural remodeling is characterized by increased collagen synthesis and decreased collagen degradation in the myocardium, resulting in disproportionate deposition of types collagen I and III.4 Type I collagen has a strong anti-tensile strength and small extension resilience, while type III collagen belongs to embryo-type collagen and has largely extended resilience. The ratio of type I and type III collagen is increased during the process of remodeling, and myocardial stiffness is increased, eventually leading to failure of function.4 In our study, picrosirius red staining and the hydroxyproline assay showed that CCM reduced deposition of collagen. This finding is consistent with our previous study that CCM therapy downregulated types collagen I and III expression by inhibiting the transforming growth factor-β1/Smad3 signaling pathway.19 Further studies are required to investigate the change in other collagen types during CCM therapy of CHF.
As mediators of myocardial fibrosis, CTGF and Gal-3 are involved in decreasing collagen deposition in myocardial interstitium and adverse structural remodeling of CHF. Overexpression of CTGF induces cardiac hypertrophy and collagen production as a downstream mediator of the transforming growth factor-β1/Smad3 signaling pathway during fibrotic response.25,26 Additionally, a Gal-3 inhibitor alleviated collagen deposition and significantly decreased fibrosis.14 In our study, CTGF and Gla-3 protein levels were suppressed by CCM in rabbits with CHF, which may be a potential mechanism by which CCM attenuated structural remodeling.
Electrophysiological remodeling includes changes in a variety of ion channels, the sarcoplasm reticulum calcium cycle, and gap junctions between cells. These changes lead to reduced cardiac electrical stability, slow conduction, and a prolonged action potential duration, making the heart prone to arrhythmia.27 Cx43 is the main component of gap junction channels and is the main conductor of current conduction between ventricular myocytes. Studies have shown that Cx43 is downregulated with HF.15,28 Decreased Cx43 expression contributes to slow conduction and increases the inducibility of reentrant arrhythmia.29 In the current study, we found that rabbits with CHF had prolongation of the QTc interval and ERP, and increased inducibility of VT. The underlying mechanism of these findings may be downregulation of the Ito subunit Kv4.3, the Ikr subunit KCNH2, the Iks subunit KCNQ1, and Cx43.30–32 CCM can shorten the duration of ADP in isolated rabbit hearts and is partially dependent on activation of Iks.33 A previous study on the isolated heart of healthy rabbits showed that CCM may increase the risk for arrhythmia.33 An increase in Cx43 expression is associated with protection from arrhythmias.34 Recently, a study reported that a reduction in fibrosis and upregulation of Cx43 rendered the heart less susceptible to malignant arrhythmias in the isoproterenol model of heart failure.35 In the present study, we found that CCM partially restored the prolonged QTc interval and ERP, and reduced the susceptibility to ventricular arrhythmia. At the molecular level, CCM reversed downregulation of Kv4.3, KCNQ1, KCNH2 and Cx43 protein expression, which may contribute to attenuation of electrical remodeling in HF.
Some limitations of this study should be noted. First, the sample size was relatively small. Second, HF is a complex process, and it remains unclear whether HF from other models, such as volume overload or tachycardia, leads to similar results.
In conclusion, this study shows that CCM treatment attenuates myocardial structural and electrical remodeling in a rabbit model of trans-aortic constriction-induced CHF. The beneficial effects of CCM may be related to downregulation of CTGF and Gal-3, and upregulation of Kv4.3, KCNQ1, KCNH2, and Cx43 in the heart. These findings provide experimental evidence for the clinical use of CCM in treating CHF, but further studies are required to confirm this conclusion.
