Abstract
Background
Testing of vaccine candidates to prevent infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an older population is important, since increased incidences of illness and death from coronavirus disease 2019 (Covid-19) have been associated with an older age.
Methods
We conducted a phase 1, dose-escalation, open-label trial of a messenger RNA vaccine, mRNA-1273, which encodes the stabilized prefusion SARS-CoV-2 spike protein (S-2P) in healthy adults. The trial was expanded to include 40 older adults, who were stratified according to age (56 to 70 years or ≥71 years). All the participants were assigned sequentially to receive two doses of either 25 μg or 100 μg of vaccine administered 28 days apart.
Results
Solicited adverse events were predominantly mild or moderate in severity and most frequently included fatigue, chills, headache, myalgia, and pain at the injection site. Such adverse events were dose-dependent and were more common after the second immunization. Binding-antibody responses increased rapidly after the first immunization. By day 57, among the participants who received the 25-μg dose, the anti–S-2P geometric mean titer (GMT) was 323,945 among those between the ages of 56 and 70 years and 1,128,391 among those who were 71 years of age or older; among the participants who received the 100-μg dose, the GMT in the two age subgroups was 1,183,066 and 3,638,522, respectively. After the second immunization, serum neutralizing activity was detected in all the participants by multiple methods. Binding- and neutralizing-antibody responses appeared to be similar to those previously reported among vaccine recipients between the ages of 18 and 55 years and were above the median of a panel of controls who had donated convalescent serum. The vaccine elicited a strong CD4 cytokine response involving type 1 helper T cells.
Conclusions
In this small study involving older adults, adverse events associated with the mRNA-1273 vaccine were mainly mild or moderate. The 100-μg dose induced higher binding- and neutralizing-antibody titers than the 25-μg dose, which supports the use of the 100-μg dose in a phase 3 vaccine trial. (Funded by the National Institute of Allergy and Infectious Diseases and others; mRNA-1273 Study ClinicalTrials.gov number, NCT04283461.)
The coronavirus disease 2019 (Covid-19) pandemic is an international public health emergency with major social and economic disruptions and devastating health consequences. The rapid development of vaccines is imperative. More than 30 vaccine candidates against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes Covid-19, have entered clinical trials.1 Recently, in a phase 1, open-label trial, we reported that a messenger RNA vaccine, mRNA-1273, which encodes SARS-CoV-2 prefusion-stabilized spike protein, had an acceptable safety and reactogenicity profile and was immunogenic in participants between the ages of 18 and 55 years.2 Two injections of this vaccine, spaced 28 days apart, resulted in neutralizing-antibody responses similar to those elicited by convalescent serum obtained from patients who had recovered from Covid-19 infection. Mice and rhesus macaques that were given mRNA-1273 and were subsequently challenged with high-dose intranasal SARS-CoV-2 rapidly cleared the virus from the upper and lower airways.3,4
The immune response to many other vaccines has been shown to decrease with increasing age.5 Thus, the testing of SARS-CoV-2 vaccine candidates in older populations is of paramount importance, since these persons account for the majority of serious Covid-19 cases and associated deaths.6,7 Here, we report preliminary safety and immunogenicity data for the mRNA-1273 vaccine in an expansion of the phase 1 trial among healthy participants who were 56 years of age or older.
Methods
Trial Design and Participants
We initially conducted a phase 1, dose-escalation, open-label clinical trial of mRNA-1273 involving participants between the ages of 18 and 55 years2 in which we evaluated doses of 25 μg, 100 μg, and 250 μg. We subsequently expanded the trial to include 40 participants who were 56 years of age or older and who were stratified into two subgroups: those between the ages of 56 and 70 years and those who were 71 years of age or older. Because of clinically significant systemic reactogenicity observed in participants between the ages of 18 and 55 years at the 250-μg dose, we administered doses of 25 μg or 100 μg to the older participants.
The trial was conducted at Kaiser Permanente Washington Health Research Institute in Seattle, the Emory University School of Medicine in Atlanta, and the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center in Bethesda, Maryland. Enrolled adults were healthy and provided written informed consent before undergoing any study procedures. We did not screen for evidence of past or current SARS-CoV-2 infection by testing blood or nasal specimens before enrollment. Full eligibility criteria, along with details of the trial design, conduct, oversight, and statistical analyses, are described in the protocol, which is available with the full text of this article at NEJM.org.
mRNA-1273 Vaccine
The mRNA-1273 vaccine was codeveloped by researchers at the NIAID Vaccine Research Center and Moderna in Cambridge, Massachusetts. This vaccine encodes a stabilized version of the SARS-CoV-2 full-length spike glycoprotein trimer, S-2P, which has been modified to include two proline substitutions at the top of the central helix in the S2 subunit. The mRNA is encapsulated in lipid nanoparticles at a concentration of 0.5 mg per milliliter and diluted with normal saline to achieve the final target vaccine concentrations.
