Abstract
Our recent study showed a high rate of HBsAg seroconversion in inactive HBsAg carriers (IHCs) treated with pegylated IFN (PEG-IFN). To understand the immune-mediated component of the HBsAg seroconversion better, this study investigated the role of NK cells. A total of 44 IHCs were given 48 wk of PEG-IFN. Fifteen cases achieved HBsAg seroconversion (R group), whereas 29 failed (NR group). The proportion and activity (CD107α and IFN-γ production) of NK cells were measured before and during treatment. We found that the proportion of NK cells in the R group was higher than in the NR group at baseline and during PEG-IFN treatment, even when patients were matched for age, sex and treatment period. IFN- γ secretion and CD107α expression from NK cells in cases who achieved HBsAg seroconversion were significantly higher than patients matched for age, sex, HBsAg and treatment period in the NR group at baseline and during PEG-IFN treatment. We also found that in HBsAg seroconversion cases, NK cells activity increased after PEG-IFN treatment, especially before HBsAg seroconversion. These effects were not found in non-responders. In conclusion, we demonstrated that the increase of NK cells accompanied by enhanced activity during PEG-IFN treatment favoured HBsAg seroconversion for IHC, and that NK cells may play a role in HBV seroconversion.
Keywords: Pegylated IFN-α-2a, inactive HBsAg carriers, HBsAg, seroconversion, NK cells
Introduction
Inactive HBsAg carriers (IHCs) are characterised by effective control of hepatitis B virus (HBV) replication and sustained HBeAg seroconversion. Patients in this phase have detectable circulating HBsAg, absence of HBeAg and the presence of anti-HBe, as well as undetectable or low levels of HBV DNA. Our recent study showed a high rate of HBsAg seroconversion for IHCs treated with pegylated IFN (PEG-IFN).1 Li et al. also reported a similar finding in a small retrospective cohort study.2 To understand the immune-mediated component to the HBsAg loss better, we analysed the changes in selected immune parameters. We found a significant increase of NK cells in patients who achieved HBsAg seroconversion. We hypothesised that NK cells may play a role in HBsAg seroconversion.
With the deepening of research, accumulating evidence supported that the liver is an immunological organ, enriched with innate immune cells, including Kupffer cells, NK cells and NK T cells.3,4 In humans, almost 50% of all intra-hepatic lymphocytes are NK cells. NK cells have been proved to play an important role in chronic HBV infection by producing cytokines and by exerting cytotoxic functions against virus-infected cells.5,6
PEG-IFN achieves sustained off-treatment responses in many chronic hepatitis B (CHB) patients because of its direct antiviral effects and regulation of the immune response. However, the exact mechanism of how PEG-IFN promotes HBsAg seroconversion remains unknown. One study found that NK cells increased markedly during PEG-IFN therapy in HBV control cases.7 PEG-IFN could also induce NK activation in HIV-1/HCV co-infected patients, and is associated with HIV-1 DNA decline.8 However, studies on NK cells of HBsAg seroconversion are still in the exploratory stage. We therefore explored the contribution of NK cells to HBsAg seroconversion in inactive HBsAg carriers following PEG-IFN therapy.
Methods
Patients
Patients were included if they met the following criteria: HBsAg-positive for > 6 mo, serum HBsAg levels < 1000 IU/ml, HBeAg negative, anti-HBe positive, anti-HBc positive, HBV DNA < 20 IU/ml and the absence of previous antiviral therapy. Patients were excluded if they met any of the following criteria: HIV or hepatitis C virus co-infections, a history of autoimmune diseases, hepatic dysfunction, serious metabolic disease, cardiac, pulmonary or renal disease, evidence of liver carcinoma or other neoplastic diseases, or contraindication for IFN.
PEG-IFN-α-2a (135 μg/wk) was administered for 48 wk. At baseline and every 12 wk thereafter during the treatment, detection of HBsAg levels, liver function, routine blood tests and HBV DNA was performed, and PBMCs were separated and stored at –80°C. Patients were categorised into one of three groups: the responder (R) group who achieving seroconversion of HBsAg; the non-responder (NR) group who failed to achieve HBsAg seroconversion; and the healthy control (HC) group who were HBsAg negative, vaccinated against HBV and had anti-HBs levels > 200 IU/ml.
