Abstract
Drought stress as one of the most devastating abiotic stresses affects agricultural and horticultural productivity in many parts of the world. The application of melatonin can be considered as a promising approach for alleviating the negative impact of drought stress. Modeling of morphological responses to drought stress can be helpful to predict the optimal condition for improving plant productivity. The objective of the current study is modeling and predicting morphological responses (leaf length, number of leaves/plants, crown diameter, plant height, and internode length) of citrus to drought stress, based on four input variables including melatonin concentrations, days after applying treatments, citrus species, and level of drought stress, using different Artificial Neural Networks (ANNs) including Generalized Regression Neural Network (GRNN), Radial basis function (RBF), and Multilayer Perceptron (MLP). The results indicated a higher accuracy of GRNN as compared to RBF and MLP. The great accordance between the experimental and predicted data of morphological responses for both training and testing processes support the excellent efficiency of developed GRNN models. Also, GRNN was connected to Non-dominated Sorting Genetic Algorithm-II (NSGA-II) to optimize input variables for obtaining the best morphological responses. Generally, the validation experiment showed that ANN-NSGA-II can be considered as a promising and reliable computational tool for studying and predicting plant morphological and physiological responses to drought stress.
Introduction
It is well documented that drought significantly affects agricultural and horticultural productivity in many parts of the world [1]. Indeed, drought and heat stresses as a direct effect of global climate change lead to the disruptions in morphological, physiological, biochemical, and molecular responses of the plants [2, 3]. Therefore, plant growth and development, as well as agricultural productivity, are remarkably influenced by water scarcity as a major environmental restriction [4]. Drought stress also decreases canopy size, photosynthetic potential due to the acceleration of the leave senescence and chlorophyll degradation which ultimately leads to the lower crop yield [5–7]. In recent years, genetic engineering methods have been suggested as promising approaches for improving plant tolerance to different stresses in particular drought stress. However, genetic manipulation (GM) methods are unacceptable in many parts of the world due to GM product restriction and can be also considered as complicated, time-consuming, and expensive methods [8]. Nowadays, the application of inexpensive, economic, efficient, and promising compounds such as melatonin have been significantly attracted many plant biologists for improving plant tolerance to different abiotic and biotic stresses. Melatonin is categorized as a novel plant growth regulator (PGR) which exists in various levels in different plant cells and tissues [9, 10]. Melatonin has a direct function in alleviating various stresses via scavenging both reactive nitrogen species (RNS) and reactive oxygen species (ROS) and plays also an indirect role in improving the photosynthesis, recovering leaf ultrastructure, increasing antioxidant activities, and stimulating and regulating plant growth regulators in plants [10, 11]. Therefore, it is necessary to apply exogenous melatonin for coping adverse environmental conditions because, sometimes, the endogenous level of melatonin is insufficient for this aim. Several studies have shown that the application of exogenous melatonin could significantly improve stress tolerance in different plants such as tobacco [12], Camellia sinensis [13], cucumber [14], soybean [15], alfalfa [16], rice [17], maize [18], cotton [19], grape [20], and kiwi [21]. Therefore, applying melatonin can be considered as a short-term and promising approach for alleviating drought stress in various economically important plants such as citrus. Citrus plants are widely cultivated in different tropical and subtropical parts of the world, where water scarcity is a main environmental limiting factor in agricultural productivity [22]. Decreasing in leaf area and photosynthetic potential as well as increasing in root length are the most common morphological responses of citrus to drought stress [10, 11]. However, the effect of melatonin on the plant morphological and physiological responses to drought stress can be considered as a multivariable process and viewed by different factors such as genotype, environmental conditions, and the concentration of melatonin, and the level of water scarcity. These factors lead to categorize this process as a complex and non-linear biological process. Thus, traditional statistical and computational methods such as simple regression cannot be considered as an appropriate approach for studying the effect of melatonin on plant biological responses to drought stress [23]. Hence, there is a serious need to use nonlinear statistical methodology such as artificial neural networks (ANNs). The reliability and accuracy of different ANNs such as Radial basis function (RBF), Multilayer Perceptron (MLP), and Generalized Regression Neural Network (GRNN) have been previously proven in different fields of science and technology such as in vitro culture, prediction of microRNAs and transcription factors (TFs), analysis of plant promoters, remote sensing studies, genome prediction, and phenomics studies [24–27]. ANNs are a type of nonlinear computational methods, which is applied for different aims such as clustering, predicting, and classifying the complex systems [28]. ANNs are able to identify the relationship between output and input variables and recognize the inherent knowledge existent in the datasets without previous physical considerations [29]. However, ANNs do not present a neat mathematical formula that illustrates the relative relationship of each independent variable in the model. Hence, ANNs are considered as a “black box”. ANNs consist of numerous highly interconnected processing neurons that work in parallel to find a solution for a particular problem. ANNs are learned by example. The examples should be carefully chosen otherwise time is wasted or even worse the model might be working inaccurately [23]. However, difficulty in achieving an optimized solution can be considered as one of the demerit points of most machine learning algorithms [23, 30, 31]. To overcome this bottleneck, Zhang et al. [32] employed the genetic algorithm (GA) as one of the common optimization algorithms for optimizing relative humidity, light duration, agar concentration, and culture temperature in order to maximize indirect shoot organogenesis in Cucumis melo. In another study, Non-dominated Sorting Genetic Algorithm-II (NSGA-II) was employed to optimize different types and concentrations of disinfectants as well as immersion time for maximizing explant viability and minimizing in vitro contamination in chrysanthemum [33]. However, most studies have found the optimized solution by trials and error [23, 34, 35]. NSGA-II is the search algorithms inspired by natural selection and genetics concepts. The fundamental principles of NSGA-II are the creation of an initial population of search solutions and then elite search solutions were selected for crossover using a roulette wheel selection method, which will ultimately be the best solution among them [36]. Therefore, this study has attempted to apply the NSGA-II to find the optimal levels of different factors involved in morphological responses to drought stress.
Considering the dominance of water scarcity in the south of Iran, which can affect the yield, growth, development, and quality of citrus fruits in these regions, the current study was aimed to investigate the effect of foliar melatonin on morphological parameters under different levels of water scarcity in Mexican lime (Citrus aurantifolia Swingle) and Persian lime (Citrus latifolia Tanaka) as the most commercially important Citrus plants by using ANNs. Three ANNs including, MLP, RBF, and GRNN were employed to compare their prediction accuracy and introduce the appropriate ANN for modeling and predicting morphological parameters under drought stress. Also, GA was linked to the best model to find the optimal condition for achieving the best morphological parameters. According to the best of our knowledge, this investigation is the first comparing ANNs study in the field of stress physiology.
