Abstract
Infectious Bronchitis (IB) is an economically important avian disease that considerably threatens the global poultry industry. This is partly, as a result of its negative consequences on egg production, weight gain as well as mortality rate.The disease is caused by a constantly evolving avian infectious bronchitis virus whose isolates are classified into several serotypes and genotypes that demonstrate little or no cross protection. In order to curb the menace of the disease therefore, broad based vaccines are urgently needed. The aim of this study was to develop a recombinant DNA vaccine candidate for improved protection of avian infectious bronchitis in poultry. Using bioinformatics and molecular cloning procedures, sets of monovalent and bivalent DNA vaccine constructs were developed based on the S1 glycoprotein from classical and variants IBV strains namely, M41 and CR88 respectively. The candidate vaccine was then encapsulated with a chitosan and saponin formulated nanoparticle for enhanced immunogenicity and protective capacity. RT-PCR assay and IFAT were used to confirm the transcriptional and translational expression of the encoded proteins respectively, while ELISA and Flow-cytometry were used to evaluate the immunogenicity of the candidate vaccine following immunization of various SPF chicken groups (A-F). Furthermore, histopathological changes and virus shedding were determined by quantitative realtime PCR assay and lesion scoring procedure respectively following challenge of various subgroups with respective wild-type IBV viruses. Results obtained from this study showed that, groups vaccinated with a bivalent DNA vaccine construct (pBudCR88-S1/M41-S1) had a significant increase in anti-IBV antibodies, CD3+ and CD8+ T-cells responses as compared to non-vaccinated groups. Likewise, the bivalent vaccine candidate significantly decreased the oropharyngeal and cloacal virus shedding (p < 0.05) compared to non-vaccinated control. Chickens immunized with the bivalent vaccine also exhibited milder clinical signs as well as low tracheal and kidney lesion scores following virus challenge when compared to control groups. Collectively, the present study demonstrated that bivalent DNA vaccine co-expressing dual S1 glycoprotein induced strong immune responses capable of protecting chickens against infection with both M41 and CR88 IBV strains. Moreso, it was evident that encapsulation of the vaccine with chitosan-saponin nanoparticle further enhanced immune responses and abrogates the need for multiple booster administration of vaccine. Therefore, the bivalent DNA vaccine could serve as efficient and effective alternative strategy for the control of IB in poultry.
Keywords: Bivalent DNA vaccine, Plasmid, Infectious bronchitis virus, Coronavirus, Immunogenicity, Poultry, Chitosan, Saponin, Nanoparticle
1. Introduction
Infectious bronchitis (IB) causes significant economic loss in the poultry industry worldwide. The disease is associated with a decrease in egg production, poor weight gain, low carcass score as well as increased morbidity and mortality rates [1,2]. IB is caused by the avian infectious bronchitis virus which is an RNA virus classified under gamma coronavirus, family coronaviridae [3]. There are growing number of emerging IBV genotypes that usually shows limited cross protection, thus making control of the disease, a daunting challenge [4]. Conventionally, chickens are vaccinated against IB at an early age as well as at point of lay (breeders) using live attenuated vaccine (LAV) and/or killed vaccines [5,6]. However, there are several limitations associated with the use of live attenuated IB vaccines, notably, reversion to virulence, ability to cause disease, tissue damages; interference by maternally derived antibodies as well as loss of immunogenicity as a result of over attenuation [7,8]. Live attenuated vaccines have also been linked with mutation and recombination events, thus, leading to the emergence of new viruses probably having diverse tissue tropism and failure of the vaccinated chickens to respond to the commercially availlable vaccines [9,10]. On the other hand, vaccination with inactivated vaccines often requires multiple boosting and results in poor cellular immune response [8]. These challenges might complicates the use of conventional IB vaccines and invariably hampers the control and prevention of the disease [9,11,12]. The need for broad and safe vaccine alternatives, therefore become highly important. DNA vaccines are new generation, recombinant based vaccines with the ability to induce both humoral and cell mediated immune responses against intending pathogen such as coronaviruses [[13], [14], [15]]. Compared to live attenuated vaccines, DNA vaccines are considered safe, thermostable and highly efficacious. They could be produced within a short period of time without the need to invest huge capital. Interestingly, one or more antigenic proteins or epitopes from the same virus or different serotypes could be incorporated in a single plasmid to achieve broad immunization [16,17]. While there are some developments in DNA vaccines in poultry, not many studies have focused on dual or multiple serotype-based IB vaccines [18,19]. Moreover, improvement in the delivery and immunogenicity of DNA vaccines is needed to reduce the effect of enzymatic degradation as well as enhances their ability to evoke effective immune response. Recently, advances in nanotechnology have pave way to a significant progress in the use of non-viral vectors for gene and vaccine deliveries. In this regards, cationic polymers such as chitosan have been used as biodegradable gene delivery alternatives [20]. On the other hand, immunomodulators such as saponin; a compound with amphiphilic properties have been incorporated to several vaccines with the view to enhance the resultant immune response [21]. This study therefore, aimed at developing nanoparticle encapsulated DNA vaccine as well as determining its immunogenicity and protective capacity against infection with the two widely distributed avian infectious bronchitis viruses, namely M41 and CR88 serotypes.
2. Materials and methods
2.1. Viruses used
A classical Massachussets strain (M41-IBV) [4] was commercially obtained from the American Type Culture Collection (ATCC: VR-21™), while the Europe originating IBV variant designated CR88 (similar to IBV 4/91 or 793B strains) [22] was kindly donated by the Rhone Ma Holdings Berhad, Malaysia. Both strains were selected based on their wide distributions and reported virulence. M41 belongs to GI-1 lineage and mainly associated with the respiratory infections. On the other hand, CR88 is classified as member of G1-13 lineage commonly found in many countries of the world [23]. The two viruses were individually propagated via the allantoic cavity in 10-day-old Specific Pathogen Free (SPF) embryonated chicken eggs. Viruses were harvested 36 hs post inoculation and either kept at -20 degree centigrade [24] or used to determine the 50% Egg Infection Dose (EID50) by inoculating serial 10-fold dilutions of each virus into embryonated SPF chicken eggs and quantified as previously described by Reed and Munch [25].
3. Amplification, bioinformatic analysis and construction of plasmid DNA vaccines
The full-length S1 gene from M41 and CR88 IBV strains were amplified by RT-PCR using gene specific primers, as listed in Table 1 . Briefly, viral RNA was extracted from the harvested allantoic fluid using the QIAamp viral RNA mini kit (QIAGEN, Germany). First strand cDNA was synthesized using the RevertAid H-minus Reverse Transcriptase kit (ThermoScientifics, USA), and PCR amplification of the S1 gene was carried out in a final volume of 25 μl in a gradient master cycler (Eppendorf, Germany); following an initial denaturation (95 °C: 2 min), amplification in 35 cycles of 20 s at 98 °C, 30 s at 62 °C and 2 min at 72 °C. Final extension step was achieved in 5 min at 72 °C. The amplified genes were gel purified and verified through the sequencing of the full length S1-gene. Bioinformatic and Phylogenetic comparison was carried out based on the full-length S1-gene sequences to determine the genetic relatedness between the two vaccine candidates (KMO067900[CR88]; KMO067901[M41]) and other IBV virus strains reported in different countries (appendix A). The verified S1-segments were then cloned into a bicistronic mammalian expression vector pBudCE4.1 (Invitrogen, USA) with the view to obtain three different DNA plasmids, namely, pBudM41-S1 (encoding M41 S1 protein); pBudCR88-S1 (encoding CR88 S1 protein) and pBudCR88-S1/M41-S1 (bivalent plasmid co-expressing M41 and CR88 S1 proteins). After transformation into Escherichia coli (E. coli) Top 10 competent cells and screened on LB agar, recombinant plasmids were confirmed by colony PCR and restriction enzyme mapping. Further, integrity and nucleotide sequence of the inserted genes were verified by double-stranded sequencing approach (Medigene, Malaysia).
