In spring 2020 paediatricians working across Europe and the USA [1] alerted colleagues to clusters of previously healthy children presenting with unremitting fever, multisystem inflammation and pancarditis. This syndrome, initially termed Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 infection (PIMS-TS), or by its US variation Multisystem Inflammatory Syndrome in Children (MIS-C; Table 1), appears to be a rare complication of (largely) asymptomatic SARS-CoV-2 infection in children [8]. However, the overlap with other paediatric inflammatory syndromes such as Kawasaki disease (KD) and optimum treatments remain unknown. We provide a narrative overview of PIMS-TS and highlight important knowledge gaps.
Table 1.
Abbreviate case definitions from the UK Royal College of Paediatrics and Child Health (RCPCH) for PIMS-TS, the US Centre for Disease Control and Prevention (CDC) for MIS-C, and the World Health Organization (WHO) for multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19
| Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS; RCPCH 2020) | Multisystem Inflammatory Syndrome in Children (MIS-C; CDC 2020) | Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 (WHO 2020) |
|---|---|---|
| A child presenting with persistent fever, inflammation and evidence of single or multi-organ dysfunction | An individual aged < 21 years presenting with fever, inflammation, and severe illness requiring hospitalization, with multisystem (> 2) organ involvement | Children and adolescents 0–19 years of age with fever > 3 days |
| This may include children meeting full or partial criteria for Kawasaki disease | No alternative plausible diagnoses |
AND two of the following: - Rash or bilateral non-purulent conjunctivitis or muco-cutaneous inflammation signs . Hypotension or shock . Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities . Evidence of coagulopathy . Acute gastrointestinal problems |
| Exclusion of any other microbial cause | Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms |
AND Elevated markers of inflammation |
| SARS-CoV-2 PCR testing may be positive or negative | Some individuals may fulfil full or partial criteria for Kawasaki disease but should be reported if they meet the case definition for MIS-C |
AND No other obvious microbial cause of inflammation |
| Consider MIS-C in any paediatric death with evidence of SARS-CoV-2 infection |
AND Evidence of COVID-19, or likely contact with patients with COVID-19 |
What is PIMS-TS?
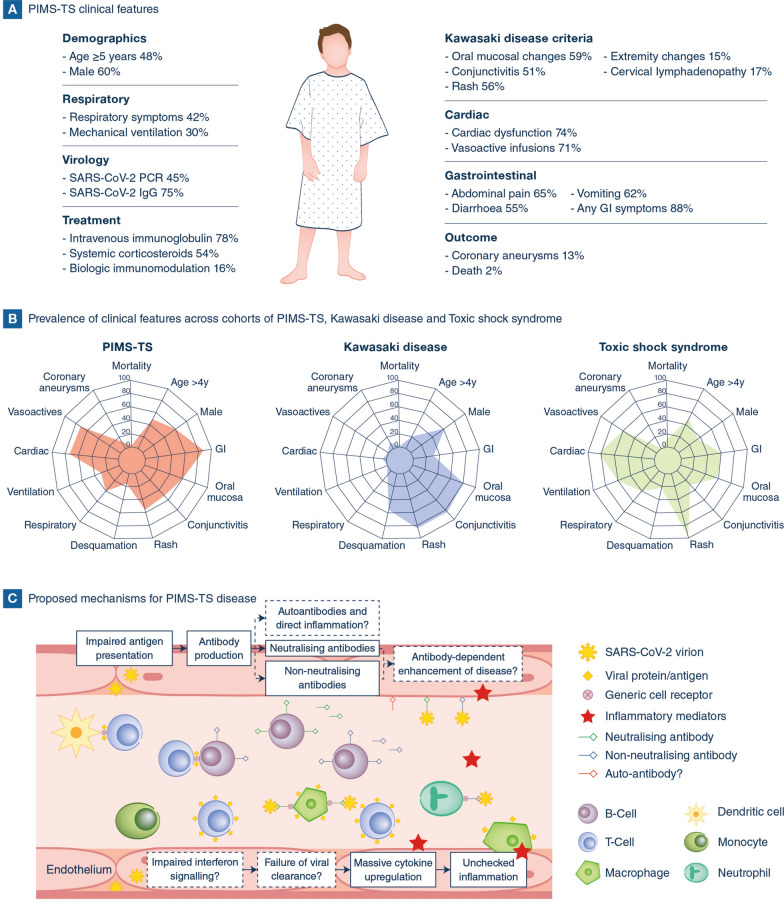
Cardinal signs of PIMS-TS include fever, stigmata of inflammation (rash, conjunctivitis, and oral mucosal changes), gastrointestinal symptoms, and cardiac dysfunction (Fig. 1A). These features are accompanied by laboratory evidence of significant inflammation: neutrophilia, lymphopaenia, elevated serum CRP and ferritin concentrations; hypercoagulable state; and non-ST elevation pancarditis. Echocardiograms typically reveal left ventricular dysfunction, and hyperechoic coronary arteries. Complications of PIMS-TS include systemic thrombosis [1] and coronary artery aneurysms in approximately 13% of children in published cohorts [4]. Nearly 2% of affected children have died [4].
