Abstract
More than half of patients undergoing hematopoietic cell transplantation at our institution are ethnic or racial minorities, making the search for matched unrelated donors more challenging. Since the introduction of haploidentical bone marrow transplant (haplo-BMT) into our pediatric BMT program in 2015, 69.2% of the recipients have been minorities. Herein, we describe our experience with the first thirteen pediatric and young adult patients with hematologic malignancies who have undergone T-cell replete haplo-BMT following myeloablative conditioning (MAC) at our institution. We have previously documented that in experimental haplo-BMT, post-transplant bendamustine (PT-BEN) is at least as effective as post-transplant cyclophosphamide (PT-CY) against graft versus host disease (GvHD) and elicits superior graft versus leukemia (GvL) effects. We report on, for the first time in humans, four patients treated with PT-CY with PT-BEN (PT-CY/BEN) following haplo-BMT as part of our ongoing institutional phase I/II study (NCT02996773). The remaining nine patients reviewed in this report received PT-CY. Our findings indicate that MAC haplo-BMT is well-tolerated by children and young adults with advanced hematologic malignancies with no observed non-relapse mortality (NRM) or grade III-IV GvHD. All patients who underwent haplo-BMT remain alive and disease-free with a median follow-up of 15.6 months (1.5-31.2 months). Preliminary findings from our ongoing clinical trial demonstrate that partial substitution of PT-BEN for PT-CY is feasible and safe following haplo-BMT as an immune modulatory strategy to alleviate GvHD and potentially more effectively preserve GvL.
Keywords: haploidentical BMT, PT-cyclophosphamide, PT-bendamustine
Introduction
Haploidentical hematopoietic cell transplantation (haplo-HCT) has emerged as a safe and effective treatment for patients with hematologic malignancies. The most widely used approach, particularly in North America, is T-cell replete haplo-HCT with post-transplant cyclophosphamide (PT-CY) 1–5. While there have been numerous reports, of haplo-HCT with PT-CY in adults, there have been very limited primarily pediatric publications to date 6–8. Jaiswal et al. reported on the use of PT-CY following a chemotherapy based myeloablative conditioning (MAC) regimen and unmanipulated peripheral blood hematopoietic cell transplant in pediatric leukemia patients in India7. Berger et al. described their pediatric haploidentical bone marrow transplantation (haplo-BMT) experience from five Italian centers, with 58% of patients receiving MAC and the remaining reduced intensity conditioning (RIC), all followed by PT-CY6. Klein et al. from Johns Hopkins recently summarized their pediatric and young adult outcomes following haplo-BMT for advanced hematologic malignancies conditioned with their well-known RIC regimen followed by PT-CY8. The one- or two-year disease-free survival (DFS) in these studies ranged from 32-61%.
Haplo-HCT has particularly benefited minority patients who are often unable to find a matched unrelated donor (MUD). About half of the patients undergoing HCT at our institution are ethnic or racial minorities. Thus, our pediatric BMT program started offering haplo-BMT to our patients in 2015. We describe our experience with the first 13 pediatric and young adult patients with hematologic malignancies who underwent T-cell replete haplo-BMT following myeloablative conditioning. We have previously documented that in experimental haplo-BMT, post-transplant bendamustine (PT-BEN) is at least as effective as PT-CY against graft versus host disease (GvHD) and improves graft versus leukemia (GvL) effects9. We report on, for the first time in humans, four patients treated with a combination of PT-CY and PT-BEN (PT-CY/BEN) following haplo-BMT as part of our ongoing institutional phase I/II study. The remaining nine patients in this report received PT-CY. Impressively, all 13 patients that underwent haplo-BMT remain alive and disease-free with a median follow-up of 15.6 months (1.5 to 31.2 months).
Methods
From October 2015 to March 2018, thirteen pediatric and young adult patients with hematologic malignancies underwent haplo-BMT at Banner University Medical Center in Tucson, Arizona. University of Arizona institutional review board approval was obtained to review and report our findings. All patients were treated by the pediatric BMT service except for the oldest patient (26 years) who was transplanted by our adult BMT colleagues.
Seven patients with acute lymphoblastic leukemia (ALL) were conditioned with fractionated total body irradiation (TBI) of 200 BID given on days −8, −7 and −6 (1200 cGy total dose with lungs shielded to 900 cGy by custom cerrobend blocking), followed by fludarabine (FLU) 30 mg/m2 on days −5, −4, −3, and −21. The remaining six patients (four with myeloid leukemias, one with acute undifferentiated leukemia and two with lymphomas) received busulfan (BU) at 0.8 mg/kg IV every 6 hours for a total of 12 doses (days −8 to −6), targeting an average area under the curve (AUC) of 1000-1100 μMol/min for the duration of the course. BU pharmacokinetics of the first dose were performed at the Seattle Cancer Care Alliance Laboratory. The seventh and remaining doses were modified to achieve the average exposure of 1000-1100 μMol/min. BU was followed by FLU 30 mg/m2 on days −5, −4, −3, and −2 and melphalan (MEL) 100 mg/m2 on day −2 10.
Nine patients received PT-CY 50 mg/kg on days +3 and +4. The other four patients were treated on an IRB-approved phase I/II single institution clinical trial through the University of Arizona Cancer Center (NCT02996773). Two patients (cohort 1) received PT-CY 50 mg/kg on day +3. On day +4, they received 40 mg/kg PT-CY, immediately followed by PT-BEN 20 mg/m2. Two additional patients (cohort 2), received PT-CY 50 mg/kg on day +3 and 20 mg/kg on day +4 immediately followed by PT-BEN 60 mg/m2. For GvHD prophylaxis, all patients were started on mycophenolate mofetil on day +5 at 15 mg/kg/dose q 8 hr IV or PO with a max dose of 1000 mg until day +28. Tacrolimus was initiated on day +5 IV or PO q12 hr adjusting the dose to maintain levels of 7-12 ng/mL. G-CSF was started on day +5 at 5 μg/kg/day until an absolute neutrophil count (ANC) of 2.5 × 109/L was achieved for three consecutive days.
