Abstract
The hypoxic tumor microenvironment is associated with malignant progression and poor treatment response. The glucose transporter Glut-1 is a prognostic factor and putative hypoxia marker. So far, studies of Glut-1 in cancer have utilized conventional immunohistochemical analysis in a series of individual biopsy or surgical specimens. Tissue microarrays, however, provide a rapid, inexpensive means of profiling biomarker expression. To evaluate hypoxia markers, tissue cores must show the architectural features of hypoxia; i.e. viable tissue surrounding necrotic regions. Glut-1 may be a useful biomarker to validate tissue microarrays for use in studies of hypoxia-regulated genes in cancer. In this study, we carried out immunohistochemical detection of Glut-1 protein in many tumor and normal tissue types in a range of tissue microarrays. Glut-1 was frequently found in peri-necrotic regions, occurring in 9/34 lymphomas, 6/12 melanomas, and 5/16 glioblastomas; and in 43/54 lung, 22/84 colon, and 23/60 ovarian tumors. Expression was rare in breast (6/40) and prostate (1/57) tumors, and in normal tissue, was restricted to spleen, tongue, and CNS endothelium. In conclusion, tissue microarrays enable the observation of Glut-1 expression in peri-necrotic regions, which may be linked to hypoxia, and reflect previous studies showing differential Glut-1 expression across tumor types and non-malignant tissue.
Keywords: Glut-1, Tissue microarray, Tumors, Hypoxia, Expression pattern
1. Introduction
Tumor tissue shows increased uptake of glucose relative to that of normal tissue, a phenomenon first observed by Warburg in 1930 (Hatanaka, 1974). Efforts to therapeutically exploit this metabolic difference have included the use of glucose antimetabolites such as 2-deoxyglucose (Sridhar et al., 1979), which show some specificity to hypoxic cells and glucose-linked conjugates of cytotoxic agents such as gluphosphamide (Niculescu-Duvaz, 2002), which are preferentially taken up by tumors. Certain novel anticancer agents are also believed to act via modulation of Glut-1, such as the histone deacetylase inhibitors, shown recently to reduce glucose transport into multiple myeloma cells by downregulating Glut-1 expression (Wardell et al., 2009) and fasentin, a chemical sensitizer to FAS-mediated apoptosis believed to act via binding and inhibition of Glut-1 (Wood et al., 2008). The renewed interest in tumor glucose transport coincided with the discovery and characterization of the erythrocyte type facilitative glucose transporter Glut-1, and hence the structural and functional basis of cellular glucose uptake (Mueckler et al., 1985). More recently, though, there has been a significant increase in knowledge of how tumor hypoxia regulates the expression and functionality of glucose transporters. Tumor hypoxia is a well-established therapeutic problem and is an independent predictor of poor prognosis (Brizel et al., 1996). The influence of hypoxia is reflected in terms of its impact on treatment response—resulting in radiation (Hall Eric, 2000) and chemotherapy (Teicher, 1994) resistance, and morphological changes, which reduce the penetration of drugs into the tumor. Hypoxia, due to the selection of p53 mutants and the upregulation of hypoxia-inducible survival genes, which are regulated via stabilization of the transcription factor hypoxia-inducible factor (HIF-1), results in increased tumor aggressiveness (Hockel et al., 1996). This change in tumor behavior includes increased vascularity, adaptation to low tumor pH (Potter and Harris, 2003), and increased rate of anaerobic glycolysis (Seagroves et al., 2001). The use of intrinsic hypoxia markers, which may be detected by traditional immunohistochemical techniques in biopsy or surgical specimens, may prove useful in the rational selection of patients to receive hypoxia-linked therapies such as hypoxia-dependent bioreductive drugs, e.g. EO9 and AQ4N (McKeown et al., 2007), and accelerated radiotherapy with carbogen and nicotinamide (ARCON) (Hoskin et al., 2003). Glut-1 has been used as an intrinsic marker of hypoxia in patients being treated for carcinoma of the cervix (Airley et al., 2001; Airley et al., 2003). Glut-1 expression and hypoxia have also been mechanistically linked by comparison with HIF-1 expression in colorectal cancer and colorectal cell lines exposed to hypoxic conditions (Chung et al., 2009). Further, in a phase I trial involving patients with solid tumors, Glut-1 was successfully used as a hypoxia marker for the prediction of response to AQ4N (Albertella et al., 2008). Glut-1 is hypoxia-inducible, dually controlled via HIF-1 and in response to a decreased rate of oxidative phosphorylation (Behrooz and Ismail-Beigi, 1997). Depending upon the depth and duration of hypoxia, changes in Glut-1 activity may manifest as unmasking of pre-existing plasma membrane-bound Glut-1, followed by translocation to the plasma membrane of cytoplasmic Glut-1, which is bound by intracellular vesicles and finally, de novo synthesis of Glut-1 (Zhang et al., 1999). There is now an accumulation of evidence, provided by previous studies involving clinical tumor samples, that Glut-1 is ubiquitously expressed in many tumor types, but rarely expressed in corresponding benign tissue (Medina and Owen, 2002; Binder et al., 