Abstract
The human pathogen Pseudomonas aeruginosa can rapidly induce fatal sepsis, even in previously healthy infants or children treated with appropriate antibiotics. To reduce antibiotic overuse, exploring novel complementary therapies, such as probiotics that reportedly protect patients against P. aeruginosa infection, would be particularly beneficial. However, the major mechanism underlying the clinical effects is not completely understood. We thus aimed to investigate how probiotics affect IL-8 and human beta-defensin 2 (hBD-2) in P. aeruginosa–infected intestinal epithelial cells (IECs). We infected SW480 IECs with wild type PAO1 P. aeruginosa following probiotic treatment with Lactobacillus rhamnosus GG or Bifidobacterium longum spp. infantis S12, and analysed the mRNA expression and secreted protein of IL-8 and hBD-2, Akt signalling and NOD1 receptor protein expression. We observed that probiotics enhanced hBD-2 expression but suppressed IL-8 responses when administered before infection. They also enhanced P. aeruginosa–induced membranous NOD1 protein expression and Akt activation. The siRNA-mediated Akt or NOD1 knockdown counteracted P. aeruginosa–induced IL-8 or hBD-2 expression, indicating regulatory effects of these probiotics. In conclusion, these data suggest that probiotics exert reciprocal regulation of inflammation and antimicrobial peptides in P. aeruginosa–infected IECs and provide supporting evidence for applying probiotics to reduce antibiotic overuse.
Keywords: Probiotics, Pseudomonas aeruginosa, IL-8, human defensins, intestine epithelia
Introduction
Pseudomonas aeruginosa has become the most common Gram-negative bacterium associated with hospital-acquired infections, particularly in intensive care units,1–3 and represents a leading cause of infection-related fatality among critically ill patients. P. aeruginosa bacteraemia is well known for its rapid progression into fatal sepsis and is responsible for a high mortality rate, even among previously healthy infants and children,4 or patients receiving appropriate initial antimicrobial therapy.5 Several factors contribute to its high mortality rate, such as virulence factors, inappropriate initial antibiotics and multidrug-resistant P. aeruginosa strains. Indeed, antibiotic usage is a risk factor for P. aeruginosa resistance. It is thus highly important to develop a useful strategy to prevent nosocomial P. aeruginosa infection and to limit the use of antibiotics.
Any relevant invasive perturbation of the gut or alteration of its microbiota likely plays a role in promoting systemic inflammation and infection in the critically ill. Community-acquired P. aeruginosa is a reported cause of infectious diarrhoea in immunocompromised adults6 and even immunocompetent children,7,8 sometimes resulting in necrotising bowel lesions, complicated by fulminant septicaemia, and high mortality rates. This implies that intestinal epithelial cells (IECs), the first to come into contact with the pathogen, play a central role in the innate immune response against P. aeruginosa infection.
An effective immune response against microbial infection requires the activation of the innate immune response, including the expression of pro-inflammatory cytokines, chemokines and antimicrobial peptides.9 We previously demonstrated the differential regulation of the inflammatory cytokine IL-8 and antimicrobial peptide human beta-defensin 2 (hBD-2) in P. aeruginosa–infected IECs10 via PI3K/Akt signalling and NOD1 protein expression, respectively. It was suggested that secreted antimicrobial peptides from P. aeruginosa–infected IECs exert bacterial killing, while the down-regulation of the pro-inflammatory response safeguards the host from detrimental inflammation.
The number of experimental and clinical pieces of evidence increases, illustrating the benefits of probiotics in the prevention or treatment of P. aeruginosa infection, including delayed respiratory-tract colonisation/infection by P. aeruginosa in intensive care unit patients,11–13 prophylaxis against sepsis and even death in burns patients complicated with P. aeruginosa14 or fighting P. aeruginosa pulmonary infections.15 In a P. aeruginosa–induced pneumonia mouse model, the early administration of Lactobacillus rhamnosus GG (LGG) reduced lung injury following experimental sepsis.16 Thus, it is logical to postulate that probiotics might modulate innate immunity in IECs against P. aeruginosa infection and prevent its invasion, as well as a consequent sepsis syndrome. However, the major mechanisms underlying the clinical effects are not fully understood.
Therefore, we investigated the effects of probiotics on IL-8 and hBD-2 expression and expressional regulation in P. aeruginosa–infected IECs. We selected two common clinically used probiotic strains – LGG and Bifidobacterium longum (BL) – to evaluate possible strain-related differences in the potential immunomodulatory effects, as both strains provide safe means of inflammatory bowel disease, and other related diseases, prevention and treatment.17
Methods
Cell culture and infection
SW480 human colon cancer cells (BCRC Number: 60249) were purchased from the Bioresource Collection and Research Centre (Hsinchu, Taiwan) and were grown until they reached 60–80% confluence, as reported previously.10 SW480 cells (passage 10–30) were seeded on suitable plates, cultured in growth medium without antibiotics and then infected with 1 × 108 CFU of P. aeruginosa wild type strain PAO1 for 1 h in the absence or presence of 1 × 109 CFU of LGG or BL (Supplemental Figure S1).
