Abstract
Sepsis is a life-threatening disease caused by systemic dys-regulated inflammatory response to infection. We previously revealed that LL-37, a human cathelicidin antimicrobial peptide, improves the survival of cecal ligation and puncture septic mice. Ectosomes, microvesicles released from neutrophils, are reported to be elevated in sepsis survivors; however, the functions of ectosomes in sepsis remain largely unknown. Therefore, we herein elucidated the protective action of LL-37 on sepsis, by focusing on LL-37-induced ectosome release in a cecal ligation and puncture model. The results demonstrated the enhancement of ectosome levels by LL-37 administration, accompanied by a reduction of bacterial load. Importantly, ectosomes isolated from LL-37-injected cecal ligation and puncture mice contained higher amounts of antimicrobial proteins/peptides and exhibited higher antibacterial activity, compared with those from PBS-injected cecal ligation and puncture mice, suggesting that LL-37 induces the release of ectosomes with antibacterial potential in vivo. Actually, LL-37 stimulated mouse bone-marrow neutrophils to release ectosomes ex vivo, and the LL-37-induced ectosomes possessed antibacterial potential. Furthermore, administration of LL-37-induced ectosomes reduced the bacterial load and improved the survival of cecal ligation and puncture mice. Together these observations suggest LL-37 induces the release of antimicrobial ectosomes in cecal ligation and puncture mice, thereby reducing the bacterial load and protecting mice from lethal septic conditions.
Keywords: Sepsis, antimicrobial peptide LL-37, neutrophil, ectosome, antibacterial activity
Introduction
Sepsis has recently been re-defined as a life-threatening organ dysfunction resulting from dys-regulated host response to infection, involving both pro- and anti-inflammatory processes.1 Despite substantial advances in our knowledge regarding the pathophysiology of sepsis and subsequent improvements of clinical care, sepsis is still a major cause of death in the intensive care unit with an estimated mortality rate of 25–50%.1–3 This unacceptably high mortality is considered to be due to the absence of a reliable diagnostic marker and definitive therapeutic strategy that manages the disease.1
Neutrophils are phagocytes that are the first line of host defense against invading pathogens.4 Neutrophils exert antimicrobial activity through phagocytosis and subsequent killing of microbes through the action of reactive oxygen species and antibacterial granule proteins or peptides such as myeloperoxidase (MPO), lactoferrin, and cathelicidins.4 Of the antibacterial peptides, LL-37 is a sole human cathelicidin that is proteolytically cleaved from an 18 kDa cationic antimicrobial protein (CAP18).4 In addition to its potent antibacterial activity, LL-37 exerts diverse biological activities.5 We have shown that LL-37 inhibits the spontaneous apoptosis of neutrophils via P2X7 purinergic receptor and formyl peptide receptor-like 1 (FPRL1),6 and suppresses Gram-negative bacterial LPS-induced endothelial cell apoptosis by blocking the binding of LPS to cell-surface CD14 and TLR4.7 We further demonstrated the protective action of LL-37 on a murine endotoxin shock model and on a mouse cecal ligation and puncture (CLP) sepsis model.8–10
Various host cells release submicron extracellular vesicles, including microvesicles (0.1–1.0 μm in diameter) and exosomes (30–150 nm in diameter) that mediate intercellular communications.11,12 During the inflammatory response, neutrophils release microvesicles, called ectosomes, which bud off from the cell membrane.11,12 Ectosomes express the cell surface molecules originating from neutrophils such as Ly6G and phosphatidylserine (PS), and have the functional proteins of neutrophils.12,13 Neutrophil-derived ectosomes modulate the inflammatory response, either pro- or anti-inflammatory depending on the target cells. Ectosomes adhere to endothelial cells14 and enhance the expression of pro-inflammatory molecules (IL-6 and IL-8) by the cells.15 In addition, ectosomes deliver granule proteins to intestinal epithelial cells and impair their wound healing.16 In contrast, they exert an anti-inflammatory action on monocytes by inducing TGF-β1 but reducing TNF-α and IL-8.17,18 Moreover, ectosomes released from bacteria-stimulated neutrophils have bacteriostatic potential by forming aggregates with bacteria.19
Notably, ectosome levels are reported to be augmented in patients sepsis surviving;20,21 thus, ectosomes are speculated to play a protective role in sepsis. However, detailed actions of ectosomes in sepsis have not been elucidated. We previously demonstrated that LL-37 improves survival in a murine CLP sepsis model.8,9 In this study, therefore, we elucidated the protective action of LL-37 on sepsis, by focusing on the effect of LL-37 on ectosome release in the CLP model. The results demonstrated that LL-37 induced the release of ectosome-containing microvesicles with antibacterial potential and reduced the bacterial burden in CLP mice. Moreover, LL-37 stimulated mouse bone-marrow neutrophils to release antibacterial ectosomes ex vivo, and the administration of LL-37-induced ectosomes reduced the bacterial load and improved the survival of CLP mice.
Materials and methods
Reagents
A 37-mer peptide of human cathelicidin (LL-37; 1LLGDFFRKSKEKIGKEFKRIVQ RIKDFLRNLVPRTES37) and a 38-mer peptide of mouse CRAMP (1ISRLAGLLRKGGEKI GEKLKKIGQKIKNFFQKLVPQPE,38 cathelicidin-related antimicrobial peptide, a mouse ortholog of human cathelicidin peptide) were synthesized in house by the solid-phase method on a peptide synthesizer (model PSSM-8, Shimadzu, Kyoto, Japan) and purified as previously described.22 Polyclonal Ab against CRAMP was raised in rabbits by Scrum Inc. (Tokyo, Japan) and partially purified by sodium sulfate precipitation.23 The concentrations of synthesized peptides and purified Ab were determined using bicinchoninic acid (BCA) protein assay reagents (Pierce, Rockford, IL) with BSA as a standard. N-Formyl-Met-Leu-Phe (fMLF), heparin sodium salt, 4-aminobenzoic acid hydrazide (MPO inhibitor), and SB225002 (CXCR2 antagonist)24 were purchased from Sigma-Aldrich (Saint Louis, MO). WRW4 (formyl peptide receptor 2 (FPR2, a mouse ortholog of FPRL1) antagonist),25 normal rabbit IgG, 4% paraformaldehyde (PFA) phosphate buffer solution, and isoflurane were obtained from Fujifilm Wako Pure Chemical (Osaka, Japan). Anti-mouse CD16/CD32 (clone 2.4G2), phycoerythrin (PE)-rat anti-mouse Ly6G (Gr1) (clone RB6-8C5), and PE-conjugated rat IgG2b isotype control (clone LTF-2) were purchased from eBiosciences (San Diego, CA). Rabbit anti-MPO heavy chain (sc-33596) was from Santa Cruz Biotechnology (Dallas, TX). FITC-conjugated Annexin V, rabbit anti-cathelicidin (ab180760), and rabbit anti-lactoferrin (ab15811) were purchased from Abcam (Cambridge, UK). Percoll was from GE Healthcare (Chicago, IL). HRP-conjugated goat anti-rabbit IgG was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). The following reagents were tested for the endotoxin level and used for isolation and stimulation of neutrophils as well as animal experiments: distilled water (Otsuka Pharmaceutical Co. Tokyo, Japan), saline (Otsuka Pharmaceutical Co.), PBS (Nacalai Tesque, Kyoto, Japan), Hank’s balanced salt solution with (HBSS+) or without Mg2+ and Ca2+ (HBSS-) (ThermoFisher Scientific, Waltham, MA), RPMI 1640 (Sigma-Aldrich), and DMSO (Sigma-Aldrich).
Murine CLP sepsis model
Specific pathogen-free BALB/c female mice (Sankyo Labo Service, Tokyo, Japan) were allowed to acclimate for 1 wk in a temperature-controlled facility with 12/12 h light/dark cycles, and food and water ad libitum. All experimental procedures were approved by the Ethics Committee for the Use of Laboratory Animals of Juntendo University (300194, April 2018).
