Abstract
The World Health Organization (WHO) has cautioned on specific respiratory symptoms for suspecting an individual of Corona Virus Disease 2019 (COVID-19). Meanwhile, many suspects are reporting dysfunctions of smell and taste. This study aimed to investigate the percentage of positive COVID-19 who had associated loss of sensation as detected by psychophysical testing. Eight hundred and thirty two suspects were enrolled. At the time of sampling for testing COVID-19 status, olfactory dysfunction (OD) and gustatory dysfunction (GD) tested using odorants like coffee and camphor and solutions of sweet and salty solvants, respectively. The strength of the association between test results of these sensory losses and COVID-19 positivity was assessed by calculating sensitivity, specificity, and predictive values. The responses in positive and negative individuals presented as age-adjusted odds ratio with 95% CI. Seventy six (9.1%) [95% CI: 7.4%–11.3%] of 832 suspects were tested positive for COVID-19. Paediatric cases of age between 2 and 10 years could not reply appropriately, hence OD in 134 and GD in 118 could not be tested. Anosmia or hyposmia was present in 62 (81.6%) and ageusia in 64 (84.2%) of the total 76 confirmed cases. The OD and GD dysfunctions were significantly higher among confirmed COVID-19 cases compared to negative subjects [Adj OR (95% CI): Smell 3.22 (1.77–5.88); taste 3.05 (1.61–5.76), p < 0.001]. In this study, testing of smell and taste dysfunctions had higher sensitivity in identifying recent-onset loss of sensations in COVID-19 cases. Hence, it may be used as a simple and cost-effective screening test.
Keywords: COVID-19, SARS-CoV-2, Olfactory, Anosmia, Ageusia
Introduction
The World Health Organization (WHO) has declared that Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the disease caused by it known as Coronavirus Disease 2019 (COVID-19) is here to stay for long. The rapidly spreading pandemic has swept the globe and is progressing relentlessly [1].
The transmission of SARS-CoV-2 can occur through direct, indirect, or close contact with infected people through infected secretions such as saliva and respiratory secretions or their respiratory droplets, which are expelled when an infected person coughs, sneezes or talks. Respiratory droplet transmission can occur when a person is in close contact (within 1 m) with an infected person who has respiratory symptoms (e.g., coughing or sneezing) or who is talking or singing; in these circumstances, respiratory droplets that include the virus can reach the mouth, nose or eyes of a susceptible person and can result in infection. Indirect contact transmission involving contact of a susceptible host with a contaminated object or surface (fomite transmission) may also be possible.
Any person developing cough, fever, myalgia, headache, shortness of breath, Influenza-Like Illness (ILI), or non-respiratory symptoms indicates occurrence of possible viral infection. Subsequently, there were reports of anosmia/hyposmia and ageusia among many suspects from different countries. In the current situation, where high infectivity of the virus is leading to the rapid spread of infection, any diagnostic means which can detect the spread at the earliest will be valuable and can be helpful for effective containment of virus spread.
The clinical expertise of front-line otolaryngologists who have in-depth knowledge about rhinology and olfaction can diagnose these cases much early during screening and sampling suspects of COVID-19. Subjective olfactory assessment alone has poor reliability. Many clinical and research settings for the psychophysical analysis of olfactory dysfunction (OD) and gustatory dysfunction (GD) have utilized a validated olfactory test, which determines the odor threshold by identifying or discriminating one or more odor [2]. However, currently available objective psychophysical tests like Sniffin Sticks or University of Pennsylvania Smell Identification Test (UPSIT) [3] for testing this sensation are not accessible to majorities. Their application in the field set up for the mass screening of suspects may not be feasible in all the places.
Post viral olfactory dysfunction (PVOD) and gustatory dysfunction is a known phenomenon following viral upper respiratory tract infection (URTI). Many viruses, including Rhinovirus, Corona Virus, and Parainfluenza viruses, have been found in patients' nasal discharge, causing PVOD by a different mechanism. In case of COVID-19 however it is the direct damage to olfactory epithelium which leads to loss of smell sensation [4].
