It is reasonable to wonder why inhibiting dihydroorotate dehydrogenase (DHODH), a protean and vital metabolic enzyme, would be expected to solve, not exacerbate, prevalent oncotherapy problems of toxicity and resistance. Yet, in addition to ASLAN003, described in this issue of Haematologica,1 at least four other DHODH inhibitors are being developed for oncotherapy.2 DHODH is the sole mitochondrial enzyme in the pathway of de novo pyrimidine synthesis, which makes pyrimidine nucleobases from glutamine and aspartate. Pyrimidines are not just building blocks for DNA and RNA, but are also key cofactors for glycoprotein, glycolipid and phospholipid synthesis. Moreover, the reaction that DHODH executes, reduction of dihydroorotate to orotate, is coupled to mitochondrial electron transport, to manufacture ATP independently of glucose and the Krebs cycle. Not surprisingly, therefore, DHODH is vital - its knock-out is lethal. Surprisingly, however, treatment of malignant cells with clinically tolerable concentrations of DHODH inhibitors induces not the cytotoxicity (apoptosis) expected from most anti-metabolite oncotherapeutics but terminal differentiation.
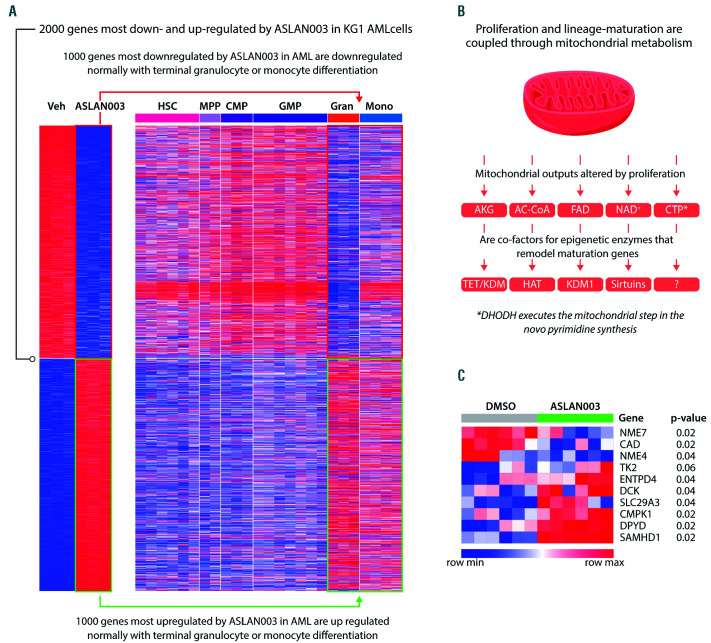
Unbiased analyses illustrate this: of the thousands of genes most significantly up- and down-regulated by ASLAN003 treatment of acute myeloid leukemia (AML) cells, most are the same genes coordinately up- and downregulated during normal myeloid differentiation into granulocytes or monocytes (Figure 1A). Such ready recapitulation of normal lineage progression is rendered less astonishing upon recognition that malignant cells express very high levels of lineage differentiation-driving master transcription factors to begin with, e.g., SPI1, CEBPA, RUNX1 in AML cells.3,4 One function of these lineage master transcription factors is to activate lineage differentiation programs, but another is to cooperate with MYC for high-grade activation of proliferation – coupling of exponential proliferation and onward differentiation in this way is a feature of metazoan biology sometimes called ‘transit amplification’. Oncogenic mutations decouple exponential proliferation from onward differentiation to create malignant self-replication.4 In short, partial DHODH inhibition reconnects circuitry already present to release malignant cells to complete lineage journeys already begun (Figure 1A).
This modality for leukemia/cancer cytoreduction is worthy of investment for three fundamental reasons. First, cell cycle exiting by terminal differentiation does not require the p53 apoptosis machinery that mediates cytoreduction by antimetabolite chemotherapeutics in general, and thus offers activity even in chemorefractory disease with p53- system mutations.4 Second, DHODH inhibitor-mediated induction of terminal differentiation is not restricted to rare AML or genetic subtypes of cancer, although differences in pyrimidine metabolism between histologically diverse cancers may influence this activity (discussed below). Last but not least, non-cytotoxic differentiation-based oncotherapy can spare normal dividing cells essential for health/normal lifespan, offering a good therapeutic index.4 To efficiently realize these fundamentals in the clinic, however, an obvious question needs an answer: how exactly does partial inhibition of DHODH, a protean metabolic enzyme, reconnect cancer cells to terminal lineage fates intended by their master transcription factor content?