Donors were parents or siblings who were HLA-haploidentical based on high-resolution typing at HLA-A, -B, -Cw, -DRB1, and -DQB1. Nine of the donors were 5 of 10 antigen matches, three were 6 of 10 and one 7/10. None of the patients had anti-donor HLA antibodies. There were seven major and one minor ABO incompatibilities that required donor RBC reduction using Hespan® (6% hetastarch in 0.9% sodium chloride injection) for RBC sedimentation 11. Day of myeloid engraftment was defined as the first of three consecutive days with an ANC of 0.5 × 109/L. Day of platelet engraftment was considered the first of three consecutive days with platelet counts of ≥20 × 109/L with no platelet transfusions administered in the previous 7 days.
Results
Patient, disease and transplant characteristics
Thirteen pediatric and young adult patients with a median age of 19.4 years (range 4.6 to 26.1 years) underwent haplo-BMT at our institution. Patient characteristics are summarized in Table 1. Ethnic and/or racial minorities constituted 69.2% of all patients, the majority of whom (53.8%) were Hispanic. There were 10 males and 3 females. Lansky/Karnofsky performance status scores ranged from 70-100 with a median of 90.
Table 1:
Patient Characteristics
| All patients n=13 | PT-CY n=9 | PT-CY/BEN20 n=2 | PT-CY/BEN60 n=2 | |
|---|---|---|---|---|
| Patient age at BMT years, median (range) | 19.4 (4.6-26.1) | 19.4 (4.6-24.4) | 20.9 (15.6-26.1) | 15.3 (9.2-21.4) |
| Males/Females | 10/3 | 7/2 | 1/1 | 2/0 |
| Race/Ethnicity (%) | ||||
| Hispanic | 7 (53.8) | 5 | 1 | 1 |
| Native American | 1 (7.7) | 1 | ||
| African American | 1 (7.7) | 1 | ||
| White | 4 (30.8) | 2 | 1 | 1 |
| Diagnosis and Disease Stage | ||||
| ALL CR1 | 1 | 1 | ||
| ALL CR2 | 5 | 3 | 1 | 1 |
| ALL CR4 | 1 | 1 | ||
| AML CR1 | 1 | 1 | ||
| AML CR2 | 1 | 1 | ||
| AUL CR1 | 1 | 1 | ||
| CML CP | 1 | 1 | ||
| NHL PR | 1 | 1 | ||
| HD PR | 1 | 1 | ||
| Pre-Transplant Conditioning (%) | ||||
| TBI-FLU | 7 (53.8) | 5 | 1 | 1 |
| BU-FLU-MEL | 6 (46.2) | 4 | 1 | 1 |
| Prior BMT (Dx, months pre-Haplo) | ||||
| UCB (ALL, 18) | 1 | 1 | ||
| Auto (HD, 11) | 1 | 1 | ||
| Lansky/Karnofsky median (range) | 90 (70-100) | 90 (80-100) | 90 (90) | 80 (70-90) |
ALL= acute lymphoblastic leukemia, CR= complete remission, AML =acute myeloid leukemia, AUL=acute undifferentiated leukemia, CML= chronic myeloid leukemia, NHL= non-Hodgkin lymphoma, HD= Hodgkins disease, FLU= fludarabine, BU= busulfan, MEL= melphalan, TBI= total body irradiation, CY= cyclophosphamide, BEN= bendamustine, Dx= diagnosis, UCB= umbilical cord blood, Auto= autologous
The most common diagnosis was acute lymphoblastic leukemia (ALL), with 5 of the 7 ALL patients receiving transplant in CR2, all of which were minimal residual disease (MRD) negative. Three patients had intrachromosomal amplification of chromosome 21 (iAMP21) and one was Philadephia chromosome positive (Ph+). The ALL patient receiving haplo-BMT in CR1 achieved MRD negative status with blinatumomab after primary induction failure following two chemotherapy regimens12. Similarly, the patient receiving haplo-BMT in CR4 had previously relapsed 75 days after an unrelated cord blood (UCB) transplant, and subsequently had four failed infusions of CAR T-cells but achieved an MRD negative status (CR4) after blinatumomab treatment 12. Two patients had acute myeloid leukemia (AML), one of whom developed a t(9;11),11q23 positive secondary leukemia eight months following completion of chemotherapy for osteogenic sarcoma 10. Three patients had measurable disease at the time of haplo-BMT, two with refractory lymphoma and the other patient with chronic myelogenous leukemia (CML) with a T315I mutation and previously failed treatment with three different tyrosine kinase inhibitors (TKIs). For two of the thirteen patients, haplo-BMT was their second transplant (Table 1).
The median age of the donors was 27 years (range 16.3 to 47.7, Table 2). Thirty-eight percent of the donors were siblings, 31% fathers and 31% mothers. Six of the ten male patients (60%) and two of the three female patients (66.6%) received cells from male donors. The median number of bone marrow CD34+ cells infused was 3.5 × 106/kg (range 1.5 to 7.45). All patients and nine donors were CMV seropositive (Table 2).
