Supplemental digital content is available in the text.
Key Words: CHILD, EXERCISE, HEAT STRESS, THERMOREGULATION, YOUTH
ABSTRACT
Prepubertal children (6–12 yr) differ from adults in various morphological and physiological factors that may influence thermoregulatory function; however, experimental evidence of meaningful child–adult differences in heat strain during exercise-heat stress is sparse, despite numerous studies. Although we appreciate the challenges associated with performing such comparisons, part of that discrepancy may be due to the methods used. Nonetheless, a focused discussion of these methodological considerations and their implications for current understanding remains unavailable. This is an important knowledge gap given the threat to health posed by rising global temperatures and the ongoing focus on improving physical activity levels in children. The aims of this methodological review were, therefore, to (i) review the theoretical basis for child–adult differences in thermoregulatory function, (ii) describe previous comparisons of exercise thermoregulation between prepubertal children and adults, (iii) discuss two methodological issues associated with that research, which, in our view, make it difficult to present empirical evidence related to child–adult differences in thermoregulatory function and associated heat strain, (iv) provide potential solutions to these issues, and (v) propose pertinent areas for further research.
With the threat to health posed by global warming and the rising demand for greater engagement in exercise for health, there is continued research interest in understanding the effects of various individual factors (e.g., age, sex, disease) on thermoregulatory function during exercise-heat stress. Perhaps due to their frequent exposure to heat stress during competitive sport and/or play (1,2), considerable emphasis has been placed on thermoregulation in prepubertal children (defined typically as age 6–12 yr [3]). However, although children differ from adults in various morphological and physiological factors that may influence thermoregulation (4–6), experimental evidence of meaningful child–adult differences in heat strain during exercise-heat stress is sparse (7–9). In our view, this discrepancy may be explained by the methodology used, although a focused discussion of these considerations and their implications for current understanding is unavailable, despite numerous comparative reviews on thermoregulation in children and adults (4–8,10–13).
The purpose of this methodological review was, therefore, to examine the approaches used to evaluate differences in exercise thermoregulation between prepubertal children and young adults. First, we discuss the theoretical basis for child–adult differences in thermoregulatory function. Our attention is then directed to previous comparisons of exercise thermoregulation between prepubertal children and adults. We then consider two common methodological issues associated with that research, which, in our view, make it difficult to interpret evidence for (or against) the presence of child–adult differences in thermoregulatory function and associated heat strain. Finally, we provide potential solutions to these issues and propose some pertinent areas for further research.
THE THEORETICAL BASIS FOR CHILD–ADULT DIFFERENCES
To optimize function, humans strive to regulate body temperature within a narrow range. This requires a balance between metabolic heat production (metabolic rate − external work) and the sum (total) of the dry (convection, radiation, conduction) and evaporative heat exchanges occurring between the body and surrounding environment. Those heat exchanges occur passively according to the prevailing environmental conditions and the body’s physical characteristics, and are also actively facilitated by several thermoeffector mechanisms that are recruited when a mismatch between metabolic heat production and total heat loss causes an increase or decrease in body heat storage and a corresponding change in body temperature. The most powerful thermoeffector is behavioral change (e.g., seeking a cooled/shaded location, pacing, changing clothing), although we are often unwilling or unable to make such adjustments (e.g., during structured activities or competition). We therefore rely heavily on the autonomic thermoeffectors of cutaneous vasodilatation and sweating to facilitate heat loss by modifying dry and evaporative heat exchange (14).
Under compensable exercise conditions, where increases in cutaneous vasodilatation and sweating can facilitate the total heat loss required to balance metabolic heat production, body temperature will eventually stabilize, albeit at an elevated level (14). However, during more intense exercise in hotter and/or more humid environments, the heat loss required to maintain heat balance can often exceed the body’s heat loss capacity, or the maximal rate of heat loss possible in the surrounding environment. In these uncompensable situations, the rate of body heat storage will remain positive and body temperature will continue to rise as exercise progresses, potentially leading to adverse health effects if left unchecked.
Compared with young adults, prepubertal children have long been thought to be at a thermoregulatory disadvantage and, therefore, more vulnerable to these adverse effects during heat stress. That notion likely stemmed from the seminal work of Bar-Or (4), who, based on theoretical grounds and a limited number of comparative studies, concluded that there are several differences between children and adults that may compromise thermoregulation in children. These primarily included (i) morphological differences, which modify passive heat exchange occurring between the body and surrounding environment; (ii) physiological differences in the control of cutaneous vasodilatation and sweating; and (iii) differences in the ratio of external work accomplished to the rate of energy expenditure (mechanical efficiency). Our attention in the following subsections will therefore be directed to how such child–adult differences could modulate exercise thermoregulation.
Passive heat exchange
When considering individual differences in thermoregulatory function, our attention is often drawn to the effectiveness of the thermoeffector responses that actively facilitate heat loss. It is important to recognize, however, that the body is also a passive structure that exchanges heat with the surrounding environment according to its mass, composition, and surface area, as well as the thermal and vapor-pressure gradients between the skin and environment. Therefore, when exposed to the same environmental conditions, child–adult differences in physical characteristics will partly determine heat exchange and, therefore, increases in body heat storage and temperature.
In objects with identical shape and composition, heat-storage capacity is a function of volume (mass), whereas heat exchange is surface-area dependent. Therefore, the ratio between body surface area and mass (specific surface area) will partly determine the rate of heat storage and exchange. Although the biophysics of human heat transfer is a far more complex topic, which has been discussed in detail elsewhere (15), a simplified example of this principle can be appreciated in Figure 1. In this experiment, two cubes (cubes A and B) of identical composition, but of differing size (Fig. 1C), were heated to a uniform central temperature (40°C) using a stirred water bath before being plunged into a cooler water bath (20°C) (Fig. 1A). Although cube B had a larger mass and surface area, cube A had the faster cooling rate (0.4°C·min−1 vs 0.9°C·min−1) (Fig. 1B), due to its smaller size, and thus, higher specific surface area.
FIGURE 1.
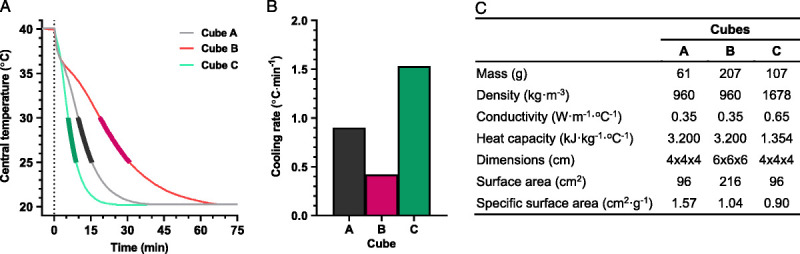
Physical models (three cubes; A, B, and C) to illustrate the influences of variations in morphology and composition on passive heat exchange, adapted with permission from Taylor and Notley (16). Each cube was first heated in a stirred water bath (40°C) to a common central (core) temperature, and then cooled (time zero) in a second water bath (20°C). Panel A shows time-dependent changes in central temperature in each object, whereas panel B shows the cooling rate of each cube over a 5°C temperature range (30°C to 25°C), as bolded in Panel A. Panel C denotes the dimensions and thermal properties of each cube. Cubes A and B (both beeswax) differed only in size, with cube B being larger than cube A. Cubes A and C differed only in composition, with cube C being made of plasticine, and thus, of higher density and thermal conductivity, but of lower specific heat capacity.
These first principles become particularly important for morphologically divergent groups, such as children and adults, who differ markedly in body size. Indeed, relative to the average young man age 20 yr (mass, 71 kg; surface area, 1.87 m2; specific surface area, 264 cm2·kg−1), a boy age 9 yr (mass, 28 kg; surface area, 1.03 m2; specific surface area, 368 cm2·kg−1) (17), would possesses a lower body mass and surface area, but a higher specific surface area for heat exchange (Fig. 2). It follows that when a thermal gradient exists for heat loss (i.e., ambient temperature cooler than the skin), children would be expected to dissipate more heat per unit mass than adults. Conversely, in hotter environments (i.e., warmer than the skin), children would gain more heat per unit mass than adults (Fig. 2).