Table 1.
Oligonucleotides used for the amplification of S-glycoprotein genes of M41 and CR88 IBV strains for cloning into pBudCE vector.
| Primer Name | Sequences (5′-3′) | Size (bp) | RE |
|---|---|---|---|
| pBudCR88S1–F | ATCCTGTCGACACCATGTTGGACAAACCGCTTTTAC | 1620 | SalI |
| pBudCR88S1-R | ACGTTGGATCCACAACGTCTAGAGCGACGTGT | BamHI | |
| pBudM41S1V5–F | TCCTGCGGCCGCACCATGTTGGTAACACCTCTT | 1614 | NotI |
| pBudM41S1V5-R | ACCGTTCTCGAGTCACGTCTAAAACGACGTGTTC | XhoI |
4. In vitro expression analysis
In six-well tissue culture plates containing a cover-slip (SPL life science, Korea), DNA plasmids were transfected into DF1 chicken fibroblast using lipofectamine LTX PLUS (Life Technologies, USA) according to manufacturer's guidelines. By 48 h post-transfection, the cells were tested for transgenes expression using commercially available kits. Transcriptional expression was assessed by RT-PCR. For this, total RNA was extracted from the cells using the RNA isolation Plus kit (Qiagen, Germany) and then treated with the RQ1 RNase-Free DNase I (Promega, USA). After verification of DNA removal by PCR, DNA-free samples were used for RT-PCR analysis using one step access RT-PCR assay kit (Promega, Germany) with gene specific primers designed in this study (Table 1).
Note: coloured sequences indicates RE; italicize sequence indicates the Kozak sequence; bold sequence represents anchor, and underlined sequences denotes the start codon. Similarly, stop codons were deleted at the 5 ʹ ends of each reverse primers and the two genes; CR88-S1 and M41-S1 were fused with myc and V5 epitopes respectively to enable the detection of the individual proteins using IIFT.
Briefly, DNA-free samples were subjected to RT-PCR. Following a reverse transcription step at 45 °C for 45 min and then denaturation at 94 °C for 2 min, gene amplification was carried out in 35 cycles of 30 s at 94 °C; 30 sec at 62 °C; 2 min at 72 °C; and a final elongation step at 72 °C for 7 min. The products were analysed on 1% agarose gel and viewed under the GelDocR illumination system.
Translational expression of the transgenes was evaluated by indirect immunoflourescence test (IIFT) as previously described [26]. The transfected cells were washed first with 1 × PBS, and fixed with 3.7% formaldehyde at room temperature (RT) for 15 min before treatment with 0.2% buffered Triton X-100 for 5 min. After blocking the cells with 1% BSA for 1 h at RT, the cells were incubated with V5-Mouse monoclonal antibody and Alexa Fluor® 568 goat anti-mouse IgG2a as primary and secondary antibodies, respectively to detect V5 epitope-fused M41-S1 glycoprotein. Mouse anti-myc monoclonal antibody and Alexa Fluor® 488 Goat anti-Mouse IgG1 antibody were used for the detection of myc-epitope fused-CR88-S1 glycoprotein. Thereafter, the cells were rinsed with PBS and stained with DAPI (4′, 6-Diamidino-2-Phenylindole, Dihydrochloride) (Life Technology, USA) for 5 min at RT. The cover-slip was carefully removed and inverted on the surface of a microscope slide containing a drop of ProLong® antifade reagent (Life Technology, USA). The mounted slides were kept in the dark and allowed to dry before visualization under confocal microscope (Nikon, Japan).
4.1. DNA vaccination in chicken
4.1.1. DNA vaccine preparation
All the DNA plasmids were purified using a large-scale endotoxin-free plasmid purification kit (Qiagen, USA) and kept at −20 °C. The bivalent DNA vaccine pBudCR88-S1/M41-S1 was either coated with chitosan-saponin nanoparticle (+Nano) or kept uncoated (-Nano) as described previously by Bande et al. [27].
4.2. Animal care and Use Committee
The present study was approved by the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (Ref.: AUP. No. RO53/2013). . All the guidelines and standards governing the approval were observed for the study.
4.3. Immunization protocol
Three-week old Specific Pathogen Free (SPF) chickens were commercially obtained from the Malaysian Vaccine and Pharmaceuticals (MVP) firm, Malaysia and kept at the control experimental facility, Institute of BioScience, UPM. The chickens were randomly assigned into six different groups A to F (Table 2 ) each comprising up of 12 chickens kept in a barrier compartment with access to a feed ration and drinking water ad libitum.
Table 2.
Experimental animals grouping and their respective vaccination protocols.
| No. | Vaccination groups |
Vaccination protocol | Number of chickens per group (n) | Prime vaccination week3 |
Booster 1 week5 |
Booster 2 week7 |
|---|---|---|---|---|---|---|
| 1 | A | PBS (pH 7.4) | 12 | NA | ✓ | ✓ |
| 2 | B | pBudCE4.1 | 12 | ✓ | ✓ | ✓ |
| 3 | C | pBudCR88-S1 | 12 | ✓ | ✓ | ✓ |
| 4 | D | pBudM41-S1 | 12 | ✓ | ✓ | ✓ |
| 5 | E | pBudCR88-S1/M41-S1+Nano | 12 | ✓ | ✓ | – |
| 6 | F | pBudCR88-S1/M41-S1- Nano | 12 | ✓ | ✓ | ✓ |
NA= Not Applicable; √ = Done; - = not done.
Chicken from groups B–F received intramuscular (i.m) injection of 100 μg plasmid DNA on the pectoral muscle, while those in the group A (negative controls) received PBS. All vaccinated chickens received booster at week 5 (booster 1), and week 7 (booster 2), except those in group E that received a single booster vaccination at week 5 only.
4.4. Evaluation of humoral immune response
Blood samples were collected weekly via the wing vein and sera were seperated and subjected to test using commercially available ELISA (IDEXX–USA) with the view to measure total serum anti-IBV IgG antibodies. Absorbance was determined in TECAN sunrise reader (TECAN, USA) at 650 nm and the results analysed by using the mean absorbance to calculate the sample positive ration (S/P) as well as the end point or titre as described by the manufacturers.
4.5. Evaluation of T cell responses
Two weeks after the second booster vaccination, about 2–5 ml of peripheral blood samples were collected from the chickens (depending on age) via the wing vein and placed in a bottle preloaded with EDTA solution (BD, USA). Subsequently, peripheral blood mononuclear cells (PBMC) were isolated using Histopaque® (Sigma, USA) according to manufacturer's guidelines. A single cell suspension was prepared at a concentration of 1 × 107 cells/ml and about 100 μl of cell suspensions (1 × 106 cells) were incubated for 2 h at 4 °C with mouse anti-chicken CD3-FITC (Southern Biotech, USA), mouse anti-chicken CD4-R-phycoerythrin (R-PE) (Southern Biotech, USA), and mouse anti-chicken CD8 cy5 (Southern Biotech, USA). Leukocytes were triply labelled with CD3, CD4 and CD8 and further processed using fluorescence activated cell sorter (Guava®, Merck Millipore, USA).