Table 2:
Patient, Donor Graft Characteristics, Engraftment and GvHD
| All patients n=13 | PT-CY n=9 | PT-CY/BEN20 n=2 | PT-CY/BEN60 n=2 | |
|---|---|---|---|---|
| Donor age years, median (range) | 27 (16.3-47.7) | 38.4 (16.3-47.7) | 35.7 (26.1-45.3) | 26.4 (25.8-27) |
| Donors Males/Females | 8/5 | 5/4 | 2/0 | 1/1 |
| Recipient/Donor (%) | ||||
| Male/Mother | 4 (30.8) | 3 | 1 | |
| Male/Father | 3 (23.1) | 2 | 1 | |
| Male/Brother | 3 (23.1) | 2 | 1 | |
| Female/Father | 1 (7.7) | 1 | ||
| Female/Brother | 1 (7.7) | 1 | ||
| Female/Sister | 1 (7.7) | 1 | ||
| Bone Marrow Cells Infused median (range) | ||||
| CD45+ 108/kg | 2.57 (1.14-6.18) | 2.50 (1.14-6.18) | 3.62 (2.41-4.82) | 4.79 (3.70-5.87) |
| CD34+ 106/kg | 3.50 (1.50-7.45) | 3.10 (1.50-6.35) | 5.0 (2.55-7.45) | 5.28 (3.67-6.89) |
| RBC incompatibility (%) | 8 (61.5) | 5 | 1 | 2 |
| CMV status Recipient/Donor | ||||
| Positive/Positive | 9 | 7 | 1 | 1 |
| Positive/Negative | 4 | 2 | 1 | 1 |
| Days post-BMT | ||||
| ANC of >500/μL | 16 (12-26) | 16 (13-26) | 16 (15-17) | 13.5 (12-15) |
| Platelets >20,000//μL | 27.5 (16-176) | 28 (25-176) | 27.5 (22-33) | 21 (16-26) |
| Transfusions Received | ||||
| Platelets | 14 (2-42) | 16 (7-42) | 17 (14-20) | 6 (2-10) |
| PRBCs | 3 (0-14) | 5 (1-14) | 3 (0-6) | 2 (1-3) |
| Day post-BMT discharged | 28 (18-39) | 28 (20-39) | 24.5 (18-31) | 29.5 (26-33) |
| GVHD (%) | ||||
| Acute grade II/IV | 4 (30.8) | 3 | 1 | 0 |
| Acute grade III/IV | 0 | 0 | 0 | 0 |
| Chronic (all) | 3 (23.1) | 3 | 0 | n/a |
| Extensive Chronic | 2 (15.4) | 2 | 0 | n/a |
GvHD=graft versus host disease, RBC= red blood cell, CMV= cytomegalovirus, ANC=absolute neutrophil count
Outcomes after haplo-BMT
Post-transplant immunosuppression, engraftment and chimerism
Nine patients received PT-CY 50 mg/kg on days +3 and +4. As part of an institutional phase I/II trial (NCT02996773), four patients received PT-BEN with de-escalation of PT-CY on day +4 (two CY 40 mg/kg with BEN 20 mg/m2 and two at CY 20 mg/kg with BEN 60 mg/m2, Table 1). Neutrophil engraftment occurred at a median of 16 days post-BMT (range 12 to 26 days, Table 2 and Figure 1). Platelet engraftment (≥20,000/μL) took place at a median of 27.5 days (range 16 to 176 days, Table 2 and Figure 2). PT-CY/BEN did not adversely affect neutrophil or platelet engraftment (Table 2). Two PT-CY patients received suboptimal numbers of CD34+ cells (1.5 and 1.55 × 106/kg). Both achieved timely myeloid engraftment (day +16 and +17), but one displayed delayed platelet engraftment (day +54). Another PT-CY patient received adequate CD34+ cells (4.62 × 106/kg) but had delayed platelet engraftment likely secondary infections including CMV reactivation requiring ganciclovir. All patients had complete donor chimerism on day 28, eleven of eleven evaluable patients on day 100 and at 6 months after haplo-BMT. No graft failures were observed.
Figure. 1.
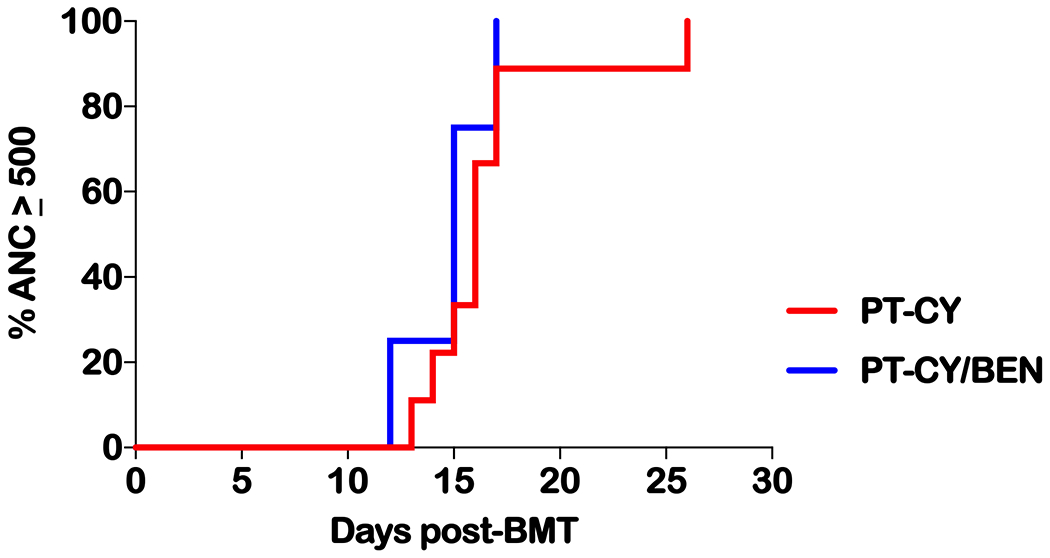
Time to an absolute neutrophil count (ANC) of 500. Time in days from haplo-BMT to achieving an ANC of at least 0.5 × 109/L. Defined as the first of three consecutive days with an ANC of 0.5 × 109/L. PT-CY vs PT-BEN P=ns
Figure. 2.