CLP was performed according to the previous description.26 Briefly, mice (aged 7–8 wk) were anesthetized with isoflurane. The abdominal skin was disinfected with 70% ethanol, then a 1 cm midline laparotomy was performed. The cecum was exteriorized, the contents massaged to the tip, and the distal 7 mm of the tip was ligated with 3-0 silk suture. The ligated portion was perforated once with an 18G needle and a small amount of stool (1 mm in length) was extruded. The cecum was replaced back into the abdominal cavity and the midline incision was closed in two layers with 3-0 nylon suture. Mice were resuscitated by subcutaneous administration of 1 ml saline in the neck. In the sham group, mice underwent the same procedure without ligation and perforation. To evaluate the effect of LL-37 on ectosome release in CLP mice, 200 μl of LL-37 solution (3 μg) or PBS as a control was intravenously administered just prior to the CLP operation. To determine the humane endpoint, we employed the murine sepsis score that can be used as a surrogate marker of mortality27 with some modifications; mice were euthanized when they manifested ruffled hair, no response to touch stimuli, completely closed eyes, and consistent labored breathing.
Preparation of the peritoneal lavage fluid and plasma from CLP mice
Then 14–16 h after the operation, mice were anesthetized with isoflurane and the blood was drawn by cardiac puncture and mixed with 3 units of heparin sodium (Mochida Pharmaceutical Co., Tokyo, Japan). The platelet-depleted plasma was obtained by centrifugation of heparinized blood at 3000 g for 20 min at 4°C. Furthermore, the skin was cut then saline or HBSS- (3.5 ml) was injected into the peritoneal cavity using a 26G needle, the abdomen gently massaged, and the peritoneal exudate was collected using a 21G needle. To count the number of peritoneal cells, the peritoneal exudate (100 μl) was mixed with the same volume of 4% PFA in PBS, incubated for 5 min at room temperature (21°C), and centrifuged at 500 g for 5 min. The pelleted cells were suspended in 100 μl of 2% PFA in PBS. Then, the fixed cells (10 μl) were mixed with 90 μl of Turk’s solution (Nacalai Tesque), and the numbers of polymorphonuclear cells and mononuclear cells (macrophages) counted under a microscope at a × 400 magnification. Peritoneal cells were also collected from unoperated mice (without sham and CLP operations) and counted. To determine the bacterial load in the peritoneal exudates and blood of CLP and sham mice, an aliquot of the peritoneal exudates or heparinized blood was serially diluted with PBS, plated (50 μl) on Luria-Bertani (LB) plates, and incubated aerobically overnight (14–16 h) at 37°C.
Isolation of microvesicles from peritoneal exudates
Microvesicles were isolated according to the previous description20 with some modification. Briefly, the peritoneal exudate was centrifuged at 400 g for 5 min at 4°C to sediment cells, and the supernatant was further centrifuged at 5000 g for 10 min at 4°C to sediment cell debris, microbes, and large-sized particles. Then the resulting supernatant was centrifuged at 100,000 g for 1 h at 4°C to sediment microvesicles. The pellet was re-suspended in PBS and centrifuged again (100,000 g, 30 min). The resulting pellet was re-suspended in PBS (300–400 μl), followed by centrifugation at 10,000 g for 5 min at 4°C to further sediment the residual bacteria. The supernatant, designated as the microvesicle fraction, was subjected to downstream analyses. The protein content of the microvesicle fractions was determined by BCA protein assay.
The microvesicles were assayed by flow cytometry immediately after their preparation from peritoneal exudates or supernatants of LL-37-stimulated bone marrow-derived neutrophils (described below), as well as after the preparation of platelet-depleted plasma. In contrast, for analyses of Western blotting and antibacterial activity, the microvesicles of peritoneal exudates and supernatants of LL-37-stimulated neutrophils (as described in the section below) were stored at -80°C after preparation, and thereafter the fractions were thawed and utilized for the analyses.
Flow cytometry analysis for microvesicles
To measure the counts of neutrophil-derived ectosomes, the microvesicle fractions (20 μl) prepared from peritoneal exudates or platelet-depleted plasma were incubated with anti-CD16/CD32 IgG (1 ng/sample) for 10 min on ice to block Fc receptors, then incubated with PE-conjugated rat anti-mouse Ly6G (20 ng/sample) and FITC-Annexin V (1 μl/sample) in the binding buffer (10 mM HEPES/NaOH, pH 7.4, 250 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) for 30 min on ice. For the gating control, microvesicles were incubated with PE-rat IgG isotype (20 ng/sample) and FITC-Annexin V (1 μl/sample) in Ca2+-free buffer. To normalize the microvesicle counts, Trucount fluorescent beads (BD Biosciences, Farnklin Lakes, NJ) suspended in PBS (1 × 102 particles/μl) were added prior to the flow cytometry analysis as an internal control. Microvesicle populations were sized by forward- and side-scatter gates using MegaMix (0.5, 0.9, and 3 μm beads) (Biocytex, Marseille, France) or Flow Cytometry Sub-micron Particle Size Reference (0.5, 1, and 2 μm beads) (Molecular Probes, Eugene, OR). Samples were loaded to FACSVerse (BD Biosciences) or LSRFortessa (BD Biosciences), and analyzed using FlowJo software (Tree Star, Ashland, OR). Ectosomes were defined as particles with a diameter of 0.5–1 μm (0.5 μm is the minimum detection limit of the flow cytometers used) and double-positive for both Annexin V (a substance with an ability to bind to phosphatidylserine) and Ly6G (a neutrophil surface marker) according to the previous publication.28
Assay for antibacterial activity of microvesicles
Bacteria isolated from the cecum of a BALB/c mouse was identified as Escherichia coli using a Microflex (Bruker, Billerica, MA) MALDI-TOF mass spectrometry system. For antibacterial assay, the E. coli was cultured in LB broth until the mid-log phase and washed three times with PBS. Then bacteria (5 × 103 cells) were incubated with the fraction containing the indicated amounts of microvesicles or vehicle (PBS) for 20 min at 37°C by shaking at 100 rpm in a total volume of 50 μl. Thereafter, bacteria were serially diluted with PBS, plated on LB plates (one to three plates per sample) and incubated overnight at 37°C. Formed colonies were counted, and the relative bacteria viability was calculated and expressed as percentage by dividing the CFU incubated with microvesicles by that with vehicle (PBS). To examine the effect of neutralizing Abs and inhibitors for neutrophil-derived granule molecules, microvesicle fractions (25 μl) were pre-incubated with anti-lactoferrin IgG (0.1 μg), anti-CRAMP IgG (0.1 μg), normal rabbit IgG (0.1 μg), 4-aminobenzoic acid hydrazide (1 μg/ml), heparin sodium (Sigma-Aldrich; 0.1 μg), or vehicle (PBS for IgGs, or DMSO for 4-aminobenzoic acid hydrazide) for 20 min at room temperature, then incubated with bacteria (5 × 103 cells, 25 μl) as described above. The antibacterial activity of microvesicle fractions in the absence or presence of an IgG or an inhibitor was calculated by the following formula: the antibacterial activity (%) = (1-(CFU when incubated with microvesicle fraction in the absence or presence of an IgG or an inhibitor/CFU when incubated only with PBS or DMSO)) × 100.
Western blotting
Microvesicle fractions (1–3 μg) prepared from CLP mice were subjected to 8% or 13% SDS-polyacrylamide gel analysis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Billerica, MA) using a Trans Blot SD Semi Dry Transfer Cell (Bio-Rad, Hercules, CA). The membrane was blocked with Block One (Nacalai Tesque), and incubated with a primary Ab (rabbit anti-MPO, anti-cathelicidin, anti-LL-37 or anti-lactoferrin; 1:1000 dilution) followed by secondary Ab (HRP-goat anti-rabbit IgG, 1:5000 dilution). The membrane was then incubated with SuperSignal West Dura Extended Duration Substrate (Pierce), and bands were visualized with an ImageQuant LAS-4000 (GE Healthcare). Furthermore, microvesicle fractions were subjected to SDS-PAGE, and the gel was stained with Coomassie Brilliant Blue to confirm the same amounts of proteins were analyzed.
Isolation of mouse bone marrow-derived neutrophils and induction of microvesicle release
Mice (11–14 wk old) were sacrificed by CO2 inhalation. Neutrophils were isolated from mouse bone marrow according to the previous description26 with some modifications. Briefly, femurs and tibias were taken out then muscles, nerves, and other tissues were removed. The bones were rinsed with 70% ethanol, followed by washing three times with PBS. The epiphyses were trimmed and bone-marrow cells were flushed with RPMI 1640 medium. After passing through a 100 μm cell strainer (PluriSelect Life Science, Leipzig, Germany), cells were centrifuged at 400 g for 7 min at 4°C. Erythrocytes were hypotonically lysed with 0.2% NaCl, and the osmolarity was restored with 1.6% NaCl. Cells were centrifuged at 400 g for 7 min at 4°C, re-suspended in 5 ml PBS, laid over 5 ml of 55% Percoll layer (100% Percoll was diluted with 0.15 M NaCl), and centrifuged at 800 g for 20 min at room temperature. Neutrophils recovered in the pellet were washed three times with PBS, and the purity of neutrophils was around 90% as determined by Giemsa staining (Fujifilm Wako Pure Chemicals).
