Abstract
Sporotrichosis is a neglected endemic mycosis with a high incidence in Latin America, mainly in Brazil. Sporothrix schenckii is the most frequent species in Latin America, whereas Sporothrix brasiliensis is the predominant species observed in Brazil and is associated with both human and animal sporotrichosis. Sporotrichosis treatment remains restricted to a few options, itraconazole being the first choice for human and animal therapy. In this work, we screened the molecular library Pathogen Box (Medicines for Malaria Venture [MMV], Switzerland) in search of compounds with anti-Sporothrix activity. Our initial screen of the 400 compounds identified five compounds that inhibited more than 80% of S. brasiliensis and S. schenkii growth. Among those, three compounds (MMV675968, MMV102872, and MMV002817 (known as iodoquinol)) not previously described as antifungals or agrochemicals, were selected for further evaluation. MMV102872 and iodoquinol showed the most promising combination of antifungal activity (lower inhibitory concentration) and fungal selectivity (lower cytotoxicity in LLC-MK2 cells). Scanning electron microscopy and flow cytometry analyses revealed that MMV102872 and iodoquinol induced changes in cell morphology, membrane integrity, and the presence of neutral lipids, impairing fungal survival. Our results indicate that MMV102872 and iodoquinol are promising molecules for use as scaffolds for the development of new antifungal agents.
Introduction
Sporotrichosis is a neglected endemic mycosis caused by thermodimorphic fungi of the genus Sporothrix that affects humans and animals [1]. In Brazil, sporothrichosis is an endemic disease and an important zoonosis, with thousands of humans and cats being infected in the last two decades [2]. Between 1992 and 2015, 782 hospitalizations and 65 deaths due to human sporotrichosis were reported in Brazil [3], and outbreaks of the disease are currently present in areas of the southeast, south, and northeast of the country [4].
Sporothrix schenckii and Sporothrix brasiliensis are the most virulent species in the Sporothrix genus and the main etiological agents of sporotrichosis in Latin America and Brazil, respectively [4, 5]. Infection with S. schenckii occurs by traumatic inoculation of fungus present in contaminated organic materials, while S. brasiliensis transmission is associated with the zoonotic route involving cats as intermediate hosts [6]. Importantly, S. brasiliensis can induce a pronounced inflammatory response, leading to more severe and/or atypical clinical presentations of sporotrichosis [7, 8].
Although sporotrichosis is endemic in several countries, treatment remains restricted to a few options, relying mainly on itraconazole [4]. The emergence of clinical and feline-borne isolates with low in vitro susceptibility to itraconazole and the constantly reported therapeutic failure highlights the need to seek new options for sporotrichosis treatment [9–11].
High-content screening of libraries containing hundreds of drug-like compounds is a powerful strategy to search for novel antifungal drugs and to explore new targets [12]. Screening of large chemical libraries aim to identify compounds able inhibit the growth of pathogenic fungi and have been the most successful approach in the discovery of chemical scaffolds with promising antifungal action [12]. However, good quality of compounds and the establishment of standardized assays for susceptibility tests are required for this approach to succeed [12].
The Pathogen Box library, developed by the Medicines for Malaria Venture organization (MMV), contains 400 compounds with repurposing potential and also new molecules (https://www.mmv.org/mmv-open/pathogen-box), selected on the basis of their promising activity against pathogens associated with neglected tropical diseases [13]. In an effort to identify new molecules with antifungal activity, the Pathogen Box library was previously screened for its antifungal activity against Candida species, Cryptococcus species, and chromoblastomycosis agents [14–17]. Thus, the purpose of this study was to screen this collection in search of compounds with potential anti-S. brasiliensis and S. schenckii activity.
Materials and methods
Microorganisms
The reference isolates S. brasiliensis CBS 133006 and S. schenckii ATCC 32286 were used in this study. In addition, eight clinical isolates, six from human sporotrichosis patients (S. brasiliensis B972 and HE06; and S. schenckii Ss22, Ss42, Ss73, and Ss110) and two from felines (S. brasiliensis: Ss53 and Ss245) were used [18]. All isolates were stored in saline solution containing 10% glycerol and 10% glucose at -20°C. The filamentous form was cultivated for 7 days in Sabouraud broth (Difco, United States), with orbital shaking (at 150 rpm), at 36°C. Then, an aliquot containing 105 CFU/ml was inoculated into brain heart infusion broth (Difco, USA) supplemented with 2% glucose (pH 7.8) and cultivated at 36°C, with orbital shaking for 7 days, in order to obtain yeast cells. All assays were carried out using the yeast form as it is the pathogenic form.
Compounds
The Pathogen Box library was obtained from Medicines for Malaria Venture (MMV, Switzerland) and contained 400 compounds diluted in dimethyl sulfoxide (DMSO) at 10 mM. A stock solution of all compounds was prepared in DMSO at 1 mM and stored at -20°C. Itraconazole (Sigma Chemical Co., USA) was used as a reference antifungal, and a stock solution (1 mM in DMSO) was kept at -20°C.
Screening the compound library for inhibition of Sporothrix spp. growth
The reference isolates S. brasiliensis CBS 133006 and S. schenckii ATCC 32286 were used to screen The Pathogen Box compounds for putative inhibitors of fungal growth according to the following protocol: dx.doi.org/10.17504/protocols.io.bje9kjh6. The final concentration of compounds was 1 μM following the MMV recommended guidelines for screening (https://www.mmv.org/mmv-open/pathogen-box/pathogen-box-supporting-information), while the final concentration of fungal cells was 1 x 105 CFU/ml. Itraconazole (reference antifungal) was also tested at 1 μM. After 48 h (at 35°C in a 5% CO2 atmosphere), fungal growth was analyzed by visual inspection in an inverted light microscope and quantified by spectrophotometric readings. Inhibitions of more than 80% were defined as the cut-off to select for the most promising compounds, corresponding to clearly visible prevention of growth when samples were initially analyzed by visual inspection. Experiments were performed in quadruplicate.