1997). Glut-1 is over-expressed and predicts poor prognosis in a wide range of tumors including those of the head and neck (Oliver et al., 2004), colorectum (Cooper et al., 2003), breast (Younes et al., 1995), cervix (Airley et al., 2001), and clear cell renal carcinoma (Ozcan et al., 2007). The influence of Glut-1 on prognosis and its use as a biomarker may be a manifestation of tumor hypoxia, and the adaptive upregulation of anaerobic glycolysis that may ultimately promote tumor cell survival (Airley and Mobasheri, 2007). However, there are other factors, such as its association with the oncogenes H-ras and C-myc, estrogen, and growth factors including Interleukin-3 (Osthus et al., 2000; Chen et al., 2001; Baron-Delage et al., 1996; Ahmed and Berridge, 1997). Glut-1 expression may be a cause or effect of malignant transformation by viruses, either by facilitating the infection of a transforming virus such as HTLV (Manel et al., 2004), or as a consequence of the energy-dependent malignant changes induced after viral transformation (Kitagawa et al., 1985). This has application in the clinic, where hypoxia driven changes in Glut-1 expression relates to the degree of malignant transformation and invasive potential of cervix metaplasia (Rudlowski et al., 2003), breast cancer (Gatenby et al., 2007), and hepatocellular carcinoma (Amann et al., 2009). Glut-1 expression correlates with tumor invasiveness (Grover-McKay et al., 1998) and promotes increased proliferative activity, which is associated with the loss of functional p53 (Schwartzenberg-Bar-Yoseph et al., 2004). Finally, Glut-1 expression may predict a response to standard chemotherapeutic agents such as lomustine, and dacarbazine (Airley et al., 2005).
Tissue microarrays (TMAs) are an ordered array of multiple (from tens to hundreds) formalin-fixed, paraffin-embedded tissue cores presented on a slide. These offer the advantage of providing a rapid and relatively inexpensive means of profiling tumors and tissues for the expression of different genes and proteins. Annotation, such as clinical characteristics of the tumors, provides a means by which a new target may be correlated with certain parameters such as grade of differentiation, or recurrence of metastasis (Mobasheri et al., 2004). This information may be used either to provide preliminary data, or supporting evidence with which to conceive and carry out comprehensive pre-clinical and clinical studies. Chronic or diffusion-limited hypoxia, which occurs at around 70–150 μm from a patent blood vessel, is typically evident as viable tissue lying adjacent to necrotic regions but distal to a patent blood vessel (Hall Eric, 2000). To enable effective evaluation of tumor hypoxia, therefore, tissue cores used in the preparation of TMA’s must contain sufficient material to adequately represent the tissue architecture existing in hypoxic regions of tumors. In the present study, we have used a range of TMA’s containing samples from a wide range of tumor and normal tissue types to evaluate Glut-1 as a potential biomarker of malignancy and hypoxia. The major objective of this study is to determine if TMA’s and the accompanying clinical information may be used to carry out translational studies of the expression of hypoxia markers such as Glut-1 that allow correlation with clinical parameters in a manner that is comparable to conventional immunohistochemical analysis. Our validation will use three criteria: examination of the extent of Glut-1 expression including the differential between malignant and corresponding normal tissue, the use of Glut-1 as an intrinsic marker to assess the spatial pattern of hypoxia in a wide range of tumor types, and the use of available clinical information on clinical characteristics to correlate Glut-1 expression with characteristics such as differentiation and estrogen receptor status. In doing so, we may also accumulate further evidence of the merit of Glut-1 as a tumor-specific biomarker and therapeutic target.
2. Methods
2.1. Normal and tumor tissue arrays
Four different TMAs of formalin-fixed, paraffin-embedded tumor samples, were obtained from the TARP Lab, National Cancer Institute, NIH, Maryland, USA. These included the TARP-4 array, which consisted of mixed normal and tumor tissue types including melanoma, lymphoma, and CNS tumors (exclusively glioblastoma multiforme), as well as breast, colon, ovarian, lung, and prostate tumors. The three further arrays were designed to offer a wider range of tumor samples within a tumor type, and included the T-CL-1 (colon and lung tumor), T-BO-1 (breast and ovarian tumors) and finally, the T-Pr-1 array, which consisted of matched tumor and normal prostate samples. Maps and annotations of the TMAs are available at www.cancer.gov/tarp.
2.2. Immunohistochemistry
Immunohistochemical staining for Glut-1 protein was carried out on at least 2 serially cut slides for each array. Staining was undertaken as per Airley et al. (2001) and Oliver et al. (2004), using an Envision kit containing an anti-rabbit mouse-labeled polymer conjugate (DAKO). The primary antibody used was an affinity-purified anti-rabbit Glut-1 (Alpha Diagnostic International, Texas, USA) at a dilution of 1/100 (10 μg/ml protein). The negative control consisted of a rabbit IgG used at the same protein concentration.