4.6. Virus challenge experiment
For virus challenge, each of the six vaccinated groups (A-F) were subdivided into M41 (A1, B1, C1, D1, E1, F1) and CR88 (A2, B2, C2, D2, E2, F2) challenged sub groups (Table 3 ). All the chickens were challenged accordingly with 105 EID50/ml of respective viruses via ocular route at two weeks after the last booster vaccination.
Table 3.
Subgrouping and experimental challenge protocol used in the evaluation of immunogenic potentials and protective capacity of the recombinant IB-DNA vaccine .
| No. | Groups | Vaccination protocol | M41 challenged-subgroup | Chickens/group |
|---|---|---|---|---|
| 1 | A1 | PBS (pH 7.4) | A1 | 6 |
| 2 | B1 | pBudCE | B1 | 6 |
| 3 | C1 | pBudCR88-S1 | C1 | 6 |
| 4 | D1 | pBudM41-S1 | D1 | 6 |
| 5 | E1 | pBudCR88S1/M41S1+ Nano | E1 | 6 |
| 6 | F1 | pBudCR88S1/M41S1- Nano | F1 | 6 |
| CR88-Challengesubgroup | ||||
| 7 | A2 | PBS (pH 7.4) | A2 | 6 |
| 8 | B2 | pBudCE | B2 | 6 |
| 9 | C2 | pBudCR88-S1 | C2 | 6 |
| 10 | D2 | pBudM41-S1 | D2 | 6 |
| 11 | E2 | pBudCR88S1/M41S1+ Nano | E2 | 6 |
| 12 | F2 | pBudCR88S1/M41S1- Nano | F2 | 6 |
Following virus challenge, clinical signs associated with IB, were monitored daily and recorded for each challenged virus group up to 15 days post challenge. Oropharyngeal and cloacal swab samples were collected at days 3, 5, 10 and 15 post challenge to determine virus shedding. Quantification of the virus shedded was carried out by a one step Sybr-green qRT-PCR assay (Kappa BioScience, South Africa) using IBV polymerase (pol) gene-specific primers set IBV-pol-F: 5′GTGGTGATGCTACTACTGCTTATGC3′ and IBV-pol-R: 5′CAACTCATACTGCAGGCTCTTAATG3′. Additionally, microscopic changes in trachea and kidney tissues in both vaccinated and control groups were evaluated two weeks after virus challenge using Hematoxylin & Eosine (H&E) method. Lesions were then scored according to the modified procedure of Grgiae et al. [28] (for trachea) and based on protocol optimised in our laboratory (for kidney tissue).
4.7. Statistical analysis
Analyses of mean antibody response as well as virus load were carried out using a repeated measures ANOVA in JMP-SAS software, version 10 (SAS Inst. Inc., USA). Data on CD3+CD4+ and CD3+CD8+ ratio were subjected to a One Way ANOVA; since the data on CD3+CD4+ cell response did not meet up with the assumption of equal variance, the data was subjected to a non-parametric Wilcoxan test. The scored tracheal and kidney lesions were analysed based on mean and standard error of the mean. All the statistical analyses were carried out using the 95% confidence interval (CI) with p-value < 0.05 considered as significant. After performing a test, significant results were further subjected to a posthoc analysis using the Turkey-HSD method.
5. Results
5.1. Phylogenetic analysis
Phylogenetic comparison between the vaccine candidates and other sequences deposited in the genebank revealved two clusters of either Mass-like or CR-like variants reported in different countries (Fig. 1 ).
Fig. 1.
A neighbour joining phylogenetic analysis of candidate vaccine virus revealved two distict clusters of either CR88-like IBV (pink) or M41-like IBV (blue) strains as reported in different countries. Phylogenetic analysis was carried out based on nucleotide sequences of S1 gene. All analyses were conducted in Geneious software version R.
5.2. Construction of DNA vaccine
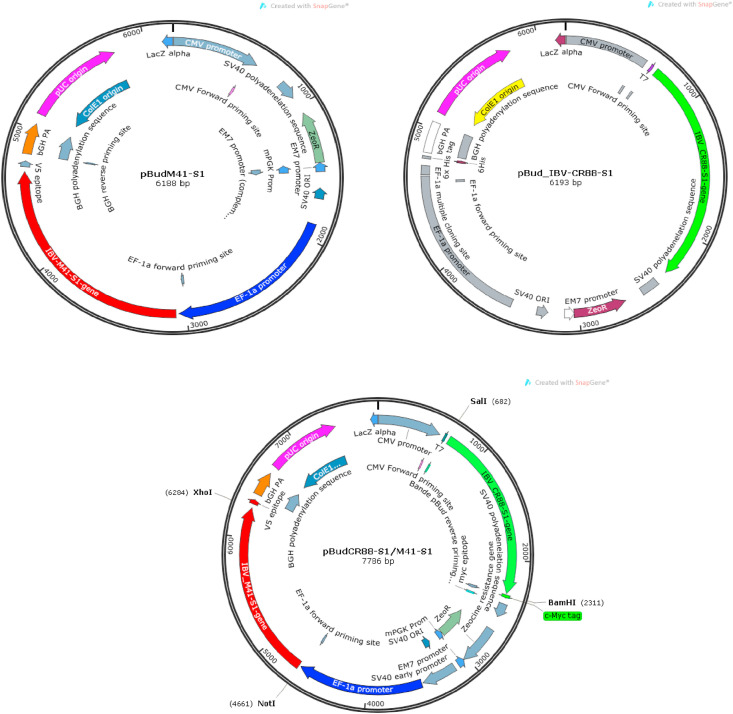
The S1-glycoprotein genes derived from two widely distributed IBV strains i.e. M41 and CR88 were amplified and cloned into a bicistronic expression vector so as to construct DNA plasmids namely pBudM41-S1, pBudCR88-S1 and a bivalent DNA plasmid pBud CR88S1/M41S1 (Fig. 2 ).
Fig. 2.
Construction of IB DNA plasmids. Three DNA plasmids were constructed using a bicistronic co-expression vector pBudCE4.1. (a) The M41 S1 gene was cloned downstream of the EF1α promoter to construct the pBudM41-S1 DNA plasmid as in the top left construct (b) The CR88 S1 gene was cloned downstream of the CMV promoter to construct the pBudCR88-S1 as in the top right DNA construct (c) The co-expressing DNA plasmid pBudCR88S1/M41S1 was constructed by inserting the S1 genes of CR88 and M41 strains downstream of the EF1α and CMV promoters, respectively as depicted in the construct placed below the two individual constructs.
