Abstract
OBJECTIVES:
Sepsis results in organ dysfunction caused by a dysregulated host response, in part related to the immune response of a severe infection. Mesenchymal stromal cells (MSCs) are known to modulate the immune response, and expression of stromal cell-derived factor-1 (SDF-1) regulates mobilization of neutrophils from the bone marrow. We are investigating the importance of MSC-derived SDF-1, and its role in promoting neutrophil function after the onset of cecal ligation and puncture (CLP)-induced sepsis. SDF-1 expression was silenced (shSDF-1) in MSCs, compared with control scrambled (shSCR) MSCs.
DESIGN:
Animal study and cell culture.
SETTING:
Laboratory investigation.
SUBJECTS:
BALB/c mice.
INTERVENTIONS:
Polymicrobial sepsis was induced by CLP. shSCR MSCs and shSDF-1 MSCs were delivered by tail vein injections to septic mice. The mice were assessed for survival, bacterial clearance, and the inflammatory response during sepsis in each of the groups. MSCs were also assessed for their ability to promote bacterial phagocytosis by neutrophils.
MEASUREMENTS AND MAIN RESULTS:
Injection of shSCR MSCs after the onset of sepsis led to an increase in mouse survival (70%) at 7 days, while survival of mice receiving shSDF-1 MSCs was significantly diminished (33%). The loss of survival benefit in mice receiving shSDF-1 MSCs was associated with less efficient bacterial clearance compared with shSCR MSCs. While shSCR MSCs, or their conditioned medium, were able to increase neutrophil phagocytosis of bacteria, this effect was significantly blunted with shSDF-1 MSCs. Assessment of peritoneal inflammation revealed that neutrophils were significantly increased and more immature in septic mice receiving shSDF-1 MSCs. This response was associated with hypocellularity and increased neutrophil death in the bone marrow of mice receiving shSDF-1 MSCs.
CONCLUSIONS:
MSC-derived SDF-1 expression enhances neutrophil function with increased phagocytosis, more efficient clearance of bacteria, and bone marrow protection from depletion of cellular reserves during sepsis.
Keywords: mesenchymal stromal cells, stromal cell-derived factor-1, sepsis, neutrophil maturity, bacterial clearance
INTRODUCTION
Sepsis is a complex and dynamic disease process, and is a leading cause of death in intensive care units (1). Sepsis is characterized by activation of the innate immune system, with a systemic inflammatory response to a severe infection (2). A crucial cell type in the innate response to sepsis is the neutrophil, a short lived cell that requires continual production in the bone marrow to supply a reservoir of mature neutrophils that can be released into the circulation at the onset of an infection (3). The high morbidity and mortality in sepsis has been attributed, in part, to derangements in the innate immune system (4, 5), including compromised neutrophil function.
Stromal cell-derived factor-1 (SDF-1) / chemokine (C-X-C motif) ligand 12 (CXCL12) is a key mediator of neutrophil homeostasis in the bone marrow. SDF-1 works predominantly through the C-X-C chemokine receptor type 4 (CXCR4), and initiates multiple cellular events including the recruitment and migration of inflammatory cells (6). In the healthy host, the SDF-1 / CXCR4 axis was originally identified for retention of neutrophils in the bone marrow (7). In contrast, SDF-1 / CXCR4 levels are reduced in the bone marrow during sepsis, and increased in peripheral tissue (spleen) (8), contributing to mobilization of bone marrow neutrophils that are critical for the host to clear an infection. Moreover, blocking SDF-1 / CXCL12 activity using antisera intraperitoneally resulted in reduced bone marrow release of granulocytes and increased mortality in murine polymicrobial sepsis (8), while systemic administration of a SDF-1 / CXCL12 peptide analogue along with antibiotic treatment improved outcome (9).
Recent initiatives for the treatment of sepsis include the investigation of cell-based therapies (10–13). Of particular interest are mesenchymal stromal cells (MSCs), an immune evasive population of cells (14) originally discovered in the bone marrow stromal compartment (15). A critical property of MSCs is modulation of the immune response to limit tissue injury during sepsis (16). While our laboratory and other groups have demonstrated that MSCs improve outcomes in the cecal ligation and puncture (CLP) model of polymicrobial sepsis in mice (17–22), much remains to be learned regarding the mechanism(s) by which MSCs control the immune response during sepsis. We are particularly interested in neutrophils, and propose that the expression of SDF-1 in MSCs plays an important role in the interaction of MSCs with neutrophils to improve the outcome in sepsis. While SDF-1 has been reported to be an important mediator of the MSC paracrine response for tissue repair (23), the role of MSC-derived SDF-1 in the therapeutic response of MSCs during sepsis, particularly as it applies to neutrophil function, is not known.
MATERIAL AND METHODS
Please see the Supplemental Material and Methods for details.
Study approval.
Studies using mice were carried out in accordance with the Public Health Service policy on the humane care and use of laboratory animals, and approved by the Institutional Animal Care and Use Committee (IACUC) of Brigham and Women’s Hospital.
Statistics.
For comparisons between two groups, we used Student’s unpaired t test. For analysis of more than two groups, one-way analysis of variance was used. When data were not normally distributed, non-parametric analyses were performed using Mann-Whitney U or Kruskal-Wallis testing, respectively. Comparisons of mortality were made by analyzing Kaplan-Meier survival curves, and then log-rank test to assess for differences in survival. Statistical significance was accepted at P<0.05.
RESULTS
Silencing of SDF-1 in MSCs leads to a loss of survival benefit and worse bacterial clearance in sepsis
Due to the important influence of SDF-1 (CXCL12) in neutrophil biology (8), and our interest in the interaction of MSCs with neutrophils, we assessed the level of SDF-1 in mouse bone-derived MSCs compared with lung fibroblasts, a control mesenchymal cell. Using quantitative real-time PCR (qRT-PCR), the level of SDF-1 mRNA was 5.7±0.9 fold higher in MSCs than fibroblasts (Fig. 1A). In addition, we exposed MSCs and fibroblasts to an inflammatory stimulus (E. coli lipopolysaccharide, LPS), and assessed SDF-1 in the cell supernatants. Supplemental Fig. 2 reveals secreted SDF-1 was significantly increased in the supernatants of MSCs exposed to LPS, compared with vehicle, at both 24 and 48 hours after stimulation. This increase in SDF-1 was not seen in lung fibroblasts exposed to LPS, with levels of SDF-1 being significantly lower in fibroblasts than MSCs. To further explore the importance of SDF-1 in MSCs during sepsis, we silenced SDF-1 using shRNA lentiviral constructs (Supplemental Material and Methods). Figure 1B demonstrates that silencing of SDF-1 resulted in decreased mRNA in cells, and decreased SDF-1 protein secreted into cell supernatants of MSCs (shSDF-1) compared with a scrambled control (shSCR). shSDF-1 and shSCR MSCs were next phenotyped using flow cytometry. shSDF-1 and shSCR MSCs showed comparable expression of mesenchymal markers, including CD73, CD105, CD90.2, CD29, CD44, and CD140b (Supplemental Fig. 1). In both lines of MSCs, a very high percentage of cells expressed Sca1, and a very low percentage expressed hematopoietic lineage markers, bone marrow lineage markers (Supplemental Table 1), or major histocompatibility complex II.