Material and method
Plant material
This study was carried out at the greenhouse of College of Agriculture, Shiraz University, Iran, in September 2019 based on a completely randomized design in a factorial arrangement with 3 factors, including citrus species (Mexican lime and Persian lime), melatonin concentrations (0, 50, 100 and 150 μM), and level of water content (100, 75 and 40% of field capacity (FC)), and four replications. One-year-old seedlings of two citrus species, Mexican lime (Citrus aurantifolia Swingle) and Persian lime (Citrus latifolia Tanaka), were prepared from a commercial nursery (Jahrom, Iran). Both species were planted in plastic pots containing soil + leaf litter (3:2 w/w) and kept in the greenhouse under natural photoperiod at 25±2°C and 80% relative humidity and irrigated three times a week with 1/2 strength Hoagland solution. Melatonin treatments were implemented three times per week for 2 months by using a manual pump (30 ml per plant). The pots were also weighed every two days for applying drought stress (40, 75, and 100% FC). The morphological parameters including leaf length (cm), number of leaves/plants, crown diameter, plant height (cm), and internode length (cm) were measured after 15, 30, 45, and 60 days of the planting.
Modeling procedures
The obtained data were used for further analysis by using three types of ANNs including MLP, RBF, and GRNN in order to predict and model the morphological responses to melatonin under drought stress. Before modeling, to achieve the minimum mean squared error (MSE), the datasets were normalized between -1 and 1.
The input variables were melatonin concentrations, days after applying treatments, citrus species, and level of drought stress. Also, morphological responses including leaf length, number of leaves/plants, crown diameter, plant height, and internode length were chosen as target variables. To train and test each model, 70 and 30% of the data lines were randomly selected, respectively.
MLP model
The MLP as one of the most well-known ANNs includes one or more hidden layers, an input layer, and an output layer. A supervised training procedure is implemented by MLP that provides input and output variables to the network; the training set continues until the following equation would be minimized:
| (1) |
where K, yk, and are the number of datapoints, the kth observed data, and the kth forecasted data, respectively. In a three-layer MLP with n inputs and m neurons in the hidden layer determined as:
| (2) |
where xi is the ith input variable, w0 represents bias related to the neuron of output, wj0 is bias of the jth neuron of hidden layer, f represents transfer functions for the output layer, g is the transfer functions for hidden layer, wji is the weight connecting the jth neuron of hidden layer and the ith input variable, and wj represents weight linking the neuron of output layer and the jth neuron of hidden layer.
Determining the construction of the MLP has the main function in its performance [37, 38]. In the construction of this model, it is necessary to determine the number of neurons in each layer and the number of hidden layers [38]. Hornik et al. [39] revealed that the MLP with a sigmoid transfer function is general approximators; which shows that it could be trained to build any construction between the input and output variables. Therefore, the number of neurons in the hidden layer plays an important role in determining the construction of the MLP. Some studies [25] have recommended the proper number of neurons (m) based on the number of data (K) or the number of input (n). For example, Tang and Fishwick [40], Wong [41], and Wanas et al., [42] suggested "n", "2n", and "log (K)" as the suitable neuron number. Eventually, the optimal number of neurons in the hidden layer should be calculated by using trial and error, however, the reported offers could be employed as an initiating point. A large number of neurons contributes to the complexity of the network while a low number of them makes for simplicity of the network, therefore should be noted that a too simple network results in under-fitting, and conversely, becoming too complex causes over-fitting [37, 43].
RBF model
RBF is a three-layer ANN consisting of an input layer, a hidden layer, and an output layer. This is the basis and principal for radial basis networks, which organizes statistical ANNs. Statistical ANNs refer to networks which in contrast to the traditional ANNs implement regression-based approaches and have not been emulated by the biological neural networks [44]. In an RBF model, Euclidean distance between the center of each neuron and the input is considered as an input of transfer function for that neuron. The most well-known transfer function in RBF is the Gaussian function, which is determined based on the following equation:
| (3) |
where Xr, Xb, and h are input with unknown output, observed inputs in time b, and spread, respectively. The output of the function close to 1 when ∥Xr − Xb∥ approaches 0 and close to 0 when ∥Xr − Xb∥ approaches a large value. Finally, dependent variable (Yr) by predictor Xr is determined as follows:
| (4) |
where w0 and wj are the bias and weight of linkage between the bth hidden layer and the output layer, respectively.
GRNN model
GRNN introduced by Specht [45] is another kind of statistical ANNs with a very fast training process. In GRNN model, the number of observed data and the number of neurons in the hidden layer are equal. This model consists of an input layer, pattern layer, summation layer, and output layer. The pattern layer is completely connected to the input layer. D-summation and S-summation neurons of the summation layer are connected to the output derived from each neuron of the pattern layer. D-summation and S-summation neurons calculate the sum of the unweighted and weighted of the pattern layer, respectively. The connection weight between S-summation neuron and a neuron of the pattern layer is equal to the target output, while the connection weight for D-summation is unity. The output layer obtains the unknown value of output corresponding to the input vector, only via dividing the output of each S-summation neuron through the output of each D-summation neuron [46]. Consequently, the following equation is used to determine the output value:
| (5) |
where Yr represents the output value, and Tb is target associated with the bth observed data.
Performance measures
To assess and compare the accuracy of mentioned models, three following performance measures including R2 (coefficient of determination), Root Mean Square Error (RMSE), and Mean Bias Error (MBE) were used:
| (6) |
| (7) |
| (8) |
Where yt , , and T are the tth observed data, the mean of observed values, the mean of predicted values, and total number of predicted values, respectively. Greater R2 and smaller RMSE and MBE indicated better performance of the constructed models [47–49].
Optimization process by Non-dominated Sorting Genetic Algorithm-II (NSGA-II)
NSGA-II was implemented to optimize the value of input variables in order to find the best morphological responses. Also, a roulette wheel selection method was applied to choose the elite population for crossover. To obtain the best fitness, the initial population, generation number, mutation rate, and crossover rate were respectively adjusted to 200, 1000, 0.5, and 0.7. In the current study, the ideal point of Pareto was selected such that studied parameters became the maximum.
Sensitivity analysis
Sensitivity analysis was conducted to identify the importance degree of input variables (melatonin concentrations, citrus species, days after applying treatments, and level of drought stress) on the target variables (leaf length, number of leaves/plants, crown diameter, plant height, and internode length). The sensitivity of these parameters was measured by the criteria including variable sensitivity error (VSE) value displaying the performance (root mean square error (RMSE)) of GRNN-GA model when that input variable is removed from the model. Variable sensitivity ratio (VSR) value was determined as ratio of VSE and GRNN-NSGA-II model error (RMSE value) when all input variables are available. A higher important variable in the model was detected by higher VSR.
MATLAB (Matlab, 2010) software was employed to write codes and run the model.
Validation experiments
The melatonin concentrations, days after applying treatments, citrus species, and level of drought stress optimized by NSGA-II were experimentally tested to evaluate the efficiency of the GRNN-NSGA-II model.
Result
The effects of exogenous melatonin on plant growth and evaluation of drought tolerance
Based on our results, in some parameter there were no remarkable differences among melatonin-treated and non-treated plantlets under the well-irrigated (control) condition. On the other hand, drought stress had adverse impacts on morphological traits. When irrigation was stopped and progressive drought stress treatments were implemented, the morphological parameters significantly changed; however, melatonin-treated plantlets exhibited greener leaf tissues than the non-treated seedlings. Also, the highest length of seedlings was observed in plantlets treated by 100 μM melatonin which was significantly longer than control plantlets (non-treated seedlings). Moreover, the plantlets under drought stress displayed a significant decrease in crown diameter and leaf length (Table 1). The leaf elongation of both genotypes was significantly promoted by application melatonin. Moreover, the maximum crown diameter as well as leaf and internode length were obtained through the application of 50 and 100 μM melatonin (Table 1). Also, a relative decrease in plant height and crown diameter was observed in 150 μM melatonin treatment, indicating the inhibitory impact of higher doses of melatonin (Table 1). Generally, most morphological traits (leaf number and length, internode length, shoot height, and crown diameter) were significantly declined under drought stress. On the other hand, our results showed that the exogenous application of appropriate concentration of melatonin can help the genotypes resist the negative impacts of drought stress.