Given the contributions of DHODH and pyrimidines to so many fundamental cellular functions, it is difficult to know where to begin to answer this question. Fortunately, work spanning decades has provided excellent clues. One important observation is that the small molecule cyclopentenyl cytosine (CPEC), which inhibits the last step in de novo pyrimidine synthesis, uridine triphosphate (UTP) amination into cytidine triphosphate (CTP) by CTP synthase 2 (CTPS2), also releases AML and solid tumor cancer cells to terminal lineage fates.5,6 Moreover, exogenous cytidine that restored CTP but not UTP pools, and exogenous uridine Given the contributions of DHODH and pyrimidines to so many fundamental cellular functions, it is difficult to know where to begin to answer this question. Fortunately, work spanning decades has provided excellent clues. One important observation is that the small molecule cyclopentenyl cytosine (CPEC), which inhibits the last step in de novo pyrimidine synthesis, uridine triphosphate (UTP) amination into cytidine triphosphate (CTP) by CTP synthase 2 (CTPS2), also releases AML and solid tumor cancer cells to terminal lineage fates.5,6 Moreover, exogenous cytidine that restored CTP but not UTP pools, and exogenous uridine
It is of course no surprise that basic building blocks such as CTP are essential for cell proliferation, including malignant proliferation. But how is CTP linked to lineage progression? Several mitochondrial products serve as cofactors for key epigenetic enzymes that remodel lineage-differentiation genes for activation or repression (Figure 1B).10 Alphaketoglutarate (AKG) is an essential cofactor for dioxygenases including TET DNA methylcytosine dioxygenases, and Jumonji domain-containing histone demethylases (KDM), which are components of multiprotein complexes (coactivators) that remodel chromatin to activate terminal differentiation genes. Acetyl-CoA is a cofactor for histone lysine acetyltransferases (HAT), which are also coactivator components. Flavin adenine dinucleotide (FAD) is a cofactor for amine oxidase domain-containing histone demethylases (KDM1A, KDM1B), which are components of corepressor complexes that repress terminal differentiation genes. Nicotinamide adenine dinucleotide (NAD+) is a cofactor for Sirtuin histone deacetylases (SIRT1, SIRT2) that participate in the regulation of tissue stem-cell genes. In short, sensing for these mitochondrial outputs is another way in which exponential proliferation and onward differentiation are coupled (Figure 1B), powerfully demonstrated by the natural experiment of neoplastic evolution: for example, recurrent mutations in isocitrate dehydrogenase genes (IDH1, IDH2) in cancer reduce or antagonize AKG to decouple exponential proliferation and onward differentiation.10
Figure 1.
The dihydroorotate dehydrogenase inhibitor ASLAN003 recapitulates in acute myeloid leukemia cells the coordinated up- and down-regulation of thousands of genes that occurs with normal terminal granulocyte or monocyte differentiation. (A) The 1,000 genes most significantly up- or down-regulated upon addition of ASLAN003 to KG1 or MOLM14 acute myeloid leukemia (AML) cells were examined for their expression pattern in normal hematopoietic stem cells (HSC), multipotent progenitors (MPP), common myeloid progenitors (CMP), granulocyte-monocyte progenitors (GMP), granulocytes (gran) and monocytes (mono) (data from BloodPool15), and found to be genes normally up- or down-regulated with terminal granulocyte or monocyte differentiation. Experimental details of ASLAN003 treatment are described by Zhou et al.,1 gene expression by RNA-sequencing Geo Database GSE128950. (B) Proliferation and lineage differentiation are coupled through mitochondrial metabolism. AKG: alpha-ketoglutarate; Ac-CoA: acetyl-CoA; FAD: flavin adenine dinucleotide; NAD+: nicotinamide adenine dinucleotide; CTP: cytidine triphosphate. The putative epigenetic protein in eukaryotic cells for which CTP is a cofactor is unknown. (C) The dihydroorotate dehydrogenase (DHODH) inhibitor ASLAN003 acutely reconfigures pyrimidine metabolism in KG1 and MOLM14 AML cells (data from GSE1289501). Analysis using Broad Institute Morpheus software, P-value Marker Selection, 100 permutations.