Study Oversight
The NIAID served as the trial sponsor and made all decisions regarding the study design and implementation. The vaccine Investigational New Drug application and the protocol amendment expanding the age subgroups were reviewed by the Food and Drug Administration and the institutional review board at Advarra, a regulatory compliance consulting company, which served as the single institutional review board for all the study sites. An independent data and safety monitoring committee reviewed interim safety reports.
Moderna provided mRNA-1273 for use in this trial but did not provide any financial support. Employees of Moderna collaborated on the development of the protocol, contributed to the Investigational New Drug application, and participated in weekly team meetings regarding the study.
Emmes, the statistical and data coordinating center for the study, developed the statistical analysis plan and performed all data analyses. Data reports, which were generated from the raw data by the statistical and data coordinating center, were provided and available to all the authors. The manuscript was written entirely by the authors, with the first two authors serving as overall lead authors. All the authors vouch for the completeness and accuracy of the data and for the adherence of the study to the protocol. No one who is not an author contributed to the writing of the manuscript.
Trial Procedures
The mRNA-1273 vaccine was administered as a 0.5-ml intramuscular injection into the deltoid on days 1 and 29 of the study; the same dose of the vaccine was administered on both days. Follow-up visits were scheduled 7 and 14 days after the administration of each dose of vaccine and on day 57. A standard toxicity scale was used to grade adverse events (Table S1 in the Supplementary Appendix, available at NEJM.org). Solicited local and systemic adverse events were collected for 7 days after each vaccination, as facilitated by the use of a memory aid. Data regarding unsolicited adverse events and the use of new medications were collected through day 57. Collection of specimens, as well as monitoring for medically attended adverse events, development of new chronic medical conditions, and serious adverse events, was scheduled to continue through 1 year after the last dose. These initial findings will be updated with final safety and immunogenicity data when the results are available.
After the initial safety data from the first phase of the study were available from participants between the ages of 18 and 55 years,2 the administration of mRNA-1273 was initiated sequentially in the subgroup of participants between the ages of 56 and 70 years at the 25-μg dose, which was followed by the initiation of the 100-μg dose. Since no halting rules were met after the participants in this subgroup had completed day 8, vaccine administration was initiated sequentially in the subgroup of participants who were 71 years of age or older at the 25-μg dose, which was followed by the initiation of the 100-μg dose.
Assessment of Antibody Responses
We performed enzyme-linked immunosorbent assays (ELISA) to quantify the binding IgG responses to S-2P containing an Asp (D) residue at position 614 (initial Wuhan-1 strain sequence8) and to the receptor-binding domain on days 1, 15, 29, 36, 43, and 57. (The receptor-binding domain is the portion of the SARS-CoV-2 virus that is located on its spike domain and that links with body receptors to infect cells.) A SARS-CoV-2 native spike-pseudotyped lentivirus reporter single-round-of-infection neutralization assay (pseudovirus neutralization assay) was used to assess vaccine-induced neutralizing activity against the 614D variant at the same time points. Vaccine-induced neutralization on day 43 was assessed with a second pseudovirus neutralization assay with the use of the 614-Gly (614G) polymorphic variant, since the 614G strain had become predominant in both the United States and worldwide.9 (Details are provided in the Methods section in the Supplementary Appendix.)
Three live-virus neutralization methods were used: first, the SARS-CoV-2 nanoluciferase high-throughput neutralization assay (nLuc HTNA), which uses a virus expressing the reporter gene nanoluciferase (nLuc)10; second, the focus reduction neutralization test mNeonGreen (FRNT-mNG), which uses recombinant SARS-CoV-2 expressing the fluorescent reporter gene mNeonGreen11; and third, a SARS-CoV-2 plaque-reduction neutralization testing (PRNT) assay, which uses wild-type virus. We used the nLuc HTNA to analyze specimens that were obtained on days 1, 29, and 43 from the participants who were 56 years of age or older and who received the 100-μg dose. We used the FRNT-mNG assay to analyze specimens obtained on days 1, 29, and 43 from all the participants in the two age and dose subgroups. For this preliminary report, because of the time-intensive nature of the PRNT assay and to maximize usable information obtained from its use, we performed PRNT assays for the presence of SARS-CoV-2 on samples obtained on days 1 and 43 from participants who received the 100-μg dose only. We used as comparators previously reported results for participants between the ages of 18 and 55 years who had been enrolled in the 100-μg subgroup, as well as results from controls who had donated convalescent serum.2 The severity of Covid-19 illness was known for 38 of these controls and was classified as mild in 63% of the participants, moderate in 22%, and severe (defined as hospitalization requiring intensive care, ventilation, or both) in 15%.