2.3. Semi-quantitative analysis
Glut-1 expression was assessed by initial examination at low power (× 100) using a light microscope to confirm the identity of the tumor sample according to the layout of the array, and to assess the tumor samples for absence or presence of Glut-1 protein. To evaluate the extent and intensity of Glut-1 staining, and to put this staining into the context of tumor histology, a further examination of the samples was made at higher magnification (× 250 and × 400). For the TARP-4 arrays, Glut-1 staining was classified as positive or negative. However, for the tissue-specific arrays (T-CL-1, T-BO-1, and T-Pr-1), where there was a wider range of tissue samples available for each site, Glut-1 expression was classified as negative, light (< 30% and mostly cytoplasmic) or heavy (> 30% and mostly membranous) staining. Although areas of necrosis were excluded, peri-necrotic areas were closely examined in order to note possible hypoxia-linked Glut-1 expression. Arrays were scored blindly by two independent observers (RA and AE) to rule out inter-observer variation.
3. Results
3.1. Pattern of Glut-1 protein staining
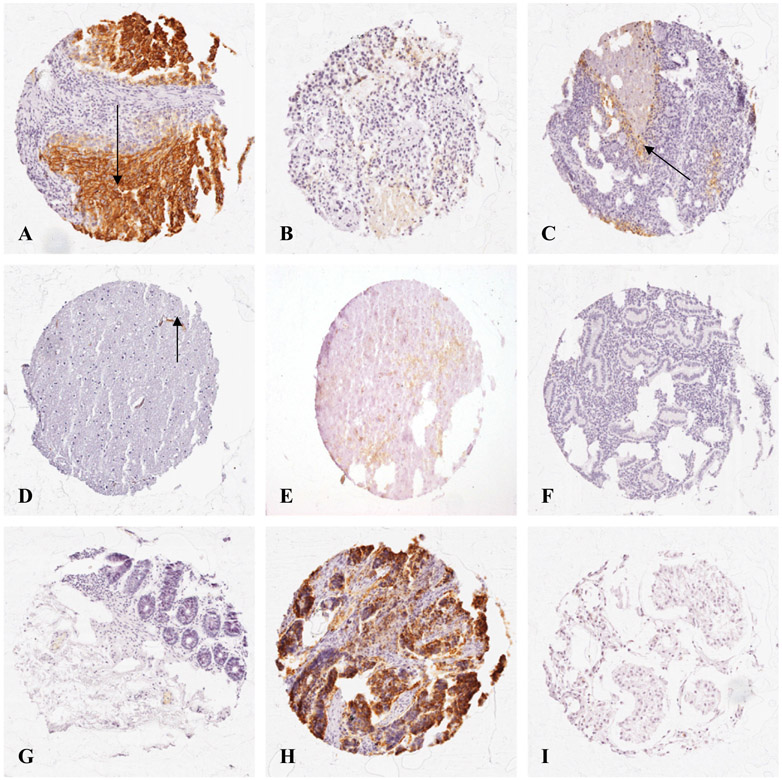
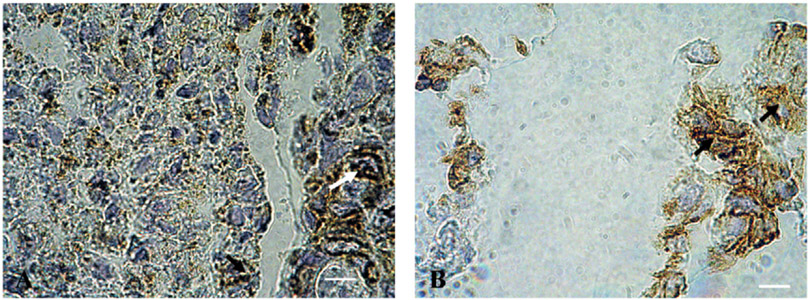
Fig. 1 shows Glut-1 expression surrounding necrosis in (A) lung, (B) breast, and (C) ovarian carcinomas. Peri-necrotic Glut-1 expression was particularly apparent in a number of samples of ovarian tumors and in squamous carcinoma of the lung. Adenocarcinomas were characteristically hard to define visually by semi-quantitative analysis, owing to the glandular nature of the tumor tissue. Here, in samples showing heavy Glut-1 staining, the protein did not appear to be expressed in the core of tumor nests, but rather in layers proximal to the stroma. However, peri-necrotic Glut-1 staining was detectable at higher magnification in colonic adenocarcinoma as well as squamous carcinoma of the lung (Fig. 2).
Fig. 1.
Glut-1 expression frequently appeared in peri-necrotic areas, which are likely to coincide with chronically hypoxic regions in malignant tissue. Glut-1 showed a pattern of staining typical of chronic or diffusion-limited hypoxia in malignant tissue from (A) lung; (B) breast, and (C) ovarian carcinomas, where arrows show oxygen gradients between vascularised stroma, and necrotic regions. Differential Glut-1 staining was observed between normal cerebellum (D), where Glut-1 staining occurred in the vascular endothelium (shown by arrow), and glioblastoma, which was positive for Glut-1 staining (E); as well as between in normal (G), and malignant (H) colon. Glut-1 expression was undetected in many normal tissues, including endometrial (F), and testicular (I) tissues. Magnification × 125.