Authors’ contributions
BN and YD participated in the study design, performed the experiment, collected the data, performed statistical analyses, and drafted the manuscript. XLS and FFZ participated in the study design, performed statistical analyses, and helped to draft the manuscript. QQH, RL, and YXL performed the experiment and collected the data. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Feifei Zhang https://orcid.org/0000-0003-0750-4263
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. DOI: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.Heusch G, Libby P, Gersh B, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014; 383: 1933–1943. DOI: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stix G, Borggrefe M, Wolpert C, et al. Chronic electrical stimulation during the absolute refractory period of the myocardium improves severe heart failure. Eur Heart J 2004; 25: 650–655. DOI: 10.1016/j.ehj.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Chan YS, Liang YJ, et al. Comparison of left ventricular reverse remodeling induced by cardiac contractility modulation and cardiac resynchronization therapy in heart failure patients with different QRS durations. Int J Cardiol 2013; 167: 889–893. DOI: 10.1016/j.ijcard.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 5.Radlberger P, Adlbrecht C, Mittermayr T. Cardiac contractility modulation in patients with heart failure refractory to drug treatment. Exp Clin Cardiol 2011; 16: 43–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Duncker D, Veltmann C. Device therapy in heart failure with reduced ejection fraction-cardiac resynchronization therapy and more. Herz 2018; 43: 415–422. DOI: 10.1007/s00059-018-4710-6. [DOI] [PubMed] [Google Scholar]
- 7.Tschope C, Kherad B, Klein O, et al. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur Heart J 2019; 21: 14–22. DOI: 10.1002/ejhf.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastogi S, Mishra S, Zaca V, et al. Effects of chronic therapy with cardiac contractility modulation electrical signals on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiology 2008; 110: 230–237. DOI: 10.1159/000112405. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, Borggrefe M, Neuser H, et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. Eur J Heart Fail 2019; 21: 1103–1113. DOI: 10.1002/ejhf.1374. [DOI] [PubMed] [Google Scholar]
- 10.Roger S, Rudic B, Akin I, et al. Long-term results of combined cardiac contractility modulation and subcutaneous defibrillator therapy in patients with heart failure and reduced ejection fraction. Clin Cardiol 2018; 41: 518–524. DOI: 10.1002/clc.22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschope C, Van Linthout S, Spillmann F, et al. Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function in HFpEF. Int J Cardiol 2016; 203: 1061–1066. DOI: 10.1016/j.ijcard.2015.10.208. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Chan JY, Zhang Q, et al. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. JACC Cardiovasc Imaging 2009; 2: 1341–1349. DOI: 10.1016/j.jcmg.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Behnes M, Brueckmann M, Lang S, et al. Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol 2014; 103: 107–116. DOI: 10.1007/s00392-013-0626-6. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Li S, Hao X, et al. Perindopril and a Galectin-3 Inhibitor Improve Ischemic Heart Failure in Rabbits by Reducing Gal-3 Expression and Myocardial Fibrosis. Front Physiol 2019; 10: 267. DOI: 10.3389/fphys.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecoraro M, Velia V, Aldo P, et al. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 2015; 768: 71–76. DOI: 10.1016/j.ejphar.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 2004; 61: 208–217. DOI: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Ning B, Qi X, Li Y, et al. Biventricular pacing cardiac contractility modulation improves cardiac contractile function via upregulating SERCA2 and miR-133 in a rabbit model of congestive heart failure. Cell Physiol Biochem 2014; 33: 1389–1399. DOI: 10.1159/000358705. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Liu S, Zhao C, et al. Cardiac contractility modulation improves left ventricular systolic function partially via miR-25 mediated SERCA2A expression in rabbit trans aortic constriction heart failure model. J Thorac Dis 2018; 10: 3899–3908. DOI: 10.21037/jtd.2018.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Dang Y, Li Y, et al. Cardiac Contractility Modulation Attenuate Myocardial Fibrosis by Inhibiting TGF-beta1/Smad3 Signaling Pathway in a Rabbit Model of Chronic Heart Failure. Cell Physiol Biochem 2016; 39: 294–302. DOI: 10.1159/000445624. [DOI] [PubMed] [Google Scholar]