Reagents
Standard laboratory reagents were obtained from Sigma–Aldrich (St Louis, MO) or Thermo Fisher Scientific (Waltham, MA).
Bacterial strains
P. aeruginosa strain PAO1, a well-characterised wild type laboratory strain, was grown in tryptic soy broth (Thomas Scientific, Swedesboro, NJ) supplemented with 10 μg/ml kanamycin until the optical density at 600 nm was 0.5. The bacteria were collected by centrifugation at 1400 g for 10 min, washed and finally suspended in PBS (Gibco, Grand Island, NY) for infection in SW480 cells (MOI = 10).
LGG (ATCC 53103) and B. longum spp. infantis S12 (BL; ATCC15697) were obtained from the Bioresource Collection and Research Centre. These strains were grown anaerobically overnight at 37°C in MRS Broth modified (Thomas Scientific) supplemented with 0.5 g/l cysteine (Sigma–Aldrich). The cultures were harvested, washed twice and suspended in sterile PBS.
Cytokine assays
IL-8 and hBD-2 were measured in the culture supernatants by ELISA, according to the manufacturer’s instructions and modified as previously described.10
Western blotting
Equal amounts of total extracted protein from the experimental cultured cells were subjected to SDS-PAGE and then transferred to membranes by semi-dry blotting, as described previously.10,18 After blocking, the membranes were probed with anti-phosphorylated (p)-Akt (#4058, 1:1000; Cell Signaling Technology, Danvers, MA) or anti-NOD1 (ab105338, 1:1000; Abcam, Cambridge, MA). After washing, the membranes were incubated with appropriate HRP-associated secondary Abs, and the signals were visualised with an enhanced chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ). The membranes were then stripped and re-probed with Abs against total GAPDH (sc-137179, 1:1000; Santa Cruz Biotechnology, Dallas, TX) as appropriate.
RNA isolation and cDNA synthesis
Total RNAs were extracted from the experimental cultured cells using TRIzol reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions. The complementary DNAs were synthesised using a reverse transcription kit, as described previously.10,18
Real-time RT-PCR
Transcripts were quantified by real-time RT-PCR in a fluorescence temperature cycler (LightCycler; Roche Diagnostics, Indianapolis, IN) using a method similar to the one previously described.10,18 The following primers were used: IL-8, 5ʹ-AAACCACCGGAAGGAACCAT-3ʹ (forward) and 5ʹ-GCCAGCTTGGAAGTCATGT-3ʹ (reverse); hBD-2, 5ʹ-ATCAGCCATGAGGGTCTTGT-3ʹ (forward) and 5ʹ-GAGACCACAGGTGCCAATTT-3ʹ (reverse); and GAPDH, 5ʹ-CCAGCCGAGCCACATCGCTC-3ʹ (forward) and 5ʹ-ATGAGCCCCAGCCTTCTCCAT-3ʹ (reverse). The number of transcripts was calculated using the comparative threshold cycle (2–ΔΔCt) formula, with the endogenous GAPDH transcript as the internal control.
RNA interference in IECs
RNA interference (RNAi) experiments were carried out using a method similar to that previously described.10,18 Briefly, cells were transfected with siRNAs by using Lipofectamine RNAiMAX (Invitrogen-Thermo Fisher Scientific) to achieve high levels of knockdown. The following siRNAs were prepared: protein kinase B (Akt) siRNA (cat. #1299001; Invitrogen-Thermo Fisher Scientific), NOD1 siRNA (cat. #105131; Ambion-Thermo Fisher Scientific) or control siRNA duplex (Santa Cruz Biotechnology). The efficiency of silencing was confirmed by Western blot analysis. After transfection, the IECs were treated with LGG, BL along with the infection of P. aeruginosa PAO1. Then, the cells were lysed, and total RNAs or proteins were extracted for further analysis.
Cell viability and morphologic features
The representative cultured cells from each experiment were subject to microscopic examination to evaluate cell viability. No significant morphological changes were observed under any experimental condition. Cell viability was calculated as the number of viable cells divided by the total number of cells by trypan blue exclusion.
Statistical analysis
All experiments were carried out at least in triplicate, with similar results. Statistical analyses were performed using the paired Student’s t-test for comparison of two means and ANOVA for three or more means (IBM SPSS Statistics for Windows v17; IBM Corp., Armonk, NY). P values < 0.05 were considered statistically significant.