This study investigated the correlation between the development of new-onset OD and GD by psychophysical testing among laboratory-confirmed COVID-19 positive cases.
Material and Method
Study Design
This is a facility-based survey performed in screening facilities of quarantine centers for COVID-19. The suspects from outpatient clinics treating the cases of Influenza-like illness (ILI) and Severe Acute Respiratory Infection (SARI) were also included.
Study Setting and Study population
The study is carried out in five facility-based quarantine centers established under Nagpur Municipal Corporation, Maharashtra. These quarantine centers cater to COVID-19 suspects identified based on the following criteria: 1—close contacts of confirmed COVID-19 positive cases 2. Patients presented with ILI from containment zones 3. International and inter-state history of travel with/out presumptive symptoms. Each quarantine center had about 150–200 occupants, and they were discharged from the quarantine centers based on a negative laboratory report or 14 days of stay, whichever is earlier. Also, the study enrolled patients attending the screening center of the tertiary care facility. Patients presenting with ILI /SARI like symptoms are subjected to further evaluations. We wanted to know the real-time frequency of OD and GDs in a cohort of suspects who have acquired COVID-19; hence the recruitment of cases from quarantine centers was done.
Administrative approval from concerned authorities and the Institute Ethics Committee is obtained before beginning the study. The patient's willingness to participate in the survey, readiness for follow-up, and agreement with the informed consent terms were confirmed beforehand.
Patients less than 2 years of age or more than 80 years of age, nasal polyposis, and any patient on intranasal corticosteroid spray are excluded from the study.
Data Collection
Data on participant demographic and clinical characteristics were collected in person and on the telephone using structured proforma. OD and GD are tested in eligible suspects using standard procedure before the sample collection for COVID-19. The status is tracked by the sample registration form (SRF), ID number extracted from the laboratory records maintained at the designated COVID-19 molecular diagnostic laboratory.
Assessment of Olfactory and Gustatory Dysfunctions
The olfaction was tested by asking subjects to identify the smell of particular odorant like crushed coffee beans and crushed camphor diskettes of a fixed weight. This was placed in a disposable test tube; the suspect was not allowed to see or touch the contents. The items so selected keeping in mind their regular usage among the population. Hence, improving the reliability of testing that particular sensation. In between the identification of two odors, the gustatory sensation was tested. The gustatory sensation was tested by asking the patient to identify the taste after instilling two drops of solution on the anterior third of the tongue by disposable 2 ml dropper. The sweet and salty solutions were prepared from glucose and salt of fixed dilution each time, respectively. The responses were documented in the datasheet.
For olfaction, if the suspect can identify either coffee or camphor affirmatively, then it is marked as identified. Those who failed to identify the smell are labeled as not identified. Hence, identifying the healthy sense of smell and anosmia, respectively. For a taste sensation, which is done in the same setup. The testing is done with sugar and salt solutions prepared and administered independently, and patients’ responses were marked as it is done for the smell. Thus, a single observer collects all the data.
Statistical Analysis Procedure
Data is entered in an Excel spreadsheet and analyzed using STATA 14 (StataCorp, College Station, Texas). Participant demographic and clinical characteristics are summarized as frequencies and percentages. Since age was not following the Gaussian distribution, it is summarised as median with Inter Quartile Range (IQR). Proportions of COVID-19 positivity rate and rate of dysfunction in smell and tastes are described as percentages with 95% CI. The rate of smell and taste disturbances between COVID-19 positive and COVID-19 negative individuals compared using the Chi-square test. The association of smell and taste dysfunction with COVID-19 status described as an adjusted odds ratio with 95% Confidence Interval. The confounding factors such as age and sex-adjusted through multivariate logistic regression analysis.
Results
Participant Characteristics
A total of eight hundred and thirty-two subjects screened at quarantine centers in 3 months between April and June 2020 were included in the study. Four hundred and twenty (50.5%) were males, and the median (IQR) age of the participants was 28 (13–40) years.