Figure 1.
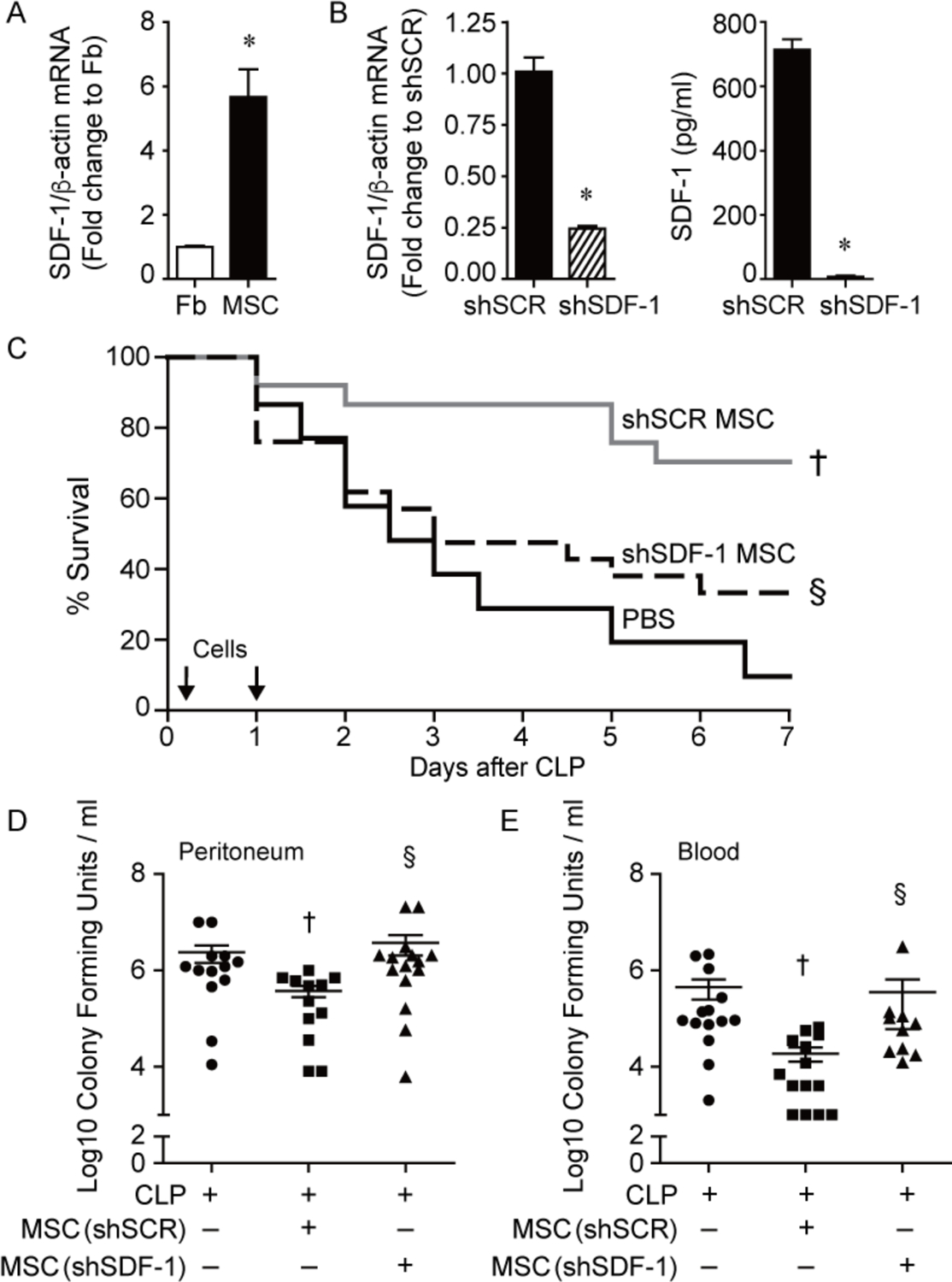
Improved survival and bacterial clearance by MSCs during polymicrobial sepsis is lost after silencing SDF-1. A) RNA was extracted from mouse lung fibroblasts (Fb, white bar) and bone-derived MSCs (black bar) and quantitative real-time PCR (qRT-PCR) was performed for SDF-1. Data are presented as fold change in RNA levels of SDF-1 normalized to β-actin, mean ± SEM, n=6 per group from three independent experiments. * versus Fb, P=0.0003. B) RNA was extracted from shSCR MSCs (black bar) and shSDF-1 MSCs (striped bar), and qRT-PCR was performed for levels of SDF-1. Data are presented as fold change in RNA levels of SDF-1 normalized to β-actin, mean ± SEM, n=9 per group from three independent experiments. * versus shSCR, P<0.0001. ELISAs for SDF-1 were also performed on cell culture supernatants of shSCR and shSDF-1 MSCs. * versus shSCR, P<0.0001. C) Septic BALB/c mice were randomly separated into 3 groups: PBS control (black solid line, n=15), shSCR MSCs (gray solid line, n=25), and shSDF-1 MSCs (black dashed line, n=25). All mice were subjected to CLP, and 2 hours after CLP the mice received PBS or cells (5 × 105) via tail vein injection. This treatment was also repeated at 24 hours (2.5 × 105) after CLP. Survival of mice was monitored over 7 days, and data are presented as Kaplan-Meier survival curves. † shSCR MSCs versus PBS, P=0.0006; § shSDF-1 versus shSCR, P=0.01. Bacteria were assessed in the peritoneum (D) and blood (E) in the PBS control (circles, n=13 or 14 respectively), shSCR MSCs (squares, n=12 or 14 respectively), and shSDF-1 MSCs (triangles, n=15 or 11 respectively). P=0.0077 and P=0.0005 respectively, with significant comparisons † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs.