Assessment of T-Cell Responses
Intracellular cytokine-staining assays were performed to quantify antigen-specific T-cell responses against the spike protein on days 1, 29, and 43. (Details are provided in the Supplementary Appendix.)
Statistical Analysis
Safety analyses included all the participants who had received at least one dose of mRNA-1273. Immunogenicity results excluded specimens that had been obtained after day 29 in a participant who had received only a single dose of vaccine. No other data points were missing. Seroconversion was defined as an increase from baseline in the antibody titer by a factor of 4 or more. Geometric means were calculated by log transforming the data points and calculating the mean and 95% confidence interval on the log-transformed data. The log-transformed mean and 95% confidence interval were then back-transformed to the original scale. We used the Student’s t-test to calculate confidence intervals. Interim analyses in the study subgroups were prespecified to inform critical decisions about vaccine development.
Results
Trial Population
Forty participants, 10 in each of the two age and dose subgroups, were enrolled between April 16 and May 12, 2020 (Fig. S1). The demographic characteristics of the participants are shown in Table 1. One participant who was between the ages of 56 and 70 years in the 25-μg subgroup did not receive a second vaccination or have blood collected after day 29. (Details regarding this participant are provided in the following section.)
Table 1. Characteristics of the Participants at Baseline.*.
| Characteristic | Age of 56–70 Years | Age of ≥71 Years | All Participants (N=40) |
||
|---|---|---|---|---|---|
| 25-μg Dose (N=10) |
100-μg Dose (N=10) |
25-μg Dose (N=10) |
100-μg Dose (N=10) |
||
| Sex — no. (%) | |||||
| Male | 3 (30) | 5 (50) | 8 (80) | 3 (30) | 19 (48) |
| Female | 7 (70) | 5 (50) | 2 (20) | 7 (70) | 21 (52) |
| Age — yr | 65.8±4.5 | 63.8±4.3 | 72.8±1.2 | 72.6±1.1 | 68.7 |
| Race or ethnic group — no. (%)† | |||||
| Asian | 0 | 0 | 1 (10) | 0 | 1 (2) |
| White | 10 (100) | 10 (100) | 9 (90) | 10 (100) | 39 (98) |
| Hispanic or Latino | 0 | 0 | 1 (10) | 0 | 1 (2) |
| Body-mass index‡ | 25.4±2.5 | 23.7±2.3 | 24.8±3.5 | 26.0±3.5 | 25.0±3.0 |
Plus–minus values are means ±SD.
Race or ethnic group was reported by the participants, who could select more than one category.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Vaccine Safety
No serious adverse events were reported, and no prespecified trial-halting rules were met. Two days after vaccination, paronychia (infection of the tissue adjacent to a nail) developed in one participant who was between the ages of 56 and 70 years in the 25-μg subgroup. This participant was treated with trimethoprim–sulfamethoxazole, and 7 days later, a diffuse maculopapular rash developed. The rash was considered by investigators to be unrelated to vaccination and was treated with systemic administration of glucocorticoids. This participant did not receive a second vaccination.
