Abstract
Aim:
This study was designed to assess the efficacy and safety of Saccharomyces cerevisiae variant boulardii CNCM I-3799 (S. boulardii CNCM I-3799) in the management of acute diarrhea in children.
Methods:
A total of 100 infants and children 3–36 months of age with acute diarrhea received medical care according to the World Health Organization guidelines on the management of acute diarrhea in children and were randomly allocated to the probiotic group (S. boulardii CNCM I-3799 at a daily dose of 5 billion CFU twice daily) or to the placebo group. Infants and children were treated for 5 days and an extended follow-up was planned 1 and 2 months after the end of the treatment period. Primary endpoint was the time of recovery from diarrhea defined as the duration of diarrhea. Other parameters, such as frequency and consistency of stools, associated with the severity of diarrhea episodes were defined as secondary endpoints.
Results:
The administration of S. boulardii CNCM I-3799 was associated with beneficial effects on duration and severity of diarrhea. The time of recovery from diarrhea was significantly shorter in the probiotic group compared with the placebo group (65.8 ± 12 hours vs. 95.3 ± 17.6 hours, P = 0.0001). Faster remission in the probiotic group was also demonstrated by a shorter time before the first episode of semisolid stool [−23.5 hours, diff (95% CI): −7.99 (−31.49 to −15.51), P = 0.0001] and the faster normalization of stool consistency. S. boulardii CNCM I-3799 was well tolerated.
Conclusion:
S. boulardii CNCM I-3799 supplementation in children with acute diarrhea was shown effective in reducing the duration and severity of diarrhea in infants and children.
Keywords: acute diarrhea, pediatric, probiotic, efficacy, safety
Diarrhea is one of the most common infectious diseases and remains a leading cause of death in children under 5 years of age in low- and middle-income countries.1 Although important reductions in the number of deaths have been reported since several decades,1,2 millions children worldwide suffer from diarrhea every year and are at risk of dehydration and malnutrition. Dehydration is the principal cause of death from diarrhea. Fluids and electrolytes replacement therapy by oral rehydration solution (ORS) is consequently central in the World Health Organization (WHO) guidelines on the management of acute diarrhea in children.3 Nevertheless, oral rehydration has no effect on the duration of diarrhea, prompting a growing interest in adjunctive solution to limit the burden of the disease. Alongside rehydration therapy, the WHO also recommends the use of zinc supplementation which has been shown to reduce the duration and severity of diarrheal episodes.4 Other interventions have also been suggested and among them, there is considerable interest in the role of probiotics in promoting recovery from acute diarrhea. Indeed, several meta-analyses have shown that probiotic supplementation, as an adjunct to rehydration therapy in the management of acute diarrhea in children, is effective in reducing diarrhea duration.4–7 These consistent pieces of evidence have led to universal recommendations in favor of the use of probiotics for the management of acute diarrhea from the European and International Societies for Pediatric Gastroenterology, Hepatology and Nutrition.8,9 More specifically, experts have recommended the use of probiotic strains with clinically proven efficacy such as Lactobacillus rhamnosus GG or S. cerevisiae variant boulardii (S. boulardii). On the contrary, recent clinical studies have found no benefit of probiotics on the duration of diarrhea.10,11 A systematic review and network meta-analysis12 designed to compare and determine the most effective pharmacologic or nutritional interventions in reducing diarrhea duration supports the recommendation towards the use of S. boulardii in the management of acute diarrhea in children. S. boulardii associated with zinc supplementation has been shown superior to both standard treatment, zinc supplementation alone or supplementation with any of the other probiotic strains tested. Although the efficacy of S. boulardii in various indications in children and adults is already well documented in the literature, it is the first time that the efficacy and safety of the S. boulardii CNCM I-3799 is evaluated in children with acute diarrhea.
MATERIALS AND METHODS
Aim
This randomized, double-blind, placebo-controlled trial was designed to evaluate the efficacy and safety of Saccharomyces cerevisiae var. boulardii CNCM I-3799 in the management of acute diarrhea in children.