We next assessed the therapeutic impact of SDF-1 on MSC function in vivo. After CLP-induced sepsis, mice received vehicle (PBS), shSCR MSCs, or shSDF-1 MSCs. MSCs (5 × 105 cells/200 μL PBS) or vehicle (PBS 200 μL) were administered intravenously 2 hours after CLP, and then again 24 hours after CLP (2.5 × 105 cells/200 μL PBS or PBS 200 μL), and survival was assessed over 7 days. Mice treated with PBS alone had a dramatic death response to CLP, with 10% survival (Fig. 1C). Injection of shSCR MSCs led to a dramatic increase in mouse survival (70%), while the survival of mice receiving shSDF-1 MSCs was significantly diminished (33%). Assessment of organ injury revealed mice receiving shSDF-1 MSCs or PBS after CLP had a similar increase in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) compared with mice receiving sham surgery, while mice receiving shSCR MSCs after CLP had a blunted increase in ALT and AST (Supplemental Fig. 3A). Plasma creatinine (Cr) was also increased in mice receiving shSDF-1 MSCs or PBS after CLP, but in mice receiving shSCR MSCs the level of Cr did not reach significance compared with sham mice (Supplemental Fig. 3B). These data showed worse liver and kidney injury in septic mice receiving MSCs silenced for SDF-1 or PBS, compared with mice receiving shSCR MSCs. In the lung, mice receiving shSCR MSCs after the onset of sepsis had very little infiltration of Ly6G-positive (+) neutrophils (Supplemental Fig. 3C–D), while mice receiving shSDF-1 MSCs had significant neutrophil infiltration, comparable with mice receiving PBS.
Bacterial clearance was also assessed at 24 hours after CLP. Mice in all sepsis groups demonstrated bacteria in the peritoneum and blood. The administration of shSCR MSCs after the onset of sepsis resulted in a significant decrease in bacteria in both the peritoneum and the blood compared with the PBS group (Fig. 1D and 1E). In contrast, mice receiving shSDF-1 MSCs had significantly higher numbers of bacteria in the peritoneum and blood compared with mice receiving shSCR MSCs, analogous to mice receiving PBS alone.
SDF-1 is important for the ability of MSCs to promote neutrophil phagocytosis
To better understand bacterial clearance, we performed bacterial phagocytosis assays using peritoneal neutrophils in conjunction with MSCs (± silencing SDF-1) or PBS. For these assays we used Gram-stain negative (E. coli) and Gram-stain positive (E. faecalis) bacteria, both prevalent in the cecum of mice (24). Exposure of neutrophils to shSCR MSCs led to an increase in the phagocytosis of E. coli (Fig. 2A) and E. faecalis (Fig. 2B), and this response was significantly less in neutrophils exposed to shSDF-1 MSCs. To determine whether the actions of SDF-1 required direct cell-to-cell interactions of MSCs with neutrophils at the site of injury, or the potential of a paracrine route, we performed in vivo tracking studies using bioluminescence to localize MSCs after CLP-induced sepsis. The images revealed an overwhelming majority of cells (both shSDF-1 and shSCR MSCs) remained in the thoracic cavity 36 hours after injection (Supplemental Fig. 4A), suggesting paracrine effects of MSCs contribute to their actions. To further confirm this at the cellular level, we fluorescently labeled shSCR and shSDF-1 MSCs (Supplemental Fig. 4B), and then injected the cells intravenously 2 hours after the onset of CLP-induced sepsis. At 36 hours the mice were sacrificed, and bone marrow was harvested and a cytospin of cells performed. Supplemental Fig. 4C shows no evidence of increased fluorescent cells in the bone marrow of mice receiving shSCR and shSDF-1 MSCs, compared with mice receiving no MSCs. These data suggest that the injected MSCs in each group did not reach the bone marrow at 36 hours after CLP.
Figure 2.
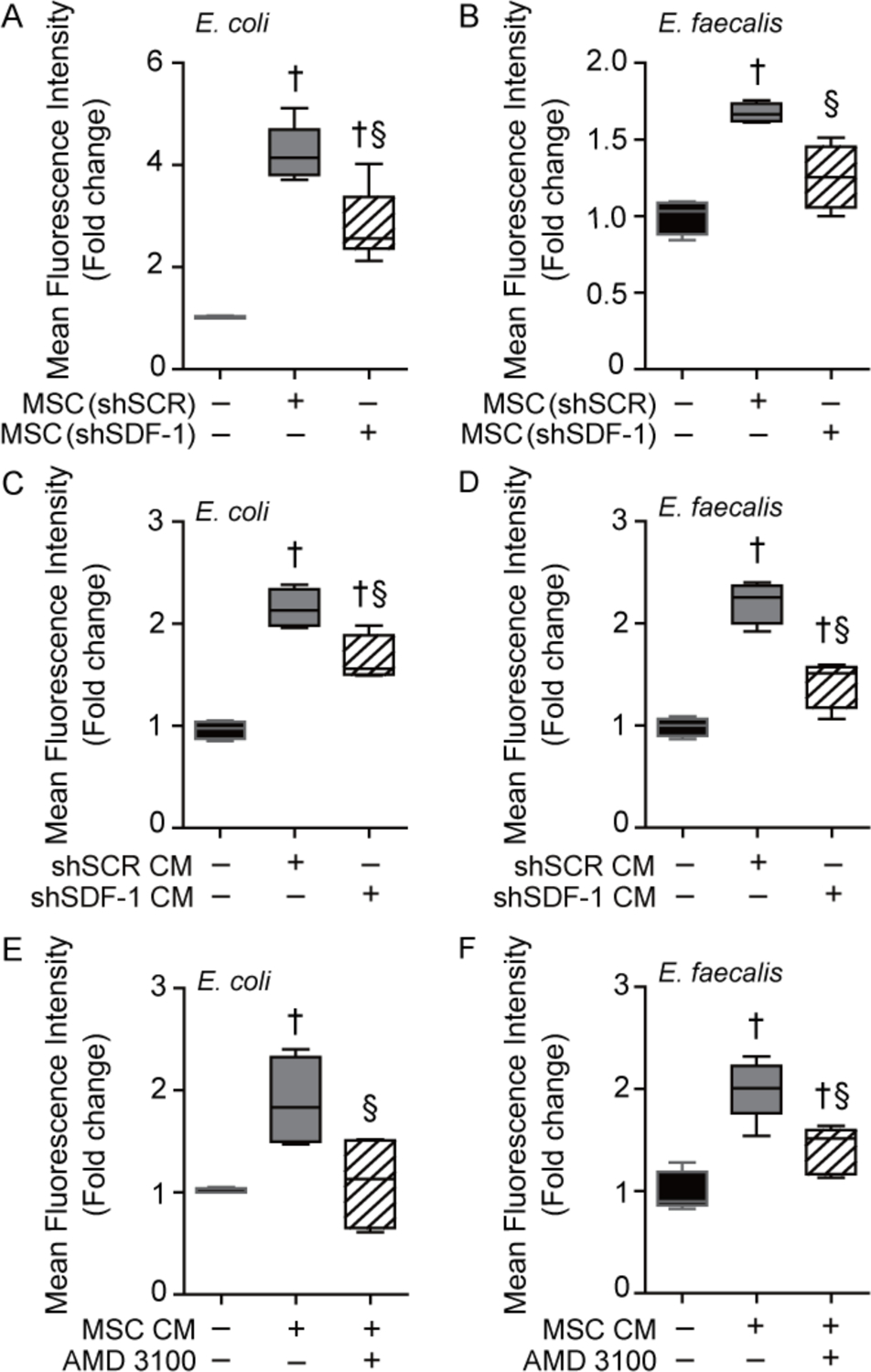