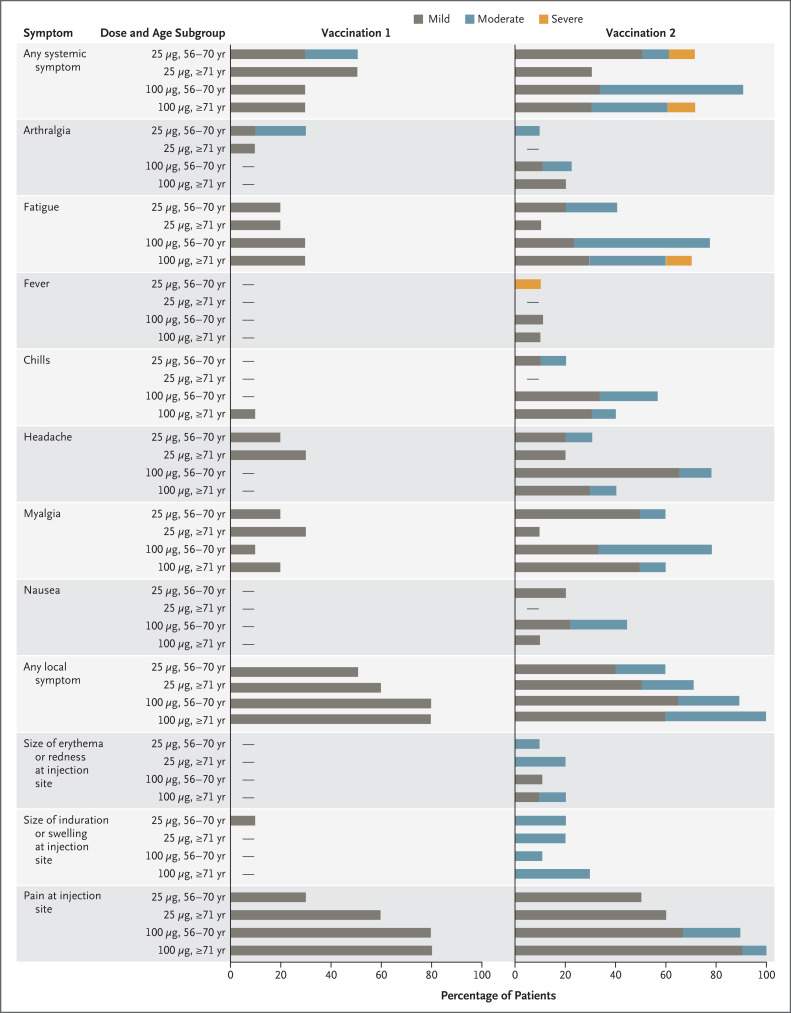
The most common solicited adverse events were headache, fatigue, myalgia, chills, and injection-site pain (Figure 1). Local and systemic reactogenicity events were more common and were predominantly moderate in severity after the administration of the second dose of vaccine. All 10 solicited local adverse events that were classified as moderate, and all but 2 moderate systemic events, occurred after the administration of the second dose (Figure 1 and Table S2). These symptoms typically occurred on the day of vaccination or 1 day afterward and resolved quickly. Three participants had erythema that lasted for 5 to 7 days; all the cases were mild and began on day 1 or 2. One participant had mild myalgia symptoms that began on day 3 and lasted for 5 days. Two solicited systemic adverse events that were classified as severe (grade 3) occurred after the second dose: fever in a participant between the ages of 56 and 70 years in the 25-μg dose subgroup and fatigue in a participant who was 71 years of age or older in the 100-μg dose subgroup. A total of 71 unsolicited adverse events were reported, of which 17 were considered by the investigators to be related to the vaccine and are detailed in Table S3. All related unsolicited adverse events were mild except for a single event of moderate severity, which was decreased appetite in a participant who was between the ages of 56 and 70 years in the 25-μg dose subgroup. The single severe (grade 3) unsolicited adverse event was hypoglycemia (glucose level, 50 mg per deciliter [2.8 mmol per liter]; reference range, 65 to 99 mg per deciliter [3.6 to 5.5 mmol per liter]), which occurred in a participant who was between the ages of 56 and 70 years in the 100-μg dose subgroup after fasting and vigorous exercise and was considered by the investigators to be unrelated to the vaccine. Clinical laboratory values of grade 2 or higher are detailed in the Supplementary Appendix.
Figure 1. Solicited Systemic and Local Adverse Events within 7 Days after Receipt of mRNA-1273.
Shown are data for older participants in the two age subgroups (56 to 70 years and ≥71 years) and the two dose subgroups (25 μg and 100 μg). The severity of solicited adverse events was graded as mild, moderate, or severe on the basis of definitions that are detailed in Table S1 in the Supplementary Appendix. Dashes indicate that the adverse event was not reported in any participant.