Determination of minimum inhibitory concentration values (MICs) against S. brasiliensis and S. schenckii
The MIC of the most active compounds were determined using the in vitro broth microdilution technique described by the CLSI [19] with modifications according to the following protocol: dx.doi.org/10.17504/protocols.io.bjgxkjxn. MIC values were compared with that of the reference drug itraconazole. Yeasts (1 x 105 CFU/ml) were treated with different concentrations of compounds (0.002–1 μM) for 48 h, at 35°C, in a 5% CO2 atmosphere. Fungal growth was analyzed by visual inspection in an inverted light microscope and quantified by spectrophotometric readings. Concentrations that inhibit 50% and 80% of fungal growth (IC50 and IC80, respectively) were estimated. Results are presented as the mean of two independent experiments, performed in duplicate.
Cytotoxicity assays
Cytotoxicity assays with MMV102872 and iodoquinol (selected as the most promising compounds) were performed using a mammalian epithelial cell line (LLC-MK2; ATCC CCL-7) according to the following protocol: dx.doi.org/10.17504/protocols.io.bjgbkjsn. Several concentrations of compounds (0.1–10 μM) were tested, concentrations that elicited 50% cytotoxicity (CC50) were estimated, and the selectivity towards Sporothrix spp. was determined using the IC80 values obtained previously. The selectivity index (SI) of compounds was calculated using the following equation: SI = CC50/ IC80 median. Results are representative of two independent experiments, performed in triplicate.
Treatment of fungal cells
The effect of MMV102872 and iodoquinol (MMV002817) on the morphology and metabolism of S. brasiliensis yeasts (CBS133006) was evaluated by scanning electron microscopy and flow cytometry. Standardized yeast suspensions (1 x 105 CFU/ml) were treated with the IC50 concentrations (0.25 μM) of MMV102872 or iodoquinol (MMV002817) in supplemented RPMI, for 48 h, at 36°C, with orbital shaking. Untreated controls were grown in the absence of drugs in parallel. Next, samples were centrifuged at 927 G for 3 min, washed three times in sterile PBS and processed for scanning electron microscopy or flow cytometry as described below.
Scanning electron microscopy
Untreated and treated cells (obtained as described in “Treatment of fungal cells”) were fixed in 2.5% glutaraldehyde and 4% formaldehyde, in 0.1 M cacodylate buffer, for 1 h and then washed three times in 0.1 M cacodylate buffer. Samples were adhered to poly-L-lysine-coated glass coverslips, dehydrated in a graded ethanol series, critical-point-dried in CO2, and coated with gold. Images were obtained in a ZEISS EVO 10 scanning electron microscope (ZEISS Company, Germany) and processed using Photoshop software (Adobe, USA). The area distribution of 100 cells was measured and the count of damaged cells was performed using ImageJ software (National Institute of Health, USA).
Flow cytometry
Neutral lipid accumulation was evaluated by flow cytometry using the fluorescent marker BODIPY 493/503 (Thermo Fisher Scientific, USA). Plasma membrane integrity was evaluated using SYTOX Green (Thermo Fisher Scientific, USA), which is impermeable to intact membranes, but penetrates compromised membranes and stains nucleic acids. Untreated and treated cells (obtained as described in “Treatment of fungal cells”) were incubated with 200 μl containing 20 μM of each fluorochrome for 30 min at room temperature in the dark. Cells were washed in PBS, lightly fixed in 2% formaldehyde, and washed again in PBS. The fluorescence intensity of stained cell populations was quantified in a BD Accuri C6 flow cytometer (BD Biosciences, USA) that counted 10,000 or 2000 events per sample for BODIPY 493/503 or SYTOX Green, respectively. Data were analyzed using BD Accuri C6 software. Experiments were repeated three times. Data depicted in the results section shows the mean and standard error of the mean of one representative experiment.
Statistical analyses
Statistical analysis was performed using Prism 6.01 software (GraphPad Software, USA), by one-way ANOVA (with Dunnett’s post hoc test). Statistical significance was considered when p < 0.05.
