Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to multi-system dysfunction with emerging evidence suggesting that SARS-CoV-2-mediated endothelial injury is an important effector of the virus. Potential therapies that address vascular system dysfunction and its sequelae may have an important role in treating SARS-CoV-2 infection and its long-lasting effects.
Keywords: Endothelium, Coagulation, Thrombosis, Inflammation, Microvessels
SARS-CoV-2 infection and vascular dysfunction
In health, the vascular endothelium maintains homeostasis through regulation of immune competence, inflammatory equilibrium, tight junctional barriers, hemodynamic stability as well as optimally balanced thrombotic and fibrinolytic pathways. In the novel coronavirus disease of 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), dysregulation of many of these pathways has emerged as a mediator of severe disease. The constellation of clinical and biomarker derangements seen in COVID-19 can be classified into disruption of the immune, renin-angiotensin-aldosterone (RAA), and thrombotic balance, all of which converge on the vascular endothelium as a common pathway. Accumulating evidence from basic science, imaging and clinical observations, has clarified the picture of COVID-19 as a vascular disease. Understanding the disease in this context may provide novel avenues of understanding COVID-19 and lead to critically needed improvements in therapeutic strategies.
SARS-CoV-2 uses the angiotensin converting enzyme 2 (ACE2) to facilitate entry into target cells and initiate infection. This viral entry into the cell is further mediated by transmembrane serine protease 2 (TMPRSS2) and cathepsin L which cleave the S protein on the viral particle to permit engagement with ACE2 [1]. Endothelial cells (ECs) in general and cardiac pericytes in particular express abundant ACE2, making them a direct target of SARS-CoV-2 infection (Fig. 1 ) [2]. Examination of the pulmonary vascular bed shows severe derangements in COVID-19, compared to control and influenza patients, particularly with widespread thrombosis and microangiopathy, endothelial activation and extensive angiogenesis [3]. These studies and pervasive findings establish the role of viral injury to the vascular system with resulting vascular dysfunction in COVID-19 patients [4].
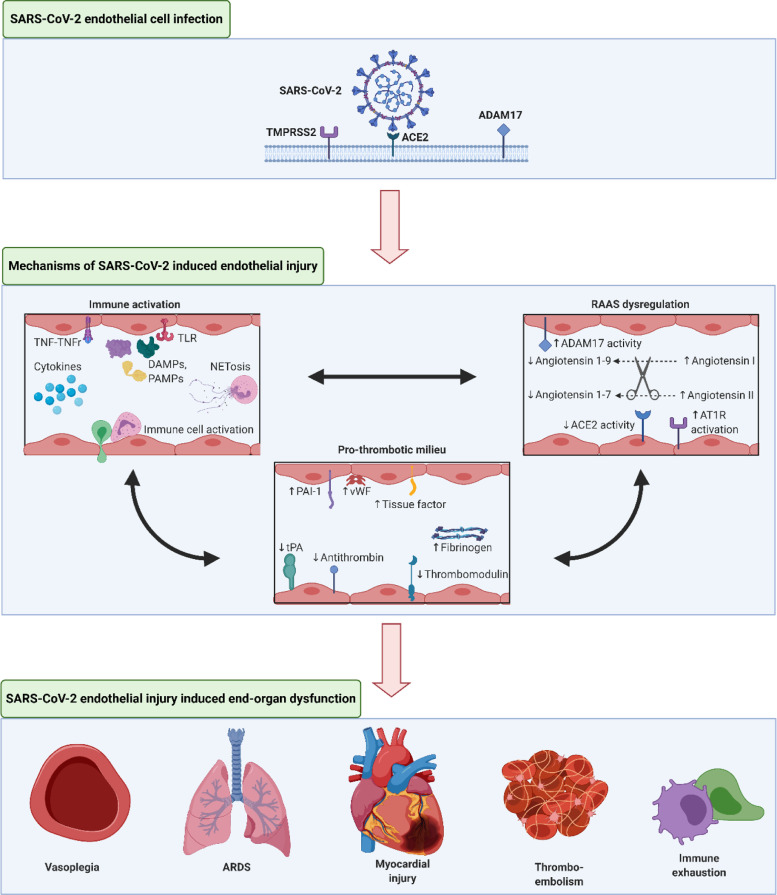
Fig. 1.