Tests of HBV infection
HBsAg and anti-HBs levels were detected using the HBsAg quantitative Elecsys (Roche Diagnostics GmbH, Mannheim, Germany). The lower limit of quantitative HBsAg determination was 0.05 IU/ml, and anti-HBs >10 IU/ml was defined as anti-HBs positive. HBV DNA levels were determined using the fluorescence quantitative PCR (FQ-PCR) method, with a lower detection limit of 20 IU/ml (Roche).
HBsAg synthetic peptides
HBsAg synthetic peptides (Sigma–Aldrich, St Louis, MO) were kindly donated by the MRC Human Immunology Unit, WIMM, University of Oxford. These polypeptides had all the aa sequences coded by the S gene region of HBV genotype C2/ACO053361, with each peptide containing 18 aa residues. Adjacent peptide fragments overlapped by 10 aa.
Flow cytometry
The mAbs were added to the separated PBMCs in this order: CD3-PerCP, CD56-APC and CD45-FITC. Isotype controls were also set up. FACS Calibur four-colour flow cytometry was performed, and the results were analysed using CELL Quest (Becton Dickinson, San Jose, CA). All mAbs were purchased from BD (San Diego, CA). NK cells were defined as CD3–CD56+, whereas NKT were CD3+CD56+ (Figure 1).
Figure 1.
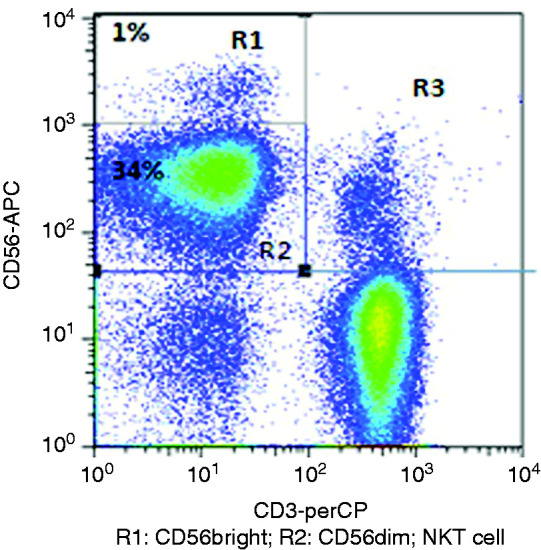
Analysis of NK cells and NKT cells. PBMCs stained with CD3-PerCP, CD56-APC and CD45-FITC. NK cells are CD3–CD56+; R1 is CD3–CD56bright; R2 is CD3–CD56dim. NKT cells are CD3+CD56+.
NK cell activity test
PBMCs were isolated from patients in the R and NR groups and co-cultured with HBsAg overlapping peptide pools and plasma samples from the patients (high anti-HBs level) at 37°C for 5 h with brefeldin A and monensin (Sigma–Aldrich). DMSO was added to the negative control, and combined SEB was added to the positive control. The addition of mAbs CD3-PerCP, CD56- FITC and CD107α-APC was performed with or without the Ab. After permeabilisation, IFN-γ-PE (BD Biosciences Pharmingen, San Jose, CA) was added to the samples. FACS Calibur four-color flow cytometry was performed, and the frequencies of IFN-γ and CD107α expression were analysed using CELL Quest (Becton Dickinson).
Statistical analysis
SPSS for Windows v11.5 (SPSS, Inc., Chicago, IL) was used for analysis. The data were normally distributed and are reported as the mean±SD. t-Tests were performed between two groups, and chi-square tests were performed between three groups. Two sided P values of < 0.05 were considered statistically significant.
Results
Patients’ clinical characterisation
A total of 44 IHC patients were given 48 wk of PEG-IFN. Fifteen cases achieved HBsAg seroconversion (R), whereas 29 failed (NR). There were no significant differences in sex, age, ALT, WBC and HBV DNA among the R, NR and HC groups. The HBsAg level in the R group was lower than that in the NR group (P < 0.001). Patients in the R group experienced different periods of time to achieve HBsAg seroconversion. One case achieved it at 12 wk after PEG-IFN therapy, and seven, two and five cases achieved it at 24, 36 and 48 wk, respectively. The patients’ demographic and clinical data are listed in Table 1.
Table 1.