FIGURE 2.
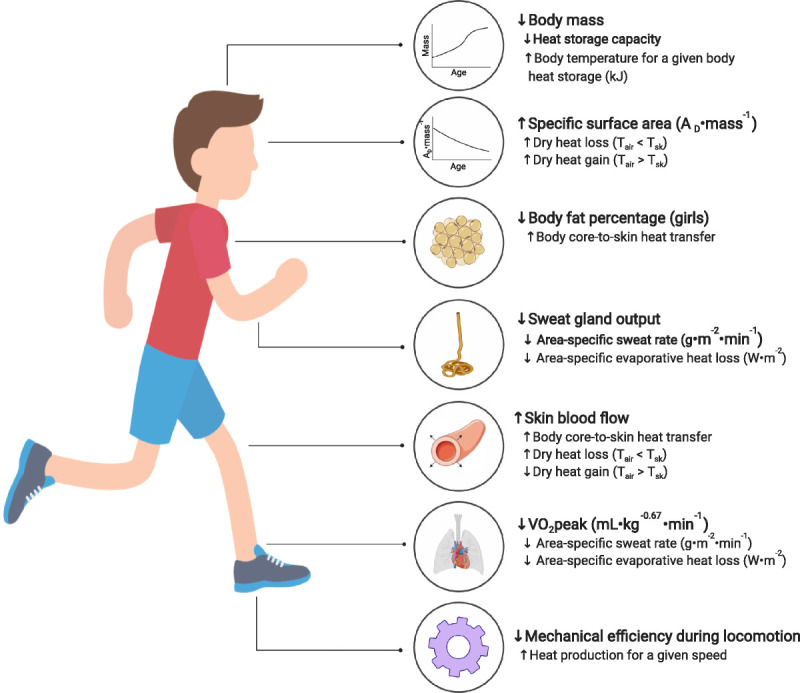
Schematic summary of the key physical and physiological factors that may modulate exercise thermoregulation in children. The up/down arrows indicate a higher/lower response relative to young adults. Please see section: “The theoretical basis for child–adult differences” for more details. AD, body surface area; Tair, air temperature; Tsk, mean skin temperature; V˙O2peak, peak oxygen consumption.
Child–adult differences in the thermal properties (heat capacity, conductivity, and density) of the tissue between deep-body structures and the skin (primarily muscle and adipose) could also alter convective heat transfer to the skin surface. This concept can be illustrated using the same simplified experiment described above (Fig. 1) by comparing two cubes of identical volume (size), but comprised of different materials reflecting the divergent thermal properties of adipose (cube A; beeswax) and muscle tissue (cube C; plasticine) (18). Due to its higher density and thermal conductivity, but lower specific heat capacity, cube C will cool more rapidly than cube A (1.5°C·min−1 vs 0.9°C·min−1; Fig. 1). Relative body adiposity is consistent between boys and young men, however it is generally higher in women relative to girls (19). Although thermal conductivity through body tissues could be independently modified by regional differences in blood flow, this may confer a minor thermoregulatory advantage to girls relative to women by enhancing body core-to-skin heat transfer.
The emphasis of this section has been on passive heat exchange; however, it is important to note that child–adult differences in body mass and to a lesser extent, body composition, can also influence heat-storage capacity. In objects of similar average composition, including humans, heat-storage capacity is a function of mass, with heavier objects requiring more thermal energy to raise their temperature than lighter ones. Therefore, if one assumes a similar average tissue specific heat capacity in children and adults (3.47 kJ·kg−1·°C−1) (14), a 139-kJ increase in body heat content would raise mean body temperature by 1.0°C in a 40-kg child but only 0.5°C in an 80-kg adult. Thus, even if the rate of body heat storage (metabolic heat production − total heat loss; kJ·min−1) during exercise was similar between a child and adult, the rate of body temperature change would be double that observed in an adult in the absence of any compensatory increases in heat loss. Because lean mass (3.66 kJ·kg−1·°C−1) and adipose tissue (2.97 kJ·kg−1·°C−1) differ in specific heat capacity, changes in body composition could also modify heat storage capacity. However, given that even a marked increase in adiposity (~20%) causes a marginal reduction in the average total body specific heat capacity (~0.15 kJ·kg−1·°C−1) (20), it is unlikely that any child–adult differences in body composition would elicit meaningful changes in heat storage capacity.
Active heat exchange
Although heat exchange can occur passively, humans rely heavily on the autonomic thermoeffector responses of cutaneous vasodilatation and sweating to actively facilitate heat exchange during exercise-heat stress. To discuss potential child–adult differences in these thermoeffector responses without the complexities associated with examining them during exercise, our attention here will be directed to mechanistic research evaluating developmental changes in the control of skin blood flow and sweating during passive heat exposure and in response to pharmacological stimulation.
Despite a limited number of studies, a relatively consistent finding has been that children demonstrate greater cutaneous vasodilatation, and thus, skin blood flow relative to adults (21–24). For instance, during lower-limb hot-water immersion, Shibasaki et al. (22) observed higher skin blood flow in children (7–11 yr) relative to young adults (21–25 yr) at the chest and back, but not the forearm or thigh region. Importantly, this occurred despite both groups displaying a similar rise in mean body temperature, and thus, a matched stimulus (i.e., afferent drive to the hypothalamus) for cutaneous vasodilatation (25,26). More recently, Hodges et al. (27) observed greater skin blood flow in boys (~9 yr) compared with young men (~21 yr) at rest (skin temperature, 33°C) and during local skin heating to 39°C, although it is important to recognize that skin temperatures of 39°C would be rare during exercise-heat stress.
Facilitating elevations in skin blood flow during exercise-heat stress, requires an increase in cardiac output, which exceeds that required to support elevated metabolic demand. However, because cardiac output is comparable between children and adults when appropriately scaled to fat free mass (9), children may not necessarily possess the cardiac output required to facilitate these proportionally greater elevations in skin blood flow. Further, during vigorous exercise in the heat, skin blood flow can be reduced to maintain arterial pressure (28). This may compromise heat loss to a greater extent in children, who may rely more heavily on this mechanism for heat loss (8). Nonetheless, these effects remain poorly understood and are potentially minor given that sweat evaporation forms the primary avenue for heat loss during vigorous exercise in the heat.
Attenuated sweat production and the consequent limitation to evaporative heat loss is considered one of the key factors that may augment exercise-induced heat strain in children compared with adults (4,5,8,12,29). Given that the number of sweat glands is consistent throughout the lifespan (30), children naturally possess a higher density of sweat glands per unit area due to their smaller surface area. However, the average size of those glands is approximately 27% smaller in children (5–7 yr) compared with young adults (18 yr) (31), causing a reduction in sweat gland output (the sweat secreted per gland) during passive heating (32). Pharmacological studies incorporating local delivery (via iontophoresis) of pilocarpine to the skin (33) also support reduced sweat gland output in children, and indicate a peripheral modification of sweat gland function. The net effect of these child–adult differences in sweat gland density and output appears to be a generalized reduction in sweat rate over a given area of the body surface with increases in body temperature (mg·cm−1·min−1·°C−1) during passive heat exposure (22). The mechanism(s) explaining such decrements, however, remain to be fully elucidated (12).