SARS-CoV-2 Induced Endothelial Injury
Legend: A schematic of SARS-CoV-2 infection and proposed resulting endothelial injury, involving immune activation, pro-thrombotic milieu, and RAAS dysregulation. These insults interact with each other to cause end-organ dysfunction that is manifest in many COVID-19 patients.
TMPRSS2 = Transmembrane protease serine 2; ADAM17 = A disintegrin and metalloproteinase 17; TNF = Tumor necrosis factor; TNFr = Tumor necrosis factor receptor; TLR = toll-like receptor; DAMPs = Damage-associated molecular patterns; PAMPs = Pathogen-associated molecular patterns; PAI-1 = plasminogen activator inhibitor-1; vWF = von Willebrand factor; eNOS = endothelial nitric oxide; tPA = tissue plasminogen activator; AT1R = angiotensin 1 receptor; ARDS = acute respiratory distress syndrome
Created with BioRender.com.
COVID-19, immune dysregulation and endothelial injury
The vascular endothelium has an intricate role in immune regulation and inflammation, an axis that SARS-CoV-2 infection disturbs. Reports from hospitalized COVID-19 patients reveal an activation of the immune system that displays findings consistent with cytokine storm, macrophage activating syndrome and subsequent immune exhaustion, most severe in the sickest patients [5,6]. This hyper-inflammatory state has deleterious effects on the vascular system with resulting EC dysfunction. In the presence of circulating inflammatory mediators such as interleukin (IL)-1, IL-6, damage-associated molecular patterns and pathogen-associated molecular patterns, ECs undergo transition to an activated state that participates in host defenses [7]. Activated ECs promote localized inflammation by inducing proinflammatory gene expression, attracting immune cells, promoting recruitment of inflammatory cells to injured or infected tissues, vascular leak by increasing endothelial permeability, and altering the thrombotic potential of the local intimal surface. Activation of neutrophils leads to formation of neutrophil extracellular traps (NETs), a process sometimes referred to as NETosis, which may contribute to responses to pathogens and thrombosis [8]. Additionally, EC injury is compounded by toll-like receptor (TLR) activation by viral RNA recognition, with resulting increased reactive oxidative species (ROS) production [9].
In a regulated and self-limited immune response, these mechanisms help to quell the local insult, with subsequent healing and return to a resting EC state. In contrast, states of a heightened innate immune response and a prothrombotic state elicited by innate immune mediators such as that seen in COVID-19 lead to an escalating cascade of these pathways that promote profound micro- and macrovascular EC dysfunction and damage, with impairment of other important functions of the endothelium (Fig. 1). Recent reports of a hyperinflammatory syndrome similar to Kawasaki disease shock syndrome in children, with elevated biomarkers of cardiac injury, development of arrythmia and progression to giant coronary aneurysms in some cases further sheds light on one manifestation of a macrovascular-focused immune phenomenon in COVID-19, an area that warrants further study [10]. The EC dysregulation imposed by COVID-19 extends beyond the circulatory system. Pulmonary ECs have an important role in immune surveillance, maintaining alveolar integrity and ensuring appropriate oxygen exchange. In COVID-19, direct viral infection and the systemic inflammatory response likely lead to severe dysfunction of these important ECs, compounding the resulting picture of severe hypoxia and acute respiratory distress syndrome frequently reported in hospitalized patients.
Endothelial injury and thrombosis in COVID-19
The endothelium, along with its key immunoregulatory functions, also plays an essential role in maintaining a dynamic interplay between the pro-coagulant and fibrinolytic factors in the vascular system. In the quiescent state, the endothelium forms a barrier between the prothrombotic subendothelial layer and pro-coagulant factors in the blood. In times of stress, activated ECs express more plasminogen activator inhibitor-1 (a key inhibitor of endogenous fibrinolysis), tissue factor (a potent procoagulant) and release von Willebrand factor (a protein that forms multimers that promote thrombus growth). Activated ECs lower activity of thrombomodulin and tissue plasminogen activator, favoring thrombus accumulation [11]. Furthermore, during stress, immune effectors potentiate the thrombotic response, including NET formation, and thus can also propagate thrombosis [8]. These changes, along with other complex regulatory pathways lead to endothelial dysfunction, activation of the coagulation cascade and suppression of fibrinolytic mechanisms, with a resulting prothrombotic milieu (Fig. 1) [11]. Such disturbances in the fine balance of endothelial health and the coagulation and fibrinolytic systems lead to both macrovascular and diffuse microvascular thrombotic disease in the patient [12].