5.3. In vitro expression analysis of the DNA plasmids
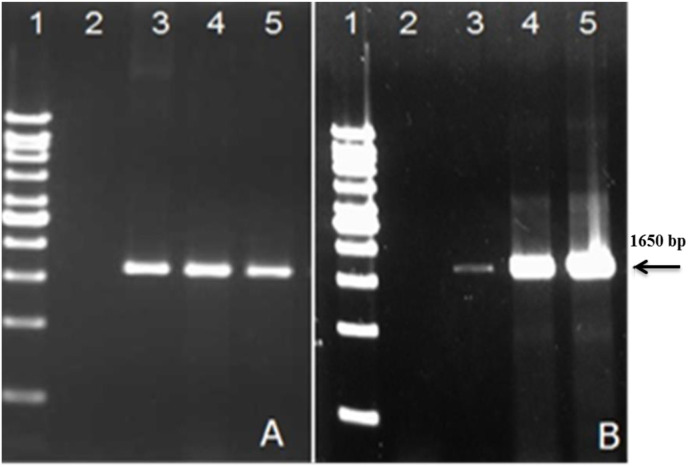
In vitro transcription of the recombinant plasmids was confirmed by the amplification of target S1-gene in the transfected cells (Fig. 3 ). This was further established by sequencing the PCR product of the transcripts and comparison of nucleotide and deduced amino acid sequence with the reference Genbank sequences.
Fig. 3.
In vitro transcription expression analysis. (A) Expression analysis of the M41-S1 and (B) CR88-S1 genes 48 h post-trasfection in DF1 cells. Transcriptional expression analysis was carried out by RT-PCR using primers specific for the M41-and CR88-S1 genes which resulted in a PCR fragment of 1650 bp. Lane 1: 1 kb DNA ladder (Viventis, Malaysia); Lane 2: negative control; Lane 3: positive S1-gene control derived from reference M41 and CR88 viruses; Lane 4: PCR product of the S1 gene from the recombinat pBudM41-S1 (A) and pBudCR88-S1 (B) plasmids; Lane 5: pBudCR88S1/M41S1-transfected DF-1 cells tested for the transcriptional expression of the M41-S1 (A) and CR88-S1 genes.
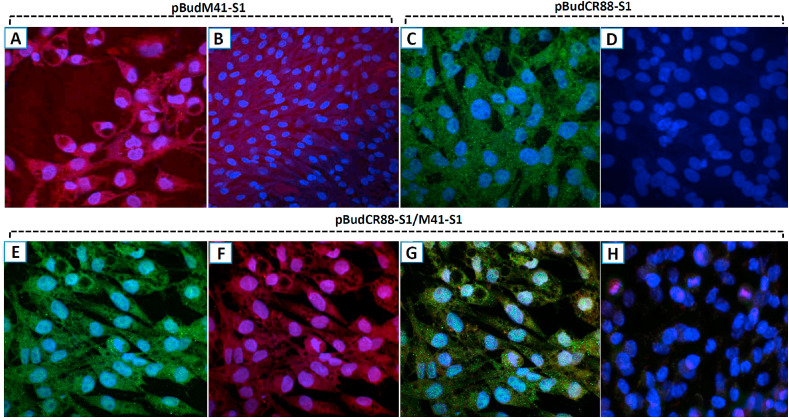
Translational expression of the constructed DNA plasmids was confirmed using indirect immunoflourescent test (IIFT) as evidenced by strong cytoplasmic fluorescence produced by individual constructs encoding S1-glycoprotein and fused to their respective epitopes. This occurred 48 h s following transfection of cells as revealed by red-coloured rhodamin signals in cells transfected with pBudM41-S1 (Fig. 4 A and 4B) and green signals in pBudM41-S1 transfected cells (Fig. 4C and 4D). Similar signals and their combinations appeared in cells transfected with the bivalent DNA plasmid pBudCR88S1/M41S1 encoding both M41 and CR88 S1 genes (Fig. 4: E-H).
Fig. 4.
(A–H): In vitro expression of the DNA plasmids by indirect immunofluorescence. DF-1 cells were transfected with each of the recombinant plasmids and at 48 h and gene expression was later detected by IIF. In pBudM41-S1-trasfected cells, anti-V5 mouse monoclonal antibody and Rhodamine conjugated anti-mouse antibody were used for the detection of V5-fused S1 protein: (A) a red-stained cytoplasm signifies the presence of the expressed protein in the cytoplasm; (B) no fluorescence signal was observed in the cytoplasm of the negative control cells. In pBudCR88S1-trasfected cells, anti-myc mouse monoclonal antibody and FITC conjugated anti-mouse antibody were used as primary and secondary antibodies for the dection of myc-fused S1 protein: (C) the appearance of the green FITC-stained cytoplasm and the blue colour DAPI stained nucleus indicated S1 protein expression; (D) no green fluorescence signal was observed in the cytoplasm of the negative control cells. In the cells transfected with the bivalent plasmid pBudCR88-S1/M41-S1, dual staining with anti myc- and anti-V5 antibodies recognizing myc and V5 epitopes respectively, was applied. Both proteins were individually detected followed by a co-staining: (E) CR88-S1 fused protein was indicated by positve FITC-stained green colouration in the cytoplasm; (F) M41-S1 protein was indicated by Rhodamine stained cytoplasm; (G) combinations of the two strainings; (H) un-transfected (negative control) cells. Mag: × 100.
5.4. In vivo evaluation of DNA vaccines
5.4.1. Evaluation of humoral immune response
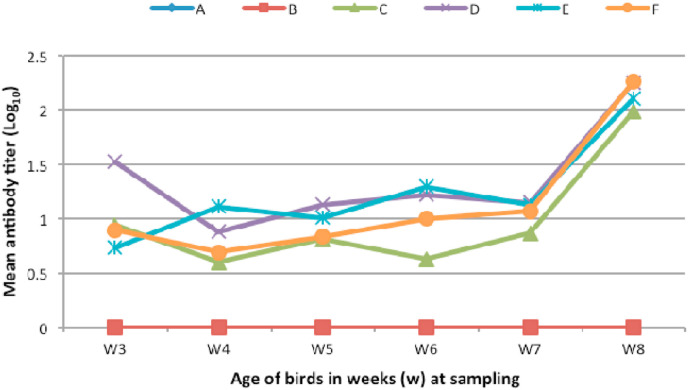
The presence of IBV-specific antisera was quantified by ELISA and humoral response was measured by taking the mean anti-IB antibody titre in vaccinated- and control groups, as shown in Fig. 5 . In contrast to the PBS and naked plasmid control groups (A and B, respectively), significant level of antibody titre (p < 0.05) was observed in chickens vaccinated with different regimens of S1-encoded DNA plasmids (groups C–F), one week post vaccination. Booster vaccination at the second week as well as the fourth week resulted in an increase in the response in both single and dual plasmid-vaccinated groups. Following second booster vaccination, a significant increase in antibody response in the dual pBudCR88-S1/M41-S1 vaccinated group was detected when compared to other groups. Chickens in group E receiving pBudCR88-S1/M41-S1 encapsulated with nanoparticle showed comparably high antibody response despite receiving a single booster vaccination.
Fig. 5.
Antibody response in the vaccinated chickens. Anti IB antibody titer was determined following vaccination with single and bivalent DNA vaccines. Serum samples were evaluated weekly by indirect ELISA using the IDEXX IBV-ELISA kit. (A) PBS; (B) pBudCE4.1; (C) pBudCR88-S1; (D) pbudM41-S1; (E) pBudCR88-S1/M41-S1+nanoparticle; (F) pBudCR88-S1/M41-S1_Nano.