- 20.Hemmeryckx B, Feng Y, Frederix L, et al. Evaluation of cardiac arrhythmic risks using a rabbit model of left ventricular systolic dysfunction. Eur J Pharmacol 2018; 832: 145–155. DOI: 10.1016/j.ejphar.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Khobragade SB, Gupta P, Gurav P, et al. Assessment of proarrhythmic activity of chloroquine in in vivo and ex vivo rabbit models. J Pharmacol Pharmacother 2013; 4: 116–124. DOI: 10.4103/0976-500X.110892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada S, Lo LW, Chou YH, et al. Renal denervation regulates the atrial arrhythmogenic substrates through reverse structural remodeling in heart failure rabbit model. Int J Cardiol 2017; 235: 105–113. DOI: 10.1016/j.ijcard.2017.02.085. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Maguy A, Duverger JE, et al. Induced KCNQ1 autoimmunity accelerates cardiac repolarization in rabbits: potential significance in arrhythmogenesis and antiarrhythmic therapy. Heart Rhythm 2014; 11: 2092–2100. DOI: 10.1016/j.hrthm.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 24.Polyák A, Kui P, Morvay N, et al. Long-term endurance training-induced cardiac adaptation in new rabbit and dog animal models of the human athlete's heart. Rev Cardiovasc Med 2018; 19: 135–142. DOI: 10.31083/j.rcm.2018.04.4161. [DOI] [PubMed] [Google Scholar]
- 25.Accornero F, Van Berlo JH, Correll RN, et al. Genetic Analysis of Connective Tissue Growth Factor as an Effector of Transforming Growth Factor beta Signaling and Cardiac Remodeling. Mol Cell Biol 2015; 35: 2154–2164. DOI: 10.1128/MCB.00199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vainio LE, Szabo Z, Lin R, et al. Connective Tissue Growth Factor Inhibition Enhances Cardiac Repair and Limits Fibrosis After Myocardial Infarction. JACC Basic Transl Sci 2019; 4: 83–94. DOI: 10.1016/j.jacbts.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007; 87: 425–456. DOI: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez JP, Ramachandran J, Xie LH, et al. Selective Connexin43 Inhibition Prevents Isoproterenol-Induced Arrhythmias and Lethality in Muscular Dystrophy Mice. Sci Rep 2015; 5: 13490. DOI: 10.1038/srep13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nisbet AM, Camelliti P, Walker NL, et al. Prolongation of atrio-ventricular node conduction in a rabbit model of ischaemic cardiomyopathy: Role of fibrosis and connexin remodelling. J Mol Cell Cardiol 2016; 94: 54–64. DOI: 10.1016/j.yjmcc.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji Y, Zicha S, Qi XY, et al. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation 2006; 113: 345–355. DOI: 10.1161/CIRCULATIONAHA.105.552968. [DOI] [PubMed] [Google Scholar]
- 31.Freundt JK, Frommeyer G, Spieker T, et al. Histone deacetylase inhibition by Entinostat for the prevention of electrical and structural remodeling in heart failure. BMC Pharmacol Toxicol 2019; 20: 16. DOI: 10.1186/s40360-019-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu SH, Hsiao YW, Chong E, et al. Rhodiola Inhibits Atrial Arrhythmogenesis in a Heart Failure Model. J Cardiovasc Electrophysiol 2016; 27: 1093–1101. DOI: 10.1111/jce.13026. [DOI] [PubMed] [Google Scholar]
- 33.Winter J, Brack KE, Ng GA. The acute inotropic effects of cardiac contractility modulation (CCM) are associated with action potential duration shortening and mediated by beta1-adrenoceptor signalling. J Mol Cell Cardiol 2011; 51: 252–262. DOI: 10.1016/j.yjmcc.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tribulova N, Szeiffova Bacova B, Benova T, et al. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J Electrocardiol 2015; 48: 434–440. DOI:10.1016/j.jelectrocard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Szeiffova Bacova B, Viczenczova C, Andelova K, et al. Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats. Antioxidants 2020; 9: 546. DOI:10.3390/antiox9060546. [DOI] [PMC free article] [PubMed] [Google Scholar]