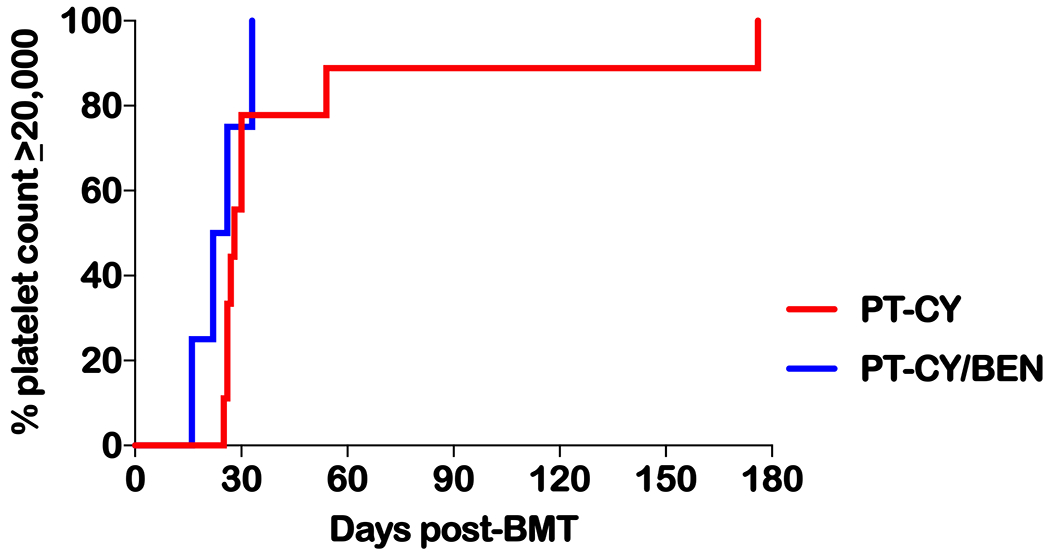
Time to a platelet count of 20,000. Time in days from haplo-BMT to achieving a platelet count of at least 20 × 109/L. Defined as the first of three consecutive days with platelet counts of ≥20 × 109/L with no platelet transfusions administered in the previous 7 days. PT-CY vs PT-BEN P=ns
Post-transplant complications
Patients were hospitalized from the start of the conditioning regimen through engraftment and were discharged when they were able to tolerate adequate oral nutrition, fluids and medications. The median time to discharge was 28 days (range 18 to 39) which was similar between patients receiving PT-CY or PT-CY/BEN (Table 2). Eight patients required hospitalization again during the first 100 days post-haplo-BMT, three requiring two admissions. Patients receiving PT-CY/BEN accounted for only one of the eleven re-hospitalizations. The most common cause of hospitalization was fever and/or infection. There were no transfers or admissions to ICU during the first 100 days post haplo-BMT.
Graft versus host disease
All patients received mycophenolate mofetil from day +5 to day +28. Tacrolimus was discontinued at a median of 149 days post-haplo-BMT (range 95 to 222). The incidence of grades II to IV and grade III to IV acute GvHD (aGvHD) was 30.8% and 0%, respectively. Of the four patients with grade II GvHD, only one required treatment with systemic steroids, while the others responded to topical creams (Figure 3). One of the three patients receiving PT-CY/BEN (cohort 1) had grade II aGvHD (stage II skin) that resolved with steroid creams and tacrolimus ointment (Table 2). The incidence of chronic GvHD (cGvHD) and extensive cGvHD to date is 23.1% and 15.4%, with 2-year probabilities estimated at 29.3% and 19.2%, respectively (Figure 4). All three patients with cGvHD responded to treatment, two are off immunosuppression and the third is on a prednisone taper. None of the PT-CY/BEN patients have developed cGvHD, but the follow-up is short for cohort 2 (1.5 and 3.8 months).
Figure. 3.
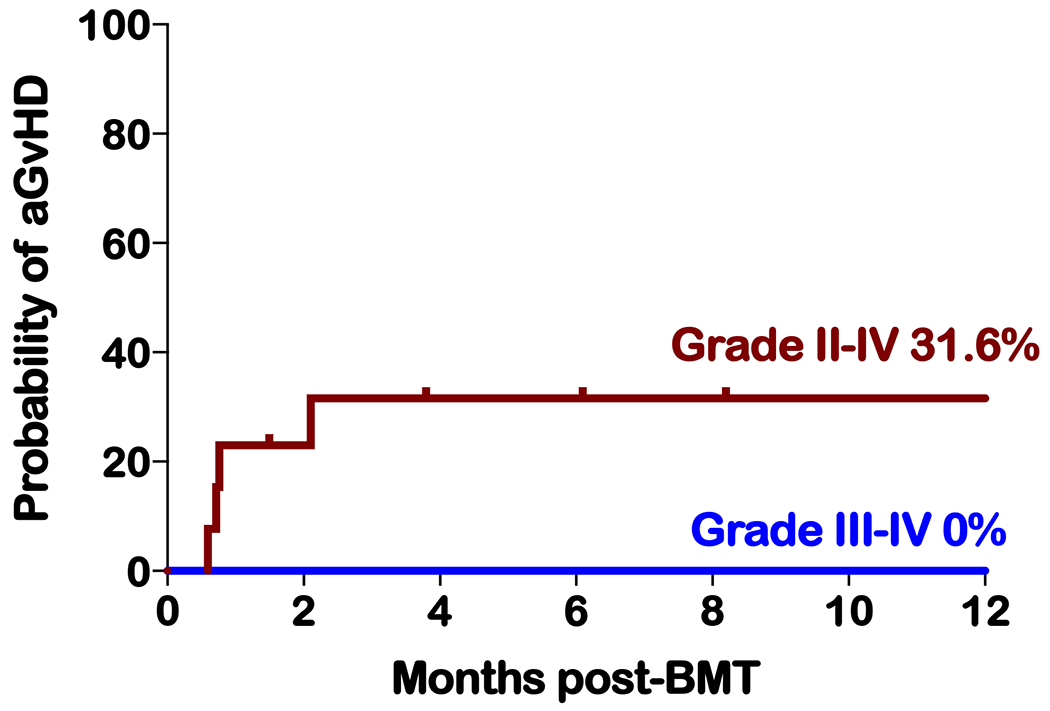
Cumulative incidences of grades II to IV and grades III to IV acute GVHD
Figure. 4.