SDF-1 is important for the paracrine actions of MSC to enhance neutrophil phagocytosis. Isolated peritoneal neutrophils were incubated with GFP-labeled E. coli (A) or E. faecalis (B) in the presence of no MSCs (black bars, n=4 in each group), shSCR MSCs (gray bars, n=5 or 4 respectively), or shSDF-1 MSCs (striped bars, n=6 or 4 respectively). P<0.0001 and P=0.0003 respectively, with significant comparisons † versus E. coli (− MSCs), § versus E. coli+shSCR MSCs. Next, isolated peritoneal neutrophils were incubated with GFP-labeled E. coli (C) or E. faecalis (D) in the presence of no conditioned medium (− CM, black bars, n=4), CM from shSCR MSCs (gray bars, n=4), or CM from shSDF-1 MSCs (striped bars, n=4). Neutrophils were also incubated with GFP-labeled E. coli (E) or E. faecalis (F) in the presence of no CM (− CM, black bars, n=4 or 5 respectively), CM from wild-type MSCs (gray bars, n=4 or 5 respectively), or wild-type MSC CM in the presence of AMD 3100, an antagonist of CXCR4 (striped bars, n=4 or 5 respectively). For (C) P<0.0001, (D) P<0.0001, (E) P=0.0165, and (F) P<0.0001, significant comparisons † versus no CM, and § versus CM from shSCR MSCs or no AMD 3100.
To confirm paracrine actions of MSCs, phagocytosis assays were carried out using peritoneal neutrophils exposed to conditioned medium (CM) from shSCR or shSDF-1 MSCs. CM from shSCR MSCs significantly increased the phagocytosis of both E. coli and E. faecalis by neutrophils, however this response was blunted in neutrophils exposed to CM from shSDF-1 MSCs (Fig. 2C and 2D respectively). When neutrophils were exposed to an antagonist (AMD 3100) of CXCR4, the main receptor of SDF-1, the enhanced phagocytic response was attenuated in the presence of CM from wild-type MSCs (Fig. 2E and 2F). Moreover, when recombinant SDF-1α was added to the conditioned medium of wild-type MSCs, this led to a further increase in the phagocytosis of E. coli and E. faecalis (Supplemental Fig. 5A–B) by neutrophils. These data verify that SDF-1 in part contributes to the paracrine actions of MSCs to promote neutrophil phagocytosis. Interestingly, exposure of neutrophil to SDF-1α in the absence of MSC conditioned medium did not result in enhanced neutrophil phagocytosis (Supplemental Fig. 5A–B). This suggests that SDF-1 in conjunction with other paracrine factors produced by shSCR MSCs, but not shSDF-1 MSCs, are necessary for the improved phagocytosis response by neutrophils.
Persistent recruitment of neutrophils from the bone marrow, and increased neutrophil death, foster bone marrow hypocellularity in mice receiving shSDF-1 MSCs during sepsis
We next evaluated inflammatory cells in the peritoneum at 24 hours after CLP-induced sepsis. All groups of mice undergoing CLP had increased white blood cells (WBCs) in the peritoneum compared with sham mice (Supplemental Fig. 6A). Mice receiving shSCR MSCs had a trend toward lower total WBCs compared with mice receiving shSDF-1 MSCs or PBS, although this difference was not statistically significant. However, there were significantly fewer Ly6G+ neutrophils in mice receiving shSCR MSCs after CLP, compared with mice receiving shSDF-1 MSCs or PBS (Fig. 3A). In contrast, there was no difference in F4/80+ macrophages (Supplemental Fig. 6B). We also performed a manual count of myeloid cells in the peritoneum (Supplemental Fig. 7A). There was heterogeneity in the maturity of neutrophils in mice after CLP, with evidence of immature myelocytes, metamyelocytes, and banded neutrophils, in addition to mature neutrophils. Even though there were fewer neutrophils in the peritoneum of mice receiving shSCR MSCs, the percentage of mature neutrophils was greater than in the shSDF-1 or PBS groups (Fig. 3B). Mice receiving shSDF-1 MSCs or PBS after CLP showed not only increased numbers of Ly6G+ neutrophils, but also more immature myeloid cells compared with mice receiving shSCR MSCs (Supplemental Fig. 7B).
Figure 3.