Ethics and Regulatory Approval
The study received institutional ethics committee approval, was performed in compliance with the Declaration of Helsinki and carried out according to the general principles of the International Conference on Harmonization guidelines for Good Clinical Practice. Parents received information on all aspects of the study and were provided with a written informed consent to be signed before participation of their child in the study.
The study was registered prospectively in the Clinical Trials Registry of India (CTRI) under the CTRI number CTRI/2017/07/009101.
Clinical Study Settings
This multicenter study was performed by Ethicare Clinical Trial Services and took place in 4 different study sites located across Ahmedabad, India.
Participants
A total of 100 infants and children 3–36 months of age with acute diarrhea, defined as 3 or more loose stools in the last 24 hours, were included between January and December 2018. Volunteers were excluded if they presented clinical signs of severe malnutrition or clinical signs of severe dehydration if the investigator reported clinical evidence of coexisting acute systemic illnesses (eg, sepsis and pneumonia) or clinical evidence of chronic disease (eg, chronic liver disease). Volunteers were also excluded in case of antibiotic medication or probiotic consumption in the week before inclusion.
Interventions
All children were given standard treatment which consisted of an ORS (Electral, FDC Limited) as per WHO recommendations3 and a daily dose of zinc (Zinconia, Zuventus Healthcare Ltd) at 10 mg for children less than 1-year-old and at 20 mg for children above 1-year-old. Children were randomly allocated to the intervention group or the control group. Participants in the intervention group received 2 sachets per day of the test product (LesunBerry Sundyota Numandis), each containing 5 billion CFU of the probiotic yeast S. cerevisiae var. boulardii CNCM I-3799, a proprietary strain of Lesaffre Group (Marcq-en-Barœul, France). S. boulardii CNCM I-3799 is stabilized by fluid-bed gentle drying technology which confers adequate viability to the probiotic together with an improved flowability. Participants in the control group were given sachet of identical appearance containing only maltodextrin and received the same instructions. Active and placebo products were similar in color and flavor.
Study Design and Procedures
This clinical study consisted of a treatment period of 5 days and an extended follow-up 1 and 2 months after the end of the treatment period. The intervention period included 4 visits to the investigation center. The first visit was a screening visit during which the eligibility of the subject was verified. Subjects fulfilling the eligibility criteria were then randomly allocated to the intervention group or to the control group. From randomization to the end of the 5-day treatment period, parents or legal guardian were asked to record the following parameters in a subject diary: the total number of stools daily as well as stool’s consistency, the date and time of the first semisolid stool and the total volume of ORS taken during the day. In addition, stool collection for detection of rotavirus antigen at baseline and the end of the 5-day treatment period was conducted in a subset of 20 subjects per group. Extended follow-up visits were planned to check the occurrence and duration of any new episode of diarrhea.
Efficacy Assessments
The primary endpoint was the time of recovery from diarrhea defined as the duration of diarrhea assessed according to the consistency of stool: 3 consecutive semisolid stool or no stool in the last 12 hours was considered as recovery from diarrhea. Time and date of semisolid stools were collected by the parents of the subjects. Secondary endpoints were the frequency and consistency of stools, the time of the first semisolid stool, the daily ORS consumption, the time of first rehydration, the number and the duration of relapses episodes. Stool consistency was evaluated using a previously described score system13 where feces were classified as 1 (normal), 2 (loose), 3 (semiliquid) or 4 (liquid). The time of first rehydration evaluation relies on clinical examination and interview with the parent of the subjects. The occurrence and the duration of any new episode of diarrhea during the 2-month follow-up period, defined as relapses episodes, were collected during the extended follow-up visits 1 and 2 months after the end of the 5-day treatment period. The presence of rotavirus antigen at baseline and the end of the treatment period is reported as an exploratory endpoint.
Safety Assessments
Safety was assessed considering the occurrence and evaluation of adverse events as well as any changes in vital signs (pulse rate, respiratory rate and temperature) from baseline. Adverse events were recorded throughout the study and investigators, blinded to treatment assignment, had to estimate their severity and to evaluate whether they could be related to the study or the tested product. Any deterioration of the health status of the children which requires either rescue medicine or hospitalization for intravenous fluid replacement therapy was considered as an adverse event and the children were classified as being in treatment failure.