Binding-Antibody Responses
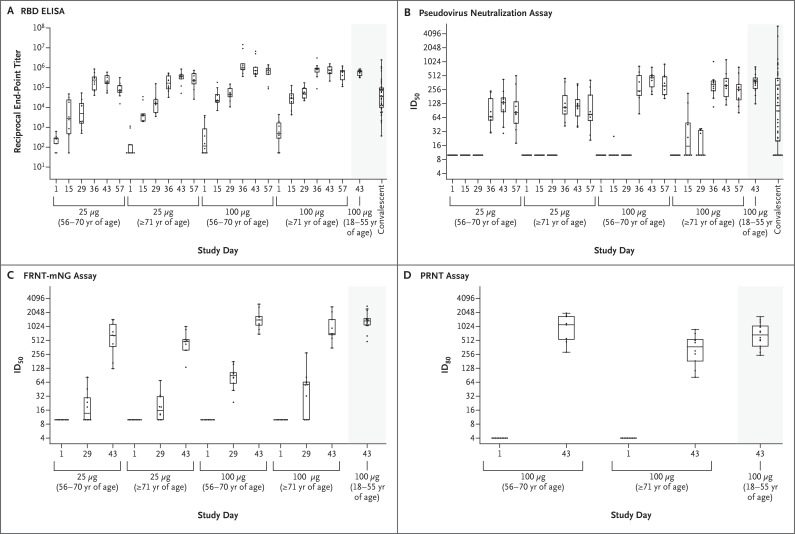
Data were available for all the participants at all time points except for the period after day 29 in the participant who did not receive a second vaccine dose. At baseline, some participants had detectable serum binding-antibody responses in the receptor-binding domain and in S-2P IgG on ELISA. Binding IgG antibody geometric mean titers (GMTs) to S-2P and receptor-binding domain increased rapidly after the first vaccination. Dose-dependent responses to the first and second doses of vaccine were evident, and after the second vaccination, responses reached the upper quarter of the distribution of responses among controls who had donated convalescent serum (Figure 2A, Figs. S2 and S3, and Tables S4 and S5). On the S-2P ELISA, the GMT at day 57 in the 25-μg subgroup was 323,945 (95% confidence interval [CI], 182,202 to 575,958) among participants who were between the ages of 56 and 70 years and 1,128,391 (95% CI, 636,087 to 2,001,717) among those who were 71 years of age or older. These responses were greater than those observed among participants who had donated convalescent serum (GMT, 138,901; 95% CI, 82,876 to 232,799). The GMTs of the participants in the 100-μg subgroup far exceeded the responses among participants who had donated convalescent serum: 1,183,066 GMT (95% CI, 379,698 to 3,686,201) among participants who were between the ages of 56 and 70 years and 3,638,522 GMT (95% CI, 1,316,233 to 10,058,130) among those who were 71 years of age or older.
Figure 2. SARS-CoV-2 Antibody-Binding and Neutralization Responses.
Shown are reciprocal end-point binding IgG titers on receptor-binding domain (RBD) enzyme-linked immunosorbent assay (ELISA) (Panel A), along with titers to 614D on the pseudovirus neutralization assay at a 50% inhibitory dilution (ID50) (Panel B), on the focus reduction neutralization test mNeonGreen assay (FRNT-mNG ID50) (Panel C), and on plaque-reduction neutralization testing (PRNT80) (Panel D).2 PRNT80 results were available only for the participants who had received the 100-μg dose of the mRNA-1273 vaccine on days 1 and 43. Boxes denote interquartile ranges, and horizontal bars denote median end-point titers. Whisker end points denote the maximum and minimum values below or above the median at 1.5 times the interquartile range. The shaded portion on the right side of each panel indicates one or two categories of reference values: antibody titers from 41 controls who had donated convalescent serum (Panels A and B) and antibody titers from participants between the ages of 18 and 55 years who had received the 100-μg dose of mRNA-1273 (Panels A through D).2
Neutralization Responses
Titers to 614D on the pseudovirus neutralization assay were undetectable before vaccination and showed dose-dependent responses that were observed as early as 7 days after the second dose (day 36) in an age-independent manner. At day 43, on the pseudovirus neutralization assay, the 50% inhibitory dilution (ID50) titers to 614D that were induced by the 100-μg dose were similar among the participants who were between the ages of 56 and 70 years (GMT, 402; 95% CI, 289 to 560) and those who were 71 years of age or older (GMT, 317; 95% CI, 198 to 508) (Figure 2B and Tables S4 and S5); the responses were also similar to those previously reported for participants between the ages of 18 to 55 years. Responses to the 100-μg dose were higher than responses to the 25-μg dose and than responses among controls who had donated convalescent serum. Robust neutralizing activity to the 614G variant was observed for the 100-μg dose regardless of the participant’s age (Fig. S5 and Tables S4 and S5). Neutralizing activity remained high through 4 weeks after the administration of the second dose in all the subgroups.
Neutralizing responses were undetectable by nLuc HTNA, FRNT-mNG, or PRNT at baseline (Figure 2C and 2D, Figs. S6 and S7, and Tables S4 and S5). Potent neutralization responses were observed in all the participants 14 days after the second dose of vaccine (on day 43). Responses to the 100-μg dose on day 43 on nLuc HTNA and FRNT-mNG were similarly high across age strata; the PRNT80 (80% inhibitory dilution) GMT was 878 (95% CI, 516 to 1494) among participants who were between 56 and 70 years of age and 317 (95% CI, 181 to 557) among those who were 71 years of age or older.