Results
Effects of probiotics on P. aeruginosa–induced pro-inflammatory IL-8 and antimicrobial peptide hBD-2 mRNA expression in SW480 cells
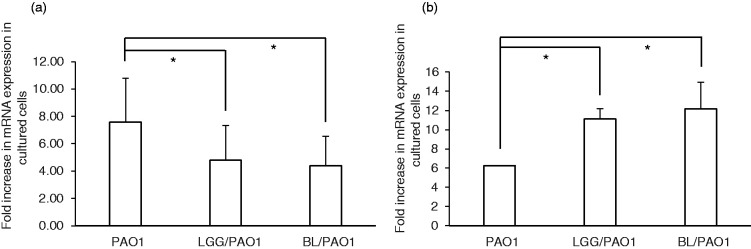
In order to demonstrate how probiotics could affect the innate immune response in P. aeruginosa–infected IECs, we analysed IL-8 and hBD-2 mRNA expression in infected SW480 cells in the presence of probiotics. As shown in Figure 1, we observed a significant reduction in P. aeruginosa PAO1-induced IL-8 mRNA expression in SW480 cells under the treatment of LGG or BL, whereas LGG or BL treatment enhanced hBD-2 mRNA expression in P. aeruginosa PAO1-infected SW480 cells. This suggests that such probiotics could suppress the inflammatory response but enhance antimicrobial peptide expression in P. aeruginosa–infected IECs.
Figure 1.
Immunomodulatory effects of probiotics on IL-8 and hBD-2 mRNA expression in Pseudomonas aeruginosa–infected SW480 cells. SW480 cells were infected or not with P. aeruginosa PAO1 under the treatment of an effective concentration (109 CFU/ml) of LGG or BL. Total RNA samples were extracted after the infection and analysed using real-time quantitative RT-PCR to estimate the number of IL-8 (a) and hBD-2 (b) transcripts. The amount of mRNA produced is shown as the fold increase over non-infected control cells. GAPDH was used as an internal control. mRNA expression levels are shown as the mean ± SEM of duplicate wells from at least three separate experiments (*P < 0.05 compared to P. aeruginosa infection only). hBD-2: human beta-defensin 2; LGG: Lactobacillus rhamnosus GG; BL: Bifidobacterium longum.
Involvement of intracellular PI3K/Akt signalling and NOD1 protein in the regulatory probiotic effects on IEC innate immunity against P. aeruginosa infection
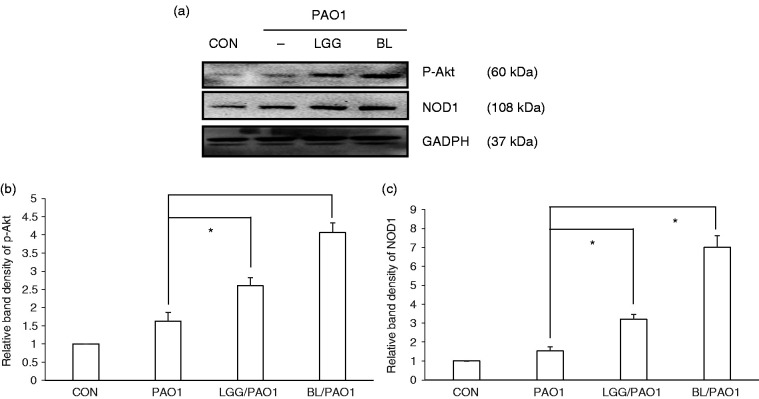
We previously reported that PI3K/Akt signalling down-regulated the expression of IL-8 and that NOD1 protein up-regulated hBD-2 expression in P. aeruginosa–infected IECs.10 In order to study the involvement of these signals in the immunomodulatory probiotic effects on IEC innate immunity against P. aeruginosa infection, SW480 cells were either not infected or infected with P. aeruginosa PAO1 for 1 h under the treatment of LGG or BL. The activation of Akt or NOD1 protein expression was assessed by Western blotting. As showed in Figure 2, we observed that the p-Akt and NOD1 protein levels were significantly up-regulated in P. aeruginosa PAO1-infected SW480 cells when the cells were treated with LGG or BL before PAO1 infection, suggesting that the Akt and NOD1 signalling proteins were involved in the immunoregulatory effects of these probiotics on the innate immunity of SW480 cells against P. aeruginosa infection.
Figure 2.
Effect of probiotics on P. aeruginosa–activated phosphorylated Akt and NOD1 protein expression. SW480 cells were either left untreated (CON) or treated with LGG or BL, along with infection with P. aeruginosa PAO1 for 1 h. Immunoblotting was performed using whole-cell lysates using an Ab to detect phosphorylated (p) Akt and NOD1 protein expressions or GAPDH for normalisation. (a) Representative immunoblots. (b) and (c) Densitometric quantification of immunoreactive bands. Relative band densities of the phosphorylated Akt (b) and NOD1 (c) proteins in the presence of probiotics were quantified as the fold increase compared to the levels in PAO1-infected cells only. Each value represents the mean ± SEM of duplicate wells from at least three separate experiments (*P < 0.05).