Statistical Analysis
Based on the results from a meta-analysis assessing the efficacy of S. boulardii in the management of diarrhea in children,6 a mean difference in the duration of diarrhea of 20 hours was assumed with a standard deviation of 30 hours. To detect this difference with a power of 80% and a significance level of 0.05, a minimum of 37 subjects per arm is needed. To make allowance for potential protocol deviations, treatment failures or premature withdrawals, a total of 50 subjects per group was considered appropriate. Clinical study endpoints depending on continuous variables were analyzed using unpaired t test, while χ2 test was used for analyzing categorical variables. Statistical significance was set at P < 0.05. Primary analysis was performed on the efficacy analysis population, which comprised subjects who completed the study in compliance with the protocol as well as subjects considered as being in treatment failure.
RESULTS
Patients
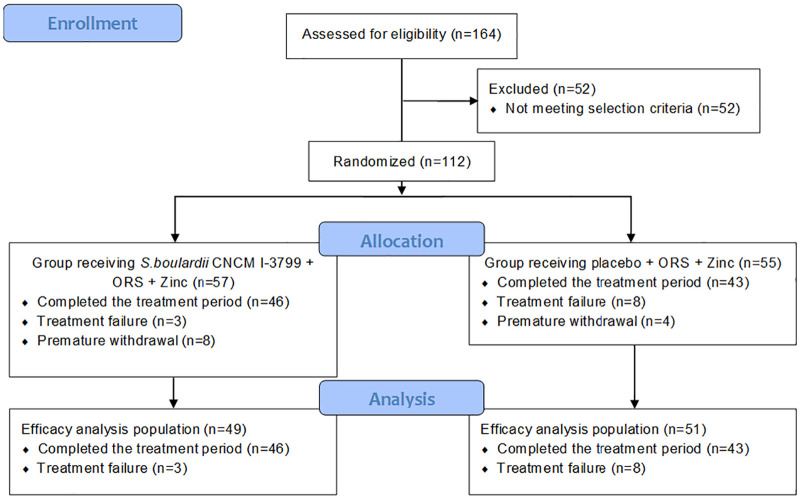
A total of 112 subjects were included in this study, received ORS and zinc supplementation and were randomly allocated to the group receiving S. boulardii CNCM I-3799 (n = 57) or the group receiving the placebo (n = 55). Twenty-three subjects did not complete the study [treatment failure (n = 11), protocol deviation (n = 3), consent withdrawal (n = 4) and lost to follow-up (n = 5)]. Efficacy analysis population (n = 100: probiotic group n = 49 and placebo group n = 51) includes subjects who completed the study in compliance with the protocol as well as subjects considered as being in treatment failure. The flow of the study is presented in Figure 1.
FIGURE 1.
Flowchart of participants through the study of Saccharomyces boulardii in acute diarrhea management.
Baseline characteristics of the study population are presented in Table 1. Both groups were similar with regard to demographic characteristics and duration of diarrhea before enrollment in the study protocol.
TABLE 1.
Baseline Characteristics of Participants: Comparison Between Saccharomyces boulardii and Placebo Groups
| S. boulardii CNCM I-3799 | Placebo | P | |
|---|---|---|---|
| Age (months ± SD) | 13.2 ± 8.1 | 13.3 ± 8. 2 | 0.77 |
| Gender (female/male) | 23/26 | 23/28 | 0.71 |
| Weight (kg ± SD) | 8.8 ± 2.8 | 9.5 ± 4.8 | 0.96 |
| Height (cm ± SD) | 75.7 ± 9.7 | 71.2 ± 11.9 | 0.13 |
| Diarrhea duration before intervention (hours ± SD) | 40.4 ± 24.3 | 43.6 ± 31.4 | 0.57 |
SD, standard deviation.