Clinical studies show that patients with COVID-19 have increased fibrinogen, fibrin degradation products, D-dimer and von Willebrand factor, and these elevations appear to correlate with severity of disease and thrombotic risk [13,14]. Early reports showed a substantial burden of myocardial injury in patients who were critically ill or died from COVID-19 [15]. Multiple mechanisms may cause cardiac injury in COVID-19 [16]. While infection and hemodynamic stresses of acute critical illness can trigger plaque rupture and resulting myocardial infarction, recent reports indicate that some COVID-19 patients show biomarker and electrocardiographic findings of myocardial infarction without evidence of acute plaque rupture on angiography [17]. Furthermore, a recent case series reported a 78% prevalence of cardiovascular involvement and myocardial inflammation without apparent left ventricular impairment on cardiac magnetic resonance imaging in recovered COVID-19 patients without cardiac symptoms post-discharge from the hospital [18]. This pattern of cardiac injury could result from endothelial dysfunction and coronary microvascular thrombosis in these patients, rather than coronary macrovascular thrombosis.
Evidence of endothelial injury also comes from autopsy reports that provide further support of this pan-vascular involvement in COVID-19, with series showing compelling evidence of a high incidence of both deep venous thrombosis as well as in situ pulmonary arterial thrombosis [3,19]. Prominent findings in these autopsies included microvascular thrombi, particularly in the pulmonary circulation, pointing to a disrupted endothelium with a resultingly pro-coagulable state in COVID-19. Multiple analyses and systematic reviews have shown a substantial burden of venous thromboembolism (VTE) in COVID-19 patients, with up to 25% incidence of VTE and 20% incidence of pulmonary embolism in hospitalized patients, particularly those with more severe illness requiring critical care and amongst those not on prophylactic or therapeutic anticoagulation [14,[20], [21], [22]]. Further support for macrovascular manifestations of endothelial dysfunction with an activation of pro-thrombotic mechanisms comes from reports highlighting the higher incidence of large-vessel stroke in COVID-19 patients as well as well as a retrospective case-control analysis showing COVID-19 to be an independent risk factor for stroke [23,24].
Recent obstetric data also add evidence to SARS-CoV-2 acting through endothelial and vascular mechanisms. In a case series of 42 pregnancies admitted with SARS-CoV-2 infection, 14% of all women and 75% of women with severe COVID-19 symptoms manifested a constellation of symptoms similar to pre-eclampsia and hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, with spontaneous resolution after COVID-19 recovery [25]. Pre-eclampsia has considerable vascular and endothelial dysfunction in its central pathobiology, and the development of this disorder at a considerable rate in women with COVID-19 suggests endothelial involvement as a sequela of viral infection.
COVID-19 and the renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone (RAA) system that crucially regulates vascular health and function also participates in COVID-19. As ACE2 contributes critically to the biology of SARS-CoV-2 infection, much attention has focused on the interplay between COVID-19 and the RAA system. Angiotensin II is the main vascular effector of the RAA system and exerts its deleterious effects on the cardiovascular system via angiotensin-II type 1 (AT1) receptors by activating vasoconstrictor, inflammatory and fibrotic pathways. Prior studies show that angiotensin II perturbs endothelial functions in multiple ways, including by monocyte recruitment, formation of ROS, activation of pro-inflammatory pathways including through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) regulation, as well as promoting plasminogen activator inhibitor-1 (PAI-1) production in ECs [26,27]. In health, ACE2 plays a counter-regulatory role in the RAA system by cleaving angiotensin II to angiotensin 1-7. The enzyme also acts on angiotensin I to convert it to angiotensin 1-9. Both of these enzymatic conversions yield peptides that have potent anti-inflammatory, anti-oxidant and anti-fibrotic properties.