Clinical Characteristics of COVID-19 Suspects
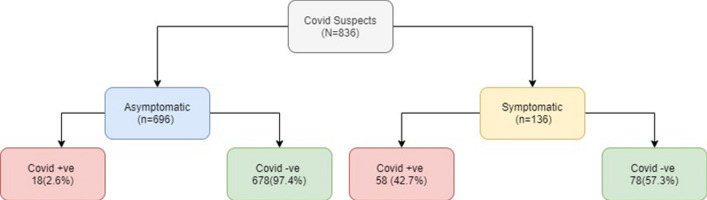
Six hundred and ninety-six suspects (83.6%) were asymptomatic. One hundred thirty-six suspects presented with at least one clinical symptom, including oto-rhinology related one. It was found that the majority of these, 57.3% (78/136) tested negative. (Fig. 1) The most frequently reported symptom was nasal blockade 62 (7.5%), followed by dry cough 54(6.5%) (Table 1).
Fig. 1.
COVID-19 status amongst symptomatic and asymptomatic quarantine suspects
Table 1.
Distribution of symptoms among COVID suspects and COVID confirmed cases in Maharashtra, 2020
| Symptom* | COVID 19 Suspects (n = 832) n (%) | COVID confirmed cases (n = 76) n (%) |
|---|---|---|
| Asymptomatic | 696 (83.6) | 18 (23.7) |
| Cough | 54 (6.5) | 30 (39.5) |
| Fever | 22 (2.6) | 16 (21.1) |
| Breathlessness | 4 (0.5) | 4 (5.3) |
| Running nose | 8 (1) | 0 (0) |
| Nasal blockade | 62 (7.5) | 24 (31.6) |
*Multiple symptoms possible in same patient
COVID-19 Positivity Rate
Seventy-six of 832 suspects tested positive for COVID-19. The overall laboratory COVID-19 positivity, as reported after reverse transcription-polymerase chain reaction (RT-PCR) test, was 9.1% (95% CI: 7.3–11.3%).
Clinical Characteristics of COVID-19 Confirmed Cases
Cough was the most common symptom accounting for 30 (39.5%) individuals, followed by nasal blockade in 24 (31.6%) cases as predominant presentations in confirmed cases. Patterns of other symptoms reported among confirmed cases are presented (Table 1).
Distribution of Smell and Taste Dysfunction among COVID-19 Suspects
One hundred and seventy eight (25.5%) and 168 (24.2%) suspect failed to identify the correct smell of coffee beans and camphor, respectively. While 90 (12.6%) and 96 (13.5%) suspects could not identify sweet and salty solutions, respectively, when asked to taste the solution to identify (Table 2).
Table 2.
Distribution of smell and taste disturbances identified during the testing among COVID-19 suspects
| Category | Smell n (%) | Taste n (%) | ||
|---|---|---|---|---|
| Camphor | Coffee | Sugar | Salt | |
| Not identified | 168 (24.2) | 178 (25.5) | 90 (12.6) | 96 (13.5) |
| Identified | 526 (75.8) | 520 (74.5) | 624 (87.4) | 618 (86.5) |
The Olfactory and Gustatory Dysfunctions in COVID-19 Positive Cases
Sixty two (81.6%) of 76 positive cases had smell dysfunction. And, 64/76 (84.2%) cases reported taste dysfunction upon psychophysical testing (Fig. 2). The confirmed cases had three times more odds of having OD and GDs compared to negative suspects (Smell—adj OR(95% CI):3.02 (1.67–5.57; taste- adj OR(95% CI):3.06 (1.61–5.88) (Table 3). This suggests that COVID-19 positive cases were more likely to have new-onset OD and GDs, which is statistically significant (Table 4).
Fig. 2.