To induce microvesicle release, neutrophils were suspended in HBSS+ (2 x 107 cells/ml, 100–300 μl) and incubated with LL-37 (1, 3, or 10 μg/ml in PBS), fMLF (10 μM in DMSO), or vehicle (PBS or DMSO) for 45 min at 37°C. To further examine the LL-37 receptors involved in the microvesicle release, neutrophils (2 × 106 cells) were pre-incubated with P2X7 antagonist (1 mM oxATP, 1 or 10 μM A438079), CXCR2 antagonist (3 μM SB225002), FPR2 antagonist (0.3 μM WRW4), or vehicle (DMSO) for 20 min at 37°C, and further incubated with LL-37 (10 μg/ml) for 45 min at 37°C. The cell suspension was centrifuged at 400 g for 5 min at 4°C to sediment cells, and the supernatant was centrifuged at 3000 g for 10 min at 4°C to sediment cell debris and large-sized particles. The resulting supernatant was subjected to flow cytometry immediately after the preparation.
To assess the antibacterial activity of neutrophil-derived microvesicles, neutrophils were stimulated with LL-37 (10 μg/ml) as described above and centrifuged at 400 g for 5 min at 4°C. The supernatant was further centrifuged at 100,000 g for 1 h at 4°C, and the pellet was suspended and centrifuged (100,000 g, 30 min). The resulting pellet was re-suspended in PBS (300–400 μl) followed by centrifugation at 3000 g for 10 min at 4°C to remove large particles. The resulting supernatant was subjected to flow cytometry. Alternatively, the microvesicles were stored at -80OC after the preparation, and thereafter the fractions were thawed and utilized for the analyses of antibacterial activity and Western blotting, and for the assessment of the protective action of microvesicles on CLLP mice.
Evaluation of action of LL-37-induced microvesicles on the CLP model
Sham or CLP-operated mice were intraperitoneally administered PBS or microvesicle fraction prepared from LL-37-stimulated neutrophils (containing 3 × 105 ectosomes) 2 h after surgery. Survival was monitored every 24 h for 10 d. In separate experiments, PBS- or microvesicle-administered mice were sacrificed 14–16 h after injection and the bacteria loads of the peritoneal exudates and blood were measured.
Statistics
Data are shown as mean ± standard deviation. Statistical test was performed by Chi-square test, two-tailed unpaired Student’s t-test, one-way ANOVA, two-way ANOVA followed by multiple comparison test, or Kaplan-Meier survival curves by using GraphPad Prism (Graph-Pad software, San Diego, CA). A P value < 0.05 was considered significant.
Results
Administration of LL-37 reduces the bacterial load and modulates inflammatory cell infiltration in CLP mice
We previously revealed that intravenous administration of LL-37 significantly improved the survival of CLP septic mice, when observed for 7 d.9 In this study, the survival rate of LL-37-injected CLP mice (LL-37-CLP) (95.2%, n = 21) was significantly higher than that of PBS-injected CLP mice (PBS-CLP) (72.7%, n = 22) (P < 0.05 by Chi-square test) at 14–16 h after surgery, confirming the protective action of LL-37 on murine sepsis for a shorter period. In contrast, the survival rate of PBS- or LL-37-injected Sham-operated mice was 100% at 16 h (n = 10 each).
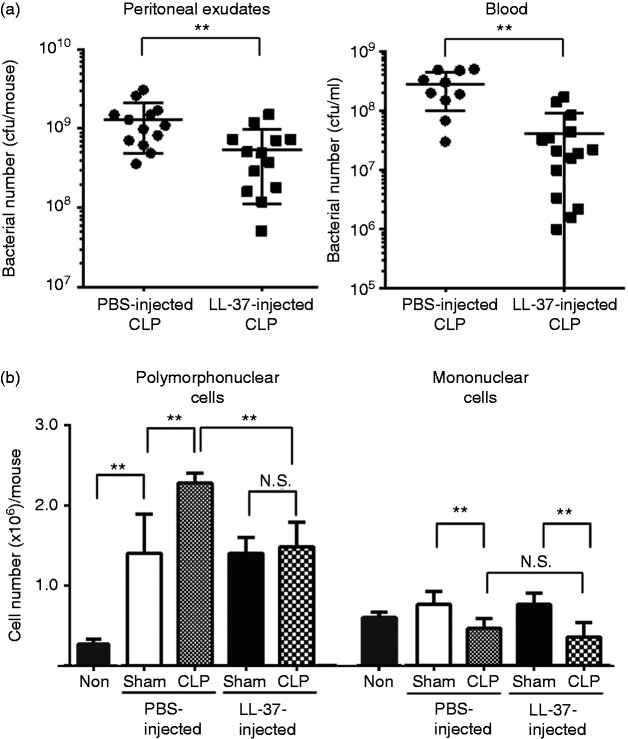
Because bacterial clearance is critical for controlling inflammatory response during sepsis,2 we next evaluated the bacterial load in the peritoneal exudates and blood of the PBS- and LL-37-injected CLP mice. LL-37 administration significantly reduced the bacterial load in the peritoneal exudates (PBS-CLP vs LL-37-CLP) (P < 0.01) as well as blood (P < 0.01) (Figure 1a). We confirmed that bacteria were not essentially counted in the peritoneal exudates and blood of PBS-injected sham mice (PBS-sham) or LL-37-injected sham mice (LL-37-sham) (n = 10 each) (data not shown).
Figure 1.
Effect of LL-37 administration on the bacterial load and inflammatory cell infiltration in cecal ligation and puncture (CLP) mice. Mice were intravenously injected with PBS (200 μl) or LL-37 (3 μg/200 μl in PBS) just prior to CLP or sham operation. Peritoneal exudates (3 ml) and blood were collected 14–16 h after the operation, and the number of bacteria and inflammatory cells was determined. Data are shown as the mean ± SD. (a) The CFU of bacteria in the peritoneal exudates and blood of PBS- or LL-37-injected CLP mice are presented. Values were compared and analyzed between PBS-injected CLP mice (n = 13) and LL-37-injected CLP mice (n = 13) for peritoneal exudates, and PBS-injected CLP mice (n = 11) and LL-37-injected CLP mice (n = 13) for blood by two-tailed unpaired Student’s t-test. (b) The number of polymorphonuclear cells and mononuclear cells (macrophages) in the peritoneal exudates are presented. Values were compared and analyzed among unoperated mice (Non) (n = 3), PBS-injected sham mice (n = 10), PBS-injected CLP mice (n = 13), LL-37-injected sham mice (n = 10), and LL-37-injected CLP mice (n = 14) by one-way ANOVA followed by Holm-Sidak’s multiple comparison tests. **P < 0.01. NS: not significant.
Inflammatory cells are recruited to the loci of infection and inflammation;29,30 thus, we measured the number of inflammatory cells, that is, polymorphonuclear cells (mostly neutrophils) and mononuclear cells (mostly macrophages) in the peritoneal exudates of sham and CLP mice as well as unoperated mice (without sham and CLP operations). The number of polymorphonuclear cells was markedly increased in sham- and CLP-operated mice compared with unoperated mice. The number of mononuclear cells was slightly increased in sham-operated mice and decreased in CLP-operated mice compared with unoperated mice, although the changes were not significant. Interestingly, the CLP procedure increased the number of polymorphonuclear cells (PBS-sham vs PBS-CLP) (P < 0.01) but decreased mononuclear cells (P < 0.01) compared with sham operation (Figure 1b). Importantly, the number of polymorphonuclear cells in LL-37-CLP was not significantly increased compared with that in LL-37-sham but was significantly decreased compared with that in PBS-CLP (P < 0.01) (Figure 1b). In contrast, the number of mononuclear cells in LL-37-CLP was significantly decreased compared with LL-37-sham (P < 0.01) but was not essentially different from that in PBS-CLP (Figure 1b). These observations suggest LL-37 administration significantly decreased the number of polymorphonuclear cells but did not essentially affect the number of mononuclear cells in the peritoneal exudates of CLP mice.