5.4.2. Evaluation of T cell response
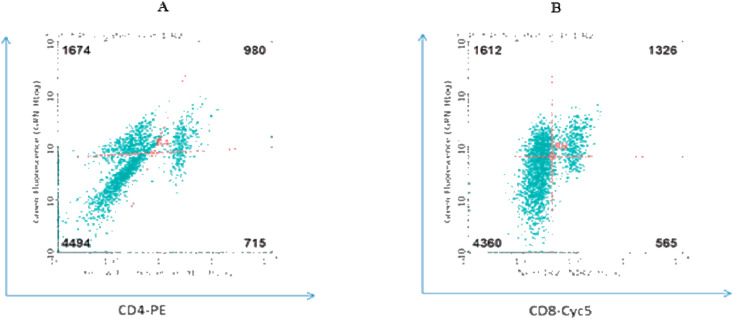
The percentage of CD3+ CD4+ and CD3+ CD8+ T-lymphocytes were enumerated by flow-cytometry using triple antibody staining methods. The representative results are shown in Fig. 6 .
Fig. 6.
Representative results showing distributions of (A) CD3+ CD4+ and (B) CD3+ CD8+ T-lymphocytes from IB DNA vaccinated chickens (group E) as determined by flow cytometry.
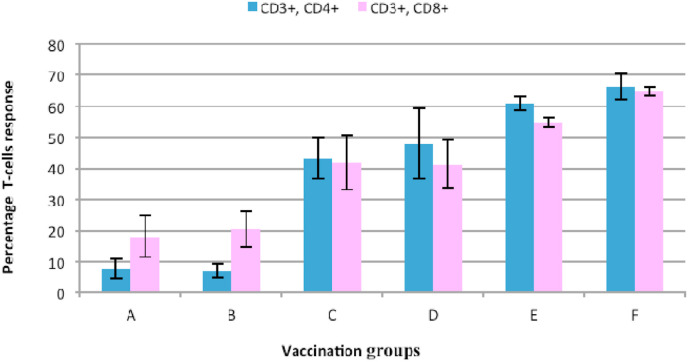
Data on CD3+ CD4+ T-cells revealed a significant (p < 0.05) increase in the CD3+ CD8+ cells in the vaccination groups compared to the controls (Fig. 7 ). Evidently, dual S1-gene plasmid-vaccinated chickens in group E and F showed significantly high CD3+ CD4+ response compared to the single S1-gene plasmid-vaccinated groups (C and D) as well as the controls (groups A and B). Similarly, analysis of the CD3+ CD8+ cell ratio, revealed significantly higher CD3+ CD8+ cell ratio in the chickens vaccinated with a dual S1-gene plasmid with or without a nanoparticle (groups E and F) as compared to the single S1-gene plasmid vaccinated chickens pBudM41-S1 (C) and pBudCR88-S1 (D) groups. Although the CD3+ CD8+ response were not significantly different (p > 0.05) between group E (encapsulated dual S1-plasmid: single booster) and Group F (dual S1-plasmid: two booster) (see Fig. 7).
Fig. 7.
Percentage CD3+CD4+and CD3+CD8+T-lymphocytes in vaccinated and control chicken groups 2 weeks after booster vaccination with IB - DNA vaccines. Vaccination regimen and control includes: (A) PBS; (B) pBudCE4.1 (naked DNA); (C) pBudCR88-S1; (D) pBudM41-S1; (E) pBudCR88S1/M41S1+Nano; (F) pBudCR88S1/M41S1- Nano. Evaluations of Clinical signs and viral shedding after challenge.
Three days post challenge, chickens in all the groups showed mild clinical signs, including general weakness, depression and ruffle feathers. These signs continued up to the 7th days but later subsided, in the vaccinated groups (C–F). However, those receiving homologous challenge (challenged with a virus similar to the vaccination plasmid) resolved earlier than those challenges with a different virus (heterologous challenge). Control chickens in groups A and B continue to show general weakness with occasional diarrhoea noticed in CR88 challenged groups up to the day-14 post challenge.
The absolute concentration of virus in viral copy number (Log10) was measured in oropharyngeal and cloacal samples. In the M41 challenged groups (A1-F1), significant decrease (p < 0.05) in oropharyngeal and the cloacal viral shedding was observed in the vaccinated groups when compared with the PBS- and pBudCE4.1-control groups (A1 and B2). The virus shedding was significantly low and consistent throughout the sampling periods in the chickens vaccinated with pBudM41-S1 (C1), pBudCR88-S1/M41-S1+Nano particle (group E1), and pBudCR88S1/M41S1-Nano (F1). Similarly, CR88 challenge study revealed a significant decrease in viral shedding throughout the sampling period in pBudCR88-S1 (D2) and pBudCR88-S1/M41-S1+Nano (E2) vaccinated chickens. The data generated is summarized in Table 4 .
Table 4.
Oropharyngeal and cloacal virus shedding from IBV-S1 plasmid vaccinated chickens following challenge of respective groups with M41 and CR88 IBV strains.
| M41-Challenged (Viral Copy No, Log10) Mean ± SD |
CR88- Challenged (Viral Copy No, Log10) Mean ± SD |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days post challenge | A1 | B1 | C1 | D1 | E1 | F1 | A2 | B2 | C2 | D2 | E2 | F2 | |
| 3 | CLO | 8.25 ± 0.34 | 7.12 ± 0.34 | 5.37 ± 0.46*** | 7.54 ± 0.34 | 6.38 ± 0.08* | 6.48 ± 1.15* | 10.85 ± 0.61 | 10.10 ± 0.18 | 8.03 ± 0.89*** | 7.83 ± 0.71*** | 5.69 ± 0.30*** | 10.62 ± 0.41 |
| ORO | 11.9 ± 0.38 | 9.31 ± 2.82 | 6.75 ± 0.15* | 8.63 ± 2.1 | 5.50 ± 0.32** | 7.34 ± 0.38* | 10.08 ± 0.47 | 9.62 ± 0.10 | 7.24 ± 0.25*** | 7.70 ± 0.69*** | 6.55 ± 0.20*** | 10.96 ± 0.49 | |
| 5 | CLO | 7.72 ± 0.66 | 6.64 ± 0.31* | 6.56 ± 0.37* | 6.64 ± 0.21* | 5.71 ± 0.10*** | 6.77 ± 0.00* | 9.94 ± 0.97 | 9.53 ± 0.33 | 7.51 ± 0.48** | 6.76 ± 0.53*** | 5.94 ± 0.05*** | 6.99 ± 0.96** |
| ORO | 9.65 ± 0.23 | 9.03 ± 0.18 | 6.56 ± 0.25*** | 7.77 ± 0.12*** | 5.27 ± 0.028*** | 6.93 ± 0.13*** | 9.55 ± 0.33 | 10.19 ± 0.75 | 7.83 ± 0.74** | 6.49 ± 0.19*** | 7.03 ± 0.02*** | 9.27 ± 0.23 | |
| 10 | CLO | 7.76 ± 0.70 | 6.60 ± 0.28* | 5.87 ± 0.39*** | 6.82 ± 0.35 | 5.49 ± 0.31*** | 5.72 ± 0.06*** | 8.40 ± 0.17 | 7.02 ± 0.76 | 9.20 ± 1.17 | 5.40 ± 0.24** | 5.98 ± 0.79** | 6.55 ± 0.32 |
| ORO | 7.55 ± 0.75 | 7.48 ± 0.68 | 6.47 ± 0.37 | 6.59 ± 0.19 | 5.46 ± 0.38** | 6.48 ± 0.37 | 8.70 ± 0.21 | 8.47 ± 0.70 | 8.79 ± 0.20 | 6.73 ± 0.38*** | 5.39 ± 0.38*** | 7.80 ± 0.146 | |
| 15 | CLO | 7.36 ± 0.43 | 7.21 ± 0.11 | 5.19 ± 0.10*** | 6.31 ± 0.29** | 5.30 ± 0.08*** | 5.18 ± 0.25*** | 9.03 ± 0.61 | 8.68 ± 0.23 | 8.55 ± 0.08 | 5.45 ± 0.35*** | 5.46 ± 0.05*** | 6.50 ± 0.74*** |
| ORO | 8.88 ± 0.54 | 7.56 ± 0.37 | 5.71 ± 0.34*** | 6.56 ± 0.70*** | 6.16 ± 0.19*** | 6.42 ± 0.64*** | 8.08 ± 0.70 | 7.42 ± 0.21 | 7.14 ± 0.32 | 6.16 ± 0.07*** | 5.04 ± 0.03*** | 6.79 ± 0.34** | |
Note: Three chickens determined as biological replicates (n = 3) were tested from each control and vaccination groups as follows: A = PBS; B = pBudCE; C = pBudM41-S1; D = pBudCR88-S1; E = pBudCR88S1/M41S1+Nano; F = pBudCR88S1/M41S1-Nano. Statistical significance was checked in relation to PBS control mean and mean values that were found to be significant are denoted with an asterisk sign either as *** = P < 0.001; **P < 0.01; * = P < 0.05. CLO = cloaca; ORO=Oropharyngeal.