Similar to the results among the participants who were between the ages of 18 to 55 years, the receptor-binding domain and S-2P ELISA titers correlated with the results on the pseudovirus neutralization assay in the older subgroups. In addition, all the neutralization assays — pseudovirus neutralization assay, nLuc HTNA, FRNT-mNG, and PRNT — correlated well with each other and with the receptor-binding domain ELISA; the results on the PRNT assay did not correlate well with the results on the S-2P ELISA (Figs. S8 through S12).
T-Cell Reponses
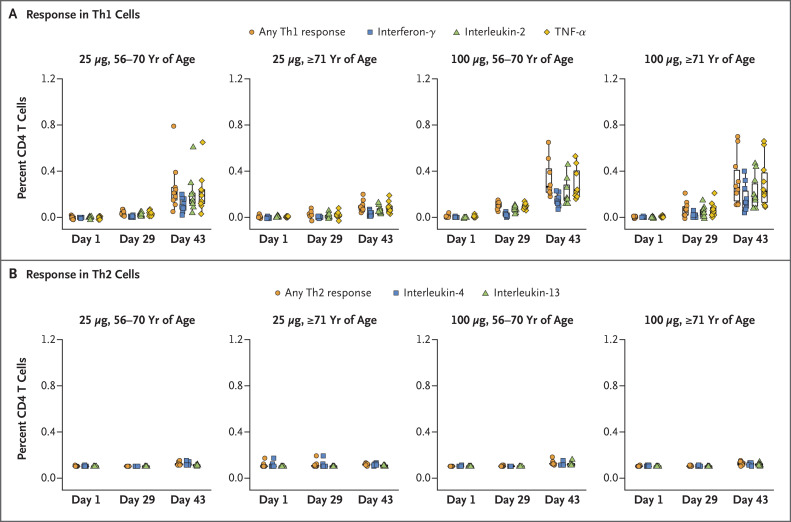
In response to S-specific peptide pools, the vaccine elicited a strong CD4 cytokine response involving type 1 helper T (Th1) cells among participants in the two age subgroups who received the 100-μg dose and among participants between the ages of 56 and 70 years who received the 25-μg dose; the tumor necrosis factor α responses were greater than the interleukin-2 responses, which in turn were greater than the interferon-γ responses (Figure 3 and Fig. S13). In the subgroup of participants who received the 25-μg dose, the cytokine response was lower among those who were 71 years of age or older than among those in the other subgroup. The response involving type 2 helper T cells (interleukin-4 and interleukin-13) was minimal regardless of age or dose. CD8 T-cell responses to S-2P were observed only at low levels after the second vaccination among the participants in the two age subgroups who received the 100-μg dose (Fig. S14).
Figure 3. CD4 T-Cell Responses to the S1 Peptide Pool after Receipt of mRNA-1273.
Shown are the percentages of CD4 T cells that produced the indicated cytokines in the two age subgroups (56 to 70 years or ≥71 years) after stimulation with the SARS-CoV-2 S1 peptide pool. Type 1 helper T (Th1) cells are shown in Panel A, and type 2 helper T (Th2) cells are shown in Panel B. A total of 40 participants who were enrolled in the study (10 participants in each of the two age and dose subgroups) were tested at each time point, except for 1 participant who was between the ages of 56 and 70 years and who did not receive the second 25-μg dose and so did not contribute data after day 29. In Panel A, the boxes indicate interquartile ranges. Details regarding the T-cell calculations are provided in the Supplementary Appendix. TNF-α denotes tumor necrosis factor α.