LL-37 enhances ectosome levels in CLP mice
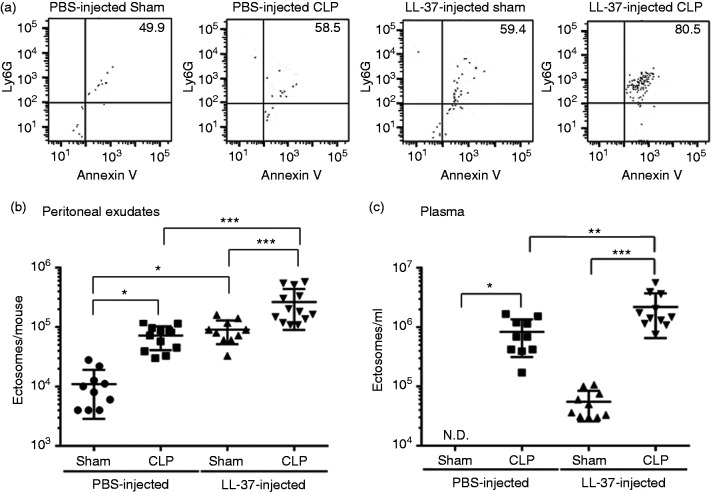
Ectosome levels are elevated in sepsis patients compared with healthy individuals31 and, interestingly, ectosome levels are augmented in patients surviving sepsis.20,21 Because LL-37 administration improved the survival of CLP septic mice as previously described,9 we speculated that ectosome levels are increased in CLP mice and further enhanced by LL-37 administration. Thus, we analyzed the ectosome level in the peritoneal exudates and plasma of sham and CLP mice by flow cytometry. Actually, the ectosome level was significantly enhanced in the peritoneal exudates of PBS-injected CLP mice compared with that of PBS-injected sham mice (PBS-sham vs PBS-CLP) (P < 0.05) (Figure 2a and b). Similarly, ectosome levels were significantly enhanced in the peritoneal exudates of LL-37-injected CLP mice compared with LL-37-injected sham mice (LL-37-Sham vs LL-37-CLP) (P < 0.001) (Figure 2a and b). Interestingly, LL-37 administration significantly enhanced the ectosome level not only in CLP mice (PBS-CLP vs LL-37-CLP; P < 0.001) but also in sham mice (PBS-Sham vs LL-37-sham; P < 0.05) (Figure 2a and b). Similar to the changes in the peritoneal exudates, the ectosome level was elevated in the blood (plasma) of CLP mice (PBS-sham vs PBS-CLP: P < 0.05; LL-37-sham vs LL-37-CLP: P < 0.001), and was further elevated by LL-37 administration in CLP mice (PBS-CLP vs LL-37-CLP; P < 0.01) (Figure 2c). These observations demonstrate the CLP procedure elevates ectosome numbers and LL-37 administration further enhances levels in the peritoneal exudates and blood.
Figure 2.
The effect of LL-37 administration on ectosome levels in the peritoneal exudates and blood of cecal ligation and puncture (CLP) mice. Mice were intravenously injected with PBS (200 μl) or LL-37 (3 μg/200 μl in PBS) just prior to CLP or sham operation. Peritoneal exudates (3 ml) and blood were collected 14–16 h after the operation. Microvesicles of the peritoneal exudates and platelet-depleted plasma were incubated with PE-anti-Ly6G IgG and FITC-Annexin V and analyzed by flow cytometry. Ectosomes are defined as Ly6G+/Annexin V+ double-positive particles, and the percentages of Ly6G+/Annexin V+ ectosomes are described in the upper-right quadrants of cytograms. (a) Representative cytograms of microvesicles from the peritoneal exudates of PBS- or LL-37-injected sham or CLP mice are shown. The counts of ectosomes in the peritoneal exudates (b) and plasma (c) are shown. Data are expressed as the mean ± SD. Values were compared and analyzed between PBS-injected sham mice (peritoneal exudates, n = 10; plasma, n = 10) and PBS-injected CLP mice (peritoneal exudates, n = 12; plasma, n = 10), LL-37-injected sham mice (peritoneal exudates, n = 10; plasma, n = 10) and LL-37-injected CLP mice (peritoneal exudates, n = 13; plasma, n = 11), PBS-injected sham mice (peritoneal exudates, n = 10; plasma, n = 10) and LL-37-injected sham mice (peritoneal exudates, n = 10; plasma, n = 10), and PBS-injected CLP mice (peritoneal exudates, n = 12; plasma, n = 10) and LL-37-injected CLP mice (the peritoneal exudates, n = 13; plasma, n = 11) by one-way ANOVA followed by Holm-Sidak’s multiple comparison tests. *P < 0.05, **P < 0.01, ***P < 0.001. ND: ectosomes could not be counted in the plasma of PBS-injected sham mice.
LL-37 induces the release of microvesicles with antibacterial potential in CLP mice
As described above, ectosome levels were elevated in LL-37-injected CLP mice compared with PBS-injected CLP mice, and the bacterial load was decreased in LL-37-injected CLP mice compared with PBS-injected CLP mice (Figures 1a and 2). These observations suggest that LL-37 induces the release of microvesicles with antibacterial capacity in CLP mice. To test the possibility, E. coli was incubated with the ectosome-containing microvesicle fractions prepared from PBS-injected CLP and LL-37-injected CLP mice. The results indicated that both fractions possessed antibacterial activity and dose-dependently reduced the bacteria viability (Figure 3). Furthermore, the microvesicle fractions from LL-37-injected CLP mice exhibited potent antibacterial activity compared with PBS-injected CLP mice (Figure 3).
Figure 3.
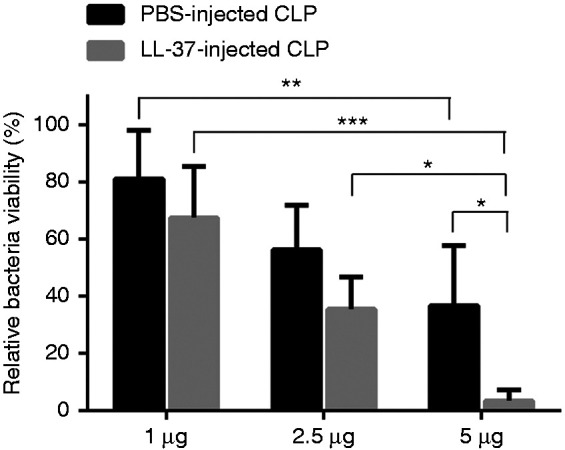
Antibacterial activity of microvesicles isolated from the peritoneal exudates of cecal ligation and puncture (CLP) mice. Microvesicles (1, 2.5, or 5 μg) isolated from the peritoneal exudates of PBS-injected CLP mice (PBS-injected CLP) or LL-37-injected CLP mice (LL-37-injected CLP) were incubated with E. coli for 20 min at 37°C. Relative bacteria viability was calculated and expressed as percentage. Data are shown as the mean ± SD (n = 7). Values were compared and analyzed between the two microvesicle fractions from PBS-injected CLP and LL-37-injected CLP mice (containing 1, 2.5, or 5 μg protein) by two-way ANOVA followed by Holm-Sidak’s multiple comparison test, and among microvesicle fractions containing 1, 2.5, and 5 μg protein, prepared from PBS-injected CLP or LL-37-injected CLP mice by two-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001.
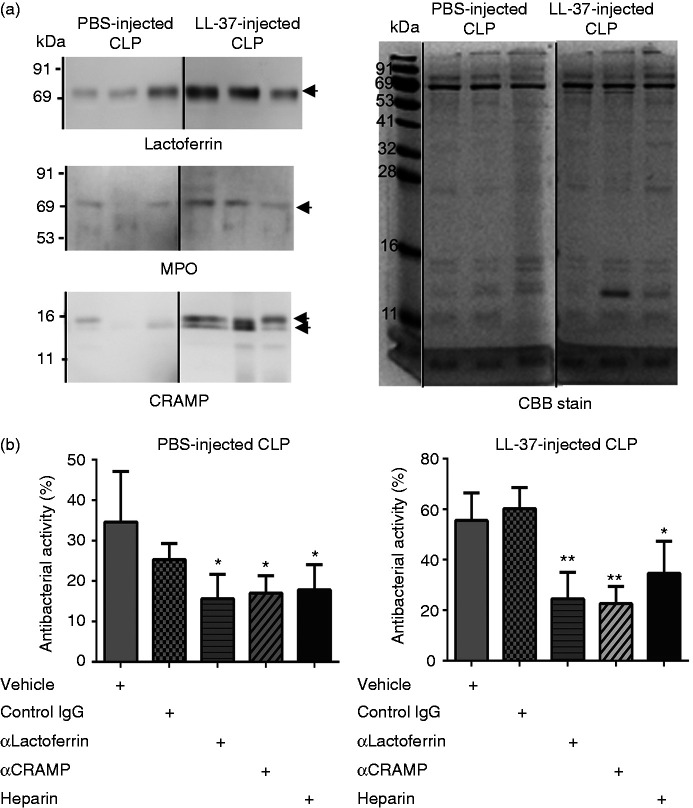
Next, we evaluated the antimicrobial molecules contained in the microvesicle fractions from PBS-injected CLP and LL-37-injected CLP mice. Western blot analysis revealed antimicrobial molecules such as lactoferrin, MPO, and cathelicidin (CRAMP) were detected in both the microvesicle fractions from PBS-injected CLP and LL-37-injected CLP mice; importantly, amounts of these molecules were increased in the microvesicle fractions from LL-37-injected CLP mice compared with those from PBS-injected CLP mice (Figure 4a).