In addition to sweat rate, evaporative cooling is dependent upon sweat composition. Greater reabsorption of electrolytes (mainly sodium and chloride) at the sweat gland is associated with more dilute sweat, which potentially evaporates more readily from the skin surface due to its lower latent heat of vaporization. Although this effect is probably relatively small, it may minimize electrolyte loss and promote fluid conservation (by reducing dripped sweat), because a greater evaporation is achieved for a given sweat rate. Although studies of sweat composition in children and adults are sparse, sweat appears to be more dilute in children than adults (34). This led to the suggestion that sweat efficiency, and thus, fluid conservation, may be greater in children relative to adults (6). It is important to note, however, that the reabsorption of electrolytes at the sweat gland is inversely related to sweat gland output, with greater sweat gland output being associated with more concentrated sweat due to a reduction in ductal transit time (i.e., permitting less electrolyte reabsorption) (35). It therefore remains uncertain whether lowered sweat electrolyte concentration in children can be ascribed to enhanced glandular reabsorption or simply to reduced sweat rate.
On balance, studies evaluating skin blood flow and sweating during passive heat exposure have generally revealed that, for a given change in body temperature, children demonstrate higher skin blood flow but lower sweat rate at certain body regions compared with young adults. This may be explained, in large part, by child–adult morphological differences. As noted above, the capacity to store heat is size dependent, whereas heat exchange is surface-area dependent. Therefore, for objects of similar composition, including humans, heat exchange and storage are tightly linked to the ratio of surface area to mass (specific surface area). Because specific surface area increases with decreasing body size, children exhibit a morphological configuration that is better suited to dry heat loss than adults in temperate environments that provide a thermal gradient between the ambient environment and skin surface. Thus, in children, skin blood flow appears to be prioritized over sweating, permitting the conservation of a relatively lower fluid volume per unit area (36), whereas adults are forced to rely more heavily on sweat evaporation. Although this theory has yet to be confirmed with direct measures of whole-body heat exchange, a similar morphological dependency has been observed in young adults spanning a wide range of body sizes (37).
Reduced reliance on sweat secretion for heat loss in children may prove beneficial in temperate environments; however, it has been suggested that this may become a liability in hotter environments that exceed skin temperature and thereby promote dry heat gain (4–6,8,12,13,29). Indeed, even though increased skin blood flow may buffer dry heat gain from the environment, sweat evaporation is the primary means of heat loss in such conditions. Thus, as ambient temperature increases, child–adult differences in heat exchange may become more apparent, especially when coupled with solar loading and/or hot surfaces (e.g., asphalt), which can amplify radiative heat gain. It is also important to consider that high ambient temperatures are also coupled with elevated humidity in many parts of the world. In such conditions, sweat rate often exceeds the maximal rate of evaporation possible in the environment, leading to greater nonevaporated (dripped) sweat relative to dry conditions, especially when wearing insulated and semipermeable protective clothing (e.g., American football uniforms), which creates a hot-humid microclimate around the wearer (38). It is therefore possible that any child–adult differences in sweat production, and thus, evaporative heat loss, may be blunted in more humid environments, because a greater rate of sweating in adults compared with children may not correspond to greater evaporative heat loss.
Mechanical efficiency
Until now, the emphasis has been on heat loss, but because body heat storage represents the difference between metabolic heat production and heat loss, the former is of equal importance when considering factors that may modify heat strain during exercise-heat stress. Human movement is relatively inefficient, with only a fraction of the energy from metabolic processes eventually being utilized to perform external work. The remaining energy is liberated as heat (metabolic heat production) and, therefore, stored within the body, unless paralleled by a matched increase in heat loss (14).
It is well established that children consume more oxygen, and therefore, produce more heat per unit mass during locomotion relative to adults (39–41). Indeed, although the mass-specific oxygen cost of one stride when walking or running at the same speed is similar between children and adults, children must take more strides due to their shorter leg length. As such, running economy (i.e., running speed for a given oxygen consumption) is lower in children during locomotion (39–41). Consequently, when walking or running at a similar speed to an adult, a child produces more heat per unit size and is thereby required to generate relatively greater heat loss to attain heat balance. During weight-supported exercise (e.g., cycling), however, mechanical efficiency is similar between children and adults (42).
CHILD–ADULT COMPARISONS DURING EXERCISE-HEAT STRESS
Given the theoretical basis for child–adult differences in thermoregulatory function, there has been ongoing interest in whether children and adults differ in their ability to regulate body temperature during exercise-heat stress. In this section, we provide a brief overview of existing child–adult comparisons of exercise thermoregulation to facilitate later discussion of the methodological considerations associated with this work. Several investigators have assessed postpubertal children, adolescents, and/or performed retrospective comparisons of data from children and adults, but often in differing exercise and/or environmental conditions (43–46). Our discussion here, however, focuses on direct comparisons of exercise thermoregulation in prepubertal children and young adults (summarized in Table 1).
TABLE 1.
Average rectal temperature change in studies of exercise thermoregulation in prepubertal children and adults.
| Study | Exercise Protocol | Environmenta | Participants | Heat Production | Tre (°C) | |||
|---|---|---|---|---|---|---|---|---|
| n | Age (yr)b | Wc | W·m−2 | Δd | Diff. vs Adultse | |||
| Wagner et al. (47) | Walking (40 min): 5.6 km·h−1, 0% grade | 49°C, 17% R.H. | 5 boys | 11–14 | 220 | 167 | 1.1 | 0.2 |
| 5 men | 25–30 | 359 | 188 | 0.9 | ||||
| 22°C, 27% R.H. | 5 boys | 11–14 | 220 | 167 | 0.6 | 0.0 | ||
| 5 men | 25–30 | 359 | 188 | 0.6 | ||||
| Drinkwater et al. (48) | Walking (50 min): 30% V˙O2peak | 28°C, 45% R.H. | 5 girls | 12 (0) | 279 | 227 | 0.9 | 0.0 |
| 5 women | 21 (2) | 517 | 297 | 0.9 | ||||
| 35°C, 65% R.H. | 5 girls | 12 (0) | 279 | 227 | 1.4 | 0.1 | ||
| 5 women | 21 (2) | 517 | 297 | 1.3 | ||||
| 48°C, 10% R.H. | 5 girls | 12 (0) | 279 | 227 | 1.3 | −0.1 | ||
| 5 women | 21 (2) | 517 | 297 | 1.4 | ||||
| Davies (10) | Running (60 min): 68% V˙O2peak | 21°C, 67% R.H. | 8 boys | 13 (1) | 548 | 435 | 1.1 | −0.4 |
| 5 girls | 14 (1) | 592 | 455 | 1.1 | −0.4 | |||
| 8 adults | 36 (7) | 1016 | 587 | 1.6 | ||||
| Smolander et al. (49) | Cycling (60 min): 30% V˙O2peak | 5°C, 40% R.H. | 8 boys | 12 (1) | 263 | 189 | 0.1 | 0.1 |
| 11 men | 25 (5) | 342 | 174 | 0.0 | ||||
| Meyer et al. (34) | Cycling (40 min): 50% V˙O2peak | 40°C, 18% R.H. | 8 boys | 9 (1) | 231 | 206 | 0.7 | −0.1 |
| 9 boys | 12 (1) | 308 | 220 | 1.1 | 0.3 | |||
| 8 men | 21 (1) | 436 | 263 | 0.8 | ||||
| 10 girls | 9 (1) | 267 | 226 | 0.9 | 0.1 | |||
| 8 girls | 11 (1) | 321 | 255 | 0.7 | −0.1 | |||
| 8 women | 23 (2) | 632 | 345 | 0.8 | ||||
| Shibasaki et al. (50) | Cycling (45 min): 40% V˙O2peak | 30°C, 45% R.H. | 7 boys | 10–11 | 240 | 203 | 0.5 | 0.0 |
| 11 men | 21–25 | 486 | 276 | 0.5 | ||||
| Inbar et al. (51) | Cycling (85 min): 50% V˙O2peak | 41°C, 21% R.H. | 8 boys | 9 (2) | 235 | 224 | 1.0 | −0.3 |
| 8 men | 23 (2) | 560 | 298 | 1.3 | ||||
| Rivera-Brown et al. (52) | Cycling (55–77 min): 60% V˙O2peak | 34°C, 55% R.H. | 9 girls | 9–12 | 329 | 279 | 0.9 | −0.2 |
| 9 women | 20–34 | 518 | 320 | 1.1 | ||||
| Rowland et al. (9) | Cycling (41–43 min): 65% V˙O2peak | 19°C, 58% R.H. | 8 boys | 12 (0) | 461 | 320 | 0.5 | 0.0 |
| 8 men | 32 (2) | 725 | 368 | 0.5 | ||||
| Cycling (29–30 min): 65% V˙O2peak | 31°C, 50% R.H. | 8 boys | 12 (0) | 461 | 320 | 0.6 | 0.0 | |
| 8 men | 32 (2) | 725 | 368 | 0.6 | ||||
| Leites et al. (53) | Cycling (80 min): fixed heat production | 35°C, 35% R.H. | 10 boys | 10–12 | 234 | 175 | 0.6 | −0.1/0.3f |
| 10 men | 19–25 | 397 | 213 | 0.7 | ||||
| 234 | 125 | 0.3 | ||||||
a Environmental conditions indicate air temperature and relative humidity (R.H.). When reported, air flow was low, ranging from <0.2 to 4.0 m·s−1.