Based on our previous report10 describing that PI3K/Akt signalling and NOD1 were involved in the regulation of IL-8 and hBD-2 expression in P. aeruginosa–infected IECs and the above-mentioned observation of the probiotic-mediated up-regulation of P. aeruginosa–induced Akt activation and NOD1 protein expression, we studied if Akt and NOD1 would be involved in the regulation of P. aeruginosa–induced IL-8 and hBD-2 expression by probiotics using an Akt or NOD1 siRNA-mediated knockdown approach. The effective knockdown of Akt or NOD1 in SW480 cells with a specific siRNA was confirmed by Western blotting, as demonstrated previously.10 The siRNA-transfected SW480 cells were then either not infected or infected with P. aeruginosa PAO1 for 1 h under the treatment of LGG or BL. We observed that the LGG- and BL-mediated suppression of P. aeruginosa–induced IL-8 mRNA expression in the SW480 cells was reversed in the Akt-silenced cells (Figure 3). Moreover, we detected that the enhanced effect of the LGG and BL probiotics on the hBD-2 mRNA expression in P. aeruginosa–infected SW480 cells was diminished (Figure 4). This suggests that Akt and NOD1 are involved in the probiotic-mediated regulation of IL-8 and hBD-2 expression in P. aeruginosa–infected SW480 cells, respectively.
Figure 3.
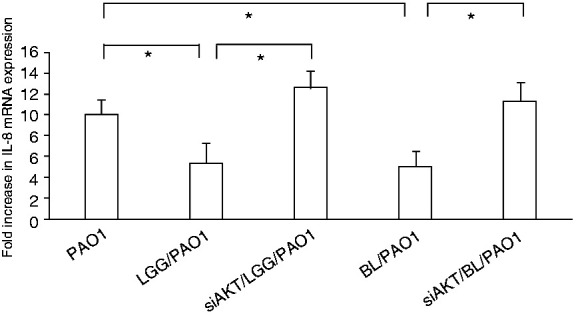
Involvement of Akt in the suppressive probiotic effects on the P. aeruginosa–induced IL-8 expression in SW480 cells. SW480 cells were transfected either with a control or AKT siRNA (siAKT = siRNA to AKT) for 48 h. The transfected cells were then either left non-infected or infected by P. aeruginosa PAO1 for 1 h under the treatment of LGG or BL. After the infection, total RNA samples were prepared and analysed using real-time quantitative RT-PCR to estimate the number of the IL-8 transcripts. The amount of the produced mRNA is shown as the fold increase over non-infected control cells. The corresponding amount of GAPDH is used as a normalised transcript. Results are shown as the mean ± SEM of duplicate wells from at least three separate experiments (*P < 0.05).
Figure 4.
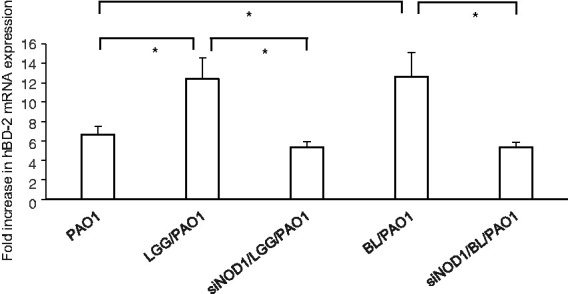
Involvement of NOD1 in the positive probiotic effects on P. aeruginosa–induced hBD-2 in SW480 cells. SW480 cells were transfected with control or NOD1 siRNA (siNOD1 = siRNA to NOD1) for 48 h. The transfected cells were then either left non-infected or infected with P. aeruginosa PAO1 for 1 h under the treatment of LGG or BL. After the infection, total RNA samples were prepared and analysed using real-time quantitative RT-PCR to estimate the number of hBD-2 transcripts. The amount of produced mRNA is shown as the fold increase over the non-infected control cells. The corresponding amount of GAPDH is used as a normalised transcript. Results are shown as the mean ± SEM of duplicate wells from at least three separate experiments (*P < 0.05).