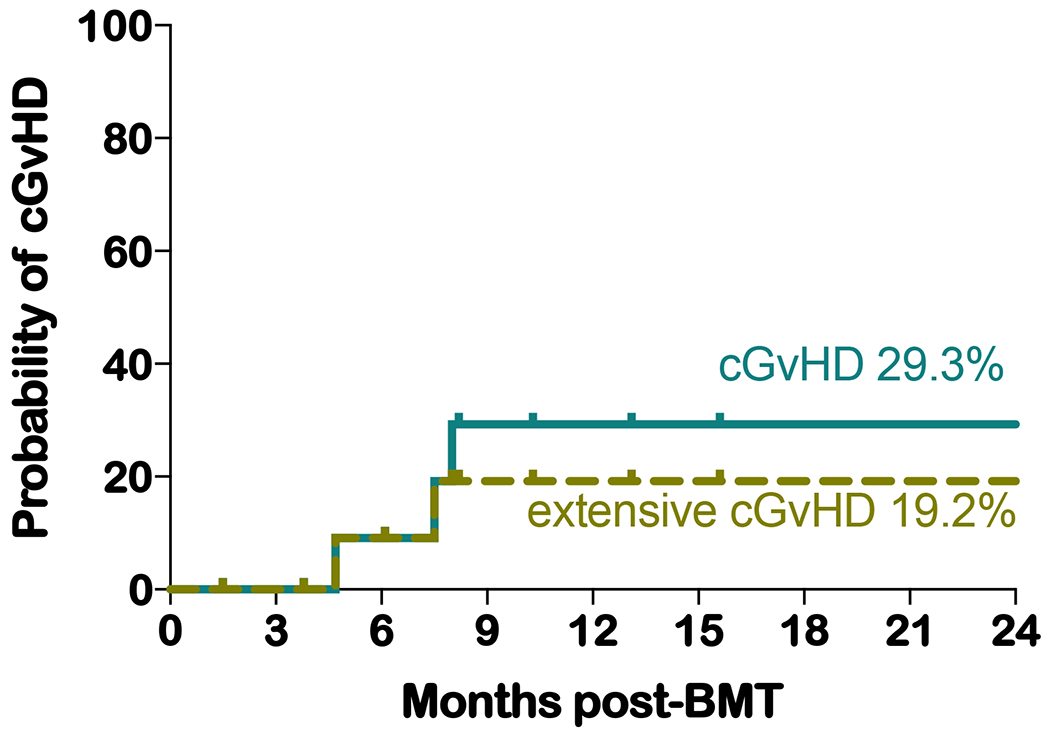
Two-year probability estimates of all chronic GVHD and of extensive cGVHD
Infections
There were five bacteremias in five patients before day +28 (3 in PT-CY and 2 in PT-CY/BEN), all of which responded to appropriate antibiotic therapy (Table 3). There were no bacteremias documented in any of the patients between days 29 and 100, while five occurred between days 101 and 365 in three patients, all of whom received PT-CY. Two patients with cGvHD had two infections each in this time period. One of these patients, with extensive cGvHD, also developed a fungal infection, coccidioidomycosis, which is endemic in Southern Arizona and responded to azoles13. This was also the only patient that required ICU admission, occurring on day +178 post BMT for stenotrophomonas bacteremia (Table 3).
Table 3:
Bacteremias and fungal infections following haplo-BMT
| Day 0-28 | Day 29-100 | Day 101-365 | PT-CY n=9 | PT-CY/BEN n=4 | |
|---|---|---|---|---|---|
| Gram positive bacteremia | |||||
| Coagulase negative staphylococcus | 1 | 1 | |||
| Streptococcus viridans group | 3 | 1 | 2 | ||
| Streptococcus pneumoniae | 2 | 2 | |||
| Rhodococcus | 1 | 1 | |||
| Gram negative bacteremia | |||||
| Escherichia coli | 1 | 1 | |||
| Klebsiella pneumoniae | 1 | 1 | |||
| Stenotrophomonas maltophilia | 1 | 1 | |||
| Fungal | |||||
| Coccidioides immitis | 1 | 1 |
CMV reactivation was common after haplo-BMT and was detected in eight patients (61.5%), six of whom were treated with ganciclovir and/or valganciclovir (Table 4). Five of the six patients requiring CMV treatment had concurrent aGvHD and/or cGvHD. Surprisingly, only one of the four patients receiving a CMV negative donor reactivated (25%), versus seven of the nine that had positive donors (77.7%). Four patients demonstrated adenovirus reactivation. One received treatment with cidofovir, while the viremia resolved spontaneously in the others. HHV6 reactivation was detected in seven patients, none of whom required treatment. Low levels of EBV DNA load (350, 800 and 1500 IU/mL) were identified in three patients, resolving in each case without intervention. Two patients developed microscopic hematuria secondary to BK viruria without viremia. Viral reactivations in patients receiving PT-CY/BEN did not appear to be more frequent. One patient was treated for CMV and was also noted to have low grade HHV6 reactivation and BK viruria. The other three patients have had no documented viral reactivations to date (Table 4).