Results
Screening of the Pathogen Box library identified five potential Sporothrix growth inhibitors
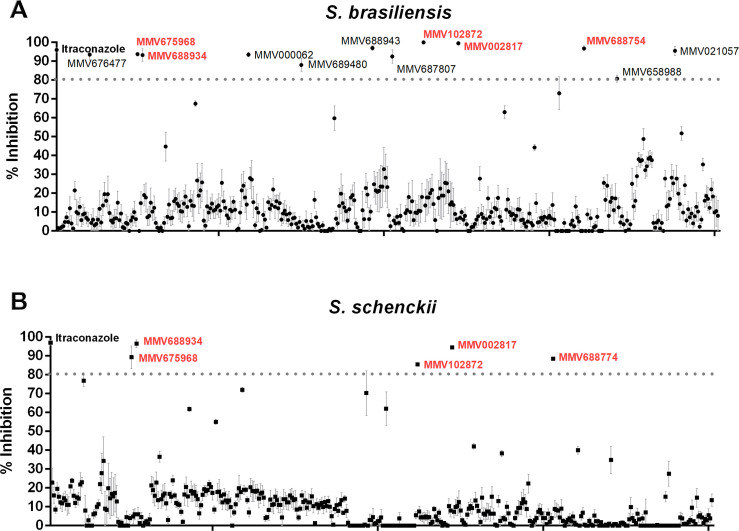
The initial screen of the 400 compounds in the Pathogen Box identified 12 compounds that inhibited S. brasiliensis CBS 133006 growth by more than 80% (Fig 1A). These compounds are (i) the antifungal drug posaconazole (MMV688754); (ii) the insecticide tolfenpyrad (MMV688934); (iii) the agricultural fungicides difenoconazole and azoxystrobin (MMV688943 and MMV021057); (iv) three commercial drugs used to treat different diseases (MMV002817 –iodoquinol, MMV000062 –pentamidine, and MMV689480 –buparvaquone); and (v) five new molecules (MMV675968, MMV102872, MMV676477, MMV687807, and MMV658988) (Fig 1A). In contrast, only five compounds were able to inhibit the growth of S. schenckii ATCC 32286 by more than 80% (Fig 1B). Interestingly, all those five compounds were also able to inhibit S. brasiliensis growth (MMV688754, MMV688934, MMV002817, MMV675968, and MMV102872).
Fig 1. High-content screening of the Pathogen Box library.
Percentage of growth inhibition of (A) Sporothrix brasiliensis and (B) Sporothrix schenckii after exposure to 1 μM of the library compounds for 48 hours. Data represent the mean ± SEM and are fully described in S1 Table.
Among the five compounds that are active for both species, compounds MMV688754 and MMV688934 were excluded from subsequent experiments as they are commercial antifungal drugs (MMV688754-posaconazol) and an agrochemical insecticide (MMV688934-tolfenpyrad). Therefore, MMV675968, MMV102872, and MMV002817 (iodoquinol) were selected for further studies exploring their potential antifungal activity and targets.
Antifungal activity of MMV675968, MMV102872, and MMV002817 (iodoquinol) against Sporothrix spp. isolates
MMV675968, MMV102872, and iodoquinol were able to inhibit 80% of S. brasiliensis growth similarly to itraconazole–i.e. similar IC80 values (Table 1); while only MMV102872 had a similar inhibitory effect against S. schenckii isolates (no statistical significance compared with itraconazole IC80) (Table 1). Thus, MMV102872 and iodoquinol were the most active compounds against S. brasiliensis and S. schenckii (Fig 2).
Table 1. Susceptibility profile of Sporothrix isolates to MMV675968, MMV102872, and iodoquinol.
| Isolates | Itraconazole | MMV675968 | MMV102872 | MMV002817 | ||||
|---|---|---|---|---|---|---|---|---|
| (Iodoquinol) | ||||||||
| S. brasiliensis | IC50 | IC80 | IC50 | IC80 | IC50 | IC80 | IC50 | IC80 |
| Reference isolate | ||||||||
| CBS133006 | 0.03 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 |
| Human isolates | ||||||||
| B972 | ND | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 0.25 | 0.5 |
| HE06 | ND | 0.25 | 0.125 | 0.5 | 0.125 | 0.25 | 0.25 | 0.5 |
| Feline-borne isolates | ||||||||
| Ss53 | 0.25 | 0.5 | 0.06 | 0.25 | 0.125 | 0.25 | 0.25 | 0.5 |
| Ss245 | 0.06 | 0.25 | 0.125 | 0.5 | 0.125 | 0.25 | 0.25 | 0.5 |
| Median | 0.06 | 0.25 | 0.125 | 0.5 | 0.125 | 0.25 | 0.25 | 0.5 |
| S. schenckii | IC50 | IC80 | IC50 | IC80 | IC50 | IC80 | IC50 | IC80 |
| Reference isolate | ||||||||
| ATCC32286 | 0.125 | 0.25 | 0.03 | 0.125 | ND | 0.25 | 0.5 | 1 |
| Human isolates | ||||||||
| Ss22 | 0.125 | 0.25 | >1 | >1 | 0.25 | 0.5 | 0.5 | 1 |
| Ss42 | ND | 0.25 | ND | 1 | ND | 0.25 | 0.25 | 1 |
| Ss73 | 0.06 | 0.25 | 1 | >1 | 0.125 | 0.25 | 0.25 | 0.5 |
| Ss110 | 0.03 | 0.125 | 0.5 | 1 | 0.125 | 0.25 | 0.25 | 0.5 |
| Median | 0.09 | 0.25 | 0.5 | 1 | 0.125 | 0.25 | 0.25 | 1 |
Minimum inhibitory concentration of different human and feline isolates of S. schenckii and S. brasiliensis to MMV675968, MMV102872, and Iodoquinol, compared with itraconazole.
All values are expressed in μM.
IC50, concentration that inhibits 50% of fungal growth. IC80, concentration that inhibits 80% of fungal growth.
ND, Not determined due to the detection limit of the microdilution technique.
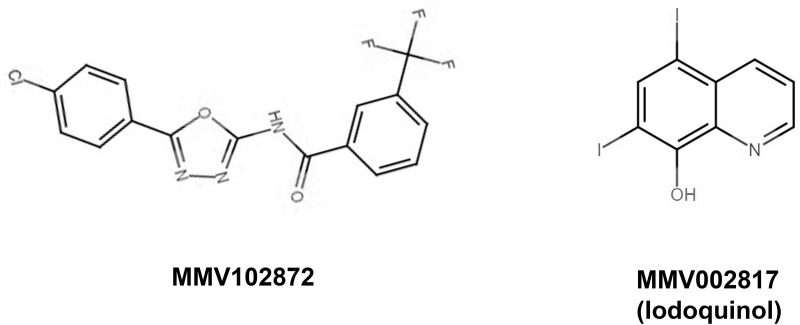
Fig 2. Molecular structure of MMV102872 and iodoquinol (MMV002817).