Primary Endpoint
At baseline, the mean duration of diarrhea before intervention was similar in both study groups (40.4 ± 24.3 hours in S. boulardii group vs. 43.6 ± 31.4 hours in the placebo group, P = 0.57). After randomization, a statistically significant difference in the overall duration of diarrhea was found between the groups. S. boulardii CNCM I-3799 shortened recovery time from diarrhea in comparison with placebo [−29.5 hours, diff (95% CI): − 6.22 [−35.74 to −23.26], P = 0.0001] (Fig. 2). This 1-day faster remission with S. boulardii supplementation was also complemented by the higher number of infants and children in the S. boulardii group considered as recovered from diarrhea over the supplementation period. Recovery from diarrhea started on day 2 in S. boulardii group with 10.9% of subjects considered as recovered from diarrhea. Within 3 days from randomization, 82.6 % of subjects in the S. boulardii group were considered as recovered, whereas only 16.3% of subjects in the placebo group were considered as recovered (descriptive statistics only). Percentage of subjects who recovered from diarrhea over the treatment period is presented in Figure 3.
FIGURE 2.
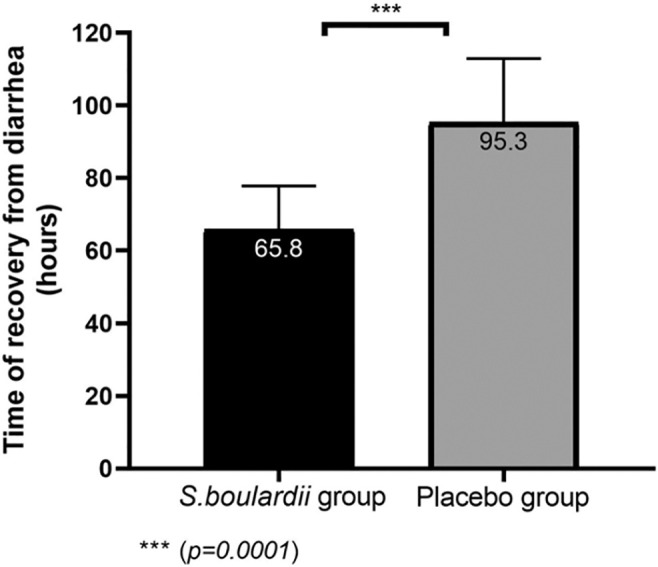
Time of recovery from diarrhea in placebo and Saccharomyces boulardii groups.
FIGURE 3.
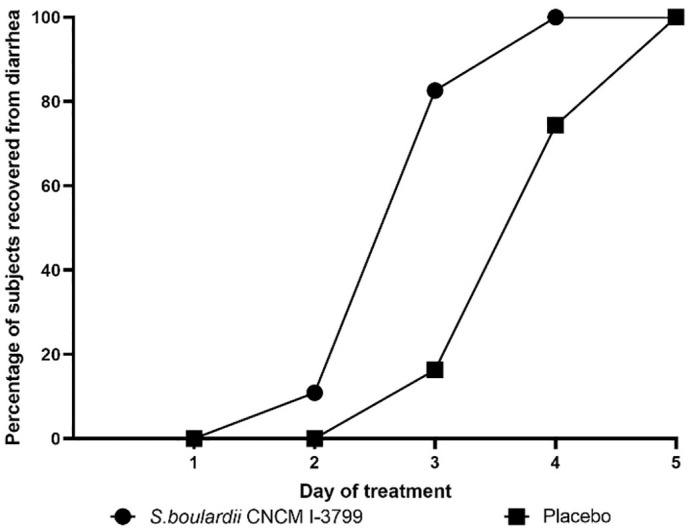
Percentage of subjects in Saccharomyces boulardii and placebo groups who recovered from diarrhea over the treatment period.
Secondary Endpoints
Several clinical features associated with diarrhea were significantly different between the groups over the course of the treatment period. The first semisolid stool appeared significantly faster in the group receiving S. boulardii [−23.5 hours, diff (95% CI): −7.99 (−31.49 to −15.51), P = 0.0001]. Similarly, faster improvements in stool consistency were reported in the probiotic group and statistically significant between-group differences in stool consistency were reported from day 3 up to the end of the intervention period. Rehydration occurred significantly faster in the group receiving S. boulardii CNCM I-3799 [−9.1 hours, diff (95% CI): −2.36 (−11.46 to −6.74), P = 0.0001) although no difference in daily total ORS consumption was reported between the groups. Daily stool frequencies were similar between the groups, the maximum intergroup difference was reported on day 2 with a statistically significant reduction in the mean number of bowel movements occurring in the S. boulardii group [−0.5, diff (95% CI): − 0.46 (−0.93 to −0.01), P = 0.044] but no statistically significant differences were observed on the days 3, 4 and 5 of treatment period. Duration of diarrhea and changes in stool consistency and frequency in placebo and S. boulardii groups are presented in Table 2. No difference in number or duration of the new episode of diarrhea was seen during the monthly observational follow-up conducted over 2 months after the end of the treatment period.