Probiotics suppressed P. aeruginosa–induced IL-8 secretion while enhancing hBD-2 secretion in SW480 cells with the involvement of AKT and NOD1
In order to examine if the use of probiotics would have any functional consequence on P. aeruginosa–infected SW480 cells, similar to their effect on mRNA expression, we investigated their effect on IL-8 and hBD-2 protein production. The cultured cells were either infected or not with wild type P. aeruginosa strain PAO1 for 1 h in the presence or absence of probiotics, LGG or BL. The supernatant of cultured cells was analysed by ELISA for IL-8 and hBD-2 secretion. As shown in Figure 5, the PAO1 infection induced both IL-8 and hBD-2 protein secretion in the SW480 cells. The use of probiotics, either LGG or BL, suppressed Salmonella-induced IL-8 production while enhancing hBD-2 production. Similar to the mRNA experiments, we used specific siRNA to knock down AKT or NOD1, and analysed IL-8 and hBD-2 protein production after the probiotic treatment and P. aeruginosa infection. We observed that the suppressed or enhanced LGG and BL probiotic effects on IL-8 or hBD-2 protein secretion in P. aeruginosa–infected SW480 cells were counteracted following the AKT or NOD1 knockdown, respectively. This confirmed the involvement of the PI3K/AKT signalling pathway and NOD1 not only in the mRNA expression but also in the probiotic-regulated IL-8 and hBD-2 protein production in P. aeruginosa–infected SW480 cells.
Figure 5.
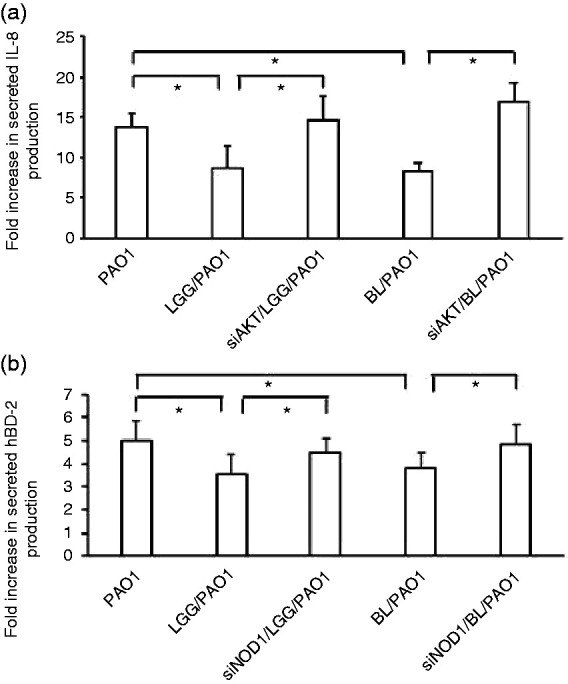
Involvement of AKT and NOD1 in the regulated probiotic effects on IL-8 and hBD-2 protein secretion in P. aeruginosa–infected SW480 cells. SW480 cells were transfected with control siRNA and NOD2 siRNA (siRNA=non-target control siRNA; siAKT=siRNA to AKT; siNOD1=siRNA to NOD1) for 48 h. The transfected cells were left uninfected or infected with P. aeruginosa PAO1 for 1 h under the treatment of LGG or BL. Supernatant was analysed by ELISA for IL-8 and hBD-2. The amount of the produced IL-8 (a) and hBD-2 (b) is shown as the fold increase over non-infected control cells. Results are represented as the mean±SEM for at least three determinations from independent experiments (*P < 0.05).
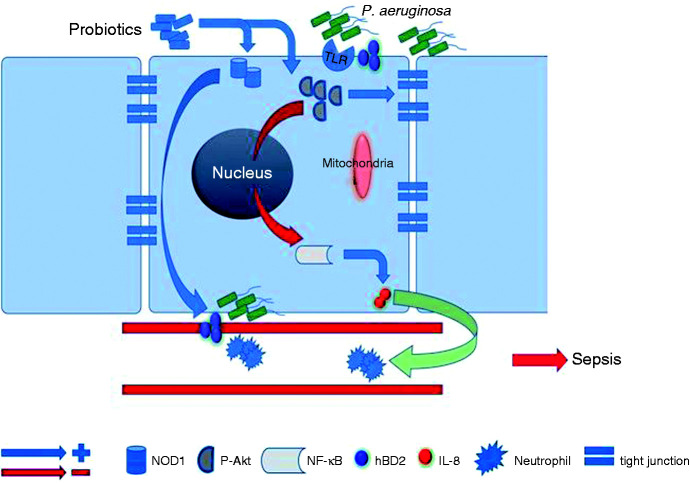
Figure 6.
Proposed mechanisms of the protective probiotic effects against the P. aeruginosa infection by enhancing pathogen elimination and abating the harmful effects of excessive IEC inflammation in order to protect the host from pathogen invasion, subsequent P. aeruginosa sepsis and progressive pneumonia.