Table 4:
Viral reactivation following haplo-BMT
| PT-CY n=9 | PT-CY/BEN n=4 | |||
|---|---|---|---|---|
| n= (%) | Treatment | No Treatment | Treatment | No Treatment |
| CMV | 5 (55.6) | 2 (22.2) | 1 (25) | |
| Adenovirus | 1 (11.1) | 3 (33.3) | ||
| HHV-6 | 6 (66.7) | 1 (25) | ||
| EBV | 3 (33.3) | |||
| BK viremia | ||||
| BK viruria | 2 (22.2) | 1 (25) | ||
CMV= cytomegalovirus, HHV6= human herpes virus-6, EBV= Epstein Barr virus
Survival
With a median follow-up of 15.6 months (range 1.5 to 31.2 months) the disease-free and overall survival remain at 100% with no relapses and no NRM (Figure 5). Follow-up for the nine patients receiving PT-CY ranges between 6.1 and 31.2 months while in the PT-CY/BEN patients it is 8.2 and 13.1 months for cohort 1 and 1.5 and 3.8 months for cohort 2
Figure. 5.
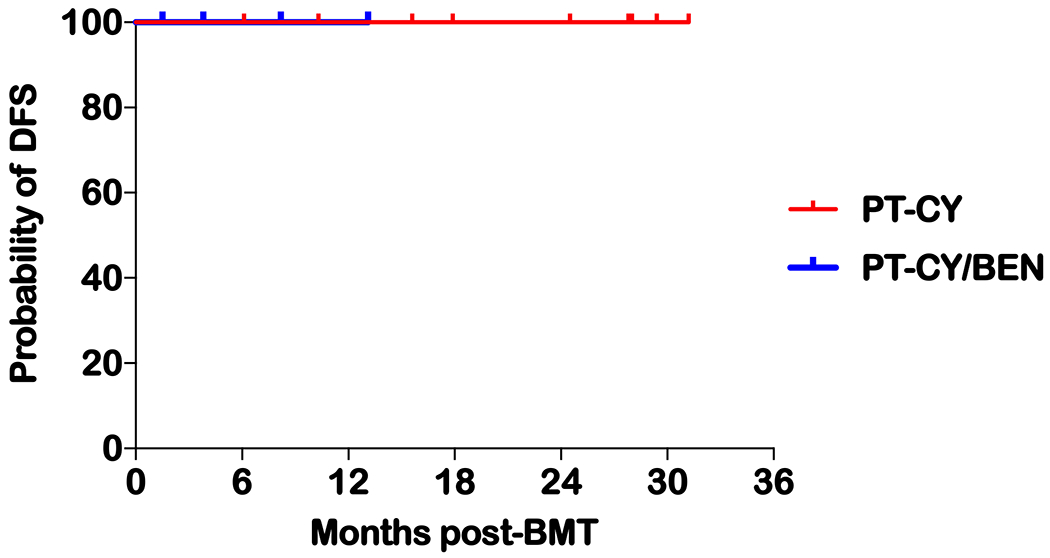
Kaplan-Meier DFS of all patients receiving haplo-BMT
Discussion
We report our initial experience with implementation of haplo-BMT in our pediatric BMT program. There are unique features to our experience compared to other pediatric studies with haplo-HCT and PT-CY. Firstly, the nearly universal donor accessibility afforded by haplo-BMT is particularly significant for our program due to our ethnic and racial minority patient demographics. Of all patients undergoing allogeneic-HCT on our pediatric blood and marrow transplant unit since 2010, 59% were minorities (49% Hispanic, 6% African American and 4% Native American). The likelihood of finding an 8/8 antigen (HLA-A, B, C and DRB1) MUD donor is only 34-40% for Hispanics, 16-19% for African Americans and 36-52% for Native Americans compared to 75% for whites of European descent14. Consequently, there was a critical need to introduce haplo-BMT to our largely minority patient population. Our pediatric BMT program therefore started offering haplo-BMT in 2015. Haplo-BMT replaced mismatched unrelated donor (MMUD) and UCB transplants for malignant diseases. Not unexpectedly, haplo-BMT has been applied more frequently in minority patients, which have comprised 69.2% of our haploidentical transplants. During the last 3 years, 56% of allogeneic transplants for hematologic malignancies have relied on haploidentical donors, compared to 28% on MSD and 16% on MUD.