Fig. 2.
Peri-necrotic Glut-1 expression in (A) colonic adenocarcinoma; and (B) squamous cell carcinoma of the lung. Where samples showed regions of necrosis, peri-necrotic Glut-1 expression was heavy and membranous, and typically adjacent to necrotic cells also positive for Glut-1 staining (shown by arrows), suggesting periods of anoxia prior to cell death. Magnification × 1000.
3.2. Glut-1 expression in normal tissue
Samples of normal tissue were provided for each array. For the T-BO-1, T-CL-1, and T-Pr-1 arrays, sections derived from identical tissue samples were used and the immunostaining achieved was reproducible. Glut-1, with few exceptions, showed differential expression between malignant and benign tissue. Fig. 1 shows that Glut-1 staining in normal cerebellum is restricted to vascular endothelium, which coincides with Glut-1 present in the blood–brain barrier (Takata, 1996) (D), and is absent in normal colon (G); whereas Glut-1 staining is present within tumor tissue in glioblastoma (E), and colorectal tumors (H). For normal tissue, Glut-1 was expressed in significant quantities in the spleen and peripheral nerve. Bone marrow also expressed Glut-1, which may have been restricted to maturing erythrocyte precursors. There was no Glut-1 protein detected in normal endometrium (F), testis (I), lung, breast or prostate. Interestingly, there was also no observable Glut-1 expression in the kidney or liver, and a section of ovary and liver obtained commercially (Abcam Laboratories), also showed no Glut-1 expression.
4. Distribution of Glut-1 staining according to tumor type and pathology
4.1. TARP-4 array
Tumor samples staining positively for Glut-1 included 9/34 (26%) lymphomas, 6/12 (50%) melanomas, 5/16 (31%) CNS, 14/37 (38%) ovarian, 12/48 (25%) breast, 14/50 (28%) colon, and 1/41 (2%) prostate tumors.
4.2. T-BO-1, T-CL-1, and T-Pr-1 arrays
Glut-1 expression was determined in colon, lung, breast, and ovarian tumors according to pathology and, where appropriate, degree of differentiation and estrogen receptor status. Details of Glut-1 staining according to tumor subtypes are shown in Table 1. Samples from 54 lung tumors and 45 colon tumors of mixed pathology were included in the T-CL-1 array. Although a high proportion of lung tumor samples stained positively for Glut-1 (43/54 (80%)), squamous cell carcinomas showed the greatest proportion of cases with heavy Glut-1 staining. Data describing the grade of differentiation were provided for 28 tumors, although in this series, there was no correlation with the grade of Glut-1 protein expression (the Spearman’s rank r=0.034, P=0.865, n = 28). For colon tumors, 22/84 (26%) samples showed Glut-1 protein expression. However, of these, 12/22 showed light and 10/22 heavy glut-1 staining. The T-BO-1 array included samples from 40 breast and 60 ovarian tumors. For breast tumors, only 6/40 (15%) samples stained positively for Glut-1, and all but one of these showed only light Glut-1 staining. Information on estrogen receptor status was available for 10 tumors, of which 7 were estrogen receptor positive. Of these, 3 tumors expressed Glut-1, whereas Glut-1 was absent in the 3 estrogen receptor negative tumors. The ovarian tumors included serous and mucinous histologies, of which 23/60 (38%) stained positively. Of these, the serous type histology (serous papillary adenoma and serous adenocarcinoma) showed the highest proportion of Glut-1 positive cases. Data describing the grade of differentiation were available for 27 tumors of mixed pathology. However, there was no significant correlation between differentiation and the level of Glut-1 expression (the Spearman’s rank r=0.106, P=0.599, n=27). The T-Pr-1 array provided 57 samples of prostate adenocarcinoma and 55 samples of normal tissue. Only one tumor sample showed Glut-1 protein expression, which was classed as heavily stained and appeared to be peri-necrotic. Unfortunately, the matched normal tissue sample was not available for this sample.
Table 1.
Distribution of Glut-1 staining according to tumor site and subtype, using T-BO, TCL, and T-Pr tissue microarrays.