b Age is presented as a range or mean (SD).
c Metabolic heat production is presented as both absolute (W) and area-specific values (W·m−2). In instances where heat production was not provided, it was estimated from group mean data assuming a respiratory exchange ratio of 0.87 (14).
d Data represent the change (Δ) in rectal temperature from baseline/resting to end-exercise (imputed when not provided).
e Difference (diff.) compared with adults.
f Difference from adults at the high (397 W) and low heat production (234 W) separated by the dash.
Perhaps, the first direct comparison of exercise thermoregulation in children and adults involved walking at a fixed speed (5.6 km·h−1) in both temperate and hot conditions (47). Children displayed an area-specific rate of evaporative heat loss (estimated from body mass change) that was approximately 40 and 50 W·m−2 lower than adults in the temperate and hot conditions, respectively. Those reductions were paralleled by elevated skin temperature in children in both environments, which caused dry heat loss (estimated from mean skin and ambient temperature) to be approximately 20 W·m−2 higher than adults in the temperate condition and reduced dry heat gain by approximately 30 W·m−2 in the hot condition. Although the net effect of these child–adult differences was a similar change in body core (rectal) temperature in children and adults (Table 1), absolute body core temperature was higher before exercise in children, causing them to reach the criteria for termination (core temperature ≥39.5°C) approximately 20 min earlier than the adults in the hot condition. Although it has been suggested that the cutoff for dangerous heat strain in children may differ from that of adults (8), this finding was considered, by the authors, to be consistent with the notion of inferior heat tolerance in children (47). After this report and the theoretical arguments put forth in Bar-Or’s landmark review (4), the American Academy of Pediatrics released a position statement in 1982 (54), which was reaffirmed in 2000 (55), supporting the view of inferior thermoregulation in children.
Although this early work showed evidence of child–adult differences in thermoregulatory function (47), it was later criticized, with some authors suggesting that such exercise conditions deviate from reality (8,13). That is, a child would rarely exercise at the same absolute external work rate to that of an adult. For this reason, most investigations in later years involved exercise at a relative percentage of mass-specific peak oxygen consumption (V˙O2peak; mL·min−1·kg−1) (9,10,34,48–52). Further, to minimize any confounding effects of child–adult differences in aerobic fitness on thermoregulatory function, the participants were selected (matched) to possess a similar mass-specific V˙O2peak.
Although several of those investigators observed higher skin temperatures in children relative to adults, which was thought to facilitate enhanced vasomotor-mediated dry heat exchange (10,48,52), the area-specific whole-body sweat rate (g·m−2·h−1) was typically reduced in children (10,34,50,51). For instance, when assessed during intermittent cycling at 50% V˙O2peak in dry heat, Inbar et al. observed an approximately 7% lower area-specific whole-body sweat rate in children relative to adults (51). In such conditions, one would expect these reductions in sweat rate to elicit reductions in evaporative heat loss that exacerbate body heat storage and consequently augment the change in body core temperature relative to adults. Interestingly, however, most of those comparisons failed to show that such child–adult differences translate into meaningful between-group differences in body core temperature, irrespective of the ambient conditions or exercise intensity (Table 1). Based on that series of work, several authors suggested that children do not necessarily possess a thermoregulatory apparatus that is inadequate or inferior to that of adults (5,7,8,13), which was followed by the revision of the American Academy of Pediatrics policy in 2011, stating that exercise-heat stress poses no greater physiological burden to children than adults (56). This policy was reaffirmed in 2015 (57), and remains the consensus relating to thermoregulation in children.
To our knowledge, only one other direct child–adult comparison of exercise thermoregulation exists (53). In that research, children and adults with similar mass-specific V˙O2peak were assessed during intermittent exercise in hot, dry conditions. However, rather than exercising at a percentage of V˙O2peak, children completed one trial consisting of exercise eliciting a fixed, mass-specific metabolic heat production (5.7 W·kg−1), whereas adults completed two trials. The first consisted of exercise eliciting the same mass-specific metabolic heat production (5.7 W·kg−1), whereas the second involved exercise eliciting the same absolute metabolic heat production as the children (234 W). Unsurprisingly, due to their higher heat-storage capacity, adults displayed a body core temperature that was lower than children when exercising at the same absolute metabolic heat production (0.3°C vs 0.6°C). At the same mass-specific metabolic heat production (5.7 W·kg−1), however, cumulative percentage body mass loss (a surrogate of whole-body sweat loss) and the resulting change in body core temperature did not differ significantly between-groups. These outcomes therefore lend further support to the existing consensus that exercise-heat stress poses no greater burden to children than adults.
METHODOLOGICAL CONSIDERATIONS
From a theoretical standpoint, there exist various child–adult differences that could exacerbate heat strain in children during exercise-heat stress, especially in hot environments. However, as noted above, this is not necessarily supported by the relatively few direct child–adult comparisons of body core temperature during exercise-heat stress (Table 1). In our view, this discrepancy between theory and observation may be ascribed to two key methodological issues, which make the existing literature difficult to interpret, and may have even masked potential child–adult differences in heat strain. These limitations and their potential solutions are discussed in the following subsections.
Normalizing (scaling) physiological responses to body size
Performing unbiased comparisons of size-dependent physiological data in morphologically diverse populations, such as children and adults, relies on normalizing (scaling) these data to body size. Traditionally, this has been achieved with ratiometric scaling, in which the physiological variable of interest is normalized to body size by dividing it by an appropriate anthropometric attribute (e.g., body mass). This practice has been used throughout the existing literature to normalize both independent (e.g., work intensity as a percentage of mass-specific V˙O2peak; mL·min−1·kg−1) and dependent variables (e.g., sweat rate per unit area; g·m−2·h−1), as well as for group matching criteria such as mass-specific V˙O2peak (mL·min−1·kg−1). However, ratiometric scaling will only completely account for the effect of body size when the y-intercept for the least-squares, linear regression relationship between body size and the physiological variable is zero (58–62); a scenario that rarely occurs in either comparative or human physiology (63). A hypothetical example of scenarios wherein ratiometric scaling will and will not facilitate size-independent comparisons is depicted in Figure 3.
FIGURE 3.