TABLE 2.
Duration of Diarrhea and Changes in Stool Consistency and Frequency in Placebo and Saccharomyces boulardii Groups
| S. boulardii CNCM I-3799 | Placebo | Treatment effect Δ Change and CI 95% |
P | |
|---|---|---|---|---|
| Duration of diarrhea | ||||
| Time of recovery from diarrhea (hours) | 65.8 ± 12 | 95.3 ± 17.6 | − 29.5 (−35.74 to −23.26) | 0.0001 |
| Changes in stool consistency | ||||
| Time of first semisolid stool (hours) | 45.5 ± 11.6 | 69 ± 24.8 | − 23.5 (−31.49 to −15.51) | 0.0001 |
| Mean daily stool consistency: | ||||
| Day 1 | 3.7 ± 0.4 | 3.7 ± 0.4 | 0 (−0.17 to 0.17) | 1 |
| Day 2 | 3.2 ± 0.6 | 3.3 ± 0.7 | − 0.1 (−0.39 to 0.11) | 0.28 |
| Day 3 | 2.4 ± 0.8 | 2.9 ± 0.7 | − 0.5 (−0.79 to −0.19) | 0.0015 |
| Day 4 | 1.7 ± 0.6 | 2.4 ± 0.7 | − 0.7 (−0.95 to −0.42) | 0.0001 |
| Day 5 | 1.3 ± 0.5 | 1.7 ± 0.7 | − 0.4 (−0.72 to −0.22) | 0.0003 |
| Changes in stool frequency | ||||
| Mean daily stool frequency: | ||||
| Day 1 | 4.2 ± 1.4 | 4.5 ± 1.4 | − 0.3 (−0.77 to 0.35) | 0.46 |
| Day 2 | 3.1 ± 0.9 | 3.6 ± 1.3 | − 0.5 (−0.93 to −0.01) | 0.044 |
| Day 3 | 2.5 ± 1.1 | 2.7 ± 1.3 | − 0.2 (−0.65 to 0.29) | 0.453 |
| Day 4 | 1.9 ± 0.9 | 2.3 ± 1.2 | − 0.4 (−0.83 to 0.44) | 0.078 |
| Day 5 | 2.0 ± 1.4 | 1.9 ± 1.1 | − 0.1 (−0.35 to 0.63) | 0.57 |
Exploratory Endpoint
In both study groups, 11 subjects were tested positive for rotavirus antigen at baseline. At the end of the 5-day treatment period, all the subjects in the probiotic group were tested negative for rotavirus antigen whereas rotavirus antigen was still detected in 2 subjects in the placebo group.
Safety Evaluation
No serious adverse event linked to the research or to the study product was recorded during the study in either study group. The frequency of subjects with at least 1 adverse event was similar in the group receiving S. boulardii and in the placebo group (14.3% vs. 17.6%, respectively). A summary of adverse events is given in Table 3. A total of 16 adverse events were recorded as follows: 3 subjects reported fever (2 in the probiotic group and 1 in the placebo group), 1 subject reported severe weakness (placebo group), 1 subject reported vomiting (placebo group) and 11 subjects reported an increase in diarrhea symptoms which required administration of intravenous fluid therapy or rescue medicine (3 in the probiotic group and 8 in the placebo group). A total of 11 subjects were then considered as treatment failure. Among the 16 adverse events recorded during this study, none were judged as possibly linked to the research or the investigational product.
TABLE 3.