Figure 4.
Detection of antibacterial molecules in microvesicles isolated from the peritoneal exudates of cecal ligation and puncture (CLP) mice. (a) Peritoneal exudates were collected from PBS-injected CLP mice (PBS-injected CLP) and LL-37-injected CLP mice (LL-37-injected CLP) 14–16 h after the CLP operation, and microvesicles were isolated by differential centrifugation, as described in the ‘Materials and methods’. Microvesicles (1 μg) isolated from PBS-injected CLP mice and LL-37-injected CLP mice were subjected to 8% or 13% SDS-PAGE, followed by Western blotting using anti-lactoferrin, myeloperoxidase (MPO), and CRAMP Abs (left panel), or Coomassie Brilliant Blue (CBB) staining (right panel). Each lane shows a microvesicle fraction prepared from each of PBS- or LL-37-injected CLP mice. CBB staining confirmed the same amounts of proteins were loaded on a gel. Gels and blots from a single experiment were spliced and joined to present the order of PBS- and LL-37-injected CLP. (b) The microvesicles (2 μg) isolated from the peritoneal exudates of PBS- or LL-37-injected CLP mice were pre-incubated with vehicle (PBS) or with 0.1 μg of rabbit control IgG, anti-lactoferrin IgG (αlactoferrin), anti-CRAMP IgG (αCRAMP), or heparin for 20 min at 37°C, and then incubated with E. coli for 20 min at 37°C. Data are expressed as the mean ± SD (n = 3–6) of eight independent experiments. Antibacterial activities are compared and analyzed between vehicle (PBS) and control IgG, αlactoferrin, αCRAMP, or heparin by one-way ANOVA followed by Holm-Sidak’s multiple comparison test. *P < 0.05, **P < 0.01.
To further determine the involvement of these antibacterial molecules in the antibacterial activities of the microvesicle fractions, we examined the effects of neutralizing Abs against lactoferrin and CRAMP, and an MPO inhibitor (4-aminobenzoic acid hydrazide). Pretreatment of the microvesicle fractions with anti-lactoferrin or anti-CRAMP Ab partially but substantially abrogated the antibacterial potential of microvesicles from PBS- and LL-37-injected CLP mice, but normal rabbit IgG did not (Figure 4b). Moreover, the antibacterial potential of the microvesicles from PBS- and LL-37-injected CLP mice was impaired by heparin (Figure 4b), an anionic substance that neutralizes the action of cationic molecules such as CRAMP.32 In contrast, the MPO inhibitor did not essentially affect the antibacterial potential of the microvesicle fractions from both PBS-injected CLP and LL-37-injected CLP mice (data not shown). In separate experiments, we confirmed that anti-lactoferrin IgG, anti-CRAMP IgG, normal rabbit IgG, heparin, and MPO inhibitor itself did not affect the bacterial viability (data not shown), and the Abs against lactoferrin and CRAMP neutralize the antibacterial activity of lactoferricin and CRAMP, respectively (data not shown). Together these observations suggest that of the antimicrobial molecules, lactoferrin and CRAMP but not MPO are involved in the antibacterial capacity of microvesicle fractions, and increased amounts of these antibacterial molecules may be associated with enhanced antibacterial activity of microvesicle fraction from LL-37-CLP mice compared with PBS-CLP mice.
Because LL-37 possesses antibacterial potential,33,34 it is possible that intravenously injected LL-37 is contaminated in the microvesicle fractions and exerts antibacterial activity. Thus, we determined the level of LL-37 contained in the microvesicle fractions (protein content of 3 μg) from LL-37-injected CLP mice. The amount of LL-37 in the fractions was below the minimum detection limit (25 pg) of Western blotting using a LL-37-specific Ab (data not shown).35 In addition, the antibacterial activity of LL-37 was negligible below 25 pg (data not shown). These observations indicate that intravenously injected LL-37 is unlikely to contribute to the antibacterial potential of the microvesicle fraction from LL-37-injected CLP mice, if there is any contamination in the microvesicle fraction.
LL-37 induces ectosome release from mouse bone-marrow neutrophils
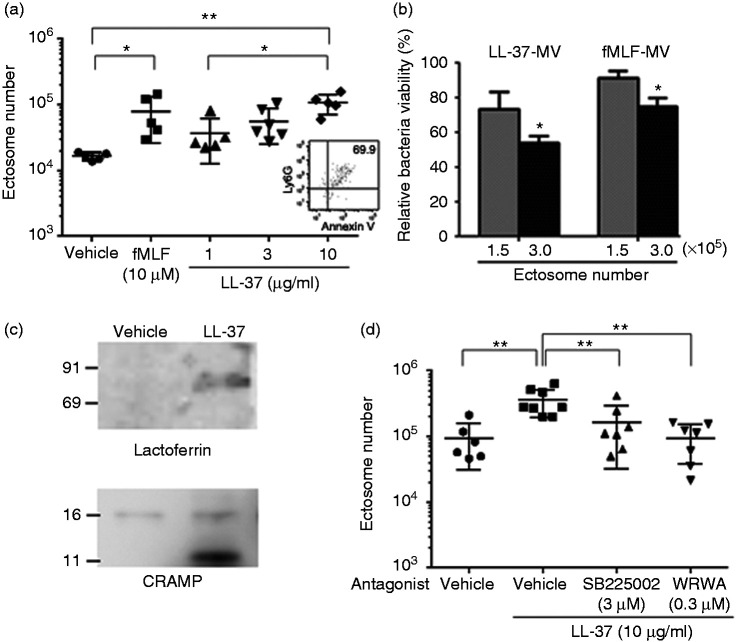
As shown in Figure 2, LL-37 was shown to induce ectosome release in CLP mice; thus, we examined whether LL-37 stimulates neutrophils to release ectosome. fMLF (used as a positive control) induced ectosome release from mouse bone-marrow neutrophils (Figure 5a), as previously reported.14,18 Importantly, LL-37 dose-dependently induced ectosome release from neutrophils (Figure 5a). Of note, the level of ectosomes released by LL-37 (10 μg/ml = 2.2 μM) was almost the same as that released by fMLF (10 μM), indicating a potent activity of LL-37 to release ectosomes from neutrophils. Furthermore, flow cytometry analysis indicated that approximately 70% of microvesicles were double positive for Ly6G and Annexin V (Figure 5a inlet), indicating the released microvesicles are mostly ectosomes. In addition, we confirmed the microvesicle fraction released from LL-37-stimulated neutrophils possessed the antibacterial activity (Figure 5b), and that the expression of lactoferrin and CRAMP was increased in the microvesicle fractions from LL-37-stimulated neutrophils compared with those from vehicle-treated neutrophils (Figure 5c). Interestingly, MPO could not be detected (data not shown) but a smaller-sized fragment of CRAMP was detected in the LL-37-induced microvesicles (Figure 5c). Furthermore, the LL-37-induced microvesicle fraction was not contaminated with a detectable amount of LL-37 based on Western blot analysis (data not shown).
Figure 5.