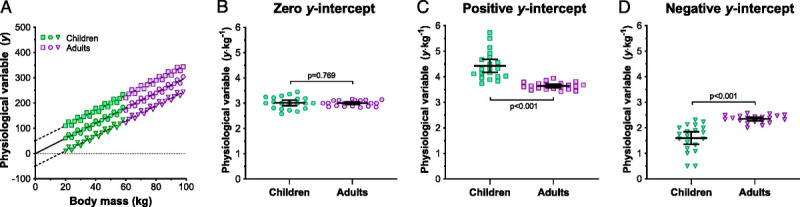
Hypothetical data to illustrate the importance of satisfying the zero y-intercept assumption when using ratiometric scaling to normalize a physiological variable (y) to body size by dividing it by an anthropometric attribute (x). Panel A shows three data sets each comprised of a physiological variable (y) that increases linearly with body size (x; body mass [kg]) in 20 children and 20 adults. The circular symbols illustrate a scenario where the y-intercept for the least-squares linear regression relationship (solid line) passes through zero and satisfies this assumption. The square and triangular symbols illustrate scenarios where that assumption is violated, with the y-intercept for this regression relationship (dashed lines) being either above or below zero, respectively. Panels B, C, and D, illustrate between-group comparisons (unpaired, two-tailed t-tests) of the ratio scaled values (y·x−1) for each data set. Ratiometric scaling removed the effect of size for data which satisfy the zero y-intercept assumption (circular symbols), as indicated by a nonsignificant difference between children and adults (Panel B). In contrast, ratiometric scaling underadjusts values for children and over-adjusts values in adults when data display a positive y-intercept, and the opposite when data display a negative y-intercept. These errors result in a significant, yet artificial, between-groups difference in the ratio scaled data, but in the opposite directions (Panel C and D).
Although the hazards of ratiometric scaling have been documented for some time (59), researchers of exercise thermoregulation in children and adults appear to be unaware of, or do not heed these warnings. Perhaps the most prevalent example of this fallacy is the normalization of oxygen consumption to body mass (mL·min−1·kg−1). Indeed, rather than a ratiometric (linear) relationship, submaximal and peak oxygen consumption share an allometric (nonlinear) relationship with body mass in both children and adults, increasing at a proportionally slower rate than does body mass (58,64–68). Therefore, expressing oxygen consumption per unit mass will overadjust values in adults, while underadjusting in children. This has two consequences. First, most of the existing literature has involved exercise eliciting a given percentage of mass-specific peak aerobic oxygen consumption (Table 1). One might, therefore, question the utility of such comparisons, and this will be discussed further in the next subsection. Second, to minimize any secondary influence of aerobic fitness, researchers have often attempted to match children and adults for mass-specific V˙O2peak (9,10,34,48–52). Doing so inadvertently ensured that aerobic fitness, as indexed by V˙O2peak, was systematically lower in adults. Given that aerobic fitness can modulate whole-body heat exchange during exercise-heat stress (69), this practice may have confounded the observed outcomes. It is our view, that the inappropriate use of ratiometric scaling has introduced unintentional bias into previous child–adult comparisons of exercise thermoregulation, which render that literature difficult to interpret.
In instances where ratiometric scaling is inappropriate, alternative statistical procedures must be used to fully account for differences in body size. Although these procedures are discussed in detail elsewhere (59–61,70), three potential alternatives to derive size-independent data include adjusted-regression analysis, ANCOVA, and allometric scaling. The most appropriate approach, however, will depend on whether the variables to be normalized satisfy the statistical assumptions associated with each procedure. A schematic representation of this selection process and a brief description of the procedures associated with each method are provided in Supplemental Digital Content 1 (Supplemental Digital Content 1, Procedures for normalizing physiological data to body size, http://links.lww.com/MSS/B993), whereas a hypothetical example of utilizing ANCOVA as an alternative to ratiometric scaling to remove size-bias is presented in Figure 4. In this example, we provide a simulated data set comprised of a physiological variable (y) that increases with body size (x; body mass [kg]) in children and adults (Fig. 4A), as well as between-group comparisons (unpaired, two-tailed t-tests) of these data in their raw form (Fig. 4B), normalized to body mass using ratiometric scaling (Fig. 4C), and normalized to body mass using ANCOVA (Fig. 4D). Given that the physiological variable of interest is dependent on body size (mass), it is inappropriate to rely on a between-group comparison on the raw, unadjusted data (Fig. 4B), which reveals a greater response in adults (P < 0.001). In such instances, one would traditionally use ratiometric scaling to normalize these data to body size (Fig. 4C) and conclude that the physiological response does not differ appreciably between groups (P = 0.394). However, because both linear regression lines display a nonzero y-intercept (children, ~50; adults, ~120; Fig. 4A), ratiometric scaling cannot fully account for the effects of body size on the physiological variable, and it becomes essential to use alternative methods to normalize these data to body size (e.g., ANCOVA). When these data are normalized appropriately with ANCOVA, we see a significantly higher response in adults compared with children (P < 0.001), which is masked if one relies upon ratiometric scaling.
FIGURE 4.
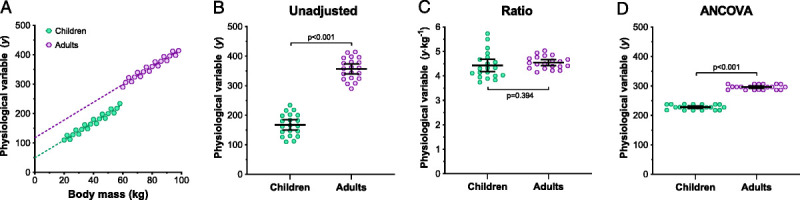
Hypothetical data to illustrate how ANCOVA (see Supplemental Digital Content 1 for procedures, http://links.lww.com/MSS/B993) can be used to account for the confounding effect of body size when data do not satisfy the zero y-intercept assumption required for ratiometric scaling. Panel A illustrates a data set comprised of a physiological variable (y) that increases linearly with body size (x; body mass [kg]) in 20 children and 20 adults, whereas the dashed lines demonstrate the least-squares linear regression relationship for each group (similar slopes). Panels B, C, and D illustrate between-group comparisons (unpaired, two-tailed t-tests) of the unadjusted, ratiometric scaled, and ANCOVA-adjusted data, respectively. Because these data display a nonzero y-intercept (panel A), ratiometric scaling cannot fully account for the effect of body size (panel C), masking the between-group difference apparent when data were normalized appropriately with ANCOVA (panel D).
Considerations for the selection of exercise intensity
As discussed above, a balance between metabolic heat production and total heat loss (dry + evaporative heat exchange) is required for body temperature to be stable. To identify between-group differences in heat exchange during exercise-heat stress, it is therefore fundamental to ensure that metabolic heat production, and the subsequent total heat loss required to balance that rise in heat production, is comparable between groups. Because heat exchange occurs between the body surface and surrounding environment, contemporary exercise studies evaluating heat exchange between groups that differ in body size often standardize the area-specific metabolic heat production rate (W·m−2) (37,71–74). Further, given that metabolic demand is known to share a nearly proportional relationship with surface area (i.e., zero y-intercept) during submaximal exercise (65,67), this approach satisfies the statistical requirements for ratiometric normalization to create a full-size independent work intensity. In addition, by standardizing metabolic heat production (metabolic heat production – external work), one can account for any potential between-group differences in mechanical efficiency (39–41). With this approach, any child–adult differences in total heat loss and the resulting changes in body heat storage would represent a true size-independent, between-group difference in heat exchange.
Notwithstanding these methodological considerations, seminal research on exercise thermoregulation in children and adults has involved treadmill walking at a fixed absolute speed (47). Because metabolic demand is proportional to body mass during weight-bearing exercise (65,67), adults would be required to dissipate more heat per unit surface area to meet the required rate of total heat loss to balance this rise in metabolic heat production (Table 1). Although non–weight-bearing exercise (e.g., cycling) at a fixed external work rate can be used to reduce this bias, the area-specific metabolic heat production now becomes larger in children, who must dissipate more heat per unit surface area to maintain heat balance. As such, both weight bearing and non–weight-bearing exercise performed at absolute speeds or external work rates are, in our view, unsuitable for performing meaningful child–adult comparisons of exercise thermoregulation.