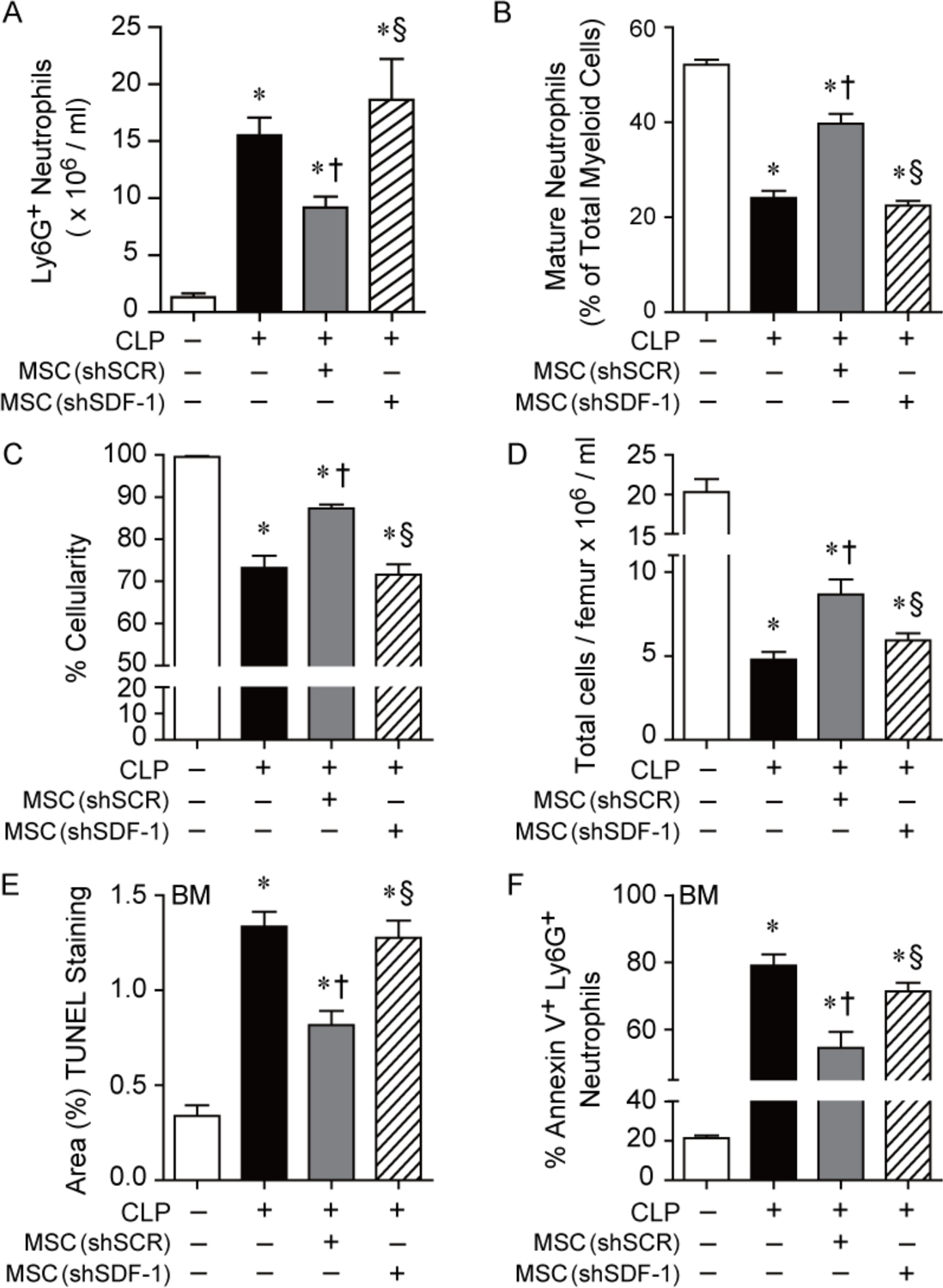
Administration of shSDF-1 MSCs to mice during sepsis leads to more total peritoneal neutrophils, a less mature phenotype and bone marrow hypocellularity, than mice receiving shSCR MSCs. A) Mice were subjected to Sham or CLP surgery, and mice receiving CLP were randomly separated into 3 groups: PBS control (black bar, n=5), shSCR MSCs (gray bar, n=5), and shSDF-1 MSCs (striped bar, n=5). Mice received PBS or cells (5 × 105) via tail vein injection 2 hours after CLP. The peritoneal fluid was assessed by flow cytometry for Ly6G+ neutrophils. P=0.0003, with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs. B) Mice were subjected to Sham or CLP surgery, and 2 hours after CLP the mice received PBS or cells (5 × 105) via tail vein injection. Manual assessment of mature neutrophils from cytospins was assessed as a % of total myeloid cells. Sham (white bar, n=9), PBS (black bar, n=14), shSCR MSCs (gray bar, n=15), and shSDF-1 MSCs (striped bar, n=16). P<0.0001, with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs. C) Bone marrow cellularity (%) and D) total number of cells was quantitated from mice undergoing Sham (white bar, n=5 in each assay), PBS control (black bar, n=5 or 6 respectively), shSCR MSCs (gray bar, n=5 in each assay), and shSDF-1 MSCs (striped bar, n=5 or 6 respectfully). P<0.0001 for (C) and (D), with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs. E) Bone marrow TUNEL staining (% area) and F) flow cytometry for % Annexin V+ Ly6G+ neutrophils in the bone marrow was quantitated from mice undergoing Sham (white bar, n=3 or 5 respectively), PBS control (black bar, n=5 in each assay), shSCR MSCs (gray bar, n=5 in each assay), and shSDF-1 MSCs (striped bar, n=5 in each assay). P<0.0001 for (E) and (F), with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs.
To evaluate a critical source of immune cells after an infectious insult, we investigated the bone marrow. Hematoxylin and eosin (H&E) staining of femurs from mice 24 hours after CLP showed decreased cellularity of the bone marrow in all sepsis groups, compared with sham mice (Supplemental Fig. 7C). However, mice receiving shSDF-1 MSCs or PBS after CLP-induced sepsis demonstrated even more hypocellularity (% area) than mice receiving shSCR MSCs (Fig. 3C). To confirm the decreased cellularity of the bone marrow, femurs of mice were flushed, and cellularity was assessed (after removal of RBCs) by automated counting. Figure 3D demonstrates decreased cellularity of the bone marrow in mice receiving shSDF-1 MSCs or PBS, compared with mice receiving shSCR MSCs, after the onset of sepsis. While recruitment of cells out of the bone marrow during sepsis contributes to the depletion of cell reserves, we also assessed cell death. TUNEL staining of femurs 24 hours after CLP showed increased TUNEL-positive staining in the bone marrow of mice receiving shSDF-1 MSCs or PBS, compared with mice receiving shSCR MSCs (Fig. 3E). To specifically determine cell death in the Ly6G+ population of the bone marrow, flow cytometry was performed (gating strategy, Supplemental Fig. 8) and revealed increased apoptosis in neutrophils (Annexin V+ Ly6G+) of septic mice receiving shSDF-1 or PBS, compared with shSCR MSCs (Fig. 3F).