Patient demographic and clinical data.
| Parameter | R | NR | HC |
|---|---|---|---|
| n = 15 | n = 15 | n = 12 | |
| Sex (M/F) | 8/7 | 17/12 | 6/6 |
| Age (yr), M ± SD | 34.47 ± 9.65 | 34.03 ± 10.03 | 30.75 ± 9.50 |
| ALT (IU/l), M ± SD | 27.25 ± 9.26 | 25.87 ± 8.80 | 30.54 ± 6.19 |
| AST (IU/l), M ± SD | 25.53 ± 5.44 | 23.49 ± 6.34 | 20.58 ± 6.31 |
| Leucocytes (109/l), M ± SD | 5.72 ± 1.90 | 5.67 ± 1.78 | 5.69 ± 1.65 |
| HBsAg (IU/ml), log10 | 1.33 ± 0.96** | 2.24 ± 0.48** | UD |
| 1st time achieved HBsAg seroconversion (n) | |||
| 12 wk | 1 | N | N |
| 24 wk | 7 | N | N |
| 36 wk | 2 | N | N |
| 48 wk | 5 | N | N |
**P < 0.01; R vs. NR.
UD: undetected.
Baseline proportion of NK cells in IHCs and HCs
The proportion of NK cells (CD3-CD56+) was analysed by flow cytometry. The proportion of NK cells was 12.07 ± 0.65% in 44 IHCs at baseline and 13.17 ± 1.29% in 12 HCs. There was no significant difference (t = 0.78, P = 0.441; Figure 2).
Figure 2.
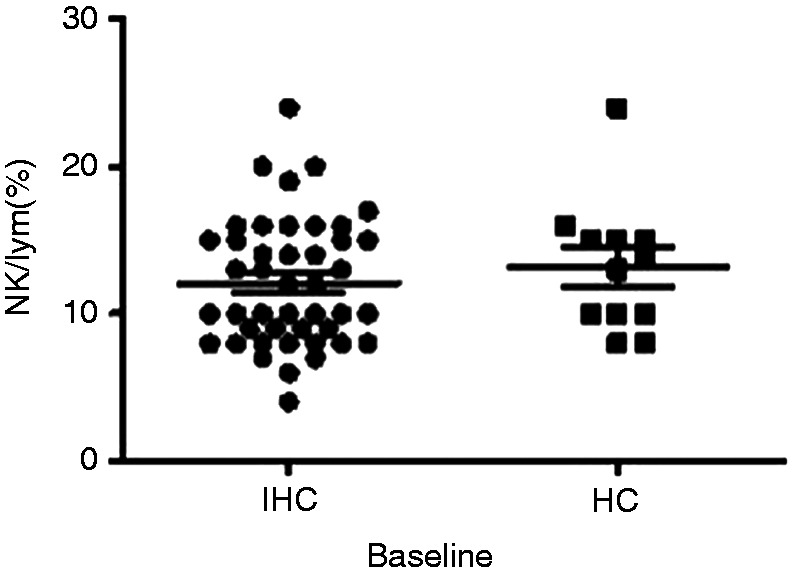
Comparison of NK cells in inactive HBsAg carriers (IHCs) and healthy control (HC) group at baseline. Comparison of proportion of NK cells between IHCs (n = 44) and healthy controls (n = 12) at baseline.
Baseline proportion of NK cells in R and NR groups
After PEG-IFN treatment of 44 IHCs, 15 cases achieved HBsAg seroconversion (R group) and 29 did not (NR group). The proportion of NK cells was 15.80±1.53% in the R group, which was significantly higher than the NR group (9.83±0.78%) at baseline (t=3.88, P<0.001; Figure 3).
Figure 3.
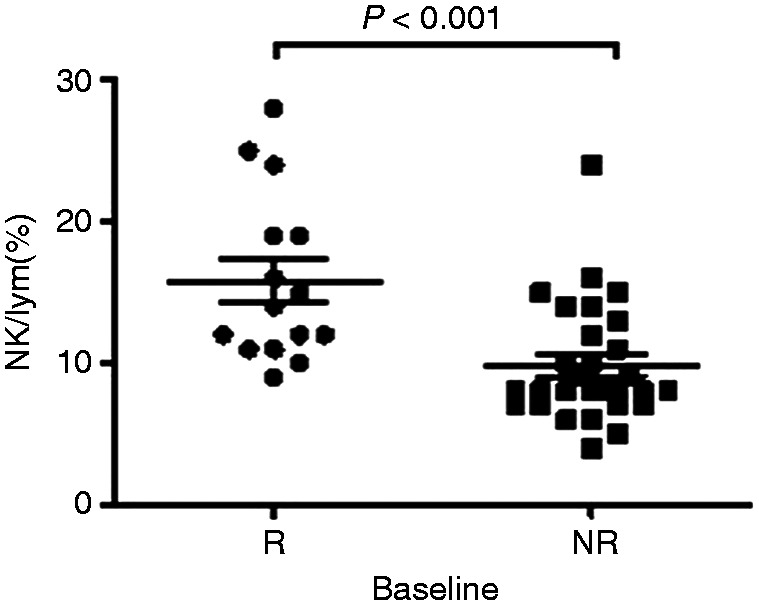
Comparison of NK cells in the R group and NR group at baseline. Comparison of NK cell levels between those who achieved seroconversion of HBsAg after 48 wk of treatment (R group; n = 15) and those who failed to achieve HBsAg seroconversion (NR group; n = 29) at baseline.