| Grade of Glut-1 immunostaining | |||
|---|---|---|---|
| Pathology | 0 (%) | 1 (%) | 2 (%) |
| Lung: total=54 | |||
| NSCCa | 0 (0) | 5 (63) | 3 (38) |
| Squamous cell Ca | 3 (12) | 6 (24) | 16 (64) |
| Adenocarcinoma | 6 (35) | 5 (29) | 6 (35) |
| Bronchioalveolar Ca | 0 (0) | 1 (50) | 1 (50) |
| Bronchioalveolar adenocarcinoma | 2 (100) | 0 (0) | 0 (0) |
| Colon: total=84 | |||
| Adenocarcinoma | 19 (46) | 12 (29) | 10 (24) |
| Mucinous adenocarcinoma | 4 (100) | 0 (0) | 0 (0) |
| Breast: total=40 | |||
| Ductal adenocarcinoma | 26 (81) | 5 (16) | 1 (3) |
| Lobular adenocarcinoma | 7 (100) | 0 (0) | 0 (0) |
| Adenocarcinoma | 1 (100) | 0 (0) | 0 (0) |
| Ovary: total=60 | |||
| Mucinous adenoma | 5 (100) | 0 (0) | 0 (0) |
| Serous papillary adenoma | 15 (58) | 6 (23) | 5 (19) |
| Clear cell carcinoma | 4 (67) | 0 (0) | 2 (33) |
| Clear cell adenocarcinoma | 4 (80) | 1 (20) | 0 (0) |
| Serous adenocarcinoma | 5 (42) | 4 (33) | 3 (25) |
| Endometrial adenocarcinoma | 3 (60) | 2 (40) | 0 (0) |
| Poorly differentiated carcinoma | 1 (100) | 0 (0) | 0 (0) |
| Prostate: total=112 | |||
| Adenocarcinoma | 56 (98) | 0 (0) | 1 (2) |
| Matched normal tissue | 55 (100) | 0 (0) | 0 (0) |
5. Discussion
The use of TMA’s to evaluate the expression of a biomarker is clearly advantageous over individual studies of several tumor types. Although the data obtained in this study were limited by issues associated with preparation, including limited sample size, missing, and poorly preserved tissue cores, these problems are countered by the number of samples analyzed, the economy, and ease and rapidity with which the study is carried out. Another consideration is that, contrary to studies involving samples of biopsy or resected tumor material from cohorts of patients, extensive ethical procedures are not necessary. Additionally, the material is collected from a large number of institutions with standard tissue handling protocols. To test the true merit of this method, it is necessary to compare the results obtained in this present study with those provided by past studies of Glut-1 expression, and to investigate the hypothesis from where similar conclusions have been drawn using TMA’s as have been from a multitude of studies involving conventional analyses of clinical samples taken from large numbers of patients. In terms of the distribution of Glut-1 expression according to tumor type, these data compare favorably to previous studies. Glut-1 has previously been found to be heavily expressed in squamous cell carcinomas of the lung relative to adenocarcinomas (Younes et al., 1997; Ito et al., 1998), observations that are similar to the present study. Also, in a previous investigation involving a large series of breast tumors, a notable majority (approximately 96%) showed no Glut-1 staining or expression in less than 50% of tumor cells (Younes et al., 1995), an observation that is echoed in this study. These differences in Glut-1 expression may be compensated by expression of alternative facilitative glucose transporters such as Glut-5 (Zamora-Leon et al., 1996) and Glut-12(Rogers et al., 2003). Another study carried out on a series of invasive ductal carcinomas of the breast that expressed Glut-1 protein in large quantities (50–100 % of tumor cells), also demonstrated moderate to high levels of Glut-2; as well as a low level of Glut-4 expression (Brown and Wahl, 1993). Both the present and previous studies show that a relatively large proportion of colorectal tumors express Glut-1. However, in previous studies variation in Glut-1 expression with depth of invasion was shown to be an important determinant of prognosis in early stage tumors (Furudoi et al., 2001; Sakashita et al., 2001), highlighting the point that any array design must include representative samples of this parameter as well as different histological subtypes. The T-Pr array adequately reflected the lack of Glut-1 expression observed in previous studies involving conventional immunohistochemical analysis of prostate tumors. In this case, although Glut-1 may have prognostic value in tumors of the prostate, where it correlates with Gleason score and is differentially expressed in benign prostatic hyperplasia, and prostate cancer (Stewart et al., 2008), in this study Glut-1 expression in malignant prostate tissue was absent.
The expression of Glut-1 in normal tissue is well characterized and occurs prolifically in the blood-brain barrier (Pardridge, 1991), and the plasma membranes of erythrocytes (Thorens, 1996), with smaller levels expressed alongside the isoform Glut-4 in muscle (Klip and Paquet, 1990). Such expression was clearly identified in this present study, in the endothelial tissue of the cerebellum, the tongue, and in areas of hemorrhage in normal as well as tumor tissue samples. Despite this, in both the present and previous studies (Medina and Owen, 2002), Glut-1 expression is extremely low or absent in a wide range of normal tissues, such as breast, colon, and lung. Interestingly, no Glut-1 was detectable in normal liver or kidney in the present study. Although Glut-1 is often described as ubiquitous, its expression, along with the other glucose transporter isoforms, is tissue-specific (Joost and Thorens, 2001). Glut isoforms specific to the liver tend to be Glut-2, due to its role in glucose sensing, and the more recently discovered Glut isoforms Glut-7, Glut-9, and Glut-10 (Medina and Owen, 2002). Whereas Glut-1 has been found in normal liver and kidney in previous studies, this may be a consequence of non-malignant but pathological states such as diabetic nephropathy (Haneda et al., 2001; Rhoads, 1994). Detectable expression of Glut-1 in the liver may also be a consequence of malignant transformation to hepatocellular carcinoma (Amann et al., 2009).