We subsequently assessed granulocyte colony-stimulating factor (G-CSF) in response to CLP-induced sepsis. Previous studies have shown that increased expression of G-CSF is responsible for the dramatic increase in neutrophil production, and the mobilization of neutrophils from the bone marrow in part through decreasing expression of SDF-1 during infection (25–31). Assessment of livers, kidneys, and lungs demonstrated that G-CSF mRNA was increased after CLP-induced sepsis compared with Sham mice (Fig. 4A–C respectively). In septic mice receiving shSDF-1 MSCs or PBS, the levels of G-CSF were increased compared with mice receiving shSCR MSCs. This translated into markedly higher plasma levels of G-CSF protein in mice receiving shSDF-1 MSCs or PBS, compared with mice receiving shSCR MSCs, during sepsis (Fig. 4D). Furthermore, immunostaining for SDF-1 in femurs/bone marrow of mice demonstrated the highest levels of SDF-1 in Sham mice. After CLP-induced sepsis, cells staining for SDF-1 were significantly reduced in mice receiving shSDF-1 MSCs or PBS, while mice receiving shSCR MSCs showed increased SDF-1 staining, comparable to Sham mice (Fig. 4E and Supplemental Fig. 9A). In contrast to bone/bone marrow expression of SDF-1, plasma levels of SDF-1 were not different between the groups of mice after CLP-induced sepsis (Supplemental Fig. 9B).
Figure 4.
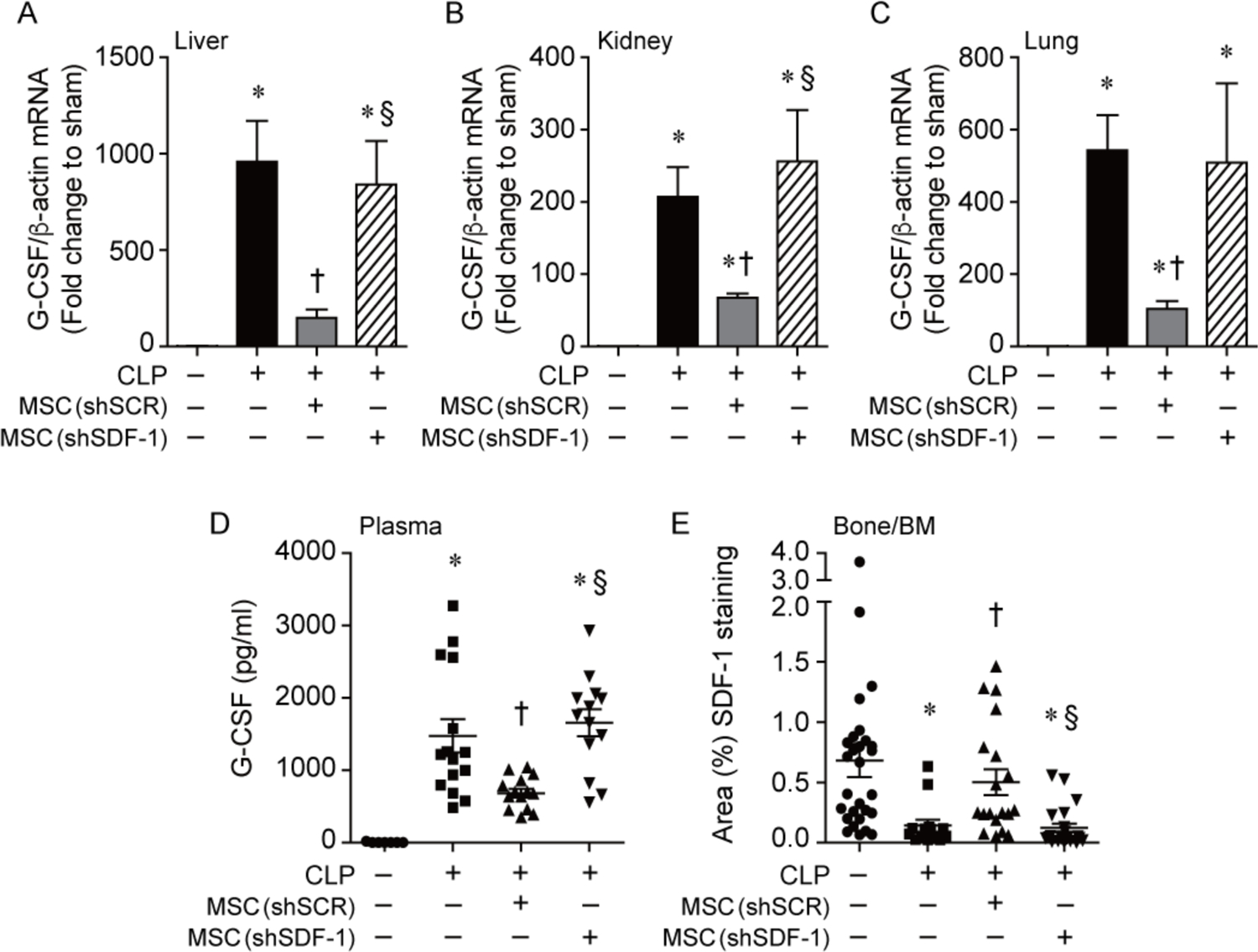
Administration of shSCR MSCs to mice during CLP-induce sepsis leads to a marked reduction in G-CSF, which is lost in mice receiving shSDF-1 MSCs. Mice were subjected to CLP, and 2 hours after CLP the mice received PBS or cells (5 × 105) via tail vein injection. At 24 hours after CLP, tissues were harvested and RNA extracted, and quantitative real-time PCR was performed for levels of G-CSF in livers (A), kidneys (B) and lungs (C). Data are presented as fold change in RNA levels of G-CSF normalized to β-actin (mean±SEM). Sham (white bars, n=4), PBS control (black bars, n=8–9), shSCR MSCs (gray bars, n=9–10), and shSDF-1 MSCs (striped bar, n=9–10). P<0.0001 in livers, kidneys, and lungs, with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs. D) G-CSF protein levels were also assessed in plasma at 24 hours after CLP in Sham (circles, n=7), PBS control (squares, n=15), shSCR MSCs (upward triangles, n=14), and shSDF-1 MSCs (downward triangles, n=13). Data are represented as pg/ml. P<0.0001 with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs. E) The area of SDF-1 positively stained cells was calculated per 20X objective of femur/bone marrow (Bone/BM) tissue, and random fields from 3 independent experiments were assessed per tissue section of Sham (circles, n=28), CLP+PBS (squares, n=14), CLP+ shSCR MSCs (upward triangles, n=19) and shSDF-1 MSCs (downward triangles, n=22). Data are represented as mean±SEM. P<0.0001, with significant comparisons * versus Sham, † versus CLP+PBS (− MSCs), § versus CLP+shSCR MSCs.