Table 1. Effect of melatonin concentrations, days after applying treatments, citrus species, and level of drought stress on morphological responses including leaf length, number of leaves/plants, crown diameter, plant height, and internode length.
| Melatonin concentration (μM) | Level of drought stress (% of field capacity) | Days after applying treatments | Citrus species (lime) | Number of leaves/plants | Leaf length (cm) | Internode length (cm) | Crown diameter (cm) | Plant height (cm) |
|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 15 | Persian | 678.00 | 12.675 | 5.390 | 3.450 | 178 |
| 0 | 100 | 30 | Persian | 664.25 | 12.925 | 5.765 | 3.447 | 178.7 |
| 0 | 100 | 45 | Persian | 651.50 | 13.075 | 6.022 | 3.435 | 180.1 |
| 0 | 100 | 60 | Persian | 644.75 | 13.075 | 6.197 | 2.130 | 180.5 |
| 0 | 75 | 15 | Persian | 168.25 | 3.650 | 1.375 | 1.180 | 110.3 |
| 0 | 75 | 30 | Persian | 162.75 | 3.375 | 1.390 | 1.145 | 110.6 |
| 0 | 75 | 45 | Persian | 161.25 | 3.325 | 1.390 | 1.127 | 111 |
| 0 | 75 | 60 | Persian | 163.75 | 2.925 | 1.397 | 1.105 | 94.9 |
| 0 | 40 | 15 | Persian | 136.25 | 3.400 | 1.197 | 1.172 | 97 |
| 0 | 40 | 30 | Persian | 134.50 | 3.475 | 1.202 | 1.172 | 98 |
| 0 | 40 | 45 | Persian | 133.25 | 3.125 | 1.202 | 1.130 | 98.1 |
| 0 | 40 | 60 | Persian | 124.50 | 2.900 | 1.202 | 1.067 | 149.7 |
| 50 | 100 | 15 | Persian | 513.50 | 6.125 | 2.892 | 3.095 | 149.7 |
| 50 | 100 | 30 | Persian | 511.00 | 5.975 | 2.892 | 3.115 | 149.9 |
| 50 | 100 | 45 | Persian | 503.75 | 5.550 | 2.955 | 3.120 | 150.1 |
| 50 | 100 | 60 | Persian | 499.50 | 5.375 | 2.897 | 3.437 | 120.2 |
| 50 | 75 | 15 | Persian | 297.25 | 5.325 | 1.825 | 2.137 | 120.6 |
| 50 | 75 | 30 | Persian | 291.25 | 5.225 | 1.845 | 2.125 | 120.8 |
| 50 | 75 | 45 | Persian | 287.00 | 4.550 | 1.852 | 2.117 | 120.8 |
| 50 | 75 | 60 | Persian | 282.75 | 4.500 | 1.852 | 3.100 | 120.8 |
| 50 | 40 | 15 | Persian | 254.50 | 4.325 | 1.592 | 2.110 | 124.9 |
| 50 | 40 | 30 | Persian | 248.75 | 3.900 | 1.595 | 2.090 | 125.7 |
| 50 | 40 | 45 | Persian | 245.00 | 3.800 | 1.595 | 2.057 | 126 |
| 50 | 40 | 60 | Persian | 239.00 | 3.525 | 1.595 | 2.147 | 126.1 |
| 100 | 100 | 15 | Persian | 774.00 | 12.625 | 8.375 | 3.642 | 174.9 |
| 100 | 100 | 30 | Persian | 772.00 | 12.975 | 8.620 | 3.597 | 178.5 |
| 100 | 100 | 45 | Persian | 768.75 | 12.875 | 8.720 | 3.607 | 179.3 |
| 100 | 100 | 60 | Persian | 766.50 | 13.00 | 8.880 | 3.600 | 179.6 |
| 100 | 75 | 15 | Persian | 622.25 | 9.700 | 7.362 | 3.487 | 175.5 |
| 100 | 75 | 30 | Persian | 619.25 | 10.075 | 7.807 | 3.452 | 178.5 |
| 100 | 75 | 45 | Persian | 626.25 | 10.175 | 7.895 | 3.432 | 180.1 |
| 100 | 75 | 60 | Persian | 625.04 | 10.325 | 8.115 | 3.425 | 189.5 |
| 100 | 40 | 15 | Persian | 540.25 | 8.675 | 5.325 | 3.437 | 165.5 |
| 100 | 40 | 30 | Persian | 539.75 | 8.525 | 5.962 | 3.307 | 166.3 |
| 100 | 40 | 45 | Persian | 535.75 | 8.475 | 5.980 | 3.310 | 166.6 |
| 100 | 40 | 60 | Persian | 538.50 | 8.325 | 6.212 | 3.305 | 166.8 |
| 150 | 100 | 15 | Persian | 511.00 | 7.625 | 3.192 | 3.262 | 156.2 |
| 150 | 100 | 30 | Persian | 508.25 | 7.400 | 3.295 | 3.340 | 156.5 |
| 150 | 100 | 45 | Persian | 503.75 | 7.200 | 3.360 | 3.232 | 156.8 |
| 150 | 100 | 60 | Persian | 499.50 | 7.075 | 3.370 | 3.265 | 157 |
| 150 | 75 | 15 | Persian | 459.00 | 5.225 | 2.650 | 2.447 | 140.2 |
| 150 | 75 | 30 | Persian | 453.50 | 5.150 | 2.710 | 2.427 | 140.2 |
| 150 | 75 | 45 | Persian | 449.75 | 4.525 | 2.732 | 2.425 | 140.6 |
| 150 | 75 | 60 | Persian | 446.50 | 4.425 | 2.750 | 2.402 | 140.6 |
| 150 | 40 | 15 | Persian | 404.25 | 4.400 | 2.120 | 2.280 | 135.2 |
| 150 | 40 | 30 | Persian | 397.50 | 3.975 | 2.340 | 2.285 | 135.8 |
| 150 | 40 | 45 | Persian | 388.00 | 3.675 | 2.420 | 2.240 | 136 |
| 150 | 40 | 60 | Persian | 382.00 | 3.350 | 2.475 | 2.185 | 136.2 |
| 0 | 100 | 15 | Mexican | 1024.50 | 8.150 | 3.240 | 2.577 | 345.5 |
| 0 | 100 | 30 | Mexican | 1017.50 | 8.275 | 3.660 | 2.590 | 347.6 |
| 0 | 100 | 45 | Mexican | 1014.75 | 8.425 | 3.830 | 2.590 | 348.2 |