Effect of LL-37 on the release of antibacterial ectosomes from mouse bone-marrow neutrophils, and effect of CXCR2 and FPR2 antagonists on the LL-37-induced ectosome release. (a) Mouse bone-marrow neutrophils (2 × 106 cells) were stimulated with N-formyl-Met-Leu-Phe (fMLF) (10 μM) or LL-37 (1, 3, or 10 μg/ml) for 45 min at 37°C, and the released ectosomes in the supernatant were analyzed by flow cytometry. Data are shown as the mean ± SD. Values were compared and analyzed between the incubation with vehicle (PBS) and fMLF (10 μM) or LL-37 (1, 3, or 10 μg/ml), and 1, 3, and 10 μg/ml of LL-37 by one-way ANOVA followed by Holm-Sidak’s multiple comparison test. An inlet shows a representative cytogram of microvesicles released from LL-37 (10 μg/ml)-stimulated neutrophils. (b) Microvesicles (containing 1.5 × 105 or 3.0 × 105 ectosomes) isolated from LL-37 (10 μg/ml) or fMLF (10 μM)-stimulated neutrophils were incubated with E. coli for 20 min at 37°C, and the relative bacteria viability was calculated and expressed as percentage. Data are shown as the mean ± SD (n = 3 each). Values were compared and analyzed between microvesicles containing 1.5 × 105 and 3.0 × 105 ectosomes isolated from LL-37-stimulated (LL37-MV) or fMLF-stimulated neutrophils (fMLF-MV) by two-tailed unpaired Student’s t-test. (c) Microvesicles (1.6 μg) isolated from the supernatant of vehicle (Hank’s balanced salt solution; HBSS+) or LL-37 (10 μg/ml) stimulated neutrophils were subjected to 8% or 13% SDS-PAGE, followed by Western blotting using anti-lactoferrin and CRAMP Abs. (d) Mouse bone-marrow neutrophils (2 × 106 cells) were pre-treated with SB225002 (CXCR2 antagonist; 3 μM), WRW4 (FPR2 antagonist; 0.3 μM), or vehicle (DMSO) for 20 min at 37°C, and then stimulated with LL-37 (10 μg/ml) for 45 min at 37°C. Released ectosomes in the supernatant were analyzed by flow cytometry. Values were compared and analyzed between the incubation with vehicle (DMSO) (n = 6) and LL-37 without antagonists (n = 8), and between the incubation with LL-37 alone and LL-37 with an antagonist (SB225002 or WRW4) (n = 7 each) by one-way ANOVA followed by Holm-Sidak’s multiple comparison test. *P < 0.05, **P < 0.01.
LL-37 is reported to act on human neutrophils via CXCR2, FPRL1, and P2X7 receptors to induce Ca2+ intracellular mobilization, chemotaxis, and inhibition of apoptosis.6,24 These receptors are functionally expressed in murine neutrophils.36–38 Thus, to determine the involvement of these receptors in the LL-37-induced ectosome release from mouse bone-marrow neutrophils, the effect of specific antagonists for the receptors were examined. The results indicated that SB225002 (CXCR2 antagonist)39 and WRW4 (antagonist for FPR2, mouse ortholog of FPRL1)25 partially but significantly reduced the LL-37-induced ectosome release from neutrophils (Figure 5d). In contrast, oxATP and A438079 (P2X7 antagonists)36,40 did not abrogate the ectosome release (data not shown). We confirmed the antagonists themselves did not affect the basal release of ectosomes from resting neutrophils (data not shown). Together these observations suggest that CXCR2 and FPR2 but not P2X7 are likely functioning as receptors for LL-37 to induce ectosome release from neutrophils.
Microvesicles released from LL-37-stimulated neutrophils ameliorate murine sepsis
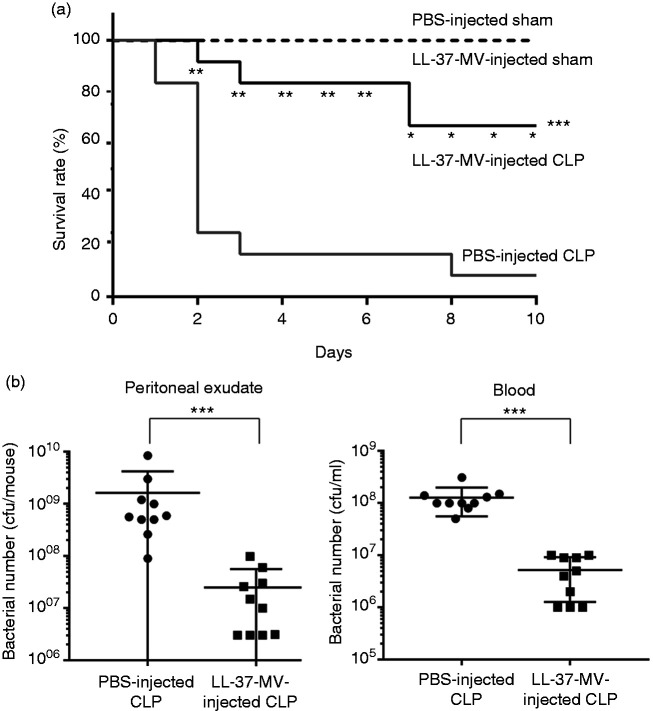
We finally determined the action of microvesicles from LL-37-stimulated neutrophils on septic CLP mice. Mice were intraperitoneally administered with PBS or microvesicles 2 h after CLP operation and the survival rates were observed for 10 d. The survival rate of microvesicle-injected CLP mice (67%) was significantly higher than that of PBS-injected CLP mice (8.3%) (P < 0.001; Figure 6a); the survival rate of PBS- or microvesicle-injected sham mice was 100% (Figure 6a). Importantly, the administration of microvesicles significantly reduced the bacterial load in the peritoneal exudates (P < 0.001) and blood (P < 0.001) of septic CLP mice (Figure 6b). These observations suggest the administration of microvesicles from LL-37-stimulated neutrophils reduces the bacterial load and protects mice from lethal sepsis in a CLP model.
Figure 6.
Effect of microvesicles isolated from LL-37-stimulated neutrophils on the survival and bacterial load of cecal ligation and puncture (CLP) mice. (a) CLP mice were intraperitoneally injected with PBS (200 μl; PBS-injected CLP, n = 12) or microvesicles (containing 3 × 105 ectosomes/200 μl) from LL-37-stimulated neutrophils (LL-37-MV-injected CLP, n = 12) 2 h after the operation. Sham mice were also injected with PBS (PBS-injected sham, n=4) or microvesicles (LL-37-MV-injected sham, n = 4) 2 h after the surgery. The survival was monitored for 10 d, and the survival rate was compared and analyzed between PBS-injected CLP and LL-37-MV-injected CLP mice by Kaplan-Meier method for the total 10 d (***) and by Chi-square analysis for each d (* or **). (b) Mice were sacrificed 14–16 h after the operation, and the number of bacteria was determined in the peritoneal exudates and blood. Data are shown as the mean ± SD (n = 10 each). Values were compared and analyzed between PBS-injected CLP and LL-37-MV-injected CLP mice by one-way ANOVA followed by Holm-Sidak’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Extracellular vesicles such as microvesicles and exosomes have recently been gathering attention as a non-soluble intercellular communication system that mediates a broad range of physiological and pathological processes.11,12 In particular, microvesicles released from neutrophils (named as ectosomes) are considered to associate with sepsis, because the ectosome level is elevated in patients of sepsis31 and of Staphylococcus aureus bacteremia19 compared with healthy individuals. Furthermore, ectosome levels are reported to increase in human sepsis survivors compared with non-survivors.20,21 We previously demonstrated that the administration of antimicrobial cathelicidin peptide LL-37 improves the survival of a murine CLP sepsis model,8,9 an established microbial sepsis model that closely resembles the pathology of human sepsis.41,42 Thus, in this study, we elucidated the protective action of LL-37 on sepsis by focusing on the effect of LL-37 on ectosome release in the CLP model.