MSCs are able to enhance phagocytosis of not only mature, but also immature neutrophils
Finally, using a mouse myeloid cell line (MPRO), which exhibits promyelocyte characteristics, we differentiated these cells into immature or mature neutrophils (Fig. 5A) and assessed the impact of conditioned medium from MSCs (shSDF-1 or shSCR) on neutrophil function. Figure 5B demonstrates that immature neutrophils (left panel) overall had less ability to phagocytize bacteria (E. coli) compared with mature neutrophils (right panel). However, in both immature and mature neutrophils, conditioned medium from shSCR MSCs was able to increase bacterial phagocytosis in vitro. This improvement in both immature and mature neutrophil phagocytic function was completely lost when conditioned medium from shSDF-1 MSCs was used (Fig. 5B). We also assessed bacterial killing by neutrophils exposed to shSCR or shSDF-1 conditioned media. Overall, bacterial killing was decreased in immature compared with mature neutrophils. However, in contrast to phagocytosis, conditioned medium from shSCR MSCs did not have an effect on the killing response of neutrophils compared with conditioned medium from shSDF-1 MSCs, or in the absence of conditioned medium (Fig. 5C).
Figure 5.

Paracrine actions of MSCs are able to enhance phagocytosis of immature neutrophils, and this response is abolished by silencing SDF-1. MPRO cells were left uninduced, or induced by all-transretinoic acid (ATRA, 10 μM) into immature (1 day) or mature (3 days) neutrophils. A) Cytospins were performed and stained with Hema 3 to assess cell morphology (top row). Flow cytometry was also performed to demonstrate the increasing expression of Ly6G as the cells matured (bottom row). B) MPRO cells were induced to become immature (left panel) or mature (right panel) neutrophils, and incubated with GFP-labeled E. coli in the presence of no MSC CM (n=3 in both groups), shSCR MSC CM (n=4 in both groups), or shSDF-1 MSC CM (n=4 in both groups). P<0.0001 and P=0.0035 in immature and mature cells respectfully, with significant comparisons * versus no MSC CM, † versus shSCR MSC CM. C) MPRO cells were induced to become immature (left panel) or mature (right panel) neutrophils as in (B). P=0.1864 and P=0.2686 in immature and mature cells respectfully, NS= not significant.
DISCUSSION
Neutrophils serve as a first line of defense to combat infectious insults. During this process, neutrophils are recruited to the site of infection, and after they exert their antimicrobial actions the majority of these cells undergo cell death (32). When the bacterial infection is controlled, resolution of inflammation occurs (33, 34), which allows a return to homeostasis and prevents collateral organ injury. Sepsis has recently been redefined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (35), and failure to control invading pathogens will result in an impaired response (36) including sustained inflammation and a prolonged neutrophil lifespan (37).
Inhibition of the CXCL12 (SDF-1)–CXCR4 axis in the bone marrow plays a critical role in the egression of neutrophils out of the bone marrow (27), thus we wanted to define the role of MSC–derived SDF-1 during sepsis, and its impact on the neutrophil response. Silencing of SDF-1 (shSDF-1) in MSCs resulted in a loss of the therapeutic benefit of these cells during CLP-induced sepsis (Fig. 1A–C and Supplemental Fig. 3), with greater mortality and worse organ dysfunction than mice receiving MSCs with intact SDF-1 (shSCR MSCs). Along with a worse outcome, mice receiving shSDF-1 MSCs during sepsis have higher peritoneal and blood bacterial counts than mice receiving shSCR MSC (Fig. 1D–E). While MSCs enhanced phagocytosis of both gram-negative (E. coli) and gram-positive (E. faecalis) bacteria by neutrophils, this enhanced phagocytosis (via cell-to-cell or paracrine actions) was blunted when SDF-1 was silenced in MSCs or by blocking CXCR4 on neutrophils (Fig. 2E–F). This altered phagocytic response likely contributed to the higher level of bacteremia and mortality.
During a severe systemic bacterial infection, circulating leukocytes are increased with a predominant neutrophilia, as the large storage pool of mature neutrophils are rapidly mobilized from the bone marrow (38). If the bacterial infection persists, the consumption of neutrophils remains high and the existing reservoir of mature cells are inadequate to control the infection (39). Thus, immature neutrophil precursor cells are released into the circulation to help fight the infection (27). However, increased numbers of circulating immature neutrophils are associated with an increased risk of death in septic shock (40), and their ability to phagocytize bacteria and promote bacterial killing is less efficient than mature neutrophils (41). In the present study, assessment of inflammatory cells in the peritoneum revealed fewer, and more mature neutrophils in the peritoneum of mice receiving shSCR MSCs after CLP-induced sepsis (Fig. 3A–B), while mice receiving shSDF-1 MSCs had more neutrophils and increased numbers of immature neutrophil precursors, including myelocytes, metamyelocytes and banded neutrophils (Supplemental Fig. 7A–B). These data suggested a prolonged inflammatory response in the presence of shSDF-1 MSCs in the setting of uncontrolled infection. Even though the phagocytic activity of immature neutrophils, and their ability to kill bacteria, were decreased compared with mature neutrophils (Fig. 5), shSCR MSCs were capable of improving phagocytic function of immature neutrophils, and this effect was lost with shSDF-1 MSCs.
To further investigate the immune response, we examined the reaction of the bone marrow to the systemic infection. The bone marrow itself appeared hypocellular (Fig. 3C), with fewer total cells (Fig. 3D). Moreover, flow cytometric analysis revealed more apoptotic Ly6G+ neutrophils (Fig. 3F) in septic mice receiving shSDF-1 MSCs, contributing to bone marrow hypocellularity. It has been suggested that tissue endothelial cells are sensors of systemic infections, and are important for the propagation of pathogen signals into G-CSF driven granulopoiesis in the bone marrow (42). Moreover, administration of G-CSF downregulates SDF-1 in the bone/bone marrow and CXCR4 in myeloid cells of the bone marrow (30, 31), allowing mobilization of cells. Organs important for production of G-CSF include the liver, kidneys, and lungs – all of which were injured or displayed an inflammatory response during CLP-induced sepsis (Supplemental Fig. 3). Compared with mice receiving shSDF-1 MSCs or PBS, the mice receiving shSCR MSCs have a marked reduction in G-CSF mRNA in these organs, decreased G-CSF total protein in the plasma, and increased expression of SDF-1 in the femur/bone marrow (Fig. 4 and Supplemental Fig. 9A). This G-CSF response was consistent with more efficient pathogen clearance (27) and resolution of peritoneal inflammation in mice receiving shSCR MSCs, while mice receiving shSDF-1 MSCs have persistent peritoneal and bloodstream bacteremia, analogous to mice receiving PBS (Fig. 1D–E). We believe that the level of SDF-1 expression in the bone/bone marrow was a reflection of bacterial clearance and G-CSF production in the different groups, and not directly related to the production and secretion of SDF-1 by MSCs.