Proportion of NK cells in patients matched for age, sex and treatment period during PEG-IFN treatment in R and NR groups
Because patients in the R group (n = 15) experienced different periods of time to achieve HBsAg seroconversion, the comparison time points were set as the last time point before HBsAg seroconversion was detected (pre-seroconversion point (Pre-P)) and the first time point when seroconversion of HBsAg was detected (post-HBsAg seroconversion point (Post-P)). Given the fact that age and sex significantly affect the distribution of NK cells, 15 patients in the NR group were compared to 15 patients in the R group after matching for age, sex and course of treatment. The results showed that the proportion of NK cells in the R group was significantly higher than in the NR group at Pre-P and Post-P (18.00±1.40 vs. 13.07±1.49, t=2.42, P=0.022; 21.87±2.18 vs. 15.87±1.22, t=2.41, P=0.023; Figure 4).
Figure 4.
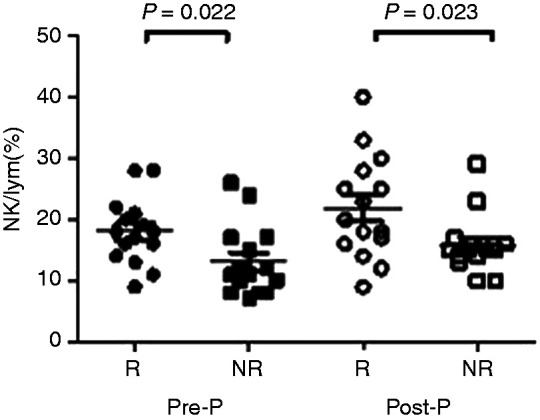
Comparison of NK cells in the R group and age-, sex- and treatment course–matched NR group during PEG-IFN treatment. Comparison of NK cell levels between those who achieved HBsAg seroconversion after 48 wk of treatment (R group; n = 15) and those who failed to achieve HBsAg seroconversion (NR group; n = 15) at the time point before HBsAg seroconversion was detected (Pre-P) and at the time point when seroconversion of HBsAg was detected (Post-P) after matching for age, sex and course of treatment.
Proportion of NK cells in selected patients matched for age, sex and treatment period in R and NR groups
Considering the differences in clinical characterisation and length of time needed to achieve HBsAg seroconversion in the R group, we further selected seven patients who achieved HBsAg seroconversion at 24 wk and seven NR patients matched for age, sex, HBsAg at baseline and treatment course for comparison (Table 2). The results also showed that the proportion of NK cells in the R group was significantly higher than in the NR group at Pre-P (12 wk of PEG-IFN treatment) and Post-P (24 wk of PEG-IFN treatment; 19.00±1.34 vs. 11.29±1.30, t=4.12, P=0.001; 24.14±2.58 vs. 14.71±0.42, t=3.61, P=0.004; Figure 5).
Table 2.
Clinical characterisation of seven patients who achieved HBsAg seroconversion at 24 wk and seven NR cases matched for age, sex, HBsAg level at baseline and treatment course.
| Patient ID | Age | Sex | ALT | HBsAg | 1st time point achieved seroconversion | |
|---|---|---|---|---|---|---|
| R (n=7) | 1 | 32 | M | 32 | 239.3 | 24 wk |
| 2 | 39 | M | 29 | 0.693 | 24 wk | |
| 3 | 39 | M | 35 | 2.85 | 24 wk | |
| 4 | 17 | F | 33 | 0.326 | 24 wk | |
| 5 | 40 | F | 31 | 13.56 | 24 wk | |
| 6 | 32 | F | 30 | 85 | 24 wk | |
| 7 | 41 | M | 28 | 70 | 24 wk | |
| NR (n=7) | 1 | 38 | M | 28 | 230.4 | NA |
| 2 | 35 | M | 29 | 1.424 | NA | |
| 3 | 40 | M | 31 | 6.7 | NA | |
| 4 | 20 | F | 34 | 0.592 | NA | |
| 5 | 36 | F | 29 | 12.9 | NA | |
| 6 | 32 | M | 30 | 89.4 | NA | |
| 7 | 40 | F | 34 | 78 | NA |
NA: not achieved.