MMV102872 and iodoquinol showed low cytotoxicity against human cell lines in vitro
The CC50 of MMV102872 and iodoquinol using the mammalian cell line LLC-MK2 were 8.7 μM and 5 μM, respectively, resulting in high selectivity index of each compound to both S. brasiliensis and S. schenckii (Table 2).
Table 2. Selectivity of compounds towards Sporothrix spp.
| Compounds | IC80 medians (μM) | Cytotoxicity against LLC-MK2 cells | Selectivity Indexa | ||
|---|---|---|---|---|---|
| S. brasiliensis | S. schenckii | CC50 (μM) | S. brasiliensis | S. schenckii | |
| MMV102872 | 0.25 | 0.25 | 8.7 | 34.8 | 34.8 |
| MMV002817 (iodoquinol) | 0.5 | 1 | 5 | 10 | 5 |
Cytotoxicity to mammalian cells in vitro and selectivity of MMV102872 and iodoquinol towards Sporothrix brasiliensis and Sporothrix schenckii cells.
a Selectivity index (SI) was defined as SI = CC50/IC80 median.
MMV102872 and iodoquinol altered fungal cell morphology and lipid metabolism and disrupted cell membrane integrity
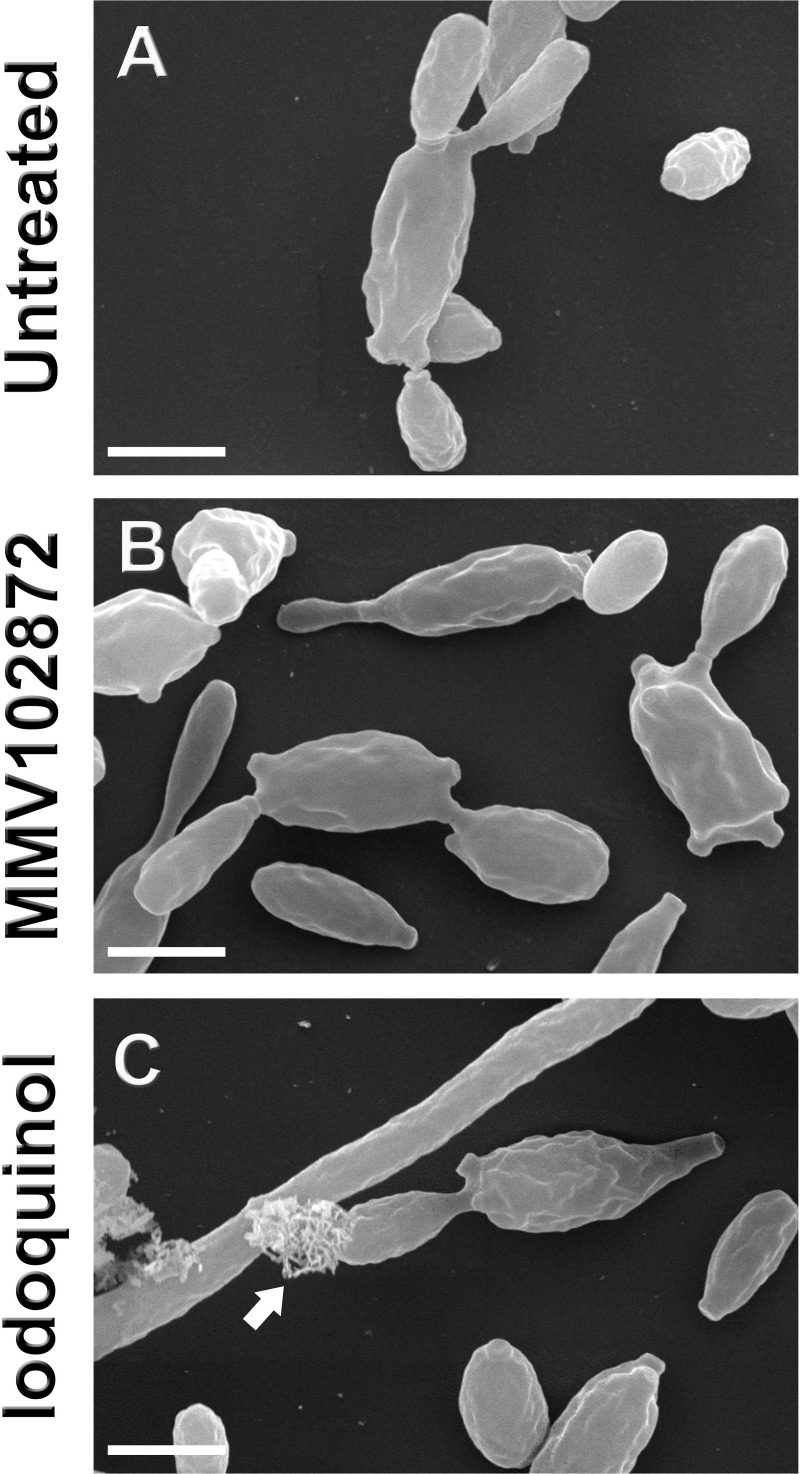
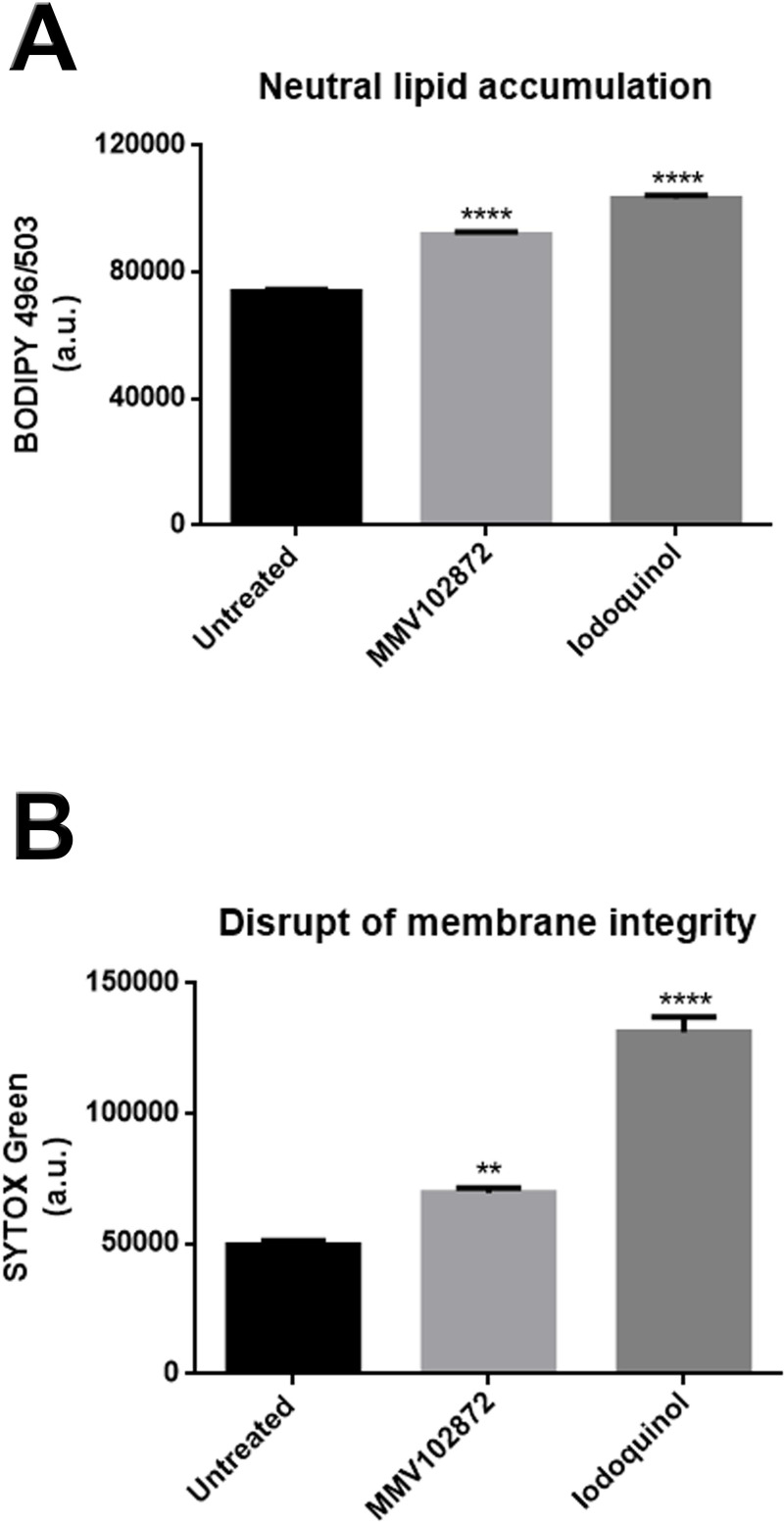
The effects of MMV102872 and iodoquinol were evaluated against S. brasiliensis as a representant of genus Sporothrix. S. brasiliensis was selected based on its epidemiological importance in Brazil, where its associated with endemic transmission and also unusual clinical