Consistent with previous reports in humans,19,31 the ectosome level was markedly elevated in the peritoneal exudates and blood of CLP mice (Figure 1). Furthermore, the ectosome level was enhanced by LL-37 administration, accompanied with the reduction of bacterial load and the increase of survival rate (Figures 1 and 2). To further confirm the antibacterial activity of ectosomes, we assessed the antibacterial activity of ectosome-containing microvesicles isolated from PBS- and LL-37-injected CLP mice. Importantly, both the microvesicle fractions possessed the antibacterial potential and, interestingly, the microvesicles from LL-37-injected CLP mice displayed higher potential than those from PBS-injected CLP mice (Figure 3). It has been reported that microvesicles are released from neutrophils by stimulation with opsonized bacteria, and the microvesicles exert bacteriostatic action by forming bacteria-microvesicle aggregates; the aggregate formation requires glucose and divalent cations.19 In contrast, the present result revealed that microvesicles isolated from CLP mice reduced bacteria viability under the conditions without containing glucose and divalent cations in PBS (Figure 3), suggesting the mechanism for the antibacterial action of microvesicles from CLP mice is likely distinct from that of opsonized bacteria-induced microvesicles. Importantly, neutrophil-granule molecules are contained in ectosomes.12,13 Further, to evaluate the involvement of neutrophil-granule molecules in the antibacterial activity of microvesicles, we analyzed the neutrophil granule-derived antimicrobial molecules (such as lactoferrin, MPO, and CRAMP) in the microvesicle fractions by Western blotting. The results indicated that lactoferrin, MPO, and CRAMP were detected in microvesicles isolated from both PBS- and LL-37-injected CLP mice and, interestingly, the microvesicles from LL-37-injected CLP mice contained increased amounts of these antimicrobial molecules compared with those from PBS-injected CLP mice (Figure 4a). In addition, the experiments using anti-lactoferrin and anti-CRAMP Abs indicated the antibacterial potential of microvesicles is attributed to the action of lactoferrin and CRAMP (Figure 4b). Collectively, these observations suggest that LL-37 induces the release of microvesicles containing a higher amount of antibacterial molecules (lactoferrin and CRAMP) in CLP mice, which exert an antibacterial action and reduce the bacterial load, thereby improving the survival of septic mice.
Furthermore, we examined whether LL-37 stimulates neutrophils to release microvesicles ex vivo, and whether the administration of microvesicles ameliorates a murine septic CLP model in vivo. Importantly, LL-37 stimulated mouse bone-marrow neutrophils to release ectosomes ex vivo (Figure 5a), supporting our observation that LL-37 administration augments the ectosome release in not only CLP mice but also sham mice in vivo (Figure 2). Phorbol myristate acetate, fMLF, C5a, and opsonized bacteria are reported to efficiently induce the release of ectosomes.14,17–19 Thus, the finding that LL-37 induces ectosome release to a similar extent to fMLF (Figure 5a) suggests a potent activity of LL-37 to release ectosomes from neutrophils. In addition, the experiments using specific antagonists for LL-37 receptors demonstrated that LL-37 likely induces the ectosome release via the action on CXCR2 and FPR2 receptors (Figure 5d). Furthermore, the LL-37-induced microvesicles possessed antibacterial potential (Figure 5b) and contained increased amounts of antibacterial molecules (Figure 5c), and the administration of microvesicles to CLP mice reduced the bacterial load and improved the survival of mice (Figure 6). These results indicate that LL-37-induced microvesicles exert a protective action in vivo against sepsis by their antibacterial potential.
In the present study, the increase of ectosomes was induced in mice by not only CLP procedures but also LL-37 administration alone (Figure 2b). However, the expression of antimicrobial molecules was higher in the ectosome fractions of LL-37-injected CLP mice (Figure 4a), and LL-37 directly stimulated bone marrow-derived neutrophils to release antibacterial ectosomes containing antimicrobial molecules (Figure 5b and c). These observations suggest LL-37 likely plays a more important role in the induction of antimicrobial molecules in ectosomes, although CLP-associated bacterial infection can induce the increase of ectosomes in mice.
In the light of sepsis resolution, reduction in the IL-6 level is suggested to associate with a better prognosis of sepsis.43 Importantly, we previously revealed that LL-37 administration reduces IL-6 levels in CLP mice.9 Furthermore, the increase in the ratio of mononuclear cells (macrophages) to polymorphonuclear cells is reported as a hallmark of sepsis resolution at an early stage.44,45 In the current study, LL-37 administration significantly reduced the number of polymorphonuclear cells without essentially affecting the number of mononuclear cells (macrophages) in the peritoneal exudates of CLP mice (Figure 1b), which results in an increased ratio of mononuclear cells to polymorphonuclear cells in LL-37-injected CLP mice (2.7 × 10−1 ± 2.5 × 10−1) compared with PBS-injected CLP mice (2.0 × 10−1 ± 1.8 × 10−1). The result indicates LL-37 is likely to have the potential to resolve sepsis at an early stage, as evidenced by the change in the inflammatory cell ratio. In this study, the mononuclear cell number was significantly reduced in CLP mice compared with sham mice (Figure 1b). Because macrophages undergo cell death (e.g., pyroptosis and apoptosis) during bacterial infection or sepsis,46,47 the reduction in mononuclear cell numbers in the peritoneal cavity of CLP mice could be explained by the macrophage cell death occurring in sepsis.
Moreover, the number of polymorphonuclear cells in the peritoneal exudates of LL-37-injected mice was not significantly increased after CLP operation compared with LL-37-injected sham mice, although the number of polymorphonuclear cells in PBS-injected mice was significantly increased after CLP operation compared with PBS-injected sham mice (Figure 1b). The present study also revealed the bacterial burden was reduced by LL-37-administration in CLP mice (Figure 1a), which is possibly caused by the increased levels of antimicrobial ectosomes in LL-37-administered CLP mice (Figure 3). Furthermore, it has been reported that bacterial clearance is critical for controlling inflammatory response during sepsis.2 Thus, we assume the infiltration of polymorphonuclear cells is suppressed in LL-37-injected CLP mice, where the bacterial burden and inflammatory response are reduced.
In this study, there may be some possible limitations. First, the effect of LL-37 on CLP mice was evaluated when LL-37 was administered just prior to CLP surgery; however, we have not investigated the effect of LL-37 when administered after an induction of sepsis. Previously, we revealed the administration of LL-37 just after CLP surgery similarly reduced the bacterial load of mice and improved survival,9 as observed when LL-37 was administered prior to CLP surgery. Furthermore, it has been reported that administration of LL-37 2 h after the LPS injection protects neonatal rats from LPS-induced lethal sepsis, although the protective effect is reduced compared with the simultaneous administration of LL-37 with LPS.48 Based on these findings, we believe LL-37 likely exerts a protective action on a sepsis model, when administered within a few h after the induction of sepsis.
Second, it is possible that in the peritoneal exudate and plasma samples, the extracellular microvesicles derived from cells other than neutrophils (such as platelets) are contaminated. In fact, a high level of platelet-derived microvesicles is detected in the blood of sepsis patients.21,49 However, in the present study, we evaluated neutrophil-derived ectosomes such as Ly6G and Annexin V double-positive microvesicles in peritoneal exudates and plasma. In fact, the percentage of Ly6G+/Annexin V+ ectosomes increased to 80.5% in CLP mice by LL-37 administration in vivo (Figure 2a), and increased to 70% by stimulation of bone marrow-derived neutrophils with LL-37 ex vivo (Figure 5a). Thus, we believe the ectosome-releasing activity of LL-37 could be evaluated by counting the Ly6G+/Annexin V+ microvesicles.
Third, we evaluated the effect of LL-37 administration on the bacterial load in CLP mice by culturing bacteria under aerobic conditions. However, obligatory anaerobes comprise more than 90% of the microflora in the mouse cecum,50 suggesting obligatory anaerobes may contribute to the development of sepsis in CLP mice. Indeed, sepsis is caused by commensal bacteria belonging to the genus Bacteroides, the most abundant obligatory anaerobe inhabiting the large intestine.51,52 The present study revealed the administration of LL-37 or LL-37-induced microvesicles improves the survival of CLP septic mice. Thus, the administration of LL-37 or LL-37-induced microvesicles is speculated to exert an antibacterial action against obligatory anaerobes in a CLP model.
Fourth, LL-37 performs multiple functions in sepsis. We previously revealed that LL-37 improves the survival of CLP mice by suppressing the macrophage pyroptosis that induces the release of pro-inflammatory cytokines (such as IL-1β) and augments inflammatory reactions in sepsis.9 Moreover, LL-37 induces the release of neutrophil extracellular traps (NETs) with potent bactericidal activity and protects mice from CLP-induced sepsis.8 Furthermore, the present study indicated that LL-37 stimulates neutrophils to release antibacterial microvesicles (ectosomes), thereby improving murine sepsis. Together these observations likely suggest that LL-37 protects CLP septic mice by not only suppressing pro-inflammatory macrophage pyroptosis and releasing antibacterial NETs from neutrophils, but also through releasing antibacterial microvesicles (ectosomes) from neutrophils. However, the precise roles of pyroptosis, NETosis (NET release), and ectosome release in the complex pathogenesis of sepsis cannot be clearly delineated at this stage.