Discussion
In our study population of older participants (≥56 years of age), the two-dose vaccine series had an acceptable safety and reactogenicity profile at doses of both 25 μg and 100 μg with mostly mild-to-moderate local and systemic adverse events of short duration, which occurred predominantly after the second dose. We did not observe systematic differences in the reactogenicity profile between this older cohort and participants between the ages of 18 and 55 years who had received the mRNA-1273 vaccine.2 Our findings were also similar to the results of other trials of mRNA vaccines involving younger adults.12,13 Owing to the small number of participants in each group, formal statistical comparisons of between-subgroup age effects are not meaningful. Our planned 13-month follow-up allows for a longer duration of assessment of vaccine-related adverse events, although the limited sample size may not capture rare serious adverse events.2
As of this writing, no correlate of protection for SARS-CoV-2 has been established. However, neutralizing-antibody levels have been shown to correlate with protection against many viruses in humans14 and have correlated with protection against SARS-CoV-2 in animal challenges.3,15 The mRNA-1273 vaccine induced high levels of both binding and neutralizing antibodies in older adults, and the time- and dose-dependent trends were similar to responses in younger adults2; the responses after the second vaccination were similar to those observed in patients who had recovered from Covid-19 and had donated convalescent serum, including some who were severely ill. We must acknowledge that these assessments are qualitative; the small number of participants in each age and dose subgroup limits the power of efforts at quantitative assessment. Antibody and T-cell responses that were observed in this older population who received the 100-μg dose exceeded the response to the 25-μg dose and were similar to the response among participants between the ages of 18 and 55 years who received the 100-μg dose.2 These data also suggest that a second dose of vaccine is needed to achieve neutralizing antibodies in participants after the age of 56 years, and titers rapidly increased by 7 days after the booster vaccination. We found strong correlations between the receptor-binding domain ELISA and all neutralization assays. These findings suggest that the receptor-binding domain ELISA and higher-throughput neutralization methods (e.g., pseudovirus neutralization assay, FRNT-mNG, and nLUC HTNA) could be used in a complementary manner to assess immunogenicity of SARS-CoV-2 vaccines and potentially to confirm an immunologic correlate of protection that would support public-health decision making regarding vaccination strategies.
Our assessments of antibody responses have focused on the 614D strain (the initial Wuhan-1 strain sequence), although the 614G polymorphism in SARS-CoV-2 spike has rapidly spread to become the globally predominant isoform,9 which suggests a selective advantage. However, we observed an increase in neutralizing activity on the pseudovirus neutralization assay when 614G was substituted for 614D. These data are consistent with results in mice that were vaccinated with mRNA-127316 and with observations from convalescent donor serum.9 These data are reassuring that immune responses to mRNA-1273 may not be compromised by the presence of the 614G strain.
Typically, immunosenescence renders older adults more susceptible to infection and hinders the development of protective immunity after immunization17 with selected vaccines (e.g., reduced immunogenicity of the live-attenuated shingles vaccine).18 Potently immunogenic vaccine designs are particularly relevant for older adults, since severe or fatal cases of Covid-19 occur more often in this population.6 Although the sample sizes in our study were limited, older participants (including those who were 71 years of age or older) had immunologic responses to the mRNA-1273 vaccine 1 month after the second dose and across multiple assays that were similar to the responses among younger participants. We did not observe systematic differences in binding-antibody responses between the two older-age subgroups and the younger population, although slight decreases in PRNT80 were observed in those who were 71 years of age or older, as has been seen with other vaccines.13 It is possible that the mRNA-1273 vaccine may activate memory B cells that recognize shared antigenic determinants between spike proteins of SARS-CoV-2 and endemic human coronaviruses, which could also account for low binding-antibody levels detected in some prevaccination specimens. These findings are consistent with those of Ledgerwood et al., who observed age-independent responses to a West Nile virus DNA vaccine.19 The mRNA-1273 platform offers several potential advantages for stimulation of protective responses in older populations, including the generation of a more immunogenic spike protein than that in wild-type virus owing to prefusion-stabilizing mutations,16,20 the ability to control innate immune stimulation through mRNA modification,10 and the efficient uptake into human cells due to its lipid nanoparticle formulation.21
Important limitations of this study include the small numbers of participants and the limited ethnic diversity. In addition, at the time of this interim report, the long-term durability of immunogenicity could not be assessed, although the magnitude of antibody, cellular, and memory responses will be followed for 12 months after the second vaccination. Waning neutralizing-antibody titers have been observed in recipients of DNA vaccines against MERS (Middle East respiratory syndrome) and SARS22,23 and in patients with SARS-CoV-2 infection.24,25 Data on the durability of responses observed after mRNA vaccines are limited. However, the administration of an mRNA H7N9 influenza vaccine resulted in detectable antibody titers in participants 6 months after vaccination.26 It is possible that early induction of high-magnitude antibody responses could extend the duration of detectable titers, as has been seen with other vaccines.27 Finally, the presence of chronic diseases (e.g., diabetes) and frailty may be better predictors of poor immunologic responses than age alone.28
Overall, these preliminary findings show that in a small group of participants, adverse events associated with the mRNA-1273 vaccine were mainly mild or moderate in older adults, a group that is particularly at risk for illness and death from Covid-19. The 100-μg dose induced higher binding- and neutralizing-antibody titers than the 25-μg dose, findings that support the continued evaluation of the 100-μg dose level and two-dose regimen in a large phase 3 trial with a more diverse population to ascertain the safety and efficacy of the mRNA-1273 vaccine and to assess its level of protection against Covid-19.