Early data indicate that SARS-CoV-2 limits ACE2 expression by promoting cleavage ACE2 by the specialized proteinase A disintegrin and metalloproteinase 17 (ADAM17) and shedding from the cell surface, leading to a reduction in ACE2’s protective role on ECs and other organs [28]. One study in COVID-19 patients showed a markedly elevated level of serum angiotensin II, further implicating an activated RAA system in the manifestations of SARS-CoV-2 infection [29]. One mechanism by which SARS-CoV-2 infection may affect vascular dysfunction is through oxidative stress. Nox2, regulated by angiotensin-II contributes to oxidative stress in the endothelium via production of reactive oxidant species, and increases in severe COVID-19, adding to the potential deleterious effects of RAA dysregulation in SARS-CoV-2 infection [30]. Overall, SARS-CoV-2 infection results in RAA system activation with its resulting profile of injurious endothelial functions (Fig. 1). This dysregulation potentiates the innate immune stimulation, oxidative stress and pro-thrombotic states previously described and fuels a vicious cycle in the vascular disease of COVID-19 [26].
Immunomodulatory therapy in COVID-19
The three pathways outlined above through which COVID-19 disrupts the vascular endothelium may provide novel targets for intervention, some using re-purposed drugs. Agents that limit endothelial dysfunction may mitigate the pro-inflammatory, pro-thrombotic and/or RAA activated state induced by COVID-19 infection and could help quell the most serious sequalae of SARS-CoV-2 infection. With the prominent role of systemic and localized inflammatory signals in disrupting vascular and EC function, therapeutic agents that attenuate the hyperinflammatory response in COVID-19 merit consideration. Several trials currently underway examine immunomodulatory therapies such as antagonists of the NLRP3 inflammasome (that generates active forms of IL-1 and IL-18), IL-1, IL-6 and GM-CSF [31]. Trials are also examining the benefit of the anti-inflammatory agent colchicine, shown to have cardioprotective effects in non-COVID-19 cohorts, in improving outcomes in COVID-19 patients.
Reducing the overall inflammatory drive in COVID-19 patients, especially before entering the severe and critical stage of disease, could alter the natural history and trajectory of illness [32]. If immunomodulatory intervention occurs too late, however, the potential infectious complications of such an approach might impact the benefit to risk ratio adversely, for example by predisposing to bacterial superinfections. It will thus be important that interventions in the NLRP3 to IL-1 to IL-6 to C-reactive protein signaling axis be initiated in those with severe enough disease to suggest early elevations in the target cytokines, but not among critically ill COVID patients where the process is unlikely to be reversible or in those without severe illness who will likely recover without intervention. Finding this balance poses a substantive clinical challenge.
Antithrombotic and anticoagulant therapy in COVID-19
Antithrombotic and anticoagulant therapy might mitigate the thrombotic risk in COVID-19. It is unclear if the hyperinflammatory state coupled with vascular injury in SARS-CoV-2 infection leads to a higher rate of thromboembolic complications compared to other acute infectious and critical illnesses. The multiple antemortem and postmortem reports of frequent microthrombi as well as venous and arterial thromboembolism leading to morbidity and mortality in COVID-19 suggest a role of agents that target the coagulation and thrombotic cascade [14,[19], [20], [21],23,24]. While any decision on choice and dosing of antithrombotic or anticoagulant agents must be accompanied by an individualized risk assessment of the bleeding profile, the reported high prevalence of thrombotic complications in COVID-19 patients highlights the potential benefit of these agents.
This concept derives further support from a recent single-center observational study which showed significantly lower mortality and risk of intubation in 4,389 hospitalized COVID-19 patients treated with anticoagulation, with no incremental benefit of therapeutic dosing over prophylactic dosing [33]. However, therapeutic dose anticoagulation was associated with an increased risk of bleeding compared to prophylactic dosing. The wide menu of options which can be tested for prevention of thromboembolic disease in SARS-CoV-2 infection includes antithrombotic agents such as aspirin, dipyridamole and P2Y12 receptor inhibitors as well as anticoagulants such as heparin, direct thrombin inhibitors and direct oral anticoagulants. With reports of persistent thrombosis despite prophylactic anticoagulation, the optimal dosing and most effective agents to use in this setting requires close study [14].