presentations of human sporotrichosis. Scanning electron microscopy analysis of S. brasiliensis (CBS 133006) revealed the presence of disrupted cells and overflow of intracellular contents after exposure to iodoquinol (Fig 3C, arrow), which was not observed for untreated or MMV102872 treated cells (Table 3). Moreover, treatments with MMV102872 and iodoquinol induced an increase in cell size of 7% and 17%, respectively (Table 3). Flow cytometry results revealed that MMV102872 and Iodoquinol induced significant accumulation of neutral lipids and disruption of the plasma membrane integrity; being such cellular alterations more pronounced after treatment with iodoquinol (Fig 4).
Fig 3. Effect of MMV102872 and iodoquinol (MMV002817) treatment on Sporothrix brasiliensis cell morphology.
Fungal cells were exposed to the IC50 of compounds (0.25 μM) for 48 h and scanning electron microscopy images showed cell disruption and overflow of intracellular contents induced by iodoquinol treatment (C, arrow). Scale bars: 5 μm.
Table 3. Cell size of Sporothrix brasiliensis and damage cells after treatments.
| Mean area (μm2) | Disrupted cells | |
|---|---|---|
| Untreated | 2.9 | 0% |
| MMV102872 | 3.1 | 0% |
| Iodoquinol | 3.4 ** | 8% |
Quantification of the area and disrupted cells performed based on scanning electron microscopy images.