It is clear that in squamous type tumors in particular, Glut-1 protein is consistently expressed around necrotic regions, reaffirming its connection with tissue hypoxia. However, the use of Glut-1 as an intrinsic marker of hypoxia remains controversial. Significant correlations have been found with both direct (by Eppendorf histography) and indirect measurements (using the bioreductive marker pimonidazole) of tumor hypoxia (Airley et al., 2001; Airley et al., 2003), although one later study found no correlation between direct oxygen measurements and Glut-1 expression detected in biopsies taken from along the oxygen electrode track (Mayer et al., 2005). This may reflect the presence of different populations of hypoxic cells, where oxygen electrodes detect both acutely and chronically hypoxic cells, but de novo synthesis of Glut-1 takes place only after chronic hypoxia via the HIF-1 transcription factor (Zhang et al., 1999). The depth and duration of hypoxia assumes importance when considering the treatment modality. For example, response to radiation therapy is now thought to be attenuated by acute hypoxia (Denekamp and Dasu, 1999), whereas outcome after surgery or chemotherapy may be more dependent upon changes in gene expression observed in chronically hypoxic cell populations. Therefore, a comprehensive validation of an intrinsic marker of hypoxia must consider correlations with clinical outcome data as well as other methods, in a variety of tumor subtypes receiving different treatment modalities. Inclusion of material representing these parameters in a TMA would facilitate this type of study. Another alternative approach in the search for hypoxia markers is the hypoxia metagene, where a gene signature composed of a wide range of hypoxia-inducible genes, e.g. Glut-1, CA9, and VEGF, is characterized using cDNA microarray analysis, and related to prognosis. This has shown potential in breast, head, and neck cancers (Winter et al., 2007). The expression pattern of Glut-1 in hypoxic tissue may also be dependent on tumor type, as well as other factors, including differentiation, proliferative potential, and oncogene expression. For example, in studies investigating Glut-1 expression in advanced carcinoma of the cervix, the typical architectural pattern representing chronic hypoxia was clearly apparent, where peri-necrotic, membranous Glut-1 staining became less intense and more cytoplasmic distal to patent blood vessels, which themselves were indicated by the presence of Glut-1 expressing erythrocytes (Airley et al., 2003). The change in subcellular location of Glut-1 from cytoplasmic to membranous expression reflects the depth and duration of hypoxia (Zhang et al., 1999), which itself may be of clinical significance in terms of the relative importance of acute and chronic hypoxia to predict treatment response and prognosis (Rofstad et al., 2007). In a different study of Glut-1 expression in oral squamous cell carcinoma, there were two distinct patterns of staining—either peri-necrotic, or in the basal and parabasal layers (Oliver et al., 2004). Therefore, although TMA’s are useful in that they contain multiple tumor types, and to provide data comparable to conventional immunohistochemical analysis of tumor hypoxia, they must also contain sufficient material to enable study of different spatial patterns of hypoxia marker staining.
Finally, in this study, no correlation was found between Glut-1 and grade of differentiation, which contradicts previous studies, where Glut-1 appears to correlate with the histological differentiation of lung (Ito et al., 1999), colon (Fogt et al., 2001), ovarian (Cantuaria et al., 2000), and breast (Younes et al., 1995) tumors. Although only a limited number of tumors were available for analysis based on differentiation, it is very unlikely that including additional tumors would have changed this, owing to the inclusion of samples from a wide range of histolopathological locations, which may have an impact on differentiation that is independent of necrosis and Glut-1 expression.
Overall, TMA’s have provided useful data on Glut-1 overexpression and spatial pattern of staining, which is comparable to previous studies and may be used in initial studies of novel biomarkers to provide rapid assessments of differential expression patterns between normal and malignant tissue, and to indicate hypoxia-inducible expression. However, to obtain statistically significant assessment of the prognostic significance of a biomarker, comprehensive annotation of TMA’s with clinical characteristics, as well as greater numbers of samples within a tumor type is necessary.
In our laboratory, we are currently validating Glut-1 as a novel therapeutic target. These data may prove useful in identifying tumor types that show differential expression between normal and malignant tissue, and therefore those that may benefit from any Glut-1-linked strategy.
Acknowledgement
This work was funded by the Association of International Cancer Research.