| 0 | 100 | 60 | Mexican | 1009.25 | 8.575 | 4.297 | 1.265 | 348.6 |
| 0 | 75 | 15 | Mexican | 458.25 | 2.375 | 1.062 | 1.057 | 191.8 |
| 0 | 75 | 30 | Mexican | 455.50 | 2.125 | 1.072 | 1.032 | 192.1 |
| 0 | 75 | 45 | Mexican | 443.75 | 2.050 | 1.072 | 1.017 | 192.4 |
| 0 | 75 | 60 | Mexican | 438.50 | 1.825 | 1.072 | 1.035 | 192.5 |
| 0 | 40 | 15 | Mexican | 370.00 | 1.650 | 0.850 | 1.080 | 179.1 |
| 0 | 40 | 30 | Mexican | 362.50 | 1.300 | 0.852 | 1.037 | 178.7 |
| 0 | 40 | 45 | Mexican | 351.50 | 1.150 | 0.855 | 1.030 | 177 |
| 0 | 40 | 60 | Mexican | 349.25 | 1.125 | 0.855 | 1.025 | 177 |
| 50 | 100 | 15 | Mexican | 826.00 | 3.750 | 1.992 | 1.822 | 330.1 |
| 50 | 100 | 30 | Mexican | 820.50 | 3.500 | 2.025 | 1.880 | 330.4 |
| 50 | 100 | 45 | Mexican | 814.50 | 3.425 | 2.042 | 1.880 | 330.5 |
| 50 | 100 | 60 | Mexican | 811.75 | 3.150 | 2.052 | 1.880 | 330.5 |
| 50 | 75 | 15 | Mexican | 485.00 | 2.775 | 1.347 | 1.233 | 220.6 |
| 50 | 75 | 30 | Mexican | 476.25 | 2.600 | 1.375 | 1.217 | 220.8 |
| 50 | 75 | 45 | Mexican | 470.00 | 2.550 | 1.387 | 1.212 | 221.3 |
| 50 | 75 | 60 | Mexican | 465.25 | 2.375 | 1.450 | 1.135 | 221.4 |
| 50 | 40 | 15 | Mexican | 482.50 | 2.700 | 1.192 | 1.235 | 215.6 |
| 50 | 40 | 30 | Mexican | 461.50 | 2.225 | 1.197 | 1.217 | 216.2 |
| 50 | 40 | 45 | Mexican | 453.00 | 2.075 | 1.200 | 1.225 | 216.5 |
| 50 | 40 | 60 | Mexican | 445.50 | 1.550 | 1.202 | 1.250 | 216.7 |
| 100 | 100 | 15 | Mexican | 1063.25 | 8.375 | 6.135 | 2.855 | 344.5 |
| 100 | 100 | 30 | Mexican | 105.50 | 8.750 | 6.410 | 2.845 | 347.2 |
| 100 | 100 | 45 | Mexican | 1059.25 | 8.900 | 6.680 | 2.810 | 347.7 |
| 100 | 100 | 60 | Mexican | 1061.00 | 9.025 | 6.785 | 2.795 | 348.4 |
| 100 | 75 | 15 | Mexican | 1022.00 | 5.700 | 5.172 | 2.640 | 345.6 |
| 100 | 75 | 30 | Mexican | 1023.50 | 5.825 | 5.580 | 2.650 | 347.4 |
| 100 | 75 | 45 | Mexican | 1019.25 | 5.975 | 5.755 | 2.630 | 348.1 |
| 100 | 75 | 60 | Mexican | 1013.75 | 6.100 | 5.955 | 2.602 | 348.7 |
| 100 | 40 | 15 | Mexican | 877.50 | 4.500 | 3.137 | 2.385 | 339 |
| 100 | 40 | 30 | Mexican | 875.25 | 4.425 | 3.650 | 2.360 | 340.6 |
| 100 | 40 | 45 | Mexican | 874.25 | 4.325 | 3.815 | 2.275 | 341.1 |
| 100 | 40 | 60 | Mexican | 827.50 | 4.175 | 4.377 | 2.260 | 341.8 |
| 150 | 100 | 15 | Mexican | 827.50 | 4.300 | 2.390 | 2.120 | 332.9 |
| 150 | 100 | 30 | Mexican | 823.00 | 4.100 | 2.375 | 2.110 | 333.3 |
| 150 | 100 | 45 | Mexican | 820.00 | 3.975 | 2.420 | 2.072 | 333.4 |
| 150 | 100 | 60 | Mexican | 815.00 | 3.825 | 2.422 | 2.070 | 333.4 |
| 150 | 75 | 15 | Mexican | 770.50 | 3.025 | 1.692 | 1.655 | 320.6 |
| 150 | 75 | 30 | Mexican | 763.50 | 2.625 | 1.715 | 1.635 | 321.2 |
| 150 | 75 | 45 | Mexican | 745.00 | 2.550 | 1.732 | 1.617 | 321.2 |
| 150 | 75 | 60 | Mexican | 757.50 | 2.100 | 1.755 | 1.612 | 321.2 |
| 150 | 40 | 15 | Mexican | 626.75 | 2.400 | 1.132 | 1.440 | 316 |
| 150 | 40 | 30 | Mexican | 617.25 | 1.925 | 1.285 | 1.405 | 316.9 |
| 150 | 40 | 45 | Mexican | 602.50 | 1.725 | 1.390 | 1.395 | 317.2 |
| 150 | 40 | 60 | Mexican | 593.00 | 1.625 | 1.447 | 1.395 | 317.5 |
Comparison of MLP, RBF, and GRNN models
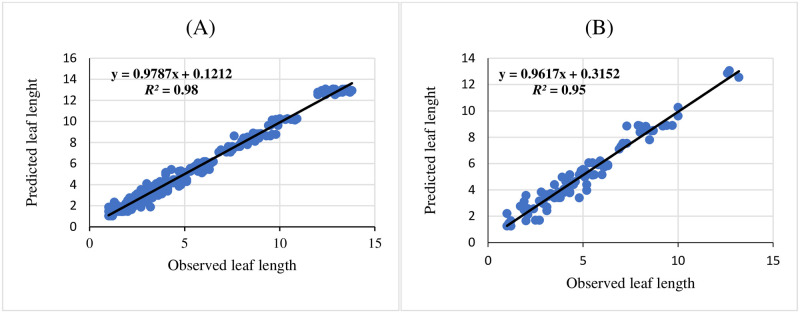
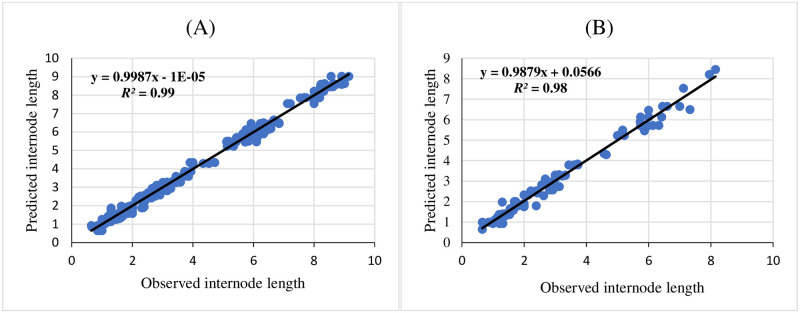
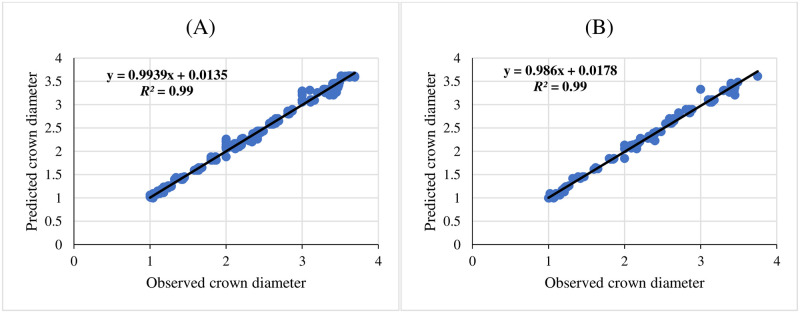
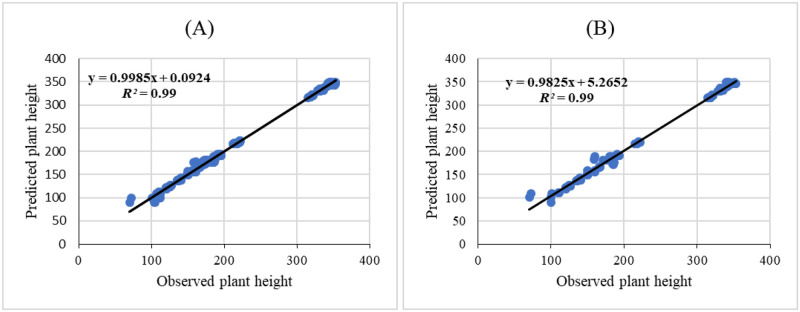
Three ANNs including MLP, RBF, and GRNN were used for modeling and predicting the effect of melatonin on morphological responses of citrus to drought stress. R2, RMSE, and MBE of each developed model were presented in Table 2. High significant R2 value and low RMSE and MBE values displayed the model capability. The results showed that GRNN and RBF had better prediction accuracy than MLP for all studied parameters. Also, comparing the results of GRNN and RBF (Table 2) revealed that GRNN is more accurate than RBF in all studied parameters. The regression lines (Figs 1–5) demonstrated that a good fit correlation between the forecasted and observed data of all studied parameters for both the training and testing sets in GRNN models.