Fig. 1 .
A PIMS-TS clinical features (mean value from published cohorts, August 2020). B Prevalence of clinical features across cohorts of PIMS-TS, Kawasaki disease and toxic shock syndrome. “Cardiac” and “Respiratory” refer to signs and symptoms of respective organ system involvement, whilst “Ventilation” and “Vasoactives” refer to types of organ support. C Proposed mechanisms for PIMS-TS disease, including altered interferon signalling, failure to clear SARS-CoV-2 and resultant cytokine excess leading to excess inflammation; or, antibody-mediated disease including potential autoantibodies or antibody-dependent enhancement of disease by enhanced viral invasion of host cells
What are the differential diagnoses of PIMS-TS?
Children with PIMS-TS were initially treated as KD or presumed toxic shock syndrome (TSS) with broad spectrum antibiotics and intravenous immunoglobulins [1, 2]. KD, TSS, occult infection, acute abdominal conditions, and rare inflammatory conditions remain important differentials (Fig. 1 B). However, there are now clinical, microbiological and immunological data describing PIMS-TS as a novel immunopathogenic illness [5, 9, 10]. Similarities between PIMS-TS and KD include ubiquity of fever and high prevalence of oral mucositis, conjunctivitis and rash. In contrast, children with PIMS-TS are often older than 5 years of age (48%), compared with children with KD (18% > five years) [11, 12], and gastrointestinal symptoms, cardiac dysfunction and need for vasoactive infusions are considerably more prevalent. A rare subset of KD patients present with shock syndrome, but these children typically have lower ferritin, troponin and less disordered coagulation than children with PIMS-TS [5]. Approximately 45% of children with PIMS-TS have a positive PCR test for SARS-CoV-2 infection. In addition, the high proportion (75%) with class-switched antibody to viral antigens, indicate that most, if not all, cases of PIMS-TS are a result of prior, or uncleared, infection with SARS-CoV-2 [9]. However, with no accurate test for the diagnosis of PIMS-TS, vigilance for alternative diagnoses must be maintained.
How is PIMS-TS treated?
In the midst of these unknowns, children presenting with fever and multisystem inflammation should be managed with parallel strategies, including careful administration of crystalloid fluids and early administration of antibiotics for TSS (typically cephalosporins, clindamycin and vancomycin) as indicated. Initial and serial laboratory investigations should include full blood count, biochemical profile (including ferritin, triglycerides, troponin, creatine kinase and proBNP), inflammatory markers (CRP, procalcitonin), coagulation profile (including d-dimers and fibrinogen), blood for culture, nasopharyngeal sampling for viral pathogens and serum saved for serological testing. In our experience, immediate and serial assessment of electro- and echocardiograms are required given the breadth of cardiac involvement, variable trajectory of disease and exquisite sensitivity to volume loading in some patients. Cardiac dysfunction requires vasoactive support in the majority of children, with mechanical ventilation instituted in approximately one third of children, and a small number requiring mechanical circulatory support [13].