5.4.3. Evaluation of microscopic changes
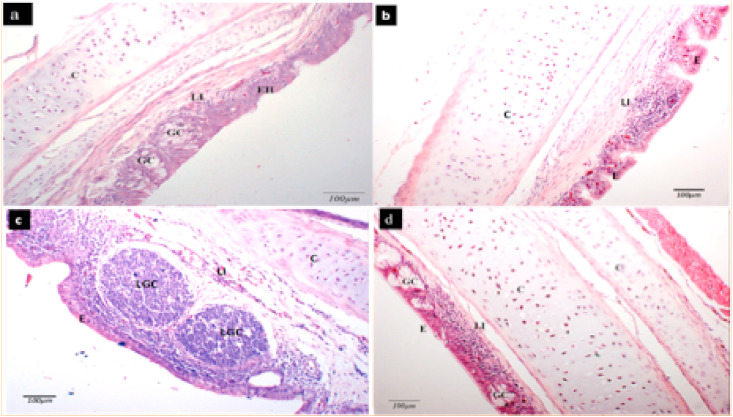
Analysis of microscopic changes revealed that in M41 challenged experiment, vaccinated chickens (C1–F1) had slightly lowered tracheal lesion scores compared to the non-vaccinated ones (A1 and B1). Likewise, mean kidney lesion scores were also lower in a pBudM41-S1 plasmid vaccinated (C1) as well as in dual S1 gene vaccinated groups (E1 and F1). However, the differences in the mean tracheal as well as mean kidney lesion scores were not statistically significant (p > 0.05). In the CR88 challenge experiment, vaccinated chickens in groups (D2-F2) had lower tracheal lesion scores compared to the control group (A2). Low kidney lesions scores was also observed in vaccinated groups compared with the non vaccinated chickens although the difference was not statistical significant in both tracheal and kidney lesions recoded in vaccinated and control chickens. In general, common microscopic changes observed in the trachea tissue include mild to moderate infiltration of lymphocytes within the lamina propria, hyperplasia of the epithelial tissue, and distention of goblet cells (Fig. 8 ).
Fig. 8.
Representative micrograph showing microscopic changes in tracheal tissue of chicken following challenge with IBV virus. A section of the trachea showing (a) mild epithelial hyperplasia (EH) with distended goblet cells (GC) and mild lymphocytic infiltration (LI) in the lamina propria (b) tracheal epithelium (E), cartilage (C) with lymphocytic infiltration (LI) in the lamina propria (c) tracheal epithelium (E) with large lymphoid germinal centers (LGC) in the lamina propria, lymphocytic infiltration (LI) can also be seen in the submucosa (d) tracheal epithelium (E) with distended goblet cells (GC), cartilage (C) and multifocal lymphocytic infiltration (LI) in the lamina propria (H&E, x200).
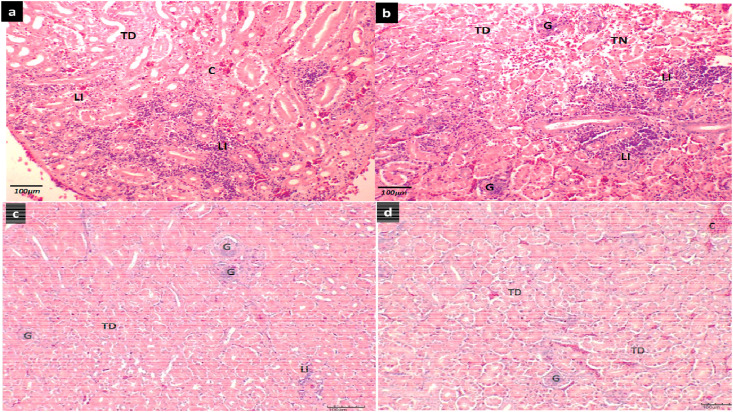
On the other hands, microscopic changes in the kidney include mild congestion, lymphocytic infiltration, tubular degeneration and necrosis of the kidney tubule (Fig. 9 ).
Fig. 9.
Representative micrograph showing microscopic changes in kidney tissue of chicken following challenge with IBV virus. A section of the kidney showing (a) tubular degeneration (TD), congestion (C) and moderate lymphocytic infiltration (LI) in the interstitium (b) an intact glomerulus (G), area of tubular degeneration (TD) and tubular necrosis (TN) with multifocal areas of lymphocytic infiltration in the interstitium. (c) glomeruli (G), tubular degeneration (TD) and mild focal lymphocytic infiltration (LI) in the interstitial tissue (d) glomerulus (G), congested (C) blood vessel and tubular degeneration (TD) (H&E, x200).