Acknowledgments
We thank the participants for their altruism and their dedication to this trial; the members of the data and safety monitoring committee (Stanley Perlman [chair], University of Iowa; Gregory Gray, Duke University; and Kawsar Talaat, Johns Hopkins Bloomberg School of Public Health) for their oversight; Huihui Mu and Michael Farzan for providing the ACE2-overexpressing 293 cells; Vineet Menachery and Pei-Yong Shi for providing the infectious-clone–derived SARS-CoV-2-mNG virus; and Dominic Esposito, director of the Protein Expression Laboratory at the Frederick National Laboratory for Cancer Research, for providing the S-2P protein for the immunologic assays. The Emory University study team thanks the Georgia Research Alliance and Children’s Healthcare of Atlanta for their support; and Heidi Bornstein of Kaiser Permanente for assistance in the preparation of a previous version of Figure 1. The Kaiser Washington study team thanks Howard Crabtree and Sheena Mangicap of Seattle Pharmacy Relief for their many years of support for vaccine trials. The NIH Vaccine Research Center study team thanks our colleagues at the NIH Clinical Center and NIAID for their contributions, including the NIH Institutional Review Board, the NIH Clinical Center Pharmacy, and colleagues at the Vaccine Research Center, including the Vaccine Immunology Program, for their support.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The views expressed in this article are those of the authors and do not necessarily reflect those of the Department of Health and Human Services, nor does any mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This article was published on September 29, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by grants (UM1AI148373, to Kaiser Washington; UM1AI148576, UM1AI148684, and NIH P51 OD011132, to Emory University; NIH AID AI149644, to the University of North Carolina; UM1Al148684-01S1, to Vanderbilt University Medical Center; and HHSN272201500002C, to Emmes) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); by a grant (UL1 TR002243, to Vanderbilt University Medical Center) from the National Center for Advancing Translational Sciences, NIH; and by the Dolly Parton Covid-19 Research Fund (to Vanderbilt University Medical Center). Laboratory efforts were in part supported by the Emory Executive Vice President for Health Affairs Synergy Fund award, the Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Covid-Catalyst-I3 Funds from the Woodruff Health Sciences Center and Emory School of Medicine, and North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill, with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly. Funding for the manufacture of mRNA-1273 phase 1 material was provided by the Coalition for Epidemic Preparedness Innovation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Draft landscape of COVID-19 candidate vaccines. September 9, 2020. (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
- 2.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. DOI: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett KS, Edwards D, Leist SR, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. June 11, 2020. (https://www.biorxiv.org/content/10.1101/2020.06.11.145920v1). preprint. [DOI] [PMC free article] [PubMed]
- 5.Lang PO, Govind S, Bokum AT, et al. Immune senescence and vaccination in the elderly. Curr Top Med Chem 2013;13:2541-2550. [DOI] [PubMed] [Google Scholar]
- 6.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020;182(4):812.e19-827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 2020;182(2):429.e14-446.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Muruato A, Lokugamage KG, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe 2020;27(5):841.e3-848.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. July 1, 2020. (https://www.medrxiv.org/content/10.1101/2020.06.30.20142570v1). preprint.
- 13.Walsh EE, Frenck R, Falsey AR, et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. August 28, 2020. (https://www.medrxiv.org/content/10.1101/2020.08.17.20176651v2). preprint.
- 14.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010;17:1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020;369:812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020. August 5 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol 2018;40:83-94. [DOI] [PubMed] [Google Scholar]
- 18.Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008;197:825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledgerwood JE, Pierson TC, Hubka SA, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis 2011;203:1396-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A 2017;114:E7348-E7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wec AZ, Wrapp D, Herbert AS, et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 2020;369:731-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis 2019;19:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JE, Louder MK, Holman LA, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine 2008;26:6338-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200-1204. [DOI] [PubMed] [Google Scholar]
- 25.Cao W-C, Liu W, Zhang P-H, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 2007;357:1162-1163. [DOI] [PubMed] [Google Scholar]
- 26.Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019;37:3326-3334. [DOI] [PubMed] [Google Scholar]
- 27.Van Damme P. Long-term protection after hepatitis B vaccine. J Infect Dis 2016;214:1-3. [DOI] [PubMed] [Google Scholar]
- 28.Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017;216:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.