The multisystem nature of PIMS-TS has invited a multidisciplinary approach to management, including intensivists, infectious disease paediatricians, rheumatologists, cardiologists and haematologists, surgeons (where acute abdominal conditions are suspected) and specialist nurses. Prompt administration of pooled intravenous immunoglobulins is advocated by many institutions [13], and is an evidence-based therapy for KD [14] and TSS [15]. As an epicentre for the UK epidemic of PIMS-TS, we also advocate transfer to a specialist paediatric cardiac centre capable of providing mechanical circulatory support. Following clinical exclusion of TSS, and dependent upon the trajectory of inflammatory markers, systemic corticosteroids, additional intravenous immunoglobulins, and/or immunomodulation with biologics (most frequently interleukin-6 blockade) have been used. Aspirin (as anti-inflammatory or prophylaxis of thrombosis) or heparins are administered to children with acute PIMS-TS. Convalescent follow-up may involve cardiac computed tomography or magnetic resonance imaging if concerns of coronary artery aneurysms or ventricular dysfunction persist [13].
Sharing of data across institutions will be crucial to understanding the best treatment and outcomes for this novel disease [13]. Globally, the Best Available Treatment Study for PIMS-TS (ISRCTN69546370) will detail retrospectively the trajectory of disease following immunomodulation in PIMS-TS. Prospectively, the UK Recovery Trial (ISRCTN50189673) has been extended to include immunoglobulins, methylprednisolone or tocilizumab for children with PIMS-TS.
What is the pathobiology of PIMS-TS?
Delayed clearance of the SARS-CoV-2 leading to unchecked inflammation is a possible mechanism of disease for PIMS-TS (Fig. 1C) [8]. Certainly, children with PIMS-TS have greatly elevated serum concentrations of pro-inflammatory interleukins (IL-1 beta, IL-17, IL-6 and IL-8), accompanied by activation of neutrophils and monocytes [9]. However, we have few data on the role of anti-viral interferons (alpha, beta and lambda) in viral clearance. Alternatively, antibody-dependent enhancement (ADE), with invasion of host cells augmented by antibody, serum proteases, or auto-antibody mediated disease [10], have been suggested. However, PIMS-TS appears to affect only children and young adults, and ADE would be expected in older adults (with more prevalent prior exposure to other coronaviruses). Treatment of adult patients with COVID-19 convalescent plasma has also not been associated with hyperinflammation. The broad effects of intravenous immunoglobulins via Fcγ-receptors, scavenging of inflammatory mediators, and attenuation of lymphocyte apoptosis preclude inferences for the pathobiology of PIMS-TS.
Into the unknown; again
Important questions remain [8]: How to tease out environmental, genetic and acquired risk factors for PIMS-TS? What are the long-term outcomes of children with PIMS-TS? Is there a cohort of unrecognised PIMS-TS cases that may suffer complications of coronary aneurysms in future? What effect will re-opening of schools have on the epidemiology of PIMS-TS? And, if PIMS-TS is associated with impaired interferon signalling, might adjunctive interferon therapy with interferons be useful? Vaccinologists will be carefully monitoring SARS-CoV-2 trial data for signals of hyperinflammation as a result of ADE. Meanwhile, and with resurgent SARS-CoV-2 transmission, paediatricians and intensivists might be wise to prepare for further clusters of PIMS-TS.
Funding
This research was supported by the National Institute for Health Research, UK.
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. 2020 doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Inf Dis. 2020 doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020 doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 10.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Cooke E, Barrios TA, Sanchez-Manubens J, et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development (2011–2016): KAWA-RACE study group. PLoS ONE. 2019;14:e0215665. doi: 10.1371/journal.pone.0215665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacke CE, Breunis WB, Pereira RR, Breur JM, Kuipers IM, Kuijpers TW. Five years of Kawasaki disease in the Netherlands: a national surveillance study. Pediatr Infect Dis J. 2014;33:793–797. doi: 10.1097/INF.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 13.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2020 doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 15.Parks T, Wilson C, Curtis N, Norrby-Teglund A, Sriskandan S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:1434–1436. doi: 10.1093/cid/ciy401. [DOI] [PMC free article] [PubMed] [Google Scholar]