More recently, researchers have assessed thermoregulation in children and adults with matched mass-specific V˙O2peak during exercise at a relative percentage of that V˙O2peak (Table 1). Although this approach provides a more realistic representation of the work intensity that a child and adult may perform during exercise, we are of the opinion that the outcomes presented are also difficult to interpret for two reasons. First, as noted above, ratiometric scaling of V˙O2peak to body mass will not create size-independent data (58–62). Therefore, exercise performed at a similar percentage of mass-specific V˙O2peak between children and adults does not necessarily indicate that both groups are exercising at the same relative intensity. In fact, expressing peak oxygen consumption per unit mass will overadjust these values in adults, while underadjusting these data in children (64,75). Further, exercise at a given percentage of V˙O2peak will elicit an area-specific metabolic heat production and subsequent requirement for total heat loss to attain heat balance that differs markedly between children and adults (Table 1).
Because the heat storage capacity of any structure is determined by its mass and thermal properties, exercise performed at a mass-specific metabolic heat production rate (W·kg−1) has recently been used to evaluate thermoregulatory function in children and adults (53). However, as noted above, mass-specific normalization (ratiometric scaling) will only fully account for differences in body size when the least-squares, linear regression relationship between that variable and body mass displays a zero y-intercept. Unfortunately, the regression relationship between metabolic demand and body mass displays a nonzero y-intercept, and more closely follows an allometric (nonlinear) association (65,67). Further, due to the larger specific surface area in children compared with adults, exercise eliciting a similar mass-specific heat production will result in a lower area-specific heat production, and thus, requirement for heat loss in children. As such, comparisons of thermoregulatory function during work performed at a fixed mass-specific heat production also become challenging to interpret.
Another potential shortcoming in the existing literature pertains to the magnitude of the exercise intensity used. As discussed above, several authors have demonstrated that relative to adults, children demonstrate higher skin blood flow (21–23,27), but lower local sweat rates (mg·cm−2·min−1) for a given rise in body temperature during passive heating (4,5,8,12,29). Given those findings and the fact that the contribution of skin blood flow to heat loss decreases with increasing exercise intensity (28), these differences might translate into detrimental reductions in evaporative heat loss during more vigorous exercise in hot environments. This is consistent with recent studies on the effects of other individual factors on whole-body heat exchange (71–74), which have used an exercise model involving short-duration exercise (~30 min) at increasing fixed metabolic heat productions (akin to an incremental exercise test) to demonstrate that such factors modulate heat loss only above specific requirements for heat loss.
Despite this, most child–adult comparisons during exercise-heat stress have involved a single exercise intensity that does not progressively increase the requirement for heat loss (Table 1). This represents an additional methodological consideration that may have masked potential child–adult differences in heat exchange and their impact on body heat storage, and thus, body temperature. To address this issue, an incremental model involving short bouts of exercise at increasing, area-specific metabolic heat productions like that described above can be used. However, although this model may permit the detection of child–adult differences in heat exchange, it does not allow one to accurately determine the extent to which those differences may exacerbate heat strain. For this purpose, separating such an incremental exercise model into independent bouts of exercise (i.e., performed on separate days), ideally of longer duration to reflect the protracted duration of many sports and/or structured activities, may be more appropriate for assessing exercise thermoregulation in children and adults. Such a model would also satisfy the steady-state requirements essential for indirectly deriving estimates of dry and evaporative heat exchange using partitional calorimetry, and when coupled with estimates of metabolic heat production using indirect calorimetry, a means to approximate body heat storage (14).
RESEARCH NEEDS
In light of the methodological issues presented, it is difficult to conclude whether exercise thermoregulation differs between children and adults. To move toward more valuable child–adult comparisons of thermoregulatory function during exercise-heat stress, we therefore propose the following. First, because heat exchange occurs between the body surface and surrounding environment, exercise must be performed at a fixed, area-specific metabolic heat production (W·m−2) to ensure that both children and adults are exposed to the same relative heat loss requirement. This approach satisfies the statistical requirements for ratiometric normalization to create a full-size independent work intensity, while also accounting for any potential between-group differences in mechanical efficiency (39–41). Second, given that child–adult differences in thermoregulatory function may depend on the magnitude of the exercise-induced heat load, it is necessary to examine these effects at increasing, area-specific metabolic heat productions, ideally reflecting the protracted duration of many sports and/or structured activities. Finally, to perform unbiased comparisons of size-dependent physiological data in children and adults, it is paramount to normalize these data to body size. For data satisfying the statistical assumptions of linear regression, one can rely on ratiometric scaling to remove body-size effects. However, this method is valid only when the linear regression relationship between the independent and dependent variable displays a zero y-intercept. If this is not the case, one must use alternative statistical methods, including adjusted-regression analysis, ANCOVA, and allometric scaling, with the most appropriate approach being dependent upon whether the variables to be normalized satisfy the assumptions associated with each procedure (see Appendix, Supplemental Digital Content 1, Procedures for normalizing physiological data to body size, http://links.lww.com/MSS/B993).
In addition to further studies specifically directed at addressing these issues, there exists an additional need to understand the interactive effects of various other interindividual (e.g., sex, disease) and intraindividual factors (e.g., aerobic fitness, hydration state) that can simultaneously modulate exercise thermoregulation in children and adults. For instance, previous child–adult comparisons of thermoregulatory function have mostly involved males (Table 1). Compared with young men, and after controlling for sex differences in secondary factors that influence heat exchange (e.g., body size, aerobic fitness, metabolic heat production), women display impaired evaporative heat loss relative to men (71). If such sex differences occur in prepubertal children, one might expect any child–adult reductions in sweating and the subsequent increases in heat strain to be more pronounced in girls. Relatedly, dehydration (≥3% reduction in body mass) can exacerbate exercise-induced hyperthermia in adults due primarily to reduced sweat secretion (76). Given their smaller fluid volume per unit surface area, one might therefore expect a given percentage of body mass loss during dehydration to cause a larger reduction in sweat secretion in children compared with adults in order to maintain arterial pressure. In the absence of any compensatory adjustments to fluid regulation, this may cause greater dehydration-induced elevations in body temperature during exercise-heat stress. However, although several investigators have examined the effects of hypohydration on thermoregulatory function in children during exercise-heat stress (77–80), no study to our knowledge has directly compared these effects between children and adults.
CONCLUSIONS
In this methodological review, we have examined the approaches used to evaluate exercise thermoregulation in prepubertal children and young adults. In our view, that research has not appropriately normalized data to body size or considered child–adult body size differences in the exercise protocols used. There also exists a paucity of evidence on the potential modifying effects of the various interindividual and intraindividual factors that can independently modulate thermoregulatory function. These limitations preclude our ability to determine whether children demonstrate meaningful reductions in thermoregulatory function that could exacerbate exercise-induced heat strain relative to adults. Subsequently, our understanding of exercise thermoregulation in children remains in its infancy, despite a long history of research interest. Given the threat to health posed by rising global temperatures and the increasing demand for children to be engaged in exercise, we hope future work in this area will consider these issues to allow better inferences to be made.