References
- Ahmed N, Berridge MV, 1997. Regulation of glucose transport by interleukin-3 in growth factor-dependent and oncogene-transformed bone marrow-derived cell lines. Leuk. Res 21, 609–618. [DOI] [PubMed] [Google Scholar]
- Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C, 2001. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin. Cancer Res 7, 928–934. [PubMed] [Google Scholar]
- Airley RE, Loncaster J, Raleigh JA, Harris AL, Davidson SE, Hunter RD, West CM, Stratford IJ, 2003. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104, 85–91. [DOI] [PubMed] [Google Scholar]
- Airley RE, Mobasheri A, 2007. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy 53, 233–256. [DOI] [PubMed] [Google Scholar]
- Airley RE, Phillips RM, Evans AE, Double J, Burger AM, Feibig HH, West CM, Stratford IJ, 2005. Hypoxia-regulated glucose transporter Glut-1 may influence chemosensitivity to some alkylating agents: results of EORTC (First Translational Award) study of the relevance of tumour hypoxia to the outcome of chemotherapy in human tumour-derived xenografts. Int. J. Oncol 26, 1477–1484. [DOI] [PubMed] [Google Scholar]
- Albertella MR, Loadman PM, Jones PH, Phillips RM, Rampling R, Burnet N, Alcock C, Anthoney A, Vjaters E, Dunk CR, Harris PA, Wong A, Lalani AS, Twelves CJ, 2008. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin. Cancer Res 14, 1096–1104. [DOI] [PubMed] [Google Scholar]
- Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Scholmerich J, Oefner PJ, Kreutz M, Bosserhoff AK, Hellerbrand C, 2009. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am. J. Pathol 174, 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Delage S, Mahraoui L, Cadoret A, Veissiere D, Taillemite JL, Chastre E, Gespach C, Zweibaum A, Capeau J, Brot-Laroche E, Cherqui G, 1996. Deregulation of hexose transporter expression in Caco-2 cells by ras and polyoma middle T oncogenes. Am. J. Physiol 270, G314–G323. [DOI] [PubMed] [Google Scholar]
- Behrooz A, Ismail-Beigi F, 1997. J. Biol. Chem 272, 5555–5562. [DOI] [PubMed] [Google Scholar]
- Binder C, Binder L, Marx D, Schauer A, Hiddemann W, 1997. Deregulated simultaneous expression of multiple glucose transporter isoforms in malignant cells and tissues. Anticancer Res. 17, 4299–4304. [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW, 1996. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 56, 941–943. [PubMed] [Google Scholar]
- Brown RS, Wahl RL, 1993. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer 72, 2979–2985. [DOI] [PubMed] [Google Scholar]
- Cantuaria G, Magalhaes A, Penalver M, Angioli R, Braunschweiger P, Gomez-Marin O, Kanhoush R, Gomez-Fernandez C, Nadji M, 2000. Expression of GLUT-1 glucose transporter in borderline and malignant epithelial tumors of the ovary. Gynecol. Oncol 79, 33–37. [DOI] [PubMed] [Google Scholar]
- Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A, 2001. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem 276, 9519–9525. [DOI] [PubMed] [Google Scholar]
- Chung FY, Huang MY, Yeh CS, Chang HJ, Cheng TL, Yen LC, Wang JY, Lin SR, 2009. GLUT1 gene is a potential hypoxic marker in colorectal cancer patients. BMC Cancer. 9, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Sarioglu S, Sokmen S, Fuzun M, Kupelioglu A, ValentineGorken IB, Airley R, West C, 2003. Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br. J. Cancer 89 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J, Dasu A, 1999. Inducible repair and the two forms of tumour hypoxia—time for a paradigm shift. Acta Oncol. 38, 903–918. [DOI] [PubMed] [Google Scholar]
- Fogt F, Wellmann A, Urbanski SJ, Noffsinger A, Poremba C, Zimmerman RL, Alsaigh N, 2001. Glut-1 expression in dysplastic and regenerative lesions of the colon. Int. J. Mol. Med 7, 615–619. [DOI] [PubMed] [Google Scholar]
- Furudoi A, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, 2001. Clinical significance of human erythrocyte glucose transporter 1 expression at the deepest invasive site of advanced colorectal carcinoma. Oncology 60, 162–169. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ, 2007. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br. J. Cancer 97, 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ, 1998. Role for glucose transporter 1 protein in human breast cancer. Pathol. Oncol. Res 4, 115–120. [DOI] [PubMed] [Google Scholar]
- Hall Eric J, 2000. Radiobiology for the Radiologist. Lippincott Williams & Wilkins, Philadelphia; London. [Google Scholar]
- Haneda M, Koya D, Kikkawa R, 2001. Mesangial cell dysfunction as a pathogenesis of diabetic nephropathy. Contrib. Nephrol, 16–29. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, 1974. Biochim. Biophys. Acta 355, 77–104. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P, 1996. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 56, 4509–4515. [PubMed] [Google Scholar]
- Hoskin PJ, Sibtain A, Daley FM, Wilson GD, 2003. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br. J. Cancer 89, 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Noguchi Y, Satoh S, Hayashi H, Inayama Y, Kitamura H, 1998. Expression of facilitative glucose transporter isoforms in lung carcinomas: its relation to histologic type, differentiation grade, and tumor stage. Mod. Pathol 11, 437–443. [PubMed] [Google Scholar]
- Ito T, Noguchi Y, Udaka N, Kitamura H, Satoh S, 1999. Glucose transporter expression in developing fetal lungs and lung neoplasms. Histol. Histopathol 14, 895–904. [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B, 2001. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol 18, 247–256. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Nishino H, Iwashima A, 1985. Analysis of hexose transport in untransformed and sarcoma virus-transformed mouse 3T3 cells by photoaffinity binding of cytochalasin B. Biochim. Biophys. Acta 821, 63–66. [DOI] [PubMed] [Google Scholar]