Discussion
Diarrhoea was reported in half of the included studies in a Cochrane review surveying the reduced incidence of ventilator-associated pneumonia by probiotics.19 Community-acquired P. aeruginosa sepsis in previously healthy infants and children frequently causes initial symptoms of fever and diarrhoea.4 The gut–lung axis has emerged as a specific axis with intensive dialogs between both organs. Lung pathogens impact the mucosal immunity and contribute to immune tolerance or influence the gut immune system through neutrophil recruitment, production of pro-inflammatory cytokines and the release of antimicrobial peptides such as hBD-2.20 However, intestinal microbial disruption has been associated with immune reaction–mediated intestinal injury after influenza virus lung infection.21 Certain gut microbiota perturbations are predictive of airway colonisation with P. aeruginosa.22 Oral administration of B. longum prevents gut-derived P. aeruginosa sepsis in mice.23 The main mechanisms involved in promoting bacterial translocation are bacterial overgrowth, disruption of the gut mucosal barrier and impairment of host defence mechanisms24 and interference of P. aeruginosa adherence to IECs.25 Besides, the ability of P. aeruginosa to adhere to the intestinal tract might contribute to the development of fatal septicaemia.26 Oral use of mixtures of probiotics prolongs survival after experimental sepsis by multidrug-resistant P. aeruginosa infection in rodents.27 All imply that the gut plays a major role in the defence mechanisms against P. aeruginosa infection, and probiotics could act as protective therapy in the case of P. aeruginosa infection. However, to our knowledge, no report has demonstrated the effects of probiotics on P. aeruginosa–induced innate immunity in IECs.
IL-8 recruits and activates neutrophils to enhance the clearance of P. aeruginosa infection. However, increased IL-8 leads to massive infiltration of neutrophils, which results in the production of proteolytic enzymes, such as elastase, and subsequent tissue destruction and sepsis. Persistent infection with P. aeruginosa leads to exuberant production of IL-8 and neutrophil subject to Pseudomonas sepsis.28 Clinically, the efficacy of erythromycin therapy for patients with chronic airway diseases might be partly mediated through an inhibitory effect of erythromycin on IL-8 release in the airways.29 The protection effect of probiotics administered at the onset of sepsis, including LGG and BL, on reducing mortality in paediatric experimental sepsis30 is perhaps attributed to improved intestinal epithelial homeostasis, diminished local and systemic inflammation and the consequent obstacle of systemic bacteraemia. Our observation that probiotics diminished P. aeruginosa–induced IL-8 expression in IECs supports the potential benefit of probiotics to suppress the overwhelming mucosal inflammation, leaky gut and bacterial translocation and finally to block sepsis.
Anti-inflammatory PI3K-Akt signalling is suppressed in cystic fibrosis (CF),31 resulting in susceptibility to P. aeruginosa infection. During P. aeruginosa infection, the membranous PI3K/Akt pathway becomes activated32 to mediate internalisation of the pathogen33 and promotes P. aeruginosa elimination.34 Chinese medicine ameliorates P. aeruginosa–induced acute lung inflammation by inhibiting the release of cytokines (TNF-α and IL-6) and chemokines (IL-8 and RANTES) and reducing leucocyte recruitment to the inflamed tissue sites via the PI3K/Akt pathway.35 The protein kinase B/Akt pathway in the colon also mediates the protective probiotic effects on experimental sepsis.30 Our observation, showing that probiotics exert Akt-mediated IL-8 inhibition in P. aeruginosa–infected IECs, might explain the benefit of using probiotics in the case of gut-derived P. aeruginosa sepsis.
There is increasing evidence supporting that the regulation of hBD-2 expression and degradation has profound implications in pulmonary infections but is rarely reported in the case of intestinal infection. The protein hBD-2 is an essential component of the innate immune system and exhibits high antibacterial activity against P. aeruginosa and other Gram-negative bacteria.36 Clinically, increased plasma defensin levels are observed in both adults and children with sepsis; these might be involved in defence against sepsis.37,38 However, inducibility of hBD-2 is impaired in patients with severe sepsis.39 In line with previous reports that several probiotics can induce hBD-2, we observed that probiotics enhanced hBD-2 expression in P. aeruginosa–infected IECs, suggesting that probiotics could be used to prevent P. aeruginosa sepsis in the host.
NOD1 plays a part in the innate immune response against P. aeruginosa. Outer membrane vesicles released by probiotic or commensal Escherichia coli activate the NOD1-dependent immune responses in intestinal epithelial cells,40 which is essential for maintaining intestinal homeostasis. The constant stimulation of NOD1 by gut microbiotic vesicles results in controlled inflammatory responses that might help pathogen eradication and host survival. Gut mucosal injury caused by apoptosis of intestinal epithelia leads to barrier dysfunction and bacterial translocation, which could result in P. aeruginosa sepsis secondary to pneumonia.41 Intestinal epithelial apoptosis is markedly up-regulated in both patients with sepsis and in preclinical models of sepsis.42 Pre-treatment with probiotic L. rhamnosus reportedly enhanced Akt (an anti-apoptotic protein) phosphorylation and tight junction integrity, thus enhancing the barrier function and restricting pathogen invasion.43 It suggests that probiotics protect IECs from pathogen-induced damage and invasion, which might occur through the innate immune synergism between Akt and NOD1.