Table 2. Comparison statistics of different ANNs including MLP, GRNN, and RBFNN for modeling and predicting the effect of melatonin on morphological responses of citrus to drought stress.
| Model | Measured parameter | Training | Testing | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE | MBE | R2 | RMSE | MBE | ||
| MLP | leaf length | 0.89 | 1.02 | -0.93 | 0.87 | 1.34 | 0.86 |
| number of leaves/plants | 0.91 | 29.61 | 6.53 | 0.89 | 36.57 | 9.11 | |
| crown diameter | 0.94 | 0.68 | -0.08 | 0.88 | 0.66 | 1.05 | |
| plant height | 0.89 | 9.09 | 1.08 | 0.88 | 11.49 | -1.87 | |
| internode length | 0.91 | 1.23 | -0.08 | 0.91 | 1.23 | -1.09 | |
| GRNN | leaf length | 0.98 | 0.41 | 0.003 | 0.95 | 0.60 | 0.13 |
| number of leaves/plants | 0.99 | 16.07 | 0.82 | 0.99 | 17.86 | 2.07 | |
| crown diameter | 0.99 | 0.06 | -0.0001 | 0.99 | 0.07 | -0.01 | |
| plant height | 0.99 | 4.11 | -0.23 | 0.99 | 7.27 | 1.35 | |
| internode length | 0.99 | 0.19 | -0.004 | 0.98 | 0.25 | 0.02 | |
| RBF | leaf length | 0.94 | 0.68 | 0.06 | 0.91 | 1.02 | -0.53 |
| number of leaves/plants | 0.98 | 18.23 | 1.36 | 0.96 | 26.32 | 6.12 | |
| crown diameter | 0.99 | 0.12 | 0.02 | 0.98 | 0.19 | -0.57 | |
| plant height | 0.96 | 6.03 | -0.76 | 0.94 | 9.43 | -1.48 | |
| internode length | 0.98 | 0.95 | -0.07 | 0.95 | 0.68 | 0.94 |
Fig 1. Scatter plot of observed vs. predicted results of leaf length obtained by GRNN model including (A) training set and (B) testing set.
Fig 5. Scatter plot of observed vs. predicted results of internode length obtained by GRNN model including (A) training set and (B) testing set.
Fig 2. Scatter plot of observed vs. predicted results of number of leaves/plants obtained by GRNN model including (A) training set and (B) testing set.
Fig 3. Scatter plot of observed vs. predicted results of crown diameter obtained by GRNN model including (A) training set and (B) testing set.
Fig 4. Scatter plot of observed vs. predicted results of plant height obtained by GRNN model including (A) training set and (B) testing set.
Optimizing morphological parameters through NSGA-II
NSGA-II was linked to the GRNN in order to determine the optimal level of melatonin concentrations, days after applying treatments, citrus species, and level of drought stress for obtaining the best morphological responses to drought stress. The results of the optimization process were presented in Table 3. Based on optimization results, applying 89.63 melatonin on Mexican lime under 94.65 FC after 53.69 days resulted in 12.35 cm leaf length, 1063.21 leaves/plant, 3.41 cm crown diameter, 324.63 cm plant height, and 8.77 cm internode length.
Table 3. The results of GRNN-NSGA-II to find the optimal level of melatonin concentrations, days after applying treatments, citrus species, and level of drought stress for obtaining the best morphological responses to drought stress.
| input variable | leaf length | number of leaves/plants | crown diameter | plant height | internode length | |||
|---|---|---|---|---|---|---|---|---|
| melatonin concentrations | days after applying treatments | citrus species | level of drought stress | |||||
| 89.63 | 53.69 | Mexican Lime | 94.65 | 12.35 | 1063.21 | 3.41 | 324.63 | 8.77 |
Sensitivity analysis of the models
The results of sensitivity analysis were summarized in Table 4. Based on sensitivity analysis, leaf length and number of leaves/plants were more sensitive to melatonin concentration, followed by level of drought stress, days after applying treatments, and species. Also, as can be seen in Table 4, melatonin concentration was the most important factor for crown diameter, followed by species, days after applying treatments, and level of drought stress, respectively. Also, plant height was more sensitive to species, followed by melatonin concentration, days after applying treatments, and level of drought stress. Moreover, melatonin concentration was the most important factor for internode length, followed by days after applying treatments, species, and level of drought stress (Table 4).