Summary of Adverse Events in Saccharomyces boulardii and Placebo Groups
| S.boulardii CNCM I-3799 | Placebo | |
|---|---|---|
| Total number of adverse events (percentage of subject with at least 1 adverse event) | 7 (14.3%) | 9 (17.6%) |
| Total number of adverse events of mild intensity | 2 | 2 |
| Total number of adverse events of moderate intensity | 5 | 7 |
| Total number of adverse events of severe intensity | 0 | 0 |
| Treatment failure | 3 | 8 |
DISCUSSION
This multicenter, randomized, double-blind and placebo-controlled study demonstrates that the strain S. boulardii CNCM I-3799 is effective in the management of acute diarrhea in children. S. boulardii, administered as an adjunctive standard treatment of ORS and zinc supplementation, was associated with beneficial effects on several clinical features of diarrhea. Faster remission in the group receiving the probiotic is consistent with previous findings of several authors reporting an approximately 1 day faster recovery from diarrhea with S. boulardii.14–18 After 3 days of treatment, more children in the probiotic group had recovered from diarrhea than those in the placebo group. Consistently, Correa et al. reported a lower frequency of patients with diarrhea within 3 days from randomization in the group supplemented with S. boulardii (RR 0.54, 95% CI: 0.38–0.77, P < 0.001). In the present study, the faster recovery in the probiotic group appears to be associated with a faster improvement in stool consistency rather than an effect of treatment in stool frequency. Similar results have been previously reported by Riaz et al. who reported a faster normalization of stool consistency in the group receiving S. boulardii [−14.6 hours, diff (95% CI): −10.85 (−25.5 to −3.79), P = 0.009] in the absence of statistically significant differences in stool frequency.17
The beneficial effect of S. boulardii CNCM I-3799 supplementation in prompting the recovery from acute diarrhea in infants and children is consistent with findings from a Cochrane review that has assessed the effects of probiotics in acute diarrhea and has reported clear beneficial effects of probiotics used alongside rehydration therapy.4 However, the effects of probiotics in specific indications are largely strain-dependent and clinical studies designed to evaluate the comparative effectiveness of different probiotic strains are lacking. In a systematic review and network meta-analysis12 designed to compare and determine the most effective interventions in reducing diarrhea duration, S. boulardii associated with zinc supplementation has been shown superior to both standard treatment, zinc supplementation alone or supplementation with any of the other probiotic strains tested. This finding is notably supported by clinical studies that have compared the efficacy of S. boulardii with lactic acid bacteria or with Bacillus clausii and which have reported positive results in favor of S. boulardii.19–21
Numerous mechanisms of action of S. boulardii in relation to the clinically demonstrated beneficial effects have been proposed and evaluated. Clinical benefits of S. boulardii may, at least partly, be related to direct and indirect anti-pathogenic effect, promotion of gut mucosal barrier integrity as well as modulation of host immunity.22 Stimulation of local immunity through the release of secretory immunoglobulin A (IgA), one of the first-line of defense against pathogens in the intestinal lumen which prevents the adhesion of pathogens and promotes their clearance, is one of the reported mechanism of action of the strain23. This is also supported by clinical evidence showing a significant increase in IgA in the group supplemented with S. boulardii.24 This stimulatory effect on the immune system has also been hypothesized as being partly responsible for long-term beneficial effect of the yeast, as previously reported. Indeed, Billo et al. reported a lower prevalence of new episodes of diarrhea in S. boulardii group over the 2 months following the end of the treatment period.14 However, the present study did not find any difference in the prevalence or clinical features of any new episode of diarrhea within 2 months after the intervention period.
The beneficial effect of S. boulardii in the management of acute diarrhea in children is substantiated by several clinical interventions and is supported by numerous preclinical evidences elucidating its mechanisms of action. Nevertheless, this clinical study is the first to date that has evaluated the efficacy and safety of S. boulardii CNCM I-3799 in this indication. Results of the present intervention support the use of S. boulardii CNCM I-3799 at a daily dose of 5 billion living cells received twice daily for 5 days in association with rehydration therapy and zinc supplementation in the management of acute diarrhea in infants and children.
Footnotes
Sundyota Numandis Probioceuticals Pvt. Ltd. funded this study.