The use of antiplatelet agents such as aspirin merits consideration in the setting of endothelial injury and microvascular inflammation, given the anti-inflammatory and anti-thrombotic effects of these agents from the collective experience in coronary artery disease. At the same time, the burden of VTE and a potentially hyper-coagulable state in some COVID-19 patients makes anticoagulant therapy an attractive candidate therapy. The use of antiplatelet and anticoagulant agents is an area of active investigation in COVID-19 with multiple trials in various stages including a significant investment by the National Institutes of Health Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) collaborative [34]. One of the goals of the ACTIV effort (ACTIV-4) is to conduct three adaptive platform clinical trials of antithrombotics (comparing aspirin, apixaban and placebo) to better understand coagulopathy in COVID-19 patients and define optimal therapeutic strategies in this population [34]. These trials, along with others currently in progress, will provide insight into this question. The use of biomarkers such as fibrinogen, d-dimer and CRP may help guide patient selection, agent, duration and dosing strategy for antithrombotic and anticoagulant therapy, since a “one-size-fits-all” strategy may not be effective in this complex disease. Trials of antithrombotic and anticoagulant therapies will also need to examine the best point in disease progression and severity at which to initiate these therapies, from ambulatory patients with mild disease, to those entering critical illness, to those requiring post-hospitalization prophylaxis. Novel agents, such as those that target P-selectin, are also under consideration.
RAA system inhibition in COVID-19
Given the involvement of the RAA system in COVID-19, the use of ACE inhibitors (ACEi) in COVID-19 has raised questions of hypothetical benefit as well as harm. Preclinical studies suggested that blockade of the RAA system could cause a counterregulatory augmentation in ACE2, the virus’ receptor, possibly raising the susceptibility to SARS-CoV-2 infection. Newly published data provide reassurance that there is not a higher risk of SARS-CoV-2 infection or progression to severe disease as a result of ACEi or angiotensin receptor blocker (ARB) use [35]. Furthermore, recent experimental data in states of cardiovascular stress show an important effect of ARBs in attenuating vascular dysfunction by reducing AT1 induced inflammatory effects on the endothelium [27]. While convincing data on therapeutic benefit in COVID-19 are lacking, those already prescribed these therapies for pre-existing cardiovascular disease should continue their prior regimens in the setting of SARS-CoV-2 infection, a position supported by multiple global societies [36].
Statins in COVID-19
Another class of commonly used cardiovascular medications that have anti-inflammatory and vasculoprotective effects include 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (statins). Given their pleiotropic and anti-inflammatory effects [37], [38], [39], some have suggested a role for statins in treatment of COVID-19. To date, a propensity matched study showed a reduction in all-cause mortality in COVID-19 in Chinese patients, while most other observational studies do not show a benefit of statins in reducing COVID-19 infection or severity [40]. Prior studies on statins in acute respiratory distress syndrome have not shown benefit in this broader context.
As new data and clinical evidence emerge about this disease, the vascular nature of COVID-19 has come into focus. Understanding the key dysregulated cascade triggered by COVID-19 in the vascular system provides us with a framework to inform interventions on key mediators of the pathologic process, and in this way reduce the short- and long-term morbidity and mortality of this pandemic disease. The picture presented here supports the view that the vasculature proves pivotal in the pathogenesis and is a potential target of therapy in COVID-19.
Declaration of Competing Interest
Hasan Siddiqi: No disclosures
Peter Libby: Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion, Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Merck, Novartis, Pfizer, Sanofi-Regeneron. Dr. Libby is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, XBiotech, Inc. Dr. Libby's laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby's interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund.
Paul Ridker: Dr Ridker has received research grant support from Kowa, Novartis, NHLBI, NCI, and Amarin (unrelated to this manuscript) and served as a consultant to Novartis, Jansen, CiviBiopharm, Corvidia, Flame, Agepha, and Inflazome, (unrelated to this manuscript). Dr. Ridker is also part of the National Institutes of Health Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) collaborative steering committee.