CONCLUSION
In the present study, we demonstrated that treatment with shSCR MSCs during sepsis resulted in improved phagocytosis of bacteria by neutrophils, both mature and immature cells, resulting in more efficient clearance of bacteria. Thus, the stimulus for persistent granulopoiesis (G-CSF) was alleviated, and fewer immature cells were released from the bone marrow and present at the site of infection (peritoneum). In addition, shSCR MSCs protected Ly6G+ neutrophils from death in the bone marrow during sepsis. All of these actions of shSCR MSCs allowed improved survival and a return of the inflammatory response to homeostasis. In contrast, neutrophils of mice receiving shSDF-1 MSCs during sepsis have less efficient phagocytosis and persistent bacteremia, resulting in continued consumption of mature neutrophils and death of Ly6G+ cells in the bone marrow. The continued granulopoietic response, and the presence of more immature neutrophils from myeloid precursors in the peritoneum, further contributed to persistent bacteremia and death in the mice. These data support the importance of SDF-1 for an efficacious MSC response and improved neutrophil function during sepsis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bonna Ith for his assistance with tissue processing, embedding, and sectioning for histology and immunostaining analyses.
Financial support: This work was supported by National Institutes of Health grants R01GM118456 and P01HL108801 (to M.A. Perrella), U01AI38318 (to M.A. Perrella and J.A. Lederer), K08GM126313 (to S. Ghanta), and T32HL007633 (to S. Ghanta and J. Ng).
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369(9):840–851. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;35:63–78. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N Neutrophils, from marrow to microbes. Immunity 2010;33(5):657–670. [DOI] [PubMed] [Google Scholar]
- 4.Danikas DD, Karakantza M, Theodorou GL, et al. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 2008;154(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature reviews Immunology 2013;13(12):862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006;20(11):1915–1924. [DOI] [PubMed] [Google Scholar]
- 7.Strydom N, Rankin SM. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 2013;5(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delano MJ, Kelly-Scumpia KM, Thayer TC, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol 2011;187(2):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan S, Guo C, Zingarelli B, et al. Combined treatment with a CXCL12 analogue and antibiotics improves survival and neutrophil recruitment and function in murine sepsis. Immunology 2014. [DOI] [PMC free article] [PubMed]
- 10.McIntyre LA, Stewart DJ, Mei SHJ, et al. Cellular Immunotherapy for Septic Shock (CISS): A Phase I Clinical Trial. American journal of respiratory and critical care medicine 2017;197(3):337–347. [DOI] [PubMed] [Google Scholar]
- 11.Kusadasi N, Groeneveld AB. A perspective on mesenchymal stromal cell transplantation in the treatment of sepsis. Shock 2013;40(5):352–357. [DOI] [PubMed] [Google Scholar]
- 12.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014;2(12):1016–1026. [DOI] [PubMed] [Google Scholar]
- 13.Wannemuehler TJ, Manukyan MC, Brewster BD, et al. Advances in Mesenchymal Stem Cell Research in Sepsis. J Surg Res 2011;173(1):113–126. [DOI] [PubMed] [Google Scholar]
- 14.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 2014;32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974;17(4):331–340. [DOI] [PubMed] [Google Scholar]
- 16.Keating A Mesenchymal stromal cells: new directions. Cell Stem Cell 2012;10(6):709–716. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009;58(7):929–939. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am J Respir Crit Care Med 2010;182(8):1047–1057. [DOI] [PubMed] [Google Scholar]
- 20.Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 2012;302(10):L1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall SR, Tsoyi K, Ith B, et al. Mesenchymal Stromal Cells Improve Survival During Sepsis in the Absence of Heme Oxygenase-1: The Importance of Neutrophils. Stem Cells 2012;31(2):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsoyi K, Hall SR, Dalli J, et al. Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators. Critical care medicine 2016;44(12):e1236–e1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Q, Zhang B, Kuang D, et al. Role of Stromal-Derived Factor-1 in Mesenchymal Stem Cell Paracrine-Mediated Tissue Repair. Curr Stem Cell Res Ther 2016;11(7):585–592. [DOI] [PubMed] [Google Scholar]
- 24.Chung SW, Liu X, Macias AA, et al. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 2008;118:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheers C, Haigh AM, Kelso A, et al. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infection and immunity 1988;56(1):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami M, Tsutsumi H, Kumakawa T, et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood 1990;76(10):1962–1964. [PubMed] [Google Scholar]
- 27.Manz MG, Boettcher S. Emergency granulopoiesis. Nature reviews Immunology 2014;14(5):302–314. [DOI] [PubMed] [Google Scholar]
- 28.Selig C, Nothdurft W. Cytokines and progenitor cells of granulocytopoiesis in peripheral blood of patients with bacterial infections. Infection and immunity 1995;63(1):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watari K, Asano S, Shirafuji N, et al. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood 1989;73(1):117–122. [PubMed] [Google Scholar]
- 30.Kim HK, De La Luz Sierra M, Williams CK, et al. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood 2006;108(3):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 2005;106(9):3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 33.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 2014;76:467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013;13(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen XF, Cao K, Jiang JP, et al. Neutrophil dysregulation during sepsis: an overview and update. Journal of cellular and molecular medicine 2017;21(9):1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008;125(3):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. Journal of leukocyte biology 1994;56(6):672–686. [DOI] [PubMed] [Google Scholar]
- 40.Demaret J, Venet F, Friggeri A, et al. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. Journal of leukocyte biology 2015;98(6):1081–1090. [DOI] [PubMed] [Google Scholar]
- 41.Drifte G, Dunn-Siegrist I, Tissieres P, et al. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Critical care medicine 2013;41(3):820–832. [DOI] [PubMed] [Google Scholar]
- 42.Boettcher S, Gerosa RC, Radpour R, et al. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 2014;124(9):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