Since CTP is disproportionately elevated in malignancy, and interventions that decrease CTP reconnect to onward differentiation, it can be theorized that CTP is a cofactor for corepressor proteins that repress terminal-differentiation genes. In fact, chromosome partitioning ParB proteins in bacteria, which also serve as platforms for DNA-condensing proteins (condensins), require CTP as a cofactor.11 The identity of the putative corepressor component in eukaryotic cells for which CTP is a cofactor is, however, unknown. An alternative theory is that CTP is a negative regulator of coactivators that activate terminal differentiation genes, whose identity is also unknown.
These observations are relevant to clinical translation. If a decrease in CTP and/or UTP mediates the induction of terminal differentiation by DHODH inhibitors, then the CTP/UTP content in cancer/leukemia cells is a candidate pharmacodynamic biomarker to guide the design of drug regimens. Moreover, the most likely mechanism for treatment failure can be anticipated: the pyrimidine metabolism network will respond automatically to preserve the amounts of CTP and/or UTP. For example, DHODH inhibitor-mediated depletion of CTP and UTP will relieve their allosteric inhibition of uridine cytidine kinase 2 (UCK2), which salvages cytidines and uridines from the extracellular environment, to automatically dampen any CTP/UTP decrease.12 CTP and/or UTP decreases can also be expected to trigger compensating shifts in the expression of key enzymes of pyrimidine metabolism: for example, ASLAN003 treatment of AML cells acutely upregulated expression of deoxycytidine kinase (DCK), which salvages deoxycytidines (Figure 1C). Treatment resistance that emerges automatically from the pyrimidine metabolism network in this way will be rapid and, importantly, will not be solved by simple escalation of DHODH-inhibitor dosages, since this would worsen the therapeutic index, which is a rationale for clinical development in the first place. Fortunately, compensatory metabolic responses can potentially be turned to advantage: DCK activates several oncotherapeutic prodrugs, e.g., decitabine that, like partial DHODH inhibition, can also operate in a non-cytotoxic, differentiation-based regime,13,14 and incorporation of DCKdependent prodrugs can potentially be timed to DHODH inhibitor-mediated DCK upregulation. Other pyrimidine metabolism responses, however, could be adverse to such combinations: the pyrimidine metabolism enzyme SAMand HD-containing deoxynucleoside triphosphate triphosphohydrolase 1 (SAMHD1) was also automatically upregulated by ASLAN003 (Figure 1C) and catabolizes the active nucleotide forms of decitabine and cytarabine which are routinely used to treat AML. Ultimately, therefore, candidate solutions for resistance will require thorough experimental evaluation.
These observations are relevant to clinical translation. If a decrease in CTP and/or UTP mediates the induction of terminal differentiation by DHODH inhibitors, then the CTP/UTP content in cancer/leukemia cells is a candidate pharmacodynamic biomarker to guide the design of drug regimens. Moreover, the most likely mechanism for treatment failure can be anticipated: the pyrimidine metabolism network will respond automatically to preserve the amounts of CTP and/or UTP. For example, DHODH inhibitor-mediated depletion of CTP and UTP will relieve their allosteric inhibition of uridine cytidine kinase 2 (UCK2), which salvages cytidines and uridines from the extracellular environment, to automatically dampen any CTP/UTP decrease.12 CTP and/or UTP decreases can also be expected to trigger compensating shifts in the expression of key enzymes of pyrimidine metabolism: for example, ASLAN003 treatment of AML cells acutely upregulated expression of deoxycytidine kinase (DCK), which salvages deoxycytidines (Figure 1C). Treatment resistance that emerges automatically from the pyrimidine metabolism network in this way will be rapid and, importantly, will not be solved by simple escalation of DHODH-inhibitor dosages, since this would worsen the therapeutic index, which is a rationale for clinical development in the first place. Fortunately, compensatory metabolic responses can potentially be turned to advantage: DCK activates several oncotherapeutic prodrugs, e.g., decitabine that, like partial DHODH inhibition, can also operate in a non-cytotoxic, differentiation-based regime,13,14 and incorporation of DCKdependent prodrugs can potentially be timed to DHODH inhibitor-mediated DCK upregulation. Other pyrimidine metabolism responses, however, could be adverse to such combinations: the pyrimidine metabolism enzyme SAMand HD-containing deoxynucleoside triphosphate triphosphohydrolase 1 (SAMHD1) was also automatically upregulated by ASLAN003 (Figure 1C) and catabolizes the active nucleotide forms of decitabine and cytarabine which are routinely used to treat AML. Ultimately, therefore, candidate solutions for resistance will require thorough experimental evaluation.