Supplementary Material
Acknowledgments
No conflict of interest, financial or otherwise are declared by the author(s). The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
This work was supported by Health Canada (all funds held by Glen P. Kenny). S. R. Notley, A. P. Akerman, and G. W. McGarr are supported by Postdoctoral Fellowships from the Human and Environmental Physiology Research Unit. R. D. Meade is supported by an Ontario Graduate Scholarship.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Yeargin SW Kerr ZY Casa DJ, et al. Epidemiology of exertional heat illnesses in youth, high school, and college football. Med Sci Sports Exerc. 2016;48(8):1523–9. [DOI] [PubMed] [Google Scholar]
- 2.Decher NR Casa DJ Yeargin SW, et al. Hydration status, knowledge, and behavior in youths at summer sports camps. Int J Sports Physiol Perform. 2008;3(3):262–78. [DOI] [PubMed] [Google Scholar]
- 3.Knoppert D Reed M Benavides S, et al. Paediatric age categories to be used in differentiating between listing on a model essential medicines list for children. World Health Organization position paper. 2007;1(5). [Google Scholar]
- 4.Bar-Or O. Climate and the exercising child-a review. Int J Sports Med. 1980;1(2):53–65. [Google Scholar]
- 5.Falk B. Effects of thermal stress during rest and exercise in the paediatric population. Sports Med. 1998;25(4):221–40. [DOI] [PubMed] [Google Scholar]
- 6.Smith CJ. Pediatric Thermoregulation: Considerations in the Face of Global Climate Change. Nutrients. 2019;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong LE, Maresh CM. Exercise-heat tolerance of children and adolescents. Pediatr Exerc Sci. 1995;7(3):239–52. [Google Scholar]
- 8.Falk B, Dotan R. Children's thermoregulation during exercise in the heat: a revisit. Appl Physiol Nutr Metab. 2008;33(2):420–7. [DOI] [PubMed] [Google Scholar]
- 9.Rowland T, Hagenbuch S, Pober D, Garrison A. Exercise tolerance and thermoregulatory responses during cycling in boys and men. Med Sci Sports Exerc. 2008;40(2):282–7. [DOI] [PubMed] [Google Scholar]
- 10.Davies CT. Thermal responses to exercise in children. Ergonomics. 1981;24(1):55–61. [DOI] [PubMed] [Google Scholar]
- 11.Gomes LH, Carneiro-Junior MA, Marins JC. Thermoregulatory responses of children exercising in a hot environment. Rev Paul Pediatr. 2013;31(1):104–10. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, Kuwahara T, Araki T. Maturation- and aging-related changes in heat loss effector function. J Physiol Anthropol Appl Human Sci. 2004;23(6):289–94. [DOI] [PubMed] [Google Scholar]
- 13.Rowland T. Thermoregulation during exercise in the heat in children: old concepts revisited. J Appl Physiol (1985). 2008;105(2):718–24. [DOI] [PubMed] [Google Scholar]
- 14.Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol. 2013;3(4):1689–719. [DOI] [PubMed] [Google Scholar]
- 15.Gagge AP, Nishi Y. Heat exchange between human skin surface and thermal environment. Compr Physiol. 2010;69–92. [Google Scholar]
- 16.Taylor NA, Notley SR. Morphological and Physiological Considerations for the Modelling of Human Heat Loss. In: Theory and Applications of Heat Transfer in Humans. 2018. pp. 463–99.
- 17.Kuczmarski R Ogden C Grummer-Strawn L, et al. CDC growth charts: United States. 2000;314:1–27. [PubMed] [Google Scholar]
- 18.Hasgall P Di Gennaro F Baumgartner C, et al. IT’IS Database for thermal and electromagnetic parameters of biological tissues. Version 4.0, May 15, 2018, DOI: 10.13099/VIP21000-04-0. itis.swiss/database [Internet]. Available from: www.itis.ethz.ch/database.
- 19.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond). 2006;30(4):598–602. [DOI] [PubMed] [Google Scholar]
- 20.Dervis S, Coombs GB, Chaseling GK, Filingeri D, Smoljanic J, Jay O. A comparison of thermoregulatory responses to exercise between mass-matched groups with large differences in body fat. J Appl Physiol. 2016;120(6):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue Y Ichinose-Kuwahara T Nakamura S, et al. Cutaneous vasodilation response to a linear increase in air temperature from 28 degrees C to 40 degrees C in prepubertal boys and young men. J Physiol Anthropol. 2009;28(3):137–44. [DOI] [PubMed] [Google Scholar]
- 22.Shibasaki M, Inoue Y, Kondo N, Aoki K, Hirata K. Relationship between skin blood flow and sweating rate in prepubertal boys and young men. Acta Physiol Scand. 1999;167(2):105–10. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JA, Robinson S, Marino RP. Age and temperature regulation of humans in neutral and cold environments. J Appl Physiol. 1974;37(4):562–5. [DOI] [PubMed] [Google Scholar]
- 24.Woloschuk A, Hodges GJ, Massarotto RJ, Dotan R, Klentrou P, Falk B. The skin blood flow response to exercise in boys and men and the role of nitric oxide. Eur J Appl Physiol. 2020;120(4):753–62. [DOI] [PubMed] [Google Scholar]
- 25.Simon E, Pierau FK, Taylor DC. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66(2):235–300. [DOI] [PubMed] [Google Scholar]
- 26.Jessen C. Interaction of body temperatures in control of thermoregulatory effector mechanisms. Compr Physiol. 2010;127–38. [Google Scholar]
- 27.Hodges GJ, Mueller MC, Cheung SS, Falk B. Cutaneous vasomotor responses in boys and men. Appl Physiol Nutr Metab. 2018;43(10):1019–26. [DOI] [PubMed] [Google Scholar]
- 28.Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(5):790–4. [DOI] [PubMed] [Google Scholar]
- 29.Bar-Or O. Effects of age and gender on sweating pattern during exercise. Int J Sports Med. 1998;19(2 Suppl):S106–7. [DOI] [PubMed] [Google Scholar]
- 30.Kawahata A. Studies on the function of human sweat organs. J Mie Med College. 1950;1:25–41. [Google Scholar]
- 31.Landing BH, Wells TR, Williamson ML. Anatomy of eccrine sweat glands in children with chronic renal insufficiency and other fatal chronic diseases. Am J Clin Pathol. 1970;54(1):15–21. [DOI] [PubMed] [Google Scholar]
- 32.Shibasaki M, Inoue Y, Kondo N. Mechanisms of underdeveloped sweating responses in prepubertal boys. Eur J Appl Physiol Occup Physiol. 1997;76(4):340–5. [DOI] [PubMed] [Google Scholar]
- 33.Huebner DE, Lobeck CC, Mcsherry NR. Density and secretory activity of eccrine sweat glands in patients with cystic fibrosis and in healthy controls. Pediatrics. 1966;38(4p1):613. [PubMed] [Google Scholar]
- 34.Meyer F, Bar-Or O, MacDougall D, Heigenhauser GJ. Sweat electrolyte loss during exercise in the heat: effects of gender and maturation. Med Sci Sports Exerc. 1992;24(7):776–81. [PubMed] [Google Scholar]
- 35.Buono MJ, Claros R, Deboer T, Wong J. Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate. J Appl Physiol (1985). 2008;105(4):1044–8. [DOI] [PubMed] [Google Scholar]
- 36.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. [DOI] [PubMed] [Google Scholar]
- 37.Notley SR, Park J, Tagami K, Ohnishi N, Taylor NA. Morphological dependency of cutaneous blood flow and sweating during compensable heat stress when heat-loss requirements are matched across participants. J Appl Physiol (1985). 2016;121(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong LE Johnson EC Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unnithan VB, Eston RG. Stride frequency and submaximal treadmill running economy in adults and children. Pediatr Exerc Sci. 1990;2(2):149–55. [Google Scholar]
- 40.Rowland TW, Green GM. Physiological responses to treadmill exercise in females: adult-child differences. Med Sci Sports Exerc. 1988;20(5):474–8. [PubMed] [Google Scholar]