Based on our previous study, describing that P. aeruginosa infection evokes the anti-inflammatory PI3K/Akt signalling pathway to block the detrimental IL-8 and NOD1 inflammatory effect to enhance bacterial elimination by hBD-2,10 here we observed that probiotic administration prior to P. aeruginosa infection enhanced the hBD-2 expression but suppressed the IL-8 response in P. aeruginosa–infected SW480 cells. This suggests that such probiotics might enhance antimicrobial peptide secretion by P. aeruginosa–infected intestinal mucosa to defend against infection and reduce detrimental inflammation to decrease the translocation of the pathogen and consequent sepsis.
In order to increase the efficacy of probiotics, synthetic biology44 is in constant development to engineer novel therapeutic functionalities into host-associated microbes and to deliver recombinant probiotics, called ‘designer probiotics’, to combat specific pathogens. It will be a next-generation challenge to oppose the increasing rate of antibiotic-resistant pathogens and the rather slow development of new and efficient antibiotics. For example, the engineered probiotic strain E. coli Nissle 1917 shows prophylactic and therapeutic activity against P. aeruginosa gut infection in two animal models.45 It supports the development of engineered probiotics to eliminate and prevent bacterial gut infections. However, the mechanistic understanding of the interaction among hosts, pathogens and probiotics would be the rationale for engineering probiotics with biomedical benefits to replace antibiotics.
In summary, we have demonstrated that probiotics exert a suppressive effect on IL-8 expression but enhance hBD-2 expression in P. aeruginosa–infected SW480 cells with the involvement of Akt and NOD1, respectively. These data suggest probiotics accomplish reciprocal regulation of IL-8 and hBD-2 expression in P. aeruginosa–infected IECs via different pathways. Upon P. aeruginosa infection, the probiotic-induced antimicrobial peptide up-regulation in the IECs does not merely enhance pathogen elimination, but also protects the host from harmful effects of excessive inflammation. Figure 5 illustrates the proposed probiotic protective effect mechanisms during P. aeruginosa infection. Probiotics could thus serve as useful complementation or alternative therapy to reduce the overuse of antibiotics.
Supplemental Material
Supplemental material, sj-pdf-1-ini-10.1177_1753425920959410 for Beneficial effect of probiotics on Pseudomonas aeruginosa–infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation by Fu-Chen Huang, Yi-Ting Lu and Yu-Hsuan Liao in Innate Immunity
Acknowledgements
The authors would like to thank An-Chi Liu for her technical support.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Ministry of Science and Technology grant MOST 105-2314-B-182-054. The role of the funding body was to support the design and accomplishment of the study.
ORCID iD
Fu-Chen Huang https://orcid.org/0000-0003-3702-6856
Supplemental material
Supplemental material for this article is available online.
References
- 1.Neuhauser MM, Weinstein RA, Rydman R, et al. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 2003; 289: 885–888. [DOI] [PubMed] [Google Scholar]
- 2.Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics 1999; 103: e39. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995; 274: 639–644. [PubMed] [Google Scholar]
- 4.Huang YC, Lin TY, Wang CH. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J 2002; 21: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 5.Kang CI, Kim SH, Park WB, et al. Clinical features and outcome of patients with community-acquired Pseudomonas aeruginosa bacteraemia. Clin Microbiol Infect 2005; 11: 415–418. [DOI] [PubMed] [Google Scholar]
- 6.Adlard PA, Kirov SM, Sanderson K, et al. Pseudomonas aeruginosa as a cause of infectious diarrhoea. Epidemiol Infect 1998; 121: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung CK, Lee KH. Community acquired fulminant Pseudomonas infection of the gastrointestinal tract in previously healthy infants. J Paediatr Child Health 1998; 34: 584–587. [DOI] [PubMed] [Google Scholar]
- 8.Tsai MJ, Teng CJ, Teng RJ, et al. Necrotizing bowel lesions complicated by Pseudomonas septicaemia in previously healthy infants. Eur J Pediatr 1996; 155: 216–218. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007; 449: 819–826. [DOI] [PubMed] [Google Scholar]
- 10.Huang FC. Differential regulation of interleukin-8 and human beta-defensin 2 in Pseudomonas aeruginosa–infected intestinal epithelial cells. BMC Microbiol 2014; 14: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med 2010; 182: 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forestier C, Guelon D, Cluytens V, et al. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care 2008; 12: R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014: CD009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argenta A, Satish L, Gallo P, et al. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS One 2016; 11: e0165294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandre Y, Le Berre R, Barbier G, et al. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol 2014; 14: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khailova L, Baird CH, Rush AA, et al. Lactobacillus rhamnosus GG improves outcome in experimental Pseudomonas aeruginosa pneumonia: potential role of regulatory T cells. Shock 2013; 40: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, et al. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int 2015; 2015: 505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang FC, Huang SC. The different effects of probiotics treatment on Salmonella-induced interleukin-8 response in intestinal epithelia cells via PI3K/Akt and NOD2 expression. Benef Microbes 2016; 7: 739–748. [DOI] [PubMed] [Google Scholar]