**p < 0.01 vs. untreated by one-way ANOVA and Dunnett’s test.
Fig 4. Levels of neutral lipids and damage to the plasma membrane in Sporothrix brasiliensis treated with MMV102872 and iodoquinol (MMV002817).
(A) Neutral lipid accumulation was observed after treatment with the IC50 (0.25 μM) of either compound for 48 h, as revealed by accumulation of BODIPY 496/503 stain. (B) SYTOX Green labelling revealed that both treatments induced damage to the plasma membrane, which was even more pronounced after iodoquinol treatment. **p < 0.01 and ****p < 0.0001.
Discussion
Over the last 50 years, several fungal infections have emerged as important public health problems [20]. In the present work, we screened the Pathogen Box library in search of promising molecules with anti-Sporothrix activity, showing that 3% (12/400) and 1.25% (5/400) of them were able to inhibit S. brasiliensis and S. schenckii, respectively.
S. brasiliensis and S. schenckii can exhibit distinct in vitro susceptibility profiles to commercial antifungals [21]. Besides, higher rates of in vivo resistance, as wells as prolonged treatment in sporotrichosis human cases, are reported in S. schenckii infections [8]. We observed that S. brasiliensis was susceptible to a higher number of compounds from the library, compared with S. schenckii. The lower sensitivity of S. schenckii to the compounds could be in part related to differences in the structure and composition of the cell wall of this species compared to S. brasiliensis [22].
Three compounds that showed activity against S. brasiliensis in the initial screening are usually used as agrochemicals: difenoconazole (MMV688943), azoxystrobin (MMV021057), and tolfenpyrad (MMV688934), the last one also being effective against S. schenckii. The expanding use of antifungal-based agrochemicals has been correlated with the emergence of antifungal resistance among pathogenic fungi [23], and Sporothrix spp. are most often found in the environment, which increases the probability of exposure to these compounds, ultimately reducing its susceptibility to them. Therefore, as our goal is to treat humans and animals, we decided to exclude those agrochemical molecules from our pool of candidates for the development of new human and/or animal antifungal agents. However, it is important to emphasize that the use of some of those compounds (mainly tolfenpyrad) could be explored for decontamination of environmental areas with high fungal loads that may be a focus of animal/human infection [6].
The initial screening also showed that posaconazole (MMV688754) and three other commercial drugs were able to inhibit S. brasiliensis growth: pentamidine (MMV000062), buparvaquone (MMV689480), and iodoquinol (MMV002817); however, only posaconazole (MMV688754) and iodoquinol (MMV002817) were also effective against S. schenckii (Fig 1). The in vitro and in vivo activity of posaconazole against Sporothrix spp. is well known [21, 24]; however, its high costs compromises usage in lower-income countries of South America, like Brazil, where sporotrichosis also figures as a higly prevalent mycosis.
Among the new molecules, five compounds (MMV675968, MMV102872, MMV676477, MMV687807, and MMV658988) exhibited high activity against S. brasiliensis; however, only MMV675968 and MMV102872 were also effective against S. schenckii (Fig 1). The new compounds that showed anti-Sporothrix activity are diverse, and the previously described mechanisms of action for several cell types and microorganisms are summarized in Table 4. The identification of five new molecules with possibly distinct cellular targets opens perspectives for future studies, because these molecules have a different mechanism of action than antifungals currently available to treat fungal infections, a relevant discovery due to the increase in fungus resistance [23].
Table 4. New compounds with anti-Sporothrix activity.
| Compound | Class | Known antimicrobial activity | Described in this work | Mechanism of action |
|---|---|---|---|---|
| MMV676477 | pyrimidinone-pyrazole | Mycobacterium tuberculosis [13] | S. brasiliensis | tyrosine kinase inhibitor [13] |
| MMV658988 | pyrimidine | Trypanosoma and Leishmania species [13] | S. brasiliensis | methionine aminopeptidases inhibitor [13] |
| MMV687807 | hydroxybenzamide | Mycobacterium tuberculosis, Candida albicans [13, 14] | S. brasiliensis | disruption of the mitochondrial proton gradient [13] |
| MMV675968 | quinazoline | Toxoplasma gondii, Cryptosporidium parvum, Pneumocystis jrovecii, Candida albicans [13, 14] | S. schenckii, S. brasiliensis | dihydrofolate reductase inhibitor [13] |
| MMV102872 | oxadiazole | Mycobacterium tuberculosis, Staphylococcus aureus, Candida auris [13, 16] | S. schenckii, S. brasiliensis | unknown |
Summary of chemical and biological information of five new compounds with antifungal activity against Sporothrix spp. described in this study.
According to the above-cited results from the initial susceptibility assays, iodoquinol (MMV002817) and the new molecules MMV675968 and MMV102872 were selected for further evaluation as anti-Sporothrix agents. Iodoquinol (MMV002817) is a quinoline derivative used as an antiparasitic drug for intestinal worms (also known as diiodohydroxyquinoline) (Fig 2) [25]. MIC values showed that iodoquinol is highly active against Sporothrix spp. at concentrations as low as 1 μM, inducing almost complete inibition of proliferation (IC80) of all 10 clinical isolates tested (Table 1). Iodoquinol has also been reported to inhibit in vitro growth of Candida species and chromoblastomycosis agents [16, 17]. The IC50 for planktonic cells of Candida spp. was similar to what we described here for Sporothrix spp. (0.25 μg/ml–corresponding to 0.63 μM), and growth inhibition of 100% of chromoblastomycosis agents was observed with 1.25 μM iodoquinol [16, 17]. Therefore, a comprehensive study including a panel of diverse fungi would be of interest to elucidate whether iodoquinol has a broad spectrum of antifungal activity.