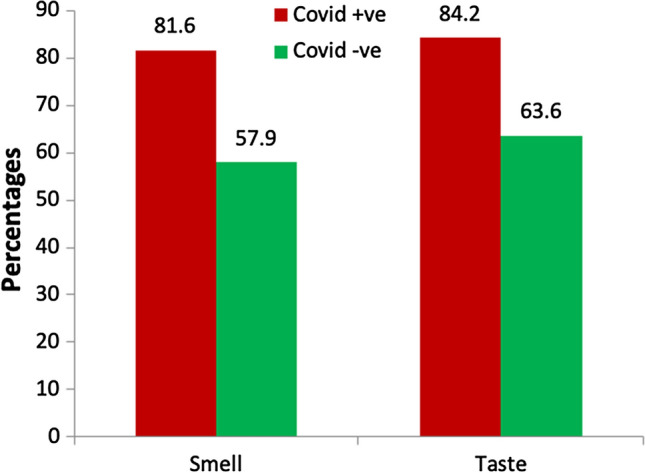
Distribution of smell and taste dysfunction among COVID-19 suspects
Table 3.
Comparison of taste and smell dysfunction based on COVID-19 status
| Dysfunctio domain | (n) | COVID-19 positive n (%) | COVID-19 negative n (%) | Odds ratio (95% CI) | p value | Adjusted odds ratio (95% CI) | p value adjusted model* |
|---|---|---|---|---|---|---|---|
| Smell | 698 | 62/76 (81.6) | 360/622 (57.9) | 3.22 (1.77–5.88) | 0.001 | 3.02 (1.67–5.57) | 0.001 |
| Taste | 714 | 64/76 (84.2) | 406/639 (63.6) | 3.05 (1.61–5.76) | 0.001 | 3.06 (1.61–5.88) | 0.001 |
*Adjusted for age and gender
Table 4.
Diagnostic utility of results of Sensory dysfunctions tests associated with COVID-19
| Sensory dysfunctions # | Sensitivity | Specificity | Positive predictive value | Negative predictive value | LR + | LR |
|---|---|---|---|---|---|---|
| 1. Olfactory dysfunction | 92.1 | 32.8 | 14.3 | 97.1 | 1.32 | 0.43 |
| 2. Gustatory dysfunction | 81.6 | 42.1 | 14.7 | 94.9 | 1.41 | 0.43 |
LR Likelihood ratio
#as tested by psychophysical testing
*True positive- zero
Discussion
Many paucisymptomatic suspects od COVID-19 have reported dysfunctions of smell and taste as presenting complaints [5]. In this study, an easy to perform and administer screening test done for assessing OD and GD had an overall sensitivity of 92.1% and 81.6% among the confirmed cases of COVID-19, respectively.
Sometimes referred to as the forgotten cranial nerve, the olfactory nerve, and testing for its function is often overlooked in clinical practice. Besides, in the perspective of normal trans-nasal airflow, the odorant molecules reaching olfactory cleft, and in the absence of clinical features of intranasal diseases like in infectious rhinosinusitis, allergic or vasomotor rhinitis, or polyposis, up until now, patients with sensorineural viral anosmia have been infrequently seen in general practice and otolaryngology practice. Historically our knowledge about the neurotropic or neurovirulent viral infection affecting the olfactory system, is incomplete. Only the most severe cases may self-recognize the ongoing neurosensory dysfunction, and in remaining cases, it might manifest itself after a prolonged latency period [5]. Therefore, up until the COVID-19 pandemic, the low prevalence of sensorineural viral anosmia in various populations as a whole has made clinical research challenging. Lack of decisive infrastructures in performing neuroimaging studies, difficulties in obtaining histopathological tissue specimens, and an absence of viral culture techniques of infected olfactory neuroepithelium compounds the problems in studying this condition [5].
The University of Pennsylvania Smell Identification Test (UPSIT) was administered to 60 confirmed COVID-19 inpatients by Shima T Moein et al. and concluded that the ODs of the variable extent was a reliable biomarker affecting 98% of the cohort studied [6]. In the current study, a psychophysical evaluation of these sensory functions was performed. Even though it is not confirmatory, baseline information about the alteration of these sensations can be easily obtained. The classification described in this study broadly categorizes the losses of olfaction and gustatory functions.
Using the COVID-19 anosmia tool developed by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS), after analyzing 273 entries, it was suggested by Rachel Kaye and colleagues that anosmia may be a critical initial symptom and it may help to alert particular individual infected with SARS-CoV2 who may unknowingly transmit the virus [7]. This tool may be helpful to the attending healthcare worker to submit data regarding OD and GD in COVID-19 patients effectively. Further, testing of olfaction and taste, as suggested in this study along with the above COVID-19 anosmia tool, could fortify the evidence.