Figure 5.
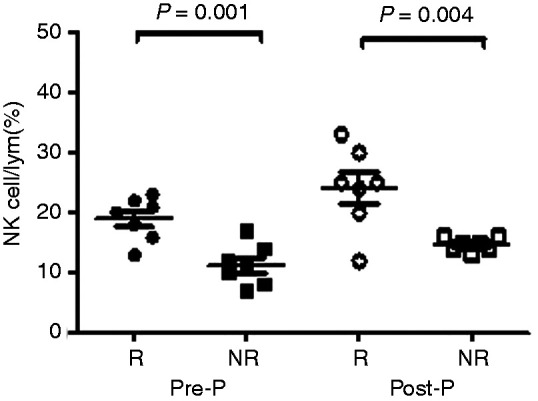
Comparison of NK cells proportion in R group and those matched for age, sex, HBsAg level at baseline and treatment course in the NR group during PEG-IFN treatment. Comparison of proportion of NK cells between IHCs who achieved seroconversion of HBsAg at 24 wk of PEG-IFN treatment (n=7) and those patients who failed to achieve seroconversion of HBsAg (n=7) after matching for age, sex, HBsAg level at baseline and course of treatment.
NK cell activity in patients matched for age, sex, HBsAg level at baseline and treatment period in R and NR groups
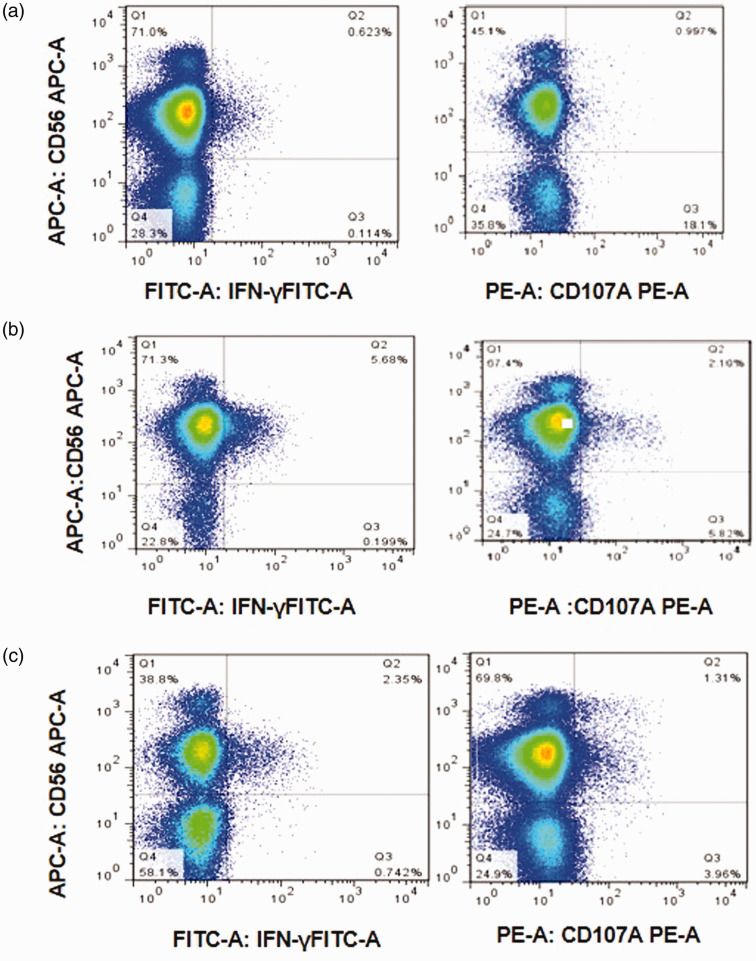
To evaluate NK cells activity for the 14 patients described previously, we used overlapping peptides spanning the entire HBsAg protein of the HBV C2 genotype, which is one of the most common genotypes in China. Example FACS data are shown in Figure 6. This patient (number 5 in R group, table 2) achieved HBsAg seroconversion at 24 wk after PEG-IFN treatment. At baseline, the HBsAg level was 13.56 IU/ml, and anti-HBs was negative (1.29 IU/ml). At 12 wk, although the HBsAg level was still positive (8 IU/ml), anti-HBs was also positive (22.13 IU/ml). At 24 wk, HBsAg loss was achieved, and the anti-HBs level rose to 72.9 IU/ml.