- 41.Cureton KJ, Sloniger MA, Black DM, McCormack WP, Rowe DA. Metabolic determinants of the age-related improvement in one-mile run/walk performance in youth. Med Sci Sports Exerc. 1997;29(2):259–67. [DOI] [PubMed] [Google Scholar]
- 42.Rowland TW, Staab JS, Unnithan VB, Rambusch JM, Siconolfi SF. Mechanical efficiency during cycling in prepubertal and adult males. Int J Sports Med. 1990;11(6):452–5. [DOI] [PubMed] [Google Scholar]
- 43.Macek M, Vavra J, Novosadova J. Prolonged exercise in prepubertal boys. I. Cardiovascular and metabolic adjustment. Eur J Appl Physiol Occup Physiol. 1976;35(4):291–8. [DOI] [PubMed] [Google Scholar]
- 44.Gullestad R. Temperature regulation in children during exercise. Acta Paediatr Scand. 1975;64(2):257–63. [DOI] [PubMed] [Google Scholar]
- 45.Falk B, Bar-Or O, MacDougall JD, Goldsmith CH, McGillis L. Longitudinal analysis of the sweating response of pre-, mid-, and late-pubertal boys during exercise in the heat. Am J Hum Biol. 1992;4(4):527–35. [DOI] [PubMed] [Google Scholar]
- 46.Falk B, Bar-Or O, Calvert R, MacDougall JD. Sweat gland response to exercise in the heat among pre-, mid-, and late-pubertal boys. Med Sci Sports Exerc. 1992;24(3):313–9. [PubMed] [Google Scholar]
- 47.Wagner JA, Robinson S, Tzankoff SP, Marino RP. Heat tolerance and acclimatization to work in the heat in relation to age. J Appl Physiol. 1972;33(5):616–22. [DOI] [PubMed] [Google Scholar]
- 48.Drinkwater BL, Kupprat IC, Denton JE, Crist JL, Horvath SM. Response of prepubertal girls and college women to work in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(6):1046–53. [DOI] [PubMed] [Google Scholar]
- 49.Smolander J, Bar-Or O, Korhonen O, Ilmarinen J. Thermoregulation during rest and exercise in the cold in pre- and early pubescent boys and in young men. J Appl Physiol (1985). 1992;72(4):1589–94. [DOI] [PubMed] [Google Scholar]
- 50.Shibasaki M, Inoue Y, Kondo N, Iwata A. Thermoregulatory responses of prepubertal boys and young men during moderate exercise. Eur J Appl Physiol Occup Physiol. 1997;75(3):212–8. [DOI] [PubMed] [Google Scholar]
- 51.Inbar O, Morris N, Epstein Y, Gass G. Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males. Exp Physiol. 2004;89(6):691–700. [DOI] [PubMed] [Google Scholar]
- 52.Rivera-Brown AM, Rowland TW, Ramirez-Marrero FA, Santacana G, Vann A. Exercise tolerance in a hot and humid climate in heat-acclimatized girls and women. Int J Sports Med. 2006;27(12):943–50. [DOI] [PubMed] [Google Scholar]
- 53.Leites GT, Cunha GS, Obeid J, Wilk B, Meyer F, Timmons BW. Thermoregulation in boys and men exercising at the same heat production per unit body mass. Eur J Appl Physiol. 2016;116(7):1411–9. [DOI] [PubMed] [Google Scholar]
- 54.Shaffer TE Flynn TG Coryllos E, et al. Climatic heat stress and the exercising child. Pediatrics. 1982;69(6):808–9. [PubMed] [Google Scholar]
- 55.Committee on Sports Medicine Fitness Climatic heat stress and the exercising child and adolescent. Pediatrics. 2000;106(1):158–9. [PubMed] [Google Scholar]
- 56.Bergeron MF, Devore C, Rice SG. Policy statement-climatic heat stress and exercising children and adolescents. Pediatrics. 2011;128(3):e741–7. [DOI] [PubMed] [Google Scholar]
- 57.American Academy of Pediatrics AAP publications reaffirmed or retired. Pediatrics. 2015;135(2):e558-e. [Google Scholar]
- 58.Toth MJ, Goran MI, Ades PA, Howard DB, Poehlman ET. Examination of data normalization procedures for expressing peak VO2 data. J Appl Physiol (1985). 1993;75(5):2288–92. [DOI] [PubMed] [Google Scholar]
- 59.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol. 1949;2(1):1–15. [DOI] [PubMed] [Google Scholar]
- 60.Packard GC, Boardman TJ. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol Part A Mol Integr Physiol. 1999;122(1):37–44. [Google Scholar]
- 61.Nevill AM, Ramsbottom R, Williams C. Scaling physiological measurements for individuals of different body size. Eur J Appl Physiol Occup Physiol. 1992;65(2):110–7. [DOI] [PubMed] [Google Scholar]
- 62.Katch VL. Use of the oxygen/body weight ratio in correlational analyses: spurious correlations and statistical considerations. Med Sci Sports Exerc. 1973;5(4):253–7. [PubMed] [Google Scholar]
- 63.Schmidt-Nielsen K, Knut S-N. Scaling: why is animal size so important? Cambridge university press; 1984. p.14-28. [Google Scholar]
- 64.Welsman J, Armstrong N. Interpreting aerobic fitness in youth: the fallacy of ratio scaling. Pediatr Exerc Sci. 2019;31(2):184–90. [DOI] [PubMed] [Google Scholar]
- 65.Rogers DM, Olson BL, Wilmore JH. Scaling for the VO2-to-body size relationship among children and adults. J Appl Physiol. 1995;79(3):958–67. [DOI] [PubMed] [Google Scholar]
- 66.Loftin M, Sothern M, Abe T, Bonis M. Expression of VO2peak in children and youth, with special reference to allometric scaling. Sports Med. 2016;46(10):1451–60. [DOI] [PubMed] [Google Scholar]
- 67.Bergh U, Sjödin B, Forsberg A, Svedenhag J. The relationship between body mass and oxygen uptake during running in humans. Med Sci Sports Exerc. 1991;23(2):205–11. [PubMed] [Google Scholar]
- 68.Lolli L, Batterham AM, Weston KL, Atkinson G. Size exponents for scaling maximal oxygen uptake in over 6500 humans: a systematic review and meta-analysis. Sports Med. 2017;47(7):1405–19. [DOI] [PubMed] [Google Scholar]
- 69.Lamarche DT, Notley SR, Louie JC, Poirier MP, Kenny GP. Fitness-related differences in the rate of whole-body evaporative heat loss in exercising men are heat-load dependent. Exp Physiol. 2018;103(1):101–10. [DOI] [PubMed] [Google Scholar]
- 70.Winter EM. Scaling: partitioning out differences in size. Pediatr Exerc Sci. 1992;4(4):296–301. [Google Scholar]
- 71.Gagnon D, Kenny GP. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol (1985). 2012;113(5):746–57. [DOI] [PubMed] [Google Scholar]
- 72.Meade RD Notley SR D'Souza AW, et al. Interactive effects of age and hydration state on human thermoregulatory function during exercise in hot-dry conditions. Acta Physiol (Oxf). 2019;226(1):e13226. [DOI] [PubMed] [Google Scholar]
- 73.Notley SR Poirier MP Sigal RJ, et al. Exercise heat stress in patients with and without type 2 diabetes. JAMA. 2019;322(14):1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Notley SR, Poirier MP, Yardley JE, Sigal RJ, Kenny GP. Impaired whole-body heat loss in type 1 diabetes during exercise in the heat: a cause for concern? Diabetologia. 2019;62(6):1087–9. [DOI] [PubMed] [Google Scholar]
- 75.Welsman JR, Armstrong N, Nevill AM, Winter EM, Kirby BJ. Scaling peak VO2 for differences in body size. Med Sci Sports Exerc. 1996;28(2):259–65. [DOI] [PubMed] [Google Scholar]
- 76.Sawka MN, Cheuvront SN, Kenefick RW. Hypohydration and human performance: impact of environment and physiological mechanisms. Sports Med. 2015;45(1 Suppl):S51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bar-Or O, Dotan R, Inbar O, Rotshtein A, Zonder H. Voluntary hypohydration in 10- to 12-year-old boys. J Appl Physiol Respir Environ Exerc Physiol. 1980;48(1):104–8. [DOI] [PubMed] [Google Scholar]
- 78.Meyer F, Volterman KA, Timmons BW, Wilk B. Fluid balance and dehydration in the young athlete: assessment considerations and effects on health and performance. Am J Lifestyle Med. 2012;6(6):489–501. [Google Scholar]
- 79.Wilk B, Meyer F, Bar-Or O, Timmons BW. Mild to moderate hypohydration reduces boys' high-intensity cycling performance in the heat. Eur J Appl Physiol. 2014;114(4):707–13. [DOI] [PubMed] [Google Scholar]
- 80.Wilk B, Timmons BW, Bar-Or O. Voluntary fluid intake, hydration status, and aerobic performance of adolescent athletes in the heat. Appl Physiol Nutr Metab. 2010;35(6):834–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