Funding Statement
Funding National Heart, Lung and Blood Institute PO1 HL146372; National Cancer Institute P30 CA043703; National Cancer Institute RO1 CA204373
References
- 1.Zhou J, Quah JY, Ng Y, et al. ASLAN003, a potent dihydroorotate dehydrogenase inhibitor for differentiation of acute myeloid leukemia. Haematologica. 2020;105(9):2286-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madak JT, Bankhead A, 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol Ther. 2019;195:111-131. [DOI] [PubMed] [Google Scholar]
- 3.Gu X, Ebrahem Q, Mahfouz RZ, et al. Leukemogenic nucleophosmin mutation disrupts the transcription factor hub that regulates granulomonocytic fates. J Clin Invest. 2018;128(10):4260-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velcheti V, Schrump D, Saunthararajah Y. Ultimate precision: targeting cancer but not normal self-replication. Am Soc Clin Oncol Educ Book. 2018;38:950-963. [DOI] [PubMed] [Google Scholar]
- 5.Huang M, Wang Y, Collins M, Graves LM. CPEC induces erythroid differentiation of human myeloid leukemia K562 cells through CTP depletion and p38 MAP kinase. Leukemia. 2004;18(11):1857-1863. [DOI] [PubMed] [Google Scholar]
- 6.Bierau J, van Gennip AH, Helleman J, van Kuilenburg AB. The cytostatic- and differentiation-inducing effects of cyclopentenyl cytosine on neuroblastoma cell lines. Biochem Pharmacol. 2001;62(8):1099-1105. [DOI] [PubMed] [Google Scholar]
- 7.Huang M, Wang Y, Collins M, Mitchell BS, Graves LM. A77 1726 induces differentiation of human myeloid leukemia K562 cells by depletion of intracellular CTP pools. Mol Pharmacol. 2002;62(3):463-472. [DOI] [PubMed] [Google Scholar]
- 8.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1-22. [DOI] [PubMed] [Google Scholar]
- 9.Williams JC, Kizaki H, Weber G, Morris HP. Increased CTP synthetase activity in cancer cells. Nature. 1978;271(5640):71-73. [DOI] [PubMed] [Google Scholar]
- 10.Nieborak A, Schneider R. Metabolic intermediates - cellular messengers talking to chromatin modifiers. Mol Metab. 2018;14:39-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soh YM, Davidson IF, Zamuner S, et al. Self-organization of parS centromeres by the ParB CTP hydrolase. Science. 2019;366(6469):1129-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Graves LM. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell Mol Life Sci. 2003;60(2):321-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunthararajah Y, Sekeres M, Advani A, et al. Evaluation of noncytotoxic DNMT1-depleting therapy in patients with myelodysplastic syndromes. J Clin Invest. 2015;125(3):1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng KP, Ebrahem Q, Negrotto S, et al. p53 independent epigeneticdifferentiation treatment in xenotransplant models of acute myeloid leukemia. Leukemia. 2011;25(11):1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapin N, Bagger FO, Jendholm J, et al. Comparing cancer vs normal gene expression profiles identifies new disease entities and common transcriptional programs in AML patients. Blood. 2014;123(6):894-904. [DOI] [PubMed] [Google Scholar]