Figure 6.
NK cells activity in a patient who achieved HBsAg seroconversion at 24 wk after PEG-IFN treatment. IFN-γ secretion and CD107α expression detected by flow cytometry analysis at (a) baseline, (b) the time point before HBsAg seroconversion was detected (Pre-P) and (c) the time point when seroconversion of HBsAg was detected (Post-P).
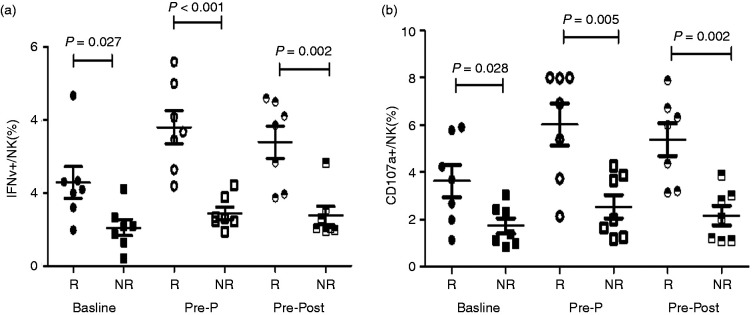
IFN-γ secretion from NK cells in the R group was 2.29±0.44%, 3.80±0.46% and 3.39±0.44% at baseline, Pre-P (12 wk) and Post-P (24 wk), respectively, which was significantly higher than in seven matched patients in the NR group (1.05±0.22%, 1.30±0.24%, 1.32±0.27%; t=2.52, P=0.027; t=4.83, P<0.001; t=4.00, P=0.002; Figure 7a). Similarly, CD107α expression was significantly higher in the R group at baseline, Pre-P (12 wk) and Post-P (24 wk; 3.62±0.69% vs. 1.72±0.33%, t=2.50, P=0.028; 6.02±0.89% vs. 1.61±0.31%, t=4.69, P=0.005; 5.37±0.69 vs. 1.49±0.35, t=5.00, P<0.001; Figure7b).
Figure 7.
IFN-γ secretion and CD107α expression of NK cells in the R group and NR group at baseline and during PEG-IFN treatment. (a) Comparison of IFN-γ secretion of NK cells between IHCs who achieved seroconversion of HBsAg at 24 wk of PEG-IFN treatment (n=7) and the patients who failed (n=7) after matching for age, sex, HBsAg level at baseline and course of treatment. (b) Comparison of CD107αexpression of NK cells between IHCs who achieved seroconversion of HBsAg at 24 wk of PEG-IFN treatment (n=7) and those who failed (n=7) after matching for age, sex, HBsAg level at baseline and course of treatment.
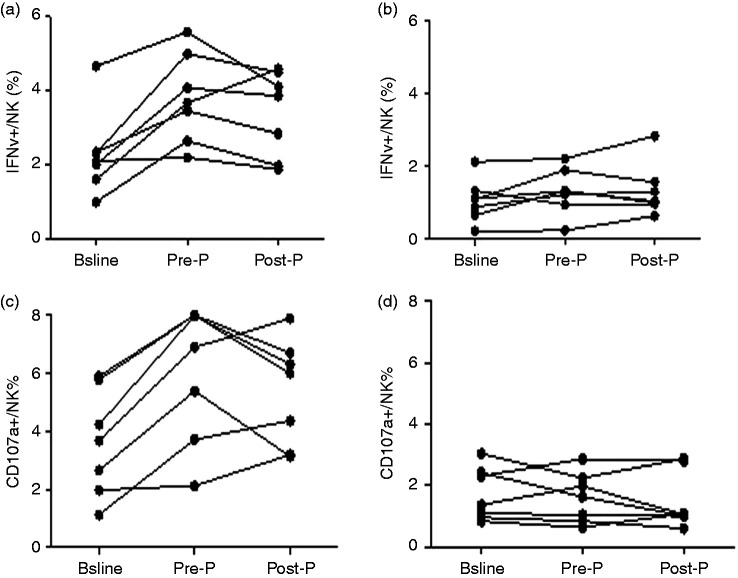
IFN-γ secretion and CD107α expression were increased from baseline to Pre-P, and then decreased in Post-P, but still higher than baseline in the R group. It is suggested that the function of NK cells was enhanced during Peg-IFN treatment with the removal of HBsAg, although there were no significant differences among the three points (F=3.12, P=0.069; F=2.64, P=0.099), which might be related to the small sample size (Figure 8a and c). In contrast, IFN-γ secretion and CD107α expression did not change much in patients in the NR group after PEG-IFN therapy (Figure 8b and d).