6. Discussion
The emergence of different IBV strains and lack of effective vaccine for the control of the disease is of great concern to poultry industry worldwide [27]. Moreover, realization of a broad IB vaccine will not only benefit the poultry industry but may serve as a model for the needed vaccines against other emerging coronavirus diseases such as the COVID-19. The IBV S1 glycoprotein play important role for the induction of neutralizing antibodies and cell mediated immune response, thus it serves as an excellent candidate for the recombinant coronavirus vaccine [29,30]. In the present study, mono and bivalent DNA vaccines encoding the full-length S1-glycoprotein of two IBV serotypes, namely M41 and CR88 were generated and evaluated in SPF chickens. The S1 segment from selected vaccine candidates showed phylogenetic relatedness with several IBV isolates reported in different countries of the world, thus suggesting the likely application of the vaccine in the control of variously matched IBV variants reported in parts of the world [31]. In vitro expression study revealed the successful expression of the target genes in chicken DF-1 cells. Following immunization of SPF chickens, the monovalent (pBudCR88-S1 and pBudM41-S1) and bivalent (pBudCR88S1/M41S1) DNA plasmids produced higher IBV-specific antibodies as compared with the negative control groups. Our findings also revealed that encapsulated bivalent DNA vaccine given in addition to one booster is able to generate high antibody titre in vaccinated chickens which were comparable with the bivalent plasmid alone given with two booster vaccinations. This findings signifies the importance of encapsulating the DNA plasmid with nanoparticles to enhance its delivery, protects the DNA from enzymatic degradation and probably, improve its release [27]. Earlier studies demonstrated the incorporation of chitosan nanoparticle with DNA plasmid to enhance mucosal delivery of influenza virus vaccine in a mouse model. These studies reported that the humoral as well as cell mediated immune responses were all found to be significantly increased after immunization [32]. Likewise, encapsulation of live attenuated IB vaccine with chitosan nanoparticle has been shown to improve antibody and cell mediated responses which was characterized by the increased mucousal IgA and increased expression of interferon gamma related genes at the primary sites of viral replication [33]. The saponin used in this study might act as immunostimulant as reported in other studies. In this regards, Berezin et al. [34], demostrated significant increase in antibody- (IgG, IgM and IgA) and cell mediated (IFN-gamma and IL2) immune responses following single intranasal immunization against influenza using saponin nanoparticle based delivery. Similarly, Yu et al. [35], reported the use of oral ginseng stem-leaf saponins (GSLS) to enhanced vaccine induce response in immunosuppressed chickens. Both chitosan and saponin therefore might as well acted as adjuvants or immunomodulators that positively improves the desired host immune responses as revealed in the present study [36]. Our analysis of CMI response, by taking the CD4+, CD8+ ratios, revealed that a single booster of chitosan-saponin DNA encapsulated bivalent plasmid (pBudM41-S1/CR88-S1) was able to induce comparably high response compared to the bivalent plasmid (without nanoparticle) given with additional two consecutive boosters. The same scenario was observed when CD3+ CD4+ and CD3+ CD8+ T cell responses were assessed. In all the cases, bivalent DNA vaccinated groups showed significantly higher CMI response as compared to the monovalent types. In a similar study, effective cellular response as well as good protective capacity was achieved following prime immunization of chickens with attenuated vaccine followed by further administration of a multivalent DNA vaccine [37].
Virus challenge experiment revealed that the bivalent IB-DNA vaccine used in this study was able to significantly caused reduction in oronasal and cloacal viral shedding in both M41 and CR88 IBV challenge experiments, thus, demostrating its potential use in IBV vaccinations against the classical M41 and variant CR88 like virus strains. interestingly, bivalent DNA plasmid encapsulated with chitosan-saponin nanoparticle showed comparable reduction in virus shedding despite given in single booster as well. This result suggests that, vaccination with a bivalent IB DNA vaccine (pBudCR88S1/M41S1) could protect chickens against challenge with the two virus strains (dual virus protection) as demonstrated by the reduction in virus shedding. Also, encapsulation of the said DNA plasmid aboragates the need for multiple booster vaccination and thus showed potentials in relieving farmers from the burden and cost associated with repeated vaccine applications. In another related study Jiao et al. [38] used oral vaccination approach to administer a recombinant DNA vaccine encoding the S1 gene to induce significant levels of virus eradication in vaccinated chickens when compared to control groups. Similarly, another study reported that a DNA plasmid encoding S1 gene resulted in 75% protection in challenged chickens, thus further demostrating the immunogenic potentials of IBV S1 glycoprotein based DNA vaccine [39]. The protection observed in this study interms of immununological responses, reduced virus sheddings, low kidney and tracheal lesion scores as well as reduced clinical disease might be associated with the abundance of protective epitopes within the S1 glycoprotein of the two virus serotype and might signifies the potential uses of the vaccine evaluated in this study for the control of IBV for the [[29], [40], [41]]. In conclusion, the present study, demonstrated that a bivalent IB DNA vaccine co-expressing the M41 and CR88 S1 glycoproteins is capable of inducing significant humoral and CMI responses targeting M41 and CR88 IBV serotypes and perhaps any other genetically similar strain. These response could be enhanced through the use of chitosan and saponin nanoparticles as vaccine delivery vehicles as well as adjuvants to achieve the desired protection.
Further studies are needed to assess the applications of the present findings in broilers and layers chicken types as well as to explore other delivery methods especially the intranasal route of administering the candidate vaccines to ease applications in large scale poultry settings.
Acknowledgment
The authors would like to acknowledge support from the Virology Laboratory, Faculty of Veterinary Medicine, Chemical Characterization Laboratory, Faculty of Engineering as well as Vaccine and Immunotherapeutic Laboratory, Institute of Bioscience, Universiti Putra Malaysia. This project was co-funded under the following grants (i) Grant Putra: GP/2017/9551800 (Project Tittle: Transcriptome Regulation and protein-protein interaction during Japanese Enchephalitis Virus infection in vector and host), (ii) Universit Putra Grant: GP-IPS/2016/9500500 (Project Tittle: Molecular Detection of Bovine Viral Diarrhea Virus [BVDV] in Cattle in Selected Farms in Selangor ; (iii) Industry Grant: 6300899 (Project Tittle: Inactivated fowl adenovirus vaccine UPM1137 FADV Serotype 8 Strain) and (iv) Higher Institution Centre of Excellence (HICoE) grant: 369,101.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.micpath.2020.104560.
Author contribution
FB: conceptualized the idea, designed and carried out the experiment. He also drafted manuscript for publication; SSA,MHB, ARO, HM: contributed in the research design and experiment; SK, TSW, YSK,YB, AIA: all participated in conducting the research and were involved in data analysis. All authors read and agreed with the final submission of the manuscript.