Secondly, we report for the first time that other chemotherapeutic agents such as bendamustine may be introduced following BMT to control GvHD. More than half a century ago, it was demonstrated that a single dose of CY was able to prolong the survival of a skin allograft from a haploidentical donor if given between the first and fourth day following implantation of the graft15. The use of PT-CY originated from experimental HCT in murine models performed at Johns Hopkins University. This approach has been critical in the development of T-replete haplo-HCT16. PT-CY is effective for several reasons, including its ability to target rapidly dividing alloreactive donor T-cells that are responsible for GvHD, while not affecting quiescent hematopoietic stem cells due to their relatively high levels of aldehyde dehydrogenase17,18. Additional benefits of PT-CY are that it is simple to use and can be applied by any center performing allogeneic HCT. T-cell replete haplo-HCT with PT-CY has therefore emerged as the most widely applied regimen, at least in the U.S., as it circumvents the need to manipulate stem cell grafts. While many of the initial studies focused primarily on adult patients, T-replete haplo-HCT with PT-CY is becoming an increasingly utilized transplant approach in pediatric patients afflicted by both malignant and nonmalignant diseases. Based on our recently published preclinical study in haplo-BMT demonstrating that PT-BEN, when compared to PT-CY, is equally protective against early GvHD and has advantages in late GvHD and GvL9, our phase I/II trial seeks to challenge the PT-CY dogma. Although our findings are preliminary and limited, we have not observed any differences in engraftment, toxicity, GvHD, infections or relapse between patients receiving PT-CY and PT-CY/BEN. The trial continues to enroll both pediatric and adult patients evaluating clinical parameters and immune reconstitution.
A third unique feature to our experience is that we have employed MAC regimens for all patients, rather than using the Johns Hopkins RIC protocol with PT-CY. For patients with ALL, our institution uses a TBI-based regimen previously described in adult patients by Bacigalupo et al. from Genoa 1. For myeloid leukemia and lymphoma, we have applied a chemotherapy-based MAC regimen comprised of busulfan, fludarabine and melphalan. Coincidently, this regimen is similar to the one recently reported by Jaiswal et al. with regards to busulfan, but we use four days of fludarabine (30 mg/m2) instead of five, given following busulfan rather than concurrently, and a lower dose of melphalan (100 mg/m2 instead of 140) 7. Both of our regimens have been well-tolerated with no transplant related mortality or admissions to ICU.
Fourthly, our outcomes thus far have been excellent, with no grade III-IV aGvHD, no NRM and 100% DFS. These findings have to be interpreted with caution as our follow-up is short with a median of 15.6 months (Figure 5). Moreover, although our study included patients with advanced disease, comparatively, our cohort appeared to be of lower risk than the previously published pediatric haplo-HCT studies with PT-CY. Jaiswal et al. reported on the use of unmanipulated PBSCs in pediatric patients with detectable leukemia at transplant 7. The NRM in this study was high at 20%, with relapse low at 25% and a two-year DFS of 59%. In an Italian multi-institutional study using the Johns Hopkins RIC regimen in most patients, NRM was 9%, with relapse at 24% and a one-year DFS of 61%6. The pediatric group from Johns Hopkins treated pediatric and young adult patients (1-25 years) using their haplo-HCT RIC regimen (CY 14.5 mg/kg ×2, FLU 30 mg/m2 ×5 and 200 cGy of TBI) with PT-CY8. Engraftment occurred in 94% of patients, while grade III-IV aGvHD and cGvHD developed in 13% and 24%, respectively, with a NRM of 13% and relapse rate of 52%. The one- and two-year DFS was only 43% and 32%, respectively. This underscores the need for MAC regimens for disease control in pediatric haplo-HCT for refractory and advanced hematologic malignancies.
Haplo-HCT is becoming an accepted transplant modality in pediatrics. Various options exist with respect to the choice of conditioning regimen, graft manipulation and GvHD prophylaxis. The Europeans have more commonly used T-cell depletion strategies, rather than T-replete haplo-HCT with PT-CY. A more refined approach to T-cell depletion consists of haplo-HCT with αβ T- and B-cell depleted grafts, allowing the transfer of CD34+ stem cells with γδ T- and NK cells, both of which are capable of eliciting anti-leukemic and anti-pathogenic effects. A German group reported their pediatric results with this approach using a MAC regimen 19. Engraftment occurred in 88%, grade III-IV aGvHD in 15%, cGvHD in 19% (9% extensive) and relapse in 47% of patients. With a median follow-up of 19 months, the disease-free survival was 51%. Of note, the ten patients who received their first transplant in CR showed a DFS of 100% at one year, illustrating the importance of disease status at the time of transplantation. Locatelli et al. from Italy added to the investigation of αβ T-cell/CD19 B-cell depleted haplo-HCT with 80 acute leukemia pediatric patients in complete remission 20. All patients received a myeloablative preparative regimen (75% TBI-based) and no post-transplant GvHD prophylaxis. Two patients experienced primary graft failure (98% engraftment), there was no grade III-IV aGvHD and only 5% limited cGvHD was observed. NRM was only 5% and relapse rates were 24%. With a median follow-up of 46 months, the 5-year probability of DFS was 71%. As part of this report, they compared the outcomes of haplo-HCT to their patients receiving MSD or MUD HCT during the same time period. Haplo-HCT was associated with a lower incidence of grade III-IV aGvHD and cGvHD and no significant difference in DFS was seen amongst the three transplant groups.
Taken together with the aforementioned published reports, our results strongly indicate that MAC haplo-BMT with PT-CY or PT-CY/BEN is well-tolerated by pediatric and young adult patients and offers an excellent alternative to matched donor allogeneic HCT. It is clear that patients in remission have lower rates of relapse. However, research is still needed to determine the optimal donor and graft characteristics, as well as to refine conditioning regimens and GvHD prophylaxis, including agents that may replace PT-CY, such as PT-BEN, thus improving immune reconstitution for enhanced pathogen and disease surveillance.