Table 4. The results of sensitivity analysis to find the importance of each input in morphological responses to drought stress.
| Output | Item | Melatonin concentrations | Days after applying treatments | Citrus species | Level of drought stress |
|---|---|---|---|---|---|
| leaf length | VSR | 3.36 | 2.76 | 1.97 | 2.94 |
| Rank | 1 | 3 | 4 | 2 | |
| number of leaves/plants | VSR | 1.94 | 1.28 | 1.09 | 1.75 |
| Rank | 1 | 3 | 4 | 2 | |
| crown diameter | VSR | 2.09 | 1.82 | 1.95 | 1.65 |
| Rank | 1 | 3 | 2 | 4 | |
| plant height | VSR | 2.31 | 1.73 | 2.65 | 1.66 |
| Rank | 2 | 3 | 1 | 4 | |
| internode length | VSR | 2.03 | 1.74 | 1.36 | 1.15 |
| Rank | 1 | 2 | 3 | 4 |
Validation experiment
According to the validation experiment, the differences between experimental validation data and predicted data via GRNN-NSGA-II were not significant. Therefore, it can be concluded that GRNN-NSGA-II with better performance than RBF or MLP can be employed for accurately predicting and optimizing the effects of melatonin on morphological responses under drought stress.
Discussion
Drought can be considered as one of the most common abiotic stresses that restricts plant growth and development as well as productivity through changing different morphological, biochemical, physiological, and molecular pathways [50]. Continuous drought stress leads to a water shortage inside the different plant cells, tissues, and organs. Moreover, the effects of drought stress are usually first observed in the leaf tissues which gradually fold and curl, and then, are seen in whole plant growth and development [51]. By losing water content, the leaves under drought stress have initiated to roll up and turn yellow and necrosis. Also, drought stress resulted in the decline in plant height which might be due to the decreases in cell turgor, cell growth, cell elongation, and cell volume [52]. Previous studies showed that drought stress plays a pivotal role in decreasing water contents, inhibiting cell expansion and division, increasing osmotic stress, and, generally declining plant growth [53]. In the current study, drought stress led to inhibition of citrus seedlings growth. However, the results of the current study indicated that the negative impacts of drought stress in both citrus genotypes could be mitigated by the application of melatonin through improving morphological responses. Also, our results elucidated that the melatonin-treated plants in comparison with untreated plants exhibited larger plant height, leaf number and length, crown diameter and internode length with better tolerance potential under the water scarcity due to the assimilates of water and osmolytes to growing cells and tissues. A high growth of leaf (length / number) during drought stress might be due to the improved stomatal conductance related to the foliar-sprayed melatonin. Therefore, leaf growth via cell proliferation and expansion may be influenced by exogenous application of melatonin. Indeed, the duration and rate of cell division, as essential parameters of cell proliferation, play an important role in the leaf size [54]. In line with our results, previous studies revealed that the exogenous application of melatonin can improve morphological responses under drought stress in different plants such as tobacco [12], Camellia sinensis [13], cucumber [14], soybean [15], alfalfa [16], rice [17], maize [18], cotton [19], grape [20], and kiwi [21]. It seems that melatonin as a novel plant growth regulator, independently of auxin signaling, plays a pivotal role in shoot and root growth and development [10, 11]. Although melatonin at a lower level improves growth and development, a higher level of this plant growth regulator has an inhibitory function; therefore, melatonin can be considered as a dose-dependent growth regulator [55].
Morphological changes to drought stress are a multivariable process that is influenced by several factors. Also, morphological responses to drought stress are a highly complex and nonlinear process. Therefore, there is a serious need to employ robust nonlinear computational methods for analyzing plant biological processes. The efficiency of a good statistical approach depends on the neat understanding of the variable structure, experimental design, and using the appropriate model [56]. One of the most important primary requirements to identify suitable statistical approaches is comprehending the type of data [57, 58]. Variables can be clustered into two groups including quantitative (continuous and discrete) and qualitative (ordinal and nominal). Names with two or more classes without a hierarchical order are categorized as nominal variables, while ordinal data have distinct order (level X is more intense than level Y) [57, 59]. Counts that include integers are classified as discrete data, while measurements along a continuum, which could be included smaller fractions are categorized as continuous variables [60]. Plant biological data can be categorized as ordinal (plant quality rated as weak, moderate, and good), nominal (type of morphological responses such as normal and necrosis), continuous (height of shoots or roots), and discrete (number of leaf). Traditional linear methods such as regression and ANOVA must be just applied with continuous variables that demonstrate a linear relationship between the explanatory and dependent variables [23, 61, 62]. Hence, the conventional computational approaches are not appropriate for analyzing plant biological processes [57]. Recently, different machine learning algorithms have been successfully utilized to predict and optimize various plant physiological processes. Several studies [24, 58, 63] used MLP to predict various plant biological processes. However, they only applied the MLP model and did not compare this well-known algorithm with other models. In the current study, different ANNs including GRNN, RBF, and MLP, for the first time, were used to develop a suitable model for prediction morphological responses to various concentrations of melatonin under different levels of drought stress and compare their prediction accuracy. According to our results, RBF and GRNN had more accuracy than MLP for modeling and predicting the system. Also, NSGA-II was linked to GRNN as the most suitable model for the optimization process. Based on our results, GRNN-NSGA-II can be considered as an efficient computational methodology for modeling and predicting morphological responses in the field of stress physiology. Also, according to the current results, the developed model presented here would allow more accurate estimations without the requirements for the availability of all data.
Conclusion
In the current study, different ANNs including GRNN, MLP, and RBF along with NSGA-II were used for studying and predicting the morphological responses of citrus under drought stress for the first time. The great accordance between the predicted and observed data support the high efficiency of ANNs. Overall, ANNs effectively model complex input-output patterns and show a supreme ability for studying and predicting different plant morphological, physiological, and biochemical responses to abiotic stresses. Therefore, ANN-NSGA-II can be employed as a new computational strategy in the field of stress physiology. While ANNs usually have good performance in prediction, they are limited to explicitly illustrate the effects of explanatory variables on the response variable, due to their “black box” feature. Therefore, it would be suggested to compare ANNs with other machine-learning methods (e.g., Random Forest, Gradient Boosting, support vector machine), to allow a more thorough appreciation of the relative potential of ANNs applied to the presented problem.