- 19.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014; 10: CD009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enaud R, Prevel R, Ciarlo E, et al. The gut–lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 2020; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Li F, Wei H, et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 2014; 211: 2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoen AG, Li J, Moulton LA, et al. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr 2015; 167: 138–147.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto T, Ishikawa H, Tateda K, et al. Oral administration of Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa sepsis in mice. J Appl Microbiol 2008; 104: 672–680. [DOI] [PubMed] [Google Scholar]
- 24.Hirakata Y, Furuya N, Tateda K, et al. In vivo production of exotoxin A and its role in endogenous Pseudomonas aeruginosa septicemia in mice. Infect Immun 1993; 61: 2468–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto T, Tateda K, Miyazaki S, et al. Effect of immunisation with Pseudomonas aeruginosa on gut-derived sepsis in mice. J Med Microbiol 1998; 47: 295–301. [DOI] [PubMed] [Google Scholar]
- 26.Furuya N, Hirakata Y, Tomono K, et al. Mortality rates amongst mice with endogenous septicaemia caused by Pseudomonas aeruginosa isolates from various clinical sources. J Med Microbiol 1993; 39: 141–146. [DOI] [PubMed] [Google Scholar]
- 27.Machairas N, Pistiki A, Droggiti DI, et al. Pre-treatment with probiotics prolongs survival after experimental infection by multidrug-resistant Pseudomonas aeruginosa in rodents: an effect on sepsis-induced immunosuppression. Int J Antimicrob Agents 2015; 45: 376–384. [DOI] [PubMed] [Google Scholar]
- 28.Shen XF, Cao K, Jiang JP, et al. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med 2017; 21: 1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi K, Sonoda F, Kobayashi S, et al. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun 1994; 62: 4145–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khailova L, Frank DN, Dominguez JA, et al. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology 2013; 119: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang PX, Cheng J, Zou S, et al. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun 2015; 6: 6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel J, Eran Y. Subversion of mucosal barrier polarity by Pseudomonas aeruginosa. Front Microbiol 2011; 2: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kierbel A, Gassama-Diagne A, Mostov K, et al. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol Biol Cell 2005; 16: 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riquelme SA, Hopkins BD, Wolfe AL, et al. Cystic fibrosis transmembrane conductance regulator attaches tumor suppressor PTEN to the membrane and promotes anti Pseudomonas aeruginosa immunity. Immunity 2017; 47: 1169–1181.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Y, Nie Y, Cheng B, et al. Qingfei Xiaoyan Wan, a traditional Chinese medicine formula, ameliorates Pseudomonas aeruginosa–induced acute lung inflammation by regulation of PI3K/AKT and Ras/MAPK pathways. Acta Pharm Sin B 2016; 6: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature 1997; 387: 861. [DOI] [PubMed] [Google Scholar]
- 37.Panyutich AV, Panyutich EA, Krapivin VA, et al. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med 1993; 122: 202–207. [PubMed] [Google Scholar]
- 38.Thomas NJ, Carcillo JA, Doughty LA, et al. Plasma concentrations of defensins and lactoferrin in children with severe sepsis. Pediatr Infect Dis J 2002; 21: 34–38. [DOI] [PubMed] [Google Scholar]
- 39.Book M, Chen Q, Lehmann LE, et al. Inducibility of the endogenous antibiotic peptide beta-defensin 2 is impaired in patients with severe sepsis. Crit Care 2007; 11: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canas MA, Fabrega MJ, Gimenez R, et al. Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front Microbiol 2018; 9: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia-induced sepsis. Crit Care Med 2000; 28: 2573–2577. [DOI] [PubMed] [Google Scholar]
- 42.Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 2574–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Zhu YH, Yang JC, et al. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS One 2015; 10: e0125717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bober JR, Beisel CL, Nair NU. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu Rev Biomed Eng 2018; 20: 277–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang IY, Koh E, Wong A, et al. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat Commun 2017; 8: 15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ini-10.1177_1753425920959410 for Beneficial effect of probiotics on Pseudomonas aeruginosa–infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation by Fu-Chen Huang, Yi-Ting Lu and Yu-Hsuan Liao in Innate Immunity