F.M., C.T., and A.D. are full-time employees of Gnosis by Lesaffre (Lesaffre Group). V.S. and D.K. are full-time employees of Sundyota Numandis Probioceuticals Pvt. Ltd. Dr. M.S. and D.S. are full-time employees of Ethicare Clinical Trial Services. Ethicare Clinical Trial Services received fees from Sundyota Numandis Probioceuticals Pvt. Ltd. to set up and conduct the clinical trial. Dr. P.S., Dr. D.P. and Dr. U.U. were investigators of the study and received fees from Ethicare Clinical Trial Services.
REFERENCES
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016; 388:3027–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003; 81:197–204 [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. 2005
- 4.Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szajewska H, Skórka A. Saccharomyces boulardii for treating acute gastroenteritis in children: updated meta-analysis of randomized controlled trials. Aliment Pharmacol Ther. 2009; 30:960–961 [DOI] [PubMed] [Google Scholar]
- 6.Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014; 134:e176–e191 [DOI] [PubMed] [Google Scholar]
- 7.Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children – a 2019 update. Aliment Pharmacol Ther. 2019; 49:1376–1384 [DOI] [PubMed] [Google Scholar]
- 8.Guarino A, Ashkenazi S, Gendrel D, et al. ; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014; 59:132–152 [DOI] [PubMed] [Google Scholar]
- 9.Guarino A, Lo Vecchio A, Dias JA, et al. Universal recommendations for the management of acute diarrhea in nonmalnourished children. J Pediatr Gastroenterol Nutr. 2018; 67:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med. 2018; 379:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman SB, Williamson-Urquhart S, Farion KJ, et al. ; PERC PROGUT Trial Group. Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med. 2018; 379:2015–2026 [DOI] [PubMed] [Google Scholar]
- 12.Florez ID, Veroniki AA, Al Khalifah R, et al. Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: a systematic review and network meta-analysis. PLoS One. 2018; 13:e0207701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007; 335:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billoo AG, Memon MA, Khaskheli SA, et al. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J Gastroenterol. 2006; 12:4557–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burande MA. Comparison of efficacy of Saccharomyces boulardii strain in the treatment of acute diarrhea in children: a prospective, single-blind, randomized controlled clinical trial. J Pharmacol Pharmacother. 2013; 4:205–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S, Gupta PK, Das RR. Efficacy and safety of Saccharomyces boulardii in acute rotavirus diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediatr. 2016; 62:464–470 [DOI] [PubMed] [Google Scholar]
- 17.Riaz M, Alam S, Malik A, et al. Efficacy and safety of Saccharomyces boulardii in acute childhood diarrhea: a double blind randomised controlled trial. Indian J Pediatr. 2012; 79:478–482 [DOI] [PubMed] [Google Scholar]
- 18.Dinleyici EC, Kara A, Dalgic N, et al. Saccharomyces boulardii CNCM I-745 reduces the duration of diarrhoea, length of emergency care and hospital stay in children with acute diarrhoea. Benef Microbes. 2015; 6:415–421 [DOI] [PubMed] [Google Scholar]
- 19.Asmat S, Shaukat F, Asmat R, et al. Clinical efficacy comparison of Saccharomyces boulardii and lactic acid as probiotics in acute pediatric diarrhea. J Coll Physicians Surg Pak. 2018; 28:214–217 [DOI] [PubMed] [Google Scholar]
- 20.Bhat S, Shreekrishna GN, Savio CD. Efficacy of probiotics in acute diarrhoea in children. Int J Contemp Pediatr. 2018; 5:1646–1650 [Google Scholar]
- 21.Vineeth S, Saireddy S, Keerthi T, et al. Efficacy of Bacillus clausii and Saccharomyces boulardii in treatment of acute rotaviral diarrhea in pediatric patients. Indones J Clin Pharm. 2017; 6:91–98 [Google Scholar]
- 22.Czerucka D, Rampal P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J Gastroenterol. 2019; 25:2188–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stier H, Bischoff SC. Influence of Saccharomyces boulardii CNCM I-745 on the gut-associated immune system. Clin Exp Gastroenterol. 2016; 9:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozkan TB, Sahin E, Erdemir G, et al. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J Int Med Res. 2007; 35:201–212 [DOI] [PubMed] [Google Scholar]