Figure 8.
Longitudinal analysis of IFN-γ secretion and CD107αexpression of NK cells in the R group and NR group at baseline and during PEG-IFN treatment. (a) IFN-γ secretion in the R group (n=7). (b) IFN-γ secretion in the NR group (n=7). (c) CD107α expression in the R group (n=7). (d) CD107α expression in the NR group (n=7).
Discussion
In our recent study, we observed a high proportion of cases of HBsAg seroconversion in IHC patients with low HBsAg levels treated with Peg-IFN.1 The exact mechanism of how PEG-IFN promotes HBsAg clearance remains unknown, and studies on predictors of HBsAg loss are still in the exploratory stage.9–11 However, there is accumulating evidence that PEG-IFN predominantly expands the NK cells compartment without boosting virus-specific T cells in HBV.12,13 NK cells respond quickly to stimulants. They can gather in large quantities to attack virus-infected or malignant cells without prior stimulation.14 Aside from the strong cytotoxic activity against tumour cells and viruses, NK cells secrete a variety of cytokines (IFN-γ, TNF-α, GM-CSF and chemokines), participate early in the innate immune response, modulate the acquired immune response and act as a bridge linking the two immune responses.15–17 Here, we first performed longitudinal analysis of lymphocyte subsets in IHCs undergoing PEG-IFN treatment, focusing on NK cells.
In our study, we found that the proportion of total NK cells in patients with HBsAg seroconversion was higher than in those who failed to achieve seroconversion. Even when patients were matched for age, sex and treatment period, the conclusion was the same. We directly measured NK cell activity by using overlapping peptides spanning HBsAg, and we detected degranulation and IFN-γ secretion. We found IFN- γ secretion and CD107α expression from NK cells in patients who achieved HBsAg seroconversion were all significantly higher than in patients matched for age, sex and treatment period in the NR group at baseline, and at the last time point before HBsAg seroconversion and the first time point when seroconversion of HBsAg was detected. This result suggests that NK cells from HBsAg seroconversion patients are more functional. In addition, we also found that in HBsAg seroconversion cases, NK cells activity (IFN-γ secretion and CD107α expression) increased after PEG-IFN treatment, especially at the time point before HBsAg seroconversion. It is suggested that the function of NK cells was enhanced during PEG-IFN treatment with the removal of HBsAg, although there was no significant difference, which might be related to the small sample size. In contrast, there were no such trends in the NR group.
Our findings suggest that the elevation of NK cells is associated with response and that it favours HBsAg clearance. It has been reported that in chronic HBV infection patients, increased NK cell function is associated with HBsAg seroconversion following structured NA cessation.18 CD56 bright NK cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to PEG-IFN.19 Combination therapy significantly influences NK cell phenotype and function. Differences between patients with CHB with HBsAg clearance and non-responders suggest that NK cells play a role in the clearance of HBsAg during IFN-based combination therapy.20 All of these data indicate that NK cell levels are closely correlated with the control of HBV infection. In agreement with the previously mentioned reports, we found that PEG-IFN can increase NK cells levels and function, which favours the seroconversion of HBsAg.
In conclusion, we demonstrated that for IHCs, the increase of NK cells during PEG-IFN treatment favoured HBsAg seroconversion, and NK cells may play a role in HBV seroconversion. Because it is very difficult to acquire HBsAg seroconversion for chronic HBV infection patients, our sample size was small. Of course, larger studies are therefore needed.
Supplemental Material
Supplemental material, sj-pdf-1-ini-10.1177_1753425920942580 for Contribution of NK cells to HBsAg seroconversion in inactive HBsAg carriers following pegylated IFN therapy by Zhenhuan Cao, Sha Meng, Yanhong Zheng, Junli Wang, Rui Wang and Xinyue Chen in Innate Immunity
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The Thirteenth Five-Year Major Science and Technology Projects (2017ZX10202201; 2017ZX10201021-001-008; 2017ZX10302201-004-003; 2017ZX10202203-006), Chinese National Natural Science Foundation (81900537), Capital Health Research and Development Projects (2018-2-2183, 2020-1-2181), The Capital Characteristic Clinical Application Research (Z171100001017062), Beijing Municipal Natural Science Foundation (7182073), Beijing Outstanding Talent Training Funding (2017000021469G253).