Acknowledgements
The authors wish to thank the inpatient and outpatient nursing and other staff on the pediatric BMT unit at Banner University Medical Center in Tucson for their outstanding care of our patients. We thank Vanessa Frisinger, MA, our BMT program coordinator for her excellent administrative assistance. This work was supported by in part by pilot research funding, from the University of Arizona Cancer Center Support Grant P30 CA023074, the Leukemia and Lymphoma Society Translational Research Program, Hyundai Hope on Wheels, Tee up for Tots, Angel Charity for Children and PANDA.
Footnotes
Conflict of interest disclosure
There are no conflicts of interest, financial or otherwise, involving any of the authors regarding the submission or publication of this manuscript.
References
- 1.Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, Bregante S, Van Lint MT, Geroldi S, Luchetti S, Grasso R, Pozzi S, Colombo N, Tedone E, Varaldo R, Raiola AM. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50 Suppl 2:S37–39. [DOI] [PubMed] [Google Scholar]
- 2.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, Morris LE, Holland HK, Solomon SR. Comparison of Outcomes of Hematopoietic Cell Transplants from T-Replete Haploidentical Donors Using Post-Transplantation Cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 Allele-Matched Unrelated Donors and HLA-Identical Sibling Donors: A Multivariable Analysis Including Disease Risk Index. Biol Blood Marrow Transplant. 2016;22(1):125–133. [DOI] [PubMed] [Google Scholar]
- 3.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, Antin JH, Chen J, Devine SM, Fowler DH, Luznik L, Nakamura R, O'Donnell PV, Perales MA, Pingali SR, Porter DL, Riches MR, Ringden OT, Rocha V, Vij R, Weisdorf DJ, Champlin RE, Horowitz MM, Fuchs EJ, Eapen M. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolanos-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolanos-Meade J, Rosner GL, Kanakry CG, Perica K, Symons HJ, Brodsky RA, Gladstone DE, Huff CA, Pratz KW, Prince GT, Dezern AE, Gojo I, Matsui WH, Borrello I, McDevitt MA, Swinnen LJ, Smith BD, Levis MJ, Ambinder RF, Luznik L, Jones RJ, Fuchs EJ, Kasamon YL. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M, Lanino E, Cesaro S, Zecca M, Vassallo E, Faraci M, De Bortoli M, Barat V, Prete A, Fagioli F. Feasibility and Outcome of Haploidentical Hematopoietic Stem Cell Transplantation with Post-Transplant High-Dose Cyclophosphamide for Children and Adolescents with Hematologic Malignancies: An AIEOP-GITMO Retrospective Multicenter Study. Biol Blood Marrow Transplant. 2016;22(5):902–909. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal SR, Chakrabarti A, Chatterjee S, Bhargava S, Ray K, O'Donnell P, Chakrabarti S. Haploidentical Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide in Children with Advanced Acute Leukemia with Fludarabine-, Busulfan-, and Melphalan-Based Conditioning. Biol Blood Marrow Transplant. 2016;22(3):499–504. [DOI] [PubMed] [Google Scholar]
- 8.Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D, Zambidis E, Llosa NJ, Huo JS, Robey N, Holuba MJ, Kasamon YL, McCurdy SR, Ambinder R, Bolanos-Meade J, Luznik L, Fuchs EJ, Jones RJ, Cooke KR, Symons HJ. Nonmyeloablative Haploidentical Bone Marrow Transplantation with Post-Transplantation Cyclophosphamide for Pediatric and Young Adult Patients with High-Risk Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23(2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes J, Hoffman EA, Zeng Y, Larmonier N, Katsanis E. Post-transplant bendamustine reduces GvHD while preserving GvL in experimental haploidentical bone marrow transplantation. Br J Haematol. 2016;174(1):102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsanis E, Sapp LN, Pelayo-Katsanis L, Whitney K, Zeng Y, Kopp LM. Alternative Donor Hematopoietic Cell Transplantation Conditioned With Myeloablative Busulfan, Fludarabine, and Melphalan is Well Tolerated and Effective Against High-risk Myeloid Malignancies. J Pediatr Hematol Oncol. 2016;38(8):e315–e318. [DOI] [PubMed] [Google Scholar]
- 11.Dinsmore RE, Reich LM, Kapoor N, Gulati S, Kirkpatrick D, Flomenberg N, O'Reilly RJ. ABH incompatible bone marrow transplantation: removal of erythrocytes by starch sedimentation. Br J Haematol. 1983;54(3):441–449. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Y, Katsanis E. Potential niche indications for blinatumomab as a bridge to hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(12):1671–1673. [DOI] [PubMed] [Google Scholar]
- 13.Bravo R, Pelayo-Katsanis LO, Shehab ZM, Katsanis E. Diagnostic and treatment challenges for the pediatric hematologist oncologist in endemic areas for coccidioidomycosis. J Pediatr Hematol Oncol. 2012;34(5):389–394. [DOI] [PubMed] [Google Scholar]
- 14.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenbaum MC, Brown IN. Prolongation of Homograft Survival in Mice with Single Doses of Cyclophosphamide. Nature. 1963;200:84. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. [DOI] [PubMed] [Google Scholar]
- 17.Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, Colvin OM, Hilton J. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 18.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–1950. [PubMed] [Google Scholar]
- 19.Lang P, Feuchtinger T, Teltschik HM, Schwinger W, Schlegel P, Pfeiffer M, Schumm M, Lang AM, Lang B, Schwarze CP, Ebinger M, Urban C, Handgretinger R. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015;50 Suppl 2:S6–S10. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, Rondelli R, Lucarelli B, Brescia LP, Masetti R, Milano GM, Bertaina V, Algeri M, Pinto RM, Strocchio L, Meazza R, Grapulin L, Handgretinger R, Moretta A, Bertaina A, Moretta L. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood. 2017;130(5):677–685. [DOI] [PubMed] [Google Scholar]