It is observed that traditional clinical features, as seen in other URTI (e.g., Influenza, Rhinovirus, and Adenovirus), are often absent in patients with COVID-19. The silent progress of viremia fails to produce clinically significant nasal congestion or rhinorrhoea—i.e., a red, runny, stuffy, itchy nose. Instead, many cases have presented with newer onset loss of smell and taste. Hence, prompting this observation that the SARS-CoV-2 is a neurotropic virus that is site-specific for the olfactory system. Although known as a respiratory virus, coronaviruses are known to be neurotropic and neuroinvasive [8, 9].
In any typical viral URIs, the resultant nasal obstruction caused by rhinitis precludes an individual from perceiving the flavor. However, in COVID-19, there is a direct viral injury to the olfactory neuroepithelium. Retro nasal olfaction, which is a combination of ortho-nasal smell and taste, and is a complex sensory process allowing us to perceive flavor, anything beyond the five tastes of the food: sweet, salty, bitter, sour, and umami [10]. The congestion in the nasal cavity during the ongoing episode physically blocks the entry of odor and flavor molecules to the olfactory cleft. In the current study, fifty-four positive cases of the 76 cases did not complain of nose blockage, suggesting that unlike other URTI, COVID-19 cases do not exhibit nasal congestion.
European multicentre study including 417 mild-to-moderate patients with COVID-19, Lechien et al. reported 85.6% and 88.0% OD and GD, respectively. In 11.8% cases, the loss of smell sensation was the first symptom to appear even before the appearance of any other symptom, suggesting OD may be an early indicator for early COVID-19 detection [11]. The onset and duration of these sensory losses could not be tracked in this study as data collection is done while screening suspects due to shortage of time. This may be a shortcoming in itself of this study. An objective analysis of olfaction and gustatory sensation and associated clinical characteristics with timely follow up will further enlighten the role of investigating chemo-sensory losses among COVID-19 patients.
During a naïve pandemic, using appropriate screening tools is an imperative need. The tool should be useful enough not to miss the positive cases as well as not to tag the unaffected individuals as cases in the context of prevailing stigma and exclusion of affected individuals. This study identifies the potential symptoms for inclusion in the checklist, and simple screening tests, as described, can be used in masses as an adjunct. Similarly, the application of this feasible smell and taste dysfunction tests could help the individual become alert and come forward for testing, especially in the containment region. Proactively getting isolated from others in the family when these symptoms arise or dysfunction occurs can help in limiting the spread.
Conclusion
In this study, testing of smell and taste dysfunctions had higher sensitivity in identifying recent-onset loss of sensations in COVID-19 cases. Hence, it may be used as a simple and cost-effective screening test.
Funding
None.
Data Availability
Data transparency.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors provide consent to publish.
Ethical Approval
Institutional Ethical Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki.
Consent to participate
Verbal informed consent of participant taken.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan WJ, Zhong NS. Clinical Characteristics of Covid-19 in China. N Engl J Med. 2020;382(19):1861–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 2.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 3.Hugh SC, Siu J, Hummel T, et al. Olfactory testing in children using objective tools: comparison of Sniffin' Sticks and University of Pennsylvania Smell Identification Test (UPSIT) J Otolaryngol Head Neck Surg. 2015;44(1):10. doi: 10.1186/s40463-015-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, Murakami S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e.PMID:17277621;PMCID:PMC7165544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xydakis MS, Belluscio L. Detection of neurodegenerative disease using olfaction. Lancet Neurology. 2017;16:415–416. doi: 10.1016/S1474-4422(17)30125-4. [DOI] [PubMed] [Google Scholar]
- 6.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachel Kaye CW, Chang D, Kazahaya K, Brereton J, Denneny JC. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 8.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepherd GM. Neurogastronomy: how the brain creates flavor and why it matters. New York: Columbia University Press; 2012. [Google Scholar]
- 11.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data transparency.