ORCID iD
Zhenhuan Cao https://orcid.org/0000-0003-4204-1049
References
- 1.Cao Z, Liu Y, Ma L, et al. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology 2017; 66: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 2.Li MH, Xie Y, Zhang L, et al. Hepatitis B surface antigen clearance in inactive hepatitis B surface antigen carriers treated with peginterferon alfa-2a. World J Hepatol 2016; 8: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- 4.Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol 2018; 36: 247–277. [DOI] [PubMed] [Google Scholar]
- 5.Mikulak J, Bruni E, Oriolo F, et al. Hepatic natural killer cells: organ-specific sentinels of liver immune homeostasis and physiopathology. Front Immunol 2019; 10: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisicaro P, Rossi M, Vecchi A, et al. The good and the bad of natural killer cells in virus control: perspective for anti-HBV therapy. Int J Mol Sci 2019; 20: 5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen X, Fu B, Liu Y, et al. NKp30+ NK cells are associated with HBV control during pegylated-interferon-alpha-2b therapy of chronic hepatitis B. Sci Rep 2016; 6: 38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua S, Vigano S, Tse S, et al. Pegylated interferon-α-induced natural killer cell activation is associated with human immunodeficiency virus-1 DNA decline in antiretroviral therapy-treated HIV-1/hepatitis C virus-coinfected patients. Clin Infect Dis 2018; 66: 1910–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana RJ, Avigan MI, Janssen HLA, et al. Liver safety assessment in clinical trials of new agents for chronic hepatitis B. J Viral Hepat 2020; 27: 96–109. [DOI] [PubMed] [Google Scholar]
- 10.Ma Z, Zhang E, Gao S, et al. Toward a functional cure for hepatitis B: the rationale and challenges for therapeutic targeting of the B cell immune response. Front Immunol 2019; 10: 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Y, Wu D, Zhou L, et al. Present and future therapies for chronic hepatitis B. Adv Exp Med Biol 2020; 1179: 137–186. [DOI] [PubMed] [Google Scholar]
- 12.Penna A, Laccabue D, Libri I, et al. Peginterferon-α does not improve early peripheral blood HBV-specific T-cell responses in HBeAg-negative chronic hepatitis. J Hepatol 2012; 56: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 13.Shen X, Fu B, Liu Y, et al. NKp30+ NK cells are associated with HBV control during pegylated-interferon-alpha-2b therapy of chronic hepatitis B. Sci Rep 2016; 6: 38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoy SKA, Boudreau JE. The evolutionary arms race between virus and NK cells: diversity enables population-level virus control. Viruses 2019; 11: 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Male V, Stegmann KA, Easom NJ, et al. Natural killer cells in liver disease. Semin Liver Dis 2017; 37: 198–209. [DOI] [PubMed] [Google Scholar]
- 16.Abel AM, Yang C, Thakar MS, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol 2018; 9: 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villalba M, Alexia C, Bellin-Robert A, et al. Non-genetically improving the natural cytotoxicity of natural killer (NK) cells. Front Immunol 2020; 10: 3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmer CL, Rinker F, Höner Zu Siederdissen C, et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J Infect Dis 2018; 217: 1656–1666. [DOI] [PubMed] [Google Scholar]
- 19.Shi A, Zhang X, Xiao F, et al. CD56bright natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat 2018; 25: 1352–1362. [DOI] [PubMed] [Google Scholar]
- 20.Stelma F, De Niet A, Tempelmans Plat-Sinnige MJ, et al. Natural killer cell characteristics in patients with chronic hepatitis B virus (HBV) infection are associated with HBV surface antigen clearance after combination treatment with pegylated interferon alfa-2a and adefovir. J Infect Dis 2015; 212: 1042–1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ini-10.1177_1753425920942580 for Contribution of NK cells to HBsAg seroconversion in inactive HBsAg carriers following pegylated IFN therapy by Zhenhuan Cao, Sha Meng, Yanhong Zheng, Junli Wang, Rui Wang and Xinyue Chen in Innate Immunity