- Klip A, Paquet MR, 1990. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 13, 228–243. [DOI] [PubMed] [Google Scholar]
- Manel N, Kinet S, Kim FJ, Taylor N, Sitbon M, Battini JL, 2004. [GLUT-1 is the receptor of retrovirus HTLV]. Med. Sci. (Paris). 20, 277–279. [DOI] [PubMed] [Google Scholar]
- Mayer A, Hockel M, Wree A, Vaupel P, 2005. Microregional expression of glucose transporter-1 and oxygenation status: lack of correlation in locally advanced cervical cancers. Clin. Cancer Res 11, 2768–2773. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ, 2007. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol). 19, 427–442. [DOI] [PubMed] [Google Scholar]
- Medina RA, Owen GI, 2002. Biol. Res 35, 9–26. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Airley R, Foster CS, Schulze-Tanzil G, Shakibaei M, 2004. Post-genomic applications of tissue microarrays: basic research, prognostic oncology, clinical genomics and drug discovery. Histol. Histopathol 19, 325–335. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF, 1985. Sequence and structure of a human glucose transporter. Science 229, 941–945. [DOI] [PubMed] [Google Scholar]
- Niculescu-Duvaz I, 2002. Glufosfamide (Baxter Oncology). Curr. Opin. Investig. Drugs 3, 1527–1532. [PubMed] [Google Scholar]
- Oliver RJ, Woodwards RT, Sloan P, Thakker NS, Stratford IJ, Airley RE, 2004. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection; results of EORTC Translational Research Fund studies. Eur. J. Cancer 40, 503–507. [DOI] [PubMed] [Google Scholar]
- Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV, 2000. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem 275, 21797–21800. [DOI] [PubMed] [Google Scholar]
- Ozcan A, Shen SS, Zhai QJ, Truong LD, 2007. Expression of GLUT1 in primary renal tumors: morphologic and biologic implications. Am. J. Clin. Pathol 128, 245–254. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, 1991. Advances in cell biology of blood-brain barrier transport. Semin. Cell Biol 2, 419–426. [PubMed] [Google Scholar]
- Potter CP, Harris AL, 2003. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br. J. Cancer 89, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DB, 1994. Liver GLUT-1 expression: an enigma deepens. Hepatology. 19, 540–542. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Galappathi K, Mathiesen B, Ruud EB, 2007. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin. Cancer Res 13, 1971–1978. [DOI] [PubMed] [Google Scholar]
- Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD, 2003. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett. 193, 225–233. [DOI] [PubMed] [Google Scholar]
- Rudlowski C, Becker AJ, Schroder W, Rath W, Buttner R, Moser M, 2003. GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am. J. Clin. Pathol 120, 691–698. [DOI] [PubMed] [Google Scholar]
- Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M, 2001. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur. J. Cancer 37, 204–209. [DOI] [PubMed] [Google Scholar]
- Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E, 2004. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 64, 2627–2633. [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS, 2001. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol. Cell. Biol 21, 3436–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar R, Stroude EC, Inch WR, 1979. Cytotoxicity of glucose analogues in V79 multicell spheroids. In Vitro 15, 685–690. [DOI] [PubMed] [Google Scholar]
- Stewart GD, Gray K, Pennington CJ, Edwards DR, Riddick AC, Ross JA, Habib FK, 2008. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol. Rep 20, 1561–1567. [PubMed] [Google Scholar]
- Takata K, 1996. Glucose transporters in the transepithelial transport of glucose. J. Electron. Microsc. (Tokyo) 45, 275–284. [DOI] [PubMed] [Google Scholar]
- Teicher BA, 1994. Hypoxia and drug resistance. Cancer Metastasis Rev. 13, 139–168. [DOI] [PubMed] [Google Scholar]
- Thorens B, 1996. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am. J. Physiol 270, G541–G553. [DOI] [PubMed] [Google Scholar]
- Wardell SE, Ilkayeva OR, Wieman HL, Frigo DE, Rathmell JC, Newgard CB, McDonnell DP, 2009. Glucose metabolism as a target of histone deacetylase inhibitors. Mol. Endocrinol 23, 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, Shah KA, Cox GJ, Corbridge RJ, Homer JJ, Musgrove B, Slevin N, Sloan P, Price P, West CM, Harris AL, 2007. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 67, 3441–3449. [DOI] [PubMed] [Google Scholar]
- Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD, 2008. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol. Cancer Ther 7, 3546–3555. [DOI] [PubMed] [Google Scholar]
- Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R, 1995. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 15, 2895–2898. [PubMed] [Google Scholar]
- Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT, 1997. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer 80, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Zamora-Leon SP, Golde DW, ConchaII DW, Rivas CI, Delgado-Lopez F, Baselga J, Nualart F, Vera JC, 1996. Expression of the fructose transporter GLUT5 in human breast cancer. Proc. Natl. Acad. Sci. USA 93, 1847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Behrooz A, Ismail-Beigi F, 1999. Regulation of glucose transport by hypoxia. Am. J. Kidney Dis 34, 189–202. [DOI] [PubMed] [Google Scholar]