Compound MMV675968 (5-chloro-6-[(2,5-dimethoxyanilino) methyl] quinazoline-2,4-diamine) was designed as a dihydrofolate reductase inhibitor, an enzyme conserved in prokaryotes and eukaryotes that catalyzes the NADPH-dependent reduction of dihydrofolate into tetrahydrofolate [13]. Previous studies demonstrated that MMV675968 also exhibited an inhibitory effect against some protozoa (Toxoplasma gondii and Cryptosporidium parvum) and fungi (Pneumocystis jrovecii and Candida albicans) [13, 14] (Table 4). Our results showed that although MMV675968 presented high activity against S. brasiliensis, it was less effective at preventing proliferation of S. schenckii at concentrations lower than 1 μM (Table 1). The IC50 of MMV675968 against planktonic cells of C. albicans was also higher than 1 μM (3.12 μM), and inhibition of biofilms was only achieved at 50 μM [14].
Finally, MMV102872 (N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-3-(trifluoromethyl) benzamide) is an azole derivative (Fig 2) with previously reported antimycobacterial and anti-Staphylococcus activities [13]. Our data revealed that Sporothrix spp. was more susceptible to MMV102872 than to MMV675968, being able to prevent fungal growth of both Sporothrix species at concentrations lower than 0.5 μM, similarly to itraconazole (Table 1). Wall and co-workers recently showed that, at 20 μM, MMV102872 inhibits 88% of growth in the emerging pathogen Candida auris [16]. The anti-Sporothrix activity observed was remarkably higher than that observed for C. auris.
Despite its demonstrated antimicrobial activities, no mechanism of action has been reported for MMV102872 in the literature, while the amoebicidal activity of iodoquinol has been associated with its ability to chelate iron [13]. MMV102872 is an azole-derivative, and previous reports have shown that inhibition of ergosterol biosynthesis by azoles leads to accumulation of neutral lipids; therefore, the accumulation of neutral lipids was quantified in Sporothrix-treated and non-treated cells, as well as cell membrane integrity effects given its central role in maintaining cellular homeostasis [26]. Disruption in the membrane was observed to a lesser extent after treatment with MMV102872, as well as neutral lipid accumulation, when compared to iodoquinol (Fig 4). Since this compound is an azole-derivative, we speculate that, in Sporothrix spp., MMV102872 may be interfering with ergosterol biosynthesis [26]. Alterations in iron absorption could disrupt enzymatic activity and metabolic pathways, culminating in irreversible cell damage and rupture, as observed by SEM in Sporothrix cells after treatment with iodoquinol (Fig 3C). However, further studies are needed to elucidate the mechanism(-s) involved in the antifungal activity exerted by MMV102872 and iodoquinol.
Importantly, the cytotoxicity results reported here demonstrate that MMV102872 and iodoquinol are more selective towards fungi than mammalian cells (Table 2). Previous reports using HepG2 cells showed a CC50 for MMV102872 of 9 μM; and a CC20 for iodoquinol of 2.5 μM (reported in Pathogen Box documents: https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box#composition). Together, these data suggest that both compounds have reduced toxicity and are potential candidates to advance to in vivo and clinical studies.
Iodoquinol has been used for the treatment of protozoal infections of the gastrointestinal tract; however, it is not able to penetrate into the intestinal epithelium [25]. Topical formulations containing 1% iodoquinol are available to treat dermatoses and exhibit antifungal and antibacterial properties [27] and could be repurposed as an adjuvant topical treatment for sporotrichosis in association with oral itraconazole therapy. Thus, future in vivo and clinical studies are required to evaluate the effectiveness of iodoquinol in the treatment of sporotrichosis.
Conclusion
In this work we screened the Pathogen Box library and identified two compounds, MMV102872 and iodoquinol, with promising anti-Sporothrix activity. These compounds were effective against both Sporothrix species endemic in Latin America: S. schenkii and S. brasiliensis and demonstrated reduced toxicity to mammalian cell lines in vitro. Mechanistic studies indicated that both compounds induced neutral lipid accumulation and disruptions in cell membrane homeostasis. Finally, based on the described results we suggest that MMV102872 and iodoquinol are promising molecules and could be used as a scaffold for the development of new antifungal agents.
Supporting information
Inhibition of Sporothrix brasiliensis CBS 133006 and Sporothrix schenckii ATCC 32286 after exposure to 1 μM of the library compounds for 48 hours.