References
- 1.Liu T., Luo S., Libby P., Shi G.-P. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol Therapeut. Sep 2020;213 doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. Jun 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020 Aug 21;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. Jun 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. .e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. Reduction and functional exhaustion of T Cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020 May 1;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pons S., Arnaud M., Loiselle M., Arrii E., Azoulay E., Zafrani L. Immune consequences of endothelial cells’ activation and dysfunction during sepsis. Crit Care Clin. Apr 2020;36(2):401–413. doi: 10.1016/j.ccc.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Moreno D., Adrover J.M., Hidalgo A. Neutrophils as effectors of vascular inflammation. Eur J Clin Invest. Nov 2018;48:e12940. doi: 10.1111/eci.12940. [DOI] [PubMed] [Google Scholar]
- 9.To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat Commun. Dec 2017;8(1):69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet. May 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M., Hao H., Leeper N.J., Zhu L. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol [Internet] Jun 2018;38(6) doi: 10.1161/ATVBAHA.118.310367. https://www.ahajournals.org/doi/10.1161/ATVBAHA.118.310367 [cited 2020 Jul 28] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. Jun 19 2020;1358863X2093264 doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. The Lancet Haematol. Jun 2020;S2352302620302167 doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. Jun 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. Jul 1 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atri D., Siddiqi H.K., Lang J.P., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist. JACC: Basic Transl Sci. May 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B. ST-segment elevation in patients with Covid-19 — a case series. N Engl J Med. Jun 18 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol [Internet] Jul 27 2020 doi: 10.1001/jamacardio.2020.3557. https://jamanetwork.com/journals/jamacardiology/fullarticle/2768916 [cited 2020 Aug 30]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Internal Med. May 14 2020:M20–2566. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkeland K., Zimmer R., Kimchi A., Kedan I. Venous thromboembolism in hospitalized COVID-19 patients: a systematic review (Preprint) Interact J Med Res [Internet] Jul 22 2020 doi: 10.2196/22768. http://preprints.jmir.org/preprint/22768/accepted [cited 2020 Aug 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Shen L., Le K.-J., Pan M.-M., Kong L.-C., Gu Z.-C. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta-analysis. Front Cardiovasc Med. Aug 6 2020;7:151. doi: 10.3389/fcvm.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poyiadi N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K. Acute pulmonary embolism and COVID-19. Radiology. May 14 2020;201955 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K. COVID-19 is an independent risk factor for acute ischemic stroke. AJNR Am J Neuroradiol. Jun 25 2020;ajnr;ajnr.A6650v1 doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. New Engl J Med. Apr 28 2020;0(0):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza M., Garcia‐Ruiz I., Maiz N., Rodo C., Garcia‐Manau P., Serrano B. Preeclampsia‐like syndrome induced by severe COVID‐19: a prospective observational study. BJOG: Int J Obstet Gy. Jun 2020;1471-0528:16339. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasier A.R., Recinos A., Eledrisi M.S. Vascular inflammation and the renin-angiotensin system. ATVB. Aug 2002;22(8):1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 27.Molitor M., Rudi W.-S., Garlapati V., Finger S., Schüler R., Kossmann S. Nox2+ myeloid cells drive vascular inflammation and endothelial dysfunction in heart failure after myocardial infarction via angiotensin II receptor type 1. Cardiovasc Res. Jan 31 2020;cvaa042 doi: 10.1093/cvr/cvaa042. [DOI] [PubMed] [Google Scholar]
- 28.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. Aug 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. Mar 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R. Nox2 activation in Covid-19. Redox Biol. Sep 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley L.F., Wohlford G.F., Ting C., Alahmed A., Van Tassell B.W., Abbate A. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit Care Exp. Aug 2020;2(8):e0178. doi: 10.1097/CCE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. Mar 2020;S105324982031473X doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P., Pujadas E. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Am Coll Cardiol. Aug 2020;S0735109720364081 doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institutes of Health. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) [Internet]. 2020 [cited 2020 Aug 27]. Available from: https://www.nih.gov/research-training/medical-research-initiatives/activ
- 35.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. Jun 18 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID-19. J Cardiac Fail. May 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laufs U., La Fata V., Plutzky J., Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. Mar 31 1998;97(12):1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 38.Undas A., Brummel-Ziedins K.E., Mann K.G. Statins and blood coagulation. ATVB. Feb 2005;25(2):287–294. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 39.Kunieda Y., Nakagawa K., Nishimura H., Kato H., Ukimura N., Yano S. HMG CoA reductase inhibitor suppresses the expression of tissue factor and plasminogen activator inhibitor-1 induced by angiotensin II in cultured rat aortic endothelial cells. Thrombosis Res. Jun 2003;110(4):227–234. doi: 10.1016/s0049-3848(03)00346-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X.-J., Qin J.-J., Cheng X., Shen L., Zhao Y.-C., Yuan Y. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. Jun 2020;S1550413120303168 doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]