The poor outcomes of sepsis patients are attributed to the aberrant immune responses that compromise the elimination of infecting pathogen and predispose the host to recurrent infection.1 Thus, a novel therapeutic approach has been anticipated to regulate the uncontrolled immune response in sepsis. However, strategies using anti-TNF-α Abs, TNF-α receptor proteins, and MD2-TLR4 antagonist have been ineffective in protecting against sepsis.53,54 In the present study, LL-37 is shown to stimulate neutrophils to release antibacterial microvesicles (ectosomes), thereby eliminating bacteria and protecting mice from lethal sepsis. In this regard, agents such as LL-37, which potently induce the release of antibacterial microvesicles (ectosomes), would be potentially useful for providing a better outcome of sepsis.
Acknowledgements
We thank Yuka Hiraoka (Division of Proteomics and Biomolecular Science) for synthesizing the CRAMP peptide, and Tamani Sakanishi and Akemi Koyanagi (Division of Cell Biology) for advising on flow cytometric analysis in BioMedical Research Center, Juntendo University, Graduate School of Medicine.
Declaration of conflicting interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants-in-Aid (17K08840 and 19K07562) from Ministry of Education, Culture, Sport, Science, and Technology, Japan, and grants (E2916 and E3021) from Institute for Environmental & Gender-specific Medicine, Juntendo University, Japan.
ORCID iD
Isao Nagaoka https://orcid.org/0000-0003-0824-7406
References
- 1.Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018; 392: 75–87. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: A roadmap for future research. Lancet Infect Dis 2015; 15: 581–614. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34: 15–21. [DOI] [PubMed] [Google Scholar]
- 4.Amulic B, Cazalet C, Hayes GL, et al. Neutrophil function: From mechanisms to disease. Annu Rev Immunol 2012; 30: 459–489. [DOI] [PubMed] [Google Scholar]
- 5.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 2006; 1758: 1408–1425. [DOI] [PubMed] [Google Scholar]
- 6.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol 2006; 176: 3044–3052. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Murakami T, Kuwahara-Arai K, et al. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int Immunol 2011; 23: 185–193. [DOI] [PubMed] [Google Scholar]
- 8.Hosoda H, Nakamura K, Hu Z, et al. Antimicrobial cathelicidin peptide LL37 induces NET formation and suppresses the inflammatory response in a mouse septic model. Mol Med Rep 2017; 16: 5618–5626. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z, Murakami T, Suzuki K, et al. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int Immunol 2016; 28: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaoka I, Hirota S, Niyonsaba F, et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol 2002; 9: 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68: 2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int J Mol Sci 2016; 17: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol 2014; 15: 602–611. [DOI] [PubMed] [Google Scholar]
- 14.Gasser O, Hess C, Miot S, et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res 2003; 285: 243–257. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y, Eleftheriou D, Hussain AA, et al. Anti-neutrophil cytoplasmic Abs stimulate release of neutrophil microparticles. J Am Soc Nephrol 2012; 23: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater TW, Finkielsztein A, Mascarenhas LA, et al. Neutrophil microparticles deliver active myeloperoxidase to injured mucosa to inhibit epithelial wound healing. J Immunol 2017; 198: 2886–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004; 104: 2543–2548. [DOI] [PubMed] [Google Scholar]
- 18.Eken C, Sadallah S, Martin PJ, et al. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology 2013; 218: 382–392. [DOI] [PubMed] [Google Scholar]
- 19.Timar CI, Lorincz AM, Csepanyi-Komi R, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 2013; 121: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalli J, Norling LV, Montero-Melendez T, et al. Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis. EMBO Mol Med 2014; 6: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lashin HMS, Nadkarni S, Oggero S, et al. Microvesicle subsets in sepsis due to community acquired pneumonia compared to faecal peritonitis. Shock (Augusta, Ga ) 2018; 49: 393–401. [DOI] [PubMed] [Google Scholar]
- 22.Nagaoka I, Hirota S, Niyonsaba F, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14+ cells. J Immunol 2001; 167: 3329–3338. [DOI] [PubMed] [Google Scholar]
- 23.Page M, Thorpe R. Purification of IgG by precipitation with sodium sulfate or ammonium sulfate In:Walker JM. (eds) The protein protocol handbook. Totowa, NJ: Humana Press, 2002, pp. 721–722. [Google Scholar]
- 24.Zhang Z, Cherryholmes G, Chang F, et al. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol 2009; 39: 3181–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin EH, Lee HY, Kim SD, et al. Trp-Arg-Trp-Trp-Trp-Trp antagonizes formyl peptide receptor like 2-mediated signaling. Biochem Biophysical Res Comms 2006; 341: 1317–1322. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Murakami T, Tamura H, et al. Neutrophil extracellular traps induce IL-1beta production by macrophages in combination with lipopolysaccharide. Int J Mol Med 2017; 39: 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai SHC, Sharma N, Kwong AC, et al. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med Exp 2018; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson BL, 3rd, Midura EF, Prakash PS, et al. Neutrophil derived microparticles increase mortality and the counter-inflammatory response in a murine model of sepsis. Biochim Biophys Acta 2017; 1863: 2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 30.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash PS, Caldwell CC, Lentsch AB, et al. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg 2012; 73: 401–406; discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baranska-Rybak W, Sonesson A, Nowicki R, et al. Glycosaminoglycans inhibit the antibacterial activity of LL-37 in biological fluids. J Antimicrob Chemother 2006; 57: 260–265. [DOI] [PubMed] [Google Scholar]
- 33.Nagaoka I, Hirota S, Yomogida S, et al. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res 2000; 49: 73–79. [DOI] [PubMed] [Google Scholar]
- 34.Travis SM, Anderson NN, Forsyth WR, et al. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 2000; 68: 2748–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Murakami T, Hu Z, et al. Human host defense cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake by liver sinusoidal endothelial cells without cell activation. J Immunol 2016; 196: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar M, Katsnelson MA, Dubyak GR, et al. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat Commun 2016; 7: 10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurosaka K, Chen Q, Yarovinsky F, et al. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol 2005; 174: 6257–6265. [DOI] [PubMed] [Google Scholar]
- 38.Tsai WC, Strieter RM, Mehrad B, et al. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 2000; 68: 4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White JR, Lee JM, Young PR, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 1998; 273: 10,095–10,098. [DOI] [PubMed] [Google Scholar]
- 40.Hibell AD, Thompson KM, Simon J, et al. Species- and agonist-dependent differences in the deactivation-kinetics of P2X7 receptors. Naunyn Schmiedebergs Arch Pharmacol 2001; 363: 639–648. [DOI] [PubMed] [Google Scholar]
- 41.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: Setting the stage. Nat Rev Drug Discov 2005; 4: 854–865. [DOI] [PubMed] [Google Scholar]
- 42.Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009; 4: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013; 27: 669–684. [PMC free article] [PubMed] [Google Scholar]
- 44.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009; 461: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiang N, Fredman G, Backhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012; 484: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luan Y, Yao Y, Xiao X, et al. Insights into the apoptotic death of immune cells in sepsis. J Interferron Cytokine Res 2015; 35: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinheiro da Silva F, Nizet V. Cell death during sepsis: Integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis 2009; 14: 509–521. [DOI] [PubMed] [Google Scholar]
- 48.Rhys HI, Dell’Accio F, Pitzalis C, et al. Neutrophil microvesicles from healthy control and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. EBioMedicine 2018; 29: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehner GF, Harler U, Haller VM, et al. Characterization of microvesicles in septic shock using high-sensitivity flow cytometry. Shock (Augusta, Ga) 2016; 46: 373–381. [DOI] [PubMed] [Google Scholar]
- 50.Gu S, Chen D, Zhang JN, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 2013; 8: e74957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura I, Aoki K, Miura Y, et al. Fatal sepsis caused by multidrug-resistant Bacteroides fragilis, harboring a cfiA gene and an upstream insertion sequence element, in Japan. Anaerobe 2017; 44: 36–39. [DOI] [PubMed] [Google Scholar]
- 52.Garcia M, Bouvet P, Petitpas F, et al. First case report of a human sepsis involving a recently identified anaerobic agent: Bacteroides faecis. Anaerobe 2016; 42: 74–77. [DOI] [PubMed] [Google Scholar]
- 53.Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013; 309: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 54.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: Update on clinical trials and lessons learned. Crit Care Med 2001; 29: S121–125. [DOI] [PubMed] [Google Scholar]