(XLSX)
Acknowledgments
The authors would like to thank Medicine for Malaria Venture (MMV, Switzerland) for kindly providing the Pathogen Box library and Evotec SE (Hamburg, Germany) for synthesizing the compounds. We thank Professor Anderson Messias Rodrigues and Professor Zoilo Pires de Camargo (Universidade Estadual de São Paulo, São Paulo, SP, Brazil) for providing Sporothrix isolates. The authors also thank Professor Wanderley de Souza and colleagues from the Laboratório de Ultraestrutura Celular Hertha Meyer for flow cytometry analysis and Centro Nacional de Biologia Estrutural e Bioimagem (CENABIO, UFRJ, Rio de Janeiro, Brazil) for providing support for the use of SEM equipment.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by the Brazilian funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto C. Neglected endemic mycoses. Lancet Infect Dis. 2017. July 31;e367–e377. 10.1016/S1473-3099(17)30306-7 [DOI] [PubMed] [Google Scholar]
- 2.Gremião IDF, Oliveira MME, Miranda LHM, Freitas DFS, Pereira SA. Geographic expansion of sporotrichosis, Brazil. Emerg Infect Dis. 2020;26:621–624. 10.3201/eid2603.190803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falcão EMM, de Lima Filho JB, Campos DP, Valle ACFD, Bastos FI, et al. Hospitalizations and deaths related to sporotrichosis in Brazil (1992–2015). Cad Saude Publica. 2019;35:e00109218 [Article in Portuguese]. 10.1590/0102-311X00109218 [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues AM, Terra PPD, Gremião ID, Pereira SA, Orofino-Costa R, de Carmargo ZP. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020. February 12 10.1007/s11046-020-00425-0 [DOI] [PubMed] [Google Scholar]
- 5.Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Mariné M, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651–655. 10.1111/j.1469-0691.2009.02824.x [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLOS Pathog. 2016;12:e1005638 10.1371/journal.ppat.1005638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batista-Duarte A, Téllez-Martínez D, de Roberto Andrade C, Portuondo DL, Jellmayer JÁ, et al. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells response than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018;122:1168–1170. 10.1016/j.funbio.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Almeida-Paes R, de Oliveira MM, Freitas DF, do Valle AC, Zancope-Oliveira RM, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:e3094 10.1371/journal.pntd.0003094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borba-Santos LP, Rodrigues AM, Gagini TB, et al. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol. 2015;53:178–188. 10.1093/mmy/myu056 [DOI] [PubMed] [Google Scholar]
- 10.Waller SB, Dalla Lana DF, Quatrin PM, Ferreira MRA, Fuentefria AM, Mezzari A. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2020; 10.1007/s42770-020-00307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakasu CCT, Waller SB, Ripoll MK, Ferreira MRA, Conceição FR, Gomes AR, et al. Feline sporotrichosis: a case series of itraconazole-resistant Sporothrix brasiliensis infection. Braz J Microbiol. 2020. May 9 10.1007/s42770-020-00290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Wang C, Su H, Zhang W, Sheng C. Strategies in the discovery of novel antifungal scaffolds. Future Med Chem. 2016;8:1453–1454. 10.4155/fmc-2016-0020 [DOI] [PubMed] [Google Scholar]
- 13.Veale CGL. Unpacking the Pathogen Box: an open source tool for fighting neglected tropical disease. Chem Med Chem. 2019;14:386–453. 10.1002/cmdc.201800755 [DOI] [PubMed] [Google Scholar]
- 14.Vila T, Lopez-Ribot JL. Screening the Pathogen Box for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother. 2016;61: e02006–16. 10.1128/AAC.02006-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer FL, Kronstad JW. Discovery of a novel antifungal agent in the Pathogen Box. mSphere 2017;2:e00120–17. 10.1128/mSphere.00120-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall G, Herrera N, Lopez-Ribot JL. Repositionable compounds with antifungal activity against multidrug resistant Candida auris identified in the Medicines for Malaria Ventures’s Pathogen Box. J Fungi (Basel). 2019;5:92 10.3390/jof5040092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho RA, Joffe LS, Alves GM, Figueiredo-Carvalho MHG, Brito-Santos F, Amaral ACF, et al. A screening of the MMV Pathogen Box® reveals new potential antifungal drugs against the etiologic agents of chromoblastomycosis. PLoS ONE 2020;15:e0229630 10.1371/journal.pone.0229630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borba-Santos LP, de Sá LFR, Ramos JÁ, Rodrigues AM, Camargo ZP, et al. Tacrolimus increases the effectiveness of itraconazole and fluconazole against Sporothrix spp. Front Microbiol. 2017;8:1759 10.3389/fmicb.2017.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. CLSI standard M27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 20.Bongomin F, Gago S, Oladele RO, Denninng DW. Global and multi-national prevalence of fungal diseases: Estimate precision. J Fungi (Basel). 2017;3:57 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chackrabarti A, et al. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother. 2017;61:e01057–17. 10.1128/AAC.01057-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes-Bezerra LM, Walker LA, Niño-Vega G, Mora-Montes HM, Neves GWP, Villalobos-Duno H, et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. PLoS Negl Trop Dis. 2018; 12: e0006169 10.1371/journal.pntd.0006169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrickson JA, Hu C, Aitken SL, Beyda N. Antifungal resistance: a concerning trend for the present and future. Curr Infect Dis Rep. 2019;21:47 10.1007/s11908-019-0702-9 [DOI] [PubMed] [Google Scholar]
- 24.Mario DN, Guarro J, Santurio JM, Alves SH, Capilla J. In vitro and in vivo efficacy of amphotericin B combined with posaconazole against experimental disseminated sporotrichosis. Antimicrob Agents Chemother. 2015;59:5018–5021. 10.1128/AAC.00052-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta YK, Gupta M, Kohlir AK. Current drug therapy of protozoa diarrhea. Indian J Pediatr. 2004;71:55–58. 10.1007/BF02725657 [DOI] [PubMed] [Google Scholar]
- 26.Robbins N, Wright GD, Cowen LE. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr. 2016;4:1–20. 10.1128/microbiolspec.FUNK-0002-2016 [DOI] [PubMed] [Google Scholar]
- 27.Burnett BP, Mitchell CM. Antimicrobial activity of iodoquinol 1%–hydrocortisone acetate 2% gel against ciclopirox and clotrimazole. Cutis 2008;82:273–280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of Sporothrix brasiliensis CBS 133006 and Sporothrix schenckii ATCC 32286 after exposure to 1 μM of the library compounds for 48 hours.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript.