Abstract
This work is devoted to a stochastic model on the spread and control of corona virus (COVID-19), in which the total population of a corona infected area is divided into susceptible, infected, and recovered classes. In reality, the number of individuals who get disease, the number of deaths due to corona virus, and the number of recovered are stochastic, because nobody can tell the exact value of these numbers in the future. The models containing these terms must be stochastic. Such numbers are estimated and counted by a random process called a Poisson process (or birth process). We construct an SIR-type model in which the above numbers are stochastic and counted by a Poisson process. To understand the spread and control of corona virus in a better way, we first study the stability of the corresponding deterministic model, investigate the unique nonnegative strong solution and an inequality managing of which leads to control of the virus. After this, we pass to the stochastic model and show the existence of a unique strong solution. Next, we use the supermartingale approach to investigate a bound managing of which also leads to decrease of the number of infected individuals. Finally, we use the data of the COVOD-19 in USA to calculate the intensity of Poisson processes and verify our results.
Keywords: COVID-19 epidemic, Stochastic process, Stability, Unique strong solution, Poisson process
Disease COVID-19, named after the attack of coronavirus in China at the end of 2019, spread world wide and killed more than 0.6 million individuals in initial eight months. This virus transmits person to person through respiratory droplets produced when an infected person coughs or sneezes. Infected droplets land in the noses and mouths of people who are nearby or possibly are inhaled into the lungs. It also spreads through touching a surface or object that has the virus on it and then touching your own mouth, nose, or possibly your eyes. It infects the respiratory system, and the infected person faces fever, cough, shortness of breath, and breathing difficulties. The infection and the onset of symptoms ranges from one to fourteen days. An infectious person shows symptoms within five to six days. To prevent the infection spread, one needs regular hand washing, covering mouth and nose when coughing and sneezing, and avoiding contact with effected individuals.
Mathematical modeling is a tool to study the structure of spread and control of various infectious diseases. Mathematical models have potential to educate persons about the control techniques of such diseases. It can also be used to predict the expected number of patients in the future under any controlling strategy and to set their goals. Fundamental work is done by researchers to model viral diseases and used by policy makers to control them. For example, in 2009, Pang et al. [1] considered dynamical behavior of a Hepatitis B virus transmission model with vaccination, and Zou et al. [2] discussed modeling the transmission dynamics and control of Hepatitis B virus in China. These models were implemented in China to study the number of HBV patients and to control HBV virus. To understand the spread and control of coronavirus, Chen et al. [3] proposed a mathematical model to understand the transmissibility of coronavirus. Zhou et al. [4] studied pneumonia outbreak associated with coronavirus, whereas Li et al. [5] discussed the early transmission dynamics in Wuhan, China. Huang et al. [6] provided clinical features of the patients infected with coronavirus, whereas Chan et al. [7] discussed familywise transmission of the novel coronavirus. Wu et al. [8] provided newscasting and forecasting of the COVID-19 outbreak in Wuhan, China, through modeling. Zhao et al. [9] have used the model approach to estimate the unreported number of COVID-19 in China in the first half of January 2020. Chen et al. [3] proposed a mathematical model for simulating the transmission of this virus in Wuhan. Ivorra et al. [10] developed and implemented a mathematical model on the spread of COVID-19 using data from China. Sameni [11] studied the epidemic patterns of coronavirus through a mathematical model. Kochańczyk et al. [12] studied constant and time-dependent contact rates of COVID-19 pandemic. For more detail on mathematical modeling of such viral disease, we refer the readers to [13–22]. Besides the integer-order models, fractional calculus and stochastic differential equations play an important role in the epidemic models; see [23–26]. A Poisson process is a random process that counts the number of occurrences of certain events that happen at certain rate called the intensity of the Poisson process. For more detail on this process, we refer the readers to [27].
In this work, we study a stochastic model on the spread and control of coronavirus in which the total population of an infected area is divided into susceptible, infected, and recovered classes. Generally, the exact number of individuals who get the disease, the number of deaths due to coronavirus, and the number of recovered are random and unknown, and thus these numbers are stochastic. Any model containing these terms must be stochastic. These numbers are estimated and counted by a Poisson process. We construct an SIR type model in which the above numbers are stochastic and counted by a Poisson process. To understand the flow and prevent of coronavirus in a better way, we first study the stability of the corresponding deterministic model, calculate the unique nonnegative strong solution, and investigate an inequality managing of which leads to control of the virus. After this, we pass to the stochastic model and show the existence of a unique strong solution. Next, we use the supermartingale approach to investigate a bound managing of which also leads to decrease of the number of infected individuals. Finally, we use COVID-19 data from USA (April 1 to July 19, 2020) to calculate the intensity of Poisson processes and verify our results.
The rest of the paper is organized as follows. In Sect. 2, we give a deterministic model, discus the stability, calculate solution of the model, and investigate an inequality managing of which leads to control the virus. In Sect. 3, we pass to a stochastic model and show the existence and uniqueness of a nonnegative strong solution. Next, we use COVID-19 data from USA, calculate the parameters of the Poison process, verify our results, and sketch the number of effected individuals. To control the virus, this work provides useful information to policy makers. Moreover, the results can be used to predict the number of patients in any future time to set the future goals.
Deterministic model and stability
In this section, we consider an SIR deterministic model on the spread and control of coronavirus, discuss the local and global stability of the model and the reproductive number, calculate the unique strong solution, which can be used to calculate the future number of infected individuals, and investigate an inequality depending on some parameters of the model and the number of susceptible individuals, which would be managed to control the virus and to set the future goals.
Let us divide the total population in three categories, susceptible, infected, and recovered individuals, and denote by , the number of susceptible individuals, the number of infected individuals, and by the number of recovered individuals. The flow and control are shown in the Fig. 1. The mathematical model on these classes is expressed by the following system of differential equations:
| 1 |
where a is the rate of new born and migrated individuals, b is the transmission rate from susceptible to infected, c is the contact rate of susceptible with infected, μ is the natural death rate, k is the recovery rate, and λ is the death rate of infected class due to virus.
Figure 1.
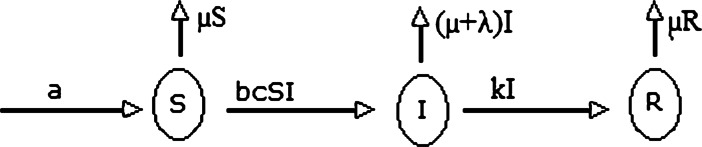
Figure shows the flow and control of the coronavirus
In the following result, we discuss the local and global stability of the model and calculate the reproductive number .
Theorem 1
The proposed model is locally asymptotically stable at the free virus equilibrium point if the reproductive number , whereas it is globally asymptotically stable at the positive virus point if , where .
Proof
The free virus equilibrium point is , whereas the positive virus point is . The Jacobian matrix of system (1) is
Putting the point in the Jacobean matrix J gives the eigenvalues and . Putting , we get the reproductive number . Thus all the eigenvalues are negative if and only if , and hence the model is locally asymptotically stable.
Similarly, at point , all the eigenvalues are negative if . Hence the proposed model is globally stable if . □
Next, we come to the nonnegative unique strong solution of the model, formula for calculating the future number of infected individuals, and an inequality that would be managed to control the virus.
Theorem 2
Solution of model (1) is nonnegative, and the number decreases if the transmission function satisfies the upper bound
Proof
As coefficients in the model are locally Lipschitz, the solution of the model is strong and unique (see Arnold [28]). Using integrating factors, we can express the solution of the proposed model as
| 2 |
From solution (2) we first observe that is nonnegative, and using this, we find that and , are also nonnegative for all t.
The future value of the infected number can be calculated from the expression of .
For the second part, we put the exponent into less than zero and get the answer. □
Note that the product bc can be easily calculated by using past information in the expression of .
Stochastic model formulation
In this section, we pass from deterministic to stochastic model as the number of infected population, deaths due to virus, and recovered individuals are generally not deterministic. These numbers are random and can be counted by a Poisson process. To do this, let us consider Ω a sample space, F a sigma algebra, a filtration of F, and P a probability measure. On a filtered probability space , we consider three classes, the susceptible class , the infected individuals , the recovered class , and three independent Poisson processes , , and with intensities λ, γ, and η, respectively. Let be the number of individuals who got disease in the time interval due to I, be the number of deaths, and be the number of recovered in this interval. We replace the transmission term in a deterministic form by a Poisson random variable , the death term by , and the recovery term by . For simplicity, we drop the natural death rate μ from as it can be added in the number .
The flow in the model is shown in Fig. 2.
Figure 2.
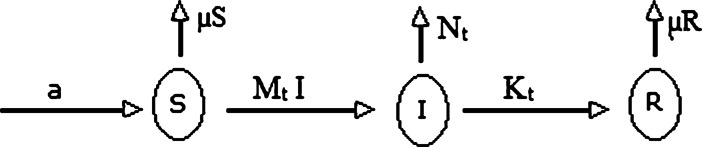
Figure shows the flow and control of coronavirus
The sizes of S, I, and R can be formulated by the system of stochastic equations (see Asmussen and Albrecher [29]) as
| 3 |
with bounds
Generally, a Poison process is right continuous with left-hand limits (see Lamberton and Lapeyre [27]), and therefore the increment △ in a Poisson random process can be given as
and the derivative of the Poisson process coincides with the increment (see Lamberton and Lapeyre [27]). Thus the Poisson random processes , , and must satisfy
Since μ is the natural death rate, using the later expressions, the differential form of system (3) is (see Shreve [30] in case of assets modeling)
| 4 |
Since most of the data is available daywise, so if t is time in days, then if , , and , , denote the numbers of infected individuals, deaths, and recovered individuals, respectively, at the ith day since the starting day , then the Poisson processes , , and satisfy
| 5 |
where the random variables , , and are independent and identically distributed with uniform distribution.
If t is time in days, then , , and satisfy
where
| 6 |
Similarly,
where
| 7 |
The expected values of the above expressions can be expressed (see Lamberton and Lapeyre [27] and Karatzas and Shreve [31]) as
| 8 |
with the following quadratic variations (see Karatzas and Shreve [31], p. 31)
| 9 |
Moreover, the random processes , , and are martingales (see Shreve [30]).
Existence of the solution of the stochastic model
Here, we show the stability of the stochastic model and investigate an inequality managing of which leads to decrease of the number of COVID-19 patients.
Theorem 3
For initial positive values , , and in formulation (3), there exists a unique nonnegative solution , , with probability one.
Proof
As all the coefficients in stochastic system (4) are locally Lipschitz continuous (see Mao [32]), there exists a unique maximum local solution on , where is the explosion time.
To show that the solution is global, we will show that the explosion time a.s. Otherwise, we suppose that there is some bounded time such that the solution cannot explode to infinity. Let N be a large positive number such that the initial values , , and belong to the interval . For each integer , let us define the sequence of stopping times
and let . Note that the above sequence increases and denote ; then a.s.
If a.s., then also a.s. Thus all the values (, , and ) are positive a.s. for all t. Otherwise, suppose there exists a pair , and , such that the probability . Thus there exists an integer such that the probability for all integers .
Next, let us define the smooth function as
| 10 |
Using Itô’s formula (see Karatzas and Shreve [31]), we calculate
| 11 |
where and are the first- and second-order partial derivatives of W, with respect to the space variable respectively, and , , and are the quadratic variations.
Using (3), (4), and (9), we calculate
| 12 |
Using the martingale property of , ,and , this stochastic equation becomes
where
| 13 |
with .
Further, as the positive space variables S, I, and R represent the numbers of individuals, we have , , and . Using this, we write
| 14 |
Thus by Theorem 2.2 in [33] we deduce that , which completes the proof. □
Now we come to the inequality among some parameters of the stochastic model, managing of which leads to decrease of the number of the infected COVID-19 patients.
Theorem 4
The expected value of the infected class decreases with time, that is, (or is supermartingale) if
| 15 |
Proof
From (3) we write
Applying mathematical expectation to both sides and simplifying, we obtain
and differentiating with respect to t, we find
Using inequality (15), we get the result. □
Note that in this work the parameter γ is calculated from by using equations (5) and (8), which is replaced by transmission and contact rate parameters. These parameters decrease by applying lock down, using a sanitizer, washing hands, and keeping social distance and awareness campaign. Moreover, η is calculated from replaced by the recovery term, and we know that recovery terms increase through strong immunity system of the body and good treatment. Decrease of γ and increase of η strick inequality (15). The strictness of (15) leads to control of disease COVID-19.
A controlling strategy is optimal if it decreases the value of and strick inequality (15).
Finally, we use relations (5) in and graph using the COVID-19 data of USA from first April to 19th July, 2020 taken from [34]. We also calculate the values of γ, λ, and η to verify Theorem 4.
The following table shows the values of γ, λ, and η for April, May, June, and July, 2020, in case of COVID-19 data of USA [34].
In Table 1 the parameter γ is calculated from
Similarly, the parameters λ and η are calculated from
and
Table 1.
Values of the stochastic parameters calculated from data of USA during COVID-19 for four months
| Month | γ | λ | η |
|---|---|---|---|
| April | 2.794370191 | 8.161600786 | 83.71889805 |
| May | 1.844039647 | 2.211604232 | 348.0864949 |
| June | 2.820176949 | 0.48994873 | 318.1660605 |
| July | 11.76093832 | 0.325367682 | 706.6299277 |
From the Table it is clear that the condition of Theorem 4 is not satisfied for every month, and therefore the value of increases (see the data of the months of April, May, June, and July 2020 in [34]).
Conclusion
In this work, we analyze a stochastic model on the spread and control of coronavirus. First, we considered an SIR model to understand the spread and control of current infectious disease, then investigated the stability of the proposed model. Further, we investigated an inequality managing of which leads to decrease of the number of infected individuals. After this, we presented the nonnegative unique strong solution of the stochastic model and showed an inequality among some parameters of the model through martingale theory, managing of which leads to minimizing the number of COVID-19 patience. We found that decreasing the number of susceptible individuals, transmission, and contact parameters and increasing the recover parameter strick the inequalities. We found that applying lock down, use of sanitizer, washing hands, keeping social distance and awareness campaign decrease the value of susceptible individuals, transmission, and contact parameters, whereas a strong immunity system of the body and good treatment increase the value of recover parameter. We concluded that managing these terms leads to dropping the number of COVID-19 patients. The results are verified through the data of USA and shown in Fig. 3, and the managing parameters are calculated in the Table 1. Analysis of the results leads to understanding the flow and control of coronavirus in a clear way.
Figure 3.
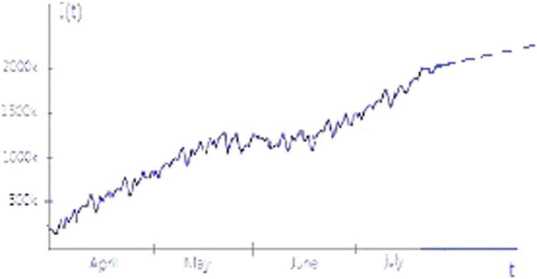
The sketch of Infected class of coronavirus
Acknowledgments
Acknowledgements
The authors thank to the editor and anonymous referees for useful comments, which led to a great improvement of the paper and also thank to the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia for funded this project, under grant no. (FP-73-42).
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the paper cited therein.
Authors’ contributions
Authors have equally contributed in preparing this manuscript. All authors read and approved the final manuscript.
Funding
This project is funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. (FP-73-42).
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Contributor Information
Sultan Hussain, Email: tausef775650@yahoo.co.in.
Anwar Zeb, Email: anwar@cuiatd.edu.pk.
Akhter Rasheed, Email: akhter@cuiatd.edu.pk.
Tareq Saeed, Email: tsalmalki@kau.edu.sa.
References
- 1.Pang J., Cui J.A., Zhou X. Dynamical behavior of a Hepatitis B virus transmission model with vaccination. J. Theor. Biol. 2010;265(4):572–578. doi: 10.1016/j.jtbi.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Zou L., Zhang W., Ruan S. Modeling the transmission dynamics and control of Hepatitis B virus in China. J. Theor. Biol. 2010;262(2):330–338. doi: 10.1016/j.jtbi.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Chen T.-M., Rui J., Wang Q.-P., Zhao Z.-Y., Cui J.-A., Yin L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty. 2020;9:24. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X., Wang X., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person to person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S., Musa S.S., et al. Estimating the unreported number of novel coronavirus (2019-nCoV) cases in China in the first half of January 2020: a data-driven modelling analysis of the early outbreak. J. Clin. Med. 2020;9(2):338. doi: 10.3390/jcm9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivorra B., Ferrández M.R., Vela-Pérez M., Ramos A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) considering its particular characteristics. The case of China. Commun. Nonlinear Sci. Numer. Simul. 2020;88:105303. doi: 10.1016/j.cnsns.2020.105303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sameni, R.: Mathematical modeling of epidemic diseases; A case study of the COVID-19 coronavirus. March 2020. Draft version
- 12.Kochańczyk M., Grabowski F., Lipniacki T. Dynamics of COVID-19 pandemic at constant and time-dependent contact rates. Math. Model. Nat. Phenom. 2020;15:28. doi: 10.1051/mmnp/2020011. [DOI] [Google Scholar]
- 13.Chen S., Yang J., Yang W., Barnighausen T. COVID-19 control in China during mass population movements at new year. Lancet. 2020;395:764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivorra B., Martínez-López B., Sánchez-Vizcaíno J.M., Ramos A.M. Mathematical formulation and validation of the Be-FAST model for classical swine fever virus spread between and within farms. Ann. Oper. Res. 2014;219(1):25–47. doi: 10.1007/s10479-012-1257-4. [DOI] [Google Scholar]
- 15.Kucharski A.J., Russell T.W., et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lekone P., Finkenstädt B. Statistical inference in a stochastic epidemic SEIR model with control intervention: Ebola as a case study. Biometrics. 2006;62(4):1170–1177. doi: 10.1111/j.1541-0420.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27(2):02. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S.A., Baloch M., Ahmed N., Ali A.A., Ayman I. The outbreak of Coronavirus Disease 2019 (COVID-19)—an emerging global health threat. J. Infect. Public Health. 2020;13:644–646. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z. A novel covid-19 mathematical model with fractional derivatives: singular and nonsingular kernels. Chaos Solitons Fractals. 2020;139:110060. doi: 10.1016/j.chaos.2020.110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeb, A., Alzahrani, E., Erturk, V.S., Zaman, G.: Mathematical model for Coronavirus Disease 2019 (COVID-19) containing isolation class. BioMed Res. Int. 10.1155/2020/3452402 [DOI] [PMC free article] [PubMed]
- 21.Yousaf M., Muhammad S.Z., Muhammad R.S., Shah H.K. Statistical analysis of forecasting COVID-19 for upcoming month in Pakistan. Chaos Solitons Fractals. 2020;138:109926. doi: 10.1016/j.chaos.2020.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K., Abdeljawad T., Mahariq I., Jarad F. Qualitative analysis of a mathematical model in the time of COVID-19. BioMed Res. Int. 2020;2020:5098598. doi: 10.1155/2020/5098598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdon A. Blind in a commutative world: simple illustrations with functions and chaotic attractors. Chaos Solitons Fractals. 2018;114:347–363. doi: 10.1016/j.chaos.2018.07.022. [DOI] [Google Scholar]
- 24.Abdon A. Fractional discretization: The African’s tortoise walk. Chaos Solitons Fractals. 2020;130:109399. doi: 10.1016/j.chaos.2019.109399. [DOI] [Google Scholar]
- 25.Abdon A. Fractal-fractional differentiation and integration: connecting fractal calculus and fractional calculus to predict complex system. Chaos Solitons Fractals. 2017;102:396–406. doi: 10.1016/j.chaos.2017.04.027. [DOI] [Google Scholar]
- 26.Ghanbari B., Atangana A. Some new edge detecting techniques based on fractional derivatives with non-local and non-singular kernals. Adv. Differ. Equ. 2020;2020:435. doi: 10.1186/s13662-020-02890-9. [DOI] [Google Scholar]
- 27.Lamberton D., Lapeyre B. Stochastic Calculus Applied to Finance. UK: Chapman & Hall; 1997. [Google Scholar]
- 28.Arnold L. Stochastic Differential Equations: Theory and Applications. New York: Wiley; 1972. [Google Scholar]
- 29.Asmussen S., Albrecher H. Ruin Probabilities. Singapore: World Scientific; 2010. [Google Scholar]
- 30.Shreve S.E. Stochastic Calculus for Finance Vol. II. New York: Springer; 2004. [Google Scholar]
- 31.Karatzas I., Shreve S.E. Brownian Motion and Stochastic Calculus. New York: Springer; 1991. [Google Scholar]
- 32.Mao X. Stochastic Differential Equations and Applications. 2. Chichester: Horward; 1997. [Google Scholar]
- 33.Mao X., Marion G., Renshaw E. Environmental Brownian noise suppresses explosions in population dynamics. Stoch. Process. Appl. 2002;97(1):95–110. doi: 10.1016/S0304-4149(01)00126-0. [DOI] [Google Scholar]
- 34.https://www.worldometers.info/coronavirus/country/us/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the paper cited therein.