Acknowledgments
The authors thank the anonymous reviewers for their valuable comments and suggestions. We also thank College of Agriculture, Shiraz University, Iran for their help and efforts.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Swann ALS. Plants and Drought in a Changing Climate. Current Climate Change Reports. 2018;4(2):192–201. 10.1007/s40641-018-0097-y [DOI] [Google Scholar]
- 2.Nawaz A, Farooq M, Cheema SA, Yasmeen A, Wahid A. Stay green character at grain filling ensures resistance against terminal drought in wheat. International Journal of Agriculture & Biology. 2013;15(6):1272–6. [Google Scholar]
- 3.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2008;103(4):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161(11):1189–202. 10.1016/j.jplph.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 5.Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiologiae Plantarum. 2016;38(4):82 10.1007/s11738-016-2101-2 [DOI] [Google Scholar]
- 6.Kamran M, Wennan S, Ahmad I, Xiangping M, Wenwen C, Xudong Z, et al. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Scientific Reports. 2018;8(1):4818 10.1038/s41598-018-23166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naeem M, Naeem MS, Ahmad R, Ahmad R, Ashraf MY, Ihsan MZ, et al. Improving drought tolerance in maize by foliar application of boron: water status, antioxidative defense and photosynthetic capacity. Archives of Agronomy and Soil Science. 2018;64(5):626–39. 10.1080/03650340.2017.1370541 [DOI] [Google Scholar]
- 8.Moshelion M, Altman A. Current challenges and future perspectives of plant and agricultural biotechnology. Trends in Biotechnology. 2015;33(6):337–42. 10.1016/j.tibtech.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Fleta-Soriano E, Díaz L, Bonet E, Munné-Bosch S. Melatonin may exert a protective role against drought stress in maize. Journal of Agronomy and Crop Science. 2017;203(4):286–94. 10.1111/jac.12201 [DOI] [Google Scholar]
- 10.Alam MN, Wang Y, Chan Z. Physiological and biochemical analyses reveal drought tolerance in cool-season tall fescue (Festuca arundinacea) turf grass with the application of melatonin. Crop and Pasture Science. 2018;69(10):1041–9. 10.1071/CP18394 [DOI] [Google Scholar]
- 11.Manchester LC, Coto-Montes A, Boga JA, Andersen LPH, Zhou Z, Galano A, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. Journal of Pineal Research. 2015;59(4):403–19. 10.1111/jpi.12267 [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Li D, Ma Y, Shen H, Zhao S, Wang Y. Combined Application of Arbuscular Mycorrhizal Fungi and Exogenous Melatonin Alleviates Drought Stress and Improves Plant Growth in Tobacco Seedlings. Journal of Plant Growth Regulation. 2020. 10.1007/s00344-020-10165-6 [DOI] [Google Scholar]
- 13.Li J, Yang Y, Sun K, Chen Y, Chen X, Li X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules. 2019;24(9):1826 10.3390/molecules24091826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H-J, Zhang N, Yang R-C, Wang L, Sun Q-Q, Li D-B, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). Journal of Pineal Research. 2014;57(3):269–79. 10.1111/jpi.12167 [DOI] [PubMed] [Google Scholar]
- 15.Wei W, Li Q-T, Chu Y-N, Reiter RJ, Yu X-M, Zhu D-H, et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Journal of Experimental Botany. 2014;66(3):695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cen H, Wang T, Liu H, Tian D, Zhang Y. Melatonin Application Improves Salt Tolerance of Alfalfa (Medicago sativa L.) by Enhancing Antioxidant Capacity. Plants. 2020;9(2):220 10.3390/plants9020220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Yu B, Cui Y, Yin Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regulation. 2017;83(3):441–54. 10.1007/s10725-017-0310-3 [DOI] [Google Scholar]
- 18.Sun L, Song F, Guo J, Zhu X, Liu S, Liu F, et al. Nano-ZnO-Induced Drought Tolerance Is Associated with Melatonin Synthesis and Metabolism in Maize. International Journal of Molecular Sciences. 2020;21(3):782 10.3390/ijms21030782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, Cao Y, Loka DA, Harris-Shultz KR, Reiter RJ, Ali S, et al. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiology and Biochemistry. 2020;151:579–88. 10.1016/j.plaphy.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Meng J-F, Xu T-F, Wang Z-Z, Fang Y-L, Xi Z-M, Zhang Z-W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. Journal of Pineal Research. 2014;57(2):200–12. 10.1111/jpi.12159 [DOI] [PubMed] [Google Scholar]
- 21.Xia H, Ni Z, Hu R, Lin L, Deng H, Wang J, et al. Melatonin Alleviates Drought Stress by a Non-Enzymatic and Enzymatic Antioxidative System in Kiwifruit Seedlings. International Journal of Molecular Sciences. 2020;21(3):852 10.3390/ijms21030852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J. Chapter 10—Lasiodiplodia theobromae in Citrus Fruit (Diplodia Stem-End Rot) In: Bautista-Baños S, editor. Postharvest Decay. San Diego: Academic Press; 2014. p. 309–35. [Google Scholar]
- 23.Silva JCF, Teixeira RM, Silva FF, Brommonschenkel SH, Fontes EPB. Machine learning approaches and their current application in plant molecular biology: A systematic review. Plant Science. 2019;284:37–47. 10.1016/j.plantsci.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 24.Hesami M, Alizadeh M, Naderi R, Tohidfar M. Forecasting and optimizing Agrobacterium-mediated genetic transformation via ensemble model- fruit fly optimization algorithm: A data mining approach using chrysanthemum databases. Plos One. 2020;15:e0239901 10.1371/journal.pone.0239901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesami M, Jones AMP. Application of Artificial Intelligence Models and Optimization Algorithms in Plant Cell and Tissue Culture. Applied Microbiology and Biotechnology. 2020;104:1–37. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Q, Huang H, Pei X, Wong SC, Gao M. Rule extraction from an optimized neural network for traffic crash frequency modeling. Accident Analysis & Prevention. 2016;97:87–95. 10.1016/j.aap.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 27.Zeng Q, Huang H, Pei X, Wong SC. Modeling nonlinear relationship between crash frequency by severity and contributing factors by neural networks. Analytic Methods in Accident Research. 2016;10:12–25. 10.1016/j.amar.2016.03.002 [DOI] [Google Scholar]
- 28.Niazian M, Shariatpanahi ME, Abdipour M, Oroojloo M. Modeling callus induction and regeneration in an anther culture of tomato (Lycopersicon esculentum L.) using image processing and artificial neural network method. Protoplasma. 2019;256(5):1317–32. 10.1007/s00709-019-01379-x [DOI] [PubMed] [Google Scholar]
- 29.Hesami M, Naderi R, Yoosefzadeh-Najafabadi M, Rahmati M. Data-driven modeling in plant tissue culture. J Appl Environ Biol Sci. 2017;7(8):37–44. [Google Scholar]
- 30.Moravej M, Amani P, Hosseini-Moghari S-M. Groundwater level simulation and forecasting using interior search algorithm-least square support vector regression (ISA-LSSVR). Groundwater for Sustainable Development. 2020:100447 10.1016/j.gsd.2020.100447 [DOI] [Google Scholar]
- 31.Araghinejad S, Fayaz N, Hosseini-Moghari S-M. Development of a Hybrid Data Driven Model for Hydrological Estimation. Water Resources Management. 2018;32(11):3737–50. 10.1007/s11269-018-2016-3 [DOI] [Google Scholar]
- 32.Zhang Q, Deng D, Dai W, Li J, Jin X. Optimization of culture conditions for differentiation of melon based on artificial neural network and genetic algorithm. Sci Rep. 2020;10(1):3524 10.1038/s41598-020-60278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesami M, Naderi R, Tohidfar M. Modeling and Optimizing Medium Composition for Shoot Regeneration of Chrysanthemum via Radial Basis Function-Non-dominated Sorting Genetic Algorithm-II (RBF-NSGAII). Scientific Reports. 2019;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moravej M. Discussion of “Modified Firefly Algorithm for Solving Multireservoir Operation in Continuous and Discrete Domains” by Irene Garousi-Nejad, Omid Bozorg-Haddad, and Hugo A. Loáiciga. Journal of Water Resources Planning and Management. 2017;143(10):07017004 10.1061/(ASCE)WR.1943-5452.0000836 [DOI] [Google Scholar]
- 35.Hesami M, Naderi R, Yoosefzadeh-Najafabadi M. Optimizing sterilization conditions and growth regulator effects on in vitro shoot regeneration through direct organogenesis in Chenopodium quinoa. BioTechnologia. 2018;99(1):49–57. 10.5114/bta.2018.73561 [DOI] [Google Scholar]
- 36.Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M. Application of Adaptive Neuro-Fuzzy Inference System-Non-dominated Sorting Genetic Algorithm-II (ANFIS-NSGAII) for Modeling and Optimizing Somatic Embryogenesis of Chrysanthemum. Frontiers in Plant Science. 2019;10(869). 10.3389/fpls.2019.00869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domingues GF, Soares VP, Leite HG, Ferraz AS, Ribeiro CAAS, Lorenzon AS, et al. Artificial neural networks on integrated multispectral and SAR data for high-performance prediction of eucalyptus biomass. Comput Electron Agric. 2020;168:105089 10.1016/j.compag.2019.105089 [DOI] [Google Scholar]
- 38.Niazian M, Sadat-Noori SA, Abdipour M. Modeling the seed yield of Ajowan (Trachyspermum ammi L.) using artificial neural network and multiple linear regression models. Ind Crop Prod. 2018;117:224–34. 10.1016/j.indcrop.2018.03.013 [DOI] [Google Scholar]
- 39.Hornik K, Stinchcombe M, White H. Multilayer feedforward networks are universal approximators. Neural Networks. 1989;2(5):359–66. [Google Scholar]
- 40.Tang Z, Fishwick PA. Feedforward neural nets as models for time series forecasting. Orsa J Comput. 1993;5(4):374–85. 10.1287/ijoc.5.4.374 [DOI] [Google Scholar]
- 41.Wong FS. Time series forecasting using backpropagation neural networks. Neurocomputing. 1991;2(4):147–59. 10.1016/0925-2312(91)90045-D [DOI] [Google Scholar]
- 42.Wanas N, Auda G, Kamel MS, Karray F, editors. On the optimal number of hidden nodes in a neural network. Conference Proceedings IEEE Canadian Conference on Electrical and Computer Engineering (Cat No 98TH8341); 1998: IEEE.