Declaration of competing interest
The Authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bande F., Arshad S.S., Omar A.R., Bejo M.H., Abubakar M.S., Abba Y. Pathogenesis and diagnostic approaches of avian infectious bronchitis. Adv. Virol. 2016;2016 doi: 10.1155/2016/4621659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molenaar R.J., Dijkman R., de Wit J.J. Characterization of infectious bronchitis virus D181, a new serotype (GII-2) Avian Pathol. 2020;9457 doi: 10.1080/03079457.2020.1713987. [DOI] [PubMed] [Google Scholar]
- 3.Britton P., Cavanagh D. Nidovirus genome organization and expression mechanisms. Nidoviruses. 2008:29–46. [Google Scholar]
- 4.De Wit J.J., Cook J.K.A., der Heijden H.M.J.F. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finney P.M., Box P.G., Holmes H.C. Studies with a bivalent infectious bronchitis killed virus vaccine. Avian Pathol. 1990;19:435–450. doi: 10.1080/03079459008418698. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.J., Youn H.N., Kwon J.S., Lee Y.J., Kim J.H., Lee J.B., Park S.Y., Choi I.S., Song C.S. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine. 2010;28:2887–2894. doi: 10.1016/j.vaccine.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondal S.P., Naqi S.A. Maternal antibody to infectious bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Vet. Immunol. Immunopathol. 2001;79:31–40. doi: 10.1016/S0165-2427(01)00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bande F., Arshad S.S., Hair Bejo M., Moeini H., Omar A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinley E.T., Jackwood M.W., Hilt D.A., Kissinger J.C., Robertson J.S., Lemke C., Paterson A.H. Attenuated live vaccine usage affects accurate measures of virus diversity and mutation rates in avian coronavirus infectious bronchitis virus. Virus Res. 2011;158:225–234. doi: 10.1016/j.virusres.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanagh D., Davis P.J., Cook J.K.A. Infectious bronchitis virus: evidence for recombination within the Massachusetts serotype. Avian Pathol. 1992;21:401–408. doi: 10.1080/03079459208418858. [DOI] [PubMed] [Google Scholar]
- 11.McKinley E.T., Hilt D.A., Jackwood M.W. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008;26:1274–1284. doi: 10.1016/j.vaccine.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abro S.H., Renström L.H.M., Ullman K., Isaksson M., Zohari S., Jansson D.S., Belák S., Baule C. Emergence of novel strains of avian infectious bronchitis virus in Sweden. Vet. Microbiol. 2012;155:237–246. doi: 10.1016/j.vetmic.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin J.E., Louder M.K., Holman L.S.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saade F., Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines. 2012 doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapczynski D.R., Hilt D.A., Shapiro D., Sellers H.S., Jackwood M.W. Protection of chickens from infectious bronchitis by in ovo and intramuscular vaccination with a DNA vaccine expressing the S1 glycoprotein. Avian Dis. 2003;47:272–285. doi: 10.1637/0005-2086(2003)047[0272:POCFIB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Bijlenga G., Cook J.K.A., Jr J.G., De Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from The Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraro B., Morrow M.P., Hutnick N.A., Shin T.H., Lucke C.E., Weiner D.B. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapczynski D.R., Hilt D.A., Shapiro D., Sellers H.S., Jackwood M.W. Protection of chickens from infectious bronchitis by in ovo and intramuscular vaccination with a DNA vaccine expressing the S1 glycoprotein. Avian Dis. 2003;47:272–285. doi: 10.1637/0005-2086(2003)047[0272:POCFIB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Tian L., Wang H., Lu D., Zhang Y., Wang T., Kang R. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem. Biophys. Res. Commun. 2008;377:221–225. doi: 10.1016/j.bbrc.2008.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao H.-Q., Roy K., Troung-Le V.L., Janes K.A., Lin K.Y., Wang Y., August J.T., Leong K.W. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J. Contr. Release. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 21.Oda K., Matsuda H., Murakami T., Katayama S., Ohgitani T., Yoshikawa M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000;381:67–74. doi: 10.1515/BC.2000.009. [DOI] [PubMed] [Google Scholar]
- 22.Jones R.C. Europe: history, current situation and control measures for infectious bronchitis. Rev. Bras. Ciência Avícola. 2010;12:125–128. [Google Scholar]
- 23.Valastro V., Holmes E.C., Britton P., Fusaro A., Jackwood M.W., Cattoli G., Monne I. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect. Genet. Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy J.S. Springer; 2008. Isolation and Propagation of Coronaviruses inEmbryonated Eggs; pp. 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- 26.Richter E., Wick G. Fluoro-immuno-cytoadherence (FICA): a new method for the identification and enumeration of antigen-binding cells. Zeitschrift Für Immunitätsforsch. Immunobiol. 1977;152:351–362. [PubMed] [Google Scholar]
- 27.Bande F., Arshad S.S., Hair Bejo M., Abdullahi Kamba S., Omar A.R. Synthesis and characterization of chitosan-saponin nanoparticle for application in plasmid DNA delivery. J. Nanomater. 2015;2015 doi: 10.1155/2015/371529. [DOI] [Google Scholar]
- 28.Grgiæ H., Hunter D.B., Hunton P., Nagy É. Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Can. J. Vet. Res. 2008;72:403. [PMC free article] [PubMed] [Google Scholar]
- 29.Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T., Wang H.N., Wang X., Tang J.N., Lu D., Zhang Y.F., Guo Z.C., Li Y.L., Gao R., Kang R.M. The protective immune response against infectious bronchitis virus induced by multi-epitope based peptide vaccines, Biosci. Biotechnol. Biochem. 2009;73:1500–1504. doi: 10.1271/bbb.80864. [DOI] [PubMed] [Google Scholar]
- 31.Bande F., Arshad S.S., Omar A.R., Hair-bejo M., Mahmuda A., Nair V. Global distributions and strain diversity of avian infectious bronchitis virus: Rev. 2017;18:70–83. doi: 10.1017/S1466252317000044. [DOI] [PubMed] [Google Scholar]
- 32.Sawaengsak C., Mori Y., Yamanishi K., Srimanote P., Chaicumpa W., Mitrevej A., Sinchaipanid N. Intranasal chitosan-DNA vaccines that protect across influenza virus subtypes. Int. J. Pharm. 2014;473:113–125. doi: 10.1016/j.ijpharm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Lopes P.D., Okino C.H., Fernando F.S., Pavani C., Casagrande V.M., Lopez R.F.V., Montassier M. de F.S., Montassier H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine. 2018;36:2630–2636. doi: 10.1016/j.vaccine.2018.03.065. [DOI] [PubMed] [Google Scholar]
- 34.Berezin V.E, Bogoyavlenskiy A.P, Turmagambetova A.S, Alexuk P.G, Zaitceva I.A., Omirtaeva E.S, Abitaeva M., Sokolova N.S. Nanoparticles from plant saponins as delivery system for mucosal influenza vaccine. Am. J. Infect. Dis. Microbiol. 2013;1:1–4. doi: 10.12691/ajidm-1-1-1. [DOI] [Google Scholar]
- 35.Yu J., Shi F.S., Hu S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015;167:147–155. doi: 10.1016/j.vetimm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Greenland J.R., Letvin N.L. Chemical adjuvants for plasmid DNA vaccines. Vaccine. 2007;25:3731–3741. doi: 10.1016/j.vaccine.2007.01.120. [DOI] [PubMed] [Google Scholar]
- 37.Yan F., Zhao Y., Hu Y., Qiu J., Lei W., Ji W., Li X., Wu Q., Shi X., Li Z. Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine. J. Vet. Sci. 2013;14:53–60. doi: 10.4142/jvs.2013.14.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao H., Pan Z., Yin Y., Geng S., Sun L., Jiao X. Oral and nasal DNA vaccines delivered by attenuated Salmonella enterica serovar Typhimurium induce a protective immune response against infectious bronchitis in chickens. Clin. Vaccine Immunol. 2011;18:1041–1045. doi: 10.1128/CVI.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen J., Zhao S., He D., Yang Y., Li Y., Zhu S. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antivir. Res. 2012;93:154–159. doi: 10.1016/j.antiviral.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Bru T., Vila R., Cabana M., Geerligs H.J. Protection of chickens vaccinated with combinations of commercial live infectious bronchitis vaccines containing Massachusetts, Dutch and QX-like serotypes against challenge with virulent infectious bronchitis viruses 793B and IS/1494/06 Israel variant 2. Avian Pathol. 2017;46:52–58. doi: 10.1080/03079457.2016.1203393. [DOI] [PubMed] [Google Scholar]
- 41.Bande Faruku, Arshad Siti Suri, Hair Bejo Mohd, Kadkhodaei Saeid, Omar Abdul Rahman. Prediction and in silico identification of novel B-cells and T-Cells epitopes in the S1-Spike glycoprotein of M41 and CR88 (793/B) infectious bronchitis virus serotypes for application in peptide vaccines. Adv. Bioinf. 2016;2016:1–5. doi: 10.1155/2016/5484972. 5484972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.