- 43.Hosseini-Moghari SM, Araghinejad S. Monthly and seasonal drought forecasting using statistical neural networks. Environ Earth Sci. 2015;74(1):397–412. 10.1007/s12665-015-4047-x [DOI] [Google Scholar]
- 44.Lin J, Zhao Y, Watson D, Chen C. The radial basis function differential quadrature method with ghost points. Math Comput Simul. 2020;173:105–14. 10.1016/j.matcom.2020.01.006 [DOI] [Google Scholar]
- 45.Specht DF. A general regression neural network. IEEE T Neur Net. 1991;2(6):568–76. 10.1109/72.97934 [DOI] [PubMed] [Google Scholar]
- 46.Lan T, Tong C, Yu H, Shi X, Luo L. Nonlinear process monitoring based on decentralized generalized regression neural networks. Expert Syst Appl. 2020;150:113273 10.1016/j.eswa.2020.113273 [DOI] [Google Scholar]
- 47.Dong B, Ma X, Chen F, Chen S. Investigating the Differences of Single-Vehicle and Multivehicle Accident Probability Using Mixed Logit Model. Journal of Advanced Transportation. 2018;2018:2702360 10.1155/2018/2702360 [DOI] [Google Scholar]
- 48.Chen F, Chen S, Ma X. Analysis of hourly crash likelihood using unbalanced panel data mixed logit model and real-time driving environmental big data. Journal of Safety Research. 2018;65:153–9. 10.1016/j.jsr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Chen F, Chen S, Ma X. Crash Frequency Modeling Using Real-Time Environmental and Traffic Data and Unbalanced Panel Data Models. International Journal of Environmental Research and Public Health. 2016;13(6):609 10.3390/ijerph13060609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta N, Thind SK, Bains NS. Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought stressed wheat. Plant Growth Regulation. 2014;72(3):221–8. 10.1007/s10725-013-9853-0 [DOI] [Google Scholar]
- 51.Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, et al. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Frontiers in Plant Science. 2018;9(393):393 10.3389/fpls.2018.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bañon S, Ochoa J, Franco JA, Alarcón JJ, Sánchez-Blanco MJ. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environmental and Experimental Botany. 2006;56(1):36–43. 10.1016/j.envexpbot.2004.12.004 [DOI] [Google Scholar]
- 53.Alam MM, Nahar K, Hasanuzzaman M, Fujita M. Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics. 2014;7(4):271–83. [Google Scholar]
- 54.Zeng L, Cai J-s, Li J-j, Lu G-y, Li C-s, Fu G-p, et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. Journal of Integrative Agriculture. 2018;17(2):328–35. 10.1016/S2095-3119(17)61757-X [DOI] [Google Scholar]
- 55.Dai L, Li J, Harmens H, Zheng X, Zhang C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiology and Biochemistry. 2020;149:86–95. 10.1016/j.plaphy.2020.01.039 [DOI] [PubMed] [Google Scholar]
- 56.Goudarzi A, Li Y, Xiang J. A hybrid non-linear time-varying double-weighted particle swarm optimization for solving non-convex combined environmental economic dispatch problem. Applied Soft Computing. 2020;86:105894 10.1016/j.asoc.2019.105894 [DOI] [Google Scholar]
- 57.Zielinska S, Kepczynska E. Neural modeling of plant tissue cultures: a review. BioTechnologia. 2013;94(3):253–68. 10.5114/bta.2013.46419 [DOI] [Google Scholar]
- 58.Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M. Development of support vector machine-based model and comparative analysis with artificial neural network for modeling the plant tissue culture procedures: effect of plant growth regulators on somatic embryogenesis of chrysanthemum, as a case study. Plant Methods. 2020;16(1):112 10.1186/s13007-020-00655-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akin M, Eyduran SP, Eyduran E, Reed BM. Analysis of macro nutrient related growth responses using multivariate adaptive regression splines. Plant Cell, Tissue and Organ Culture. 2020;140:661–70. 10.1007/s11240-019-01763-8 [DOI] [Google Scholar]
- 60.Akin M, Eyduran E, Reed BM. Use of RSM and CHAID data mining algorithm for predicting mineral nutrition of hazelnut. Plant Cell, Tissue and Organ Culture. 2017;128(2):303–16. 10.1007/s11240-016-1110-6 [DOI] [Google Scholar]
- 61.Akin M, Hand C, Eyduran E, Reed BM. Predicting minor nutrient requirements of hazelnut shoot cultures using regression trees. Plant Cell, Tissue and Organ Culture. 2018;132(3):545–59. 10.1007/s11240-017-1353-x [DOI] [Google Scholar]
- 62.Hesami M, Condori-Apfata JA, Valencia MV, Mohammadi M. Application of Artificial Neural Network for Modeling and Studying In Vitro Genotype-Independent Shoot Regeneration in Wheat. Applied Sciences. 2020;10:5370 10.3390/app10155370 [DOI] [Google Scholar]
- 63.Hesami M, Naderi R, Tohidfar M. Modeling and optimizing in vitro sterilization of chrysanthemum via multilayer perceptron-non-dominated sorting genetic algorithm-II (MLP-NSGAII). Front Plant Sci. 2019;10:282 10.3389/fpls.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.